Abstract
Niemann-Pick Type C disease is an autosomal recessive neurodegenerative disorder with abnormal lipid storage as the major cellular pathologic hallmark. Genetic analyses have identified mutations in NPC1 gene in the great majority of cases, while mutations in NPC2 account for the remainders. Yet, little is known regarding the cellular mechanisms responsible for NPC pathogenesis, especially for neurodegeneration, which is the usual cause of death. To identify critical steps that could account for the pathological manifestations of the disease in one of the most affected brain structures, we performed global gene expression analysis in the cerebellum from three-week old Npc1+/+ and Npc1-/- mice with two different microarray platforms (Agilent and Illumina). Differentially-expressed genes identified by both microarray platforms were then subjected to KEGG pathway analysis. Expression of genes in six pathways was significantly altered in Npc1-/- mice; functionally, these signaling pathways belong to the following three categories: 1) steroid and terpenoid biosynthesis, 2) immune response, and 3) cell adhesion/motility. In addition, the expression of several proteins involved in lipid transport was significantly altered in Npc1-/- mice. Our results provide novel molecular insight regarding the mechanisms of pathogenesis in NPC disease and reveal potential new therapeutic targets.
Keywords: cell adhesion, cholesterol, gene expression, microarray, Niemann-Pick type C disease
Introduction
Niemann-Pick disease type C (NPC) is an autosomal recessive neurovisceral disorder with accumulation of unesterified cholesterol and glycolipids in late endosomal/lysosomal compartments as the cellular pathological hallmark. While it is widely recognized that the final cause of death in the disease is severe progressive brain degeneration, there is no overt cholesterol accumulation in brain (Schedin et al., 1995; Xie et al., 2000). The paradoxical lack of changes in brain cholesterol content in NPC might be due to the extensive demyelination of axons associated with the disease (Xie et al., 2000). On the other hand, it may also reflect our lack of understanding of the mechanisms underlying NPC-type neurodegeneration. Genetic studies have demonstrated that the etiology of the disease resides in mutations in two genes, NPC1 and NPC2, with mutations in the former accounting for 95% of cases (Carstea et al., 1997) and in the latter for 5% (Naureckiene et al., 2000). Mutations in either gene result in NPC phenotype suggesting that both genes are necessary for maintaining cholesterol homeostasis, possibly by facilitating cholesterol efflux from the endosomal/lysosomal system [see (Storch and Xu, 2009) for a recent review]. How these two proteins regulate cholesterol egress from this system is largely unknown despite a decade-long intensive research. Furthermore, NPC1 and NPC2 may have broader functions, as a recent report indicated that copper secretion from hepatoma cells was also NPC1-dependent (Yanagimoto et al., 2009).
Recent research has made tremendous progress at characterization of the pathological features of the disease, especially in the central nervous system. Several hypotheses have emerged regarding the molecular/cellular mechanisms underlying NPC pathogenesis. For instance, abnormal activity of autophagic/lysosomal systems, which are closely associated with cholesterol accumulation in the endosomal/lysosomal system, has been implicated in NPC pathogenesis (Bi and Liao, 2007; Pacheco et al., 2007). Early onset inflammation, mediated by microglia and astrocytes, may also contribute to the progression of the disease (Baudry et al., 2003; German et al., 2001). Because a single systemic injection of the neurosteroid allopregnanolone with cyclodextrin in Npc1-/- mice at postnatal day 7 significantly delayed the onset of the disease and prolonged mice survival, abnormal neurosteroid synthesis has also been implicated in NPC progressive neurodegeneration (Ahmad et al., 2005; Griffin et al., 2004; Liao et al., 2009). Considering the complexity of the disease, it is not surprising that several mechanisms might be involved.
Microarray analysis designed to provide a genome-wide view of gene expression has proven to be a powerful tool for elucidating the etiology and pathogenesis of a number of complex diseases, including psychiatric and neurodegenerative disorders (Kadish et al., 2009; Rollins et al., 2009; Sutherland et al., 2009). However, very few microarray studies have been applied to the understanding of NPC pathology. Two studies have performed gene expression profiling in NPC fibroblasts (De Windt et al., 2007; Reddy et al., 2006). While they provided important information regarding features of NPC, they were not targeted at brain tissues. Another study profiled gene expression in primary murine cortical neuronal cultures with NPC phenotype elicited by treatment with the cholesterol transport inhibitor, U18666A (Koh et al., 2007); however, there is evidence that U18666A-induced phenotype differs in several aspects from that found in NPC (Karten et al., 2003). To further elucidate the molecular basis of neurodegeneration elicited by NPC1 mutation, we performed global gene expression analysis of cerebellum, the most affected brain structure with the earliest onset in Npc1-/- mice (Ong et al., 2001), from 3 week-old Npc1-/- mice and their littermate wild-type controls using two different microarray platforms in combination with functional signaling pathway analysis.
Methods and Materials
Animals
Npc1NIH(BALB/cNctr-Npc1m1N/J) heterozygous male and female mice on BALB/c background were purchased from Jackson Laboratory (Bar Harbor, MN) and housed in the vivarium under protocols approved by the local IACUC. Mice had free access to water and food; Npc1-/- mice were produced by in-house breeding and were genotyped using tail DNA with PCR methods as previously described (Baudry et al., 2003).
RNA isolation
Cerebelli were collected from 8 mice (4 Npc1+/+mice, and 4 Npc1-/- mice) at 3 weeks postnatal and were snap-frozen in liquid nitrogen. Total RNA was isolated with TRIzol reagent (Invitrogen, Carlsbad, CA) and purified with RNeasy Mini Kit according to the manufacturer instructions (Qiagen, Valencia, CA). RNA was quantified by measuring optical density at 260 nm using a spectrophotometer (Beckman, Fullerton, CA), and the quality of RNA was assessed using a 2% agarose gel stained with ethidium bromide.
Microarray gene expression analysis
Gene expression profiling for the same set of 8 RNA samples was performed using microarray platforms from Illumina (San Diego, CA) and Agilent (Santa Clara, CA). For the Illumina platform, we used the multi-sample format MouseWG-6 BeadChip with over 46,000 probes simultaneously profiling six samples on a single chip, and the microarray data were collected at the Microarray Core Facility of the University of Texas Southwestern Medical Center (Dallas, TX), as previously described (Guo et al., 2009). Briefly, 200 ng of total RNA was labeled using a MessageAmp II-biotin enhanced kit (Applied Biosystems, Foster City, CA). Double stranded cDNA was synthesized using T7-oligo (dT) primers followed by an in vitro transcription (IVT) reaction to amplify antisense-RNA (aRNA), while biotin was incorporated into the synthesized aRNA target. The biotinylated cRNA target was hybridized to the MouseWG-6 BeadChip. Hybridization, washing, and scanning were performed according to the manufacturer's instructions with the addition of a 10-min washing in high-temp wash buffer (Illumina) at 55 °C in a Hybex Microarray Incubation System (SciGene Corportaion, Sunnyvale, CA) following the overnight hybridization. The chips were scanned using a BeadScan 2.3.0.10 (Illumina) at a multiplier setting of “2.” The microarray images were registered and gene expression data were extracted automatically according to the manufacturer's default settings. After background subtraction, raw microarray intensity data were normalized using the cubic spline normalization method (Illumina). Intensity data were then adjusted to set the minimum intensity of an array equal to 1; this converts every data point into a positive value and allows for logarithmic transformation of the data before statistical analysis (Shi et al., 2006).
For the Agilent platform, the Mouse Genome microarrays with 45,018 spots were used and microarray data were collected at Cogenics (Morrisville, NC). The quantity of each of the total RNA samples was determined by spectrophotometry and the size distribution was assessed using an Agilent Bioanalyzer. Briefly, 500 ng of total RNA was converted into labeled cRNA with nucleotides coupled to the fluorescent dye Cy3 using the Low RNA Input Linear Amplification Kit (Agilent Technologies, Palo Alto, CA) following the manufacturer's protocol. Cy3-labeled cRNA (1.65 μg) from each sample was hybridized to an Agilent Mouse Genome microarrays in a 4x44k format, i.e., four samples were simultaneously processed on one chip. The hybridized chip was then washed and scanned. Intensity data were extracted from the scanned image with background subtracted and normalized using Feature Extraction 9.5 (Agilent Technologies) followed by global scaling so that the median intensity for each array is 1000.
Microarray data analysis and statistical analysis
Gene expression data from the Illumina and Agilent platforms were imported to ArrayTrack, a software system developed by the U.S. Food and Drug Administration's National Center for Toxicological Research for the management, analysis, visualization and interpretation of microarray data (http://www.fda.gov/ScienceResearch/BioinformaticsTools/Arraytrack/)(Fang et al., 2009; Tong et al., 2003). Statistical testing and clustering analysis were conducted within ArrayTrack. Additional calculations were performed within JMP 7 (SAS Institute, Cary, NC). For each probe, log2-transformed intensity data were used in a two-sample t-test to obtain a p value and a fold change (FC). Genes with a fold change ≥ 1.3 and between groups p <0.05 were considered to be differentially-expressed genes (DEGs). This simple gene selection method, which combines a p-value cutoff and a fold-change ranking, was demonstrated to result in higher concordance degree of DEGs between different microarray platforms or test laboratories, as compared to genes selected solely based on p-value ranking (Canales et al., 2006; Guo et al., 2006; Shi et al., 2006), and was therefore recommended by previous studies (Guo et al., 2006; Pedotti et al., 2008; Shi et al., 2006; Shi et al., 2008). DEGs were used in the KEGG pathway significance analysis with Fisher test, and those with p values <0.05 were considered significantly altered, i.e., enriched with genes on the list of DEGs. For hierarchical clustering, we used the DEGs identified by both platforms (154 genes from comparison of Npc1-/- mice with Npc1+/+ mice), and the log2 intensity data were Z-score transformed on a per gene basis. That is, for each of the 154 genes in the cluster, the average of the log2 intensity values across the 8 samples (mice) was subtracted from each of the log2 intensity values and the results were then divided by the standard deviation of the mean of the 8 log2 intensity values for the gene. The Z-score values were then hierarchically clustered using Euclidean distance metric and average linkage. Each column represents the gene expression profile across the 154 genes from a particular mouse and each row represents the gene expression profile (Z-scores) for a gene across the 8 mice.
