Abstract
In this investigation, two cell-permeable synthetic analogs of cAMP, dibutyryl-cAMP (db-cAMP) and 8-bromo-cAMP, which are widely used to elevate intracellular cAMP levels under experimental conditions, were investigated for their ability to dose-dependently improve histological and functional outcomes following continuous delivery in two models of incomplete spinal cord injury (SCI). The cAMP analogs were delivered via osmotic minipumps at 1–250 mM through an indwelling cortical cannula or by intrathecal infusion for up to 4 weeks after either a T8 unilateral over-hemisection or a C2-3 dorsolateral quadrant lesion, respectively. In both SCI models, continuous db-cAMP delivery was associated with histopathological changes that included sporadic micro-hemorrhage formation and cavitation, enhanced macrophage infiltration and tissue damage at regions beyond the immediate application site; no deleterious or beneficial effect of agent delivery was observed at the spinal injury site. Furthermore, these changes were accompanied by pronounced behavioral deficits that included an absence of progressive locomotor recovery, increased extensor tone, paralysis, and sensory abnormalities. These deleterious effects were not observed in saline-treated animals, in animals in which the db-cAMP dose did not exceed 1 mM, or in those animals that received a high dose (250 mM) of the alternative cAMP analog, 8-bromo-cAMP. These results demonstrate that, for continuous intraparenchymal or intrathecal administration of cAMP analogs for the study of biological or therapeutic effects within the central nervous system (CNS), consideration of the effective concentration applied as well as the potential toxicity of chemical moieties on the parent molecule and/or their activity needs to be taken into account.
Key Words: dibutyryl cAMP, locomotor recovery, paralysis, regeneration, spinal cord injury
Introduction
Few therapeutic options are available currently for spinal cord injury (SCI) in humans. Although approved interventions such as decompression surgery, the steroid methylprednisolone, and rehabilitation regimens may be of some clinical benefit, treatments that provide significant functional restoration after SCI are lacking (Sipski and Pearse, 2006). A number of axon growth inhibitors have been identified in the adult mammalian spinal cord; only by modifying or providing a permissive environment can central neurons regenerate axons after injury (Silver and Miller, 2006). In addition to targeting these extrinsic inhibitors of axon growth, another promising strategy has been to overcome growth failure by altering the intrinsic signaling programs for axon regeneration in neurons through the elevation of intracellular messengers, such as cyclic adenosine monophosphate (cAMP) (Pearse, 2004). Exogenously applied cAMP can enable mature neurons to extend axons on growth inhibitory myelin (Cai et al., 2001; Qiu et al., 2002). Presentation of cAMP at the growth cone can also influence growth cone turning behavior (Song et al., 1997) as cyclic nucleotide levels within the growth cone are important for their guidance towards chemo-attractive cues (Ming et al., 1997). Furthermore, increasing cAMP enhances axonal transport (Nuydens et al., 1993), increases intra-axonal Ca2+ (Kilmer and Carlsen, 1987), reduces scar formation (Hermanns et al., 2001), and potentiates the action of trophic factors (Cui et al., 2003), all of which should increase an axon's regenerative ability. One widely employed approach to increase intracellular cAMP levels has been the application of cell-permeable and stable, synthetic analogs, in particular, dibutyryl-cAMP (db-cAMP; Neumann et al., 2002; Qiu et al., 2002). Recently, we reported that, in rats with a moderate contusion injury to the spinal cord, the elevation of cAMP in combination with Schwann cell implantation could produce significant axonal sparing, promote myelination, and enhance regeneration of serotonergic fibers into and beyond the grafts, in parallel with significantly improved locomotion (Pearse et al., 2004). The cAMP elevation was achieved by the dual-use, first, of an agent able to inhibit cAMP hydrolysis, the phosphodiesterase inhibitor, Rolipram, given continuously, and then additionally, a one-time spinal cord application of db-cAMP. Although Rolipram alone was effective in attenuating an observed injury-induced cAMP decrease, only the use of both agents in the described fashion produced an elevation of cAMP above that of uninjured controls, resulting in supraspinal axon growth beyond the lesion as well as persistent functional improvements. These and other exciting findings (Nikulina et al., 2004; Lu et al., 2004; Kajana and Goshgarian, 2008; Whitaker et al., 2008) have indicated that elevating or maintaining cAMP within the injured CNS could be a promising intervention for treating human SCI.
In the present study, we investigated whether the continuous administration of different doses of the commonly employed cAMP analog, db-cAMP, when given alone for up to 2 weeks following incomplete SCI, could alter the extent of ensuing tissue damage and locomotor recovery. The ability of a single agent to provide comparable benefits to the previously employed dual agent paradigm (Pearse et al., 2004) would provide a more amenable therapeutic strategy. Therefore two independent experiments were performed, one examining the effects of subdural cAMP analog infusion at a spinal lesion site (C3), and the other examining the effects of its infusion to the cell body of thoracically lesioned corticospinal axons.
