Abstract
Purpose
Activation of Toll-like Receptors (TLR) 7 and 8 by engineered agonists has been shown to aid in combating viruses and tumors. Here, we wished to test the effect of TLR7/8 activation on monocyte Fcγ receptor (FcγR) function, as they are critical mediators of antibody therapy.
Experimental Design
The effect of the TLR7/8 agonist R-848 on cytokine production and antibody-dependent cellular cytotoxicity (ADCC) by human peripheral blood monocytes (PBM) was tested. Affymetrix microarrays were done to examine genomewide transcriptional responses of monocytes to R-848, and Western blots were done to measure protein levels of FcγR. Murine bone marrow-derived macrophages (BMM) from wild-type and knockout mice were examined to determine the downstream pathway involved with regulating FcγR expression. The efficacy of R-848 as an adjuvant for antibody therapy was tested using a CT26-HER2/neu solid tumor model.
Results
Overnight incubation with R-848 increased FcγR-mediated cytokine production and ADCC in human PBM. Expression of FcγRI, FcγRIIa and the common γ-subunit was increased. Surprisingly, expression of the inhibitory FcγRIIb was almost completely abolished. In BMM, this required TLR7 and MyD88, as R-848 did not increase expression of the γ-subunit in TLR7−/− nor MyD88−/− cells. In a mouse solid tumor model, R-848 treatment superadditively enhanced the effects of antitumor antibody.
Conclusions
These results demonstrate an as-yet undiscovered regulatory and functional link between the TLR7/8 and FcγR pathways. This suggests that TLR7/8 agonists may be especially beneficial during antibody therapy.
Keywords: Toll-like receptor, Fc-gamma receptor, immunotherapy, antibody, tumor
Introduction
Monocyte Fcγ receptors (FcγR) mediate clearance of IgG-immune complexes and IgG-coated tumor targets. Binding of IgG complexes to FcγR results in receptor clustering, which activates downstream events such as phagocytosis (1), release of reactive oxygen species (2) and cytokine production (3).
The strength of FcγR response is largely determined by the ratio of activating (FcγRI, FcγRIIa, FcγRIII and the γ-subunit) to inhibitory (FcγRIIb) receptors, as mice genetically deleted for FcγRIIb show markedly enhanced antibody-mediated tumor clearance in vivo (4). Conversely, mice lacking the common γ-subunit show very poor antibody-dependent cytotoxicity as mice do not express the γ-subunit-independent FcγRIIa (5). It has also been shown that Toll-like receptor (TLR) activation can enhance FcγR expression and function. For example, the TLR4 ligand lipopolysaccharide (LPS) has been shown to increase FcγR-mediated phagocytosis (6) and tumor cell lysis (7). Unmethylated DNA (CpG oligonucleotides), which activates TLR9, has also proven effective, enhancing antibody-dependent cellular cytotoxicity against tumors (8).
Agonists of TLR7 and TLR8 have come to light as an effective means of enhancing immune responses. The TLR7 agonist imiquimod has been shown in vivo to reduce the growth of MC-26 tumor cells (9), an effect abolished by blocking Interferon-α. Both TLR7 and TLR7/8 agonists show antitumor (10) and antiviral (11) activities. Their major mode of action seems to be induction of cytokine production, leading to stronger proinflammatory responses (12).
Here, we have studied the effects of the TLR7/8 agonist R-848 on human monocytes within the context of FcγR expression and function. Results show that R-848 regulates FcγR transcript and protein, upregulating the activating FcγR and downregulating the inhibitory FcγRIIb. Studies using BMM from wild-type and knockout mice showed that TLR7 and MyD88 are required for the changes in FcγR. Functional assays showed that R-848 treatment synergizes with FcγR function both in vitro and in a murine solid tumor model. Hence, TLR7/8 is a novel regulator of FcγR expression and function, suggesting that TLR7/8 agonists may be especially effective as adjuvants for antibody therapy.
Materials and Methods
Antibodies and Reagents
R-848 (Resiquimod) was purchased from Alexis Biochemicals and dissolved to 10 mM in DMSO, then to 1 mM in RPMI-1640 for working stock. Brefeldin A was purchased from BioLegend (San Diego, CA) and used according to manufacturer instructions. PCR primer sets (FcγRIa, QT00013475; FcγRIIa, QT01667099; FcγRIIb, QT00086842; γ-subunit, QT00055853; TRAF3, QT00080990; GAPDH, QT01192646) were from Qiagen (Valencia, CA). Trizol was purchased from Invitrogen (Carlsbad, CA). Reverse transcriptase, random hexamers and SYBR Green PCR mix were purchased from Applied Biosystems (Foster City, CA). F(ab’)2 of anti-FcγRI (32.2) and anti-FcγRIIa (IV.3) were obtained from Medarex (Annandale, NJ). The anti-FcR-γ-subunit was from Upstate Cell Signaling (Lake Placid, NY). Rabbit polyclonal antibodies specific to hFcγRIIa and hFcγRIIb were generated as previously described (13). Actin, GAPDH and HRP-conjugated antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA).
Western blotting and ELISAs
Cells were lysed in TN1 buffer (50 mM Tris (pH 8.0), 10 mM EDTA, 10 mM Na4P2O7, 10 mM NaF, 1% Triton X-100, 125 mM NaCl, 10 mM Na3VO4, 10 µg/ml each aprotinin and leupeptin). Postnuclear protein-matched lysates were boiled in Laemmli sample buffer and separated by SDS-PAGE, transferred to nitrocellulose membranes, probed with the antibody of interest, then developed by ECL (GE Healthcare, Buckinghamshire, UK). Cell supernatants were collected, centrifuged at full speed to clear cellular debris, then assayed for cytokine via sandwich ELISA (R & D Systems, Minneapolis, MN) according to manufacturer protocol.
Real-time RT-PCR
RNA was extracted from PBM using Trizol, reverse transcribed to cDNA, then run in triplicate for each donor on an Applied Biosystems Step One Plus system, with automatically-calculated thresholds. Relative expression was calculated as 2^−ΔCt, with ΔCt calculated by subtracting the average Ct of 2 housekeeping controls (TRAF3 and GAPDH) from the experimental sample Ct (14).
BMM isolation and culture
L929 cells, generously provided by Dr. Stéphanie Seveau (The Ohio State University), were used to generate conditioned media for culturing of murine bone marrow-derived macrophages (BMM) (15). L929 were incubated in minimum essential media (Invitrogen) containing 10% heat-inactivated FBS (Hyclone, Logan, UT), nonessential amino acids, sodium pyruvate and penicillin / streptomycin (Invitrogen). Conditioned media from the L929 cells was collected after 7 days, passed through a 0.22 µm filter and added to the BMM media. BMM were cultured from femurs of wild-type, TLR7−/−, MyD88−/−, TRIF−/− and Cryopyrin−/− C57/Bl6 mice (16) by flushing the marrow from femurs and plating cells on plastic dishes in D-MEM (Invitrogen, Carlsbad, CA) containing 10% FBS, 0.1% β-mercaptoethanol (BioRad, Hercules, CA) and 30% conditioned media from L929 cells. After 6–7 days, nonadherent cells were washed away using PBS, then the remaining BMM used for experiments.
PBM isolation
Peripheral blood monocytes (PBM) were isolated from Red Cross leukopaks via Ficoll centrifugation (Mediatech, Manassas, VA) followed by CD14-positive selection using MACS (Miltenyi Biotec, Inc.) as previously described (14). PBM were resuspended in RPMI-1640 containing 10% heat-inactivated FBS (Hyclone), penicillin / streptomycin and L-glutamate (Invitrogen). The purity of monocytes obtained was >97%, as determined by flow cytometry with CD14 antibody.
