Abstract
Hepatocellular carcinoma (HCC) is known to be associated with both HBV and HCV and HVC. While epigenetic changes have been previously reported to be associated with hepatocellular carcinoma (HCC), whether the epigenetic profile of HBC associated HCC differs from that of HCV associated HCC is unclear. We analyzed DNA methylation of ten genes (APC, CCND2, CDKN2A, GSTP1, HOXA9, RARB, RASSF1, RUNX, SFRP1, and TWIST1) using MethyLight assays on 65 archived liver tissue blocks. Three genes (APC, CCND2, and GSTP1) were frequently methylated in normal liver tissues. Five genes (APC, CDKN2A, HOXA9, RASSF1, and RUNX) were significantly more frequently methylated in malignant liver tissues than normal liver tissues. Among HCC cases, HOXA9, RASSF1 and SFRP1 were methylated more frequently in HBV positive HCC cases, while CDKN2A were significantly more frequently methylated in HCV positive HCC cases. Our data support the hypothesis that HCC resulting from different viral etiologies are associated with different epigenetic changes.
Keywords: hypermethylation, HBV, HCV, hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC), the predominant form of human liver cancer, is the fifth most common solid tumor worldwide and the fourth leading cause of cancer-related death, accounting for approximately 600,000 deaths per year (Bosch et al., 2005; Thomas and Zhu, 2005). Hepatitis B virus (HBV) and hepatitis C virus (HCV) infections are the major etiological factors for HCC, accounting for 80% of all HCC cases. It is hypothesized that HBV and HCV infection lead to hepatocarcinogenesis through increased hepatocyte regeneration and turnover leading to inflammation, oxidative DNA damage and chronic hepatitis. This microenvironment sets the stage for malignant transformation of hepatocytes through accumulation of both genetic and epigenetic changes (Levrero, 2006). Alternatively, specific viral proteins can directly lead to cell transformation, including X-protein encoded by HBV (HBx), core, NS3, NS4B and NS5A proteins encoded by HCV (Koike, 2002; Koike, 2005; Levrero, 2006).
Since HBV is a DNA virus, which integrates into the genome, while HCV is an RNA virus, which does not involve a DNA intermediate, it is likely that different mechanisms are involved in hepatocarcinogenesis caused by these two viruses (Farazi and DePinho, 2006; Kirk et al., 2006; Levrero, 2006). Epidemiologically, HBV infection in childhood is more likely to cause HCC in young adults, while HCV infection rarely causes HCC in adults younger than 50 years old (Kirk et al., 2006). The male-to-female ratio is higher in HBV-caused HCC than HCV-caused HCC (Shiratori et al., 1995). Patients co-infected with HBV and HCV are at increased risk for developing HCC compared to singly infected patients, further supporting the hypothesis of different pathways between the two viruses. Morphologically, HBV-caused HCCs tend to be infiltrative and multinodular, while HCV-caused HCCs tend to be solitary, smaller in size and encapsulated (But et al., 2008). However, at the molecular level, both loss of heterozygosity (LOH) and microarray analyses have failed to consistently identify specific genetic alterations and gene expression changes associated with specific viral infection (Thorgeirsson et al., 2006).
Epigenetic alterations, such as DNA methylation, play an important role in tumorigenesis (Jones and Baylin, 2002; Laird, 2003). DNA methylation, referring here to the addition of a methyl group to the cytosine in the CpG dinucleotides, when occurring at the promoter region of a gene, leads to gene silencing. Consequently, DNA methylation of a tumor suppressor gene has the equivalent effect as genetic mutations to inactivate the gene. Recent studies have reported the detection of DNA methylation of various panels of genes during the stepwise progression of HCC. For example, DNA methylation of four genes (Col1A2, IGFBP2, CTGF, fibronectin 1) has been shown to progressively increase from normal liver, chronic hepatitis, liver cirrhosis to hepatoma (Chiba et al., 2005; Kwon et al., 2005). Similarly, methylation of p16 (CDKN2A), p15 and SFRP1 is present not only in HCC, but is present also at low frequencies in chronic hepatitis and liver cirrhosis samples (Fukai et al., 2005; Shih et al., 2006), all supporting the hypothesis that CpG island methylation of tumor-related genes is an early and frequent event, and methylation changes accumulate during a multistep hepatocarcinogenesis (Lee et al., 2003). In addition, several studies have investigated the methylation patterns associated with specific viral infection without definitive conclusion. Jicai reported finding that p16 methylation preferentially occurred in liver cancerous tissues with HBV infections, as compared to those without HBV infection (Jicai et al., 2006), while others have reported higher frequency of p16 methylation in HCV-HCCs than HBV-HCCs (Katoh et al., 2006; Li et al., 2004; Narimatsu et al., 2004). These studies were confounded by other risk factors such as age, ethnic background and geographic locations, which might have lead to differential methylation patterns.