Methods
Experimental plan
The studies described below were initially performed as two independent investigations that were run concurrently to investigate the therapeutic efficacy of continuous cAMP analog delivery after SCI, as opposed to a previously used and more complicated dual-agent paradigm (Pearse et al., 2004). The first experiment sought to examine if cAMP elevation through continuous db-cAMP delivery, at a pre-determined, single concentration of 250 mM to neuronal cell bodies in the motor cortex, could promote corticospinal axon growth across the injured spinal cord following a T8 over-hemisection. The second experiment, using the same dose of db-cAMP but given instead at the lesion site via continuous intrathecal administration, was initiated to identify if cAMP elevation could promote corticospinal tract (CST) axon growth and improve functional recovery following a dorsal quadrant lesion of the C3-4 cervical spinal cord. Upon observation of serious adverse effects related to the treatment in the second experiment, resulting in its premature termination, further investigations were initiated to test lower cAMP analog dosing (1 and 50 mM) as well as an alternative analog bearing a different sub-group, 8-bromo-cAMP, to determine if the effects observed were related to the dose, elevated cAMP levels and/or the analog's reactive moiety. As the highest dose of the cAMP analog was expected to exhibit the greatest non-cAMP-specific effects, i.e., those related to the butyryl reactive group on db-cAMP, 8-bromo-cAMP was used at a single dose of 250 mM.
Animals and experimental groups
All experiments were approved by the University of Alberta Animal Care Committee and conducted in accordance with rules set by the Canadian Council on Animal Care. Adult female Lewis rats (n = 56; 180–200 g; Charles River, Wilmington, MA) were used in two experimental paradigms. In the first experiment, we examined the effects of immediate db-cAMP (1, 10, 50, and 250 mM), 8-bromo cAMP (250 mM), or saline application sub-durally for up to 12 days to a spinal cord lesion at the cervical level (n = 42, 7 per group). In another experiment, saline or db-cAMP (250 mM) were infused to the hindlimb area of the motor cortex for 4 weeks following thoracic spinal cord injury (n = 14, 7 per group). All surgical procedures were performed under Hypnorm (Janssen Pharmaceutics, Beerse, Belgium; 120 μL per 200 g body weight) and Midazolam (Sabex, Boucherville, QC, Canada; 0.75 mg in 150 μL/200 g body weight, 750 μL total volume diluted with H2O). Eye lubricant (Tears Naturale; Alcon Canada, Inc, Mississauga, ON, Canada) was applied to protect the eyes from dehydration.
Spinal cord injury and cAMP analog administration
For cervical lesions, rats were first mounted into a stereotaxic frame and their head tilted so that the spinal cord between the C2-3 cervical vertebrae could be exposed without performing a laminectomy. This was followed by a dorsolateral quadrant lesion of the right side using a customized micro-blade (Girgis et al., 2007). Following the lesion, the dorsal back musculature and the skin were sutured. During the same operation as the lesion procedure, the animals were randomly assigned into the six treatment groups. The animals (n = 7 per group) received their assigned treatment (saline, dibutyryl cAMP dissolved in saline at 1, 10, 50, and 250 mM, or 8-bromo cAMP 250 mM) by subcutaneous implantation of an osmotic mini-pump (Alzet, model 2002, Durect Corp., Cupertino, CA), attached to a fine (32-gauge) intrathecal catheter (Recathco, LLC, Allison Park, PA; part no. 0046), which was directed sub-durally to a region immediately caudal and anterior to the lesion site so as to provide a continuous supply of the agent as described previously (Fouad et al., 2005). Due to the early termination of many of the animals involved in this part of the study, catheters remained in place until after perfusion. For thoracic lesions, rats received a dorsal over-hemisection lesion at Th8 (Vavrek et al., 2006). During the same surgery, rats were randomly divided into two groups: the control (saline) treated group and the (250 mM) db-cAMP-treated group (n = 7 per group). Treatments were administered by subcutaneous implantation of an osmotic mini-pump attached to a brain infusion kit (Alzet, model 2002) as described elsewhere (Vavrek et al., 2006). The brain infusion cannula was implanted 1.5 mm lateral and 1.5 mm caudal from bregma in the left (unlesioned) motor cortex for cortical infusion. At 2 weeks, upon the completion of cAMP delivery, animals were anesthetized as previously described, the cannula and pump were removed, and the animal then closed.
Post-operative care and behavioral analysis
After surgery all animals received post-operative care as previously described (Girgis et al., 2007). The muscle and skin overlaying the injury site was sewn in layers. Directly following surgery, rats were placed on a heating pad until awake and received 0.03 mg/kg of the analgesic buprenorphine and 4 ml of saline. This was repeated following 8 h and repeated daily up to 72 h if the rats showed signs of pain or dehydration. For the first week post-surgery, health assessment was performed three times a day, thereupon twice a day. Animals were examined for signs of pain, changes in muscle tone of the injured forelimbs or paralysis. Rats with thoracic lesions were tested weekly for 4 weeks using the Basso, Beattie, and Bresnahan (BBB) open field locomotor score (Basso et al., 1996). The BBB scores given represent an average score from the evaluation of both hindlimbs. Due to severe health concerns in the cervically injured rats receiving 250 mM db-cAMP, animals were euthanized as early as 5 days following surgery. The experiment was terminated for all remaining rats at 12 days following lesion and agent administration.
Tissue preparation and histological quantification
To allow histological processing of spinal cord and brain tissues, rats were deeply anesthetized with an overdose of Pentobarbital and perfused transcardially as described previously (Barakat et al., 2005). The dura and the intrathecal catheters were removed following perfusion but prior to tissue sectioning. Their removal was performed under a surgical microscope and the process did not produce tissue damage as the catheters generally do not attach to spinal tissue. Dissected brain and spinal cord samples were post-fixed in formalin and cryoprotected in 30% sucrose before being embedded in Tissue Tek for cryosectioning. For rats receiving a Th8 lesion and cortical treatment, the entire cortex was transverse sectioned at 30 μm (every second section was saved) and then stained with 0.1% cresyl violet to permit visualization of cortical architecture (Girgis et al., 2007).