Real-time Quantitative Reverse Transcription-PCR
Total RNA (1-2 μg) was reversed transcribed into cDNA with oligo (dT) using SuperScript III first-strand synthesis system according to the manufacturer instruction (Invitrogen). cDNA was amplified by primer pairs specific for Icam1 (5’-AGC ACC TCC CCA CCT ACT TT-3’ forward, 5’-AGC TTG CAC GAC CCT TCT AA-3’ reverse), Cxcl16 (5’-GGG AAG AGT TTT CAC CAC CA-3’ forward, 5’-GGT TGG GTG TGC TCT TTG TT-3’ reverse), Ifitm1 (5’-CTT CAA AAG CCG AGA GAT GC-3’forward, 5’-TCA GGC ATG TTG ATT GTG GT-3’reverse), Fdps (5’- TCC AGG TCC AGG ACG ACT AC-3’forward, 5’- CGC CTC ATA CAG TGC TTT CA-3’ reverse), Cd86 (5’- CAG TGC TGG CAA ATC AAG AA-3’ forward, 5’- TTG CAC AGC ATT CTC CAG AC-3’ reverse), Mag (5’- GGA CCC CAT CCT TAC CAT CT-3’ forward, 5’-AGG ATT ATG GGG GCA AAC TC-3’ reverse), Vav1 (5’- CTA CGG GAT CTG CTG ATG GT-3’ forward, 5’- CTG CCG TAG GGT TTC ATT GT-3’ reverse), and Itgb7 (5’- CCT ACG ACT CTG GGC TCT TG-3’ forward, 5’- ACA GGT CAG CCT CAG AGC AT-3’ reverse). GAPDH (5’- AAC TTT GGC ATT GTG GAA GG-3’forward, 5’- ACA CAT TGG GGG TAG GAA CA-3’reverse) was used as internal control. Quantitative real-time PCR was done with Applied Biosystems 7300 real-time PCR system (Foster City, CA) using SYBR-Green. Each sample was analyzed in triplicate and the relative quantification of gene expression was performed with the comparative cycle number measured with the threshold (CT) method.
Immunoblotting
One-month-old mice were sacrificed by decapitation and cerebelli were dissected out on ice and immediately frozen. Total protein was extracted with Tris lysis buffer (50 mM Tris-HCl, pH 7.4, 1 mM EDTA, 1 mM EGTA, protease inhibitor cocktail, phosphatase inhibitor Cocktails 1 and 2). Protein aliquots (40 μg) were denatured and separated by electrophoresis on 6-14% SDS-polyacrylamide gels and transferred to PVDF membranes (GE Healthcare, UK). After blocking, membranes were incubated overnight at 4 °C with goat anti-ApoE antibody (1:1000, Santa Cruz Technology, Santa Cruz, CA) or anti-GAPDH antibody (1:10,000, Chemicon, Carlsbad, CA; used as a loading control). Membranes were then washed and incubated with HRP-conjugated secondary antibodies, and immunoreactivity was visualized by using enhanced chemiluminescence (ECL plus kit and reagents; GE Healthcare). Labeled bands were scanned and quantitatively analyzed using NIH ImageJ software. Mean densities from different groups were shown as means ± SEM following normalization with GAPDH band intensity and were compared using one-way ANOVA.
Results
Differential gene expression in cerebellum of Npc1-/- mice
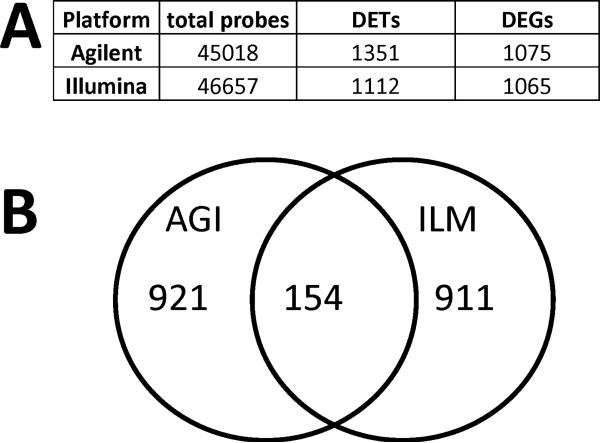
Previous studies showed that the most severe neuropathology was first observed in the cerebellum in Npc1-/- mice (Ong et al., 2001); therefore cerebellum tissues from 3-week old Npc1-/- mice and their wildtype littermates were used in the present study. A large number of genes were found to be differentially expressed in cerebellum of Npc1-/- mice, as compared with Npc1+/+ mice by either array platform. With the Agilent platform, 1,351 transcripts were differentially expressed (Fig. 1A), whereas with the Illumina platform, 1,112 transcripts were differentially expressed. Because one gene may have several transcripts, the corresponding number of differentially expressed genes (DEGs) was 1,075 with the Agilent platform and 1,065 with the Illumina one (Fig. 1A). Among these DEGs, 154 genes were common to both platforms (Fig. 1B).
Figure 1. Differentially expressed genes identified by Agilent and Illumina platforms.
A. Total differentially expressed genes (DEGs) identified by Agilent and Illumina platforms. DEGs are genes with absolute fold change ≥1.3 and p <0.05 in Npc1-/- mice as compared with Npc1+/+ mice. B. 154 DEGs were identified by both Agilent and Illumina platforms. AGI, Agilent; ILM, Illumina. The animals used in both Agilent and Illumina platforms consisted of 4 wild-type and 4 Npc1-/- mice.
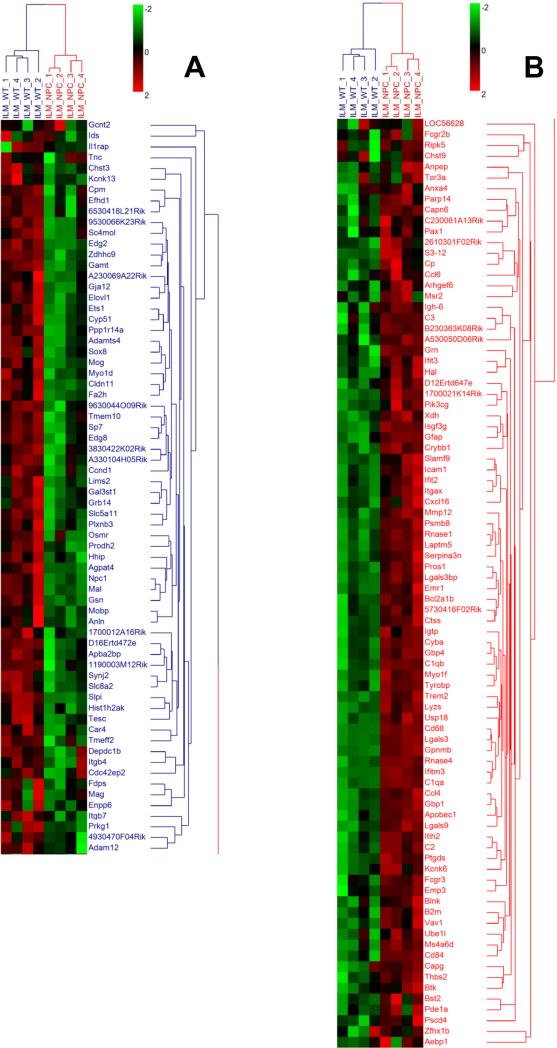
Hierarchical cluster analysis using the 154 DEGs showed that the 8 mice were clustered into two groups that corresponded to the two experimental groups: Npc1+/+ and Npc1-/- (Fig. 2), indicating that the gene expression profile of Npc1-/- mice was clearly separated from that of Npc1+/+ mice. Among these DEGs, 67 genes were down-regulated (colored in blue) and 87 genes were up-regulated (colored in red) in Npc1-/- mice as compared with Npc1+/+ mice.
Figure 2. Hierarchical clustering of differentially expressed genes.
Gene clustering was based on the Z-scores obtained in the expression profiles of the 154 DEGs in Npc1-/- (NPC) and Npc1+/+ (WT) mice. In the color-coded map, a red color indicates a higher than average expression and a green color a lower than average expression across all 8 mice. A: genes down-regulated in Npc1-/- (NPC) mice; B: genes up-regulated in Npc1-/- (NPC) mice. See Methods section for details.
Gene expression changes in 6 common KEGG pathways in cerebellum from Npc1-/- mice
To further analyze the functional significance of changes in gene expression, DEGs identified by both platforms were separately mapped to the KEGG (Kyoto Encyclopedia of Genes and Genomes) signaling pathways using the FDA's ArrayTrack software. DEGs identified by the Agilent microarray were mapped to 15 pathways and those identified by the Illumina platform were included in 9 pathways (Table 1). Mapping of the 154 DEGs identified by both platforms revealed 6 common KEGG pathways with significantly altered gene expression in Npc1-/- mice as compared with Npc1+/+ mice (Fisher test, Table 2). Detailed changes in these pathways are described in the following sections.
