Abstract
Traumatic brain injury (TBI) has been demonstrated to induce cerebral vascular dysfunction that is reflected in altered responses to various vasodilators. While previous reports have focused primarily on the short-term vascular alterations, few have examined these vascular changes for more than 7 days, or have attempted to correlate these alterations with any persisting behavioral changes or potential therapeutic modulation. Accordingly, we evaluated the long-term microvascular and behavioral consequences of experimental TBI and their therapeutic modulation via hypothermia. In this study, one group was injured with no treatment, another group was injured and 1 h later was treated with 120 min of hypothermia followed by slow rewarming, and a third group was non-injured. Animals equipped with cranial windows for visualization of the pial microvasculature were challenged with various vasodilators, including acetylcholine, hypercapnia, adenosine, pinacidil, and sodium nitroprusside, at either 1 or 3 weeks post-TBI. In addition, all animals were tested for vestibulomotor tasks at 1 week post-TBI, and animals surviving for 3 weeks post-TBI were tested in a Morris water maze (MWM). The results of this investigation demonstrated that TBI resulted in long-term vascular dysfunction in terms of altered vascular reactivity to various vasodilators, which was significantly improved with the use of a delayed 120-min hypothermic treatment. In contrast, data from the MWM task indicated that injured animals revealed persistent deficits in the spatial memory test performance, with hypothermia exerting no protective effects. Collectively, these data illustrate that TBI can evoke long-standing brain vascular and spatial memory dysfunction that manifest different responses to hypothermic intervention. These findings further illustrate the complexity of TBI and highlight the fact that the chosen hypothermic intervention may not necessarily exert a global protective response.
Key words: animal studies, behavioral assessment, hypothermia, TBI, vascular reactivity
Introduction
The brain's response to traumatic brain injury (TBI) is only partially understood. While numerous studies have focused on the acute brain parenchymal, behavioral, and vascular changes associated with TBI, few have followed these changes over a more prolonged, chronic course of injury, or have attempted to correlate these changes with any enduring morbidity. On the vascular front, our laboratory has made significant contributions to the understanding of the brain's microvascular response to injury, illustrating that in the early hours post-injury the cerebral microcirculation shows impaired vascular reactivity to known vasodilator challenges (Wei et al., 1980; Kontos et al., 1980; Kontos et al., 1981; Suehiro et al., 2003; Ueda et al., 2003; Wei et al., 1981). Further, researchers in our laboratory have suggested that this impaired vasoreactivity may persist for at least 1 week post-injury (Ueda et al., 2004); however, beyond this period neither our laboratory nor others have followed the persistence of these vascular abnormalities.
In parallel with our descriptions, as well as those of others reporting the microvascular abnormalities associated with TBI, there has also been an attempt to therapeutically modulate these vascular changes to protect the injured brain from microvascular dysfunction that might predispose the brain to secondary insult. In this vein, our laboratory has also shown that these microvascular abnormalities could be attenuated through the use of early post-traumatic hypothermic intervention (Suehiro et al., 2003; Ueda et al., 2003; Povlishock et al., 2004). However, like the natural course of these microvascular changes, little information has been obtained about whether this hypothermic intervention provides enduring microvascular protection, again with only the suggestion that this protection may persist for at least 1 week after TBI (Povlishock et al., 2004; Ueda et al., 2004). Because of these gaps in our understanding of these critical issues, the current study was initiated to address the more chronic course of traumatically induced microvascular change and its potential enduring modification by hypothermia.
Additionally, because questions arose over the relevance of these vascular changes and their relation to any ongoing changes in the brain parenchyma either induced by TBI and/or modified by hypothermia, it also appeared rational to address these issues. To date, despite indirect evidence that the same hypothermic interventions that initially protect the cerebral circulation also attenuate traumatically induced axonal damage (Koizumi and Povlishock, 1998), no direct corollary information exists on any other form of brain protection. This issue is of more than mere academic interest, as multiple laboratories have demonstrated that the structural and behavioral changes associated with TBI can be significantly attenuated via hypothermic intervention. In seminal work, Bramlett and colleagues (1995, 1997) have shown that post-traumatic hypothermic intervention provides protection from sensorimotor and cognitive behavioral deficits, while also providing enduring neuroprotection in terms of brain atrophy and parallel ventricular enlargement. Accordingly, based upon these observations, it appears reasonable and rational to probe the potential relationship between the above-described vascular and behavioral changes, examining their chronic course and potential modification by hypothermic intervention.
To this end, we utilized cranial windows to follow the course of microvascular change over a 3-week period post-injury to determine if traumatically induced microvascular change persists, and if so to determine what relation, if any, it bears to concomitant behavioral dysfunction. Additionally, using the same approaches, the potential protective effects of hypothermic intervention were explored, now considering if such hypothermic intervention provides long-term, enduring microvascular protection that parallels behavioral recovery.
Methods
Experimental design
In the current study, two basic paradigms were used, employing three groups of animals for each paradigm, evaluating 1- and 3-week survival time points. In the first paradigm, after the induction of TBI and no treatment, one group (n = 8), injured and non-treated, was re-anesthetized and prepared for cranial window placement 1 week post-injury. In these animals, the microvessels were physiologically assessed, evaluating their response to the chosen vasodilators that included hypercapnia, acetylcholine, pinacidil, adenosine, and sodium nitroprusside (SNP). Another group of animals (n = 8) was injured and then treated with 2 h of hypothermia initiated 60 min post-TBI. At 1 week post-injury these animals underwent the same vascular physiological assessments described above. A third group of animals (n = 8) was subjected to a sham injury and consequently no treatment. Vestibulomotor tasks were also assessed in these three groups of animals in the first week after TBI. Seven days after the sham injury, these animals were assessed for their vascular reactivity in response to several vasodilators.
In the second paradigm, three groups of rats were again utilized, now examining vascular reactivity at 3 weeks after the induction of TBI. Similarly to the above paradigm, one group of animals (n = 8) was injured with no treatment. Another group (n = 8) was injured and subjected to 2 h of hypothermia initiated 1 h post-injury. The third group (n = 8) was subjected to a sham injury and no treatment. Vestibulomotor tasks were assessed in these animals in the first week after TBI, similarly to the animals surviving for 1 week, and the vascular assessments were performed at 3 weeks post-injury. Additional animals surviving for 3 weeks after TBI were tested in the Morris water maze for a spatial memory task. As for the vascular studies, one group (n = 8) included injured-untreated animals, another group (n = 7) included injured hypothermia-treated animals, and the last group (n = 9) included sham-injured animals.
