Abstract
Children who spend early portions of their lives in institutions or those maltreated in their families of origin are at risk for developing emotional and behavioral problems reflecting disorders of emotion and attention regulation. Animal models may help explicate the mechanisms producing these effects. Despite the value of the animal models, many questions remain in using the animal data to guide studies of human development. In 1999, the National Institute of Mental Health in the United States funded a research network to address unresolved issues and enhance translation of basic animal early experience research to application in child research. Professor Seymour Levine was both the inspiration for and an active member of this research network until his death in October of 2007. This review pays tribute to his legacy by outlining the conceptual model which is now guiding our research studies.
Keywords: Animal models, caregiving, child development, deprivation, emotion, foster care, institutional care, regulation, stress
Early life stress (ELS) in the form of adverse care from parents and other caregivers increases the risk of psychopathology, particularly disorders of emotion and attention regulation (Kreppner et al., 2001; Provence and Lipton, 1962, Rogosch and Cicchetti, 2005; Roy et al., 2004, Shields et al., 1994; Stevens et al., 2008). While numerous studies document these increased risks, we have only a limited understanding of the psychobiological processes underlying them. Beginning with the pioneering work of Seymour Levine (e.g. Levine, 1957), animal models have provided insight into the mechanisms through which ELS alters the development of stress- and threat-response systems (De Kloet et al., 1988; Heim et al., 2004; Meaney and Szyf, 2005; Sanchez et al., 2001). Animal models also provide evidence that alterations in stress- and threat-response systems may compromise the development of emotion-and attention-regulatory systems (Brake et al., 2004; Vedhara et al., 2000). These animal data have often been used to explain the heightened risk of behavioral and affective disorders in human children exposed to ELS (e.g., Heim and Nemeroff, 2001; Nemeroff, 2004). Nonetheless, the bridge between the animal studies and human development is still more hypothetical than empirically grounded (Bremner and Vermetten, 2001). Integration of the animal and human research requires a conceptual model that is general enough to apply across species, but specific enough to guide empirical investigations that allow studies of children to inform animal models and in turn allow animal model research to impact the design and interpretation of human developmental research.
This paper describes the conceptual model developed by the Early Experience, Stress, and Neurobehavioral Development Research Network. This network is composed of basic researchers studying the neurobiological processes involved in transducing the effects of ELS in rodent and non-human primates, researchers studying human development, and prevention science researchers designing and examining early interventions. The work of first the network and now the Center was inspired by Seymour (Gig) Levine’s pioneering “early handling” research. Over the course of over half a century, Dr. Levine established a rich body of research on the role of early experience in the development of stress- and threat-regulatory systems. From its inception, he interpreted his animal model work as relevant to our understanding of human development, particularly the development of depression and anxiety disorders.
When the opportunity arose to establish an NIMH-network, Dr. Levine was one of the first to champion the effort. At our first meeting, he gave a tour-de-force lecture reviewing the history early experience stress research and noting how at each turn, new methods gave opportunities to understand early experience effects at more molecular levels of analysis, while also arguing that developmental science, as a field, has continued to move between describing early experiences as producing permanent (defining) effects on development to describing them as being of little import, being completely modifiable by later experiences. The tension between these extreme positions became a common theme for our network, and now our center, as we move forward to understand both how ELS impact the developing organism and how best to intervene to support optimal development for children exposed to ELS during their earliest years.
Conceptualizing Early Life Stress
There is no agreed upon definition of ELS; indeed, there is considerable controversy about the definition of stress more generally (Levine and Ursin, 1991). Our research network adopted a working definition of ELS based on the following arguments. Stressors are events or conditions that threaten, or are perceived to threaten, physiological equilibrium (Weinstock, 2005). Stress responses involve activity in the central nervous system to mobilize endocrine, autonomic, and behavior systems to support protection from and/or adaptation to threat. Recently the concept of allostasis has been introduced to describe the dynamic interaction of multiple systems of equilibrium maintenance (McEwen, 1998, 2003). ELS or early life allostasis refers to responses to stressors experienced during pre-pubertal development. While acknowledging that ELS can involve physical stressors, we chose to focus on adverse caregiving in order to ground our work in the early experience animal data. Furthermore, we focused on stressors experienced during the first years of life when the child is nearly wholly dependent on caregivers for its survival.
Although the early experience animal data have been used to explain the sequelae of childhood physical and sexual abuse (e.g. Heim and Nemeroff, 2001), the animal models rest heavily on the lack or loss of expectable parental care. These models more closely approximate human conditions of deprivation and neglect than those of physical or sexual abuse. However, while animal models can be designed to focus on circumscribed types of ELS, human ELS is messier. Studies of children in the child welfare system, for example, note that it is rare to find children exposed to only one type of maltreatment. Especially for children under the age of five, physical and/or sexual abuse is typically accompanied by neglect, with neglect constituting the most frequent form of maltreatment for young children (chapter 5 in Barnett et al., 2005). Consistent with the human data, even in non-human primates, physical abuse tends to co-occur with high rates of maternal rejection and failure to protect the infant (McCormack et al., 2006). Moreover, when both the frequency of physical abuse and rejection are used to predict the lower levels of CSF serotonin concentrations noted among abused Rhesus infants, the results indicate that it is neglect/rejection that predicts this neurobiological effect (Maestripieri et al., 2006a; Maestripieri et al., 2006b). Among young children placed in foster care because of maltreatment, markedly atypical cortisol diurnal rhythms are associated with the child’s history of severe neglect, as compared to physical or sexual abuse (Bruce, Fisher et al., 2009), with similar findings noted for children living within the markedly deprived conditions of an institution (e.g. orphanage; Carlson and Earls, 1997). These findings, plus the prominence of deprivation/disruption of parental care in the early experience rodent models, led our network to focus on variations in the amount and quality of parental care in conceptualizing ELS. This is not to say that physical abuse or commission of harm does not have an impact on development; however, these effects are likely more related to trauma (see Yehuda & LeDoux, 2007). Therefore, in our work on ELS, we have chosen to focus on the loss or lack of typical parental care.
This focus is consistent with Levine’s (2005) argument that lack or loss of species typical parental stimulation is among the most potent stressors early in life. A focus on deprivation or loss as a potent stressor is consistent with Hofer’s (1994) concept of hidden regulators embedded in parent-offspring relationships. He has argued that a number of sensorimotor, thermal, and nutrient-based events that are components of typical parent-offspring interactions have long-tem regulatory effects on specific components of infant behavior and physiology. Loss of these hidden regulators results in wide-spread dysregulation of physiological and behavioral responses during development resulting in disturbances in circadian rhythms, growth (including brain growth factors, e.g. Cirulli et al., 2000) and hormone levels (including activity of the hypothalamic-pituitary-adrenocortical [HPA] axis, e.g. Rosenfeld et al.,1992). Animal studies also point to circuits involved in threat- and stress-system functioning as particularly sensitive to disturbances in parental nurturance (see review, Sanchez et al., 2001). Importantly, though, recent rodent studies also indicate that later interventions may help ameliorate some (but not all) of the impact of poor early nurturance (Bredy et al., 2003; Francis et al., 2002). These findings along with our interest in translating basic research to treatments led our network to give equal consideration to both the impacts of disturbances in early parental care and possibility that improvement in care might support recovery from ELS.
Early Life Stress Model
As pictured in Figure 1, this model notes that caregiving experienced early in life regulates the activity of critical stress-sensitive systems, which in turn influence the development of systems involved in rapid appraisal and response to threat. Low parental nurturance results in chronic stress to the infant. This biases the developing threat system to rapidly orchestrate larger defense responses (fight/flight/freeze). Overactivity of both stress-response and threat-response systems may then impact the development of prefrontal regulatory systems, hence increasing the risk for both attention- and emotion-regulatory problems. The neural systems that orchestrate endocrine, autonomic, and behavioral rapid defense responses are expected to be plastic during early childhood. If the child’s care improves, stress- and threat- systems have the possibility to re-organize in order to become less reactive and more modulated. However, children exposed to particularly severe and prolonged inadequate nurturance may be less capable of reorganizing with improved care and this, in turn, may make it difficult for caregivers to sustain appropriate responsiveness to the child’s needs. One hypothesis is that re-organization of the stress- and threat-response systems requires that the child experience safety in his or her world. In early development, this requires that the child develop a relationship with a consistently responsive, caring adult. Therefore, this model also offers suggestions for intervention efforts. Furthermore, while this model may apply to most children (and to most developing mammals), vulnerability to early adverse care and recovery in response to improved care are expected to be influenced by the genetic differences among individuals.
Figure 1.