Microarray analysis
RNA was extracted from PBM using Trizol (Invitrogen), then labeled and hybridized to Affymetrix (Santa Clara, CA) hgu133plus2 chips at The Ohio State University Medical Center Microarray-Genetics core facility. Resulting data files were preprocessed and analyzed using R (17) and BioConductor (18), testing for differentially expressed genes using the “limma” package (19).
Antibody-dependent cellular cytotoxicity (ADCC) assay
ADCC assays were performed as described previously (20;21). Briefly, PBM were incubated overnight with or without 1 µM R-848 or 10 ng/ml IFNγ and were used as the effector cells. MDA-MB-468 breast cancer cells were used as the targets. These cells were incubated at 37°C with 0.3 mCi 51Cr for 30–60 minutes, then incubated with no antibody, 10 µg/ml of Rituximab (negative-control antibody), or with cetuximab. Monocytes and MDA cells were then coincubated at a 50:1 ratio in v-bottom 96-well plates for 18 hours. Supernatants were harvested and counted for radiolabeled Chromium using a gamma counter. Percent cytotoxicity was calculated as [(sample − minimum control) / (maximum control − minimum control) * 100], where minimum controls were target cells incubated alone and maximum controls were target cells incubated alone and lysed using 10% SDS. To derive values for antibody-dependent cytotoxicity, values from no-antibody controls were subtracted from values from cetuximab-treated targets.
Murine solid tumor model
CT26-HER2/neu colon carcinoma cells (22) were grown in RPMI-1640 media supplemented with 10% FBS, penicillin / streptomycin and L-glutamate, washed to remove non-adherent cells, then resuspended using enzyme-free cell dissociation buffer (Invitrogen). Cells were centrifuged and resuspended at 10×106 per ml in RPMI-1640. The murine tumor model was performed in accordance with Penichet et al., 1999 (22) and Roda et al., 2006 (21). Briefly, 5-week-old female Balb/cJ mice (Jackson Laboratories, Bar Harbor, ME) were injected subcutaneously with 1×106 of syngeneic CT26-HER2/neu cells. Mice were left for 7 days to allow tumors to develop. Intraperitoneal injections with treatments were then performed 3 times per week, and tumor measurements done on each treatment day. Tumor volumes were calculated as [0.5 × (length measurement) * (height measurement)2], where length was the longest diameter of the tumor. Treatments consisted of 4D5 anti-HER2 antibody at 20 mg/kg, R-848 at 2 mg/kg, 4D5 plus R-848, or DMSO vehicle control. All in vivo experiments were performed in strict accordance to guidelines set by the Institutional Animal Care and Use Committee.
Statistical analyses
For all experiments performed in vitro, Student’s t-tests were used to test for statistically significant differences. Statistics for the murine solid tumor model experiment were done by the Center for Biostatistics at The Ohio State University. Briefly, data were transformed by cube root, then a linear mixed model was applied, followed by an interaction contrast to test for synergy. SAS (SAS Institute, Inc., Cary, NC) software was used to analyze the in vivo experiment.
Results
R-848 enhances FcγR function
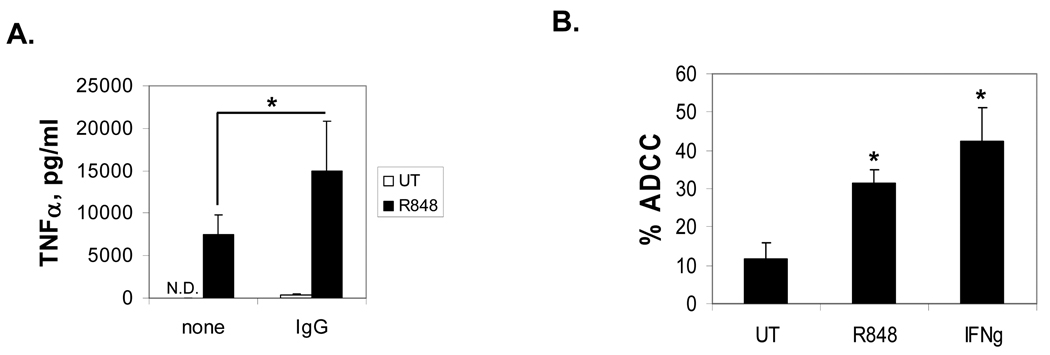
The TLR7/8 agonist R-848 has been shown to increase cytokine production (23), so we asked whether it would lead to an additive or synergistic increase in FcγR-mediated cytokine production. To test this, we incubated PBM overnight with 1 µM R-848, then plated them on immobilized IgG to cluster the FcγR. As shown in Figure 1A, R-848 treatment alone caused PBM to secrete TNFα. However, when R-848 treatment and FcγR activation were combined, there was a superadditive level of TNFα secretion. This suggests that the TLR7/8 pathways functionally synergize with FcγR.
Fig. 1.
R-848 enhances FcγR function. (A) PBM were treated overnight with 1 µM R-848 (R848) or were left untreated (UT). Following this, they were incubated in 96-well plates with (IgG) or without (none) immobilized IgG for 24 hours. Supernatants were collected and analyzed for TNFα by sandwich ELISA. Error bars represent standard deviation of 3 separate donors. Asterisks denote statistical significance at p≤0.05. N.D. is Not Detected. (B) R-848 enhances monocyte ADCC. Human PBM from 4 donors were incubated overnight with 1 µM R-848 or 10 ng/ml IFNγ, then tested in an ADCC assay (described in Methods) with cetuximab-coated MDA-MB-468 cells. The percent cytotoxicity after subtraction of no-antibody controls is plotted. Error bars represent standard deviation. Asterisks denote statistical significance versus UT (p<0.01).
Next, we examined the ability of R-848 to enhance destruction of antibody-coated tumor cells in vitro. We treated human PBM overnight with or without 1 µM R-848, then tested them in an ADCC assay (24;25) using cetuximab-coated MDA-MB-468 cells. Because it has been shown that IFNγ can enhance ADCC in human monocytes (26–28), we also used 10 ng/ml IFNγ as a positive control. Results showed that R-848 treatment significantly increased antibody-dependent cytotoxicity, even to levels approaching those seen after IFNγ treatment (Figure 1B). Hence, R-848 promotes destruction of antibody-coated tumor target cells in vitro.
Microarray analysis of R-848
We next examined the genomewide transcriptional responses of monocytes to R-848 in an effort to gain insights on how it enhanced monocyte function. To do this, we incubated PBM with 1 µM R-848 for 18 hours, extracted RNA and performed Affymetrix microarray analysis. We then searched for significantly different transcripts within the “immune response” or “inflammatory” ontologies that were upregulated 2-fold or more and with an average log2 expression of 3 or higher. There were a total of 119 unique transcripts, shown in Table 1. As expected based on previous literature, we found upregulation of cytokines such as IL-6, IL-12 p40 and TNFα. Fcγ receptors are critical for antibody-mediated clearance of tumor cells, such as that seen in Figure 1B (29). It was possible that part of the R-848-mediated enhancement of this clearance was due to upregulation of these receptors, and this was found in the analysis results (Table 1, gray highlights). Unlike this upregulation of activating FcγR, the array data showed a 6-fold downregulation of the inhibitory FcγRIIb (data not shown). These results suggest that R-848 regulates FcγR at the transcriptional level and that this may largely account for the increased FcγR-mediated cytokine production and ADCC.
Table 1.