In the present study we compared the epigenetic profile of normal liver tissue, HBV-associated HCC, and HCV-associated HCC using archived liver tissues biopsies, after adjusting for relevant co-factors.
Materials and Methods
Collection of clinical tissue specimens
Archived formalin-fixed paraffin-embedded tissue blocks were obtained from University of Washington Medical Center and Harborview Medical Center. A total of 62 cancerous liver tissue blocks from 40 HCC patients had sufficient material for the study, with 30 subjects having a single block, and 10 subjects having 2–8 blocks each. In addition, 25 normal liver biopsy samples from 25 liver transplant donors were used as normal control tissue for this study. Age, gender, race, and HBV, HCV serology test results were obtained from patients’ medical records. The study was approved by the institutional review board of the University of Washington.
DNA isolation from paraffin blocks
Six 20 µm sections were cut from each block and deparaffined by xylene extraction. The resulting tissue pellets were digested with proteinase K at 48°C overnight. The genomic DNA was isolated by phenol/chloroform extraction and ethanol precipitation. Finally the DNA was purified using QIAamp DNA mini-column according to the manufacturer protocol (Qiagen, Valencia, CA).
Sodium bisulfite conversion
Unmethylated human sperm DNA (U-DNA) and in-vitro fully methylated DNA (M-DNA) were converted with clinical samples as described before (Weisenberger et al., 2005). Briefly, about 1 µg DNA was modified by 5M sodium bisulfite, desulfonated with NaOH, then purified and resuspended in 80 µl EB buffer (10 mM Tris-HCl, pH 8.0).
DNA Methylation (MethyLight) Analysis
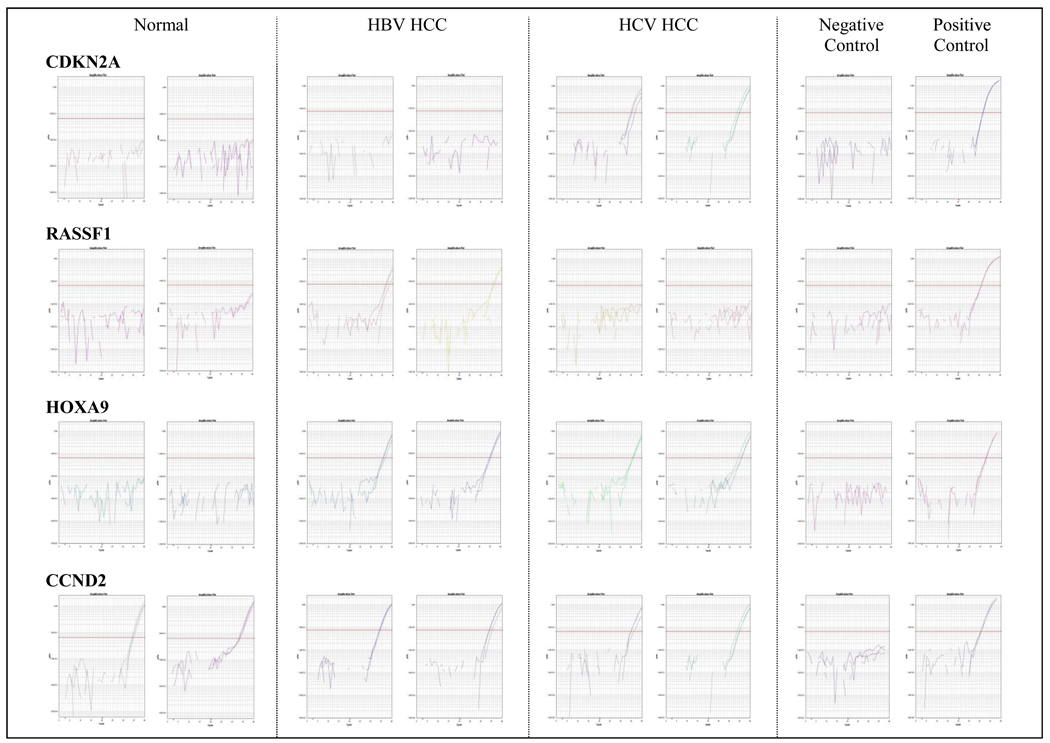
Methylation of ten genes (APC, CCND2, CDKN2A, GSTP1, HOXA9, RARB, RASSF1, RUNX, SFRP1, and TWIST1) was analyzed in this study. These ten genes were shown by previous studies to be specifically methylated in HCC tissues. For each gene, the primers and probe were designed specifically for bisulfite-converted fully methylated DNA (Supplemental Table). Quantitative PCR was performed on ABI PRISM HT 7900 thermocycler (ABI, Foster City, CA). Amplification of bisulfite converted β-Actin (ACTB) was used to normalize for input DNA. Samples that were negative for ACTB were excluded in the methylation analysis. A plasmid containing bisulfite converted ACTB gene of known concentration was diluted and used as the standard curve for quantification. The assay for a given set of samples was considered valid only if the converted U-DNA was not amplified, while the converted M-DNA was amplified. Examples of amplification curves were shown in Figure 1. The Percentage Methylated Reference (PMR) for each locus was calculated by dividing the GENE: reference ratio of a sample by the GENE: reference ratio of M-DNA and multiplying by 100 (Eads et al., 2001).
