Abstract
Failure of chemotherapy in the treatment of pancreatic cancer is often due to resistance to therapy-induced apoptosis. A major mechanism for such resistance is the expression and activity of inhibitors of apoptosis proteins (IAPs). Smac is a mitochondrial protein that inhibits IAPs. We show that JP1201, a Smac mimetic, is a potent enhancer of chemotherapy in robust mouse models of pancreatic cancer. Combination of JP1201 with gemcitabine reduced primary and metastatic tumor burden in orthotopic xenograft and syngenic tumor models, induced regression of established tumors, and prolonged survival in xenograft and transgenic models of pancreatic cancer. The effect of JP1201 was phenocopied by XIAP siRNA in vitro and correlated with elevated levels of TNFα protein in vivo. The continued development of JP1201 and other strategies designed to enhance therapy-induced apoptosis in pancreatic cancer is warranted.
Keywords: pancreatic cancer, smac, apoptosis, gemcitabine, IAPs
Introduction
Pancreatic cancer is a deadly disease with a dismal 5-year survival (1). Surgery offers a chance for a cure, but most patients have advanced disease and are not candidates for resection and must rely on chemotherapy. Resistance to chemotherapy-induced apoptosis is a significant factor contributing to poor prognosis of these patients (2). Tumor cells often circumvent the apoptotic cascade and proliferate in the face of apoptotic stimuli, which facilitates tumor progression and metastasis (3). Thus, restoring apoptotic response in tumor cells is an attractive strategy to improve the prognosis of pancreatic cancer patients (2).
Two major pathways induce apoptosis: the extrinsic or death receptor mediated pathway and the intrinsic or mitochondrial pathway (4). The extrinsic pathway is activated by the binding of molecules such as TRAIL or TNFα to their cognate receptors (3). Members of the TNF receptor superfamily induce apoptosis by recruitment of FADD and formation of the death inducing signaling complex (5). The intrinsic pathway is initiated when cellular stress causes a change in mitochondrial membrane permeability, which releases proteins such as cytochrome c and second mitochondria-derived activator of caspase (Smac) resulting in the recruitment of Apaf-1 and formation of the apoptosome (4). Executioner caspases are activated by both pathways resulting in subsequent cell death (5).
Chemotherapy and radiation ultimately cause tumor cell death by inducing apoptosis (5), which is compromised by tumor cell resistance to apoptosis (2). Many cancer cells express elevated levels of inhibitor of apoptosis proteins (IAPs) and through the activity of IAPs escape apoptosis (4). IAPs prevent the activation of caspases and, as such, block the extrinsic and intrinsic apoptotic cascades (5). X-linked IAP (XIAP) is one of the best characterized IAPs and has been shown to be expressed at a higher level in pancreatic cancer cell lines (n=19) (6) and pancreatic tumors (14/18) compared to normal pancreas (7, 8). XIAP is an attractive target for anti-cancer therapy as it functions as a “gatekeeper” of caspase activation (4). The mitochondrial protein Smac inhibits IAPs, including XIAP, thus promoting caspase activation and subsequent cell death. Smac has been shown to bind to XIAP, cIAP-1 and cIAP-2 and Smac mimetics sensitize tumors to programmed cell death (7, 9–11).
In this series of experiments, we explore the effect of a novel Smac mimetic, JP1201, in combination with chemotherapy. We show that JP1201 enhances the efficacy of chemotherapy and improves survival in multiple animal models of pancreatic cancer. These effects are mediated in part by inhibition of XIAP and induction of TNFα.
Materials and Methods
Cell Lines
Human pancreatic cancer cell lines (MIA PaCa-2, PANC-1, BxPC-3, AsPC-1, Capan-1, Capan-2, Hs 766T, and Hs 700T) were obtained from ATCC (Manassas, VA). The murine pancreatic cancer cell line Pan02 (also known as Panc02) was obtained from NCI (Frederick, MD). Cell lines were confirmed to be pathogen free and human cell lines were authenticated to confirm origin prior to use. Cell lines were grown in DMEM (Invitrogen, Carlsbad CA) containing 10% FBS and maintained at 37°C in a humidified incubator with 5% CO2 and 95% air.