Microarray analysis of monocytes treated with R-848. PBM from 3 different donors were incubated overnight with or without 1 µM R-848 and then subjected to Affymetrix microarray analysis. Transcripts with fold-increases of 2 or greater, with average log2 expression of 3 or greater and with ontologies of “immune response” or “inflammatory” were selected. Highlighted in gray are the activating FcγR.
| Symbol | FC | p val | Description | Symbol | FC | p val | Description |
|---|---|---|---|---|---|---|---|
| ADA | 4.317346 | 1.4E-05 | adenosine deaminase | IL1RN | 25.6737 | 4.5E-07 | interleukin 1 receptor antagonist |
| ADORA2A | 40.55086 | 8.8E-12 | adenosine A2a receptor | IL2RA | 43.53276 | 1.6E-07 | interleukin 2 receptor, alpha |
| ADRB2 | 16.73249 | 8.7E-06 | adrenergic, beta-2-, receptor, surface | IL2RG | 2.428115 | 0.004 | interleukin 2 receptor, gamma |
| AGER | 3.71607 | 9.8E-05 | adv glycosylation end product-specific receptor | IL4R | 13.91337 | 1.3E-06 | Interleukin 4 receptor |
| APOL3 | 2.290186 | 0.045 | apolipoprotein L, 3 | IL6 | 489.0295 | 5.4E-09 | interleukin 6 (interferon, beta 2) |
| AQP9 | 5.560471 | 1.2E-08 | aquaporin 9 | IL6ST | 3.797162 | 0.001 | interleukin 6 signal transducer |
| B4GALT1 | 7.023606 | 1.5E-06 | beta 1,4- galactosyltransferase, polypeptide 1 | IL7R | 45.28874 | 1.8E-07 | interleukin 7 receptor |
| BCL3 | 2.973849 | 1.4E-06 | B-cell CLL/lymphoma 3 | IL8 | 3.7873 | 0.005 | interleukin 8 |
| BCL6 | 4.485563 | 2.8E-11 | B-cell CLL/lymphoma 6 | IRAK2 | 25.49053 | 3.2E-08 | interleukin-1 receptor-associated kinase 2 |
| CCL18 | 8.512269 | 4.8E-06 | chemokine (C-C motif) ligand 18 | ITCH | 6.845941 | 0.005 | itchy homolog E3 ubiquitin protein ligase |
| CCL19 | 190.8174 | 4.1E-09 | chemokine (C-C motif) ligand 19 | ITGAL | 3.649082 | 6.4E-05 | integrin, alpha L (antigen CD11A (p180) |
| CCL2 | 6.25498 | 0.024 | chemokine (C-C motif) ligand 2 | LAIR1 | 7.521857 | 2.5E-06 | leukocyte-associated Ig-like receptor 1 |
| CCL20 | 55.74429 | 3.0E-07 | chemokine (C-C motif) ligand 20 | LCP2 | 2.979506 | 3.8E-04 | lymphocyte cytosolic protein 2 |
| CCL3 | 20.34984 | 1.4E-06 | chemokine (C-C motif) ligand 3 | LILRA1 | 27.92517 | 3.2E-12 | leukocyte immunoglobulin-like receptor, A1 |
| CCL4 | 18.7361 | 1.6E-06 | chemokine (C-C motif) ligand 4 | LILRA2 | 2.971885 | 0.003 | leukocyte immunoglobulin-like receptor, A2 |
| CCL7 | 4.251229 | 0.040 | chemokine (C-C motif) ligand 7 | LILRA3 | 35.13229 | 2.9E-09 | leukocyte immunoglobulin-like receptor, A3 |
| CD163 | 28.96202 | 1.2E-06 | CD163 molecule | LILRB1 | 6.353475 | 7.9E-08 | Leukocyte immunoglobulin-like receptor, B1 |
| CD274 | 31.7408 | 3.6E-09 | CD274 molecule | LILRB2 | 7.090295 | 7.9E-08 | leukocyte immunoglobulin-like receptor, B2 |
| CD40 | 3.132347 | 0.028 | CD40 molecule | LILRB3 | 3.073823 | 1.5E-06 | leukocyte immunoglobulin-like receptor, B3 |
| CD55 | 4.733903 | 1.3E-06 | CD55 molecule | LILRB4 | 2.554202 | 0.016 | leukocyte immunoglobulin-like receptor, B4 |
| CD59 | 19.06185 | 2.9E-09 | CD59 molecule | LOC65387 | 3.415538 | 0.030 | similar to Complement C3 precursor |
| CD80 | 18.26656 | 1.9E-05 | CD80 molecule | MEFV | 14.03661 | 0.001 | Mediterranean fever |
| CD83 | 2.445838 | 0.003 | CD83 molecule | MGLL | 2.640648 | 0.023 | monoglyceride lipase |
| CFB | 59.44535 | 1.4E-08 | complement factor B | MS4A1 | 3.285111 | 0.006 | Membrane-spanning 4-domains, A1 |
| CHST2 | 4.048418 | 0.001 | carbohydrate sulfotransferase 2 | NFE2L1 | 3.47481 | 1.3E-04 | nuclear factor (erythroid-derived 2)-like 1 |
| CLEC4D | 5.729237 | 3.9E-05 | C-type lectin domain family 4, member D | NLRP3 | 3.529458 | 0.026 | NLR family, pyrin domain containing 3 |
| CLEC4E | 2.414824 | 0.020 | C-type lectin domain family 4, member E | NR3C1 | 4.879714 | 3.8E-08 | nuclear receptor subfamily 3, C1 |
| CLU | 2.289794 | 0.021 | clusterin | PAG1 | 13.00331 | 2.6E-08 | p-protein assoc w/ glycosphingolipid microdomains 1 |
| COL4A3BP | 2.603515 | 4.2E-04 | collagen, type IV, alpha 3 binding protein | PDCD1LG | 7.568735 | 7.5E-06 | programmed cell death 1 ligand 2 |
| CSF3 | 36.21415 | 2.0E-07 | colony stimulating factor 3 | POMP | 2.532659 | 3.7E-04 | proteasome maturation protein |
| CXCL1 | 97.88367 | 1.2E-12 | chemokine (C-X-C motif) ligand 1 | POU2F2 | 2.35123 | 4.9E-05 | POU class 2 homeobox 2 |
| CXCL13 | 116.9714 | 2.0E-09 | chemokine (C-X-C motif) ligand 13 | PRELID1 | 3.945781 | 1.3E-05 | PRELI domain containing 1 |
| CXCL2 | 8.593548 | 1.1E-05 | Chemokine (C-X-C motif) ligand 2 | PRG2 | 3.749564 | 4.8E-04 | proteoglycan 2, bone marrow |
| CXCL3 | 17.77199 | 7.6E-07 | chemokine (C-X-C motif) ligand 3 | PRKCA | 3.283269 | 0.010 | protein kinase C, alpha |