Figure 1.
Representative MethyLight amplification curves for CDKN2A, RASSF1, HOXA9, and CCND2. CDKN2A was preferentially methylated in HCV-positive HCCs, while RASSF1 was preferentially methylated in HBV-positive HCCs. Methylation of HOXA9 was HCC specific, and methylation of CCND2 was present in both normal and HCC liver tissues.
Statistical Methods
In this study we dichotomized the semi-quantitative MethyLight data by classifying a specific gene positive for any hypermethylation if the PMR was greater than 0% (Feng et al., 2008). When multiple cancerous tissue samples were available, we randomly chose one block to be used in this analysis. Pearson’s chi-square tests and Fisher’s Exact tests were used in univariate analyses to compare dichotomous variables and Student’s t-tests were used to compare continuous variables. Continuous factors of interest that were not normally distributed were analyzed by Wilcoxon rank sum tests. Logistic regression was additionally used to determine differences in methylation frequency of each gene in relation to cancer, age, gender, serology, and odds ratios (OR) and 95% confidence intervals were calculated based upon test-based methods. Multivariable logistic regression, adjusting for age and gender, was used to determine differences in methylation frequency of each gene in subjects with cancerous tissue samples and those with normal histology. Exact logistic regression was utilized to compute risk estimates and confidence intervals when the prevalence of gene methylation was low. A two-sided 0.05 test level determined statistical significance for all analyses. All analyses were conducted using SAS version 9.1 (SAS Institute Inc., Cary, NC).
Results
Study Population
The baseline characteristics of the 25 normal and 40 HCC subjects are summarized in Table 1. Subjects with HCC were older than normal subjects (53.7 vs. 40.6; p=0.0005), but both were approximately one-quarter female. Approximately half of both cases and controls were Caucasian; however 30% of subjects with HCC were Asian, while 40% of normal subjects were either Black or Hispanic. Only 16% of normal subjects were seropositive for HBV or HCV, while 30% of HCC subjects were seropositive for HBV and 70% are seropositive for HCV. None of the study subjects were seropositive for both HBV and HCV. Among subjects with HCC, 9/12 (75%) of HBV positive subjects were Asian and only 1/12 (8%) of them was female, while 18/28 (64%) of HCV positives were Caucasian and 7/28 (25%) were female. HCC subjects who were HCV positive were moderately older than HBV positive subjects (mean age 55 vs. 50; p=0.10).
Table 1.
Demographic Characteristics of study population
| Tissue Diagnosis | ||
|---|---|---|
| Normal (n=25) |
HCC (n=40) |
|
| Age (mean years ± sd) | 40.6 ± 15.6 | 53.7 ± 9.3 |
| Female Gender | 9 (36%) | 8 (20%) |
| Race | ||
| Caucasian | 13 (52%) | 19 (48%) |
| Asian | 0 (0%) | 12 (30%) |
| Black | 6 (24%) | 3 (8%) |
| Hispanic | 4 (16%) | 0 (0%) |
| Other/Missing | 2 (8%) | 6 (15%) |
| Serology | ||
| Negative | 21 (84%) | 0 (0%) |
| HBV | 2 (8%) | 12 (30%) |
| HCV | 2 (8%) | 28 (70%) |
Gene specific methylation in normal and cancerous liver tissues
We compared the methylation frequency of the selected genes in normal liver tissues and HCC liver tissues (Table 2). Two genes (HOXA9 and RASSF1) were methylated at low frequencies in normal liver tissues, but at higher frequencies in HCC, while the remaining five genes (CDKN2A, RARB, RUNX, SFRP1, and TWIST1) were never methylated in normal liver tissues but were methylated in HCC. Five genes (APC, CDKN2A, HOXA9, RASSF1, and RUNX) were methylated significantly more frequent in malignant liver tissues compared to normal liver tissues (p<0.05). Among samples that were methylated for the specific gene, the average PMR in normal and cancerous tissues was 0.21 vs. 0.7 for APC (p=0.01), 0.16 vs. 0.25 for CCND2 (p=0.25), 0.22 vs. 1.09 for GSTP1 (p=0.0054). We did not calculate the average PMR for the remaining genes, because they were methylated in less than five normal liver tissue samples.
Table 2.