Experimental traumatic brain injury
Fluid percussion injury (FPI)
All injuries were carried out in a fashion consistent with the policies of our institution's animal care and use committee. Male Sprague-Dawley rats weighing approximately 300–350 g were initially anesthetized with 2% isoflurane in a gas mixture of 70% N2O and 30% O2 and prepared for the surgery necessary for the completion of the lateral FPI. The injury procedure itself has been described in detail elsewhere (McIntosh et al., 1989). In brief, after the animals were anesthetized, they were placed in a stereotaxic frame to ensure stability during the procedure. After the scalp was incised, a craniectomy 4.8 mm in diameter was performed over the right parietal cortex lateral to the midline consistent with previous descriptions (McIntosh et al., 1989). A modified needle base tube was placed over the exposed dura and secured to the scalp with dental acrylic. Two burr holes were drilled to hold two screws, thus ensuring that the acrylic was bonded to the skull and the tube, which was filled with saline.
Injury was produced by a fluid percussion injury device (Dixon et al., 1987; McIntosh et al., 1989) that consisted of a saline-filled cylinder with a rubber cover on one end and a transducer on the other end of the cylinder. As the pendulum struck the rubber cover on one end of the cylinder, a brief, moderate fluid pressure pulse (1.8–2.1 atm) was applied to the exposed intact dura of the rat connected to the injury device on the side of the transducer, which recorded the atmospheric pressure of the injury. Once the injury was completed and the animal's scalp was sutured, anesthesia was supplemented with sodium pentobarbital (60 mg/kg) for all subsequent studies including the induction of therapeutic hypothermia.
Hypothermia
In the present experiment, those animals treated with hypothermia were monitored after the injury and brain and body temperature were maintained at 37°C for 1 h. Hypothermia was initiated 60 min after TBI with ice packs and maintained at 32°C for 120 min. In all studies, thermistors placed in the temporalis muscle were used to monitor brain temperature (Jiang et al., 1991), while rectal thermometers were used to monitor body temperature. Upon the completion of the hypothermia, the animals were slowly rewarmed with a heat lamp to 37°C over a 90-min period, consistent with previously published protocols demonstrating that such slow rewarming assures the optimal effects of hypothermic intervention (Suehiro et al., 2003; Povlishock et al., 2004). After the animals were rewarmed to normothermic temperatures and the animals recovered from anesthesia, the rats were returned to the incubator and monitored for postoperative recovery before returning to the animal facility. Animals were randomly chosen to survive for either 1 or 3 weeks after injury for completion of neurobehavioral and vascular studies.
Neurobehavioral studies
For behavioral analyses, several tests were used including the beam balance and the beam walk for testing vestibulomotor function, and the Morris water maze for testing cognitive function (Smith et al., 1991; Hicks et al., 1993, Hamm et al., 1993).
The vestibulomotor testing started on day 1 after the injury, and the cognitive testing was assessed starting on day 11 after TBI. Only animals surviving 3 weeks post-TBI were included in the Morris water maze (MWM) task, since early TBI-induced motor deficits typically alter the animal's MWM task performance, which requires good swimming skills. Therefore, animals surviving for 1 week after the induction of injury were excluded from this cognitive test based on their compromised swimming abilities.
Vestibulomotor assessments
Beam balance
The beam balance task employed a suspended wooden beam 30 cm long and 2 cm wide. In this task, animals were placed on a wooden beam and the duration the animal was able to remain on the beam was recorded. Rats were pre-trained 1 day prior to injury, and then tested for 5 days, beginning the first day after injury. An average of three trials per day, with each trial not exceeding 60 sec, was used for the statistical analysis.
Beam walk
The beam walk task included an elevated wooden beam 100 cm long and 2 cm wide, which was suspended between a table with a bright lamp on one end and a black box on the other end. Similarly to the beam balance task, the animals were pre-trained 1 day before injury, and then tested for 5 days post-injury with three trials per day. The animals were placed on the beam, where they tried to escape the bright light accompanied by loud white noise, by walking on the beam, maneuvering through the pegs positioned on both sides of the beam 20 cm apart. As soon as the animal reached the black box, the light and noise were turned off, and the animal was allowed to remain in the box for 30 sec. The time for the animal to reach the black box was recorded. An average of three daily trials was used for the statistical analysis.
Cognitive assessment
Morris water maze
The MWM protocol has been described in detail elsewhere (Morris et al., 1982). In brief, the task is comprised of a large circular tank 180 cm in diameter and 45 cm in height, filled with warm (28°C) water to a depth of 30 cm. A clear acrylic glass platform, submerged in the water 2 cm below the water level, was the object of the animal's search. The room contained numerous cues on the walls, which remained constant throughout the experiment, in order to aid animals in this spatial memory task. The test consisted of four trials per day for 5 consecutive days.
On each trial day the animal was placed in the tank starting from one of four locations conveniently labeled east, west, south and north. The order of the starting place was randomly chosen for each trial. The animals swam freely about the tank to find and escape to the hidden platform, which remained in a constant position. The animals were allowed 120 sec to locate the platform. Once the platform was found, the animal was allowed to remain in place on the platform for 30 sec, and then was transferred to a heated incubator for 5 min until the next trial. Rats that did not find the platform after 120 sec were placed on the platform for 30 sec by the investigator and later placed in a heated incubator.
Maze performance was measured by a computerized video tracking system (Poly Track 4.01; San Diego Instruments, San Diego, CA). Three indexes of maze performance were measured, including the animal's latency to reach the goal platform and the animal's distance or path length to get to the goal. In addition, every 0.2 sec, the system calculated the distance between the animal and the goal platform, adding these distances together to indicate how far away the animal was from the platform during a given trial.
Vascular studies
General surgical preparation
Vascular studies were carried out at designated times 1 or 3 weeks after TBI by an investigator blinded to the animal's injury and treatment status. The rats were anesthetized with sodium pentobarbital (60 mg/kg IV). In these vascular studies, sodium pentobarbital anesthesia was now used in place of the initial isoflurane anesthesia to avoid any gas anesthesia-induced vasodilation. The femoral vein and artery were cannulated for the administration of medication and for the monitoring of arterial blood pressure and blood gases, respectively. After completion of tracheotomy, each rat was ventilated on a Harvard rodent respirator. Ventilation was adjusted so that the partial arterial carbon dioxide pressure (Paco2) was kept constant at 35–40 mm Hg in the resting state. This Paco2 level, unless modified for pial vessel assessment, was maintained throughout each experiment by changing the rate and/or volume of the respirator. The temporalis muscle and rectal temperatures were also monitored.