Immunohistochemical identification of macrophages was achieved by using overnight incubation at 4°C with a mouse anti-rat CD68 primary antibody (1:50; Serotec, Raleigh, NC) and then a secondary goat anti-mouse IgG conjugated to Fluorescein (Sigma, St. Louis, MO) using an earlier described protocol (Pearse et al., 2007). To quantify the density of macrophage infiltration within distinct regions of tissue injury at the cortical infusion site, fluorescent microscopy (Leica DM6000) was used. Pictures from three different areas were obtained (50 × magnification) from three saline and four db-cAMP-treated animals. Analysis was performed using Adobe Photoshop 7 software (Adobe Systems, San Jose, CA). For each image, three areas of the cortex were selected: one from the center of the lesion, one from the perilesional region, and one from the contralateral cortex to control for background fluorescence. These regions were analyzed to obtain levels of fluorescent intensity on the green channel by use of the Photoshop histogram function, a previously reported method for this type of measurement (Emoto et al., 2001). Cresyl violet stained cortical sections were analyzed using light microscopy. To estimate the extent of cortical tissue damage, the number of cortical cross sections in which tissue damage was evident was multiplied by section thickness (30 μm). For visualization of the extent of the macrophage tissue infiltration, fluorescence microscopy was used to detect CD68-fluorescein positive cells. Once images were obtained from infiltrated regions, density measurements were performed with Scion Image (NIH).
For spinal cord analysis, tissue from rats receiving direct spinal cord treatment, C3-4 cord (spanning the lesion site and caudal delivery region) was transverse sectioned at 25 μm. The sections were stained with 0.1% cresyl violet and evaluated for the extent of tissue damage as described above.
Statistical analysis
Statistical analysis was performed using a Mann–Whitney U-Test (GraphPad Prism, San Diego, CA) unless otherwise noted in the results. If variances between two groups were significantly (p < 0.05) different, a Welch's correction was performed. All data are presented as means ± SE.
Results
Cortical delivery of cAMP analogs
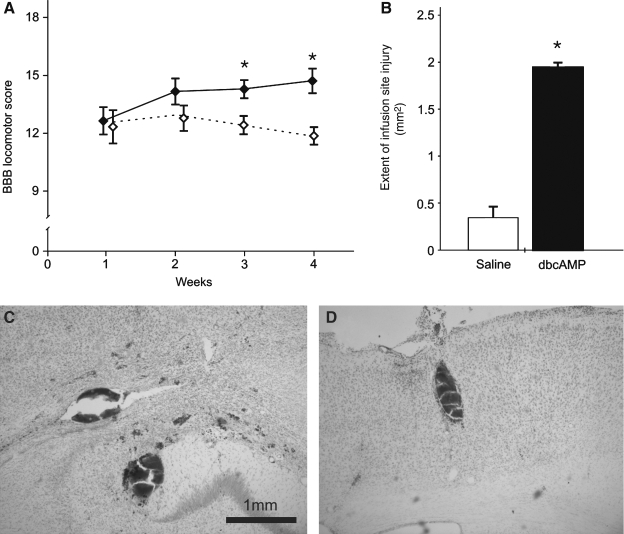
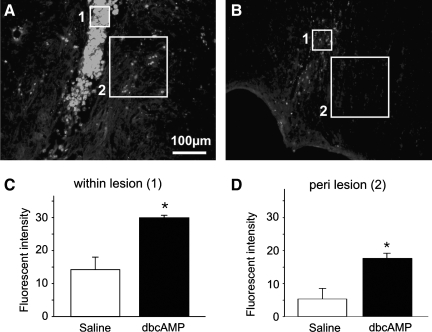
In animals receiving cortical application of db-cAMP or saline for a period of 2 weeks following a spinal lesion at the thoracic level, no difference in the motor performance between the saline and db-cAMP-treated groups was initially observed (average ± SE: 12.8 ± 0.8 versus 12.3 ± 0.9, respectively; Fig. 1A). With continued time post-injury, db-cAMP-treated rats exhibited no additional functional recovery compared to those treated with saline in which motor performance significantly improved up to 4 weeks post-injury (11.8 ± 0.2 and 14.6 ± 0.5; p < 0.05). At the site of cannula placement, tissue damage produced by the implantation of the brain infusion kit was found in both groups of animals. When the anterior-posterior lesion extent was quantitatively measured however, lesion size was significantly larger in rats that received db-cAMP as compared to those treated with saline (1.94 ± 0.12 mm2 versus 0.35 ± 0.05 mm2, respectively; 400% increase; p < 0.01; Fig. 1B). Furthermore, the db-cAMP-treated animals only exhibited extensive tissue damage and micro-hemorrhages that went beyond the immediate cannula implantation site (Fig. 1C,D). This included regions beyond the motor cortex such as the corpus callosum, the hippocampus and, in one animal receiving a 250 mM dose, damage was observed within the thalamus. Immunohistochemical detection of macrophage infiltration using CD68 immunostaining revealed significantly greater cortical tissue penetration by infiltrating immune cells had occurred in db-cAMP-infused rats (Fig. 2A,B). When compared to saline infused controls, CD68 fluorescent intensity in and around the cannula site was significantly increased (controls, 14.2 ± 3.7 arbitrary units [a.u.] versus 250 mM db-cAMP, 29.7 ± 0.8 a.u. within the implantation site, p < 0.05; controls, 5.3 ± 3.3 a.u. versus 250 mM db-cAMP, 17.6 ± 1.7 a.u. within the perilesional region, p < 0.05; Fig. 2C,D).