Promoter Hypermethylation in normal and cancerous liver tissues
| Gene | Normal (n=25) |
HCC (n=40) |
P-Value* |
|---|---|---|---|
| APC | 12 (48%) | 31 (78%) | 0.01 |
| CCND2 | 14 (56%) | 26 (65%) | 0.47 |
| CDKN2A | 0 (0%) | 18 (45%) | <.0001** |
| GSTP1 | 13 (52%) | 24 (60%) | 0.53 |
| HOXA9 | 2 (8%) | 15 (38%) | 0.0085 |
| RARB | 0 (0%) | 2 (5%) | 0.52** |
| RASSF1 | 1 (4%) | 10 (25%) | 0.04** |
| RUNX | 0 (0%) | 7 (18%) | 0.04** |
| SFRP1 | 0 (0%) | 4 (10%) | 0.15** |
| TWIST1 | 0 (0%) | 4 (10%) | 0.15** |
Chi-Square
Fisher’s Exact Test
Three genes (APC, CCND2, and GSTP1) were methylated at high frequencies in both normal and malignant liver tissues. APC was methylated in 12/25 (48%) of normal liver tissues, CCND2 was methylated in 14/25 (56%) of normal liver tissues, and GSTP1 was methylated in 13/25 (52%) of normal liver tissues. However, the average PMR in cancerous tissues was significantly higher than in normal tissues for APC and GSTP1 among methylation positive samples.
CDKN2A was significantly and HOXA9 was moderately associated with increased age and TWIST1 was moderately associated with female gender (Table 3). In multivariate models adjusting for age and gender, four genes (APC, CDKN2A, HOXA9 and RUNX) were significantly associated with malignant liver tissues (Table 4).
Table 3.
Univariate logistic regression analysis of promoter hypermethylation in normal and cancerous liver tissues (OR and 95% CI)
| Gene | Age per 10 years | Female vs. Male |
|---|---|---|
| APC | 1.1 (0.8–1.6) | 0.5 (0.1–1.4) |
| CCND2 | 0.9 (0.6–1.3) | 0.6 (0.2–1.9) |
| CDKN2A | 1.9 (1.1–3.3) | 1.6 (0.5–5.4) |
| GSTP1 | 0.9 (0.6–1.3) | 2.2 (0.7–7.2) |
| HOXA9 | 1.6 (0.98–2.63) | 2.7 (0.8–8.8) |
| RARB | 2.2 (0.6–8.0) | 1.2 (0–15.2)** |
| RASSF1 | 1.2 (0.7–2.1) | 0.6 (0.1–3.0) |
| RUNX | 1.2 (0.6–2.2) | 2.4 (0.5–11.8) |
| SFRP1 | 1.0 (0.5–2.1) | 3.1 (0.4–23.7) |
| TWIST1 | 1.3 (0.6–2.8) | 10.1 (0.97–104.6) |
Exact Logistic Regression
Table 4.
Multivariate logistic regression analysis of promoter hypermethylation in normal and cancerous liver tissues (OR and 95% CI)
| Gene | HCC vs. Normal | Age per 10 years | Female vs. Male |
|---|---|---|---|
| APC | 4.2 (1.2–14.9) | 0.9 (0.6–1.3) | 0.6 (0.2–1.9) |
| CCND2 | 1.8 (0.5–5.8) | 0.8 (0.5–1.3) | 0.7 (0.2–2.1) |
| CDKN2A | 22.3 (3.2–∞)** | 1.3 (0.6–2.7) | 4.5 (0.8–26.7) |
| GSTP1 | 2.3 (0.7–7.7) | 0.8 (0.5–1.2) | 2.6 (0.7–8.8) |
| HOXA9 | 8.7 (1.3–58.6) | 1.3 (0.7–2.3) | 5.2 (1.2–23.1) |
| RARB | *** | *** | *** |
| RASSF1 | 8.7 (0.8–93.3) | 0.9 (0.5–1.8) | 0.8 (0.1–4.5) |
| RUNX | 12.2 (1.2–∞)** | 0.5 (0.2–1.6) | 6.3 (0.8–46.6) |
| SFRP1 | 11.1 (0.8–∞)** | 0.2 (0.0–1.3) | 13.5 (0.9–212.9) |
| TWIST1 | 2.5 (0.3–∞)** | 0.4 (0.1–2.1) | 37.3 (1.8–768.0) |
Exact Logistic Regression
model is degenerate, not enough positives
Gene specific methylation associated with HBV or HCV infection in HCC
We next determined whether specific gene methylation was associated with different viral infections among patients with HCC (Table 5). Among cancerous liver tissues, three genes (HOXA9, RASSF1, and SFRP1) were somewhat more frequently methylated in HBV-positive HCC cases, and two genes (CDKN2A and RARB) were somewhat more frequently methylated in HCV-positive HCC cases. However, only the association with CDKN2A (methylated in 17% of HBV compared to 57% of HCV tissues) reached statistical significance (OR=6.7, 95% CI 1.2–36.2). Among samples that were methylated for the specific gene, the average PMR in HBV-positive HCCs and HCV-positive HCCs was 0.64 vs. 0.88 for APC (p=0.18), 0.25 vs. 0.24 for CCND2 (p=0.28), 0.80 vs. 1.67 for GSTP1 (p=0.44), 0.73 vs. 0.63 for HOXA9 (p=0.60), and 0.27 vs. 0.39 for RASSF1 (p=1.00). We did not calculate average PMR for the remaining genes, because they were methylated in less than five samples in either group.