Table 1.
Significantly changed KEGG pathways identified by the Agilent and illumina platforms
| Fisher P value |
|||
|---|---|---|---|
| Map title | Category | AGI* | ILMΔ |
| Complement and coagulation | |||
| cascades(mmu04610)*Δ | Regulatory pathway | 0.000 | 0.002 |
| Biosynthesis of steroids(mmu00100)*Δ | Energy Metabolism/Metabolic pathway | 0.000 | 0.001 |
| B cell receptor signaling pathway(mmu04662)*Δ | Regulatory pathway | 0.001 | 0.038 |
| ECM-receptor interaction(mmu04512)* | Regulatory pathway | 0.001 | 0.055 |
| Cell Communication(mmu01430)* | Regulatory pathway | 0.002 | 0.076 |
| Leukocyte transendothelial migration(mmu04670)*Δ | Regulatory pathway | 0.005 | 0.019 |
| Cytokine-cytokine receptor interaction(mmu04060)* | Regulatory pathway | 0.007 | 0.084 |
| Focal adhesion(mmu04510)* | Regulatory pathway | 0.009 | 0.232 |
| Glycosaminoglycan degradation(mmu00531)* | Metabolism of Other Amino Acids/Metabolic pathway | 0.011 | - |
| Hedgehog signaling pathway(mmu04340)* | Regulatory pathway | 0.012 | 0.494 |
| Natural killer cell mediated cytotoxicity(mmu04650)* | Regulatory pathway | 0.017 | 0.166 |
| Glycan structures - degradation(mmu01032)* | Regulatory pathway | 0.031 | 0.494 |
| Terpenoid biosynthesis(mmu00900)*Δ | Metabolism of Cofactors and Vitamins/Metabolic pathway | 0.045 | 0.022 |
| Fc epsilon Rl signaling pathway(mmu04664)* | Regulatory pathway | 0.045 | 0.174 |
| Aminosugars metabolism(mmu00530)* | Metabolism/Metabolic pathway | 0.047 | - |
| Cell adhesion molecules (CAMs)(mmu04514)Δ | Regulatory pathway | 0.094 | 0.005 |
| Nitrogen metabolism(mmu00910)Δ | Carbohydrate Metabolism/Metabolic pathway | 0.172 | 0.015 |
| Type I diabetes mellitus(rnmu04940)Δ | Regulatory pathway | 0.264 | 0.028 |
| Antigen processing and presentation(mmu04612)Δ | Regulatory pathway | 0.550 | 0.049 |
significantly changed pathway identified by the Agilent (AGI) platform
significantly changed pathway identified by the Illumina (ILM) platform.
Table 2.
Common KEGG pathways identified by both the Agilent and Illumina platforms using the common DEGs from these two platforms.
| KEGG pathway | Size* | AGI | DEGs | ILM | DEGs |
|---|---|---|---|---|---|
| Complement and coagulation cascades | 71 | 0.000 | 17 | 0.002 | 7 |
| B cell receptor signaling pathway | 65 | 0.000 | 12 | 0.038 | 7 |
| Biosynthesis of steroids | 23 | 0.002 | 6 | 0.001 | 6 |
| Leukocyte transendothelial migration | 115 | 0.003 | 15 | 0.019 | 10 |
| Terpenoid biosynthesis | 5 | 0.035 | 2 | 0.022 | 2 |
| Cell adhesion molecules (CAMs) | 156 | 0.036 | 14 | 0.005 | 15 |
Size: number of total genes in each pathway.
Reduced expression of genes involved in steroid/terpenoid biosynthesis in cerebellum of Npc1-/- mice
Among the 23 genes included in the KEGG steroid biosynthesis pathway, 4 DEGs were identified as down-regulated by both the Agilent and Illumina platforms (cytochrome P450, family 51(Cyp51), farnesyl diphosphate synthetase (Fdps), mevalonate kinase (Mvk), and sterol-C4-methyloxidase-like (Sc4mol)) (Table 3). The Agilent microarray identified two additional genes (24-dehydrocholesterol reductase (Dhcr24), and idopentenyl-diphosphate delta isomerase (Idil)) whose expression was significantly reduced in NPC1-/- mice; the Illumina microarray identified two other genes (mevalonate decarboxylase (Mvd) and squalene epoxidase (Sqle)) with reduced expression in NPC1-/- mice (Table 3). Fdps, Idil, and Sqle are also key genes in terpenoid biosynthesis, a pathway known to be significantly altered in Npc1-/- mice (Schedin et al., 1998) (marked gray in Table 3). The total overlapping rate of the DEGs between the two platforms is 50% (4/8) for the steroid biosynthesis pathways and 33.3% (1/3) for the terpenoid biosynthesis pathway. Down-regulation of these genes in Npc1-/- mice indicates potential dysfunction of these two pathways.
Table 3.
DEGs in steroid and terpenoid biosynthesis pathway in Npc1−/− mice.
| AGI* |
ILMΔ |
|||||
|---|---|---|---|---|---|---|
| Gene symbol | Gene name | Description | FC | P | FC | P |
| NM_020010 | Cyp51*Δ | cytochrome P450, family 51 | −1.5 | 0.026 | −1.4 | 0.023 |
| NM_134469 | Fdps*Δ | farnesyl diphosphate synthetase | −1.4 | 0.042 | −1.3 | 0.045 |
| NM_023556 | Mvk*Δ | mevalonate kinase | −1.4 | 0.039 | −1.4 | 0.005 |
|
NM_025436 |
Sc4mol*Δ |
sterol-C4-methyl oxidase-like |
−1.5 |
0.013 |
−1.3 |
0.021 |
| AK129036 | Dhcr24* | 24-dehydrocholesterol reductase | −1.3 | 0.047 | −1.3 | 0.134 |
| NM_177960 | Idi1* | isopentenyl-diphosphate delta isomerase | −1.5 | 0.031 | −1.3 | 0.072 |
| NM_138656 | MvdΔ | mevalonate (diphospho) decarboxylase | −1.3 | 0.103 | −1.5 | 0.010 |
| NM_009270 | SqleΔ | squalene epoxidase | −1.3 | 0.129 | −1.4 | 0.034 |
| NM_010191 | Fdftl | farnesyl diphosphate farnesyl transferase 1 | −1.2 | 0.106 | −1.3 | 0.118 |
DEGs identified by Agilent (AGI) platform
DEGs identified by Illumina (ILM) platform.
Genes also belonging to the terpenoid biosynthesis pathway are indicated in gray.
Altered expression of genes involved in cholesterol/lipid transporters in cerebellum of Npc1-/- mice
We next examined the expression of genes encoding lipid transporters or associated proteins, using the “mouse lipoprotein signaling and cholesterol metabolism pathway” (SABiosciences Corporation, Frederick, MD) as a platform. Among the genes evaluated, three genes were significantly up-regulated in Npc1-/- mice (Table 4): chemokine (C-X-C motif) ligand 16 (Cxcl16), apolipoprotein D (ApoD), and apolipoprotein E (ApoE). Increase in ApoD mRNA level in cerebellum of Npc1-/- mice as compared to Npc1+/+ mice is consistent with previously reported results (Yoshida et al., 1996). The overlapping rate of DEGs between the two platforms in this pathway is 40%. In addition, analyses with the Agilent platform showed an increase in the expression of Stabilin 1 (Stab1) and zinc finger MYND-type containing 15 (Zmynd15) genes, while results from the Illumina platform indicated an increased expression of very low density lipoprotein receptor (Vldlr) and cytochrome P450 family 7 subfamily b polypeptide 1 (Cyp7b1) genes.
Table 4.
DEGs in lipid transport and cholesterol metabolism pathway in Npc1−/− mice.
| AGI* |
ILMΔ |
|||||
|---|---|---|---|---|---|---|
| Gene symbol | Gene name | Description | FC | P | FC | P |
| NM_007470 | Apod*Δ | apolipoprotein D | 1.4 | 0.003 | 1.3 | 0.009 |
| NM_023158 | Cxcl16*Δ | chemokine (C-X-C motif) ligand 16 | 2.0 | 0.005 | 1.6 | 0.028 |
| NM_009696 | Apoe*Δ | apolipoprotein E | 1.3 | 0.013 | 1.3 | 0.002 |
|
NM_145942 |
Hmgcs1*Δ |
3-hydroxy-3-methylglutaryl-Coenzyme A synthase 1 |
−1.4 |
0.045 |
−1.3 |
0.030 |
| NM_153565 | Pcsk9* | proprotein convertase subtilisin/kexin type 9 | −1.6 | 0.045 | −1.1 | 0.536 |
| NM_138672 | Stab1* | stabilin 1 | 1.3 | 0.000 | 1.0 | 0.900 |
| NM_013703 | VldlrΔ | very low density lipoprotein receptor | −1.2 | 0.287 | 1.5 | 0.033 |
| NM_001029929 | Zmynd15* | zinc finger, MYND-type containing 15 | 1.8 | 0.036 | N.A. | N.A. |
| NM_007825 | Cyp7b1Δ | cytochrome P450, family 7, subfamily b, polypeptide 1 | 1.2 | 0.170 | 1.3 | 0.009 |
| NM_133748 | Insig2* | insulin induced gene 2 | −2.0 | 0.004 | −1.0 | 0.874 |
Several genes in this pathway were also found to be down-regulated in Npc1-/- mice. Among these, 3-hydro-3methylglutaryl-coenzyne A synthase 1 (Hmgcs1) was identified by both microarray platforms, while protein convertase subtilisin-kexin like-9 (Pcsk9) and insulin-induced gene 2 (Insig2) were detected only by the Agilent platform (Table 4). Pcsk9, a protease belonging to the mammalian proprotein convertase family, has recently been reported to degrade LDL receptor, very low density lipoprotein (VLDL) receptors, and the ApoE receptor 2 (Poirier et al., 2008). Whether down-regulation of Pcsk9 contributes to the increase in LDL receptor Cxcl16, and cholesterol transporters ApoD and ApoE is an interesting question that remains to be determined.