FIG. 1.
Effects of cortical dibutyryl-cAMP (db-cAMP; 50 mM) application in spinal cord injured rats. One week following a thoracic over-hemisection spinal cord injury (SCI), the treated and the control groups exhibited comparative locomotor performance in the open field (as measured using the Basso, Beattie, and Bresnahan [BBB] score). At 3 and 4 weeks, however, the control animals (diamond, filled) showed a progressive improvement in BBB scores, while the db-cAMP-treated animals (diamond, open) did not (A). Examination of the lesion size produced by the brain infusion catheter revealed that the extent of the lesion in db-cAMP infused rats was significantly increased (B). Data is expressed as the mean ± SEM; asterisks indicate p < 0.05. Furthermore, micro-hemorrhages were observed for up to 2 mm away from the infusion site (C,D).
FIG. 2.
The administration of dibutyryl-cAMP (db-cAMP) increases macrophage infiltration at the site of analog application. A representative image of CD68 staining, to identify macrophages, shows increased numbers at the infusion site within the cortex of animals receiving db-cAMP (A) as compared to saline controls (B). Measurement of fluorescent intensity within the site of cannula implantation (C; area 1 in A and B) and around (D; perilesional region; area 2) demonstrates that there is a significant increase in CD68 immunoreactive intensity following db-cAMP application. Data is expressed as the mean ± SEM; asterisks indicate p < 0.05.
Spinal cord delivery of cAMP analogs
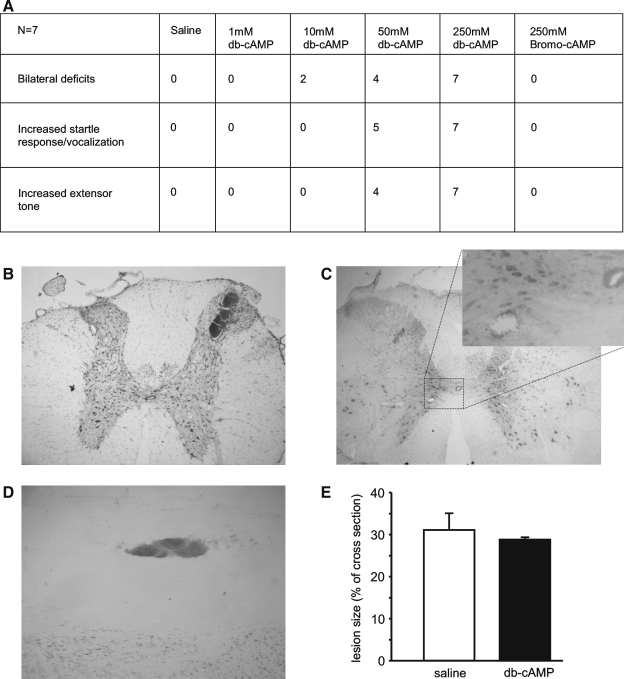
For spinal cord application, initial experiments employed subdural spinal cord administration of four db-cAMP concentrations (1, 10, 50, and 250 mM) versus a saline (vehicle) control in animals receiving unilateral C3 upper quadrant lesions. In saline-infused controls, a characteristic unilateral forelimb deficit was observed for up to 12 days following surgery (Girgis et al., 2007); there was no evidence of bilateral deficits (paralysis in wrist, elbow, and shoulder), altered sensory responses such as an increased startle response or vocalization to ambulation or extensive forelimb paralysis as exhibited by an increased extensor tone in the forelimbs (Fig. 3A). These features, however, were present in a dose-dependent fashion, absent at 1 mM but strongly evident at 250 mM, in those injured animals which received db-cAMP. All animals within the 250 mM treatment cohort showed bilateral deficits, increased startle response and extensor tone in the forelimbs. These deficits were so severe that, although there was no morbidity in the studies, the animals had to be euthanatized prior to the pre-determined experimental endpoint of study. To determine whether the observed functional deficits were directly related to an increased intracellular concentration of cAMP, we compared another treatment group that received 250 mM 8-bromo-cAMP, a synthetic analog in which the reactive and cell permeable side group is a bromo rather than a dibutyryl. Unlike the 250 mM db-cAMP-treated animals, no deficits were observed with 250 mM 8-bromo-cAMP infusion, suggesting that the deleterious effects of db-cAMP were not due to elevated levels of cAMP but rather the dose-dependent toxicity of the butyryl chemical group. Similar to cortical application, spinal cord delivery of db-cAMP at the higher concentrations, but not 8-bromo-cAMP, induced micro-hemorrhage formation and triggered tissue damage, evident particularly within gray (Fig. 3B,C) but also white matter. In the most pronounced of cases, up to seven regions of micro-hemorrhage formation could be identified in a single cross-section of spinal cord tissue from a 250 mM db-cAMP-treated animal (Fig. 3D). No evidence of comparable tissue damage outside of the immediate lesion site was observed in either saline or 8-bromo-cAMP-treated animals.
FIG. 3.
