Abstract
Purpose: The purpose of this work is to demonstrate that higher amplitude of ultrashort laser induced photoacoustic signal can be achieved by multiple-pulse excitation when the temporal duration of the pulse train is less than the minimum of the medium’s thermal relaxation time and stress relaxation time. Thus, improved signal-to-noise ratio can thus be attained through multiple-pulse excitation while minimizing the energy of each pulse.
Methods: The authors used a Michelson interferometer together with a picoseconds laser system to introduce two 6 ps pulses separated by a controllable delay by introducing a path length difference between the two arms of the interferometer. The authors then employed a series of three interferometers to create a pulse train consisting of eight pulses. The average pulse energy was 11 nJ and the temporal span of the pulse train was less than 1 ns.
Results: The detected peak-to-peak amplitude of the multiple-pulse induced photoacoustic waves were linearly dependent on the number of pulses in the pulse train and such a linearity held for different optical absorption coefficients. The signal-to-noise ratio improved when the number of pulses increased. Moreover, nonlinear effects were not detected and no photoacoustic saturation effect was observed.
Conclusions: The authors have shown that multiple-pulse excitation improves the signal-to-noise ratio through an accumulated energy deposition effect. This method is invaluable for photoacoustic measurements that require ultrashort laser pulses with minimized pulse energy to avoid laser damage.
Keywords: photoacoustic imaging, ultrasfast optics, multiple pulses
Photoacoustic (PA) imaging is a powerful means of obtaining both anatomical and functional information of biological tissues1, 2, 3, 4, 5 due to its unique optical-absorption-based contrast mechanism and capability to achieve high spatial resolution in deep tissue. PA imaging also plays an increasingly important role in molecular imaging and has been applied to study gene expression,6 deeply seated fluorescent proteins,7 and labeled proteins.8 However, only linear optical absorption is currently used to produce PA signals, where the illuminating laser pulses have a typical pulse duration of a few nanoseconds and the spectral range covers from visible to near-infrared wavelengths.
When laser light irradiates biological tissue, linear optical absorption occurs simultaneously in many different molecules. As a result, the detected PA signal contains contributions from all optical-absorbing molecules and the contributions from different molecules are hard to separate. Although multiwavelength PA imaging shows potential in separating different optical-absorbing molecules, it still has limited applications for two reasons: First, optical absorption of hemoglobin and melanin can be a few orders of magnitude stronger than those of other molecules, depending on the optical wavelength. Second, many molecules have broad, overlapping absorption spectra without distinguishable “signature” absorption features∕peaks. These two factors can make the inverse calculation of the relative concentrations of weak optical-absorbing pigments ill-posed.9 As a result, existing endogenous-contrast PA imaging is primarily limited to vascular imaging.
One possible solution to achieve endogenous molecular selectivity is to take advantage of the nonlinear optical absorption by using ultrashort (picosecond and femtosecond) pulse excitation and image within the so-called “optical window” (700 nm–900 nm),10 where the linear optical absorption of hemoglobin is minimized. The nonlinear optical absorption includes, for example, two-photon absorption11 and stimulated Raman absorption;12 however, since PA generation relies on the total absorbed optical energy (regardless of linear or nonlinear absorption), the difficulty of implementing nonlinear absorption excited PA imaging arises from the potential tissue damage due to the extremely high light intensity,13 which limits the efficiency of the nonlinear absorption and, thus, the overall PA signal.
In this letter, we experimentally demonstrated that high PA signal amplitude can be achieved by multiple low-energy picoseconds pulses excitation. Both the single pulse and the pulse train satisfied the ANSI laser safety regulations. Although PA waves were generated by linear optical absorption in the current studies, we anticipate that this method can be extended to nonlinear-optical-absorption-based PA imaging (nonlinear PA imaging).
