Abstract
Traumatic brain injury (TBI) produces αII-spectrin breakdown products (SBDPs) that are potential biomarkers for TBI. To further understand these biomarkers, the present study examined (1) the exposure and kinetic characteristics of SBDPs in cerebrospinal fluid (CSF) of adults with severe TBI, and (2) the relationship between these exposure and kinetic metrics and severity of injury. This clinical database study analyzed CSF concentrations of 150-, 145-, and 120-kDa SBDPs in 38 severe TBI patients. Area under the curve (AUC), mean residence time (MRT), maximum concentration (Cmax), time to maximum concentration (Tmax), and half-life (t1/2) were determined for each SBDP. Markers of calpain proteolysis (SBDP150 and SBDP145) had a greater median AUC and Cmax and a shorter MRT than SBDP120, produced by caspase-3 proteolysis in the CSF in TBI patients (p < 0.001). AUC and MRT for SBDP150 and SBDP15 were significantly greater in patients with worse Glasgow Coma Scale (GCS) scores at 24 h after injury compared to those whose GCS scores improved (AUC p = 0.013, MRT p = 0.001; AUC p = 0.009, MRT p = 0.021, respectively). A positive correlation was found between patients with longer elevations in intracranial pressure (ICP) measurements of 25 mm Hg or higher and those with a greater AUC and MRT for all three biomarkers. This is the first study to show that the biomarkers of proteolysis differentially associated with calpain and caspase-3 activity have distinct CSF exposure profiles following TBI that suggest a prominent role for calpain activity. Further studies are being conducted to determine if exposure and kinetic metrics for biofluid-based biomarkers can predict clinical outcome.
Key words: biomarker, CSF, exposure, spectrin, traumatic brain injury
Introduction
In the United States, there are more than 1 million traumatic brain injury (TBI) cases annually, resulting in more than 230,000 hospitalizations, 50,000 deaths, and 80,000 patients with disabilities (Langlois et al., 2005; Binder et al., 2005). To date, there are no effective neuroprotective agents for TBI patients, but biochemical markers could provide confirmation of the injury mechanism and identify candidate drug therapy targets (Ghanem et al., 2001; Foerch et al., 2005; Korfias et al., 2006; Wunderlich et al., 2006; Tanaka et al., 2007).
In a prospective case-control study, we recently determined that αII-spectrin proteolysis, as assessed in CSF, is a potentially reliable biomarker for severe TBI in humans (Pineda et al., 2007). This study examined changes in 150-kDa and 145-kDa αII-spectrin breakdown products (SBDPs) produced primarily by calpain (SBDP150 and SBDP145), and 120-kDa SBDPs produced by caspase-3. Calpain proteolysis is primarily, but not exclusively, associated with oncotic necrosis, while caspase-3 proteolysis is primarily associated with apoptosis (Wang, 2000). However, there is currently no literature available on the actual cerebrospinal fluid (CSF) exposure and kinetic characteristics of the potential brain injury biomarkers for calpain- and caspase-3–related cell death, SBDP150, SBDP140, and SBDP120. Since these biomarkers are released from the brain into the CSF and then potentially distributed to other body compartments (e.g., blood), it is extremely important to define the exposure and kinetic characteristics in the first compartment in which they are measurable in most patients, the CSF.
This is the first study to describe the exposure and kinetic metrics of SBDPs in the CSF of TBI patients. In addition, exposure and kinetic metric changes in relation to severity of injury are described.
Methods
Database sample values for this study came from a prospective, case-control study that included data from two sources: from a prospective study conducted at the University of Florida (n = 19), and from a CSF bank at Baylor College of Medicine (n = 19). The overall study was approved by the Virginia Commonwealth University and the University of Florida Institutional Review Board (IRB). Patients included in the prospective, case-control study presented to the University of Florida Trauma System (Shands Hospitals in Gainesville and Jacksonville) following a severe head injury, as defined by a GCS score of ≤ 8, and by requiring ventricular intracranial pressure (ICP) monitoring as part of their routine clinical care. This was a convenience sample, as not all consecutive patients were enrolled. Patients were enrolled in the prospective study for a 16-month period, starting in April of 2003, and were followed for 6 months after study entry. The banked samples were from patients admitted to Ben Taub General Hospital in Houston, Texas, between August 29, 2003 and August 30, 2004 with a GCS < 8, under an IRB-approved prospective study protocol from Baylor College of Medicine. De-identified CSF samples and clinical information were provided to the investigators at the University of Florida. All patient identifiers were kept confidential by the study principal investigator (PI) at the University of Florida, and de-identified data from the study database were provided to the PI at Virginia Commonwealth University for this analysis. The clinical management of patients enrolled in both centers was guided by the published “Guidelines for Management and Prognosis of Severe Traumatic Brain Injury” (2000).