Effects of intrathecal cAMP analog application in spinal cord injured rats. Following unilateral cervical lesions, dibutyryl-cAMP (db-cAMP) application at concentrations in excess of 1 mM produced bilateral functional deficits in some of the animals and at higher doses (>10 mM), increased startle responses and extensor tone (A). These effects were not seen in saline controls, at lower db-cAMP doses (1 mM), or with a high dose (250 mM) of an alternative cAMP analog, 8-bromo cAMP. Similar to cortical application, tissue damage and micro-hemorrhages beyond the application site of db-cAMP (>1 mM) were found (B–D). The size of the original spinal lesion size was not affected by the application of db-cAMP (E).
Analysis of the primary spinal cord injury site (calculated as a percentage of the entire spinal cord cross-sectional area), revealed that the delivery of db-cAMP (250 mM, 28.8 ± 1.1%) neither reduced nor exacerbated the extent of primary lesion tissue damage over that of saline-treated controls (31.1 ±4.3%, p > 0.05; Fig. 3D,E).
Discussion
In this study, we show that the continuous application of greater than 1 millimolar concentrations of db-cAMP by indwelling cortical cannula or a 250 millimolar concentration by intrathecal spinal cord infusion after SCI induces micro-hemorrhage formation and enhances macrophage infiltration within regions at and nearby the site of delivery; however, the extent of tissue damage at the lesion site is unaffected. Histopathological changes outside the primary lesion following db-cAMP delivery were accompanied by exacerbated behavioral deficits including paralysis and increased extensor tone as well as sensory abnormalities, such as an enhanced startle response and vocalization to touch. These deleterious effects were not observed in saline-treated animals, in animals which received db-cAMP at a dose of 1 mM or in those animals which received a high dose (250 mM) of an alternative cAMP analog, 8-bromo-cAMP, indicating that for continuous in vivo administration, consideration of the effective concentration applied as well as the potential toxicity of the specific reactive moieties on the parent cAMP molecule and/or their activity must be taken into account.
Few studies have investigated the effects of the continuous in vivo delivery of cAMP analogs and, to our knowledge, no studies have been conducted to examine direct and continuous db-cAMP or 8-bromo-cAMP delivery to the CNS; a lack of data on this type of administration regimen, therefore, was a major impetuous in driving the current investigation. To select the dosing range to be employed in the current study, we relied upon previous reports that have used continuous db-cAMP administration, primarily systemic, to other organ systems (Delbarre et al., 1977; Hoffmann et al., 1991; Mizunashi et al., 1994) as well as work involving the single administration of an analog to the CNS (Neumann et al., 2002; Qiu et al., 2002; Lu et al., 2004; Monsul et al., 2004; Pearse et al., 2004). In those paradigms employing continuous in vivo delivery of db-cAMP to other organs, e.g., systemic delivery to the lungs (for a 1–5-day infusion period), analog concentrations ranged from 0.5 to 140 mM (Delbarre et al., 1977; Hoffmann et al., 1991), including a 2 mM dose that was used in humans (Mizunashi et al., 1994). For direct CNS injections, db-cAMP concentrations from 1 to 100 mM have been employed (Neumann et al., 2002; Qiu et al., 2002; Lu et al., 2004; Monsul et al., 2004; Pearse et al., 2004), although most studies have used 50 mM as a single dose. To date, from the limited number of studies performed, there have been no reports of adverse effects or morbidities related to the single or continuous delivery of these analogs and these doses have been shown to be well tolerated and effective.
Although cAMP analogs have seen widespread use in both in vivo and in vitro studies to mimic cAMP signaling in biological processes or as a therapeutic modality, there are few reports on the potential toxicity of these analogs across commonly employed micro- or millimolar concentrations (Pastan et al., 1975; Friedman, 1976; Yusta, 1988; Schwede et al., 2000). The exogenous application of cAMP analogs such as db-cAMP is known to induce a more significant biological effect compared to cAMP itself (Schwede et al., 2000). This could be because the activation of its downstream effectors is more pronounced and persists for a much longer duration due to the higher metabolic stability of these analogs to enzymatic degradation by cAMP hydrolyzing phosphodiesterases within the intracellular environment (Hilz and Kaukel, 1973). However, based on the literature and the disparity in effects we obtained between db-cAMP and 8-bromo-cAMP, it is unlikely that persistent cAMP elevation was responsible for the observed pathophysiological changes associated with the continuous CNS administration of db-cAMP. On the contrary, targeted elevation of cAMP through a variety of approaches from β-adrenoreceptor activation (Lorenowicz, 2007), to analog supplementation (Pearse et al., 2004) or phosphodiesterase inhibition (Rickards, 2001; Nikulina et al., 2004; Kajana and Goshgarian, 2008; Whitaker et al., 2008) has been demonstrated to retard immune cell chemotaxis and tissue infiltration, prevent endothelial hyper-permeability (Lorenowicz et al., 2007) and blood-brain barrier dysfunction (Van Nieuw Amerongen and Van Hinsbergh, 2002) as well as retard cell death (Lee et al., 1993; Whitaker et al., 2008).
The disparity between db-cAMP and 8-bromo cAMP in their effects may be explained by differences in their lipophilicity and/or resistance to phosphodiesterase degradation (Schwede et al., 2000). However, while the lipophilicity of db-cAMP is greater than that of 8-bromo-cAMP, (two fold higher; Schwede et al., 2000) meaning more of the analog will pass through the membrane into the intracellular compartment, 8-bromo-cAMP exhibits greater resistance to phosphodiesterase degradation (Schwede et al., 2000), so that less is needed to cross the membrane to potentially obtain the same effect on downstream signaling. Therefore it is hard to discern whether one of these analogs would be able to achieve a greater stimulation of cAMP signaling in vivo when used at the same dose. Additional studies using recently developed cAMP analogs possessing labels for tracking their mobility and stability (Moll et al., 2008) are required to shed light on the temporal stability and activity of cAMP analogs in vivo and to help answer questions on how the duration of cAMP elevation with the different analogs can alter specific biological processes.