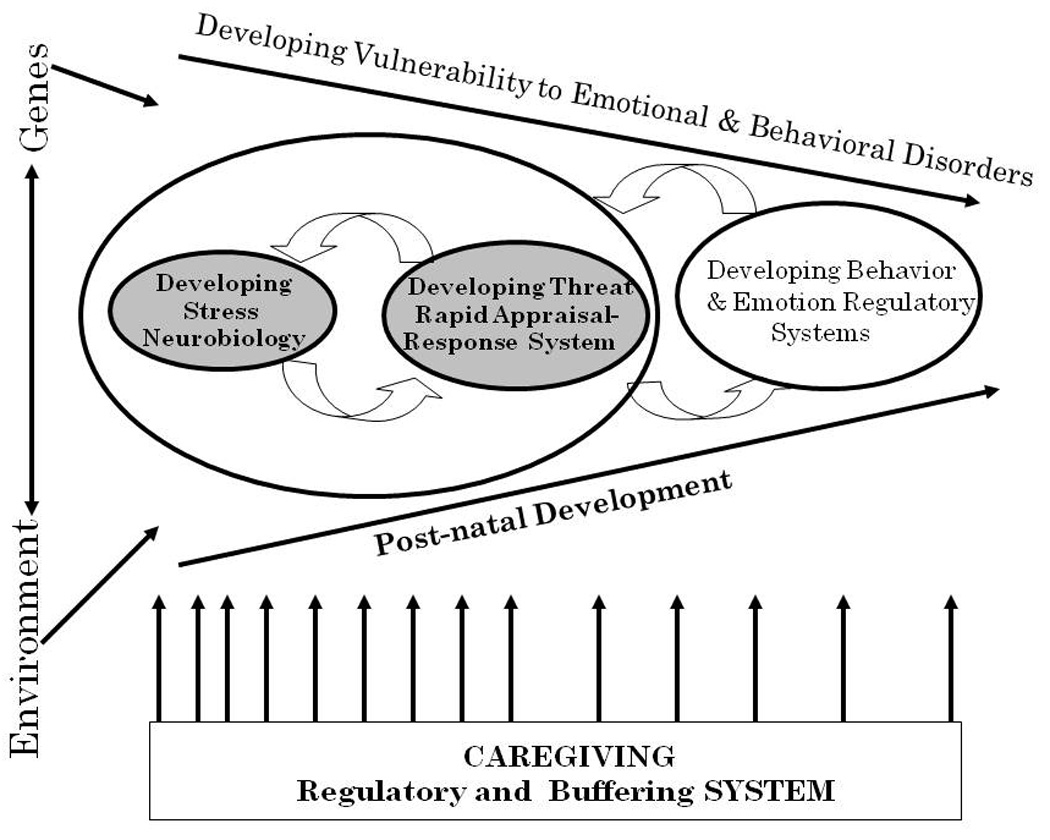
This represents the Center’s working model. It is purposely general enough to apply to the various model systems (rodent, non-human primate, human) studied by Center faculty. The model assumes that both genes and environment will influence developing vulnerability to emotional and behavioral disorders. The partially converging arrows running from left to right are meant to suggest diminishing (but continuing) plasticity of the neurobiological systems underlying risks for emotional and behavioral disorders. The facets of neurobiology depicted in the model are the neurobiology of stress and the neurobiology of rapid threat appraisal and response, along with developing behavioral and emotional regulatory systems. Stress- and Threat-response systems are depicted to the left within a larger circle to indicate their earlier emergence in development. The circular arrows connecting these two systems are meant to reflect their mutual influence on one another. Emotional and behavioral regulatory systems reflect cortico-limbic systems whose development is depicted to the right to indicate that it is somewhat later developing. The circular arrows drawn between emotional and behavioral regulatory systems and the circle containing the stress- and threat-response systems indicate mutual influence across development. Finally, the aspect of the environment most notable to our Center’s model is shown along the bottom of the figure. This is the caregiving regulatory system. The spacing of the arrows is designed to indicate that this system has more influence earlier in development, but depending on the species, may continue to play a role well for prolonged periods of the organism’s life.
Stress-Response System
As noted above, the biology of stress involves the interaction of multiple systems of equilibrium maintenance (i.e. allostasis); therefore, the box within Figure 1 labeled “stress neurobiology” refers conceptually to the multiple systems. However, for most of the work relevant to this discussion, this box refers to activity of the hypothalamic-pituitary-adrenocortical (HPA) axis and its neuroactive petides and hormones. Note that the other major effector arm of the mammalian stress system is the sympatho-adrenal system (Sapolsky et al., 2000). Although HPA activity is sometimes measured solely as an index of stress, this neuroendocrine system has multiple effects on brain development that make it an attractive target for ELS research. Additionally, animal models that form the basis of the preclinical information on ELS began with a focus on the HPA system (see review, Levine, 2005). Glucorticoids (GCs; cortisol in primates; corticosterone in rodents) are gene transcription factors (i.e., influence gene expression; Meijer, 2006), that can be measured non-invasively in young children. GCs are permissive, their presence allowing or enhancing other neural, molecular, or biochemical events. Acutely elevated GCs help to terminate fight/flight physiological and behavioral responses. However, when GCs and/or CRH remain elevated for prolonged periods, this threatens neuronal viability and increases the risk of stress-related disorders (De Kloet et al., 1996; McEwen, 2000).
GCs are produced by the adrenal cortex in response to adrenocorticotropic hormone (ACTH) from the anterior pituitary. Corticotropin-releasing hormone (CRH), produced by cells in the hypothalamus, is the main stimulus of ACTH production. Hypothalamic CRH, in turn, is regulated by neurocircuits conveying day/night information (i.e., diurnal rhythm of the HPA system) and the state of the internal and external milieu (Herman and Cullinan, 1997; Herman et al., 2005; Herman et al., 2002). Stressors (actual or perceived threats) initiate a cascade beginning with hypothalamic CRH release, increased pituitary ACTH secretion, and adrenal production of GCs.
Throughout this discussion, we will sometimes use the acronym L-HPA. The “L” in this case stands for limbic and conveys the importance of limbic system in regulation of HPA responses to psychological stressors (see review, Herman et al., 2005). Activation of the HPA axis to psychological stressors involves the amygdala and perhaps the infralimbic cortex, while inhibition of the HPA response involves the hippocampus and medial prefrontal cortex (Diorio et al., 1993; Herman et al., 2005; Sullivan and Gratton, 2002). These limbic sites have minimal direct projections to CRH-producing regions in the hypothalamus. Instead, they relay to these neurons via neurons in the bed nucleus of the stria terminalis and regions in the hypothalamus and brainstem that have access to hypothalamic CRH-producing neurons. The central nucleus of the amygdala also has projections to brainstem regions which activate sympatho-adrenal responses to stressors (Pitkanen et al., 1997). Thus, HPA and sympatho-adrenal activational pathways overlap with the threat-response system described next.
While elevated GCs have become almost synonymous with “stress,” there is increasing evidence (Fries et al., 2005; Gunnar and Vazquez, 2006) that chronic stress (i.e. prolonged, repeated elevations in GCs and CRH drive on the pituitary) results in hypocortisolism --specifically, low early morning levels of cortisol and blunted ACTH and cortisol responses to stressors. Notably, evidence from the rodent indicates that hyporeactivity of the HPA axis to stressors immediately following chronic stress is more often observed to psychological as opposed to systemic (e.g. viral) stressors (Ostrander et al., 2006). This suggests the hypothesis that some aspects of hypocortisolism, specifically hyporeactivity to stressors, may be organized via impacts on limbic regulatory pathways and is a signature of chronic psychosocial stress (Fries et al., 2005). Prolonged or repeated elevations in glucocorticoids can have opposing effects on hypothalamic (down-regulation) and extra-hypothalamic (up-regulation) CRH (see review, Rosen and Schulkin, 1998). In addition, prolonged hypothalamic CRH drive on the pituitary can down-regulate pituitary sensitivity to ACTH. The result can be low cortisol basal production in the context of increased sensitivity of the amygdala/extended amygdala system (Yehuda, 2000).
Hypocortisolism in the form of low early morning cortisol levels has been found in a number of studies of children who have experienced conditions of chronic deprivation and neglect (see review, Gunnar and Vazquez, 2001). For example, a study involving infants in foster care revealed low early morning cortisol levels in these children relative to non-maltreated infants from families of similar socioeconomic standing (Dozier et al., 2006). Even lower early morning cortisol levels and atypical patterns of cortisol production over the day have been reported for toddlers in institutional care in Romania (Carlson and Earls, 1997). These results were similar to findings noted by both Gunnar and colleagues’ small study of post-institutionalized infants assessed 2 months post-adoption (Bruce et al., 2000) and Fisher and colleagues’ study of preschoolers entering a new foster placement (Bruce, Fisher et al., 2009). In all these studies, 30–40% of the children had early morning cortisol levels that were one standard deviation or more below the mean of children who had not experienced ELS.
To date, there is little evidence that “hypocortisolism” patterns continue once young children are removed from ELS conditions. Indeed, there is evidence that with time in more supportive care environments, not only is the typical pattern of diurnal HPA activity observed, but there is some evidence of elevated basal cortisol levels. This has been shown in several studies of children who spent the early portions of their lives in the depriving conditions of institutions (i.e. post-institutionalized children) prior to being adopted into supportive, well-providing homes (Wismer Fries et al., 2005; Gunnar et al., 2001; Kertes et al., 2008). Elevated levels of basal cortisol several years post-removal from ELS conditions may be particularly likely for children who experience severe stunting in linear growth consistent with psychosocial low stature (e.g. deprivation dwarfism; Kertes et al., 2008; Gunnar et al., 2009). Stunting of physical growth due to early neglect may reflect chronic elevations in CRH and GCs and their impact on the growth axis (Albanese et al., 1994). In sum, severe deprivation of early nurturance may up-regulate HPA activity even though the initial signature of chronic early deprivation or neglect may reveal itself in patterns of hypocortisolism.