| CXCL5 | 15.40997 | 6.8E-05 | chemokine (C-X-C motif) ligand 5 | PTGS2 | 121.221 | 2.5E-10 | prostaglandin-endoperoxide synthase 2 |
| CYBB | 3.719852 | 0.002 | Cytochrome b-245, beta polypeptide | PTX3 | 34.45957 | 6.8E-14 | pentraxin-related, rapidly induced by IL-1 beta |
| DPP8 | 3.208713 | 0.021 | dipeptidyl-peptidase 8 | RAC1 | 3.784847 | 0.005 | Ras-related C3 botulinum toxin substrate 1 |
| EBI3 | 25.25581 | 9.2E-07 | Epstein-Barr virus induced gene 3 | RELA | 11.36527 | 4.4E-04 | V-rel homolog A, p65 |
| EREG | 4.980403 | 3.3E-04 | epiregulin | RIPK2 | 2.190812 | 0.034 | receptor-interacting serine-threonine kinase 2 |
| ETS1 | 13.56834 | 4.3E-04 | v-ets E26 homolog 1 | RNF19B | 10.31492 | 6.0E-05 | ring finger protein 19B |
| F3 | 18.19057 | 5.3E-06 | coagulation factor III | S100A12 | 3.133215 | 0.014 | S100 calcium binding protein A12 |
| FCER1G | 2.391636 | 0.001 | Fc fragment of IgE, gamma polypeptide | S100A8 | 15.18746 | 2.1E-04 | S100 calcium binding protein A8 |
| FCGR1A | 21.46627 | 1.8E-07 | Fc fragment of IgG, high affinity Ia, receptor (CD64) | S100A9 | 3.26703 | 0.006 | S100 calcium binding protein A9 |
| FCGR1B | 3.287157 | 0.042 | Fc fragment of IgG, high affinity Ib, receptor (CD64) | SEMA3C | 22.85418 | 1.0E-11 | sema, immunoglobulin, short basic, secreted, 3C |
| FCGR2A | 2.545392 | 0.001 | Fc fragment of IgG, low affinity IIa, receptor (CD32) | SLC11A1 | 10.12225 | 7.2E-08 | solute carrier family 11, member 1 |
| FOXP1 | 3.44095 | 3.0E-06 | forkhead box P1 | SNF1LK | 7.138636 | 0.001 | SNF1-like kinase |
| FPR1 | 3.575507 | 3.0E-04 | formyl peptide receptor 1 | SPN | 3.129464 | 0.027 | sialophorin (leukosialin, CD43) |
| FPRL1 | 34.54009 | 5.9E-09 | formyl peptide receptor-like 1 | TARP | 11.3274 | 0.011 | T cell receptor gamma constant 2 |
| FUS | 6.003565 | 0.016 | Fusion (in t(12;16) in malignant liposarcoma) | TBK1 | 2.765583 | 3.2E-05 | TANK-binding kinase 1 |
| HAMP | 59.32565 | 1.4E-12 | hepcidin antimicrobial peptide | TGM2 | 16.02947 | 4.5E-08 | Transglutaminase 2 |
| HDAC7A | 2.141959 | 0.045 | histone deacetylase 7A | TLR1 | 2.055846 | 0.005 | toll-like receptor 1 |
| HRH1 | 10.03894 | 7.2E-06 | histamine receptor H1 | TLR4 | 2.422299 | 0.033 | toll-like receptor 4 |
| HSPC111 | 6.28524 | 0.008 | hypothetical protein HSPC111 | TNF | 12.9597 | 3.4E-07 | tumor necrosis factor, member 2 |
| IFNG | 26.61823 | 1.4E-04 | interferon, gamma | TNFAIP1 | 2.2312 | 1.9E-04 | tumor necrosis factor, alpha-induced protein 1 |
| IL10 | 39.7384 | 3.3E-08 | interleukin 10 | TNFAIP6 | 14.66536 | 6.8E-07 | tumor necrosis factor, alpha-induced protein 6 |
| IL12B | 3.38804 | 2.6E-04 | interleukin 12B, p40 | TNFRSF9 | 2.658242 | 0.020 | tumor necrosis factor receptor, member 9 |
| IL1A | 61.94209 | 8.4E-08 | interleukin 1, alpha | TNFSF9 | 3.521049 | 8.9E-05 | tumor necrosis factor, member 9 |
| IL1B | 26.07393 | 7.7E-09 | interleukin 1, beta | TREM1 | 6.539373 | 6.1E-06 | triggering receptor expressed on myeloid cells 1 |
| IL1F9 | 18.53308 | 3.7E-05 | interleukin 1 family, member 9 | VAV1 | 7.233896 | 1.8E-07 | vav 1 guanine nucleotide exchange factor |
| IL1RAP | 3.382441 | 1.2E-04 | interleukin 1 receptor accessory protein |
R-848 alters FcγR protein expression
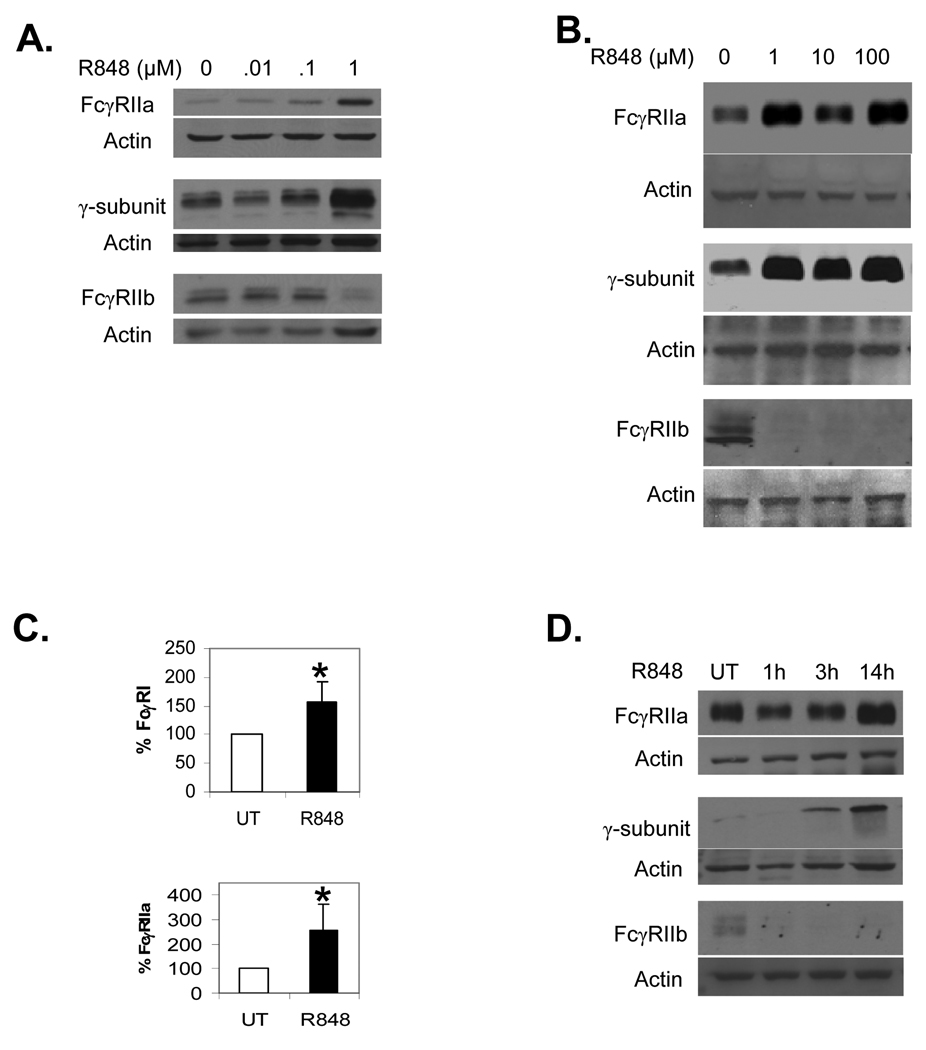
We next verified that the altered transcription of FcγR led to changes in protein expression and tested for the lowest required dosage. We treated PBM overnight with 0, 0.01, 0.1 or 1.0 µM R-848, or with 0, 1, 10 or 100 µM R-848. Western blots were done to measure expression of FcγRIIa, the common γ-subunit, and FcγRIIb. As shown in Figures 2A and 2B, a dosage of 1 µM was sufficient to alter FcγR expression and higher dosages did not lead to greater changes.
Fig. 2.
Dose response to R-848. (A–B) PBM were incubated with 0, 0.01, 0.1 or 1.0 µM (A) or 0, 1, 10 or 100 µM (B) R-848, with DMSO concentrations equalized across all treatments. Western blotting was done to measure FcγRIIa (top panels), the γ-subunit (middle panels) and FcγRIIb (bottom panels). Blots represent 3 independent experiments. (C) Flow cytometry was done to measure surface expression of FcγRI (top panel) and FcγRIIa (bottom panel) using F(ab’)2 fragments of 32.2 and IV.3 antibodies respectively, followed by F(ab’)2 goat anti-mouse FITC. The percent increases over untreated (UT) were plotted as bar graphs. Asterisks denote statistical significance at p≤0.05 and error bars represent standard deviation, n=3. (D) Time course of R-848 influence on FcγR expression. PBM were incubated for 0, 1, 3 or 14 hours with 1 µM R-848, then protein lysates extracted and analyzed by Western blotting for FcγRIIa (top panel), the γ-subunit (middle panel) or FcγRIIb (bottom panel). Blots represent 3 independent experiments.