It is more likely that the different effects observed with db-cAMP and 8-bromo-cAMP is related to the chemical moieties that have been added to the cAMP analogs, particularly when the observed pathological effects of db-cAMP were dose-dependent. Following passive diffusion into the cell, db-cAMP undergoes intracellular hydrolysis of the 2’-O-butyryl-residue with the concurrent production of N6-monobutyryl-cAMP and a free butyrate moiety (Kitaoka et al., 1982, Swislocky, 1970) the latter of which has been reported to produce adverse biological effects by interfering with the normal functioning of a number of important proteins and/or inducing tissue damage. Butyrate has been shown to interfere at several levels of the cAMP signaling pathway, by stimulating gene expression of the β-adrenergic receptor (Stadel et al., 1987), estrogen receptor (Graham et al., 1988) and chromatin-associated protein kinase (Kitzis et al., 1980; Feng et al., 1996), among others, as well as by modulating PKC signaling (Rivero et al., 1998; Cuisset et al., 1998) and abolishing the mitogenic action of the adenylyl cyclase activator, forskolin (Yusta et al., 1988). It also acts as an inhibitor of histone deacetylation (Salminen, 1998) as well as induces and/or potentiates apoptotic cell death (Salminen, 1998; Soldatenkov, 1998), which may explain the pathological effects that were observed following the administration of high concentrations of db-cAMP.
In conclusion, the current study highlights the importance of identifying optimal chemical moieties and dosing regimens for the translation of promising biological findings or therapeutic modalities based on modifying cAMP signaling to the whole organism. In addition, by identifying potential risks associated with a cAMP elevating strategy, it would appear that the comparative implementation of different methodologies for cAMP elevation from cAMP analogs, to phosphodiesterase inhibitors and/or adenylyl cyclase activators would allow better dissection of cAMP versus non-cAMP effects when seeking to therapeutically enhance cAMP signaling after CNS injury or disease.
Acknowledgments
We thank Marliss Wolfe for technical assistance. This work was supported by The Christopher Reeve Foundation (support to K.F. and D.D.P.) and NIH NINDS (grant R01NS056281 to D.D.P.).
Author Disclosure Statement
No conflicting financial interests exist.
References
- Barakat D.J. Gaglani S.M. Neravetla S.R. Sanchez A.R. Andrade C.M. Pressman Y. Puzis R. Garg M.S. Bunge M.B. Pearse D.D. Survival, integration, and axon growth support of glia transplanted into the chronically contused spinal cord. Cell Transplant. 2005;14:225–240. doi: 10.3727/000000005783983106. [DOI] [PubMed] [Google Scholar]
- Basso D.M. Beattie M.S. Bresnahan J.C. Anderson D.K. Faden A.I. Gruner J.A. Holford T.R. Hsu C.Y. Noble L.J. Nockels R. Perot P.L. Salzman S.K. Young W. MASCIS evaluation of open field locomotor scores: effects of experience and teamwork on reliability. Multicenter Animal Spinal Cord Injury Study. J. Neurotrauma. 1996;13:343–359. doi: 10.1089/neu.1996.13.343. [DOI] [PubMed] [Google Scholar]
- Cai D. Qiu J. Cao Z. McAtee M. Bregman B.S. Filbin M.T. Neuronal cyclic AMP controls the developmental loss in ability of axons to regenerate. J. Neurosci. 2001;21:4731–4739. doi: 10.1523/JNEUROSCI.21-13-04731.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuisset L. Tichonicky L. Delpech M. A protein phosphatase is involved in the inhibition of histone deacetylation by sodium butyrate. Biochem. Biophys. Res. Commun. 1998;246:760–764. doi: 10.1006/bbrc.1998.8698. [DOI] [PubMed] [Google Scholar]
- Cui Q. Yip H.K. Zhao R.C. So K.F. Harvey AR. Intraocular elevation of cyclic AMP potentiates ciliary neurotrophic factor-induced regeneration of adult rat retinal ganglion cell axons. Mol. Cell Neurosci. 2003;22:49–61. doi: 10.1016/s1044-7431(02)00037-4. [DOI] [PubMed] [Google Scholar]
- Cui Q. So K.F. Involvement of cAMP in neuronal survival and axonal regeneration. Anat. Sci. Int. 2004;79:209–212. doi: 10.1111/j.1447-073x.2004.00089.x. [DOI] [PubMed] [Google Scholar]
- Emoto M. Langille S.E. Czech M.P. A role for kinesin in insulin-stimulated GLUT4 glucose transporter translocation in 3T3-L1 adipocytes. J. Biol. Chem. 2001;276:10677–10682. doi: 10.1074/jbc.M010785200. [DOI] [PubMed] [Google Scholar]