Threat-Response System
The pathways that activate HPA and sympatho-adrenal responses to stressors overlap extensively with pathways that orchestrate fear and other behavioral responses to threat. Nonetheless, behavioral and cardiac reactions to threat (i.e. fear grimaces, freezing, distress vocalization, elevated heart rates) can be elicited under conditions that do not increase adrenocortical or adrenomedullary activity. For this reason, although the threat-response and stress-response systems have numerous interconnections, we choose to treat them as separate, but interconnecting systems (see Figure 1). Their inter-connections are reflected in the importance of CRH in both. CRH, critical in regulating activity of the HPA axis, is also synthesized and secreted in extra-hypothalamic sites. Extra-hypothalamic CRH is involved in coordinating neuroendocrine, autonomic, and behavioral responses to threat (Brunson et al., 2001; Gray and Bingaman, 1996; Nemeroff, 1996). These defensive actions are partially orchestrated via CRH type 1 (CRH-1) receptors that are widely expressed in threat-transducing (i.e. fear) pathways and in systems that activate counter-regulatory activity (e.g., autonomic nervous system; Bale and Vale, 2004). Importantly, extra-hypothalamic CRH is produced largely by CRH-producing neurons in the central nucleus of the amygdala (Rosen and Schulkin, 1998). There is substantial evidence that the central nucleus of the amygdala (CeA) and bed nucleus of the stria terminalis (BNST) are key regions in organizing fear and defensive responses to psychological stressors (Davis et al., 1997; LeDoux and Phelps, 2000). We refer to this as the “threat system” and further define it as a rapid appraisal-response system due to its capacity to orchestrate rapid reactions with little cortical processing (LeDoux & Phelps, 2000). The amygdala/extended amygdala system functions to modulate cognition, behavior, and endocrine/autonomic activity through extensive afferent and efferent connections with cortical and subcortical structures (Adolphs, 2003). The amygdala is also a target of GCs. Chronic or frequent elevations in GCs are involved in remodeling of the dendritic arborization of the amygdala (Vyas et al., 2002) and in the up-regulation of CRH activity in the central nucleus (Makino et al., 1994), potentially increasing reactivity of the threat response system. In contrast, chronic or frequent elevations of GCs have a restraining or down-regulating impact on CRH-producing cells in the hypothalamus, potentially maintaining normal to low GCs in response to chronic stress even in the face of lower thresholds for activating defensive behavior (i.e. fight/flight/freeze).
Stress- and threat-response systems: Relations with PFC development
The rapid threat appraisal-response system has bidirectional connections with regions of the prefrontal cortex (PFC) and anterior cingulate cortex (ACC; Adolphs, 2003). There is increasing evidence that development and individual differences on cognitive tasks involving dorsolateral-ACC pathways are negatively correlated with measures of negative emotionality (Posner et al., 2001; Rueda et al., 2004). With regard to threat-system modulation, there is evidence that the amygdala is modulated by the PFC, particularly pathways involving the orbital/medial PFC although this is a rapidly evolving area of study (see Davidson, 2004; Gemar et al., 2007). The orbital/medial PFC is also rich with glucocorticoid and CRH-1 receptors. In mature organisms, this region is involved in control of the HPA system and in autonomic responses to psychological stressors (Sullivan and Gratton, 2002).
There is also evidence that ELS impacts PFC development. Specifically, maternal separation in rats affects the development of the medial PFC by reducing glucocorticoid receptors, increasing synaptic density, and enlarging this region which is consistent with the stress vulnerability and emotionality observed in maternally separated non-human primates (Sanchez et al., 2001; Sullivan and Gratton, 2002). Recently, the medial PFC has been implicated in the inoculation effect of previous control over stressful stimulation on later responses to stressors (Amat et al., 2006). It has been argued that adverse early experiences may disrupt the development of medial and related regions in the PFC, thus increasing the risk of both helpless responses to stressors and attention regulatory problems (Sullivan and Brake, 2003). For example, low parental nurturance has been associated with both right-biased anterior EEG asymmetry and elevated glucocorticoid levels among human toddlers and preschoolers (Dawson and Ashman, 2000; Gunnar and Donzella, 2002; Hane and Fox, 2006). In addition to parent and teacher reports that children who have experienced ELS demonstrate attention and self-regulation problems (Kreppner et al., 2001; Provence and Lipton, 1962; Roy et al., 2004; Shields et al., 1994; Stevens et al., 2008), both children in foster care and those adopted from institutions demonstrate difficulties on neuropsychological tasks that rely on prefrontal attention regulatory circuits (Bruce, McDermott et al., 2009; Colvert et al., 2008; Pears and Fisher, 2005). A recent study found that post-institutionalized children performed more poorly than non-institutionalized children on a visual attention task related to locating a target from an array of stimuli (Pollak et al., in press). Similarly, isolate-reared monkeys were found to display deficits on executive tasks which correlated with reduced white matter tracts in the PFC and ACC (Sanchez et al., 1998). Thus, it is likely that ELS and the processes that support recovery both influence development of PFC-limbic pathways associated with frontal EEG asymmetry and emerging executive attention capacities.
Development
The stress-response and threat-response systems and their afferent and efferent pathways that extend into the PFC are immature in young mammals. There is substantial evidence that these developing systems are particularly plastic and open to modification by early life experiences (Levine, 1994; Meaney et al., 1996; Suomi, 1997). Inadequate parental care shapes a more reactive stress-response system in infants of many species and might contribute to hypersensitivity of the developing threat appraisal system, thus increasing vulnerability to stressors throughout life. Conversely, impacts of early caregiving disruptions on the developing threat system, particularly on amygdalar CRH and CRH-receptor systems may result in a lower threshold for activation of the HPA axis. Hence, there are bi-directional and transactional arrows between the developing stress- and threat-response systems in Figure 1. The developmental anatomy and physiology of these systems is only beginning to be understood (Bachevalier, 1998; Vazquez, 1998). The maturity of these systems relative to parturition also differs markedly among species. In general, however, as shown in Figure 1, the developing stress- and threat-response systems develop earlier than systems involved in behavioral and emotional regulation. There is also only emerging data on the impact that disruptions in each of these systems have on one another. For example, there is some evidence from work with Rhesus monkeys that lesions to amygdalar, hippocampal, or orbital frontal regions altered behavioral responses to social threat; however, the effect on HPA axis functioning requires further study (Machado and Bachevalier, 2006, 2008). Regardless, it is clear that damage to these stress-defense systems early in life has profound effects on socioemotional development (e.g. decreased social interaction; Bachevalier, 1998; Malkova et al., 1997).
Caregiving
Rodent early experience studies demonstrate how early parental care profoundly influences brain development, regulates gene expression, and shapes the neural systems involved in reactivity and regulation of the HPA system to subsequent stressors (Levine, 2005). In the rat, the L-HPA system is relatively hyporesponsive to stressors during the first few weeks of life (stress hyporesponsive period [SHRP]; Brunson et al., 2001). Specific maternal stimuli of licking/grooming and nursing maintain this SHRP. While brief separations from these maternal stimuli result in a more adaptive animal, perhaps due to the subsequent increases in licking and grooming upon return, removing these stimuli for longer periods of time produces HPA axis hypereactivity (Levine, 2005). These stimuli also regulate CRH activity in extrahypothalamic sites (Brunson et al., 2001). Critically, parental care not only buffers the pup from many stressors and regulates basal activity of the axis, but also influences the development of the stress- and threat-response systems. Inadequate or disorganized parental care influences stress system hyperreactivity to stressors that might persist throughout life and influences hyper-defensive behavior. These parenting effects are produced, at least in part, through epigenetic processes (i.e., methylation of the glucocorticoids receptor gene in negative feedback pathways of the L-HPA system; Meaney and Szyf, 2005). In addition, there is increasing evidence that CRH, in synergy with GCs, mediates some of these parenting effects (Brunson et al., 2001). Early adverse parental care also increases CRH-1 receptor expression in limbic and cortical regions, thus increasing sensitivity to the fear-/stress-organizing effects of CRH over time (Sanchez et al., 2001). In Figure 1, we have depicted the importance of caregiving, with the close spacing of the arrows from the caregiving box indicating the high dependence of infant mammals on parental support and the decreasing, but still evident, dependence on such support with development.
One of the challenges of translating the rodent models to human (and non-human primate) development is to identify the pattern of parenting that might serve a function similar to “licking and grooming.” While this might be physical contact, including “massage,” in our model we have chosen to emphasize caregiver sensitivity and responsiveness to the infant’s signals. The reason for this adjustment is that the human data indicate that attachment security is a critical “buffer” of HPA axis reactivity in infants and young children (Gunnar et al., 1996; Suomi, 1997), and secure attachment emerges out of a history of sensitive and responsive caregiver-infant relationships (Susman-Stillman et al., 1996).
There is increasing evidence that “sensitive and responsive care” is the critical dimension of care in ELS models. Multiple studies have demonstrated that it is difficult to elevate cortisol in young children when a parent with whom they have a secure attachment relationship is present. In contrast, infants in insecure relationships appear to have difficulty using their parent’s presence to prevent cortisol increases to emotionally distressing events (Ahnert et al., 2004; Nachmias et al., 1996; Spangler and Schieche, 1998). Notably, infants with disordered/ disorganized (i.e. atypical) attachment relationships are both most at risk for behavioral and emotional problems (Van Ijzendoorn et al., 1999), and are most likely to exhibit more prolonged cortisol elevations to threat (Hertsgaard et al., 1995; Spangler, 1997, but see also Spangler and Grossmann, 1999). Parental sensitivity and responsiveness reduces the probability of disorganized/disordered attachment in high risk infants (Bakermans-Kranenburg et al., 2003; Lyons-Ruth et al., 1987), and months later predicts the capacity of the parent’s presence to buffer toddler cortisol responses to a pain stimulus (Gunnar et al., 1996).
Similar to the human findings, in studies with monkeys, the presence of the mother buffers elevations of cortisol to threat, even in the absence of physical contact (Wiener et al., 1990). Disturbing the attachment relationship in Rhesus can produce long-term changes in ACTH and GC activity and in cerebral spinal fluid concentrations of CRH (Coplan et al., 1996). The few studies with monkeys that have examined naturally-occurring variations in parental care and GC responses to stressful stimulation implicate variations in maternal responsiveness as critical in determining whether the presence of the mother is capable of buffering the infant’s HPA responses to stressors. Following a two week separation, for example, Rhesus infants whose mothers had been non-responsive (rejecting) of the infants’ bids prior to the separation exhibited elevations in cortisol to reunion, while for those whose mothers were responsive to their signals, reunion produced a marked decrease in cortisol levels (Gunnar et al., 1981). Additionally, as noted earlier, CSF serotonin metabolites in maltreated and control Rhesus infants varied with how much the mother rejected their bids for attention, not with the amount of abuse the infants experienced (Maestripieri et al., 2006a; Maestripieri et al., 2006b). There is also evidence that contact with the mother fails to buffer cortisol increases to capture and handling in rejecting/maltreating Rhesus dyads (personal communication, Kai McCormack, 4/20/2009), a finding highly comparable to evidence that the presence of the parent in insecure attachment relationships fails to buffer cortisol increases to fear-eliciting events (Nachmias et al., 1996). Although we cannot assume that the maltreating Rhesus dyads have insecure attachment, preliminary findings suggest this may be the case. Using an adaption of the Attachment Q-sort designed for the study of human children for use with maltreating Rhesus dyads, McCormack and colleagues (2007) found that infants of abusive Rhesus mothers scored lower on attachment security than did offspring of non-abusive Rhesus mothers.