Altered expression of genes in cell adhesion pathway in cerebellum of Npc1-/- mice
Mapping DEGs from both Agilent and Illumina platforms to KEGG pathways identified cell adhesion molecule pathway as another significantly altered signaling pathway in Npc1-/- mice. Among the 154 genes analyzed, expression of 7 genes in this pathway was statistically modified in Npc1-/- mice (Table 5), with 3 being significantly up-regulated [CD86 antigen (Cd86), histocompatililty 2, T region locus 23 (H2-T23) and intercellular adhesion molecule (Icam1)], and 4 down-regulated [claudin 11 (Cldn11), claudin 14 (Cldn14), intergrin beta 7 (Itgb7), and myelin-associated glycoprotein (Mag)]. The overlapping rate is 31.8% (7 genes in total 22 genes) for the DEGs detected by either AGI or ILM platform. Additionally, 7 genes were identified as significantly modified by the Agilent platform [up-regulated for CD22 antigen (Cd22), histocompatibility 2, blastocyst (H2-Bl), histocompatibility 2, K1, K region (H2-K1), integrin beta 2 (Itgb2), and protein tyrosine phosphatase, receptor type C (Ptprc), and down-regulated for CD28 antigen (Cd28), junction adhesion molecule 3 (Jam3)], and another 7 genes by the Illumina platform [up-regulated for 4930468A15Rik, claudin 3 (Cldn3), histocompatibility 2, Q region locus 6 (H2-Q6), histocompatibility 2, Q region locus 8 (H2-Q8) and myelin protein zero-like 1 (Mpzl1), and down-regulated for histocompatibility 2, O region beta locus (H2-Ob) and sialophorin (Spn)] (Table 5).
Table 5.
DEGs in cell adhesion molecule (CAM) pathway in Npc1−/− mice.
| AGI* |
ILMΔ |
|||||
|---|---|---|---|---|---|---|
| Gen symbol | Gene name | Description | FC | P | FC | P |
| NM_010388 | Cd86*Δ | CD86 antigen | 1.8 | 0.016 | 1.5 | 0.015 |
| NM_008770 | Cldn11*Δ | claudin 11 | −1.5 | 0.020 | −1.3 | 0.004 |
| NM_019500 | Cldn14*Δ | claudin 14 | −1.4 | 0.046 | −1.4 | 0.013 |
| NM_010398 | H2-T23*Δ | histocompatibility 2, T region locus 23 | 1.3 | 0.021 | 1.5 | 0.001 |
| BC008626 | Icam1*Δ | intercellular adhesion molecule | 2.5 | 0.001 | 1.8 | 0.003 |
| NM_013566 | Itgb7*Δ | integrin beta 7 | −1.6 | 0.013 | −15 | 0.032 |
|
NM_010758 |
Mag*Δ |
myelin-associated glycoprotein |
−1.5 |
0.006 |
−1.4 |
0.022 |
| XM_135954 | 4930468A15RikΔ | RIKEN cDNA4930468A15 gene | N.A. | N.A. | 1.6 | 0.000 |
| NM_009845 | Cd22* | CD22 antigen | 7.5 | 0.000 | N.A. | N.A. |
| NM_007642 | Cd28* | CD28 antigen | −18 | 0.018 | N.A. | N.A. |
| NM_009902 | Cldn3Δ | claudin 3 | N.A. | N.A. | 1.7 | 0.005 |
| NM_008199 | H2-Bl* | histocompatibility 2, blastocyst | 1.9 | 0.012 | N.A. | N.A. |
| NM_010380 | H2-D1Δ | histocompatibility 2, D region locus 1 | N.A. | N.A. | 1.9 | 0.002 |
| BC080756 | H2-K1* | histocompatibility 2, K1, K region | 1.5 | 0.016 | N.A. | N.A. |
| NM_010389 | H2-ObΔ | histocompatibility 2, O region beta locus | N.A. | N.A. | −16 | 0.014 |
| NM_207648 | H2-Q6Δ | histocompatibility 2, Q region locus 6 | N.A. | N.A. | 1.5 | 0.030 |
| NM_023124 | H2-Q8Δ | histocompatibility 2, Q region locus 8 | N.A. | N.A. | 1.3 | 0.006 |
| NM_008404 | Itgb2* | integrin beta 2 | 2.0 | 0.002 | N.A. | N.A. |
| NM_023277 | Jam3* | junction adhesion molecule 3 | −1.4 | 0.013 | −1.2 | 0.218 |
| XM_129565 | Mpzl1Δ | myelin protein zero-like 1 | N.A. | N.A. | 1.3 | 0.029 |
| NM_011210 | Ptprc* | protein tyrosine phosphatase, receptor type, C | 2.0 | 0.015 | −1.2 | 0.503 |
| AK041480 | SpnΔ | sialophorin | N.A. | N.A. | −1.4 | 0.022 |
Abnormal expression of genes involved in cell motility and inflammation responses in cerebellum of Npc1-/- mice
Several genes involved in either cell motility or immune/inflammation function were identified as differentially expressed in cerebellum of mutant mice by both platforms. These include 5 genes [B-cell linker (Blnk), Bruton agammaglobulinemia tyrosine kinase (Btk), Fc receptor, IgG, low affinity IIb (Fcgr2b), interferon induced transmembrane protein 1(Ifitm1), and vav 1 oncogene (Vav1)] from the B cell receptor signaling pathway (out of 65 genes analyzed), 5 genes [complement component 1, q subcomponent alpha polypeptide (C1qa), complement component 1, q subcomponent beta polypeptide (C1qb), complement component 2 (C2), complement component 3 (C3) and serine (or cysteine) peptidase inhibitor clade G member 1 (Serping1)] from the complement and coagulation cascades (among 71 genes), and 5 genes [Cldn11, Cldn14, cytochrome b-245 alpha polypeptide (Cyba), Icam1, and Vav1] involved in leukocyte transendothelial migration (out of 115 genes) (Table 6). Expression of most of the genes in these pathways was up-regulated, except for Cldn11 and Cldn14 in the leukocyte transendothelial migration pathway.
Table 6.
DEGs in immune signaling pathways in Npc1−/− mice
| AGI* |
ILMΔ |
|||||
|---|---|---|---|---|---|---|
| Gene symbol | Gene name | Description | FC | P | FC | P |
| B cell receptor signaling pathway | ||||||
| NM_008528 | Blnk*Δ | B-cell linker | 2.4 | 0.000 | 1.6 | 0.027 |
| NM_013482 | Btk*Δ | Bruton agammaglobulinemia tyrosine kinase | 2.0 | 0.008 | 1.3 | 0.037 |
| NM_010187 | Fcgr2b*Δ | Fc receptor, IgG, low affinity IIb | 2.5 | 0.000 | 1.6 | 0.000 |
| NM_026820 | Ifitm1*Δ | interferon induced transmembrane protein 1 | 1.5 | 0.001 | 1.8 | 0.001 |
|
NM_011691 |
Vav1*Δ |
vav1 oncogene |
2.2 |
0.005 |
1.4 |
0.005 |
| complement and coagulation cascades pathway | ||||||
| NM_007572 | C1qa*Δ | complement component 1, q subcomponent, alpha polypeptide | 2.3 | 0.000 | 1.9 | 0.000 |
| NM_009777 | C1qb*Δ | complement component 1, q subcomponent, beta polypeptide | 2.2 | 0.001 | 2.3 | 0.001 |
| NM_013484 | C2*Δ | complement component 2 (within H-2S) | 2.3 | 0.000 | 1.9 | 0.001 |
| NM_009778 | C3*Δ | complement component 3 | 2.9 | 0.001 | 1.3 | 0.036 |
|
NM_009776 |
Serping1*Δ |
serine (or cysteine) peptidase inhibitor, clade G, member 1 |
1.3 |
0.010 |
1.4 |
0.015 |
| Leukocyte transendothelial migration pathway | ||||||
| NM_008770 | Cldn11*Δ | claudin 11 | −1.5 | 0.020 | −1.3 | 0.004 |
| NM_019500 | Cldn14*Δ | claudin 14 | −1.4 | 0.046 | −1.4 | 0.013 |
| NM_007806 | Cyba*Δ | cytochrome b-245, alpha polypeptide | 1.7 | 0.006 | 1.7 | 0.001 |
| BC008626 | Icam1*Δ | intercellular adhesion molecule | 2.5 | 0.001 | 1.8 | 0.003 |
| NM_011691 | Vav1*Δ | vavl oncogene | 2.2 | 0.005 | 1.4 | 0.005 |
Validation of microarray data by quantitative real-time PCR and immunoblotting analyses
To validate the microarray data, mRNA and protein levels of some of the aforementioned DEGs were analyzed by either quantitative real-time PCR or immunoblotting, respectively. Nine DEGs from the six KEGG pathways with a fold-change from 1.3 to 2.5 or -1.4 to -1.6 were chosen for the validation. Quantitative real-time PCR results (Fig. 3A) confirmed the microarray data for both up-regulation and down-regulation of the selected DEGs; one DEG, ApoE was validated by immunoblotting and the results were in agreement with the gene array analysis (Fig. 3B).
Figure 3. Verification of microarray data with quantitative real-time PCR and immunoblotting.