Patient data
Thirty-eight patients had CSF data available for exposure and kinetic metric analysis. CSF data from samples directly collected from the ventriculostomy catheter at 6, 12, and 24 h post-TBI, and then every 24 h for up to 12 days were used for the exposure and kinetic analysis of SBDP150, SBDP145, and SBDP120. A standard protocol for CSF collection was used in order to minimize external influences on biomarker concentration. In addition, a control group of 11 patients without TBI who required CSF sample collection from ventriculoperitoneal shunts or intraoperatively as part of their routine care during the study time period were also evaluated to verify that a change did occur in biomarker concentrations in the TBI patients. Exposure and kinetic evaluation of the control group CSF samples could not be done, as only one sample was available per patient. Approximately 3–4 mL of CSF were collected from each subject at each time point. αII-spectrin is a non-erythrocytic protein, so it is unlikely that the presence of blood in the CSF would confound measurements of αII-spectrin or its degradation products. However, to limit possible confounders, samples were immediately centrifuged for 10 min at 4°C to separate CSF from blood cells and other debris, and immediately frozen and stored at 70° as aliquots until the time of analysis. The time frame from sample collection to analysis varied up to 1 year, but this should not impact concentrations, as our analysis technique has produced stable results for up to 2 years (data not shown).
An immunoblot assay was used in the prospective study to determine the concentrations of SBDPs in the CSF samples. The CSF samples (7 μL) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis for 2 h. Following electrophoresis, separated proteins were laterally transferred to polyvinylidene fluoride for 2 h at ambient temperature in a semi-dry transfer unit (Bio-Rad, Hercules, CA). After electrotransfer, blotting membranes were blocked for 1 h at ambient temperature, and then incubated as recommended by the manufacturer at 4°C overnight. This was followed by three washes with tris-buffered saline tween and 2 h incubation at ambient temperature. After additional washes of the blots, electrochemoluminescent reagents (Amersham, Pittsburgh, PA) were used to visualize the immunolabeling on x-ray film. Molecular weights of intact αII-spectrin and SBDPs were assessed by running alongside rainbow-colored molecular weight standards (Amersham). Quantitative evaluations of SBDP levels were performed via computer-assisted densitometric scanning (Epson XL3500 high-resolution flatbed scanner; Epson America, Inc., Long Beach, CA) and image analysis with Image-J software (National Institutes of Health). Additional details of the immunoblot assay are described elsewhere. Arbitrary densitometric units (ADU) were used to describe the CSF biomarker concentrations. The lower limit of detection for this assay was 0.1 ADU.
Exposure and kinetic analysis
Exposure metrics describe the amount and duration of biomarker exposure, and the metrics evaluated in this study were area under the curve (AUC), mean residence time (MRT), maximum concentration (Cmax), and time to maximum concentration (Tmax). Half-life (t1/2) was the kinetic metric evaluated in this study and it describes the rate of decline of the biomarker in the CSF. These exposure and kinetic metrics were determined for each biomarker. Non-compartmental kinetic equations as described in Pharmacokinetics (Gibaldi and Peirrier, 1982) were used to determine AUC, MRT, and t1/2. AUC and area under the first moment curve (AUMC) were calculated by the linear trapezoidal rule to the last observed time point and these were used to determine MRT (MRT = AUMC/AUC). The rate constant for decline (λ) was estimated from log linear regression of two points between which the greatest decrease in concentration occurred. The rate constant for decline was used to determine the biomarker half-life (t1/2 = ln(2)/λ). Biomarker Cmax and Tmax were determined from the observed CSF concentrations. All kinetic equations used to determine these exposure and kinetic characteristics can be found in Table 1. Baseline levels for the brain injury biomarkers studied were assumed to be zero for these analyses. However, the concentration of these biomarkers in each patient prior to their brain injury and ventriculostomy catheter placement is unknown. In addition, control patients from our original clinical trial showed significantly lower baseline concentrations compared to our TBI patients (Pineda et al., 2007). Patients had a variable number of samples available for analysis due to early discontinuation of the ventriculostomy catheter in patients who improved, making it medically unnecessary, or patient death. All patients with data available for a time period of more than 24 h were included in the exposure metric analyses; however, only patients with at least two measurable CSF biomarker levels during the study period were included in the half-life kinetic analysis. Thirty-two patients (84%) in this study had at least four data points available for exposure and kinetic analyses for the biomarkers analyzed. Seventy-eight percent of study patients had sample data available up to 7 days post-injury and were distributed as follows: 2 days, n = 3; 3 days, n = 8; 4 days, n = 2; 5 days, n = 4; 6 days, n = 2; 7 days, n = 11. The remainder of the study patients (n = 8) had samples available up to 12 days post-injury.
Table 1.