Genetic processes
Termed multifinality in developmental psychopathology, similar adverse experiences produce a variety of outcomes; for example, while some individuals develop anxiety and depression, others develop conduct problems, and many exhibit comorbidity (Cicchetti and Rogosch, 1996). Numerous factors likely contribute to these individual differences, including one potential source of variation: genetics. While we recognize that evocative effects of genes might contribute to child behaviors that elicit more adverse parent behavior (O'Connor et al., 1998), more relevant to this discussion is exploration of the moderating effects of several candidate genes on the impact of ELS. Of particular relevance is the increasing evidence that the serotonin system plays a critical role. Individuals who are either heterozygous or homozygous for the short form of the 5’ promoter region polymorphism (SLC6A4) in the serotonin transporter (SERT) gene might be particularly vulnerable to ELS/early maltreatment. In humans, the short SERT allele is associated with increased risk of early onset depression in response to maltreatment in childhood (Caspi et al., 2003) thus demonstrating a gene by ELS interaction. In Rhesus monkeys, Bennett et al. (2002) showed that animals carrying one “short” SERT gene were more sensitive to adverse early care. There are numerous interactions between serotonin and GCs during development (Lopez et al., 1998; Mitchell et al., 1992). Furthermore, there is evidence that disturbances to the serotonin system produced by early parental deprivation in the rat may be mediated by activity of the L-HPA system (Vazquez et al., 2002). Studies of the interaction of polymorphisms in genes regulating the serotonin system and stressors affecting the L-HPA system during development are needed in both animal models and studies of human development.
The mesocortical dopamine system plays a critical role in the development and functioning of frontal systems involved in attention and emotion regulation (Berridge and Robinson, 1998; Diamond, 1998). Adverse early caregiving disturbs the development of the dopamine system in the nucleus accumbens (Hall et al., 1998), and the effects of stressful stimulation on this system are enhanced in the presence of elevated GCs (Piazza and LeMoal, 1996). Though, there is a highly coupled dopaminergic circuit, the mesocorticolimbic system, of which the nucleus accumbens is only one part (Pierce and Kalivas, 1997). The prefrontal cortex essentially puts the brakes on dopamine secretion in the nucleus accumbens. However, although acute stressors stimulate neurons in the ventral tegmental area to secrete dopamine in both the nucleus accumbens and the prefrontal cortex, repeated stressors facilitate dopamine secretion in the nucleus accumbens, but inhibit it in the prefrontal cortex (Sorg and Kalivas, 1991; Sorg and Kalivas, 1993). It is quite possible that the stimulatory effect of the GCs on dopamine secretion in the nucleus accumbens is an indirect consequence of their actions on amygdalar function and the strong input from the extended amygdala to the nucleus accumbens (Swanson, 2000)
Several studies now indicate that inefficient activity of genes regulating mesocortical dopamine confer heightened risk of poor outcomes in animals and humans exposed to ELS. For example, polymorphisms in genes in the dopamine receptor family (e.g. DRD4), increase the risk of attention problems (Schmidt et al., 2001) and disordered patterns of relationship functioning in children (Lakatos et al., 2002; Lakatos et al., 2000). Similar to the studies involving serotonin, however, gene by ELS interactions have been noted. For example, the 7-repeat DRD4 polymorphism is associated with disorganized attachment behavior only when infants are reared by mothers who suffer from unresolved trauma and loss (Van Ijzendoorn and Bakermans-Kranenburg, 2006). Given evidence that the DRD4 polymorphism impacts mesocortical dopamine activity, further research is called for to examine whether DRD4 interacts with ELS to impact development of attention-and emotion-regulatory systems. In fact, given the rapidly evolving nature of the field of molecular behavioral genetics, consideration of the moderating effects of genes beyond SERT and DRD4 are warranted.
Sex Differences
Sex differences are frequently noted in the animal models of early neglect/deprivation (see for review, Gunnar and Vazquez, 2006). Unfortunately, whether the differences favor males or females is not consistent. Sex differences in cortisol responses to stressors are also reported for adults (Kirschbaum et al., 1999; Stroud et al., 2002); but these differences may emerge with puberty (Gunnar and Vazquez, 2006). Regarding the rapid threat-response system, evidence for significant sex differences is remarkably rare, despite clinical evidence that females may be more at risk for anxiety and trauma-induced anxiety disorders than males (Breslau et al., 1997; Jorm, 1987; Renard et al., 2005). In studies of the impact of ELS on fear-potentiated startle in rodents and non-human primates, few sex differences are reported, despite significant increases in startle as a function of ELS (De Jongh et al., 2005; Sanchez et al., 2005). Regarding measures of PFC systems, there is some evidence from adult studies of frontal EEG asymmetry that when participants are asked to generate an emotion state, females show a right-frontal EEG bias while males do not; however, these effects may be fairly task specific (Davidson et al., 1976). Under resting conditions, on the other hand, sex differences are not typically found for adults (Davidson et al., 1976; Sutton and Davidson, 2000) or infants and young children (Buss et al., 2003; Fox et al., 1995). Given this inconsistent pattern of findings, possible sex/gender effects are worth considering; it is likely that ELS effects on boys versus girls are moderated by numerous factors, not all of which are understood.
Model in Action
Many of the studies described as supporting evidence for each of the components of the ELS model present examples of the feasibility of this model for translational ELS research. By drawing connections across each of the sections, one can start to see the utility of using this model to enhance our understanding of the how early caregiving experiences impact the neuroendocrine and neurobiological processes that contribute to the development of emotion-and attention-regulatory systems. For example, Gunnar et al. (1996) previously demonstrated that toddlers of parents rated as low in sensitivity when the children were infants (i.e. two- to six-months-old) exhibited larger cortisol stress responses (Figure 2–Panel A). Compared to the children of normal to high responsive parents, those of low responsive parents (bottom 25%) continued to produce elevations in cortisol at 18 months. Hane and Fox (2006) extended these findings to include measures of frontal EEG asymmetry. Right frontal EEG asymmetry is associated with withdrawal emotions (e.g., fear, sadness), and Davidson (2004) argues that it reflects constant vigilance to threat. As shown in Figure 2–Panel B, Hane and Fox found that low responsive mothers had nine-month-old infants who exhibited more right (negative numbers) frontal EEG asymmetry, whereas the infants of high responsive mothers exhibited a left frontal EEG pattern. Notably, Hane and Fox found that infants with low responsive mothers were also more inhibited (fearful). Taken together, these studies demonstrate how caregiving in the form of low-responsiveness influences the stress-response (e.g. cortisol), threat-response (behaviorally inhibited), and frontal-regulatory systems (EEG asymmetry) throughout development.
Figure 2.
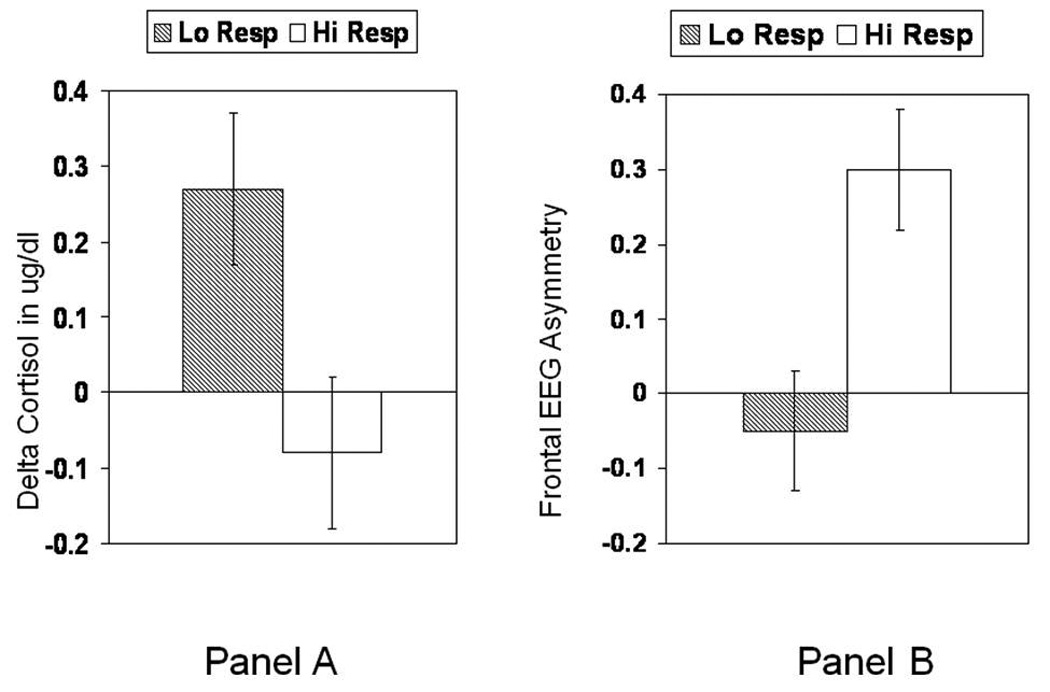
Relation between low and high maternal responsivity and reactivity of stress and emotion sensitive systems. Panel A. Cortisol responses to inoculations at 18 months in children whose mothers were low or normal to high responsive during the first 6 months of the infants lives. Adapted from Gunnar et al. (1996). Panel B. EEG asymmetry in infants of low versus high responsive mothers. Note that negative EEG asymmetry scores indicate right-biased activity associated with increased withdrawal affects, including depression. Adapted from Hane and Fox (2006).