Cranial window installation
The cranial window procedure has been explained in detail elsewhere (Levasseur et al., 1975; Ellis et al., 1983). In brief, a midline sagittal scalp incision was made over the parietal bone and the skull exposed. A 2 × 4-mm craniectomy was made over the left parietal bone, and the dura was cut. A cranial window consisting of a stainless steel ring with a glass plate on top was placed over the craniectomy and fixed to the skull with bone wax and dental acrylic to create a tight seal. The cranial window, filled with artificial CSF, allowed for the direct visualization of the pial microvessels, using a Vicker's image-splitting device. The cranial window contained three ports: one each for the inflow and outflow pathways of the drugs and/or vehicle used, and the third one was used for intracranial pressure (ICP) measurement. ICP was set at 5 mm Hg by connecting to a fluid column whose free end was placed at a predetermined height. The pH of bathing CSF was adjusted to approximately 7.35 by equilibration with a gas mixture containing 6% O2 and 6% CO2 balanced with N2. At least four arterioles were measured in each animal at resting state as well as after the challenges with vasodilators.
Hypercapnia
As the cerebrovascular response to hypercapnia helps maintain adequate cerebral blood flow (CBF; Brian, 1998), and as impaired CO2 reactivity is a common finding in clinical TBI (Cold et al., 1977; Enevoldsen and Jensen, 1978), we tested the cerebrovascular response to hypercapnia. Arterial hypercapnia was induced by ventilating the animal with a gas mixture containing 3% and 5% CO2 in air. Since several minutes were required for arteriolar adjustments to reach a new steady state, each level of hypercapnia was maintained for at least 7 min prior to measurement of vessel caliber.
Vasoreactivity to acetylcholine, adenosine, pinacidil, and sodium nitroprusside
As endothelial-dependent dysfunction has also been described with TBI (Kontos and Wei, 1992), we explored the acetylcholine (ACh)-dependent pial vascular responses. ACh (Sigma Chemical Co., St. Louis, MO) was dissolved in artificial CSF to reach a final concentration of either 0.1 or 10.0 μmol/L. Vessel diameters were measured 2–4 min following the topical application for each concentration of ACh. Under normal physiological conditions, ACh is known to dilate cerebral vessels via generation of an endothelium-derived relaxing factor of the NO type (Furchgott, 1983; Kontos et al., 1988; Wei et al., 1992). However, this normal vasodilator response caused by ACh is either reduced or converted to vasoconstriction following experimental TBI.
Adenosine (Sigma) was dissolved in artificial CSF for a final concentration of either 50 or 100 μmol/L. Adenosine causes relaxation of smooth muscle tissue in the animals under normal physiological conditions. However, under the pathological conditions of TBI, microvessels fail to dilate (Golding et al., 1999). Another vasodilator, pinacidil (Sigma), a KATP-channel opener, was dissolved in alcohol and artificial CSF to reach a final concentration of either 1 or 2 μmol/L. Under normal physiological conditions, pinacidil causes transient vasodilation by targeting KATP channels located on the arteriolar smooth muscle (Faraci and Heistad, 1998; Quayle et al., 1997). Similarly to other vasodilators in the pathological state of injury, pinacidil also fails to cause normal dilation (Kontos and Wei, 1998; Ueda et al., 2004). The last vasodilator used in the experiment, sodium nitroprusside (SNP) (Sigma), was dissolved in artificial CSF to reach a final concentration of either 0.5 or 1 μmol/L. SNP in the sham animals causes relaxation of the microvessels by the diffusion of nitric oxide, liberated by SNP, into the vascular smooth muscle causing subsequent vasodilation, which was altered after TBI (Kitazono et al., 1995; Ueda et al., 2004). Responses to topically-applied drugs were determined 2 and 4 min after each application.
Statistical analyses
For the vascular studies, the data were tested with a one-way analysis of variance (ANOVA) followed by a Duncan post-hoc test for multiple comparisons between groups. Differences were considered to be statistically significant at p < 0.05. For the Morris water maze studies, the daily mean group goal latencies for each group were analyzed by a split-plot analysis of variance (group × day). Again, differences were considered to be statistically significant at p < 0.05. All values in the provided tables and charts are expressed as ±SEM.
Results
General observations
At least four vessels were recorded per animal. Arterial blood samples were collected for determination of blood gases and pH values before measuring vessel diameter. Resting diameters were measured in all animals before the vasodilator challenge with hypercapnia, ACh, adenosine, pinacidil, or SNP at each time point. The physiological parameters of mean arterial blood pressure and blood gases did not differ significantly between groups (data not shown).
Neurobehavioral studies
Vestibulomotor responses
Beam walk and beam balance walk tests were completed during the first week after TBI in all groups of animals, including those surviving for both 1 and 3 weeks post-injury. The animals in the sham group revealed no deficits in the performance of either of the vestibulomotor tasks in comparison to those animals in both the injured-untreated and injured hypothermia-treated groups, both of which, in the first days of testing, displayed dysfunction in the completion of the tasks. However, by the fifth day the performance on the beam balance and beam walk tasks was not significantly different between all groups, as motor deficits of the injured animals improved and did not differ from those of the sham animals. In sum, there were no significant differences in vestibulomotor responses between all groups (data not shown).
Cognitive assessment
Morris water maze
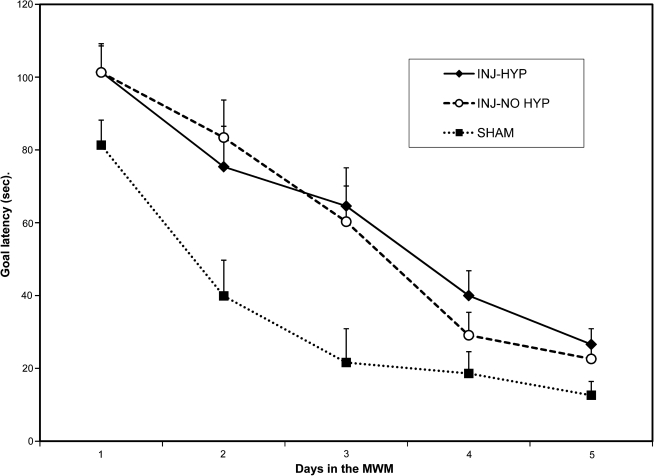
Animals surviving 3 weeks after TBI were assessed using the spatial memory task (Fig. 1). At 3 weeks post-injury, no differences could be detected between the injured hypothermia-treated and injured-untreated animals. An ANOVA (F(2, 21) = 7.65; p < 0.01) was performed with the Duncan post-hoc test, and the results indicated that while sham animals performed significantly better (p < 0.01) than both injured hypothermia-treated and injured-untreated animals, there was no significant difference between the last two groups. In the injured-untreated and injured hypothermia-treated groups, the latency to find the platform was not significantly different despite the use of hypothermia in post-injury management.
