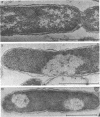
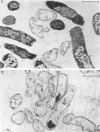
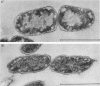
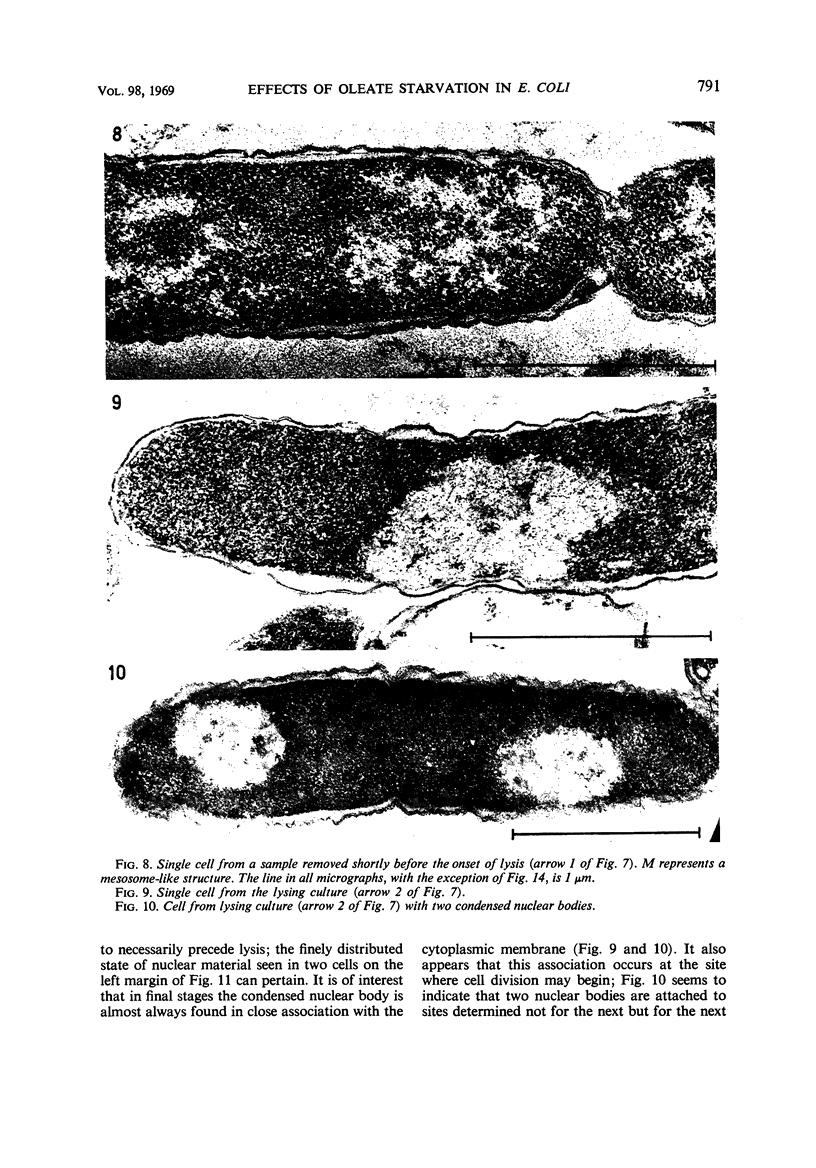
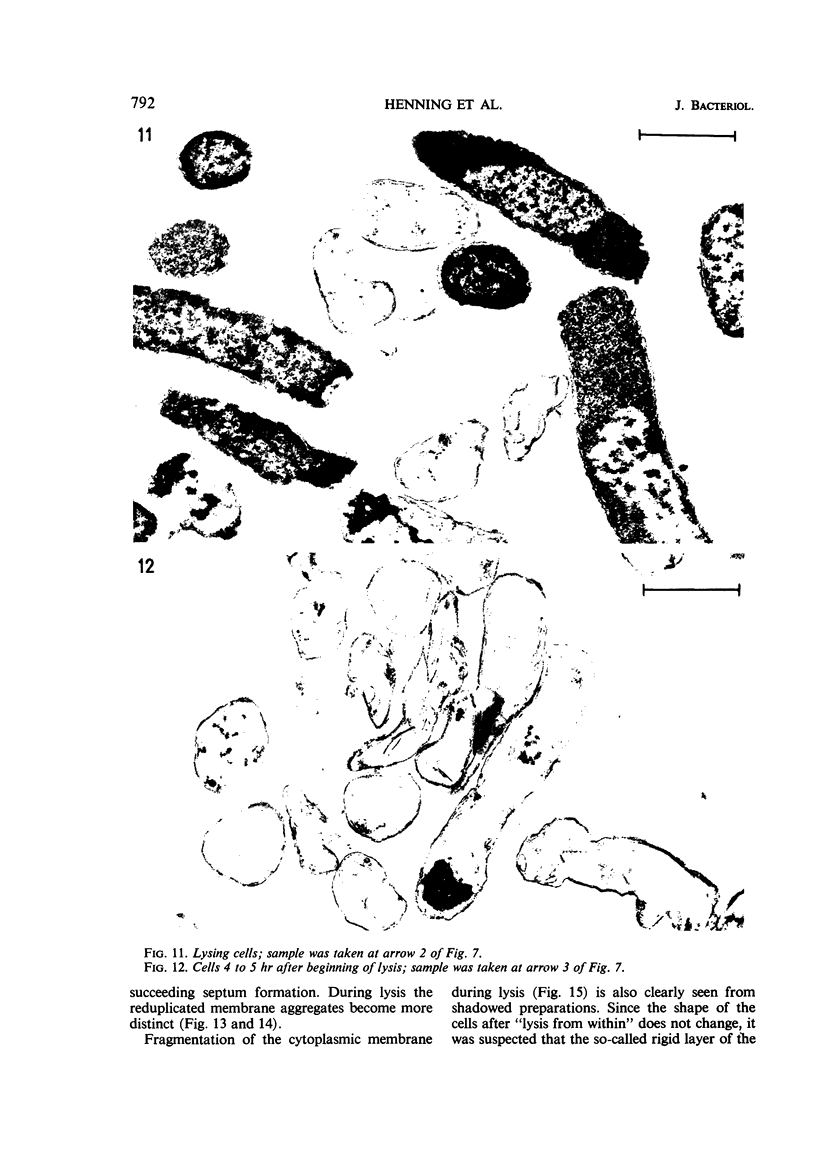
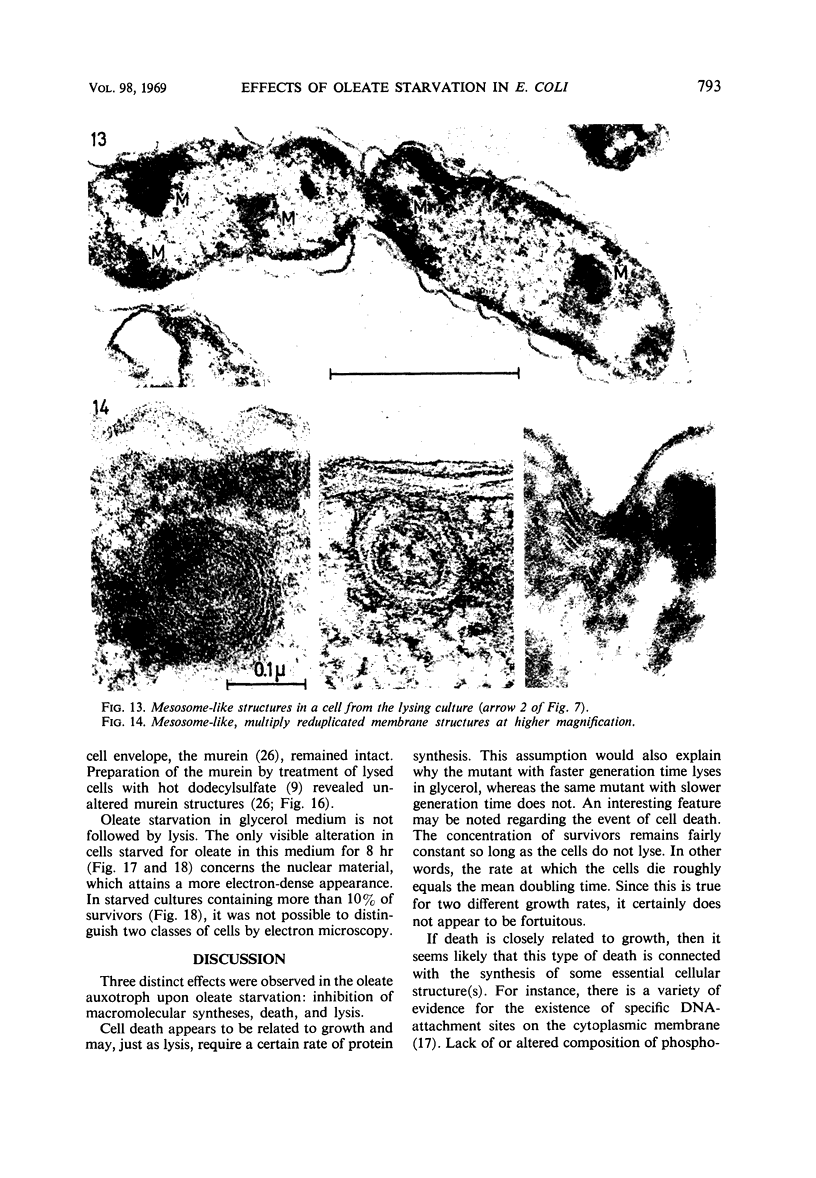
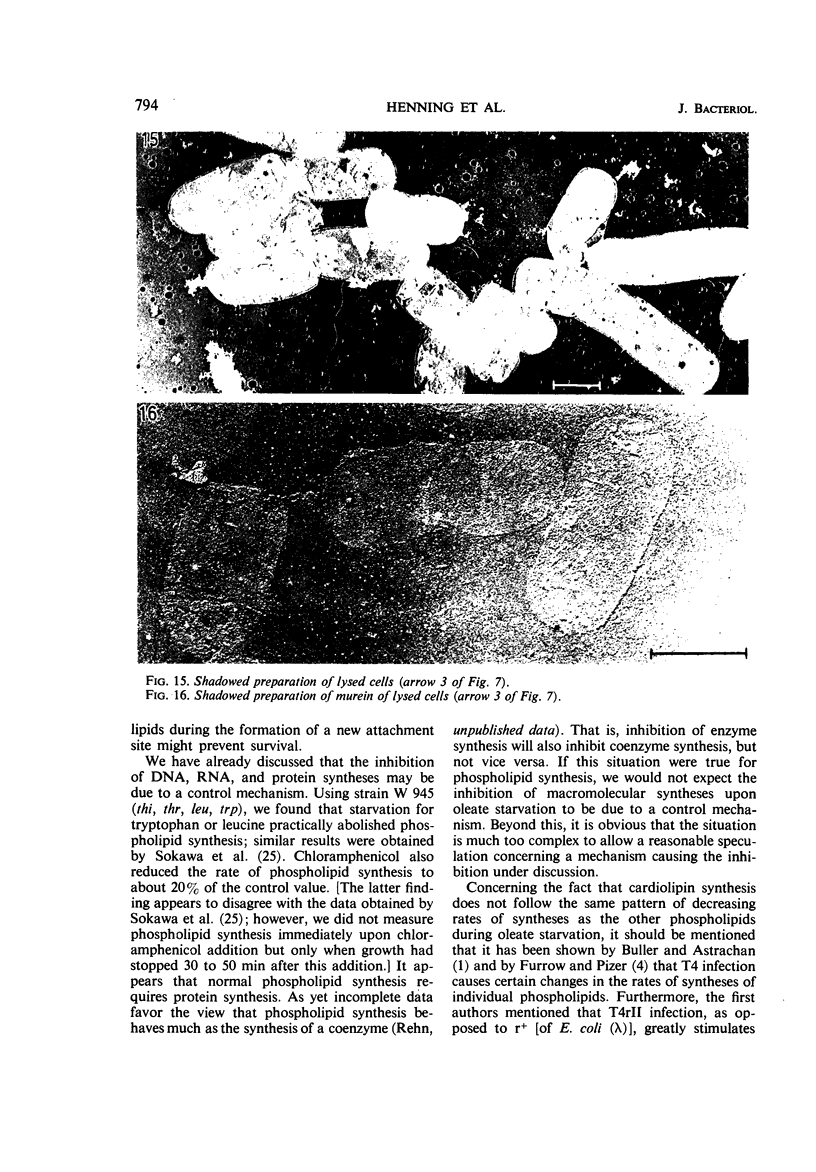
Abstract
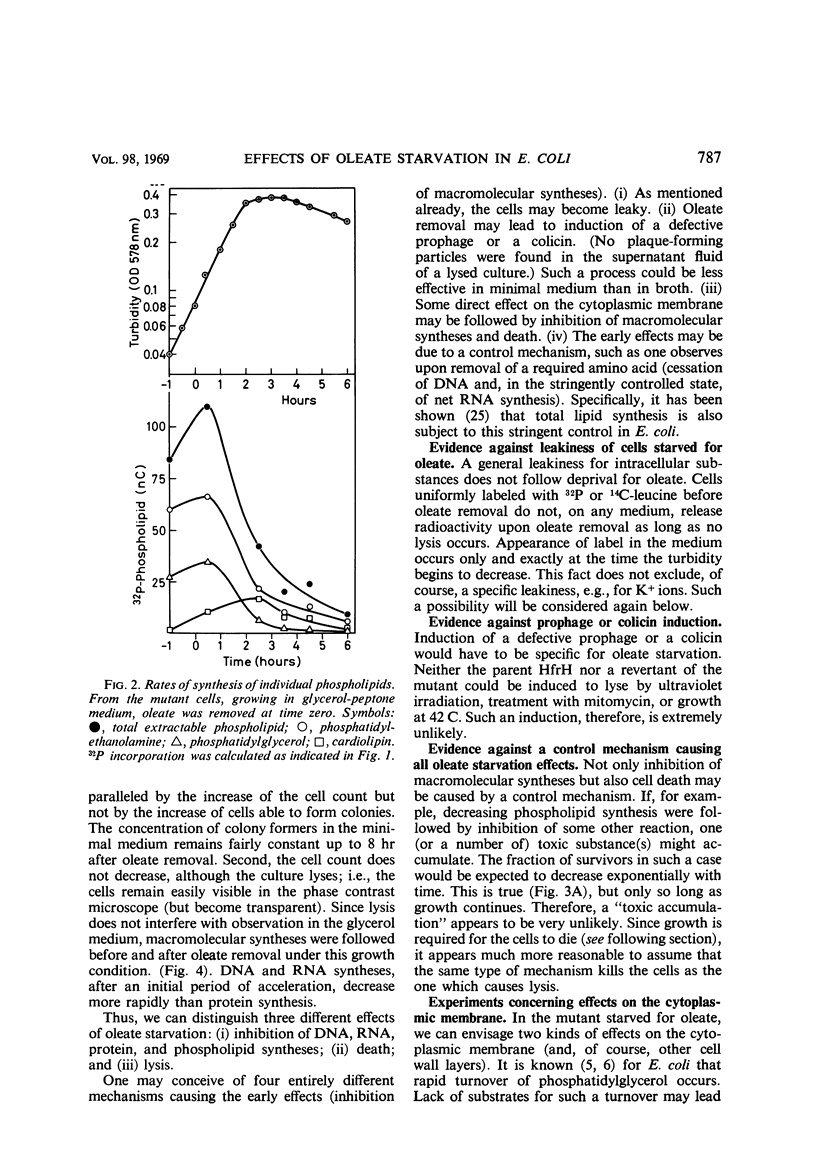
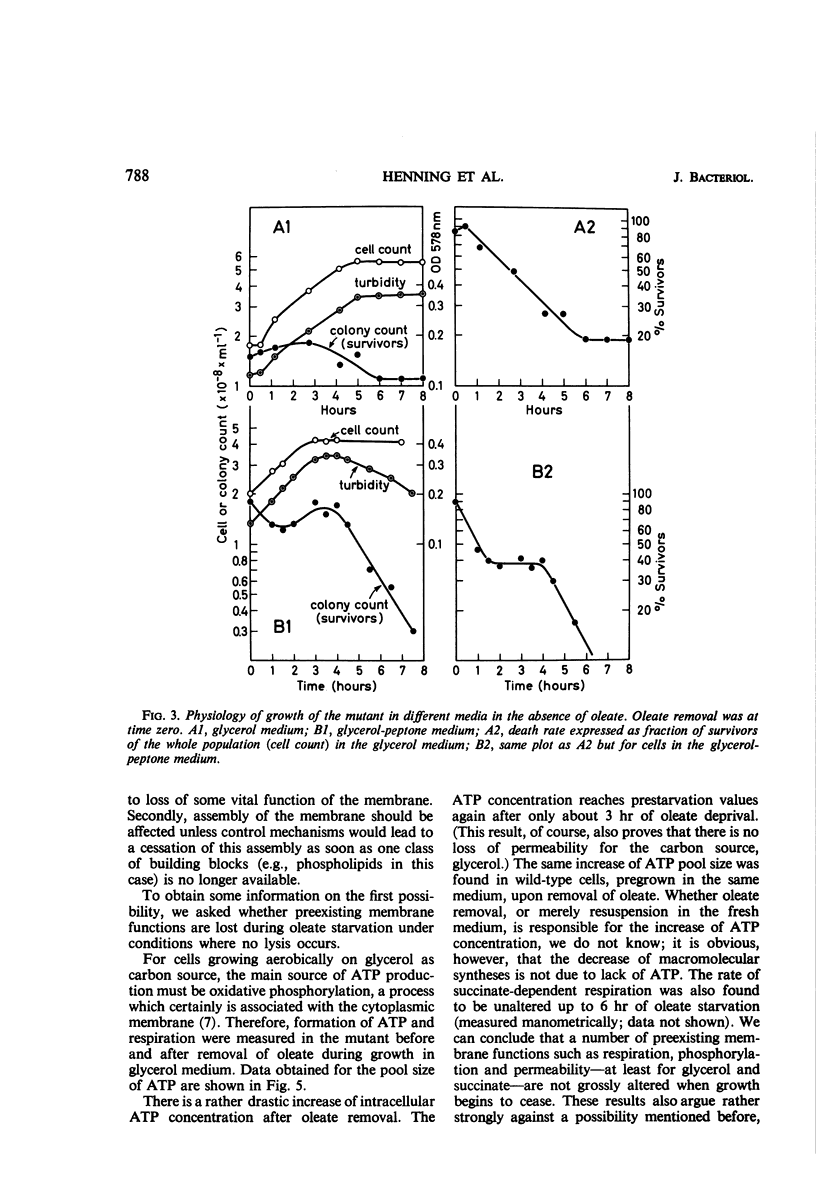
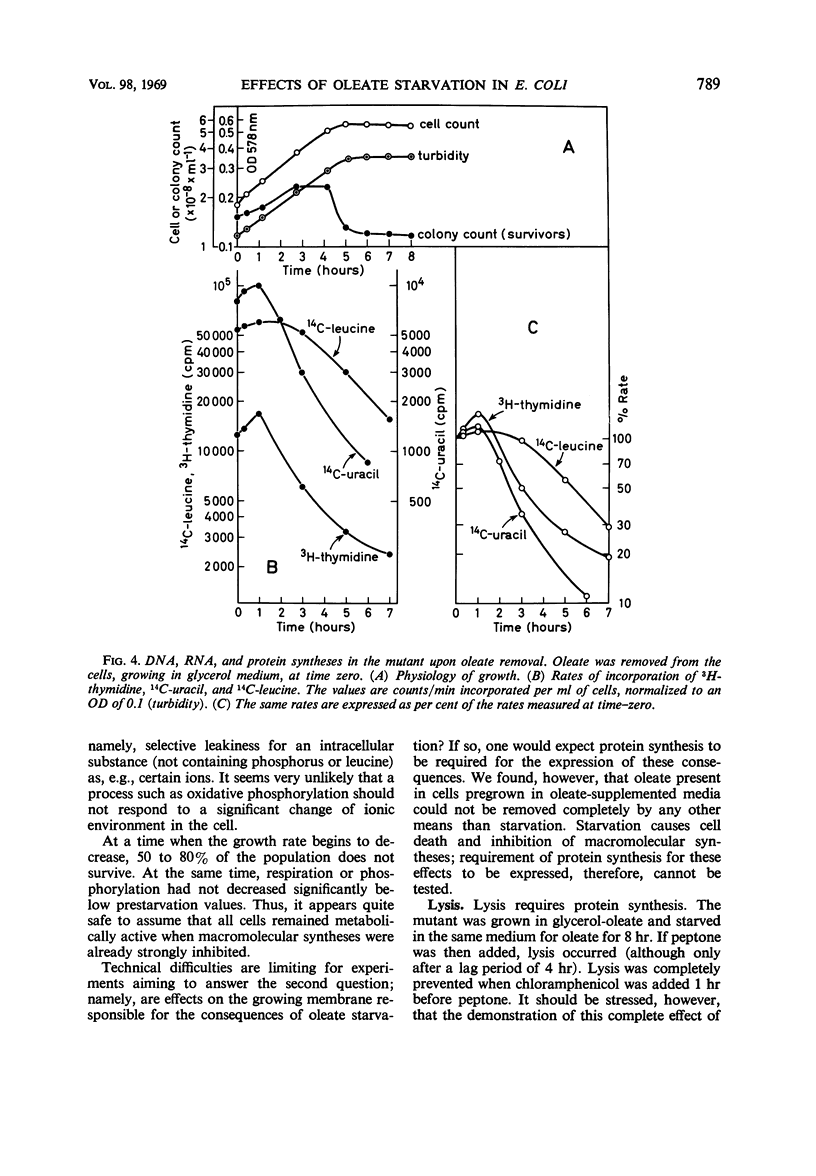
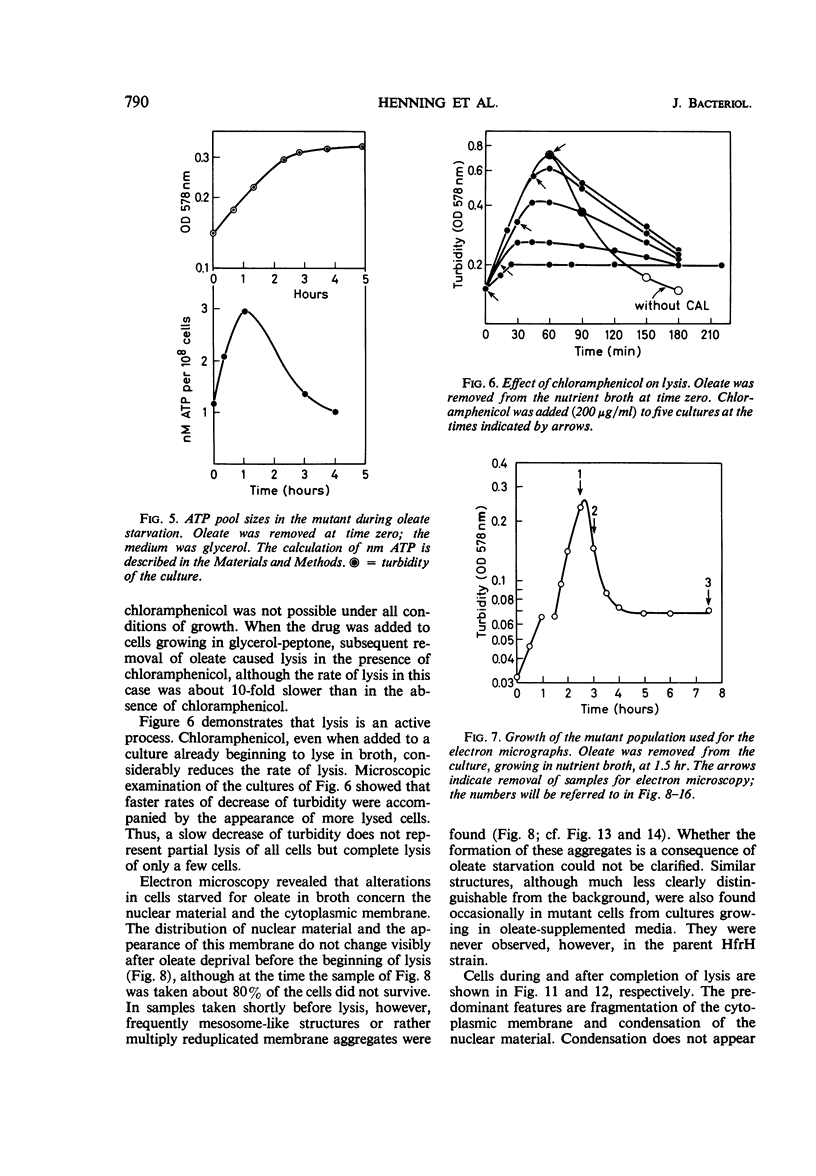
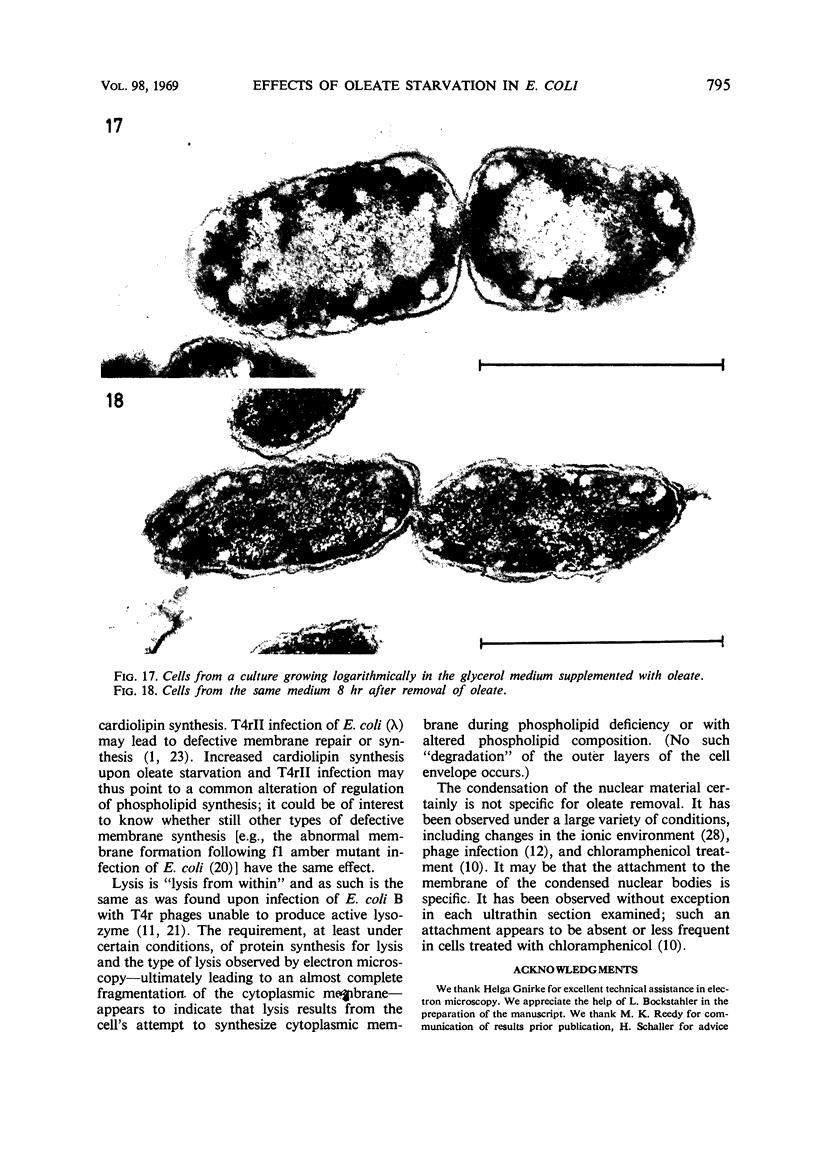
The effects of oleate starvation on an oleate auxotroph of Escherichia coli K-12 were investigated. Following removal of oleate from the mutant growing in a minimal glycerol-peptone medium, the cells stopped making deoxyribonucleic acid, ribonucleic acid, protein, and phospholipids; they began to die exponentially and finally lysed. During oleate starvation in minimal medium minus peptone, inhibition of macromolecular syntheses and death occurred; however, lysis did not follow. When