Kinetic Equations Used to Determine Biomarker Exposure and Kinetic Metrics
| AUCtrap(tn) = sum i = 1 to n ((ci + ci − 1) * (ti − ti − 1)/2) |
| AUMCtrap(tn) = sum i = 1 to n ((ci * ti + ci − 1 * ti − 1) * (ti − ti − 1)/2) |
| MRT = AUMCtrap/AUCtrap |
| t1/2 = ln(2)/lambda |
Relationships between biomarker exposure and kinetic metrics and clinical variables
The relationship between select exposure and kinetic metrics of SBDPs and clinical variables was also evaluated. These studies focused on (1) relationships with injury magnitude, and (2) secondary insults associated with ICP increases. Severity of injury was assessed using the post-resuscitation GCS score. The number of hours ICP measurements were 25 mm Hg or higher as documented hourly in the medical record up to 7 days post-injury was used to identify patients who had severely elevated ICP. We hypothesized that patients with frequent severe elevations in ICP (secondary insult) would have a greater AUC. AUC represents the concentration of biomarker in CSF over time, and is expected to increase with secondary insults as with increases in ICP. For the purpose of this analysis, we used an ICP measurement breakpoint of ≥25 mm Hg, the recommended threshold for treatment of ICP in severe TBI patients as set forth in the “Guidelines for Management and Prognosis of Severe Traumatic Brain Injury.”
Statistical analysis
For statistical analysis, biomarker exposure and kinetic metrics were expressed as median (range), as the data were not normally distributed. Analysis was performed using the Mann-Whitney U test and Kruskall-Wallis test. Correlations were performed using the Spearman Rho. Statistical significance was set at a p < 0.05. The number of subjects at each time point (n values) represent patients who had both clinical information and CSF samples available for analysis. All analyses were performed using the statistical software package SPSS version 10.0.5 (SPSS, Inc., Chicago, IL).
Results
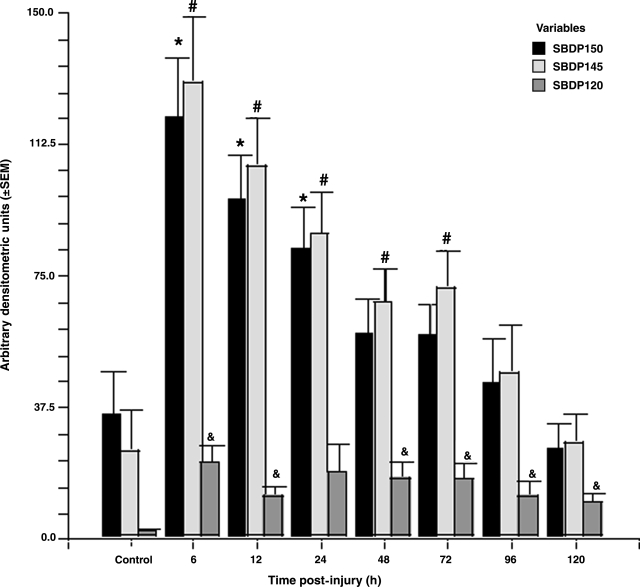
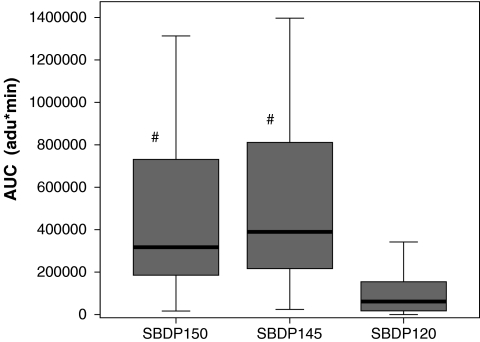
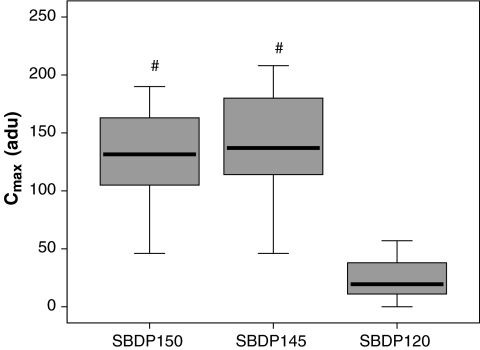
Patient demographics are shown in Table 2 and a comparison of levels of SBDPs in patients with TBI versus controls at all time points are shown in Figure 1. Calpain-mediated SBDP150 and SBDP145 were found to have different exposure characteristics than the caspase-3–mediated SBDP120 marker in the CSF of TBI patients. Table 3 shows a summary of the median patient exposure and kinetic characteristics for the patients included in the study. When compared to SBDP120, the median AUC (Fig. 2) and Cmax (Fig. 3) were significantly greater, and MRT was significantly shorter for SBDP150 and SBDP145.