A. Quantitative real-time PCR analysis of gene expression in the cerebellum of Npc1-/- mice compared with Npc1+/+ mice. Shown are means + SEM for mRNA levels relative to that of Npc1+/+ mice (values >1 indicate increased expression while those <1 represent decreased expression). N=4 for each group. * p<0.05, ** p<0.01. GAPDH mRNA was used as endogenous control. B. Immunoblots of ApoE and GAPDH (used as loading control) with samples from cerebellum of Npc1+/+ and Npc1-/- mice confirmed that ApoE levels were significantly increased in cerebellum from Npc1-/- mice (n=4, p <0.05).
Discussion
Genetic studies have revealed that impairment in intracellular cholesterol/lipid trafficking in NPC results from mutations in NPC1 and NPC2 genes (Millard et al., 2005; Millat et al., 2001; Ribeiro et al., 2001; Watari et al., 2000). However, the mechanisms underlying neurodegeneration in Npc1-/- mice or in human NPC patients are still largely unknown. In the present study, both Agilent and Illumina microarray platforms identified a large number of genes with significantly altered expression in Npc1-/- mice as compared to Npc1+/+ mice. However, only a small fraction (around 14%) of these genes was identified by both platforms. Several factors may contribute to the discordance, including different sensitivity between the two platforms and different protocols and different array designs, etc. Another important factor that contributed to the relatively low cross-platform concordance in terms of DEGs is the relatively small magnitude of differential gene expression (i.e., fold change) that was observed in this study, consistent with what has been appreciated when brain samples are used in microarray analysis (Miller et al., 2004). It is also noteworthy that in the present study animals were used before the onset of overt neurological symptoms or neuropathology. To increase the reliability of our results and to strengthen the biological functional interpretation of the DEGs, we chose to focus on genes identified as differentially expressed by both platforms. We note that it is therefore possible that our data under-estimate the number of genes with altered expression in Npc1-/- mice, but the subset of DEGs identified by both platforms should be less likely false positives. It is worth mentioning that the level of overlap (~14%) of the DEG lists between the two platforms is much higher than that (~2.5%) expected by randomly selecting the same number of genes from the two platforms (P < 0.05) (Shi et al., 2005). Importantly, KEGG pathway enrichment analysis of the 154 common DEGs revealed 6 signaling pathways that are either involved in cholesterol homeostasis maintenance, cell adhesion and motility regulation, or immune/inflammation responses. The overlapping rates of the DEGs detected by both microarray platforms were in the range of 25% to 50% for the 6 signaling pathways, indicating the higher level of validation by pathway analysis than by individual gene analysis. The biological significance of these findings for NPC pathogenesis is discussed in the following sections.
Reduced gene expression in sterol biosynthesis pathway in cerebellum of Npc1-/- mice
Cholesterol is an essential membrane component and plays important roles in maintaining normal brain function (Korade and Kenworthy, 2008). Unlike peripheral tissues that obtain cholesterol from both de novo synthesis within the cells and uptake of cholesterol-containing lipoprotein particles from serum, brain acquires cholesterol predominantly from local synthesis (Turley et al., 1996). Animal studies have indicated that during early development neurons rely heavily on de novo cholesterol synthesis (Jurevics et al., 1997; Turley et al., 1996), whereas uptake of exogenous cholesterol provided by glia may be critical for mature neurons (Cruz and Chang, 2000; Pitas et al., 1987; Weisgraber et al., 1994). However, the mechanism underlining cholesterol regulation in brain has not been thoroughly investigated.
Our results showed that gene expression in the sterol biosynthesis pathway was significantly down-regulated in cerebellum of Npc1-/-mice, which is consistent with earlier studies indicating that brain cholesterol levels and cholesterol synthesis were significantly reduced in Npc1-/- mice (Quan et al., 2003; Schedin et al., 1998; Xie et al., 2000). Reduction in cholesterol synthesis may represent a negative feedback response of cells to accumulation of free cholesterol in late endosomes/lysosomes. However, Karten et al. recently reported that the amount of cholesterol secreted by astroglia cultured from Npc1-/- mice was comparable to that in wild-type mice (Karten et al., 2005). Similarly, using radio-labeled acetate to measure brain sterol biosynthesis, Reid and coll. did not find significant differences between wild-type and Npc1-/- mice at postnatal day 10 (Reid et al., 2008). Whether these discrepancies regarding cholesterol biosynthesis in Npc1-/- mouse brain are due to different assays or to the age of the animals remains to be determined.
Reduction in farnesyl diphosphate synthetase (Fdps) expression in Npc1-/- mice is noteworthy as this enzyme controls the synthesis of farnesyl pyrophosphate, a key intermediate in both sterol and non-sterol isoprenoid synthesis pathways. Recently, Fdps has been identified as a direct target of liver X receptors (LXRs) in astrocytes (Fukuchi et al., 2003), and mice deficient in LXRs have decreased expression of Fdps (Wang et al., 2002). Furthermore, Fdps mediates the effect of fibroblast growth factor-2 (FGF-2) on cell growth and neurogenesis in developing brains (Reilly et al., 2002; Ribeiro et al., 2001). Since dysregulation of LXRs has recently been implicated in NPC pathogenesis (Repa et al., 2007), it would be interesting to determine whether increased Fdps expression could be neuroprotective in NPC.
Increased expression of genes involved in cholesterol transport in Npc1-/- mice
The main lipoproteins responsible for cholesterol transport in brain consist of ApoE, ApoJ, ApoD, and ApoA1 (Gong et al., 2002); the majority of cholesterol in brain is transported by ApoE (Boyles et al., 1985) and a small amount by the other lipoproteins (Patel et al., 1995). Brain ApoE is mostly synthesized by astrocytes and microglia and to a much smaller extent by neurons (Brecht et al., 2004). ApoD, a component of the high-density lipoprotein complex in plasma, is also expressed in neurons, oligodendrocytes, and astrocytes in the central nervous system (Patel et al., 1995). Our results showed that the expression of brain lipoproteins (ApoE and ApoD) and lipoprotein receptor Cxcl16 was significantly increased in Npc1-/- mice, which is in agreement with previous studies indicating increased levels of ApoD mRNA and protein in Npc1-/- mice (Li et al., 2005; Ong et al., 2002; Suresh et al., 1998). Increased expression of lipoproteins and their receptors can result in potential increase in brain lipid transport, which may represent a compensational reaction to “membrane cholesterol starvation”, especially in distal axons (Karten et al., 2002). On the other hand, increased uptake of exogenous cholesterol can further exaggerate late endosomal/lysosomal cholesterol accumulation in neurons.
Another protein involved in brain lipid transport and exhibiting altered expression in Npc1-/- mice is protein convertase subtilisin-kexin like-9 (Pcsk9), also known as neural apoptosis-regulated convertase-1 (NARC-1). Pcsk9 can degrade LDL receptor, VLDL receptor, and ApoE receptor 2 (Poirier et al., 2008) and is expressed in cerebellum (Poirier et al., 2006). Knockdown of Pcsk9 in zebrafish induced embryonic death with neuronal disorganization in midbrain, hindbrain and cerebellum, suggesting a critical role of Pcsk9 in brain development (Poirier et al., 2006). However, Pcsk9 knockout mice are viable but with increased liver LDL receptor protein levels and decreased plasma cholesterol levels (Rashid et al., 2005). Whether down-regulation of Pcsk9 plays any role in NPC-type neurodegeneration needs to be further investigated.
Changes in cell adhesion/motility and immune response gene expression suggest increased inflammation response and a potential mechanism for hypomyelination in Npc1-/- mice
In addition to genes involved in cholesterol homeostasis, another major category of DEGs in Npc1-/- mice are those participating in the regulation of cell adhesion, cell motility, and immune/inflammation responses. The three genes in the cell adhesion pathway that were significantly up-regulated in Npc1-/- mice were intercellular adhesion molecule 1 (Icam1), H2-T23, and CD86; these genes are also related to immune/inflammation function. Icam1 is critical for initiation of immune reaction (Makgoba et al., 1988) and has been shown to be highly expressed in reactive astrocytes in brain of patients with Alzheimer's disease (Akiyama et al., 1993) and Parkinson's disease (Miklossy et al., 2006). CD86, a cell surface glycoprotein, functions as a T-cell co-stimulatory molecule and is involved in interactions between T cells and antigen-presenting cells (Mondino and Jenkins, 1994). CD86 expression has been reported in activated macrophages, B cells, a subpopulation of T cells, and microglia but not in astrocytes (Satoh et al., 1995). Up-regulation of these genes is consistent with increased inflammation in cerebellum of Npc1-/- mice, as we and others previously reported (Baudry et al., 2003; German et al., 2002). This notion is further supported by the abnormal expression of genes in the complement and coagulation cascades and leukocyte transendothelial migration pathway. As expression of most genes in these pathways was up-regulated, it is likely that inflammation responses are enhanced in brains of Npc1-/- mice. Although these pathways are well characterized in the immune system, their functions in brain are not completely clarified. It is possible that some of the pathways have similar functions in microglia, the brain resident macrophages. We previously showed that the number of CD11b-immunopositive reactive microglia was significantly increased in Npc1-/- mice (Baudry et al., 2003). Therefore, increased expression of this class of genes may reflect increased glia-mediated inflammation in mutant mice.
Our results showed, for the first time, that expression of genes (C1q, C2, C3) in the complement and coagulation cascades was increased in Npc1-/- mice. C1q is the initiating protein in the classical complement cascade; however, its role in the CNS is not clearly defined. Our results indicate that gene expression in the complement cascade pathway in Npc1-/- mice is associated with glia-mediated brain inflammation, and strongly suggest a pro-inflammation role for this cascade in NPC disease.