- Feng P. Ge L. Akyhani N. Liau G. Sodium butyrate is a potent modulator of smooth muscle cell proliferation and gene expression. Cell Dev. Biol. 1996;29:231–241. doi: 10.1046/j.1365-2184.1996.00998.x. [DOI] [PubMed] [Google Scholar]
- Fouad K. Schnell L. Bunge M.B. Schwab M.E. Liebscher T. Pearse D.D. Combining Schwann cell bridges and olfactory-ensheathing glia grafts with chondroitinase promotes locomotor recovery after complete transection of the spinal cord. J. Neurosci. 2005;25:1169–1178. doi: 10.1523/JNEUROSCI.3562-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgis J. Merrett D. Kirkland S. Metz G.A. Verge V. Fouad K. Reaching training in rats with spinal cord injury promotes plasticity and task-specific recovery. Brain. 2007;130:2993–3003. doi: 10.1093/brain/awm245. [DOI] [PubMed] [Google Scholar]
- Graham K.A. Buick R.N. Sodium butyrate induces differentiation in breast cancer cell lines expressing the estrogen receptor. J. Cell. Physiol. 1988;136:63–71. doi: 10.1002/jcp.1041360108. [DOI] [PubMed] [Google Scholar]
- Hermanns S. Reiprich P. Müller H.W. A reliable method to reduce collagen scar formation in the lesioned rat spinal cord. J. Neurosci. Methods. 2001;30:141–146. doi: 10.1016/s0165-0270(01)00427-7. [DOI] [PubMed] [Google Scholar]
- Hilz H. Kaukel E. Divergent action mechanism of cAMP and dibutyryl cAMP on cell proliferation and macromolecular synthesis in HeLa S3 cultures. Mol. Cell Biochem. 1973;1:229–239. doi: 10.1007/BF01659332. [DOI] [PubMed] [Google Scholar]
- Kajana S. Goshgarian H.G. Spinal activation of the cAMP-PKA pathway induces respiratory motor recovery following high cervical spinal cord injury. Brain Res. 2008;26:1206–1213. doi: 10.1016/j.brainres.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilmer S.L. Carlsen R.C. Chronic infusion of agents that increase cyclic AMP concentration enhances the regeneration of mammalian peripheral nerves in vivo. Exp. Neurol. 1987;95:357–367. doi: 10.1016/0014-4886(87)90144-0. [DOI] [PubMed] [Google Scholar]
- Kitaoka H. Ohya K. Determination of sodium N6, O-2'-dibutyryladenosine cyclic 3',5'-hydrogenphosphate) and its hydrolysis products by high-performance liquid chromatography. J. Chromatogr. 1982;238:495–499. [Google Scholar]
- Kitzis A. Tichonicky L. Defer N. Kruh J. Localization of phosphoproteins and of protein kinases in chromatin from butyrate-treated HTC cells. Biochem. Biophys. Res. Commun. 1980;97:530–537. doi: 10.1016/0006-291x(80)90296-x. [DOI] [PubMed] [Google Scholar]
- Lee M.R. Liou M.L. Liou M.L. Yang Y.F. Lai M.Z. cAMP analogs prevent activation-induced apoptosis of T cell hybridomas. J. Immunol. 1993;15:5208–5217. [PubMed] [Google Scholar]
- Lorenowicz M.J. Fernandez-Borja M. Hordijk P.L. cAMP signaling in leukocyte transendothelial migration. Arterioscler. Thromb. Vasc. Biol. 2007;27:1014–1022. doi: 10.1161/ATVBAHA.106.132282. [DOI] [PubMed] [Google Scholar]
- Lu P. Yang H. Jones L.L. Filbin M.T. Tuszynski M.H. Combinatorial therapy with neurotrophins and cAMP promotes axonal regeneration beyond sites of spinal cord injury. J. Neurosci. 2004;24:6402–6409. doi: 10.1523/JNEUROSCI.1492-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming G.L. Song H.J. Berninger B. Holt C.E. Tessier-Lavigne M. Poo M.M. cAMP-dependent growth cone guidance by netrin-1. Neuron. 1997;19:1225–1235. doi: 10.1016/s0896-6273(00)80414-6. [DOI] [PubMed] [Google Scholar]
- Mizunashi K. Furukawa Y. Yoshinaga K. The effect of endogenous parathyroid hormone, exogenous calcitonin, and dibutyryl cyclic AMP on urinary excretion of N-acetyl-beta-d-glucosaminidase. Calcif. Tissue Int. 1994;54:186–194. doi: 10.1007/BF00301676. [DOI] [PubMed] [Google Scholar]
- Moll D. Prinz A. Brendel C.M. Berrera M.B. Guske1 K. Zaccolo M. Genieser H.G. Herberg F.W. Biochemical characterization and cellular imaging of a novel, membrane permeable fluorescent cAMP analog. BMC Biochem. 2008;9:1–13. doi: 10.1186/1471-2091-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann S. Bradke F. Tessier-Lavigne M. Basbaum A.I. Regeneration of sensory axons within the injured spinal cord induced by intraganglionic cAMP elevation. Neuron. 2002;13:885–893. doi: 10.1016/s0896-6273(02)00702-x. [DOI] [PubMed] [Google Scholar]