Table 2.
Patient Demographics
| Characteristic | Total (n = 35) |
|---|---|
| Age, mean years (SD) | 36 (16) |
| Gender, % male | 77% |
| Median scene GCS (range) | 4 (3–8) |
| Median post-resuscitation GCS (range) | 5 (3–8) |
| Marshall classification | |
| Diffuse injury class I | 2 |
| Diffuse injury class II | 14 |
| Diffuse injury class III | 4 |
| Diffuse injury class IV | 0 |
| Evacuated mass lesion | 14 |
| Non-evacuated mass lesion | 1 |
| Pre-ICU hypoxia | 31% |
| Pre-ICU hypotension | 9% |
Only 35 patients had clinical data available for analysis.
FIG. 1.
Comparison of levels of SBDPs in patients with TBI versus controls at all time points up to 5 days post-injury. Levels of SBDP150 and SBDP145 were predominantly elevated in the first 24–72 h post-injury, while SBDP120 levels were significantly elevated at all time points except 24 h post-injury in TBI patients compared to controls (n = 22–47 for each time point; *, #, &p < 0.05 compared to controls). Values represent arbitrary densitometry units means ± SEM. Reproduction of authors' work (Pineda et al., 2007).
Table 3.
Summary of Median Exposure and Kinetic Characteristics for Patients with Severe TBI
| SBDP150 (n = 38) | SBDP145 (n = 38) | SBDP120 (n = 38) | p Value | |
|---|---|---|---|---|
| Median AUC [arbitrary densitometric units * minutes] (range) | 317384 (16766–1313176) |
389759 (24275–1396481) |
61530 (0–635663) |
< 0.001 |
| Median MRT [hours] (range) | 52 (16–160) |
55 (17–191) |
65 (0–179) |
< 0.001 |
| Mean t1/2# [hours] (range) | 24 (5–108) |
23 (4–217) |
40 (4–347) |
< 0.176 |
| Median Cmax [arbitrary densitometric units * minutes] (range) | 132 (3–190) |
137 (10–208) |
20 (0–146) |
< 0.001 |
| Median Tmax [hours] (range) | 34 (3–240) |
34 (3–240) |
56 (3–240) |
< 0.086 |
SBDP150 (n = 34); SBDP145 (n = 35); SBDP120 (n = 25).
FIG. 2.
Comparison of median area under the curve (AUC) for SBDP150, SBDP145, and SBDP120. The differences in AUC were statistically significant between SBDP150 and SBDP120 (p < 0.001), and also between SBDP145 and SBDP120 (p < 0.001). There was no significant difference between SBDP150 and SBDP145. AUC is expressed in arbitrary densitometric units (adu)*min. The line in the box indicates the median value of the data, the upper edge of the box indicates the 75th percentile of the data set, and the lower edge indicates the 25th percentile. The ends of the vertical lines indicate the minimum and maximum data values (#p < 0.05 compared to SBDP120).
FIG. 3.
Comparison of maximum concentrations (Cmax) for SBDP150, SBDP145, and SBDP120. The differences in median Cmax were significant between SBDP150 and SBDP120 (p < 0.001), and also between SBDP145 and SBDP120 (p <0.001). There was no significant difference between SBDP150 and SBDP145. Cmax is expressed in arbitrary densitometric units (adu). The line in the box indicates the median value of the data, the upper edge of the box indicates the 75th percentile of the data set, and the lower edge indicates the 25th percentile. The ends of the vertical lines indicate the minimum and maximum data values (#p < 0.05 compared to SBDP120).