To confirm that changes in FcγR also occurred on the cell surface, we treated PBM overnight with 1 µM R-848 and measured surface expression of FcγRIa and FcγRIIa using flow cytometry. Compared to untreated PBM, there were significant increases after R-848 treatment (Figure 2C). Overnight treatments (14–18 hours) with R-848 elicited changes in FcγR, so we next tested whether short (1–3 hours) treatment times would be sufficient. Hence, we treated PBM for 1, 3 or 14 hours, then measured FcγR by Western blotting. Results showed that increases in FcγRIIa occurred at the late stage (Figure 2D, top panel), while small increases in the γ-chain appeared at 3 hours but were higher at 14 hours (Figure 2D, middle panel). However, decreases in FcγRIIb protein were seen within one hour (Figure 2D, bottom panel), while the transcript for FcγRIIb remained to 4 hours (data not shown). This suggests that R-848 triggered an immediate degradation of the FcγRIIb protein, followed by a later reduction in FcγRIIb transcript.
Secreted factors mediate increases in activating FcγR
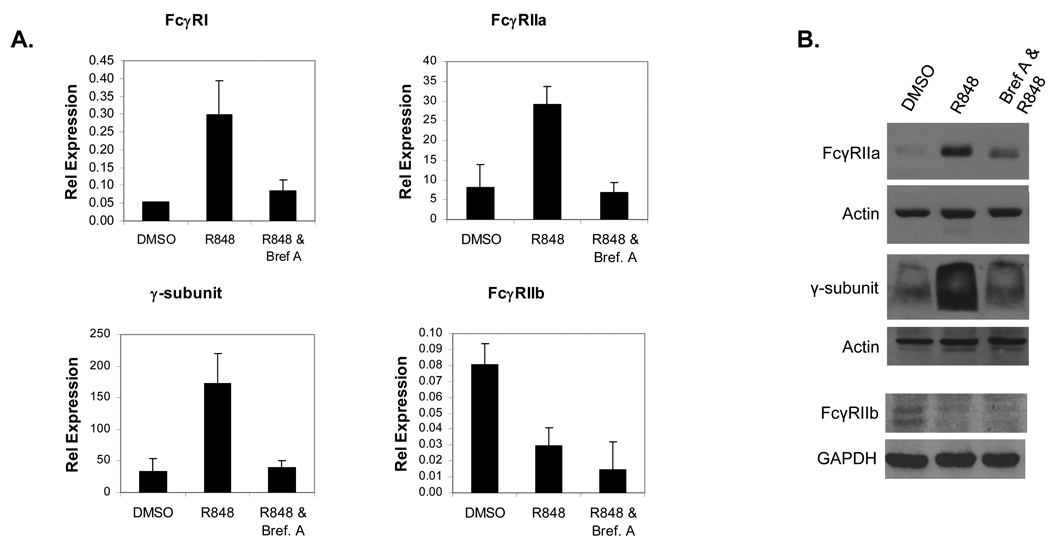
Results from the microarray analysis showed that numerous cytokines were upregulated, many of which were known to influence FcγR expression. To test whether secretion of these cytokines may have been responsible for the R-848-mediated changes in FcγR, we pretreated PBM for 30 minutes with Brefeldin A, an inhibitor of secretion. Following this, PBM were treated for 12 hours with R-848 and FcγR expression was measured by both real-time RT-PCR and Western blotting. Results showed that pretreatment with Brefeldin A prevented the R-848-mediated increases in FcγRI, FcγRIIa and γ-subunit transcripts, while the decrease in FcγRIIb was not affected (Figure 3A). Similarly, Western blotting showed that Brefeldin A pretreatment inhibited the R-848-mediated increases in FcγRIIa and the γ-subunit, but did not prevent the reduction in FcγRIIb (Figure 3B). These results suggest that R-848 drives production of secreted factors that act in an autocrine / paracrine fashion to increase expression of the activating FcγR, but that the effect of R-848 on FcγRIIb is mediated through a different mechanism.
Fig. 3.
Requirement of secreted factors for R-848-mediated changes in FcγR expression. (A) PBM were pretreated with or without Brefeldin A for 30 minutes and treated for 12 hours with or without 1 µM R848. RNA was extracted and FcγR expression measured by real-time RT-PCR. DMSO (vehicle control), R-848 alone and R-848 plus Brefeldin A pretreatment were compared for FcγRI (top left), FcγRIIa (top right), the γ-subunit (bottom left) and FcγRIIb (bottom right). Graphs are representative of 3 independent experiments. Error bars represent standard deviation. (B) PBM (n=3) were pretreated with or without Brefeldin A for 30 minutes, then treated for 12 hours with or without 1 µM R848. Protein lysates were collected and Western blots done to detect FcγRIIa (top), the γ-subunit (middle) and FcγRIIb (bottom). DMSO (vehicle control), R-848 alone and R-848 plus Brefeldin A pretreatment were compared. Actin or GAPDH reprobes were done for each blot to verify equivalent loading.
Mechanisms of R-848-mediated regulation of FcγR
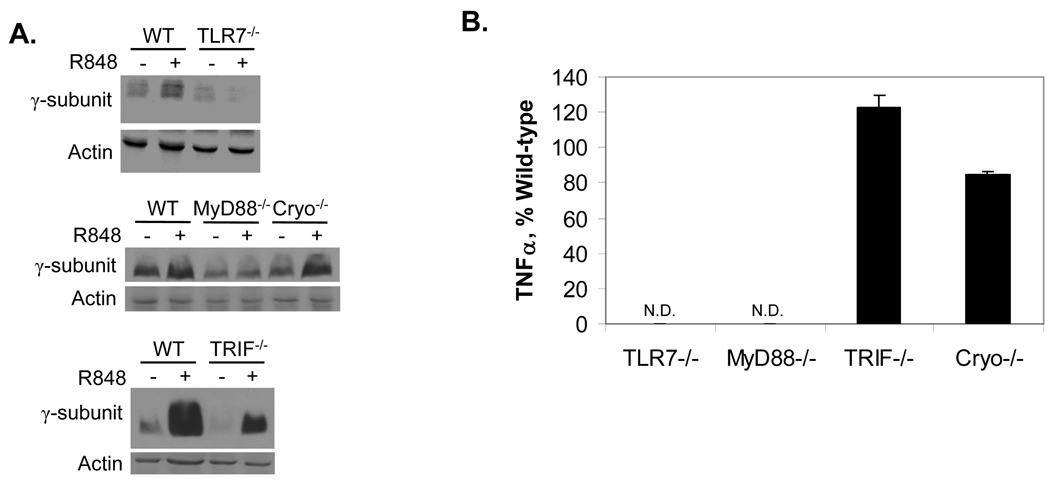
We next wished to test whether a similar effect of R-848 on FcγR expression would occur in mice, and determine the mechanism by which R-848 mediates its effects. R-848 signals through the MyD88 adapter protein (30) and the Nalp3/Cryopyrin cytosolic sensor (31). To determine which were required for regulation of FcγR and to rule out a role for the MyD88-independent TRIF, we isolated bone marrow macrophages (BMM) from wild-type, TLR7−/−, MyD88−/−, TRIF−/− and Cryopyrin−/− mice. BMM were treated overnight with or without R-848, then expression of the γ-subunit was measured by Western blotting. Of note, FcγRIIa is not expressed in mice (32). As shown in Figure 4A (top and middle panels, respectively), neither TLR7−/− nor MyD88−/− cells showed R-848-mediated increases in the γ-subunit. However, R-848 did increase γ-subunit expression in Cyropyrin- and TRIF- knockout BMM (Figure 4A, top and bottom panels, respectively). These results indicate that R-848-mediated effects on FcγR require TLR7 and MyD88. As a functional control, TNFα production was also examined in supernatants from BMM treated with or without R-848. Results were in accordance with those previously reported by Hemmi et al. (30), showing that TLR7−/− and MyD88−/− cells did not produce TNFα in response to R-848 (Figure 4B). Because R-848 does not appear to activate mouse TLR8 (33), all of its effect would be expected to require TLR7. In humans, however, R-848 can activate both TLR7 and TLR8 (33) and can signal through MyD88-independent pathways (31). Hence, further experiments will be required to rule out the involvement of a MyD88-independent pathway or a possible interaction between TLR7 and TLR8 activation in humans.