Table 5.
Univariate Logistic Regression analysis of promoter hypermethylation in cancerous liver tissues (OR and 95% CI)
| Gene | HBV (n=12) | HCV (n=28) | HCV vs. HBV |
|---|---|---|---|
| APC | 8 (67%) | 23 (82%) | 2.3 (0.5–10.7) |
| CCND2 | 8 (67%) | 18(64%) | 0.9 (0.2–3.8) |
| CDKN2A | 2 (17%) | 16 (57%) | 6.7 (1.2–36.2) |
| GSTP1 | 8 (67%) | 16 (57%) | 0.7 (0.2–2.7) |
| HOXA9 | 6 (50%) | 9 (32%) | 0.5 (0.1–1.9) |
| RARB | 0 (0%) | 2 (7%) | 1.1 (0.1–∞)** |
| RASSF1 | 5 (42%) | 5 (18%) | 0.3 (0.1–1.4) |
| RUNX | 2 (17%) | 5 (18%) | 1.1 (0.2–6.6) |
| SFRP1 | 2 (17%) | 2 (7%) | 0.4 (0.0–3.1) |
| TWIST1 | 1 (8%) | 3 (11%) | 1.3 (0.1–14.1) |
Exact Logistic Regression
We also determined methylation status of these genes in HBV (n=2) or HCV (n=2) infected normal liver tissues. While GSTP1, CCND2, and HOXA9 were methylated in one HBV-positive normal liver tissue, no genes were methylated in the other HBV-positive normal liver tissue. Similarly, GSTP1, CCND2, and APC were methylated in one HCV-positive normal liver tissue, while no genes were methylated in the other HCV-positive normal liver tissue (data not shown).
Discussion
We determined the methylation status by the MethyLight assay of ten genes in both normal and malignant liver tissues. We have identified three genes that were frequently methylated (in approximately 50% of patients) in normal liver tissues. Five additional genes were significantly more frequently methylated in HCC tissues compared to normal liver tissues. Finally, we observed that methylation of CDKN2A was frequent in HCV, but not HBV-associated HCC. These data suggest that epigenetic alterations play an important role in hepatocarcinogenesis, with different pathways affected in HBV or HCV associated HCCs.
In analyzing our DNA hypermethylation results, we dichotomized semi-quantitative MethyLight data in two different ways: as positive for any hypermethylation at PMR > 0% and as positive for high levels of hypermethylation at PMR ≥ 4% (data not shown). Earlier studies have reported that PMR > 4% is associated with loss of gene expression and best discriminates between normal and malignant or premalignant tissues (Eads et al., 2000; Ogino et al., 2006). However, other researchers have used qualitative PCR methods that report samples as positive with any observable hypermethylation signal (Virmani et al., 2003), while other researchers have used three PMR intervals in their analyses, with no methylation at PMR = 0, low methylation at PMR < median for a particular gene, and high methylation at PMR > median for that gene (Tsou et al., 2005). We have previously confirmed that low levels of hypermethylated gene are present in samples with 0% < PMR < 4% (unpublished data), but the biological significance of this level of methylation is unclear. In this present study, we performed univariate and multivariate analyses using PMR > 0% as the criterion for hypermethylation positivity. Our basic conclusions remained the same, regardless of the PMR cutoff chosen.