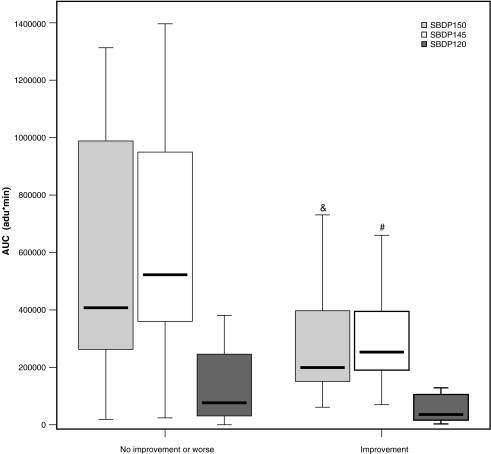
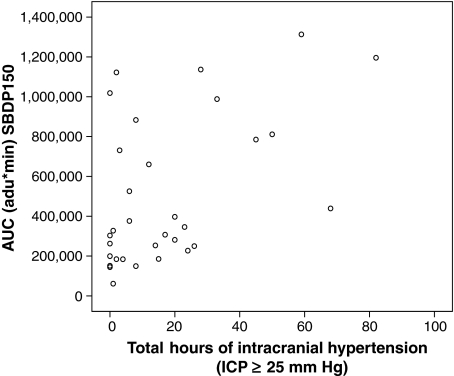
Median AUC and Cmax for SBDP150 and SBDP145 were significantly greater in patients with no improvement or declining (worse) GCS scores at 24 h post-injury compared to those whose GCS scores improved (AUC p = 0.013, Cmax p = 0.001; AUC p = 0.009, Cmax p = 0.021, respectively) (Fig. 4). MRT was shorter for SBDP120 in patients whose GCS improved versus those who did not. Evaluation of the number of hours ICP elevations were 25 mm Hg or higher and AUC showed that the longer the level in the ICP remained elevated, the greater the AUC for SBDP150. As noted in Table 3, the median Tmax and MRT were longer for SBDP120 than SBDP145 and SBDP150, but the differences were not significant. The rates of decline (expressed as the half-life, t1/2) were similar for all three biomarkers (p = 0.207). There was a positive correlation between the number of hours the ICP was 25 mm Hg or higher and (1) AUC (n = 33) for SBDP150 [correlation coefficient 0.5; p = 0.03] (Fig. 5), SBDP145 [correlation coefficient 0.2; p = 0.015], and SBDP120 [correlation coefficient 0.74; p = 0.005]; (2) MRT for SBDP150 [correlation coefficient 0.41; p = 0.02], SBDP145 [correlation coefficient 0.41; p = 0.02], and SBDP120 [correlation coefficient 0.48; p = 0.005]; and (3) Tmax for SBDP120 [correlation coefficient 0.48; p = 0.005].
FIG. 4.
Comparison of area under the curve (AUC) and changes in GCS scores from post-resuscitation to day 1. There was a statistically significant difference in median AUC for SBDP150 (p = 0.013) and SBDP145 (p = 0.009), but not SBDP120 (p = 0.322), between patients who had no improvement or worsening GCS (n = 22), compared to those who improved (n = 13) during this time period. AUC is expressed in arbitrary densitometric units (adu)*min. The line in the box indicates the median value of the data, the upper edge of the box indicates the 75th percentile of the data set, and the lower edge indicates the 25th percentile. The ends of the vertical lines indicate the minimum and maximum data values (&p < 0.05 compared to SBDP150 in the improved group; #p < 0.05 compared to SBDP145 in the improved group).
FIG. 5.
Correlation between the number of hours ICP elevations were 25 mm Hg or higher and area under the curve (AUC) for SBDP150. This figure shows the positive correlation between the number of hours ICP was 25 mm Hg or higher and AUC (n = 33) for SBDP150 [correlation coefficient 0.5; p = 0.003]. AUC is expressed in arbitrary densitometric units (adu)*min.
Discussion
αII-spectrin breakdown products are potential biomarkers of injury severity in severe TBI patients (Pineda et al., 2007), and the exposure and kinetic characteristics of SBDP150, SBDP145, and SBDP120 were described for the first time in this study. The results of this analysis show that exposure characteristics differ for caspase-3–mediated SBDP120 and calpain-mediated SBDP150 and SBDP145, and suggested a potentially greater contribution of calpain activation to the acute pathophysiology of TBI. These data support our original study results that showed differences in the mean concentrations of these biomarkers at various time points after injury versus control patients (Pineda et al., 2007). Associations between mean concentration of these biomarkers at selected time points and severity of injury, such as best GCS score 1 day post-injury, or secondary insults such as pre-ICU hypotension were also elucidated (Pineda et al., 2007).
The amount of each biomarker present in the CSF over time is reflected in the AUC. The AUCs for SBDP150 and SBDP145 were statistically significantly greater than the AUC for SBDP120. We found a five and sixfold increase in the AUC of SBDP150 and SBDP145, respectively, compared to SBDP120, during the study period. This was possibly due to the earlier and higher peak concentrations (Tmax and Cmax) in SBDP150 and SBDP145 that were observed compared to SBDP120. The median Cmax for SBDP150 and SBDP145 was approximately 6.5-fold higher than the Cmax observed for SBDP120 (p < 0.001), with the highest Cmax observed for SBDP120 being similar to the median Cmax for SBDP150 and SBDP145, and occurring 10 days post-injury. The median Tmax (time to Cmax) was 1.5-fold longer for SBDP120 compared to the other two biomarkers; however, statistically significant differences were not found during the short time period of this study (p = 0.086). The increased amount of exposure indicated by calpain- versus caspase-3–biomarker AUC and Cmax suggest more calpain than caspase-3 activity acutely after TBI. This suggests a predominance of necrosis versus apoptotic cell death acutely following TBI. However, it is possible that activation of these proteases may be related to other functions of activation of these proteases, including synaptic remodeling (Wu and Lynch, 2006; Louneva et al., 2008; Liu et al., 2006). To fully understand caspase-3 activity and changes in SBDP120, studies with a longer duration of evaluation need to be conducted.