FIG. 1.
Animals surviving 3 weeks after TBI were assessed for cognitive function using the MWM. The daily mean group goal latencies for each group were analyzed by a split-plot analysis of variance (group × day). This analysis yielded a significant group effect (F2,21 = 7.65; p < 0.01). Duncan post-ANOVA tests indicated that the sham-injured group (SHAM; n = 9) had shorter goal latencies than the injured hypothermia-treated (INJ-HYP; n = 7) and the injured-untreated (INJ-NO HYP; n = 8) groups (p < 0.05). However, the goal latencies of injured hypothermia-treated and the injured-untreated groups were not significantly different.
Vasoactive responses
One week post-injury
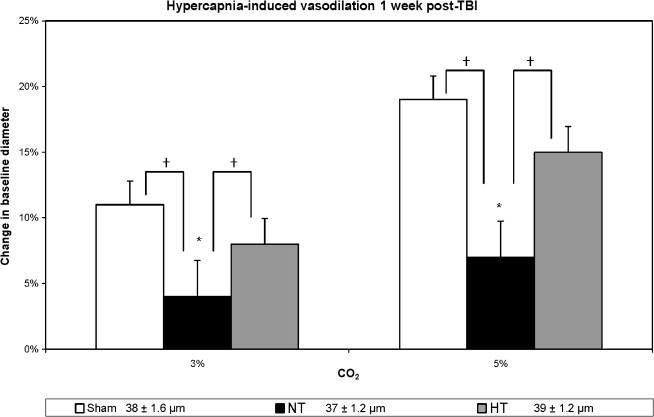
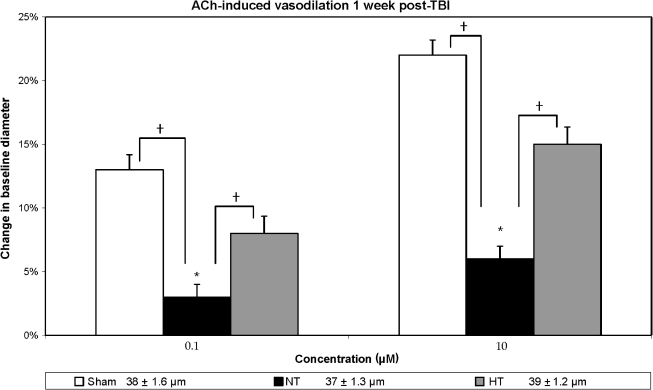
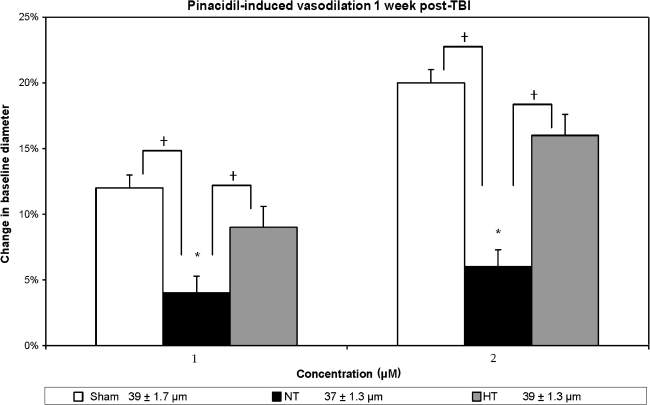
In contrast to the injured-untreated normothermic group, those animals treated with the post-traumatic hypothermia revealed vascular protection in terms of the vasodilator agents used. Hypercapnia-induced (Fig. 2) vasodilation demonstrated significant differences between the sham, injured-untreated, and injured hypothermia-treated for the 3% CO2 (F(2, 21) = 5.15; p < 0.02) and 5% CO2 (F(2, 21) = 5.62; p < 0.01) groups. Subsequent Duncan post-hoc analysis showed that the injured-untreated group had significantly decreased responses compared to the sham group (p < 0.05) for both concentrations of CO2, and there were no significant differences between injured hypothermia-treated and sham animals for both levels of CO2 as well. Similarly, ACh-induced vasodilation (Fig. 3) differed significantly between the three groups for both low (F(2, 21) = 26.43; p < 0.001) and high (F(2, 21) = 11.89; p < 0.001) levels of ACh. Based on a Duncan post-hoc analysis, this response was significantly improved in the injured hypothermia-treated (p < 0.05) rats in comparison to injured untreated rats, while no significant differences were noted between the sham and the injured hypothermia-treated groups. In terms of vasodilation response to pinacidil (Fig. 4), the data also showed significant differences between all groups (F(2, 21) = 6.82, p < 0.05 and F(2, 21) = 6.34, p < 0.05, for both concentrations, respectively). Further analysis showed significantly enhanced response in the injured hypothermia-treated group (p < 0.05), in comparison to injured-untreated rats, again with no significant differences in comparison to the sham group for both concentrations of pinacidil.
FIG. 2.
Hypercapnia-induced vasodilation is depicted in three groups: sham, non-treated (NT) and hypothermia-treated (HT) groups in terms of percent change in vessel diameter from the baseline measurement. Sham animals demonstrated an approximately 11% increase in vessel diameter in response to 3% CO2, and 19% increase in response to 5% CO2. In the injured non-treated rats the response was compromised in comparison to sham rats (p < 0.05). In the injured hypothermia-treated animals, the hypercapnia response was restored, representing no significant difference from the sham animals' response (*p < 0.05 in comparison to baseline measurement; †p < 0.05 in comparison to the injured non-treated rats). At the bottom of the figure are the baseline resting diameter measurements.
FIG. 3.