Another goal of this ELS model is to bridge connections across the human and animal conditions of ELS. While each of the differing examples of ELS undoubtedly has a unique neurobiological signature, there are commonalities across findings. For example, both young children in foster care and those living in institutional settings exhibit disturbances in the diurnal cortisol rhythm (see review, Gunnar and Fisher, 2006) which is similar to findings obtained on abused Rhesus infants (Sanchez et al., 2005). Like Rhesus infants reared under conditions of maternal deprivation (Sanchez et al., 1998), both young children in foster care and post-institutionalized children exhibit problems on tasks dependent on development of prefrontal attention regulatory circuits (Bruce, McDermott et al., 2009; Colvert et al., 2008; Pears and Fisher, 2005) And, as in children in foster care and children adopted from institutions (O'Connor et al., 1999; O'Connor and Rutter, 2000; Stovall and Dozier, 2000), Rhesus infants exposed to maternal maltreatment exhibit patterns of insecure attachment and a failure of maternal contact to buffer elevations in cortisol to stressful events (personal communication, Kai McCormack, 4/20/2009; McCormack et al., 2007). Although one must be careful about direct translation across species and specific ELS conditions, these commonalities support the idea of utilizing other models to inform further hypotheses and support research findings.
Implications and Future Directions
Despite the prevalence of conditions of ELS and knowledge of the resultant behavioral difficulties, because researchers are just starting to better understand the impact of deprived conditions on development, there are relatively few studies of interventions. The cohesive neurobehavioral model presented here has large implications for consideration of intervention efforts. For example, intervention could target aspects of parenting in order to have a direct effect on the caregiving portion of this model, which may then influence the other model components. In randomized clinical trials, Fisher et al. (2000) have shown that supporting foster parents’ ability to provide consistent, nonhostile, supportive care improves the behavioral functioning of preschoolers. Further study of these interventions provides evidence that enhancing the quality of care by foster parents might also help normalize basal L-HPA axis activity. Fisher et al. (2007) examined changes in the HPA activity for preschoolers with low morning cortisol levels and small decreases in daytime diurnal cortisol. Following a six-month period, preschoolers receiving the foster care intervention demonstrated increases in morning cortisol levels, resulting in a more typical decrease in cortisol over the day. This did not occur for those in the regular foster care condition (Fisher et al., 2007). What remains unclear, however, is how these impacts are mediated and exactly what type of parenting behavior is best. Because children experiencing early adverse care often confront their caregivers with disordered attachment behaviors and disruptive behavior problems, these children may need parenting that “gently challenges” them by providing the nurturance the child needs but does not demand (Dozier, 2003). However, intervention efforts can occur within any of the model components and perhaps may need to occur at multiple levels (i.e. level of behavioral problems and level of caregiving) in order to be most effective. It is possible that behavior problems may need to be brought under control before warm and supportive parent-child relationships can develop. The foster care intervention program, for example, provides evidence that focusing on training parents to manage behavior problems in a non-hostile, consistent, and effective manner also increases secure attachment behavior for preschooler (Fisher et al., 2006). It is conceivable that different aspects of threat- and stress-response system activity are responsive to different facets of parental care in children exposed to ELS conditions early in life. Clearly, further study conducted within the context of the presented ELS model is needed in order to finely analyze how parental care is associated with activity of stress- and threat-response systems at different points in development. This information should allow us to develop interventions that better target aspects of neurobehavioral development that increase risk of psychopathology.
Acknowledgements
This manuscript reflects work of the Early Experience, Stress, and Prevention Science (now Neurobehavioral Development) network (now Center) supported by grant number R21 MH65046 from the National Institute of Mental Health in the United States. The members of the network were (in alphabetical order): Mary Dozier, Philip Fisher, Nathan Fox, Megan Gunnar, Paul Plotsky, Seth Pollak, Mar Sanchez, and Stephen Suomi. In addition to these network members, the Center now also includes (in alphabetical order): Jacqueline Bruce, Mary Dallman, Kai McCormack, Katherine Pears, and James Ritchie who have also contributed to the work in this manuscript.
We gratefully acknowledge the work on this network by our late colleague, Seymour “Gig” Levine. He was not only its inspiration, but his courage in challenging us to not worry about protecting his legacy and instead to critically evaluate what we needed to know in order to improve outcomes for neglected and abused children set the tone for our work.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This NIMH Interdisciplinary Developmental Science Center members are Jacqueline Bruce, Mary Dallman, Mary Dozier, Philip Fisher, Nathan Fox, Megan Gunnar, Kai McCormack, Katherine Pears, Paul Plotsky, Seth Pollak, James Ritchie, Mar Sanchez, and Stephen Suomi. Seymour Levine was a member of the research network that gave rise to this NIMH Center. He passed away as we were in the process of producing the grant revision that was funded.
References
- Adolphs R. Is the human amygdala specialized for processing social information? Ann. N. Y. Acad. Sci. 2003;985:326–340. doi: 10.1111/j.1749-6632.2003.tb07091.x. [DOI] [PubMed] [Google Scholar]
- Ahnert L, Gunnar MR, Lamb ME, Barthel M. Transition to child care: Associations with infant-mother attachment, infant negative emotion, and cortisol elevations. Child Dev. 2004;75:639–650. doi: 10.1111/j.1467-8624.2004.00698.x. [DOI] [PubMed] [Google Scholar]
- Albanese A, Hamill G, Jones J, Skuse D, Matthews DR, Stanhope R. Reversibility of physiological growth hormone secretion in children with psychosocial dwarfism. Clin. Endocrinol. 1994;40:687–692. doi: 10.1111/j.1365-2265.1994.tb03022.x. [DOI] [PubMed] [Google Scholar]
- Amat J, Paul E, Zarza C, Watkins L, Maier SF. Previous experience with behavioral control over stress blocks the behavioral and dorsal raphe nucleus activating effects of later uncontrollable stress: role of the ventral medial prefrontal cortex. J. Neurosci. 2006;26:13264–13272. doi: 10.1523/JNEUROSCI.3630-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachevalier J. Development, brain function, and social behavior. In: Kalin N, Davidson RJ, editors. Madison, Wisconsin. Proceedings of the Affective Neuroscience: Fourth Annual Wisconsin Symposium on Emotion.1998. [Google Scholar]
- Bakermans-Kranenburg MJ, Van IJzendoorn MH, Juffer F. Less is more: meta-analyses of sensitivity and attachment interventions in early childhood. Psychol. Bull. 2003:195–215. doi: 10.1037/0033-2909.129.2.195. [DOI] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu. Rev. of Pharmacol. Toxicol. 2004;44:525–527. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Barnett OW, Miller-Perrin CL, Perrin R. Family violence across the lifespan: an introduction. second ed. California: Sage Publications; 2005. [Google Scholar]
- Bennett AJ, Lesch KP, Heils A, Long JC, Lorenz JG, Shoal SE, Champoux M, Suomi SJ, Linnoila MV, Higley JD. Early experience and serotonin transporter gene variation interact to influence primate CNS function. Mol. Psychiatry. 2002;7:118–122. doi: 10.1038/sj.mp.4000949. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res. Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Brake WG, Zhang TY, Diorio J, Meaney MJ, Gratton A. Influence of early postnatal rearing conditions on mesocorticolimbic dopamine and behavioural responses to psychostimulants and stressors in adult rats. Eur. J. Neurosci. 2004;19:1863–1874. doi: 10.1111/j.1460-9568.2004.03286.x. [DOI] [PubMed] [Google Scholar]
- Bredy TW, Humpartzoomian RA, Cain DP, Meaney MJ. Partial reversal of the effect of maternal care on cognitive function through environmental enrichment. Neuroscience. 2003;118(2):571–576. doi: 10.1016/s0306-4522(02)00918-1. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E. Stress and development: Behavioral and biological consequences. Dev. Psychopathol. 2001;13:473–490. doi: 10.1017/s0954579401003042. [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis GD, Andreski P, Perterson EL, Schultz LR. Sex differences in posttraumatic stress disorder. Arch. Gen. Psychiatry. 1997;54:1044–1048. doi: 10.1001/archpsyc.1997.01830230082012. [DOI] [PubMed] [Google Scholar]
- Bruce J, Kroupina M, Parker S, Gunnar MR. Proceedings of the International Conference on Infant Studies. England: Brighton; 2000. The relationships between cortisol patterns, growth retardation, and developmental delays in post-institutionalized children. [Google Scholar]