Fig. 4.
R-848 requires TLR7 and MyD88 to regulate FcγR expression. (A) Murine bone marrow-derived macrophages (BMM) were isolated, treated overnight with or without 1 µM R-848, then protein lysates extracted and analyzed by Western blotting for expression of the γ-subunit. Top panel: BMM from wild-type (WT) versus TLR7 knockout (TLR7−/−) were compared. Middle panel: BMM from WT versus MyD88 (MyD88−/−) and Cryopyrin (Cryo−/−) knockouts were compared. Bottom panel: BMM from WT versus TRIF knockouts (TRIF−/−) were compared. All blots represent 3 independent experiments. (B) Cytokine response of BMM to R-848. BMM from wild-type, TLR7−/−, MyD88−/−, TRIF−/− and Cryopyrin−/− mice were treated overnight with 1 µM R-848 or left untreated (UT). Supernatants were analyzed by sandwich ELISA for TNFα. Graph is representative of at least 3 different experiments per genotype. N.D. Not detected.
R-848 reduces tumor growth in vivo
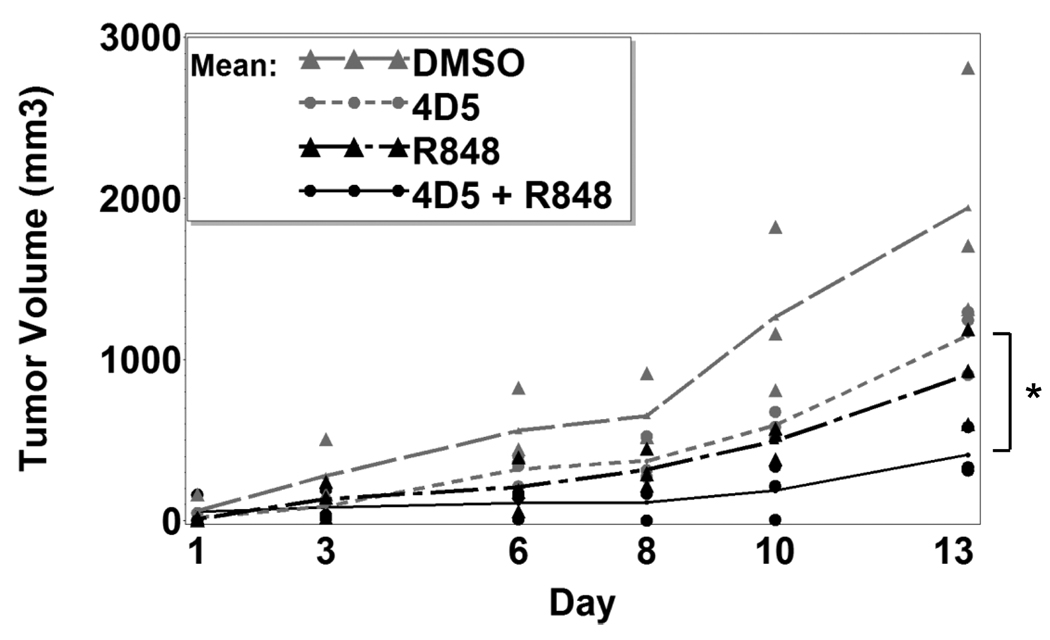
Results showed that R-848 could regulate FcγR expression and synergize with FcγR function, so we next asked whether R-848 could improve antibody therapy in vivo. To test this, we employed a solid tumor model using CT26 cells expressing human HER2/neu (21;22). Here, CT26-HER2/neu colon carcinoma cells were subcutaneously injected into syngeneic Balb/cJ mice. After 7 days to allow tumors to develop, mice were injected intraperitoneally with antibody alone, R-848 alone, R-848 plus antibody, or vehicle alone 3 times per week. Tumors were measured on each treatment day. After 13 days, there was a significantly reduced rate of growth in the mice receiving R-848 plus antibody (Figure 5). Statistical tests showed synergism between 4D5 and R-848 (p=0.03) for reducing the rate of tumor growth. Hence, R-848 plus antitumor antibody leads to synergistic reduction of tumor growth in vivo.
Fig. 5.
R-848 enhances antibody therapy in vivo. Balb/cJ mice (n=3 per group) were injected subcutaneously with 1×106 CT26-Her2/neu cells and left for 7 days for tumors to develop. Mice were then injected intraperitoneally on every other day with DMSO vehicle, anti-Her2 (4D5) plus DMSO, R-848, or 4D5 plus R-848. Tumor sizes were measured (see Methods) every other day for 2 weeks. Asterisks denote statistical significance (p≤0.05).
Discussion
Here, we have demonstrated a link between the Toll-like Receptor 7/8 and the Fcγ receptor pathways, in which TLR7/8 activation regulates FcγR expression. There is also functional synergism between these two pathways, leading to enhanced FcγR-mediated cytokine release and to a decreased rate of tumor growth. As such, TLR7/8 agonists may be of special benefit in conjunction with antibody therapy against tumors.
Several possible mechanisms may account for the functional synergism between TLR7/8 activation and FcγR activity. Firstly, as shown in Figure 2 and Table 1, there was increased expression of the activating FcγR and a marked reduction in the inhibitory FcγRIIb. This would promote superadditive responses, as the FcγR would respond more strongly to any given stimulus. With regard to the synergism seen in the murine solid tumor model (Figure 5), other factors may also contribute. T cells, B cells, dendritic cells and monocytes/macrophages can all respond to TLR7/8 ligands (34), although it has also been reported that T cells may respond only indirectly (35). Natural Killer (NK) cells are themselves unaffected by R-848 treatment but respond to monocyte-derived cytokines following R-848 treatment ((36) and data not shown). In mice it has been shown that R-848 elicits cytokine production (23), as well as driving leukocytes from circulation to peripheral organs (35). Together, these two effects might work with the increased FcγR to enhance the antitumor response. Firstly, greater cytokine production would lead to more activation of the immune cells. Secondly, since migration of leukocytes into peripheral organs is stimulated, presumably there would also be more leukocytes migrating to the tumor site as well (35). In addition, R-848 may have shifted the macrophages toward an M1 phenotype and this would have significantly enhanced their ability to combat the tumors. Tumor-associated macrophages possess an M2 phenotype and promote invasion (see (37;38) for review). However, it has been shown that treatment of squamous cell carcinoma with imiquimod, a TLR7 ligand approved for clinical use, leads to an M1 and Th1 phenotype (39). Collectively, these factors may have contributed to the synergism we observed between TLR7/8 and FcγR. Studies are ongoing to determine the precise mechanisms.
The cytokine response itself is likely responsible for the increase in activating FcγR, and might have been responsible for changes in FcγRIIb as well. Upregulation of FcγR by TLR4 has been previously shown in a murine model of arthritis, and was found to be largely mediated by IL-10 production (40). IL-10 can lead to increases in activating FcγR (41–43) as well as that of the inhibitory FcγRIIb (43;44). In contrast to this general upregulation, however, we found a striking decrease in FcγRIIb after TLR7/8 activation. Previous work has shown that IL-4 works with IL-10 to promote expression of FcγRIIb (13;44), but our microarray analysis found an almost 40-fold increase in IL-10 with no increase in IL-4 after R-848 treatment. Concurrently, TNFα and IFNγ, both known to decrease FcγRIIb expression (44;45), were strongly upregulated.