Genes in the cell adhesion pathway that were down-regulated in Npc1-/- mice included Mag, Cldn14, Cldn11, and Itgb7. Interestingly, some of these genes encode proteins that are critically involved in myelination, suggesting that hypomyelination in Npc1-/- mice could be caused by disruption of oligodendrocyte cell adhesion function, a possibility that is currently actively pursued in our laboratory. In Npc1-/- mice, brain hypomyelination was first observed at postnatal day 10 and became prominent at day 20 (Takikita et al., 2004). Further immunohistochemistry studies showed that although there were abundant premyelinating oligodendrocytes in Npc1-/- mouse brain, the number of mature oligodendrocytes was significantly reduced (Takikita et al., 2004). Understanding how axons and oligodendrocytes communicate in the process of myelination is an area of intensive research. It has been reported that electrical activity in axons induces oligodendrocyte precursor proliferation, suggesting that axonal signals are involved in oligodendrocyte development and myelination in brain (Barres and Raff, 1993; Barres and Raff, 1999). It is conceivable that changes in axonal function results in alterations in gene expression of the cell adhesion pathway in oligodendrocytes, which in turn lead to hypomyelination in Npc1-/- mice. Along this line, we previously showed that, although there is evident hypomyelination in the CNS of 4 week-old Npc1-/- mice, there is no obvious cholesterol accumulation in oligodendrocytes (Liao et al., 2009) excluding the possibility that hypomyelination is due to abnormal cholesterol accumulation in these cells.
In conclusion, our results indicated that Npc1 deficiency resulted in abnormal expression of genes involved in steroid synthesis and cholesterol transport as well as genes involved in cell adhesion, which could be responsible for neuroinflammation and hypomyelination. Importantly, our microarray data, some were validated by real-time PCR and immunoblotting analyses, represent the first implication of genes that belong to classic immune pathways (e.g. B cell receptor signaling pathway) in cerebellar pathogenesis in Npc1-/- mice. Based on these results, we suggest that treatments targeting cell adhesion and/or immune responses combined with facilitation of cholesterol egress from late endosomes/lysosomes may provide a more effective therapy for NPC.
All microarray data used in this study are available through the National Center for Biotechnology Information's Gene Expression Omnibus (series accession number: GSExxxx).
Sources of support
This work was supported by a grant from NINDS (NS048423 to XB) and by funds from Western University to X.B. Xiaoning Bi was also supported by funds from the Daljit and Elaine Sarkaria Chair.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmad I, Lope-Piedrafita S, Bi X, Hicks C, Yao Y, Yu C, Chaitkin E, Howison CM, Weberg L, Trouard TP, Erickson RP. Allopregnanolone treatment, both as a single injection or repetitively, delays demyelination and enhances survival of Niemann-Pick C mice. J Neurosci Res. 2005;82:811–21. doi: 10.1002/jnr.20685. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Kawamata T, Yamada T, Tooyama I, Ishii T, McGeer PL. Expression of intercellular adhesion molecule (ICAM)-1 by a subset of astrocytes in Alzheimer disease and some other degenerative neurological disorders. Acta Neuropathol. 1993;85:628–34. doi: 10.1007/BF00334673. [DOI] [PubMed] [Google Scholar]
- Barres BA, Raff MC. Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature. 1993;361:258–60. doi: 10.1038/361258a0. [DOI] [PubMed] [Google Scholar]
- Barres BA, Raff MC. Axonal control of oligodendrocyte development. J Cell Biol. 1999;147:1123–8. doi: 10.1083/jcb.147.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry M, Yao Y, Simmons D, Liu J, Bi X. Postnatal development of inflammation in a murine model of Niemann-Pick type C disease: immunohistochemical observations of microglia and astroglia. Exp Neurol. 2003;184:887–903. doi: 10.1016/S0014-4886(03)00345-5. [DOI] [PubMed] [Google Scholar]
- Bi X, Liao G. Autophagic-lysosomal dysfunction and neurodegeneration in Niemann-Pick Type C mice: lipid starvation or indigestion? Autophagy. 2007;3:646–8. doi: 10.4161/auto.5074. [DOI] [PubMed] [Google Scholar]
- Boyles JK, Pitas RE, Wilson E, Mahley RW, Taylor JM. Apolipoprotein E associated with astrocytic glia of the central nervous system and with nonmyelinating glia of the peripheral nervous system. J Clin Invest. 1985;76:1501–13. doi: 10.1172/JCI112130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecht WJ, Harris FM, Chang S, Tesseur I, Yu GQ, Xu Q, Dee Fish J, Wyss-Coray T, Buttini M, Mucke L, Mahley RW, Huang Y. Neuron-specific apolipoprotein e4 proteolysis is associated with increased tau phosphorylation in brains of transgenic mice. J Neurosci. 2004;24:2527–34. doi: 10.1523/JNEUROSCI.4315-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canales RD, Luo Y, Willey JC, Austermiller B, Barbacioru CC, Boysen C, Hunkapiller K, Jensen RV, Knight CR, Lee KY, Ma Y, Maqsodi B, Papallo A, Peters EH, Poulter K, Ruppel PL, Samaha RR, Shi L, Yang W, Zhang L, Goodsaid FM. Evaluation of DNA microarray results with quantitative gene expression platforms. Nat Biotechnol. 2006;24:1115–22. doi: 10.1038/nbt1236. [DOI] [PubMed] [Google Scholar]
- Carstea ED, Morris JA, Coleman KG, Loftus SK, Zhang D, Cummings C, Gu J, Rosenfeld MA, Pavan WJ, Krizman DB, Nagle J, Polymeropoulos MH, Sturley SL, Ioannou YA, Higgins ME, Comly M, Cooney A, Brown A, Kaneski CR, Blanchette-Mackie EJ, Dwyer NK, Neufeld EB, Chang TY, Liscum L, Strauss JF, 3rd, Ohno K, Zeigler M, Carmi R, Sokol J, Markie D, O'Neill RR, van Diggelen OP, Elleder M, Patterson MC, Brady RO, Vanier MT, Pentchev PG, Tagle DA. Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science. 1997;277:228–31. doi: 10.1126/science.277.5323.228. [DOI] [PubMed] [Google Scholar]
- Cruz JC, Chang TY. Fate of endogenously synthesized cholesterol in Niemann-Pick type C1 cells. J Biol Chem. 2000;275:41309–16. doi: 10.1074/jbc.M008272200. [DOI] [PubMed] [Google Scholar]
- De Windt A, Rai M, Kytomaki L, Thelen KM, Lutjohann D, Bernier L, Davignon J, Soini J, Pandolfo M, Laaksonen R. Gene set enrichment analyses revealed several affected pathways in Niemann-pick disease type C fibroblasts. DNA Cell Biol. 2007;26:665–71. doi: 10.1089/dna.2006.0570. [DOI] [PubMed] [Google Scholar]
- Fang H, Harris SC, Su Z, Chen M, Qian F, Shi L, Perkins R, Tong W. ArrayTrack: an FDA and public genomic tool. Methods Mol Biol. 2009;563:379–98. doi: 10.1007/978-1-60761-175-2_20. [DOI] [PubMed] [Google Scholar]
- Fukuchi J, Song C, Ko AL, Liao S. Transcriptional regulation of farnesyl pyrophosphate synthase by liver X receptors. Steroids. 2003;68:685–91. doi: 10.1016/s0039-128x(03)00100-4. [DOI] [PubMed] [Google Scholar]
- German DC, Quintero EM, Liang C, Xie C, Dietschy JM. Degeneration of neurons and glia in the Niemann-Pick C mouse is unrelated to the low-density lipoprotein receptor. Neuroscience. 2001;105:999–1005. doi: 10.1016/s0306-4522(01)00230-5. [DOI] [PubMed] [Google Scholar]
- German DC, Liang CL, Song T, Yazdani U, Xie C, Dietschy JM. Neurodegeneration in the Niemann-Pick C mouse: glial involvement. Neuroscience. 2002;109:437–50. doi: 10.1016/s0306-4522(01)00517-6. [DOI] [PubMed] [Google Scholar]
- Gong JS, Kobayashi M, Hayashi H, Zou K, Sawamura N, Fujita SC, Yanagisawa K, Michikawa M. Apolipoprotein E (ApoE) isoform-dependent lipid release from astrocytes prepared from human ApoE3 and ApoE4 knock-in mice. J Biol Chem. 2002;277:29919–26. doi: 10.1074/jbc.M203934200. [DOI] [PubMed] [Google Scholar]
- Griffin LD, Gong W, Verot L, Mellon SH. Niemann-Pick type C disease involves disrupted neurosteroidogenesis and responds to allopregnanolone. Nat Med. 2004;10:704–11. doi: 10.1038/nm1073. [DOI] [PubMed] [Google Scholar]
- Guo L, Lobenhofer EK, Wang C, Shippy R, Harris SC, Zhang L, Mei N, Chen T, Herman D, Goodsaid FM, Hurban P, Phillips KL, Xu J, Deng X, Sun YA, Tong W, Dragan YP, Shi L. Rat toxicogenomic study reveals analytical consistency across microarray platforms. Nat Biotechnol. 2006;24:1162–9. doi: 10.1038/nbt1238. [DOI] [PubMed] [Google Scholar]