The multiple-pulse PA excitation study is based on an observation that when two ultrashort laser pulses arrive with a small delay between each other, the resultant amplitude of the PA wave is the sum of the two waves’ amplitudes produced by each pulse independently (accumulative effect). A similar result was recently reported for two-photon fluorescence microscopy imaging, where the use of multiple-pulse excitation significantly improved the signal-to-noise ratio (SNR) of the images and reduced photobleaching.14 Multiple-pulse excitation schemes were also used to enhance molecular and material responses in spectroscopy.15
To achieve this accumulative effect for PA generation, the temporal extent of the optical excitation should be less than the minimum of the stress relaxation tstress=L∕vs and the thermal relaxation time tthermal=L2∕4D, where L is the characteristic size of the heated region,16vs is the sound velocity in the medium (∼1500 m∕s in soft tissues), and D is the thermal diffusion coefficient (for typical soft tissueD=1.4×10−3 cm2∕s). For a typical size of L=10 μm, tstress=6.7 ns and tthermal=180 μs⪢tstress.
To test the hypothesis that multiple-pulse excitation proportionally increases PA signal amplitude, we constructed optical pulse trains containing a different number of picosecond pulses to illuminate a phantom and then we evaluated the detected PA amplitudes as a function of the number of pulses.
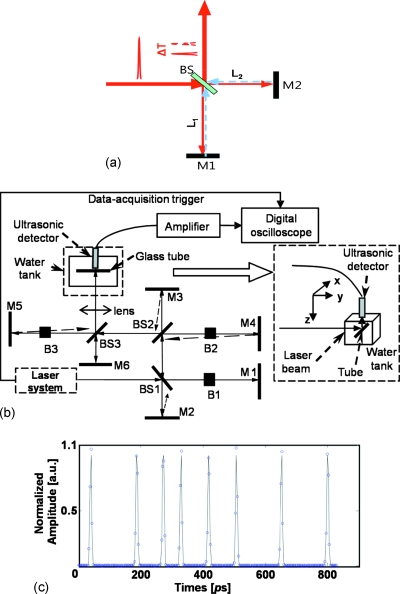
We used a Michelson interferometer [Fig. 1a] and differentiated the two arms’ path lengths by adjusting the position of the back mirror in one of the arms. The time delay between the combined two pulses was determined by Δt=2|L1−L2|∕c. Employing a series of three interferometers, we created a train consisting of eight almost identical pulses.
Figure 1.
Schematic of the experimental setup. (a) A Michelson interferometer with different path lengths introduces a delay between two pulses. (b) A series of three Michelson interferometers generate a train of eight pulses. (c) Measured temporal distribution of the pulse train containing eight pulses. BS: Beam splitter; M: Mirror; B: Beam block. M2 and M3, M4 and M5 were slightly misaligned to avoid multiple back-reflections.
A schematic diagram of the experimental setup is shown in Fig. 1b. We used a home-built, high repetition rate picosecond laser system (pulse duration: 6 ps; fundamental wavelength: 1064 nm; pulse repetition rate: 875 kHz).17 A 5 mm long KTP (phase II) crystal converted the fundamental wavelength to the 532 nm radiation.
The laser pulses were focused on a glass capillary tube (Kimax 80450-22, Kimble∕Kontes, Vineland, NJ) using a 10 cm focal length lens (BPX 080, Thorlabs, Newton, NJ). The glass capillary tube was filled with diluted red ink solution (Fiesta Red, Private Reserve Ink, Zionsville, IN). A steady flow was kept within the tube by a perfusion pump and the tube was placed inside a tank filled with distilled water. The optical absorption coefficient of the ink solution was 10.2 cm−1∕% at 532 nm.
The generated PA waves were detected by an unfocused, custom-built ultrasonic transducer with a center frequency of 40 MHz, and the waves were amplified by a wide-band, low-noise preamplifier (ZFL500LN+, Mini-Circuits, Brooklyn, NY) for 20 dB and an ultrasonic amplifier (5073PR, Olympus NDT, Kennewick, WA) for an additional 25 dB. The amplified PA signals were then digitized and stored by an oscilloscope (TDS5034B, Tektronix, Beaverton, OR). Each PA signal was averaged 400 times and the peak-to-peak PA amplitude was extracted.