Hence, it seemed likely that the specific cytokine milieu elicited by R-848 was responsible for the changes in FcγR expression. Indeed, this was likely the case for the upregulation of activating FcγR, as blocking secretion with Brefeldin A abolished the R848-mediated changes. However, Brefeldin A did not prevent the R848-mediated decrease in FcγRIIb (Figure 3B). Further, FcγRIIb protein was decreased within 1 hour (Figure 2D), while FcγRIIb transcript remained to 4 hours (data not shown). These results strongly suggest a differential regulation of activating versus inhibitory receptors by R-848, where autocrine / paracrine factors drive the upregulation of activating receptors and different mechanisms – perhaps ubiquitination and proteasomal degradation – cause the almost-immediate decrease in FcγRIIb.
There is a chance that the culture conditions (10% FBS rather than autologous sera) influenced the maturation and responses of the monocytes. For example, the quantities of many growth, survival or apoptosis factors that monocytes would normally be exposed to within circulation would have been different with the FBS culture. However, the negative controls within the experiments suggest that R-848 itself drove at least the majority of the FcγR and cytokine responses.
Results from the ADCC (Figure 1B) and the murine solid tumor (Figure 5) experiments suggest that although imidazoquinolines have previously been shown to be effective by themselves against certain tumors, they may prove especially useful as adjuvants to antibody therapy. In fact, it is plausible that part of the antitumor effect of R-848 is mediated by autoantibodies against the tumor. Such autoantibodies have been well-documented in humans (46). We found that cotreatment with antibody and R-848 led to the greatest effects (Figure 4 & Figure 5), but it is likely that more effective antibodies may elicit an even stronger TLR7/8 – FcγR synergism. Many antibodies have been engineered for better FcγR binding (47;48), and these may prove especially powerful when combined with TLR7/8 agonists.
In summary, we have identified a unique regulatory link between TLR7/8 and FcγR. This not only has implications for the clinical setting, but also uncovers a novel biological regulatory pathway of Fcγ receptors.
Acknowledgments
Grant Support: P01 CA095426 and R01 AI059406 (ST). Postdoctoral fellowship from the American Cancer Society, Ohio Division (JPB). NIH postdoctoral fellowship T32 60013191 (SEJ). AHA predoctoral fellowship 09PRE2170054 (PM). NIH postdoctoral fellowship T32 CA009338 (KDG).
Abbreviations
- TLR
Toll-like receptor
- FcγR
Fc-gamma receptor
- PBM
peripheral blood monocytes
- BMM
bone marrow-derived monocytes
- TNFα
Tumor Necrosis Factor-alpha
Footnotes
Conflict of Interest: All authors declare that no conflict of interest exists.
Statement of Translational Relevance.
Antibody therapy against tumors has proven to be a valuable tool in combating cancer, but has been shown to be only partially effective or ineffective for many patients. Because of this, there is a continued attempt to find means of enhancing the efficacy of antibody treatment. Here, we provide both functional and mechanistic evidence that activation of TLR7/8 enhances FcγR expression and activity. Treatment with the TLR7/8 agonist R-848 leads to enhanced destruction of antibody-coated tumor cells by monocytes in vitro, and to attenuated growth of solid tumors in vivo. Hence, TLR7/8 agonists may be an effective adjuvant for antibody therapy.
References
- 1.Anderson CL, Shen L, Eicher DM, Wewers MD, Gill JK. Phagocytosis mediated by three distinct Fc gamma receptor classes on human leukocytes. J.Exp.Med. 1990:1333–1345. doi: 10.1084/jem.171.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casado JA, Merino J, Cid J, Subira ML, Sanchez-Ibarrola A. The type of interaction with Fc gamma R in human monocytes determines the efficiency of the generation of oxidative burst. Immunology. 1994:148–154. [PMC free article] [PubMed] [Google Scholar]
- 3.Kindt GC, Moore SA, She ZW, Wewers MD. Endotoxin priming of monocytes augments Fc gamma receptor cross-linking-induced TNF-alpha and IL-1 beta release. Am.J.Physiol. 1993:L178–L185. doi: 10.1152/ajplung.1993.265.2.L178. [DOI] [PubMed] [Google Scholar]
- 4.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat.Med. 2000:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 5.Takai T, Li M, Sylvestre D, Clynes R, Ravetch JV. FcR gamma chain deletion results in pleiotrophic effector cell defects. Cell. 1994:519–529. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 6.Cooper PH, Mayer P, Baggiolini M. Stimulation of phagocytosis in bone marrow-derived mouse macrophages by bacterial lipopolysaccharide: correlation with biochemical and functional parameters. J.Immunol. 1984:913–922. [PubMed] [Google Scholar]
- 7.Ralph P, Nakoinz I. Antibody-dependent killing of erythrocyte and tumor targets by macrophage-related cell lines: enhancement by PPD and LPS. J.Immunol. 1977:950–954. [PubMed] [Google Scholar]
- 8.van Ojik HH, Bevaart L, Dahle CE, et al. CpG-A and B oligodeoxynucleotides enhance the efficacy of antibody therapy by activating different effector cell populations. Cancer Res. 2003:5595–5600. [PubMed] [Google Scholar]
- 9.Sidky YA, Borden EC, Weeks CE, et al. Inhibition of murine tumor growth by an interferon-inducing imidazoquinolinamine. Cancer Res. 1992:3528–3533. [PubMed] [Google Scholar]
- 10.Schon MP, Schon M. TLR7 and TLR8 as targets in cancer therapy. Oncogene. 2008:190–199. doi: 10.1038/sj.onc.1210913. [DOI] [PubMed] [Google Scholar]
- 11.Miller RL, Meng TC, Tomai MA. The antiviral activity of Toll-like receptor 7 and 7/8 agonists. Drug News Perspect. 2008:69–87. doi: 10.1358/dnp.2008.21.2.1188193. [DOI] [PubMed] [Google Scholar]
- 12.Stanley MA. Imiquimod and the imidazoquinolones: mechanism of action and therapeutic potential. Clin.Exp.Dermatol. 2002:571–577. doi: 10.1046/j.1365-2230.2002.01151.x. [DOI] [PubMed] [Google Scholar]
- 13.Joshi T, Ganesan LP, Cao X, Tridandapani S. Molecular analysis of expression and function of hFcgammaRIIbl and b2 isoforms in myeloid cells. Mol.Immunol. 2006:839–850. doi: 10.1016/j.molimm.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 14.Butchar JP, Cremer TJ, Clay CD, et al. Microarray analysis of human monocytes infected with Francisella tularensis identifies new targets of host response subversion. PLoS.One. 2008:e2924. doi: 10.1371/journal.pone.0002924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muehlbauer SM, Evering TH, Bonuccelli G, et al. Anthrax lethal toxin kills macrophages in a strain-specific manner by apoptosis or caspase-1-mediated necrosis. Cell Cycle. 2007:758–766. doi: 10.4161/cc.6.6.3991. [DOI] [PubMed] [Google Scholar]
- 16.Lamkanfi M, Moreira LO, Makena P, et al. Caspase-7 deficiency protects from endotoxin-induced lymphocyte apoptosis and improves survival. Blood. 2009:2742–2745. doi: 10.1182/blood-2008-09-178038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dudoit S, Gentleman RC, Quackenbush J. Open source software for the analysis of microarray data. Biotechniques. 2003:45–51. [PubMed] [Google Scholar]