In vitro cytotoxicity and Drug Response assay
Assays were performed in 96-well format as described (12). Briefly, cells were plated on day 0 and drug was added on day 1 in four fold dilutions. For gemcitabine (GEM, Eli Lilly and Company, Indianapolis, IN) alone and the GEM-JP1201 (100 nM) combination the highest dose of GEM given was 2000 nM. For JP1201 alone the highest concentration given was 100 µM. Relative cell number was determined by adding MTS (Promega, Madison, WI, final concentration 333 µg/ml), incubating for 1 to 3 hours at 37°C, and reading absorbance at 490 nm plate reader (Spectra Max 190, Molecular Devices, Downington, PA). Drug sensitivity curves and IC50s were calculated using in-house software.
siRNA and Gemcitabine combination therapy
For reverse transfection, 0.25 µl of 20 µM stock of each siRNA in a volume of 19.75 µl of serum free DMEM was delivered to each well of a 96 well plate. 0.125 µl of Dharmafect 1 (Dharmacon, Lafayette, CO) in 9.875 µl of serum free DMEM was then delivered into each well. RNA-lipid complexes were allowed to form (20–30 min). Following the incubation 8,000 cells were added to each well in DMEM with 5% FBS, total volume per well 100 µl. On day 1, GEM was added to each plate in DMEM with 5% FBS in four fold serial dilutions as described above. Plates were read on day five using an MTS assay as described.
Animal Studies
All animals were housed in a pathogen-free facility with 24-hour access to food and water. Experiments were approved by, and performed in accordance with, the IACUC at UT Southwestern (Dallas, TX). Athymic nu/nu mice were purchased from NCI (Frederick, MD); C57Bl/6 mice were purchased from Jackson Laboratories (Bar Harbor, MD); and SCID mice were obtained from an on campus supplier. At sacrifice, the pancreas and tumor were excised and weighed en bloc to determine primary tumor burden. Metastases were identified through visual inspection of the surface of the liver, diaphragm, peritoneal surfaces and lymph nodes. Samples were fixed in 10% formalin (Sigma, St. Louis, MO) or snap-frozen in liquid nitrogen for further studies.
Early intervention model
6–8 week old athymic nu/nu female mice were injected with MIA PaCa-2 cells (1 × 106) as described (13). Animals were randomized following tumor cell injection (TCI) into treatment groups and therapy was initiated one week following TCI. Therapy with TRAIL (20 mg/kg) alone or in combination with JP1201 (0.2, 0.6, 2.0, 6.0 mg/kg) was given three times weekly (MWF) via lateral tail vein for a total of six injections. All drugs were diluted in saline. Animals were sacrificed 2.5 weeks after the cessation of therapy.
Late Intervention model
On day 28 post TCI therapy was initiated and continued for six doses. The dosing was similar except JP1201 was only given at 6.0 mg/kg and GEM was dosed at 175 mg/kg on day 28, and 100 mg/kg on days 30, 37, 40. A replicate experiment with GEM at 25 mg/kg given 6 times over 2 weeks was also performed. The animals were sacrificed on day 42, two days following the last treatment.
Syngenic model
Pan02 cells were injected orthotopically into C57Bl/6 mice and therapy was initiated on day 21 post TCI. Therapy consisted of saline, GEM alone (25 mg/kg), JP1201 (6.0 mg/kg), and the combination of GEM and JP1201. The schedule of therapy was the same as above.
Survival Study
MIA PaCa-2 tumors were established in nude mice in an identical manner to the late intervention study. Therapy was given on the same schedule with GEM at 25 mg/kg. Following therapy, animals were weighed three times weekly and assessed for weight loss or ascites. Assessment of animals was by an experienced observer blinded to treatment group. Animals were sacrificed for humane purposes if weight loss was more than 15% of body weight or if they had ascites and weight gain of greater than 10%. These animals were counted as a death on the day of sacrifice.