- Bruce J, Fisher PA, Pears KC, Levine S. Morning cortisol levels in preschool-aged foster children: Differential effects of maltreatment type. Dev. Psychobio. 2009;51:14–23. doi: 10.1002/dev.20333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce J, McDermott JM, Fisher PA, Fox NA. Using behavioral and electrophysiological measures to assess the effects of a preventive intervention: A preliminary study with preschool-aged foster children. Prev. Sci. 2009;10(2):129–140. doi: 10.1007/s11121-008-0115-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson KL, Eghbal-Ahmadi M, Bender R, Chen Y, Baram TZ. Long-term, progressive hippocampal cell loss and dysfunction induced by early-life administration of corticotropin-releasting hormone reproduce the effects of early-life stress. Proc. Natl Acad Sci. U.S.A. 2001;98:8856–8861. doi: 10.1073/pnas.151224898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss KA, Schumacher JRM, Dolski I, Kalin NH, Goldsmith HH, Davidson RJ. Right frontal brain activity, cortisol, and withdrawal behavior in 6-month-old infants. Behav. Neurosci. 2003;117:11–20. doi: 10.1037//0735-7044.117.1.11. [DOI] [PubMed] [Google Scholar]
- Carlson M, Earls F. Psychological and neuroendocrinological sequelae of early social deprivation in institutionalized children in Romania. Ann. N. Y. Acad. Sci. 1997;807:419–428. doi: 10.1111/j.1749-6632.1997.tb51936.x. [DOI] [PubMed] [Google Scholar]
- Carrion VG, Weems CF, Ray RD, Glaser B, Hessl D, Reiss AL. Diurnal salivary cortisol in pediatric posttraumatic stress disorder. Biol. Psychiatry. 2002;51:575–582. doi: 10.1016/s0006-3223(01)01310-5. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffit T, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Equifinality and multifinality in developmental psychopathology. Dev. Psychopathol. 1996;8:597–600. [Google Scholar]
- Cirulli F, Alleva E, Antonelli A, Aloe L. NGF expression in the developing rat brain: Effects of maternal separation. Brain Res. Dev. Brain Res. 2000;123:129–134. doi: 10.1016/s0006-8993(00)02844-4. [DOI] [PubMed] [Google Scholar]
- Colvert E, Rutter M, Kreppner J, Beckett C, Castle J, Groothues C, Hawkins A, Stevens S, Sonuga-Barke E. Do Theory of Mind and Executive Function Deficits Underlie the Adverse Outcomes Associated with Profound Early Deprivation?: Findings from the English and Romanian Adoptees Study. J. Abnorm. Child Psychol. 2008;36:1057–1068. doi: 10.1007/s10802-008-9232-x. [DOI] [PubMed] [Google Scholar]
- Coplan JD, Andrews MW, Rosenblum LA, Owens MJ, Friedman S, Gorman JM, Nemeroff CB. Persistent elevations of cerebrospinal fluid concentrations of corticotropin-releasing factor in adult nonhuman primates exposed to early-life stressors: Implications for the pathophysiology of mood and anxiety disorders. Proc. Natl. Acad. Sci. U.S.A. 1996;93:1619–1623. doi: 10.1073/pnas.93.4.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ. What does the prefrontal cortex "do" in affect: perspectives on frontal EEG asymmetry research. Biol. Psychiatry. 2004;67:219–233. doi: 10.1016/j.biopsycho.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Schwartz GE, Pugash E, Bromfield E. Sex differences in patterns of EEG asymmetry. Biol. Psychol. 1976;4:119–138. doi: 10.1016/0301-0511(76)90012-0. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Lee Y. Roles of the amygdala and bed nucleus of the stria terminalis in fear and anxiety measured with the acoustic startle reflex. Ann. N. Y. Acad. Sci. 1997;821:305–331. doi: 10.1111/j.1749-6632.1997.tb48289.x. [DOI] [PubMed] [Google Scholar]
- Dawson G, Ashman S. On the origins of a vulnerability to depression: The influence of early social environment on the development of psychobiological systems related to risk for affective disorder. In: Nelson CA, editor. The Effects of Adversity on Neurobehavioral Development. Minnesota Symposia on Child Psychology. vol. 31. New York: Erlbaum & Assoc; 2000. pp. 245–278. [Google Scholar]
- De Bellis M, Baum AS, Birmaher B, Keshavan MS, Eccard CH, Boring AM, Jenkins FJ, Ryan ND. Developmental traumatology, Part 1: Biological stress systems. Biol. Psychiatry. 1999;9:1259–1270. doi: 10.1016/s0006-3223(99)00044-x. [DOI] [PubMed] [Google Scholar]
- De Jongh R, Geyer MA, Olivier B, Groenink L. The effects of sex and neonatal maternal separation on fear-potentiated and light-enhanced startle. Behav. Brain Res. 2005;161:190–196. doi: 10.1016/j.bbr.2005.02.004. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Rosenfeld P, VanEeKelen JA, Sutanto W, Levine S. Stress, glucocorticoids and development. Prog. Brain Res. 1988;73:101–120. doi: 10.1016/S0079-6123(08)60500-2. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Rots NY, Cools AR. Brain-corticosteroid hormone dialogue: Slow and persistent. Cell. Mol. Neurobiol. 1996;16:345–356. doi: 10.1007/BF02088100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. Evidence for the importance of dopamine for prefrontal cortex functions early in life. In: Roberts AC, Robbins TW, Weiskrantz L, editors. The prefrontal cortex: Executive and cognitive functions. Oxford: Oxford University Press; 1998. pp. 144–164. [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulated gyrus) in the regulation of hypothalmic-pituitary-adrenal responses to stress. J. Neurosci. 1993:13. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozier M. Attachment-based treatment for vulnerable children. Attach. Hum. Dev. 2003;5:253–257. doi: 10.1080/14616730310001596151. [DOI] [PubMed] [Google Scholar]
- Dozier M, Peloso E, Gordon MK, Manni M, Gunnar MR, Stovall-McClough KC, Levine S. Foster children's diurnal production of cortisol: An exploratory study. Child Maltreat. 2006;11:189–197. doi: 10.1177/1077559505285779. [DOI] [PubMed] [Google Scholar]
- Fisher P, Gunnar MR, Dozier M, Bruce J, Pears K. Effects of therapeutic interventions for foster children on behavior problems, caregiver attachment, and stress regulatory neural systems. Ann. N. Y. Acad. Sci. 2006;1094:215–225. doi: 10.1196/annals.1376.023. [DOI] [PubMed] [Google Scholar]
- Fisher PA, Gunnar MR, Chamberlain P, Reid JB. Preventive intervention for maltreated preschool children: Impact on children's behavior, neuroendocrine activity, and foster parent functioning. J. Am. Acad. Child Adolesc. Psychiatry. 2000;39:1356–1364. doi: 10.1097/00004583-200011000-00009. [DOI] [PubMed] [Google Scholar]
- Fisher PA, Stoolmiller M, Gunnar MR. Effects of a therapeutic intervention for foster preschoolers on daytime cortisol activity. Psychoneuoendocrinology. 2007;32:892–905. doi: 10.1016/j.psyneuen.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NA, Rubin K, Calkins SD, Marshall T, Coplan RJ, Porges SW, Long J. Frontal activation asymmetry and social competence at four years of age. Child Dev. 1995;66:1770–1784. [PubMed] [Google Scholar]
- Francis D, Diorio J, Plotsky PM, Meaney MJ. Environmental enrichment reverses the effects of maternal separation on stress reactivity. J. Neurosci. 2002;22:7840–7843. doi: 10.1523/JNEUROSCI.22-18-07840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries E, Hesse J, Hellhammer J, Hellhammer D. A new view on hypocortisolism. Psychoneuoendocrinology. 2005;30:1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Gemar MC, Segal ZV, Mayberg HS, Goldapple K, Carney C. Changes in regional cerebral blood flow following mood challenge in drug-free, remitted patients with unipolar depression. Depress. Anxiety. 2007;24:597–601. doi: 10.1002/da.20242. [DOI] [PubMed] [Google Scholar]
- Gray TS, Bingaman EW. The amygdala: Corticotropin-releasing factor, steroids, and stress. Crit. Rev. Neurobiol. 1996;10:155–168. doi: 10.1615/critrevneurobiol.v10.i2.10. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Brodersen L, Nachmias M, Buss KA, Rigatuso J. Stress reactivity and attachment security. Dev. Psychobiol. 1996;29:191–204. doi: 10.1002/(SICI)1098-2302(199604)29:3<191::AID-DEV1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27:199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Fisher P. Bringing basic research on early experience and stress neurobiology to bear on preventive interventions for neglected and maltreated children. Dev. Psychopathol. 2006;18:651–677. [PubMed] [Google Scholar]
- Gunnar MR, Frenn K, Wewerka S, Van Ryzin MJ. Moderate versus severe early life stress: Associations with stress reactivity and regulation in 10- to 12-year old children. Psychoneuoendocrinology. 2009;34:62–75. doi: 10.1016/j.psyneuen.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Gonzales C, Goodlin C, Levine S. Behavioral and pituitary-adrenal responses during a prolonged separation period in infant Rhesus macaques. Psychoneuroendocrinology. 1981;6:65–75. doi: 10.1016/0306-4530(81)90049-4. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Morison SJ, Chisholm K, Schuder M. Salivary cortisol levels in children adopted from Romanian orphanages. Dev. Psychopathol. 2001;13:611–628. doi: 10.1017/s095457940100311x. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez D. Low cortisol and a flattening of expected daytime rhythm: potential indices of risk in human development. Dev. Psychopathol. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez D. Stress neurobiology and developmental psychopathology. In: Cicchetti D, Cohen D, editors. Developmental Psychopathology: Developmental Neuroscience. vol.2. New York: Wiley; 2006. pp. 533–577. [Google Scholar]
- Hall FS, Wilkinson LS, Humby T, Inglis W, Kendall DA, Marsden CA, Robbins TW. Isolation rearing in rats: Pre-and postsynaptic changes in striatal dopaminergic systems. Pharmacol. Biochem. Behav. 1998;59:859–872. doi: 10.1016/s0091-3057(97)00510-8. [DOI] [PubMed] [Google Scholar]