- 18.Gentleman RC, Carey VJ, Bates DM, et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat.Appl.Genet.Mol.Biol. 2004 doi: 10.2202/1544-6115.1027. Article3. [DOI] [PubMed] [Google Scholar]
- 20.Johnson AJ, Wagner AJ, Cheney CM, et al. Rituximab and 17-allylamino-17-demethoxygeldanamycin induce synergistic apoptosis in B-cell chronic lymphocytic leukaemia. Br.J.Haematol. 2007:837–844. doi: 10.1111/j.1365-2141.2007.06878.x. [DOI] [PubMed] [Google Scholar]
- 21.Roda JM, Parihar R, Lehman A, et al. Interleukin-21 enhances NK cell activation in response to antibody-coated targets. J.Immunol. 2006:120–129. doi: 10.4049/jimmunol.177.1.120. [DOI] [PubMed] [Google Scholar]
- 22.Penichet ML, Challita PM, Shin SU, et al. In vivo properties of three human HER2/neu-expressing murine cell lines in immunocompetent mice. Lab Anim Sci. 1999:179–188. [PubMed] [Google Scholar]
- 23.Tomai MA, Gibson SJ, Imbertson LM, et al. Immunomodulating and antiviral activities of the imidazoquinoline S-28463. Antiviral Res. 1995:253–264. doi: 10.1016/0166-3542(95)00054-p. [DOI] [PubMed] [Google Scholar]
- 24.Poplack DG, Bonnard GD, Holiman BJ, Blaese RM. Monocyte-mediated antibody-dependent cellular cytotoxicity: a clinical test of monocyte function. Blood. 1976:809–816. [PubMed] [Google Scholar]
- 25.Shaw GM, Levy PC, LoBuglio AF. Human monocyte cytotoxicity to tumor cells. I. Antibody-dependent cytotoxicity. J.Immunol. 1978:573–578. [PubMed] [Google Scholar]
- 26.Catalona WJ, Ratliff TL, McCool RE. gamma-Interferon induced by S. aureus protein A augments natural killing and ADCC. Nature. 1981:77–79. doi: 10.1038/291077a0. [DOI] [PubMed] [Google Scholar]
- 27.Weiner LM, Steplewski Z, Koprowski H, et al. Biologic effects of gamma interferon pre-treatment followed by monoclonal antibody 17-1A administration in patients with gastrointestinal carcinoma. Hybridoma. 1986:S65–S77. [PubMed] [Google Scholar]
- 28.Shen L, Guyre PM, Fanger MW. Direct stimulation of ADCC by cloned gamma interferon is not ablated by glucocorticoids: studies using a human monocyte-like cell line (U-937) Mol.Immunol. 1984:167–173. doi: 10.1016/0161-5890(84)90132-9. [DOI] [PubMed] [Google Scholar]
- 29.Zhang M, Zhang Z, Garmestani K, et al. Activating Fc receptors are required for antitumor efficacy of the antibodies directed toward CD25 in a murine model of adult t-cell leukemia. Cancer Res. 2004:5825–5829. doi: 10.1158/0008-5472.CAN-04-1088. [DOI] [PubMed] [Google Scholar]
- 30.Hemmi H, Kaisho T, Takeuchi O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat.Immunol. 2002:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 31.Kanneganti TD, Ozoren N, Body-Malapel M, et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006:233–236. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 32.Ravetch JV, Kinet JP. Fc receptors. Annu.Rev.Immunol. 1991:457–492. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- 33.Jurk M, Heil F, Vollmer J, et al. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nat.Immunol. 2002:499. doi: 10.1038/ni0602-499. [DOI] [PubMed] [Google Scholar]
- 34.Schon M, Schon MP. The antitumoral mode of action of imiquimod and other imidazoquinolines. Curr Med.Chem. 2007:681–687. doi: 10.2174/092986707780059625. [DOI] [PubMed] [Google Scholar]
- 35.Gunzer M, Riemann H, Basoglu Y, et al. Systemic administration of a TLR7 ligand leads to transient immune incompetence due to peripheral-blood leukocyte depletion. Blood. 2005:2424–2432. doi: 10.1182/blood-2005-01-0342. [DOI] [PubMed] [Google Scholar]
- 36.Gorski KS, Waller EL, Bjornton-Severson J, et al. Distinct indirect pathways govern human NK-cell activation by TLR-7 and TLR-8 agonists. Int.Immunol. 2006:1115–1126. doi: 10.1093/intimm/dxl046. [DOI] [PubMed] [Google Scholar]
- 37.Guruvayoorappan C. Tumor versus tumor-associated macrophages: how hot is the link? Integr.Cancer Ther. 2008:90–95. doi: 10.1177/1534735408319060. [DOI] [PubMed] [Google Scholar]
- 38.Yuan A, Chen JJ, Yang PC. Pathophysiology of tumor-associated macrophages. Adv.Clin.Chem. 2008:199–223. [PubMed] [Google Scholar]
- 39.Smith KJ, Hamza S, Skelton H. Topical imidazoquinoline therapy of cutaneous squamous cell carcinoma polarizes lymphoid and monocyte/macrophage populations to a Th1 and M1 cytokine pattern. Clin.Exp.Dermatol. 2004:505–512. doi: 10.1111/j.1365-2230.2004.01593.x. [DOI] [PubMed] [Google Scholar]
- 40.van Lent PL, Blom AB, Grevers L, Sloetjes A, van den Berg WB. Toll-like receptor 4 induced FcgammaR expression potentiates early onset of joint inflammation and cartilage destruction during immune complex arthritis: Toll-like receptor 4 largely regulates FcgammaR expression by interleukin 10. Ann.Rheum.Dis. 2007:334–340. doi: 10.1136/ard.2006.057471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.te Velde AA, de Waal MR, Huijbens RJ, de Vries JE, Figdor CG. IL-10 stimulates monocyte Fc gamma R surface expression and cytotoxic activity. Distinct regulation of antibody-dependent cellular cytotoxicity by IFN-gamma, IL-4, and IL-10. J.Immunol. 1992:4048–4052. [PubMed] [Google Scholar]
- 42.van Roon J, Wijngaarden S, Lafeber FP, et al. Interleukin 10 treatment of patients with rheumatoid arthritis enhances Fc gamma receptor expression on monocytes and responsiveness to immune complex stimulation. J.Rheumatol. 2003:648–651. [PubMed] [Google Scholar]
- 43.Liu Y, Masuda E, Blank MC, et al. Cytokine-mediated regulation of activating and inhibitory Fc gamma receptors in human monocytes. J.Leukoc.Biol. 2005:767–776. doi: 10.1189/jlb.0904532. [DOI] [PubMed] [Google Scholar]
- 44.Wijngaarden S, van de Winkel JG, Jacobs KM, et al. A shift in the balance of inhibitory and activating Fcgamma receptors on monocytes toward the inhibitory Fcgamma receptor IIb is associated with prevention of monocyte activation in rheumatoid arthritis. Arthritis Rheum. 2004:3878–3887. doi: 10.1002/art.20672. [DOI] [PubMed] [Google Scholar]
- 45.Pricop L, Redecha P, Teillaud JL, et al. Differential modulation of stimulatory and inhibitory Fc gamma receptors on human monocytes by Th1 and Th2 cytokines. J.Immunol. 2001:531–537. doi: 10.4049/jimmunol.166.1.531. [DOI] [PubMed] [Google Scholar]
- 46.Houghton AN. Cancer antigens: immune recognition of self and altered self. J.Exp.Med. 1994:1–4. doi: 10.1084/jem.180.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hutchins JT, Kull FC, Jr, Bynum J, et al. Improved biodistribution, tumor targeting, and reduced immunogenicity in mice with a gamma 4 variant of Campath-1H. Proc.Natl.Acad.Sci.U.S A. 1995:11980–11984. doi: 10.1073/pnas.92.26.11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lazar GA, Dang W, Karki S, et al. Engineered antibody Fc variants with enhanced effector function. Proc.Natl.Acad.Sci.U.S A. 2006:4005–4010. doi: 10.1073/pnas.0508123103. [DOI] [PMC free article] [PubMed] [Google Scholar]