Acetylcholine-induced vasodilation is shown in three groups: sham, non-treated (NT), and hypothermia-treated (HT), in terms of percent change in vessel diameter from the baseline measurement. Sham animals demonstrated an approximately 13% increase in vessel diameter in response to 0.1 μm ACh, and a 22% increase in response to 10.0 μm ACh. In the injured non-treated rats the response was compromised and reduced in comparison to the sham and hypothermia-treated groups (p < 0.05). In the injured hypothermia-treated animals the response was improved, with no significant differences in comparison to sham animals. (*p < 0.05 in comparison to baseline measurement; †p < 0.05 in comparison to the injured non-treated rats). At the bottom of the figure are the baseline resting diameter measurements.
FIG. 4.
Pinacidil-induced vasodilation is shown in three groups: sham, non-treated (NT), and hypothermia-treated (HT), in terms of percent change in vessel diameter from the baseline measurement. Sham animals demonstrated approximately a 12% increase in the vessel diameter in response to 1.0 μm pinacidil, and a 20% increase in response to 2.0 μm pinacidil. In the injured non-treated rats the response was compromised and significantly diminished (p < 0.05). In the injured hypothermia-treated animals the vascular response improved and was no longer significantly different from the sham rats (*p < 0.05 in comparison to baseline measurement; †p < 0.05 in comparison to the injured non-treated rats). At the bottom of the figure are the baseline resting diameter measurements.
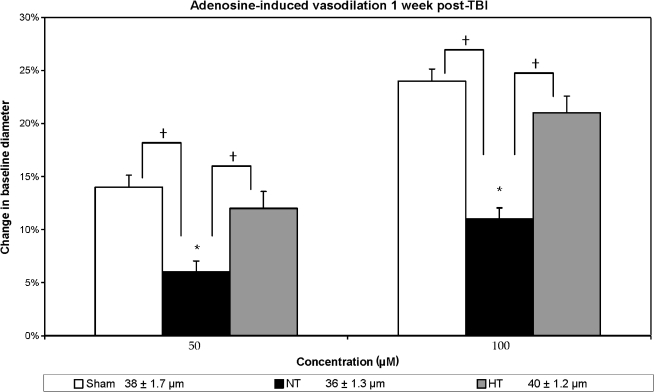
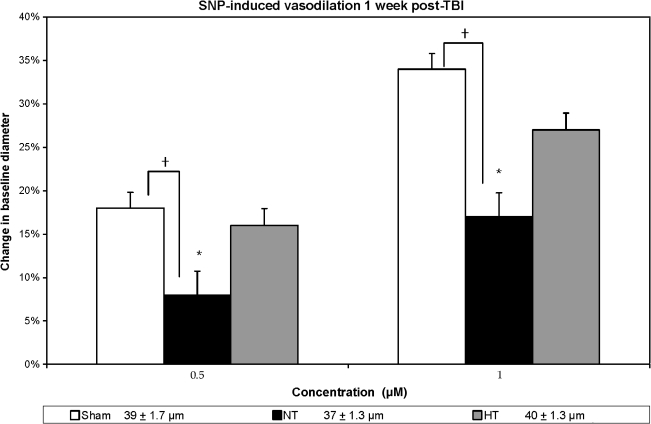
Adenosine-induced vasodilation (Fig. 5) was significantly different between the groups (F(2, 21) = 6.42, p < 0.05 and F(2, 21) = 5.08, p < 0.05, for low and high concentrations, respectively). Again, for both concentrations used, no significant differences were seen between the sham and injured hypothermia-treated groups, while the injured-untreated animals maintained significantly reduced response (p < 0.05) in comparison to both the sham and injured hypothermia-treated groups. Finally, in response to SNP (Fig. 6), the sham group again responded significantly better than the injured-untreated one (p < 0.05); however, the injured hypothermia-treated group did not respond in a manner that was statistically significantly different from both the injured-untreated and sham groups, although a trend toward improved responsiveness was observed. In sum, the vasodilator response in the cases of hypercapnia, ACh, pinacidil, and adenosine, was significantly improved in the hypothermic animals versus the normothermic group.
FIG. 5.
Adenosine-induced vasodilation is shown in three groups: sham, non-treated (NT), and hypothermia-treated (HT) groups, in terms of percent change in vessel diameter from the baseline measurement. Sham animals demonstrated approximately a 14% increase in vessel diameter in response to 50.0 μm adenosine, and a 24% increase in response to 100.0 μm adenosine. In the injured non-treated group the response was reduced (p < 0.05) compared to sham data. In the injured hypothermia-treated animals the response was increased and was no longer significantly different from the sham response (*p < 0.05 in comparison to baseline measurement; †p < 0.05 in comparison to the injured non-treated rats). The legend includes baseline resting diameter measurements (in μm).
FIG. 6.
SNP-induced vasodilation is shown in three groups: sham, non-treated (NT), and hypothermia-treated (HT) groups, in terms of percent change in vessel diameter from the baseline measurement. Sham animals demonstrated approximately an 18% increase in vessel diameter in response to 0.5 μm SNP, and a 34% increase in response to 1.0 μm SNP. In the injured non-treated group the response was compromised and was significantly reduced compared to sham animals. While the vascular response improved in the injured hypothermia-treated animals, it still did not reach sham levels (*p < 0.05 in comparison to baseline measurement; †p < 0.05 in comparison to the injured non-treated rats). At the bottom of the figure are the baseline resting diameter measurements.
Three weeks post-injury in both normothermic and hypothermic animals
At 3 weeks post-injury (Table 1), all normothermic, traumatically brain injured animals showed persisting vascular abnormalities reflected in significantly abnormal vasodilator responses to CO2, ACh, pinacidil, adenosine, and SNP. These changes were consistent across varying concentrations of vasodilator agents used, with most vessels showing 50% or greater reduction in their vasodilator response in comparison to the sham-injured animals. In contrast, those animals injured and treated with hypothermia showed significant vascular protection in response to all the chosen vasodilator challenges; however, this protection was not complete.
Table 1.