- Nikulina E. Tidwell J.L. Dai H.N. Bregman B.S. Filbin M.T. The phosphodiesterase inhibitor rolipram delivered after a spinal cord lesion promotes axonal regeneration and functional recovery. Proc. Natl. Acad. Sci. USA. 2004;8:8786–8790. doi: 10.1073/pnas.0402595101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuydens R. Nuyens R. Cornelissen F. Geerts H. The fast axonal transport in hippocampal neurones is acutely enhanced by db-cAMP. Neuroreport. 1993;4:179–182. doi: 10.1097/00001756-199302000-00016. [DOI] [PubMed] [Google Scholar]
- Pearse D.D. Pereira F.C. Marcillo A.E. Bates M.L. Berrocal Y.A. Filbin M.T. Bunge M.B. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat. Med. 2004;10:610–616. doi: 10.1038/nm1056. [DOI] [PubMed] [Google Scholar]
- Pearse D.D. Targetting intracellular signaling moleculaes within the neurons to promote repair after spinal cord injury. Topics Spinal Cord Inj. Rehabil. 2004;10:1–16. [Google Scholar]
- Pearse D.D. Sanchez A.R. Pereira F.C. Andrade C.M. Puzis R. Pressman Y. Golden K. Kitay B.M. Blits B. Wood P.M. Bunge M.B. Transplantation of Schwann cells and/or olfactory ensheathing glia into the contused spinal cord: survival, migration, axon association, and functional recovery. Glia. 2007;55:976–1000. doi: 10.1002/glia.20490. [DOI] [PubMed] [Google Scholar]
- Qiu J. Cai D. Dai H. McAtee M. Hoffman P.N. Bregman B.S. Filbin M.T. Spinal axon regeneration induced by elevation of cyclic AMP. Neuron. 2002;13:895–903. doi: 10.1016/s0896-6273(02)00730-4. [DOI] [PubMed] [Google Scholar]
- Rickards K.J. Page C.P. Lees P. Cunningham F.M. Differential inhibition of equine neutrophil function by phosphodiesterase inhibitors. J. Vet. Pharmacol. Ther. 2001;24:275–281. doi: 10.1046/j.1365-2885.2001.00344.x. [DOI] [PubMed] [Google Scholar]
- Rivero J.A. Adunyah S.E. Sodium butyrate stimulates PKC activation and induces differential expression of certain PKC isoforms during erythroid differentiation. Biochem. Biophys. Res. Commun. 1998;248:664–668. doi: 10.1006/bbrc.1998.9041. [DOI] [PubMed] [Google Scholar]
- Salminen A. Tapiola T. Korhonen P. Suuronen T. Neuronal apoptosis induced by histone deacetylase inhibitors. Mol. Brain Res. 1998;61:203–206. doi: 10.1016/s0169-328x(98)00210-1. [DOI] [PubMed] [Google Scholar]
- Schwede F. Maronde E. Genieser H. Jastorff B. Cyclic nucleotide analogs as biochemical tools and prospective drugs. Pharmacol. Ther. 2000;87:199–226. doi: 10.1016/s0163-7258(00)00051-6. [DOI] [PubMed] [Google Scholar]
- Silver J. Miller J.H. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Sipski M.L. Pearse D.D. Methylprednisolone and other confounders to spinal cord injury clinical trials. Nat. Clin. Pract. Neurol. 2006;2:402–403. doi: 10.1038/ncpneuro0221. [DOI] [PubMed] [Google Scholar]
- Song H.J. Ming G.L. Poo M.M. cAMP-induced switching in turning direction of nerve growth cones. Nature. 1997;17:275–279. doi: 10.1038/40864. [DOI] [PubMed] [Google Scholar]
- Soldatenkov V.A. Prasad S. Voloshin Y. Dritschilo A. Sodium butyrate induces apoptosis and accumulation of ubiquitinated proteins in human breast carcinoma cells. Cell Death Differ. 1998;5:30–312. doi: 10.1038/sj.cdd.4400345. [DOI] [PubMed] [Google Scholar]
- Stadel J.M. Nakada M.T. Crooke S.T. Molecular mechanisms of ß-adrenergic receptor regulation. Int. Congr. Ser. Exerpta Med. 1987;750:17. [Google Scholar]
- Swislocky N.I. Decomposition of dibutyryl cyclic AMP in aqueous buffers. Anal. Biochem. 1970;38:260–269. doi: 10.1016/0003-2697(70)90175-2. [DOI] [PubMed] [Google Scholar]
- Van Nieuw Amerongen G.P. Van Hinsbergh V.W. Targets for pharmacological intervention of endothelial hyperpermeability and barrier function. Vascul. Pharmacol. 2002;39:257–272. doi: 10.1016/s1537-1891(03)00014-4. [DOI] [PubMed] [Google Scholar]
- Vavrek R. Girgis J. Tetzlaff W. Hiebert G.W. Fouad K. BDNF promotes connections of corticospinal neurons onto spared descending interneurons in spinal cord injured rats. Brain. 2006;129:1534–1545. doi: 10.1093/brain/awl087. [DOI] [PubMed] [Google Scholar]
- Whitaker C.M. Beaumont E. Wells M.J. Magnuson D.S. Hetman M. Onifer S.M. Rolipram attenuates acute oligodendrocyte death in the adult rat ventrolateral funiculus following contusive cervical spinal cord injury. Neurosci. Lett. 2008;438:200–204. doi: 10.1016/j.neulet.2008.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusta B. Ortiz-Caro J. Pascual A. Aranda A. Comparison of the effects of forskolin and dibutyryl cyclic AMP in neurolastoma cells: evidence that some of the actions of dibutyryl cyclic AMP are mediated by butyrate. J. Neurochem. 1988;51:1808–1818. doi: 10.1111/j.1471-4159.1988.tb01162.x. [DOI] [PubMed] [Google Scholar]