growth ceased, no further dying was observed. It is shown that none of the early effects (inhibition of macromolecular syntheses and death) can be due to leakiness of the cells, induction of a prophage or a colicin, or lack of energy sources. The cause of inhibition of macromolecular syntheses remained unknown. Since the rate of death was the same as the generation time under different conditions, it appears that death is due to the defective synthesis of some cellular structure (quite possibly, cytoplasmic membrane) during phospholipid deficiency. Lysis was found to require protein synthesis; electron microscopy revealed a peculiar type of “lysis from within”; i.e., the shape of the cells did not change but fragmentation of the inner layer of the cell envelope occurred. The murein was found to be unaltered. Most likely, lysis was a consequence of the cell's attempt to synthesize cytoplasmic membrane with altered phospholipid composition or during phospholipid deficiency. Several membrane functions (respiration, adenosine triphosphate formation, permeability) existing before oleate removal were not lost during starvation. Therefore, general damage to the membrane did not occur, and it could be that most, if not all, described effects were due to defective de novo membrane synthesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buller C. S., Astrachan L. Replication of T4rII bacteriophage in Escherichia coli K-12 (lambda). J Virol. 1968 Apr;2(4):298–307. doi: 10.1128/jvi.2.4.298-307.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr H. S., Rosenkranz H. S. H3-thymidine and the conservation of deoxyribonucleic acid. J Bacteriol. 1966 Dec;92(6):1840–1841. doi: 10.1128/jb.92.6.1840-1841.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole H. A., Wimpenny J. W., Hughes D. E. The ATP pool in Escherichia coli. I. Measurement of the pool using modified luciferase assay. Biochim Biophys Acta. 1967;143(3):445–453. doi: 10.1016/0005-2728(67)90050-3. [DOI] [PubMed] [Google Scholar]