The pulse train was characterized by measuring its cross-correlation function. We used the residual 1064 nm radiation and combined it with the 532 nm radiation in a thin (1 mm thick) BBO crystal. The full cross-correlation function (representing the pulse sequence) was recorded [Fig. 1c] by varying the time delay between the 1064 and 532 nm pulses and by recording the power of the UV radiation as a function of the time delay between two pulses. All pulses were well separated from each other and the whole pulse train had a time span of less than 1 ns. By blocking one arm in one of the Michelson interferometer assemblies, as shown in Fig. 1b, the number of pulses decreased by a factor of 2, leading to a total of eight, four, two, or one pulse in the respective pulse trains. The average energy for each pulse was 11 nJ.
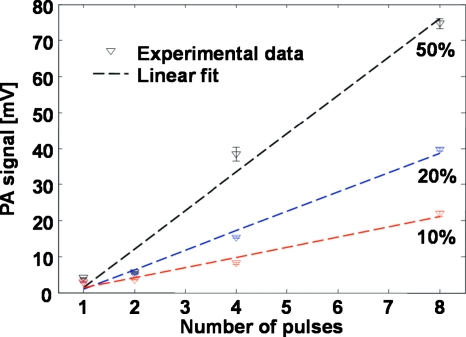
We measured the amplitudes of the PA signals excited by different numbers of pulses in the incident pulse train. Ink dilutions at 10%, 20%, and 50% were used to make solutions with different optical absorption coefficients. The results shown in Fig. 2 clearly exhibit the linear dependence of PA amplitude on the number of pulses. Moreover, this linear dependency is also valid for samples with different optical absorption coefficients. The mean random noise level was 4 mV. Note that when the illumination pulse train contained only one pulse, the measured PA amplitudes were close to the noise level for all the ink dilutions; however, as the number of pulses increased, the induced signal amplitude and, thus, the SNR increased accordingly, which clearly demonstrated the advantage of multiple-pulse excitation.
Figure 2.
PA signal amplitude as a function of the number of the excitation pulses.
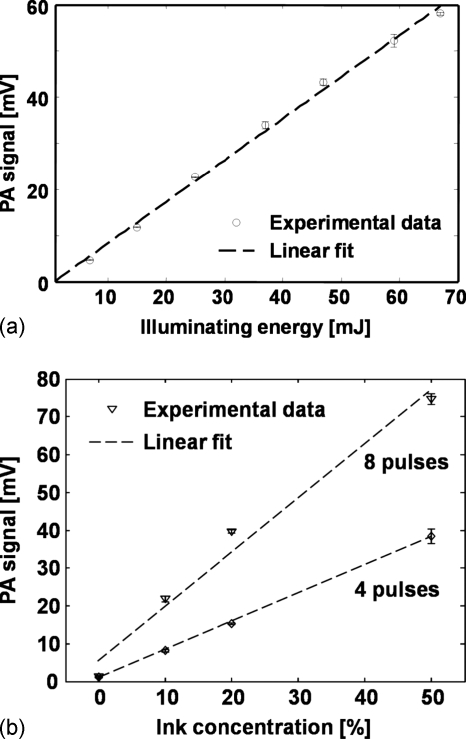
To exclude any possible nonlinear effects, we measured the linearity of the induced PA amplitude on the incident pulse energy. We used the eight-pulse train and attenuated the incident light energy by a set of neutral density filters (5215, New Focus, Santa Clara, CA). As shown in Fig. 3a, the PA signal amplitude is propositional to the incident pulse energy and no nonlinear effect is evident.
Figure 3.
PA signal amplitude (a) as a function optical illuminating energy and (b) as a function of optical absorption coefficient.