Previous studies examining methylation of tumor suppressor genes in normal liver tissues have reported conflicting results, with most studies reporting no or low methylation of several tumor suppressor genes in normal liver tissues (Lee et al., 2003), but several studies reporting high frequency of DNA methylation of these genes in normal liver tissues. For example, Lehmann et al reported high frequency of DNA methylation of APC, CCND2, GSTP1, RASSF1A, SOCS-1 in normal liver biopsies obtained from organ donors, although the methylation levels were lower than in HCC samples (Lehmann et al., 2005; Lehmann et al., 2007). Harder et al reported 100% methylation of APC but no methylation of GSTP1 in 16 normal liver tissues (Harder et al., 2008). Although Nishida et al identified a group of seven genes (HIC-1, CASP8, SOCS-1, RASSF1A, p16, and APC) that was frequently methylated in normal liver tissues, their normal liver tissues were obtained either from colon cancer patients who had hepatic metastasis, or from patients who suffered from focal nodular hyperplasia, hepatic hemangioma, or hepatic adenoma (Nishida et al., 2008). In the present study, we observed high frequency of methylation of APC (48%), CCND2 (56%), and GSTP1 (52%) in normal liver tissues, even in the absence of HBV or HCV infection. Similar to studies by Lehmann et al reported for these three genes (Lehmann et al., 2005), we reported here that methylation levels in normal liver tissues were lower than in liver cancer tissues. Among samples that were methylated for the specific gene, the average PMR in cancerous tissues was significantly higher for APC (p=0.01) and GSTP1 (p=0.0054) than in normal liver tissues. These methylation changes present in normal tissues might represent the consequence of accumulated environmental exposures, including both cytotoxic and carcinogenic chemicals being detoxified in liver. Indeed, Zhang et al reported that methylation of GSTP1, the key enzyme involved in detoxifying aflatoxin B1, was correlated with the aflatoxin-DNA adduct levels in the HCC tissues (Zhang et al., 2005). However, in the present study we did not see a trend of increasing methylation with age among normal liver tissues, and aflatoxin levels were not available. Alternatively, these methylation changes could represent tissue-dependent differentially methylated genes, which have begun to be elucidated by several genome-wide profiling analyses on normal tissues (Kitamura et al., 2007; Nagase and Ghosh, 2008; Weber et al., 2007).
Previous studies also tried to identify specific DNA methylation patterns associated with various risk factors in hepatocarcinogenesis, including viral infections, aflatoxin exposure and alcohol consumption. Methylation of CTGF, RARB, E-cadherin and p73 was more frequently seen in HBV-associated HCCs than in HCV-associated HCCs in several studies (Chiba et al., 2005; Yang et al., 2003), while RUNX3, APC, SOCS-1 and p14 were preferentially methylated in HCV-HCC (Mori et al., 2005; Yang et al., 2003). Several recent studies also linked environmental exposures to specific DNA methylation patterns. High frequencies of p16, GSTP1, MGMT and RASSF1 methylation were significantly associated with high level of AFB1-DNA adducts in HCC tumors (Zhang et al., 2002; Zhang et al., 2005; Zhang et al., 2003; Zhang et al., 2006). CDKN2A methylation has been shown to be present in early stages of HBV-associated hepatocarcinogenesis, not only in high frequency in HCCs, but also was in cirrhotic nodules (CNs) and dysplastic nodules (DNs), known precursor lesions of HCCs (Shim et al., 2003). Further, p16 methylation has been shown to preferentially occur in liver tissues with HBV infections compared to liver tissues without HBV infection (Jicai et al., 2006). Although a few studies did not detect differences of p16 methylation between HBV-HCCs and HCV-HCCs (Fukai et al., 2005; Kaneto et al., 2001), several studies consistently observed higher frequency (although not significant) of p16 methylation in HCV-HCCs than HBV-HCCs (Katoh et al., 2006; Li et al., 2004; Narimatsu et al., 2004). In the present study, we did not detect any CDKN2A methylation in normal liver tissues, but methylation of CDKN2A was significantly higher in HCV-HCCs than in HBV-HCCs. In addition, we observed higher frequencies of methylation of RASSF1, SFRP1 and HOXA9 in HBV-HCCs than in HCV-HCCs, but these differences did not attain statistical significance.
Although our retrospective case-control study suggests that different pathways are preferentially inactivated epigenetically in HCCs caused by different etiologies, our current study has several limitations. Although our samples are all from patients enrolled at either University of Washington Medical Center or Harborview Medical Center, 64% HCV-HCC patients were Caucasians, while 75% of HBV-HCC patients were Asian. As virus type was strongly correlated with race, we can not rule out the possibility that the methylation differences we observed with respect to virus type are not confounded by the different genetic backgrounds in the HBV vs. HCV-associated cancers. Further, our sample size was small, especially for the group of HBV-HCC patients. This is due to the low frequency of HBV infection in developed countries. Our conclusions need to be further confirmed in larger retrospective studies in developing countries where both HBV and HCV infections are prevalent, where the effects of race, age and gender can be adequately adjusted for. Future prospective studies need to address these limitations to definitively delineate mechanistic pathways of hepatocarcinogenesis in relationship with environmental risk factors.
Hepatocellular carcinoma is one of the most common malignancies worldwide with very poor prognosis. Elucidating the underlying molecular mechanisms in relationship with various etiological agents is a necessary step for the development of novel and effective treatments that will ultimately improve patients’ survival.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Informed consent was obtained according to procedures approved by the Human Subjects Committee of the University of Washington
The authors have no commercial or other associations that might pose a conflict of interest.