- Furrow M. H., Pizer L. I. Phospholipid synthesis in Escherichia coli infected with T4 bacteriophages. J Virol. 1968 Jun;2(6):594–605. doi: 10.1128/jvi.2.6.594-605.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANFER J., KENNEDY E. P. METABOLISM AND FUNCTION OF BACTERIAL LIPIDS. I. METABOLISM OF PHOSPHOLIPIDS IN ESCHERICHIA COLI B. J Biol Chem. 1963 Sep;238:2919–2922. [PubMed] [Google Scholar]
- KASHKET E. R., BRODIE A. F. OXIDATIVE PHOSPHORYLATION IN FRACTIONATED BACTERIAL SYSTEMS. VIII. ROLE OF PARTICULATE AND SOLUBLE FRACTIONS FROM ESCHERICHIA COLI. Biochim Biophys Acta. 1963 Oct 8;78:52–65. doi: 10.1016/0006-3002(63)91608-1. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., ARBER W. Electron microscopical studies of phage multiplication. I. A method for quantitative analysis of particle suspensions. Virology. 1957 Apr;3(2):245–255. doi: 10.1016/0042-6822(57)90091-0. [DOI] [PubMed] [Google Scholar]
- Kanemasa Y., Akamatsu Y., Nojima S. Composition and turnover of the phospholipids in Escherichia coli. Biochim Biophys Acta. 1967 Oct 2;144(2):382–390. [PubMed] [Google Scholar]
- MURRAY R. G., WHITFIELD J. F. Cytological effects of infection with T5 and some related phages. J Bacteriol. 1953 Jun;65(6):715–726. doi: 10.1128/jb.65.6.715-726.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C., Rosenkranz H. S., Carr H. S., Rose H. M. Electron microscopy of chloramphenicol-treated Escherichia coli. J Bacteriol. 1967 Jun;93(6):1987–2002. doi: 10.1128/jb.93.6.1987-2002.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai F., Streisinger G., Miller B. The mechanism of lysis in phage T4-infected cells. Virology. 1967 Nov;33(3):398–404. doi: 10.1016/0042-6822(67)90115-8. [DOI] [PubMed] [Google Scholar]
- Mukai F., Streisinger G., Miller B. The mechanism of lysis in phage T4-infected cells. Virology. 1967 Nov;33(3):398–404. doi: 10.1016/0042-6822(67)90115-8. [DOI] [PubMed] [Google Scholar]