- Hane AA, Fox NA. Natural variations in maternal caregiving of human infants influence stress reactivity. Psychol. Sci. 2006;17:550–556. doi: 10.1111/j.1467-9280.2006.01742.x. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol. Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Heim C, Plotsky P, Nemeroff CB. The importance of studying the contributions of early adverse experiences to the neurobiological findings in depression. Neuropsychopharmacology. 2004;29:641–648. doi: 10.1038/sj.npp.1300397. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: Central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis Prog. Neuropsychopharmacol. Biol. Psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Herman JP, Tasker JG, Ziegler DR, Cullinan WE. Local circuit regulation of paraventricular nucleus stress integration glutamate-GABA connections. Pharmacol. Biochem. Behav. 2002;71:457–468. doi: 10.1016/s0091-3057(01)00681-5. [DOI] [PubMed] [Google Scholar]
- Hertsgaard L, Gunnar MR, Erickson M, Nachmias M. Adrenocortical responses to the strange situation in infants with disorganized/disoriented attachment relationships. Child Dev. 1995;66:1100–1106. [PubMed] [Google Scholar]
- Hofer MA. Hidden regulators mediating attachment, separation and loss. Monogr Soc Res. Child Dev. 1994;59:192–207. [PubMed] [Google Scholar]
- Jorm AF. Sex differences in neuroticism: a quantitative synthesis of published research. Aust. N. Z. J. Psychiatry. 1987;21:501–506. doi: 10.3109/00048678709158917. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Perel J, Dahl RE, Moreci P, Nelson B, Wells W, Ryan ND. The corticotropin-releasing hormone challenge in depressed abused, depressed nonabused, and normal control children. Biol. Psychiatry. 1997;42:669–679. doi: 10.1016/s0006-3223(96)00470-2. [DOI] [PubMed] [Google Scholar]
- Kertes DA, Gunnar MR, Madsen NJ, Long J. Early deprivation and home basal cortisol levels: A study of internationally-adopted children. Dev. Psychopathol. 2008;20:473–491. doi: 10.1017/S0954579408000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom. Med. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kreppner JA, O'Connor TG, Rutter M. Can inattention/overactivity be an institutional deprivation syndrome? J. Abnorm. Child Psychol. 2001;29:513–528. doi: 10.1023/a:1012229209190. [DOI] [PubMed] [Google Scholar]
- Lakatos K, Nemoda Z, Toth I, Ronai Z, Ney K, Sasvari-Szekely M, Gervai J. Further evidence for the role of the dopamine D4 receptor (DRD4) gene in attachment disorganization: Interaction of the exon III 48-bp repeat and the −521 C/T promoter polymorphisms. Mol. Psychiatry. 2002;7 doi: 10.1038/sj.mp.4000986. [DOI] [PubMed] [Google Scholar]
- Lakatos K, Toth I, Nemoda Z, Sasvari-Szekely M, Gervai J. Dopamine D4 receptor (DRD4) gene polymorphism is associated with attachment disorganization in infants. Mol. Psychiatry. 2000;5 doi: 10.1038/sj.mp.4000773. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Phelps EA. Emotional networks in the brain. In: Lewis M, Haviland-Jones JM, editors. Handbook of Emotions. New York: Guilford; 2000. pp. 157–172. [Google Scholar]
- Levine S. Infantile experience and resistance to physiological stress. Science. 1957;126:405–406. doi: 10.1126/science.126.3270.405. [DOI] [PubMed] [Google Scholar]
- Levine S. The ontogeny of the hypothalamic-pituitary-adrenal axis: The influence of maternal factors. Ann. N. Y. Acad. Sci. 1994;746:275–288. doi: 10.1111/j.1749-6632.1994.tb39245.x. [DOI] [PubMed] [Google Scholar]
- Levine S. Developmental determinants of sensitivity and resistance to stress. Psychoneuoendocrinology. 2005;30:939–946. doi: 10.1016/j.psyneuen.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Levine S, Ursin HT. What is stress? In: Broan MR, editor. Stress, Neurobiology and Neuroendocrinology. New York: Marcell Dekker Inc; 1991. pp. 3–21. [Google Scholar]
- Lopez JF, Chalmers DT, Little KY, Watson SJ. Regulation of seratonin 1A, glucocorticoid, and mineralocorticoid receptor in rat and human hippocampus: implications for the neurobiology of depression. Biol. Psychiatry. 1998;43:547–573. doi: 10.1016/s0006-3223(97)00484-8. [DOI] [PubMed] [Google Scholar]
- Lyons-Ruth K, Connell DB, Zoll D, Stahl J. Infants at social risk: Relations among infant maltreatment, maternal behavior, and infant attachment behavior. Dev. Psychol. 1987;23:223–232. [Google Scholar]
- Machado CJ, Bachevalier J. The impact of selective amygdala, orbital frontal cortex, or hippocampal formation lesions on established social relationships in rhesus monkeys (Macaca mulatta) Behav. Neurosci. 2006;120:761–786. doi: 10.1037/0735-7044.120.4.761. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. Behavioral and hormonal reactivity to threat: Effects of selective amygdala, hippocampal or orbital frontal lesions in monkeys. Psychoneuroendocrinology. 2008;33:926–941. doi: 10.1016/j.psyneuen.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestripieri D, Higley JD, Lindell SG, Newman TK, McCormack KM, Sanchez MM. Early maternal rejection affects the development of monoaminergic systems and adult abusive parenting in rhesus macaques (Macaca mulatta) Behav. Neurosci. 2006a;120:1017–1024. doi: 10.1037/0735-7044.120.5.1017. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, McCormack K, Lindell SG, Higley JD, Sanchez MM. Influence of parenting style on the offspring's behaviour and CSF monoamine metabolite levels in crossfostered and noncrossfostered female rhesus macaques. Behav. Brain Res. 2006b;175:90–95. doi: 10.1016/j.bbr.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Malkova L, Mishkin M, Suomi S, Bachevalier J. Socioemotional behavior in adult Rhesus monkeys after early versus late lesions of the medial temporal lobe. Ann. N. Y. Acad. Sci. 1997;807:538–540. doi: 10.1111/j.1749-6632.1997.tb51961.x. [DOI] [PubMed] [Google Scholar]
- Makino S, Gold PW, Schulkin J. Effects of corticosterone on CRH mRNA and content in the bed nucleus of the stria terminals; comparison with the effects in the central nucleus of the amygdala and the paraventricualar nucleus of the hypothalmus. Brain Res. 1994;657:141–149. doi: 10.1016/0006-8993(94)90961-x. [DOI] [PubMed] [Google Scholar]
- McCormack K, Sanchez MM, Bardi M, Maestripieri D. Maternal care patterns and behavioral development of rhesus macaque abused infants in the first 6 months of life. Developmental Psychobiology. 2006;48:537–550. doi: 10.1002/dev.20157. [DOI] [PubMed] [Google Scholar]
- McCormack KM, Warfield J, Dozier M, Gunnar M, Maestripieri D, Waters E, Sanchez MM. Maternal maltreatment is associated with lower attachment security in infant Rhesus monkeys”. Winston-Salem, NC. Proceedings of the 30th Annual Meeting of the Aerican Society of Primatologists.2007. [Google Scholar]
- McEwen B. Stress, adaptation, and disease: Allostasis and allostatic load. Ann. N. Y. Acad. Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McEwen B. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. 2000;886:171–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- McEwen B. Mood disorders and allostatic load. Biol. Psychiatry. 2003;54:200–207. doi: 10.1016/s0006-3223(03)00177-x. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Diorio J, Francis D, Widdowson J, La Plante P, Caldui C, Sharma S, Seckl J, Plotsky P. Early environmental regulation of forebrain glucocorticoid receptor gene expression: Implications for adrenocortical responses to stress. Dev. Neurosci. 1996;18 doi: 10.1159/000111395. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues Clin. Neurosci. 2005;7:103–123. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer OC. Understanding stress through the genome. Stress. 2006;9:61–67. doi: 10.1080/10253890600799669. [DOI] [PubMed] [Google Scholar]
- Mitchell JB, Betito K, Rowe W, Boksa P, Meaney MJ. Serotonergic regulation of Type II corticosteroid receptor binding in hippocampal cell cultures: Evidence for the importance of serotonin induced changes in cAMP levels. Neuroscience. 1992;48:631–639. doi: 10.1016/0306-4522(92)90407-s. [DOI] [PubMed] [Google Scholar]
- Nachmias M, Gunnar MR, Mangelsdorf S, Parritz R, Buss KA. Behavioral inhibition and stress reactivity: Moderating role of attachment security. Child Dev. 1996;67:508–522. [PubMed] [Google Scholar]
- Nemeroff CB. The corticotropin-releasing factor (CRF) hypothesis of depression: New findings and new directions. Mol. Psychiatry. 1996;1:336–342. [PubMed] [Google Scholar]
- Nemeroff CB. Neurobiological consequences of childhood trauma. J. Clin. Psychiatry. 2004;65:18–28. [PubMed] [Google Scholar]
- O'Connor TG, Bredenkamp D, Rutter M. Attachment disturbances and disorders in children exposed to early severe deprivation. Infant Ment. Health J. 1999;20:10–29. [Google Scholar]
- O'Connor TG, Deater-Deckard K, Fulker D, Rutter M, Plomin R. Genotype-environment correlations in late childhood and early adolescence: Antisocial behavioral problems and coercive parenting. Dev. Psychol. 1998;34:970–981. doi: 10.1037//0012-1649.34.5.970. [DOI] [PubMed] [Google Scholar]
- O'Connor TG, Rutter M. Attachment disorder behavior following early severe deprivation: Extension and longitudinal follow-up. J. Am. Acad. Child Adolesc. Psychiatry. 2000;39:703–712. doi: 10.1097/00004583-200006000-00008. [DOI] [PubMed] [Google Scholar]
- Ostrander MM, Ulrich-Lai YM, Choi DC, Richtand NM, Herman JP. Hypoactivity of the hypothalamo-pituitary-adrenocortical axis during recovery from chronic variable stress. Endocrinology. 2006;147:2008–2017. doi: 10.1210/en.2005-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pears K, Fisher PA. Developmental, cognitive, and neuropsychological functioning in preschool-aged foster children: associations with prior maltreatment and placement history. J. Dev. Behav. Pediatr. 2005;26:112–122. doi: 10.1097/00004703-200504000-00006. [DOI] [PubMed] [Google Scholar]