The median half-life was approximately 1 day for SBDP150 (n = 34) and SBDP145 (n = 35), and was 1.5 days for SBDP120 (n = 25). The half-life was determined from the rate of decline between only two time points in most patients, which warrants cautious interpretation. The average amount of time it takes a majority (approximately two-thirds) of the biomarkers to be eliminated from the CSF (duration of exposure) is expressed by the MRT. MRT is calculated by dividing the AUC by the AUMC (Table 1), which takes into account how fast the biomarkers are produced and how fast they decline. This delay in the Cmax suggests that caspase-3–mediated apoptotic injury and cell death continues beyond the time period of our study, and SBDP120 may not even reach its maximum concentration until much later in the course of injury. The MRT ranged from approximately 2 (SBDP150 and SBDP145) to 3 (SBDP120) days, and the differences were statistically significant (p < 0.001). The shorter MRT for calpain-associated SBDPs, in addition to the AUC and Cmax findings, support the suggestion of acute “over-activation” of calpain versus caspase-3 proteolysis after TBI. Ongoing SBDP biomarker studies evaluating these kinetic and exposure characteristics over a longer period of time will provide additional insight into the duration of CSF calpain and caspase-3 activity.
Overall, direct comparisons of the exposure and kinetic characteristics for SBDP150, SBDP145, and SBDP120, show that there is a statistically significant difference in the amount of biomarker produced over a period of time (AUC), the duration of exposure of the biomarkers (MRT), and the peak concentration (Cmax) of biomarkers observed. These exposure-characteristic differences suggest that calpain may play a relatively greater role in acute pathological responses to TBI than caspase-3, which may indicate a predominance of necrotic versus apoptotic cell death.
Exposure and kinetic characteristics of these biomarkers were also compared to initial severity of injury as assessed by GCS. There were no statistically significant differences in the biomarker exposure or kinetic characteristics for patients with a post-resuscitation GCS of 3–5 versus a GCS of 6–8. The median AUC, MRT, Cmax, and Tmax were also compared to the change in GCS from post-resuscitation until day 1 (best day 1 GCS score). Using the 24-h trend in GCS score reduces the influence of factors other than severity of injury, but inherent limitations of the GCS cannot be completely avoided. The AUC and Cmax for SBDP150 and SBDP145 were less than those for patients who improved over this short period of time, with the AUC decreasing by approximately half. There was no change in AUC or Cmax for SBDP120; however, the MRT was significantly shorter for SBDP120 in patients that improved compared to those who had no improvement or worsening GCS. As expected, patients in whom GCS improved in the first 24 h have less biochemical evidence of brain injury or SBDP150 and SBDP145. The MRT is probably the best kinetic estimate of the clinical duration of biomarker production after injury. These differences in GCS and biomarker exposure metrics could be influenced by differences in management between the two groups of patients and reflect response to management more than severity of injury.
We also compared AUC, MRT, Cmax, and Tmax to the number of hours documenting ICP of 25 mm Hg or higher to try to evaluate if those with more hours of ICP elevations had greater biomarker production than those with fewer documented hours of elevations in ICP. Patients who had more hours of elevations in ICP had statistically significant increases in AUC and MRT for all three biomarkers, indicating that patients who have longer elevations in ICP also have increases in the amount and duration of SBDP production. Tmax for SBDP120 also increased with longer durations of elevated ICP. These comparisons of severity of injury and exposure metrics suggest that production of SBDP150, SBDP145, and SBDP120 increase when the severity of injury increases, or when severe TBI is associated with repeated secondary increases in ICP, a potential additional injury adversely affecting outcome.
Overall, studies analyzing exposure and kinetic characteristics of CSF brain injury biomarkers are scarce. This pilot study provides the first rigorous assessment of SBDP exposure and kinetic metrics and lays the necessary foundation for future research. However, there are some limitations to an exposure and kinetic study of CSF biomarkers. This is an exposure and kinetic analysis of a prospective, case-control clinical study database; therefore, it is limited to the patient data that were documented for the original study and the biomarker concentrations time points available. Since there are no previous studies modeling the compartmental distribution of the biomarkers in the CSF, non-compartmental pharmacokinetic metrics were used. Baseline concentrations of these biomarkers are unknown; therefore, an assumption was made for the concentration at time zero. CSF samples were not available for all time points, as the rapidly changing clinical condition of these critically ill patients made sample collection very difficult. Therefore, all available samples were included in the exposure and kinetic analyses to maximize the sample size for this analysis. Additionally, a majority of the data available for analysis in this pilot study were from admission up to 7 days post-injury, so the full kinetic profile of SBDP120, whose peak concentration appears to be prolonged compared to SBDP150 and SBDP145, could not be fully elucidated. The AUC and MRT calculations were calculated only from the time the ventricular catheter was inserted until the time it was discontinued or the death of the patient; therefore, the time period for sample collection was not consistent between patients and the results may not be completely comparable. However, these data provide an initial assessment of relationships between exposure metrics and TBI injury processes.