- Neuhard J., Randerath E., Randerath K. Ion-exchange thin-layer chromatography. 13. Resolution of complex nucleoside triphosphate mixtures. Anal Biochem. 1965 Nov;13(2):211–222. doi: 10.1016/0003-2697(65)90191-0. [DOI] [PubMed] [Google Scholar]
- Overath P., Raufuss E. M. The induction of the enzymes of fatty acid degradation in Escherichia coli. Biochem Biophys Res Commun. 1967 Oct 11;29(1):28–33. doi: 10.1016/0006-291x(67)90535-9. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYTER A., KELLENBERGER E., BIRCHANDERSEN A., MAALOE O. Etude au microscope électronique de plasmas contenant de l'acide désoxyribonucliéique. I. Les nucléoides des bactéries en croissance active. Z Naturforsch B. 1958 Sep;13B(9):597–605. [PubMed] [Google Scholar]
- Ryter A. Association of the nucleus and the membrane of bacteria: a morphological study. Bacteriol Rev. 1968 Mar;32(1):39–54. doi: 10.1128/br.32.1.39-54.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEDAR A. W., BURDE R. M. LOCALIZATION OF THE SUCCINIC DEHYDROGENASE SYSTEM IN ESCHERICHIA COLI USING COMBINED TECHNIQUES OF CYTOCHEMISTRY AND ELECTRON MICROSCOPY. J Cell Biol. 1965 Feb;24:285–295. doi: 10.1083/jcb.24.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger M. J. Secretion of alkaline phosphatase subunits by spheroplasts of Escherichia coli. J Bacteriol. 1968 Sep;96(3):727–733. doi: 10.1128/jb.96.3.727-733.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz F. M., Zinder N. D. Morphological changes in Escherichia coli infected with the DNA bacteriophage fl. Virology. 1968 Feb;34(2):352–355. doi: 10.1016/0042-6822(68)90246-8. [DOI] [PubMed] [Google Scholar]
- Sekiguchi M. Studies on the physiological defect in rII mutants of bacteriophage T4. J Mol Biol. 1966 Apr;16(2):503–522. doi: 10.1016/s0022-2836(66)80188-2. [DOI] [PubMed] [Google Scholar]
- Silbert D. F., Vagelos P. R. Fatty acid mutant of E. coli lacking a beta-hydroxydecanoyl thioester dehydrase. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1579–1586. doi: 10.1073/pnas.58.