To further exclude possible effects due to PA saturation,18, 19 we examined the dependence of PA signal amplitude on the optical absorption coefficient of the medium subject to the same illuminating pulse energy. Different concentrations (0%, 10%, 20%, and 50%) of red ink samples were used to vary the optical absorption coefficient. Ink samples were illuminated by four and eight pulses and the light energies were kept constant. As shown in Fig. 3b, PA amplitude increases linearly with the ink concentration and shows no PA saturation.
The above results demonstrate the advantage of applying multiple-pulse excitation for PA imaging when ultrashort laser pulses are required. While there is no significant benefit for this technique in linear-optical-absorption-based PA imaging because the overall PA signal depends only on the total absorbed energy and longer laser pulses (∼ns) could be used, multiple-pulse excitation could find several potential applications in nonlinear PA imaging. In nonlinear PA imaging based on two-photon11 or stimulated Raman absorption,12 we could potentially use a large number of ultrashort pulses (each weak enough to prevent damage to the selected tissue structure) to greatly improve the SNR and, thus, higher imaging quality. In our example, we used a series of only eight pulses; however, the technology exists14, 20, 21 to increase the number of pulses to a desired level to take the full advantage of the SNR enhancement.
In summary, we demonstrated a technique that improves the amplitude of the ultrashort pulse induced PA signal by applying a train of laser pulses within 1 ns. Although this technology was demonstrated for linear-optical-absorption-based PA generation, we anticipate a greater impact on nonlinear PA imaging.
Acknowledgments
This work was supported in part by the start-up fund from the University of Wisconsin-Milwaukee and a grant from The Lynde and Harry Bradley Foundation to H.F. Zhang, and in part by the NIH∕NIBIB (Grant No. R03EB008535) to V.V. Yakovlev. The authors thank Dr. Qifa Zhou for constructing the 40 MHz ultrasonic transducer. The authors also thank Dr. Zhixing Xie and Mr. Daniel D. Zirbel for helpful discussions.
References
- Wang L. V., “Multiscale photoacoustic microscopy and computed tomography,” Nat. Photonics 3, 503–509 (2009). 10.1038/nphoton.2009.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z. X., Jiao S. L., Zhang H. F., and Puliafito C. A., “Laser-scanning optical-resolution photoacoustic microscopy,” Opt. Lett. 34, 1771–1773 (2009). 10.1364/OL.34.001771 [DOI] [PubMed] [Google Scholar]
- Zhang H. F., Maslov K., Stoica G., and Wang L. V., “Functional photoacoustic microscopy for high-resolution and noninvasive in vivo imaging,” Nat. Biotechnol. 24, 848–851 (2006). 10.1038/nbt1220 [DOI] [PubMed] [Google Scholar]
- Laufer J., Zhang E., Raivich G., and Beard P., “Three-dimensional noninvasive imaging of the vasculature in the mouse brain using a high resolution photoacoustic scanner,” Appl. Opt. 48, D299–D306 (2009). 10.1364/AO.48.00D299 [DOI] [PubMed] [Google Scholar]
- Ermilov S. A., Khamapirad T., Conjusteau A., Leonard M. H., Lacewell R., Mehta K., Miller T., and Oraevsky A. A., “Laser optoacoustic imaging system for detection of breast cancer,” J. Biomed. Opt. 14(2), 024007 (2009). 10.1117/1.3086616 [DOI] [PubMed] [Google Scholar]