It is not known how ventriculostomy drainage affects the concentration of these biomarkers in the CSF, and both intermittent and continuous drainage methods were used in the patients included in our study. Multiple confounders, such as duration of biomarker production, continuous CSF production, and the fact that TBI patients require CSF drainage during periods of elevated ICP, make it extremely difficult to determine if and/or how CSF ventriculostomy drainage impacts the exposure and kinetic characteristics of these biomarkers. Shore and colleagues suggest that continuous versus intermittent ventricular catheter drainage affects biomarker concentrations, with higher CSF biomarker concentrations occurring in patients with intermittent drainage (Shore et al., 2004). The authors of this small study point out multiple confounders that make it difficult to determine a cause-and-effect relationship between these methods of drainage. Without a consistent rate of CSF drainage from the ventriculostomy catheter, and without knowing how the biomarkers distribute into other compartments, it is difficult to determine how the biomarker concentrations are affected by CSF drainage. Ongoing studies by our group are currently addressing this question. Finally, our analysis of relationships between exposure and kinetic metrics and ICP is limited by the fact that ICP information was obtained from the medical record. Future studies using high-resolution ICP data collection may further refine this analysis.
In summary, the data presented here are the first attempt to describe CSF exposure and kinetic characteristics of biomarkers after severe TBI. These initial assessments have provided potentially important insights into differences between the accumulation of biomarkers possibly reflecting calpain activity (SBDP150 and SBDP145) versus a biomarker of caspase-3 activity (SBDP120). We consistently observed evidence of a greater production of calpain-mediated biomarkers than caspase-3–mediated biomarkers in the acute phase following severe TBI. In addition, calpain markers, but not the caspase-3 marker, were reliably associated with acute injury magnitude. Increases in both calpain and caspase-3 markers were correlated with secondary increases in ICP. Thus, calpain-mediated necrotic mechanisms may be a major contributor to acute pathophysiological responses to TBI, as well as secondary insults produced by increased ICP. However, the limited time frame of this study did not allow more definitive characterization of potentially delayed and prolonged changes in the exposure and kinetic metrics of SBDP120, a possible biomarker of apoptotic mechanisms. This delayed profile for manifestation of apoptotic processes is consistent with a number of laboratory observations suggesting a sustained and delayed profile for apoptotic cell death (Wang, 2000; Nath et al., 1999). More detailed studies will provide critical information on the temporal characteristics of these two important cell death mechanisms.
Calpain activity markers (SBDP145 and SBDP150) were found to have different exposure characteristics than the caspase-3–mediated SBDP120, in the CSF of TBI patients. These exposure differences suggest that different proteolytic mechanisms occur after severe TBI over time, with calpain “over-activation” occurring sooner than caspase-3 “over-activation.” Ongoing studies by our group are currently being conducted to more fully elucidate calpain- and caspase-3–mediated SBDP biomarker exposure and kinetic metrics in CSF and blood, and their relationships to severity of injury and clinical outcomes in severe TBI patients, as well as the contribution of CSF drainage techniques to exposure and kinetic metrics.
Acknowledgments
This study was supported in part by Department of Defense Award numbers DAMD17-03-1-0772 and DAMD17-03-1-0066; National Institutes of Health Award numbers R01 NS049175-01, R01-NS052831-01, and R01 NS051431-01; Navy grant number N00014-06-1-1029 (University of Florida); and National Institutes of Health grant #P01-NS38660 (Baylor College of Medicine).
Author Disclosure Statement
Drs. Brophy, Pineda, Papa, Lewis, Valadka, Hannay, Heaton, Demery, Tepas, Gabrielli, Robicsek, and Robertson have nothing to disclose; Dr. Liu is an employee of Banyan Biomarkers, Inc.; Drs. Wang and Hayes own stock, receive royalties from, and are executive officers of Banyan Biomarkers Inc., and as such may benefit financially as a result of the outcomes of this research or work reported in this publication.