References
- Bosch FX, et al. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2005;9:191–211. doi: 10.1016/j.cld.2004.12.009. v. [DOI] [PubMed] [Google Scholar]
- But DY, et al. Natural history of hepatitis-related hepatocellular carcinoma. World J Gastroenterol. 2008;14:1652–1656. doi: 10.3748/wjg.14.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba T, et al. Identification and investigation of methylated genes in hepatoma. Eur J Cancer. 2005;41:1185–1194. doi: 10.1016/j.ejca.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Eads CA, et al. Fields of aberrant CpG island hypermethylation in Barrett's esophagus and associated adenocarcinoma. Cancer Res. 2000;60:5021–5026. [PubMed] [Google Scholar]
- Eads CA, et al. Epigenetic patterns in the progression of esophageal adenocarcinoma. Cancer Res. 2001;61:3410–3418. [PubMed] [Google Scholar]
- Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6:674–687. doi: 10.1038/nrc1934. [DOI] [PubMed] [Google Scholar]
- Feng Q, et al. DNA methylation in tumor and matched normal tissues from non-small cell lung cancer patients. Cancer Epidemiol Biomarkers Prev. 2008;17:645–654. doi: 10.1158/1055-9965.EPI-07-2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukai K, et al. Methylation status of p14ARF, p15INK4b, and p16INK4a genes in human hepatocellular carcinoma. Liver Int. 2005;25:1209–1216. doi: 10.1111/j.1478-3231.2005.01162.x. [DOI] [PubMed] [Google Scholar]
- Harder J, et al. Quantitative promoter methylation analysis of hepatocellular carcinoma, cirrhotic and normal liver. Int J Cancer. 2008;122:2800–2804. doi: 10.1002/ijc.23433. [DOI] [PubMed] [Google Scholar]
- Jicai Z, et al. Persistent infection of hepatitis B virus is involved in high rate of p16 methylation in hepatocellular carcinoma. Mol Carcinog. 2006;45:530–536. doi: 10.1002/mc.20188. [DOI] [PubMed] [Google Scholar]
- Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3:415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- Kaneto H, et al. Detection of hypermethylation of the p16(INK4A) gene promoter in chronic hepatitis and cirrhosis associated with hepatitis B or C virus. Gut. 2001;48:372–377. doi: 10.1136/gut.48.3.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh H, et al. Epigenetic instability and chromosomal instability in hepatocellular carcinoma. Am J Pathol. 2006;168:1375–1384. doi: 10.2353/ajpath.2006.050989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk GD, et al. Molecular epidemiology of human liver cancer: insights into etiology, pathogenesis and prevention from The Gambia, West Africa. Carcinogenesis. 2006;27:2070–2082. doi: 10.1093/carcin/bgl060. [DOI] [PubMed] [Google Scholar]
- Kitamura E, et al. Analysis of tissue-specific differentially methylated regions (TDMs) in humans. Genomics. 2007;89:326–337. doi: 10.1016/j.ygeno.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike K. Hepatocarcinogenesis in hepatitis viral infection: lessons from transgenic mouse studies. J Gastroenterol. 2002;37 Suppl 13:55–64. doi: 10.1007/BF02990101. [DOI] [PubMed] [Google Scholar]
- Koike K. Molecular basis of hepatitis C virus-associated hepatocarcinogenesis: lessons from animal model studies. Clin Gastroenterol Hepatol. 2005;3:S132–S135. doi: 10.1016/s1542-3565(05)00700-7. [DOI] [PubMed] [Google Scholar]
- Kwon GY, et al. Promoter methylation of E-cadherin in hepatocellular carcinomas and dysplastic nodules. J Korean Med Sci. 2005;20:242–247. doi: 10.3346/jkms.2005.20.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird PW. The power and the promise of DNA methylation markers. Nat Rev Cancer. 2003;3:253–266. doi: 10.1038/nrc1045. [DOI] [PubMed] [Google Scholar]
- Lee S, et al. Aberrant CpG island hypermethylation along multistep hepatocarcinogenesis. Am J Pathol. 2003;163:1371–1378. doi: 10.1016/S0002-9440(10)63495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann U, et al. Distinct methylation patterns of benign and malignant liver tumors revealed by quantitative methylation profiling. Clin Cancer Res. 2005;11:3654–3660. doi: 10.1158/1078-0432.CCR-04-2462. [DOI] [PubMed] [Google Scholar]
- Lehmann U, et al. Epigenetic defects of hepatocellular carcinoma are already found in non-neoplastic liver cells from patients with hereditary haemochromatosis. Hum Mol Genet. 2007;16:1335–1342. doi: 10.1093/hmg/ddm082. [DOI] [PubMed] [Google Scholar]
- Levrero M. Viral hepatitis and liver cancer: the case of hepatitis C. Oncogene. 2006;25:3834–3847. doi: 10.1038/sj.onc.1209562. [DOI] [PubMed] [Google Scholar]