Vasodilation Responses 3 Weeks Post-TBI
| |
SHAM (n = 8; 32 vessels) |
INJURED, NT (n = 8; 32 vessels) |
INJURED, HT (n = 8; 32 vessels) |
|||
|---|---|---|---|---|---|---|
| Drug tested | Baseline diameter | Change in diameter (%) | Baseline diameter | Change in diameter (%) | Baseline diameter | Change in diameter (%) |
| CO2 | 33 ± 1.5 | 41 ± 1.6 | 38 ± 1.1 | |||
| 3% | 10 ± 0.7 | 3 ± 0.8* | 7 ± 0.8† | |||
| 5% | 18 ± 1.2 | 5 ± 1.2* | 14 ± 1.6† | |||
| ACh | 33 ± 1.1 | 41 ± 1.6 | 37 ± 1.1 | |||
| 0.1 μm | 10 ± 0.7 | 3 ± 0.7* | 8 ± 0.9† | |||
| 10 μm | 19 ± 1.2 | 6 ± 1.4* | 15 ± 1.5† | |||
| 33 ± 1.2 | 41 ± 1.7 | 36 ± 1.5 | ||||
| 10 ± 0.8 | 3 ± 0.8* | 7 ± 0.8† | ||||
| 19 ± 1.0 | 6 ± 1.3* | 14 ± 1.5† | ||||
| 33 ± 1.6 | 41 ± 1.6 | 38 ± 1.2 | ||||
| 16 ± 0.8 | 6 ± 1.3* | 11 ± 1.0† | ||||
| 29 ± 1.2 | 12 ± 2.0* | 21 ± 1.5† | ||||
| 33 ± 1.5 | 41 ± 1.6 | 38 ± 1.1 | ||||
| 21 ± 1.4 | 10 ± 1.5* | 21 ± 1.6† | ||||
| 39 ± 2.0 | 19 ± 2.6* | 40 ± 3.1† | ||||
The table illustrates pial vascular diameter at 3 weeks post-injury. Baseline diameter (μm) corresponds to the vessel measurement taken at the beginning of each experiment while the vessels were in the resting state. Change in diameter (μm) refers to the change in the vessel measurement induced by various vasodilators (hypercapnia, ACh, pinacidil, adenosine, and SNP). Note that post-traumatic vascular responses significantly differed from the baseline measurements (*p < 0.05). The vascular responses in the injured hypothermia-treated group (HT) significantly improved compared to injured non-treated animals (NT) (†p < 0.05).
Discussion
The results of this study confirm and extend previous observations regarding microvascular dysfunction following traumatic brain injury (Golding et al., 1999; DeWitt and Prough, 2003). In addition, they significantly extend previous observations by now showing that this vascular dysfunction persists not only at 1 week (Ueda et al., 2004), but continues up to 3 weeks post-injury. Of equal importance is the fact that this persistent vascular dysfunction was consistent for all vasodilator challenges utilized, except SNP at 1 week post-injury, with significant abnormalities in the vascular response to hypercapnia, ACh, adenosine, and pinacidil, and manifested at both 1 and 3 weeks post-injury. From a mechanistic perspective, it appears that the traumatically induced activation of free radicals (Ellis et al., 1983; Kontos and Wei, 1986; DeWitt et al., 1988; Smith et al., 1991; Dash et al., 2000; Wei et al., 1981) previously described in this model system, injures cerebral microcirculation on both the endothelial and smooth muscle fronts, rendering both cell types dysfunctional for a relatively prolonged post-traumatic time period. This premise is entirely consistent with the fact that ACh influences vasodilation via the endothelium (Bredt and Snyder, 1994), whereas the other agents employed act on vascular smooth muscle.
Of additional interest in the current study is the fact that post-traumatic hypothermia, in large part, demonstrated protection in attenuating the above-described microvascular dysfunction, and that such attenuation persisted over a 3-week course. This finding is entirely consistent with hypothermia's known protective effects in attenuating free-radical-mediated pathology (Globus et al., 1995), which once blocked or reduced, provides protection, at least in terms of the cerebral microcirculation. We believe these findings are important because they not only confirm the vascular protective effects of hypothermic intervention but also show that this vascular protection can remain operative for a considerable time post-injury, an issue not previously addressed in the literature.
Of additional importance in the current study are the reported behavioral studies utilizing the Morris water maze, which as employed in the current study targets spatial memory. While the behavioral tests targeting motor behavior revealed rapid recovery over 5 days post-injury, spatial memory performance exposed persistent deficits, observations entirely consistent with previous reports (Hamm et al., 1993). Hypothermic treatment, however, did not appear to offer significant enhancement of the MWM task, as demonstrated by injured hypothermia-treated animals who were not significantly different from the injured-untreated group of rats. Thus, while post-traumatic hypothermia protects vascular function, it does not protect or restore spatial memory, which is linked to hippocampal integrity. Although our laboratory has not directly assessed hippocampal structure or function, we have shown that comparable levels of hypothermia can attenuate traumatic axonal damage within the brainstem (Koizumi and Povlishock, 1998). Thus, this finding of no behavioral protection was unanticipated. This was particularly so in that previous well-executed studies utilizing hypothermic interventions (Bramlett et al., 1995; Dixon et al., 1987) reported that the use of hypothermia significantly attenuated spatial memory performance deficits. While at first blush, the validity of our negative findings would be called into question, it is important to note that these important studies used different target temperatures and/or different durations of hypothermic intervention and times of initiation, all of which have been recognized to impact upon the potential benefits of hypothermic intervention (Markgraf et al., 2001; Ueda et al., 2003; Jiang et al., 2006). Thus, although our studies illustrate the long-term vascular protective effects of the strategies used, they also illustrate the limitations of the hypothermic strategy used in terms of any cognitive protection. While this does not obviate the importance of our vascular findings, it does demonstrate that these two pathologies are not necessarily interrelated. Collectively, these findings further illustrate the complexity of TBI and call into question the utility of singular approaches to obtain generalized, enduring brain protection.
In sum, we believe that the above observations significantly enhance our understanding of the pathobiology of TBI, showing that untreated microvascular dysfunction can persist for at least up to 3 weeks post-injury. While the overall implications of these abnormal cerebral arteriolar responses remain unclear, it is most likely that these impaired vascular responses suggest that these animals are still vulnerable in making appropriate regulatory adjustments, should they encounter another injury such as secondary concussion, hypertension, and/or hypoxic insult. Obviously, these issues require further study. However, these issues have merit not only for the understanding of the process of vascular dysfunction in traumatically brain injured rodents, but also for our understanding of traumatically brain-injured patients who have sustained susceptibility to secondary insult (Chi et al., 2006; McHugh et al., 2007). In this context, it is possible to speculate that enduring impaired vasoreactivity may set the stage for a less-than-optimal outcome should secondary insult occur in traumatically brain-injured humans (Chi et al., 2006; McHugh et al., 2007).
Acknowledgments
This work was supported by National Institutes of Health grants HD055813 and NS057175 and the Commonwealth Neurotrauma Trust Fund Grant NCI 04-095.
Author Disclosure Statement
No conflicting financial interests exist.