Transgenic model of pancreatic ductal adenocarcinoma
p48-Cre: KrasG12D:Ink4a/Arflox/lox mice (14, 15) were genotyped shortly after birth and therapy with saline, GEM (25 mg/kg) or JP1201 (6 mg/kg) combined with GEM was delivered by ip injection 3x/week. Endpoint and survival studies were performed as described above.
Pharmacokinetics
MIA PaCa-2 tumor cells were implanted into the pancreas of athymic nu/nu mice. Forty one days later animals were placed in groups of three and given either GEM alone (100 mg/kg – 2 mg total, IP), JP1201 alone (6 mg/kg – 0.12 mg total, IV), or a combination of GEM and JP1201. Animals were sacrificed at varying times after dosing (5 min, 30 min, 2, 8, 12, and 24 hr) and plasma and tumor sampled. The processing and analytical methods used for detection of GEM (dFdC), JP1201, and the GEM inactive metabolite, dFdU are described in detail in the online supplemental material.
Histology
Formalin-fixed tissues were embedded in paraffin and sectioned (10 µm) by the Molecular Pathology core at UTSW where routine hematoxylin & eosin staining was also performed. Antibodies were used at 5 µg/ml and included goat anti-PCNA (SC-9857, Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti- human TNFα (ab6671, Abcam, Cambridge, MA) rat anti-mouse TNFα (506302, Biolegend, San Diego, CA). TUNEL staining was performed per manufacturer instructions (Promega, Madison, WI).
Statistics
Data were analyzed using GraphPad software (GraphPad Software, San Diego, CA). Results are expressed as mean ± SEM. Data was analyzed by t-test or ANOVA and results are considered significant at p < 0.05.
RESULTS
JP1201 + TRAIL slows the growth of orthotopic pancreatic tumors
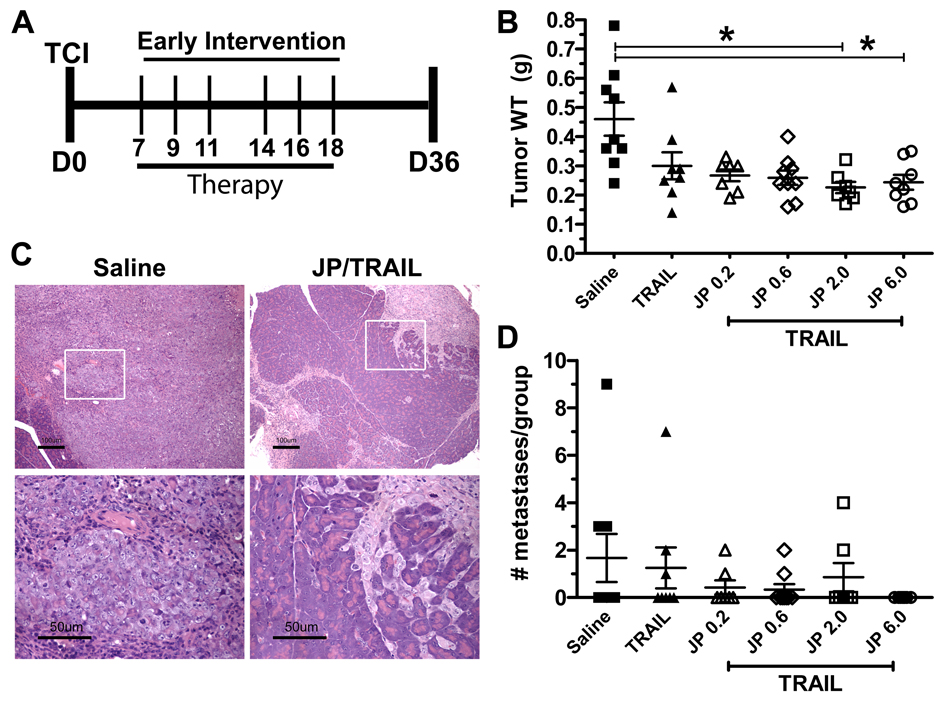
Smac mimetics induce apoptosis in vitro when combined with TRAIL or TNFα (9). JP1201 did not induce apoptosis in MIA PaCa-2 as a single agent in vitro; however, addition of JP1201 decreased the IC50 of TRAIL from 4.5 to 1.2 nM. We assessed how JP1201 in combination with TRAIL affected pancreatic cancer growth in vivo. In this early intervention model, mice bearing MIA PaCa-2 tumors were treated with saline, TRAIL or TRAIL + JP- 1201 at 0.2, 0.6, 2 or 6 mg/kg starting one week following tumor cell injection and sacrificed 2.5 weeks after the last dose of therapy (Fig. 1A). Therapy with TRAIL + JP1201 at doses of 2 mg/kg or 6 mg/kg reduced the final size of pancreatic tumors by approximately 50 percent (control, 0.46 g vs. 0.23 g and 0.24 g, respectively; Fig. 1B). TRAIL alone had a modest effect on the size of xenografts compared to saline. Tumors were removed en bloc. At the time of sacrifice, we observed significantly less tumor burden and more residual normal pancreas in mice treated with TRAIL + JP1201. Histologic analysis showed extensive tumor burden in saline treated animals. In contrast, mice treated with combination therapy using the highest doses of JP1201 showed scattered foci of tumor growth with a large amount of residual normal pancreas (Fig. 1C).