- Guo L, Li Q, Xia Q, Dial S, Chan PC, Fu P. Analysis of gene expression changes of drug metabolizing enzymes in the livers of F344 rats following oral treatment with kava extract. Food Chem Toxicol. 2009;47:433–42. doi: 10.1016/j.fct.2008.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurevics HA, Kidwai FZ, Morell P. Sources of cholesterol during development of the rat fetus and fetal organs. J Lipid Res. 1997;38:723–33. [PubMed] [Google Scholar]
- Kadish I, Thibault O, Blalock EM, Chen KC, Gant JC, Porter NM, Landfield PW. Hippocampal and cognitive aging across the lifespan: a bioenergetic shift precedes and increased cholesterol trafficking parallels memory impairment. J Neurosci. 2009;29:1805–16. doi: 10.1523/JNEUROSCI.4599-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karten B, Vance DE, Campenot RB, Vance JE. Cholesterol accumulates in cell bodies, but is decreased in distal axons, of Niemann-Pick C1-deficient neurons. J Neurochem. 2002;83:1154–63. doi: 10.1046/j.1471-4159.2002.01220.x. [DOI] [PubMed] [Google Scholar]
- Karten B, Vance DE, Campenot RB, Vance JE. Trafficking of cholesterol from cell bodies to distal axons in Niemann Pick C1-deficient neurons. J Biol Chem. 2003;278:4168–75. doi: 10.1074/jbc.M205406200. [DOI] [PubMed] [Google Scholar]
- Karten B, Hayashi H, Francis GA, Campenot RB, Vance DE, Vance JE. Generation and function of astroglial lipoproteins from Niemann-Pick type C1-deficient mice. Biochem J. 2005;387:779–88. doi: 10.1042/BJ20041694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh CH, Peng ZF, Ou K, Melendez A, Manikandan J, Qi RZ, Cheung NS. Neuronal apoptosis mediated by inhibition of intracellular cholesterol transport: microarray and proteomics analyses in cultured murine cortical neurons. J Cell Physiol. 2007;211:63–87. doi: 10.1002/jcp.20912. [DOI] [PubMed] [Google Scholar]
- Korade Z, Kenworthy AK. Lipid rafts, cholesterol, and the brain. Neuropharmacology. 2008;55:1265–73. doi: 10.1016/j.neuropharm.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Repa JJ, Valasek MA, Beltroy EP, Turley SD, German DC, Dietschy JM. Molecular, anatomical, and biochemical events associated with neurodegeneration in mice with Niemann-Pick type C disease. J Neuropathol Exp Neurol. 2005;64:323–33. doi: 10.1093/jnen/64.4.323. [DOI] [PubMed] [Google Scholar]
- Liao G, Cheung S, Galeano J, Ji AX, Qin Q, Bi X. Allopregnanolone treatment delays cholesterol accumulation and reduces autophagic/lysosomal dysfunction and inflammation in Npc1-/- mouse brain. Brain Res. 2009;1270:140–51. doi: 10.1016/j.brainres.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makgoba MW, Sanders ME, Ginther Luce GE, Dustin ML, Springer TA, Clark EA, Mannoni P, Shaw S. ICAM-1 a ligand for LFA-1-dependent adhesion of B, T and myeloid cells. Nature. 1988;331:86–8. doi: 10.1038/331086a0. [DOI] [PubMed] [Google Scholar]
- Miklossy J, Doudet DD, Schwab C, Yu S, McGeer EG, McGeer PL. Role of ICAM-1 in persisting inflammation in Parkinson disease and MPTP monkeys. Exp Neurol. 2006;197:275–83. doi: 10.1016/j.expneurol.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Millard EE, Gale SE, Dudley N, Zhang J, Schaffer JE, Ory DS. The sterol-sensing domain of the Niemann-Pick C1 (NPC1) protein regulates trafficking of low density lipoprotein cholesterol. J Biol Chem. 2005;280:28581–90. doi: 10.1074/jbc.M414024200. [DOI] [PubMed] [Google Scholar]
- Millat G, Marcais C, Tomasetto C, Chikh K, Fensom AH, Harzer K, Wenger DA, Ohno K, Vanier MT. Niemann-Pick C1 disease: correlations between NPC1 mutations, levels of NPC1 protein, and phenotypes emphasize the functional significance of the putative sterol-sensing domain and of the cysteine-rich luminal loop. Am J Hum Genet. 2001;68:1373–85. doi: 10.1086/320606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RM, Callahan LM, Casaceli C, Chen L, Kiser GL, Chui B, Kaysser-Kranich TM, Sendera TJ, Palaniappan C, Federoff HJ. Dysregulation of gene expression in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse substantia nigra. J Neurosci. 2004;24:7445–54. doi: 10.1523/JNEUROSCI.4204-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondino A, Jenkins MK. Surface proteins involved in T cell costimulation. J Leukoc Biol. 1994;55:805–15. doi: 10.1002/jlb.55.6.805. [DOI] [PubMed] [Google Scholar]
- Naureckiene S, Sleat DE, Lackland H, Fensom A, Vanier MT, Wattiaux R, Jadot M, Lobel P. Identification of HE1 as the second gene of Niemann-Pick C disease. Science. 2000;290:2298–301. doi: 10.1126/science.290.5500.2298. [DOI] [PubMed] [Google Scholar]
- Ong WY, Kumar U, Switzer RC, Sidhu A, Suresh G, Hu CY, Patel SC. Neurodegeneration in Niemann-Pick type C disease mice. Exp Brain Res. 2001;141:218–31. doi: 10.1007/s002210100870. [DOI] [PubMed] [Google Scholar]
- Ong WY, Hu CY, Patel SC. Apolipoprotein D in the Niemann-Pick type C disease mouse brain: an ultrastructural immunocytochemical analysis. J Neurocytol. 2002;31:121–9. doi: 10.1023/a:1023993405851. [DOI] [PubMed] [Google Scholar]
- Pacheco CD, Kunkel R, Lieberman AP. Autophagy in Niemann-Pick C disease is dependent upon Beclin-1 and responsive to lipid trafficking defects. Hum Mol Genet. 2007;16:1495–503. doi: 10.1093/hmg/ddm100. [DOI] [PubMed] [Google Scholar]
- Patel SC, Asotra K, Patel YC, McConathy WJ, Patel RC, Suresh S. Astrocytes synthesize and secrete the lipophilic ligand carrier apolipoprotein D. Neuroreport. 1995;6:653–7. doi: 10.1097/00001756-199503000-00017. [DOI] [PubMed] [Google Scholar]
- Pedotti P, t Hoen PA, Vreugdenhil E, Schenk GJ, Vossen RH, Ariyurek Y, de Hollander M, Kuiper R, van Ommen GJ, den Dunnen JT, Boer JM, de Menezes RX. Can subtle changes in gene expression be consistently detected with different microarray platforms? BMC Genomics. 2008;9:124. doi: 10.1186/1471-2164-9-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitas RE, Boyles JK, Lee SH, Foss D, Mahley RW. Astrocytes synthesize apolipoprotein E and metabolize apolipoprotein E-containing lipoproteins. Biochim Biophys Acta. 1987;917:148–61. doi: 10.1016/0005-2760(87)90295-5. [DOI] [PubMed] [Google Scholar]
- Poirier S, Prat A, Marcinkiewicz E, Paquin J, Chitramuthu BP, Baranowski D, Cadieux B, Bennett HP, Seidah NG. Implication of the proprotein convertase NARC1/PCSK9 in the development of the nervous system. J Neurochem. 2006;98:838–50. doi: 10.1111/j.1471-4159.2006.03928.x. [DOI] [PubMed] [Google Scholar]
- Poirier S, Mayer G, Benjannet S, Bergeron E, Marcinkiewicz J, Nassoury N, Mayer H, Nimpf J, Prat A, Seidah NG. The proprotein convertase PCSK9 induces the degradation of low density lipoprotein receptor (LDLR) and its closest family members VLDLR and ApoER2. J Biol Chem. 2008;283:2363–72. doi: 10.1074/jbc.M708098200. [DOI] [PubMed] [Google Scholar]
- Quan G, Xie C, Dietschy JM, Turley SD. Ontogenesis and regulation of cholesterol metabolism in the central nervous system of the mouse. Brain Res Dev Brain Res. 2003;146:87–98. doi: 10.1016/j.devbrainres.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Rashid S, Curtis DE, Garuti R, Anderson NN, Bashmakov Y, Ho YK, Hammer RE, Moon YA, Horton JD. Decreased plasma cholesterol and hypersensitivity to statins in mice lacking Pcsk9. Proc Natl Acad Sci U S A. 2005;102:5374–9. doi: 10.1073/pnas.0501652102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy JV, Ganley IG, Pfeffer SR. Clues to neuro-degeneration in Niemann-Pick Type C disease from global gene expression profiling. PLoS ONE. 2006;1:e19. doi: 10.1371/journal.pone.0000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid PC, Lin S, Vanier MT, Ohno-Iwashita Y, Harwood HJ, Jr., Hickey WF, Chang CC, Chang TY. Partial blockage of sterol biosynthesis with a squalene synthase inhibitor in early postnatal Niemann-Pick type C npcnih null mice brains reduces neuronal cholesterol accumulation, abrogates astrogliosis, but may inhibit myelin maturation. J Neurosci Methods. 2008;168:15–25. doi: 10.1016/j.jneumeth.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly JF, Martinez SD, Mickey G, Maher PA. A novel role for farnesyl pyrophosphate synthase in fibroblast growth factor-mediated signal transduction. Biochem J. 2002;366:501–10. doi: 10.1042/BJ20020560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repa JJ, Li H, Frank-Cannon TC, Valasek MA, Turley SD, Tansey MG, Dietschy JM. Liver X receptor activation enhances cholesterol loss from the brain, decreases neuroinflammation, and increases survival of the NPC1 mouse. J Neurosci. 2007;27:14470–80. doi: 10.1523/JNEUROSCI.4823-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro I, Marcao A, Amaral O, Sa Miranda MC, Vanier MT, Millat G. Niemann-Pick type C disease: NPC1 mutations associated with severe and mild cellular cholesterol trafficking alterations. Hum Genet. 2001;109:24–32. doi: 10.1007/s004390100531. [DOI] [PubMed] [Google Scholar]