References
- Bramlett H.M. Dietrich W.D. Green E.J. Busto R. Chronic histopathological consequences of fluid-percussion brain injury in rats: effects of post-traumatic hypothermia. Acta Neuropathol. 1997;93:190–199. doi: 10.1007/s004010050602. [DOI] [PubMed] [Google Scholar]
- Bramlett H.M. Green E.J. Dietrich W.D. Busto R. Globus M.Y. Ginsberg M.D. Posttraumatic brain hypothermia provides protection from sensorimotor and cognitive behavioral deficits. J. Neurotrauma. 1995;12:289–298. doi: 10.1089/neu.1995.12.289. [DOI] [PubMed] [Google Scholar]
- Bredt D.S. Snyder S.H. Nitric oxide: a physiologic messenger molecule. Annu. Rev. Biochem. 1994;63:175–195. doi: 10.1146/annurev.bi.63.070194.001135. [DOI] [PubMed] [Google Scholar]
- Brian J.E., Jr. Carbon dioxide and the cerebral circulation. Anesthesiology. 1998;88:1365–1386. doi: 10.1097/00000542-199805000-00029. [DOI] [PubMed] [Google Scholar]
- Chi J.H. Knudson M.M. Vassar M.J. McCarthy M.C. Shapiro M.B. Mallet S. Holcroft J.J. Moncrief H. Noble J. Wisner D. Kaups K.L. Bennick L.D. Manley G.T. Prehospital hypoxia affects outcome in patients with traumatic brain injury, a prospective multicenter study. J. Trauma. 2006;61:1134–1141. doi: 10.1097/01.ta.0000196644.64653.d8. [DOI] [PubMed] [Google Scholar]
- Cold G.E. Jensen F.T. Malmros R. The cerebrovascular CO2 reactivity during the acute phase of brain injury. Acta Anaesthesiol. Scand. 1977;21:222–231. doi: 10.1111/j.1399-6576.1977.tb01213.x. [DOI] [PubMed] [Google Scholar]
- Dash P.K. Mach S.A. Moore A.N. Regional expression and role of cyclooxygenase-2 following experimental traumatic brain injury. J. Neurotrauma. 2000;17:69–81. doi: 10.1089/neu.2000.17.69. [DOI] [PubMed] [Google Scholar]
- Dewitt D.S. Kong D.L. Lyeth B.G. Jenkins L.W. Hayes R.L. Wooten E.D. Prough D.S. Experimental traumatic brain injury elevates brain prostaglandin E2 and thromboxane B2 levels in rats. J. Neurotrauma. 1988;5:303–313. doi: 10.1089/neu.1988.5.303. [DOI] [PubMed] [Google Scholar]
- DeWitt D.S. Prough D.S. Traumatic cerebral vascular injury: the effects of concussive brain injury on the cerebral vasculature. J. Neurotrauma. 2003;20:795–825. doi: 10.1089/089771503322385755. [DOI] [PubMed] [Google Scholar]
- Dixon C.E. Lyeth B.G. Povlishock J.T. Findling R.L. Hamm R.J. Marmarou A. Young H.F. Hayes R.L. A fluid percussion model of experimental brain injury in the rat. J. Neurosurg. 1987;67:110–119. doi: 10.3171/jns.1987.67.1.0110. [DOI] [PubMed] [Google Scholar]
- Ellis E.F. Wei E.P. Cockrell C.S. Choi S. Kontos H.A. The effect of PGF2 alpha on in vivo cerebral arteriolar diameter in cats and rats. Prostaglandins. 1983;26:917–923. doi: 10.1016/0090-6980(83)90154-5. [DOI] [PubMed] [Google Scholar]
- Enevoldsen E.M. Jensen F.T. Autoregulation and CO2 responses of cerebral blood flow in patients with acute severe head injury. J Neurosurg. 1978;48:689–703. doi: 10.3171/jns.1978.48.5.0689. [DOI] [PubMed] [Google Scholar]
- Faraci F.M. Heistad D.D. Regulation of the cerebral circulation: role of endothelium and potassium channels. Physiol. Rev. 1998;78:53–97. doi: 10.1152/physrev.1998.78.1.53. [DOI] [PubMed] [Google Scholar]
- Furchgott R.F. Role of endothelium in responses of vascular smooth muscle. Circ. Res. 1983;53:557–573. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- Globus M.Y. Alonso O. Dietrich W.D. Busto R. Ginsberg M.D. Glutamate release and free radical production following brain injury: effects of posttraumatic hypothermia. J. Neurochem. 1995;65:1704–1711. doi: 10.1046/j.1471-4159.1995.65041704.x. [DOI] [PubMed] [Google Scholar]
- Golding E.M. Robertson C.S. Bryan R.M., Jr. The consequences of traumatic brain injury on cerebral blood flow and autoregulation: a review. Clin. Exp. Hypertens. 1999;21:299–332. doi: 10.3109/10641969909068668. [DOI] [PubMed] [Google Scholar]
- Hamm R.J. Lyeth B.G. Jenkins L.W. O'Dell D.M. Pike B.R. Selective cognitive impairment following traumatic brain injury in rats. Behav. Brain Res. 1993;59:169–173. doi: 10.1016/0166-4328(93)90164-l. [DOI] [PubMed] [Google Scholar]
- Hicks R.R. Smith D.H. Lowenstein D.H. Saint Marie R. McIntosh T.K. Mild experimental brain injury in the rat induces cognitive deficits associated with regional neuronal loss in the hippocampus. J. Neurotrauma. 1993;10:405–414. doi: 10.1089/neu.1993.10.405. [DOI] [PubMed] [Google Scholar]
- Jiang J.Y. Lyeth B.G. Clifton G.L. Jenkins L.W. Hamm R.J. Hayes R.L. Relationship between body and brain temperature in traumatically brain-injured rodents. J. Neurosurg. 1991;74:492–496. doi: 10.3171/jns.1991.74.3.0492. [DOI] [PubMed] [Google Scholar]
- Jiang J.Y. Xu W. Li W.P. Gao G.Y. Bao Y.H. Liang Y.M. Luo Q.Z. Effect of long-term mild hypothermia or short-term mild hypothermia on outcome of patients with severe traumatic brain injury. J. Cereb. Blood Flow Metab. 2006;26:771–776. doi: 10.1038/sj.jcbfm.9600253. [DOI] [PubMed] [Google Scholar]
- Kitazono T. Faraci F.M. Taguchi H. Heistad D.D. Role of potassium channels in cerebral blood vessels. Stroke. 1995;26:1713–1723. doi: 10.1161/01.str.26.9.1713. [DOI] [PubMed] [Google Scholar]