- Li L., Zemp R. J., Lungu G., Stoica G., and Wang L. V., “Photoacoustic imaging of lacZ gene expression in vivo,” J. Biomed. Opt. 12(2), 020504 (2007). 10.1117/1.2717531 [DOI] [PubMed] [Google Scholar]
- Razansky D., Distel M., Vinegoni C., Ma R., Perrimon N., Koster R. W., and Ntziachristos V., “Multispectral opto-acoustic tomography of deep-seated fluorescent proteins in vivo,” Nat. Photonics 3, 412–417 (2009). 10.1038/nphoton.2009.98 [DOI] [Google Scholar]
- McDonald M. A., Jankovic L., Shahzad K., Burcher M., and Li K. C. P., “Acoustic fingerprints of dye-labeled protein submicrosphere photoacoustic contrast agents,” J. Biomed. Opt. 14(3), 034032 (2009). 10.1117/1.3155519 [DOI] [PubMed] [Google Scholar]
- Maslov K., Zhang H. F., and Wang L. V., “Effects of wavelength-dependent fluence attenuation on the noninvasive photoacoustic imaging of hemoglobin oxygen saturation in subcutaneous vasculature in vivo,” Inverse Probl. 23, S113–S122 (2007). 10.1088/0266-5611/23/6/S09 [DOI] [Google Scholar]
- Tuchin V., Tissue Optics: Light Scattering Methods and Instruments for Medical Diagnosis (SPIE, Bellingham, 2007). [Google Scholar]
- Yoshihisa Y., Katsuji F., and Tetsuro T., “Improvement of depth resolution on photoacoustic imaging using multiphoton absorption,” Proc. SPIE 6631, 663102 (2007). 10.1117/12.728204 [DOI] [Google Scholar]
- Yakovlev V. V., Petrov G. I., Zhang H. F., Noojin G. D., Denton M. L., Thomas R. J., and Scully M. O., “Stimulated Raman scattering: Old physics, new applications,” J. Mod. Opt. 56, 1970–1973 (2009). 10.1080/09500340903082671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- König K., So P. T. C., Mantulin W. W., and Gratton E., “Cellular response to near-infrared femtosecond laser pulses in two-photon microscopes,” Opt. Lett. 22, 135–136 (1997). 10.1364/OL.22.000135 [DOI] [PubMed] [Google Scholar]
- Ji N., Magee J. C., and Betzig E., “High-speed, low-photodamage nonlinear imaging using passive pulse splitters,” Nat. Methods 5, 197–202 (2008). 10.1038/nmeth.1175 [DOI] [PubMed] [Google Scholar]
- Kawashima H., Wefers M. M., and Nelson K. A., “Femtosecond pulse shaping, multiple-pulse spectroscopy, and optical control,” Annu. Rev. Phys. Chem. 46, 627–656 (1995). 10.1146/annurev.pc.46.100195.003211 [DOI] [PubMed] [Google Scholar]
- Wang L. V. and Wu H. -i, Biomedical Optics: Principles and Imaging (Wiley, New York, 2007). [Google Scholar]
- Petrov G. I., Yakovlev V. V., and Minkovski N. I., “Broadband nonlinear optical conversion of a high-energy diode-pumped picosecond laser,” Opt. Commun. 229, 441–445 (2004). 10.1016/j.optcom.2003.11.009 [DOI] [Google Scholar]
- Sivaramakrishnan M., Maslov K., Zhang H. F., Stoica G., and Wang L. V., “Limitations of quantitative photoacoustic measurements of blood oxygenation in small vessels,” Phys. Med. Biol. 52, 1349–1361 (2007). 10.1088/0031-9155/52/5/010 [DOI] [PubMed] [Google Scholar]
- Wang J., Liu T., Jiao S., Chen R., Zhou Q., Shung K. K., Wang L. V., and Zhang H. F., “Saturation effect in functional photoacoustic imaging,” J. Biomed. Opt. 15, 021317 (2010). 10.1117/1.3333549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fittinghoff D. N. and Squier J. A., “Time-decorrelated multifocal array for multiphoton microscopy and micromachining,” Opt. Lett. 25, 1213–1215 (2000). 10.1364/OL.25.001213 [DOI] [PubMed] [Google Scholar]
- Weiner A. M., “Femtosecond pulse shaping using spatial light modulators,” Rev. Sci. Instrum. 71, 1929–1960 (2000). 10.1063/1.1150614 [DOI] [Google Scholar]