References
- Binder S. Corrigan J.D. Langlois J.A. The public health approach to traumatic brain injury: an overview of CDC's research and programs. J. Head Trauma Rehabil. 2005;20:189–195. doi: 10.1097/00001199-200505000-00002. [DOI] [PubMed] [Google Scholar]
- Brain Trauma Foundation. The American Association of Neurological Surgeons. The Joint Section on Neurotrauma and Critical Care. Intracranial pressure thresholds. J. Neurotrauma. 2000;17:493–495. doi: 10.1089/neu.2000.17.493. [DOI] [PubMed] [Google Scholar]
- Foerch C. Singe O.C. Neumann-Haefelin T. du Mesnil de Rochemont R. Steinmetz H. Sitzer M. Evaluation of serum S100B as a surrogate marker for long-term outcome and infarct volume in acute middle cerebral artery infarction. Arch. Neurol. 2005;62:130–134. doi: 10.1001/archneur.62.7.1130. [DOI] [PubMed] [Google Scholar]
- Ghanem G. Loir B. Morandini R. Sales F. Lienard D. Eggermont A. Lejeune F. On the release and half-life of S100B protein in the peripheral blood of melanoma patients. Int. J. Cancer. 2001;94:586–590. doi: 10.1002/ijc.1504. [DOI] [PubMed] [Google Scholar]
- Gibaldi M. Peirrier D. Pharmacokinetics. 2nd. Marcel Dekker; New York: 1982. [Google Scholar]
- Korfias S. Stranjalis G. Psachoulia C. Vasiliadis C. Pitaridis M. Boviatsis E. Sakas D.E. Slight and short-lasting increase of serum S-100B protein in extra-cranial trauma. Brain Inj. 2006;20:867–872. doi: 10.1080/02699050600832395. [DOI] [PubMed] [Google Scholar]
- Langlois J.A. Marr A. Mitchko J. Johnson R.L. Tracking the silent epidemic and educating the public: CDC's traumatic brain injury-associated activities under the TBI Act of 1996 and the Children's Health Act of 2000. J. Head Trauma Rehabil. 2005;20:196–204. doi: 10.1097/00001199-200505000-00003. [DOI] [PubMed] [Google Scholar]
- Liu M.C. Akle V. Zheng W.R. Dave J.R. Tortella F.C. Hayes R.L. Wang K.K.W. Comparing calpain- and caspase-3-degradation patterns in traumatic brain injury by differential proteome analysis. Biochem. J. 2006;394:715–725. doi: 10.1042/BJ20050905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louneva N. Cohen J.W. Han L.Y. Talbot K. Wilson R.S. Bennett D.A. Trojanowski J.Q. Arnold S.E. Caspase-3 is enriched in postsynaptic densities and increased in Alzheimer's disease. Am. J. Pathol. 2008 doi: 10.2353/ajpath.2008.080434. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath R. Scott M. Nadimpalli R. Gupta R. Wang K.K.W. Activation of apoptosis-linked caspase(s) in NMDA-injured brains in neonatal rat. Neurochem. Int. 1999;36:119–126. doi: 10.1016/s0197-0186(99)00112-6. [DOI] [PubMed] [Google Scholar]
- Pineda J.A. Lewis S.B. Valadka A.B. Papa L. Hannay H.J. Heaton S.C. Demery J.A. Liu M.C. Aikman J.M. Akle V. Brophy G.M. Tepas III J.J. Wang K.K.W. Robertson C.S. Hayes R.L. Clinical significance of αII-spectrin breakdown products in cerebrospinal fluid after severe traumatic brain injury. J. Neurotrauma. 2007;24:354–366. doi: 10.1089/neu.2006.003789. [DOI] [PubMed] [Google Scholar]
- Shore P.M. Thomas N.J. Clark R.S.B. Adelson D. Wisniewski S.R. Janesko K.L. Bayir H. Jackson E.K. Kochanek P.M. Continuous versus intermittent cerebrospinal fluid drainage after severe traumatic brain injury in children: effect on biochemical markers. J. Neurotrauma. 2004;21:1113–1122. doi: 10.1089/neu.2004.21.1113. [DOI] [PubMed] [Google Scholar]
- Tanaka Y. Koizumi C. Marumo T. Omura T. Yoshida S. Serum S100B indicates brain edema formation and predicts long-term neurological outcomes in rat transient middle cerebral artery occlusion model. Brain Res. 2007;1137:140–145. doi: 10.1016/j.brainres.2006.12.025. [DOI] [PubMed] [Google Scholar]
- Wang K.K. Calpain and caspase: can you tell the difference? Trends Neurosci. 2000;23:20–26. doi: 10.1016/s0166-2236(99)01536-2. [DOI] [PubMed] [Google Scholar]
- Wu H.Y. Lynch D.R. Calpain and synaptic function. Mol. Neurobiol. 2006;33:215–236. doi: 10.1385/MN:33:3:215. [DOI] [PubMed] [Google Scholar]
- Wunderlich M.T. Lins H. Skalej M. Wallesch C.W. Goertler M. Neuron- specific enolase and tau protein as neurobiochemical markers of neuronal damage are related to early clinical course and long-term outcome in acute ischemic stroke. Clin. Neurol. Neurosurg. 2006;108:558–563. doi: 10.1016/j.clineuro.2005.12.006. [DOI] [PubMed] [Google Scholar]