- Li X, et al. p16INK4A hypermethylation is associated with hepatitis virus infection, age, and gender in hepatocellular carcinoma. Clin Cancer Res. 2004;10:7484–7489. doi: 10.1158/1078-0432.CCR-04-1715. [DOI] [PubMed] [Google Scholar]
- Mori T, et al. Decreased expression and frequent allelic inactivation of the RUNX3 gene at 1p36 in human hepatocellular carcinoma. Liver Int. 2005;25:380–388. doi: 10.1111/j.1478-3231.2005.1059.x. [DOI] [PubMed] [Google Scholar]
- Nagase H, Ghosh S. Epigenetics: differential DNA methylation in mammalian somatic tissues. Febs J. 2008;275:1617–1623. doi: 10.1111/j.1742-4658.2008.06330.x. [DOI] [PubMed] [Google Scholar]
- Narimatsu T, et al. p16 promoter hypermethylation in human hepatocellular carcinoma with or without hepatitis virus infection. Intervirology. 2004;47:26–31. doi: 10.1159/000076639. [DOI] [PubMed] [Google Scholar]
- Nishida N, et al. Aberrant methylation of multiple tumor suppressor genes in aging liver, chronic hepatitis, and hepatocellular carcinoma. Hepatology. 2008;47:908–918. doi: 10.1002/hep.22110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogino S, et al. Precision and performance characteristics of bisulfite conversion and real-time PCR (MethyLight) for quantitative DNA methylation analysis. J Mol Diagn. 2006;8:209–217. doi: 10.2353/jmoldx.2006.050135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih YL, et al. Promoter methylation of the secreted frizzled-related protein 1 gene SFRP1 is frequent in hepatocellular carcinoma. Cancer. 2006;107:579–590. doi: 10.1002/cncr.22023. [DOI] [PubMed] [Google Scholar]
- Shim YH, et al. p16 Hypermethylation in the early stage of hepatitis B virus-associated hepatocarcinogenesis. Cancer Lett. 2003;190:213–219. doi: 10.1016/s0304-3835(02)00613-4. [DOI] [PubMed] [Google Scholar]
- Shiratori Y, et al. Characteristic difference of hepatocellular carcinoma between hepatitis B- and C- viral infection in Japan. Hepatology. 1995;22:1027–1033. doi: 10.1016/0270-9139(95)90605-3. [DOI] [PubMed] [Google Scholar]
- Thomas MB, Zhu AX. Hepatocellular carcinoma: the need for progress. J Clin Oncol. 2005;23:2892–2899. doi: 10.1200/JCO.2005.03.196. [DOI] [PubMed] [Google Scholar]
- Thorgeirsson SS, et al. Molecular prognostication of liver cancer: end of the beginning. J Hepatol. 2006;44:798–805. doi: 10.1016/j.jhep.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Tsou JA, et al. Distinct DNA methylation profiles in malignant mesothelioma, lung adenocarcinoma, and non-tumor lung. Lung Cancer. 2005;47:193–204. doi: 10.1016/j.lungcan.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Virmani A, et al. Aberrant methylation of the cyclin D2 promoter in primary small cell, nonsmall cell lung and breast cancers. Int J Cancer. 2003;107:341–345. doi: 10.1002/ijc.11393. [DOI] [PubMed] [Google Scholar]
- Weber M, et al. Distribution, silencing potential and evolutionary impact of promoter DNA methylation in the human genome. Nat Genet. 2007;39:457–466. doi: 10.1038/ng1990. [DOI] [PubMed] [Google Scholar]
- Weisenberger DJ, et al. Analysis of repetitive element DNA methylation by MethyLight. Nucleic Acids Res. 2005;33:6823–6836. doi: 10.1093/nar/gki987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, et al. Aberrant promoter methylation profiles of tumor suppressor genes in hepatocellular carcinoma. Am J Pathol. 2003;163:1101–1107. doi: 10.1016/S0002-9440(10)63469-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YJ, et al. High frequency of promoter hypermethylation of RASSF1A and p16 and its relationship to aflatoxin B1-DNA adduct levels in human hepatocellular carcinoma. Mol Carcinog. 2002;35:85–92. doi: 10.1002/mc.10076. [DOI] [PubMed] [Google Scholar]
- Zhang YJ, et al. Silencing of glutathione S-transferase P1 by promoter hypermethylation and its relationship to environmental chemical carcinogens in hepatocellular carcinoma. Cancer Lett. 2005;221:135–143. doi: 10.1016/j.canlet.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Zhang YJ, et al. Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation and its relationship to aflatoxin B1-DNA adducts and p53 mutation in hepatocellular carcinoma. Int J Cancer. 2003;103:440–444. doi: 10.1002/ijc.10852. [DOI] [PubMed] [Google Scholar]
- Zhang YJ, et al. Aflatoxin B1 and polycyclic aromatic hydrocarbon adducts, p53 mutations and p16 methylation in liver tissue and plasma of hepatocellular carcinoma patients. Int J Cancer. 2006;119:985–991. doi: 10.1002/ijc.21699. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.