- Koizumi H. Povlishock J.T. Posttraumatic hypothermia in the treatment of axonal damage in an animal model of traumatic axonal injury. J. Neurosurg. 1998;89:303–309. doi: 10.3171/jns.1998.89.2.0303. [DOI] [PubMed] [Google Scholar]
- Kontos H.A. Dietrich W.D. Wei E.P. Ellis E.F. Povlishock J.T. Abnormalities of the cerebral microcirculation after traumatic injury: the relationship of hypertension and prostaglandins. Adv. Exp. Med. Biol. 1980;131:243–256. doi: 10.1007/978-1-4684-3752-2_19. [DOI] [PubMed] [Google Scholar]
- Kontos H.A. Wei E.P. Cerebral arteriolar dilations by KATP channel activators need L-lysine or L-arginine. Am. J. Physiol. 1998;274:H974–H981. doi: 10.1152/ajpheart.1998.274.3.H974. [DOI] [PubMed] [Google Scholar]
- Kontos H.A. Wei E.P. Endothelium-dependent responses after experimental brain injury. J. Neurotrauma. 1992;9:349–354. doi: 10.1089/neu.1992.9.349. [DOI] [PubMed] [Google Scholar]
- Kontos H.A. Wei E.P. Superoxide production in experimental brain injury. J. Neurosurg. 1986;64:803–807. doi: 10.3171/jns.1986.64.5.0803. [DOI] [PubMed] [Google Scholar]
- Kontos H.A. Wei E.P. Marshall J.J. In vivo bioassay of endothelium-derived relaxing factor. Am. J. Physiol. 1988;255:H1259–H1262. doi: 10.1152/ajpheart.1988.255.5.H1259. [DOI] [PubMed] [Google Scholar]
- Kontos H.A. Wei E.P. Povlishock J.T. Pathophysiology of vascular consequences of experimental concussive brain injury. Trans. Am. Clin. Climatol. Assoc. 1981;92:111–121. [PMC free article] [PubMed] [Google Scholar]
- Levasseur J.E. Wei E.P. Raper A.J. Kontos A.A. Patterson J.L. Detailed description of a cranial window technique for acute and chronic experiments. Stroke. 1975;6:308–317. doi: 10.1161/01.str.6.3.308. [DOI] [PubMed] [Google Scholar]
- Markgraf C.G. Clifton G.L. Moody M.R. Treatment window for hypothermia in brain injury. J. Neurosurg. 2001;95:979–983. doi: 10.3171/jns.2001.95.6.0979. [DOI] [PubMed] [Google Scholar]
- McHugh G.S. Engel D.C. Butcher I. Steyerberg E.W. Lu J. Mushkudiani N. Hernandez A.V. Marmarou A. Maas A.I. Murray G.D. Prognostic value of secondary insults in traumatic brain injury: results from the IMPACT study. J. Neurotrauma. 2007;24:287–293. doi: 10.1089/neu.2006.0031. [DOI] [PubMed] [Google Scholar]
- McIntosh T.K. Vink R. Noble L. Yamakami I. Fernyak S. Soares H. Faden A.L. Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience. 1989;28:233–244. doi: 10.1016/0306-4522(89)90247-9. [DOI] [PubMed] [Google Scholar]
- Morris R.G. Garrud P. Rawlins J.N. O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Povlishock J.T. Ueda Y. Wei E.P. A review of the protective effects of hypothermia on the axonal and vascular pathobiology associated with TBI. In: Hayashi N., editor; Bullock R., editor; Dietrich D.W., editor; Maekawa T., editor; Tamura A., editor. Hypothermia for Acute Brain Damage. Springer; Tokyo: 2004. pp. 19–23. [Google Scholar]
- Quayle J.M. Nelson M.T. Standen N.B. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol. Rev. 1997;77:1165–1232. doi: 10.1152/physrev.1997.77.4.1165. [DOI] [PubMed] [Google Scholar]
- Smith D.H. Okiyama K. Thomas M.J. Claussen B. McIntosh T.K. Evaluation of memory dysfunction following experimental brain injury using the Morris water maze. J. Neurotrauma. 1991;8:259–269. doi: 10.1089/neu.1991.8.259. [DOI] [PubMed] [Google Scholar]
- Suehiro E. Ueda Y. Wei E.P. Kontos H.A. Povlishock J.T. Posttraumatic hypothermia followed by slow rewarming protects the cerebral microcirculation. J. Neurotrauma. 2003;20:381–390. doi: 10.1089/089771503765172336. [DOI] [PubMed] [Google Scholar]
- Ueda Y. Wei E.P. Povlishock J.T. Pial microcirculation evaluated by closed cranial window method 7 days after impact acceleration injury in rats: does posttraumatic hypothermia provide persistent pial vascular protection. In: Hayashi N., editor; Bullock R., editor; Dietrich D.W., editor; Maekawa T., editor; Tamura A., editor. Hypothermia for Acute Brain Damage. Springer; Tokyo: 2004. pp. 141–144. [Google Scholar]
- Ueda Y. Wei E.P. Kontos H.A. Suehiro E. Povlishock J.T. Effects of delayed, prolonged hypothermia on the pial vascular response after traumatic brain injury in rats. J. Neurosurg. 2003;99:899–906. doi: 10.3171/jns.2003.99.5.0899. [DOI] [PubMed] [Google Scholar]
- Wei E.P. Dietrich W.D. Povlishock J.T. Navari R.M. Kontos H.A. Functional, morphological, and metabolic abnormalities of the cerebral microcirculation after concussive brain injury in cats. Circ. Res. 1980;46:37–47. doi: 10.1161/01.res.46.1.37. [DOI] [PubMed] [Google Scholar]
- Wei E.P. Kontos H.A. Dietrich W.D. Povlishock J.T. Ellis E.F. Inhibition by free radical scavengers and by cyclooxygenase inhibitors of pial arteriolar abnormalities from concussive brain injury in cats. Circ. Res. 1981;48:95–103. doi: 10.1161/01.res.48.1.95. [DOI] [PubMed] [Google Scholar]
- Wei E.P. Kukreja R. Kontos H.A. Effects in cats of inhibition of nitric oxide synthesis on cerebral vasodilation and endothelium-derived relaxing factor from acetylcholine. Stroke. 1992;23:1623–1628. doi: 10.1161/01.str.23.11.1623. ; discussion 1628–1629. [DOI] [PubMed] [Google Scholar]