- Rollins B, Martin MV, Sequeira PA, Moon EA, Morgan LZ, Watson SJ, Schatzberg A, Akil H, Myers RM, Jones EG, Wallace DC, Bunney WE, Vawter MP. Mitochondrial variants in schizophrenia, bipolar disorder, and major depressive disorder. PLoS One. 2009;4:e4913. doi: 10.1371/journal.pone.0004913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh J, Lee YB, Kim SU. T-cell costimulatory molecules B7-1 (CD80) and B7-2 (CD86) are expressed in human microglia but not in astrocytes in culture. Brain Res. 1995;704:92–6. doi: 10.1016/0006-8993(95)01177-3. [DOI] [PubMed] [Google Scholar]
- Schedin S, Pentchev PG, Brunk U, Dallner G. Changes in the levels of dolichol and dolichyl phosphate in a murine model of Niemann-Pick's type C disease. J Neurochem. 1995;65:670–6. doi: 10.1046/j.1471-4159.1995.65020670.x. [DOI] [PubMed] [Google Scholar]
- Schedin S, Nilsson M, Chojnacki T, Dallner G. Alterations in the biosynthesis of cholesterol, dolichol and dolichyl-P in the genetic cholesterol homeostasis disorder, Niemann-Pick type C disease. Biochim Biophys Acta. 1998;1394:177–86. doi: 10.1016/s0005-2760(98)00108-8. [DOI] [PubMed] [Google Scholar]
- Shi L, Tong W, Fang H, Scherf U, Han J, Puri RK, Frueh FW, Goodsaid FM, Guo L, Su Z, Han T, Fuscoe JC, Xu ZA, Patterson TA, Hong H, Xie Q, Perkins RG, Chen JJ, Casciano DA. Cross-platform comparability of microarray technology: intra-platform consistency and appropriate data analysis procedures are essential. BMC Bioinformatics. 2005;6(Suppl 2):S12. doi: 10.1186/1471-2105-6-S2-S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Reid LH, Jones WD, Shippy R, Warrington JA, Baker SC, Collins PJ, de Longueville F, Kawasaki ES, Lee KY, Luo Y, Sun YA, Willey JC, Setterquist RA, Fischer GM, Tong W, Dragan YP, Dix DJ, Frueh FW, Goodsaid FM, Herman D, Jensen RV, Johnson CD, Lobenhofer EK, Puri RK, Schrf U, Thierry-Mieg J, Wang C, Wilson M, Wolber PK, Zhang L, Amur S, Bao W, Barbacioru CC, Lucas AB, Bertholet V, Boysen C, Bromley B, Brown D, Brunner A, Canales R, Cao XM, Cebula TA, Chen JJ, Cheng J, Chu TM, Chudin E, Corson J, Corton JC, Croner LJ, Davies C, Davison TS, Delenstarr G, Deng X, Dorris D, Eklund AC, Fan XH, Fang H, Fulmer-Smentek S, Fuscoe JC, Gallagher K, Ge W, Guo L, Guo X, Hager J, Haje PK, Han J, Han T, Harbottle HC, Harris SC, Hatchwell E, Hauser CA, Hester S, Hong H, Hurban P, Jackson SA, Ji H, Knight CR, Kuo WP, LeClerc JE, Levy S, Li QZ, Liu C, Liu Y, Lombardi MJ, Ma Y, Magnuson SR, Maqsodi B, McDaniel T, Mei N, Myklebost O, Ning B, Novoradovskaya N, Orr MS, Osborn TW, Papallo A, Patterson TA, Perkins RG, Peters EH, Peterson R, Philips KL, Pine PS, Pusztai L, Qian F, Ren H, Rosen M, Rosenzweig BA, Samaha RR, Schena M, Schroth GP, Shchegrova S, Smith DD, Staedtler F, Su Z, Sun H, Szallasi Z, Tezak Z, Thierry-Mieg D, Thompson KL, Tikhonova I, Turpaz Y, Vallanat B, Van C, Walker SJ, Wang SJ, Wang Y, Wolfinger R, Wong A, Wu J, Xiao C, Xie Q, Xu J, Yang W, Zhong S, Zong Y, Slikker W., Jr. The MicroArray Quality Control (MAQC) project shows inter- and intraplatform reproducibility of gene expression measurements. Nat Biotechnol. 2006;24:1151–61. doi: 10.1038/nbt1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Jones WD, Jensen RV, Harris SC, Perkins RG, Goodsaid FM, Guo L, Croner LJ, Boysen C, Fang H, Qian F, Amur S, Bao W, Barbacioru CC, Bertholet V, Cao XM, Chu TM, Collins PJ, Fan XH, Frueh FW, Fuscoe JC, Guo X, Han J, Herman D, Hong H, Kawasaki ES, Li QZ, Luo Y, Ma Y, Mei N, Peterson RL, Puri RK, Shippy R, Su Z, Sun YA, Sun H, Thorn B, Turpaz Y, Wang C, Wang SJ, Warrington JA, Willey JC, Wu J, Xie Q, Zhang L, Zhong S, Wolfinger RD, Tong W. The balance of reproducibility, sensitivity, and specificity of lists of differentially expressed genes in microarray studies. BMC Bioinformatics. 2008;9(Suppl 9):S10. doi: 10.1186/1471-2105-9-S9-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch J, Xu Z. Niemann-Pick C2 (NPC2) and intracellular cholesterol trafficking. Biochim Biophys Acta. 2009;1791:671–8. doi: 10.1016/j.bbalip.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh S, Yan Z, Patel RC, Patel YC, Patel SC. Cellular cholesterol storage in the Niemann-Pick disease type C mouse is associated with increased expression and defective processing of apolipoprotein D. J Neurochem. 1998;70:242–51. doi: 10.1046/j.1471-4159.1998.70010242.x. [DOI] [PubMed] [Google Scholar]
- Sutherland GT, Matigian NA, Chalk AM, Anderson MJ, Silburn PA, Mackay-Sim A, Wells CA, Mellick GD. A cross-study transcriptional analysis of Parkinson's disease. PLoS One. 2009;4:e4955. doi: 10.1371/journal.pone.0004955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takikita S, Fukuda T, Mohri I, Yagi T, Suzuki K. Perturbed myelination process of premyelinating oligodendrocyte in Niemann-Pick type C mouse. J Neuropathol Exp Neurol. 2004;63:660–73. doi: 10.1093/jnen/63.6.660. [DOI] [PubMed] [Google Scholar]
- Tong W, Cao X, Harris S, Sun H, Fang H, Fuscoe J, Harris A, Hong H, Xie Q, Perkins R, Shi L, Casciano D. ArrayTrack--supporting toxicogenomic research at the U.S. Food and Drug Administration National Center for Toxicological Research. Environ Health Perspect. 2003;111:1819–26. doi: 10.1289/ehp.6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turley SD, Burns DK, Rosenfeld CR, Dietschy JM. Brain does not utilize low density lipoprotein-cholesterol during fetal and neonatal development in the sheep. J Lipid Res. 1996;37:1953–61. [PubMed] [Google Scholar]
- Wang L, Schuster GU, Hultenby K, Zhang Q, Andersson S, Gustafsson JA. Liver X receptors in the central nervous system: from lipid homeostasis to neuronal degeneration. Proc Natl Acad Sci U S A. 2002;99:13878–83. doi: 10.1073/pnas.172510899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watari H, Blanchette-Mackie EJ, Dwyer NK, Sun G, Glick JM, Patel S, Neufeld EB, Pentchev PG, Strauss JF., 3rd NPC1-containing compartment of human granulosa-lutein cells: a role in the intracellular trafficking of cholesterol supporting steroidogenesis. Exp Cell Res. 2000;255:56–66. doi: 10.1006/excr.1999.4774. [DOI] [PubMed] [Google Scholar]
- Weisgraber KH, Roses AD, Strittmatter WJ. The role of apolipoprotein E in the nervous system. Curr Opin Lipidol. 1994;5:110–6. doi: 10.1097/00041433-199404000-00007. [DOI] [PubMed] [Google Scholar]
- Xie C, Burns DK, Turley SD, Dietschy JM. Cholesterol is sequestered in the brains of mice with Niemann-Pick type C disease but turnover is increased. J Neuropathol Exp Neurol. 2000;59:1106–17. doi: 10.1093/jnen/59.12.1106. [DOI] [PubMed] [Google Scholar]
- Yanagimoto C, Harada M, Kumemura H, Koga H, Kawaguchi T, Terada K, Hanada S, Taniguchi E, Koizumi Y, Koyota S, Ninomiya H, Ueno T, Sugiyama T, Sata M. Niemann-Pick C1 protein transports copper to the secretory compartment from late endosomes where ATP7B resides. Exp Cell Res. 2009;315:119–26. doi: 10.1016/j.yexcr.2008.10.022. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Cleaveland ES, Nagle JW, French S, Yaswen L, Ohshima T, Brady RO, Pentchev PG, Kulkarni AB. Molecular cloning of the mouse apolipoprotein D gene and its upregulated expression in Niemann-Pick disease type C mouse model. DNA Cell Biol. 1996;15:873–82. doi: 10.1089/dna.1996.15.873. [DOI] [PubMed] [Google Scholar]