- Piazza PV, LeMoal M. Pathophysiological basis of vulnerability to drug abuse: role of an interaction between stress, glucocorticoids, and dopaminergic neurons. Annu. Rev. Pharmacol. Toxicol. 1996;36:359–378. doi: 10.1146/annurev.pa.36.040196.002043. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Rev. 1997;25(2):192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: An emerging framework for understanding function of the amygdala. In: Smith J, Schoenwolf G, editors. Neurulation. Elsevier Science Ltd; 1997. pp. 517–523. [DOI] [PubMed] [Google Scholar]
- Pollak SD, Nelson CA, Schlaak M, Roeber B, Wewerka S, Wiik KL, Frenn K, Loman MM, Gunnar MR. Neurodevelopmental effects of early deprivation in post-institutionalized children. Child Dev. doi: 10.1111/j.1467-8624.2009.01391.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner M, Rothbart MK, Geraldi-Caulton G. Exploring the biology of socialization. Ann. N. Y. Acad. Sci. 2001;935 doi: 10.1111/j.1749-6632.2001.tb03482.x. [DOI] [PubMed] [Google Scholar]
- Provence S, Lipton RC. Infants in institutions: A comparison of their development with family reared infants during the first year of life. International. New York: Universities Press, Inc; [Google Scholar]
- Renard GM, Suarez MM, Levin GM, Rivarola MA. Sex differences in rats: effects of chronic stress on sympathetic system and anxiety. Physiol. Behav. 2005;85:363–369. doi: 10.1016/j.physbeh.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Rogosch F, Cicchetti D. Child maltreatment, attention networks, and potential precursors to borderline personality disorder. Dev. Psychopathol. 2005;17:1071–1089. doi: 10.1017/s0954579405050509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen JB, Schulkin J. From normal fear to pathological anxiety. Psychol. Rev. 1998;105:325–350. doi: 10.1037/0033-295x.105.2.325. [DOI] [PubMed] [Google Scholar]
- Rosenfeld P, Suchecki D, Levine S. Multifactorial regulation of the hypothalamic-pituitary-adrenal axis during development. Neurosci. Biobehav. Rev. 1992;16:553–568. doi: 10.1016/s0149-7634(05)80196-4. [DOI] [PubMed] [Google Scholar]
- Roy P, Rutter M, Pickles A. Institutional care: associations between overactivity and lack of selectivity in social relationships. J. Child Psychol. Psychiatry. 2004;45:866–873. doi: 10.1111/j.1469-7610.2004.00278.x. [DOI] [PubMed] [Google Scholar]
- Rueda MR, Fan J, McCandliss BD, Halparin JD, Gruber DB, Lercari LP, Posner M. Developmental of Attentional Networks in Childhood. Neuropsychologia. 2004;42:1029–1040. doi: 10.1016/j.neuropsychologia.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Hearn EF, Do D, Rilling JK, Herndon JG. Differential rearing affects corpus callosum size and cognitive function of rhesus monkeys. Brain Res. 1998;812:38–49. doi: 10.1016/s0006-8993(98)00857-9. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: Evidence from rodent and primate models. Dev. Psychopathol. 2001;13:419–450. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Noble PM, Lyon CK, Plotsky PM, Davis M, Nemeroff CB, Winslow JT. Alterations in diurnal cortisol rhythm and acoustic startle response in nonhuman primates with adverse rearing. Biol. Psychiatry. 2005;57:373–381. doi: 10.1016/j.biopsych.2004.11.032. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schmidt LA, Fox NA, Perez-Edgar K, Hu S, Hamer DH. Association of DRD4 with attention problems in normal childhood development. Psychiatr. Genet. 2001;11:25–29. doi: 10.1097/00041444-200103000-00005. [DOI] [PubMed] [Google Scholar]
- Shields AM, Cicchetti D, Ryan RM. The development of emotional and behavioral self regulation and social competence among maltreated school-aged children. Dev. Psychopathol. 1994;6:57–75. doi: 10.1017/S0954579400005885. [DOI] [PubMed] [Google Scholar]
- Sorg BA, Kalivas PW. Effects of cocaine and footshock stress on extracellular dopamine levels in the ventral striatum. Brain Res. 1991;559(1):29–36. doi: 10.1016/0006-8993(91)90283-2. [DOI] [PubMed] [Google Scholar]
- Sorg BA, Kalivas PW. Effects of cocaine and footshock stress on extracellular dopamine levels in the medial prefrontal cortex. Neuroscience. 1993;53(3):695–703. doi: 10.1016/0306-4522(93)90617-o. [DOI] [PubMed] [Google Scholar]
- Spangler G. Psychological and physiological responses during an exam and their relation to personality characteristics. Psychoneuroendocrinology. 1997;22:423–441. doi: 10.1016/s0306-4530(97)00040-1. [DOI] [PubMed] [Google Scholar]
- Spangler G, Grossmann K. Individual and physiological correlates of attachment disorganization in infancy. In: Salomon J, George C, editors. Attachment disorganization. New York, NY: Guilford; 1999. pp. 95–124. [Google Scholar]
- Spangler G, Schieche M. Emotional and adrenocortical responses of infants to the strange situation: The differential function of emotional expression. Int. J. Behav. Dev. 1998;22:681–706. [Google Scholar]
- Stevens S, Sonuga-Barke E, Kreppner J, Beckett C, Castle J, Colvert E, Groothues C, Hawkins A, Rutter M. Inattention/Overactivity Following Early Severe Institutional Deprivation: Presentation and Associations in Early Adolescence. J. Abnorm. Child Psychol. 2008;36:385–398. doi: 10.1007/s10802-007-9185-5. [DOI] [PubMed] [Google Scholar]
- Stovall KC, Dozier M. The development of attachment in new relationships: single subject analyses for 10 foster infants. Dev. Psychopathol. 2000;12:133–156. doi: 10.1017/s0954579400002029. [DOI] [PubMed] [Google Scholar]
- Stroud L, Salovey P, Epel ES. Sex differences in stress responses: social rejection versus achievement stress. Biol. Psychiatry. 2002;52:318–327. doi: 10.1016/s0006-3223(02)01333-1. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Brake WG. What the rodent prefrontal cortex can teach us about attention-deficit/hyperactivity disorder: the critical role of early developmental events on prefrontal function. Behav. Brain Res. 2003;146:43–55. doi: 10.1016/j.bbr.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Gratton A. Prefrontal cortical regulation of hypothalamic-pituitary-adrenal function in the rat and implications for psychopathology: Side matters. Psychoneuroendocrinology. 2002;27:99–114. doi: 10.1016/s0306-4530(01)00038-5. [DOI] [PubMed] [Google Scholar]
- Suomi SJ. Early determinants of behaviour: Evidence from primate studies. Br. Med. Bull. 1997;53:170–184. doi: 10.1093/oxfordjournals.bmb.a011598. [DOI] [PubMed] [Google Scholar]
- Susman-Stillman A, Kallkoske M, Egeland B, Waldman E. Infant temperament and maternal sensitivity as predictors of attachment security. Infant Behav. Dev. 1996;19:33–47. [Google Scholar]
- Sutton SK, Davidson RJ. Prefrontal brain electrical asymmetry predicts the evaluation of affective stimuli. Neuropsychologia. 2000;38:1723–1733. doi: 10.1016/s0028-3932(00)00076-2. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain Res. 2000;886(1–2):113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- Van Ijzendoorn HW, Schuengel C, Bakersmans-Kranenburg MJ. Disorganized attachment in early childhood: Meta-analysis of precursors, concommitants, and sequelae. Dev. Psychopathol. 1999;11:225–249. doi: 10.1017/s0954579499002035. [DOI] [PubMed] [Google Scholar]
- Van Ijzendoorn MH, Bakermans-Kranenburg MJ. DRD4 7-repeat polymorphism moderates the association between maternal unresolved loss or trauma and infant disorganization. Hum. Dev. 2006;8:291–307. doi: 10.1080/14616730601048159. [DOI] [PubMed] [Google Scholar]
- Vazquez DM. Stress and the developing limbic-hypothalamic-pituitary-adrenal axis. Psychoneuroendocrinology. 1998;23:663–700. doi: 10.1016/s0306-4530(98)00029-8. [DOI] [PubMed] [Google Scholar]
- Vazquez DM, Eskandari R, Zimmer CA, Levine S, Lopez JF. Brain 5-HT receptor system in the stressed infant rat: implications for vulnerability to substance abuse. Psychoneuoendocrinology. 2002;27:245–272. doi: 10.1016/s0306-4530(01)00048-8. [DOI] [PubMed] [Google Scholar]
- Vedhara K, Hyde J, Gilchrist ID, Tytherleigh M, Plummer S. Acute stress, memory, attentional and cortisol. Psychoneuroendocrinology. 2000;25 doi: 10.1016/s0306-4530(00)00008-1. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Rao BSS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J. Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock M. The potential influence of maternal stress hormones on development and mental health of the offspring. Brain Behav. Immun. 2005;19:296–308. doi: 10.1016/j.bbi.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Wiener SG, Bayart F, Faull KF, Levine S. Behavioral and physiological responses to maternal separation in squirrel monkeys (Saimiri sciureus) Behav. Neurosci. 1990;104:108–115. doi: 10.1037//0735-7044.104.1.108. [DOI] [PubMed] [Google Scholar]
- Wismer Fries AB, Ziegler TE, Kurian JR, Jacoris S, Pollak SD. Early experience in humans is associated with changes in neuropeptides critical for regulating social behavior. Proc. Natl. Acad. Sci. U.S.A. 2005;102:17237–17240. doi: 10.1073/pnas.0504767102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R. Biology of Posttraumatic Stress Disorder. J. Clin. Psychiatry. 2000;61(Suppl7):15–21. [PubMed] [Google Scholar]
- Yehuda R, LeDoux J. Response Variation following Trauma: A Translational Neuroscience Approach to Understanding PTSD. Neuron. 2007;56:19–32. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]