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokawa Y., Nakao E., Kaziro Y. On the nature of the control by RC gene in e. coli: amino acid-dependent control of lipid synthesis. Biochem Biophys Res Commun. 1968 Oct 10;33(1):108–112. doi: 10.1016/0006-291x(68)90263-5. [DOI] [PubMed] [Google Scholar]
- WEIDEL W., PELZER H. BAGSHAPED MACROMOLECULES--A NEW OUTLOOK ON BACTERIAL CELL WALLS. Adv Enzymol Relat Areas Mol Biol. 1964;26:193–232. doi: 10.1002/9780470122716.ch5. [DOI] [PubMed] [Google Scholar]
- WEIGERT M. G., GAREN A. AMINO ACID SUBSTITUTIONS RESULTING FROM SUPPRESSION OF NONSENSE MUTATIONS. I. SERINE INSERTION BY THE SU-1 SUPPRESSOR GENE. J Mol Biol. 1965 Jun;12:448–455. doi: 10.1016/s0022-2836(65)80267-4. [DOI] [PubMed] [Google Scholar]
- WHITFIELD J. F., MURRAY R. G. The effects of the ionic environment on the chromatin structures of bacteria. Can J Microbiol. 1956 May;2(3):245–260. doi: 10.1139/m56-029. [DOI] [PubMed] [Google Scholar]