Figure 1. JP1201 in combination with TRAIL is effective in controlling pancreatic tumor xenografts.
A, Early intervention treatment algorithm. 1×106 MIA PaCa-2 cells were injected into mice on Day 0. Therapy was initiated on Day 7 post tumor cell injection (TCI). Therapy was given by iv or ip injection every other day (MWF) for two weeks on the days indicated. Mice received saline, TRAIL (20 mg/kg), or TRAIL + JP1201 (JP, at 0.2, 0.6, 2.0, or 6.0 mg/kg). Animals were sacrificed 2.5 weeks after the last dose of therapy (D36). B, A scatter plot of tumor weights at the time of sacrifice showing the mean +/− SEM as well as the weight of each tumor from individual mice. *, p < 0.05 by one-way ANOVA with Dunn’s posttest. C, Representative H&E staining of formalin-fixed, paraffin embedded sections of tumor tissue from mice treated with Saline or JP/TRAIL. The upper row are images at 100× total magnification (scale bar, 100 µm) while the lower row is at 400× total magnification (scale bar, 50 µm). The white box in the upper row is the area magnified. D, The mean +/− SEM number of metastases in each treatment group as well as the number of metastatic events in each animal is shown in the scatter plot. Metastases were evaluated by careful visual inspection at the time of necropsy. Note the animals treated with JP/TRAIL at the highest dose did not have any visible metastases. The data represent a single animal experiment with 7–9 animals in each treatment group.
In addition to controlling primary tumor burden, metastases were reduced by JP1201 + TRAIL. There were no metastatic events in the TRAIL + JP1201 (6 mg/kg) group, compared to 15 total metastatic events in control animals (Fig. 1D). These results show that JP1201 sensitizes pancreatic tumors to therapy with the death receptor ligand TRAIL. Animals treated with combination therapy did not display any weight loss or other signs of systemic toxicity.
Inhibition of XIAP improves sensitivity to gemcitabine
Despite being the standard of care for pancreatic cancer, gemcitabine (GEM) is only minimally effective. Blocks in apoptosis, including overexpression of IAPs might mediate chemoresistance in pancreatic cancer (16). To determine if JP1201 affected the sensitivity in vitro to GEM, we screened a panel of cell lines against GEM alone, JP1201 alone or the combination (Table 1). We found that in general a low dose (100 nM) of JP1201 enhanced the sensitivity of pancreatic cancer cells to GEM by approximately 20-fold. However the range of fold-reduction was wide with two cell lines showing no change in the IC50 of GEM (Capan-2 and Hs 766T) while PANC-1 cells demonstrated a 170-fold reduction.
Table 1.
JP1201 sensitizes pancreatic tumor cells to gemcitabine
| GEM (nM) | GEM (nM) + JP1201 (100 nM) |
Fold reduction |
JP1201 (µM) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cell Line | Assays | Median | SD | Assays | Median | SD | Assays | Median | SD | |
| AsPC-1 | 4 | 177 | 3 | 4 | 149 | 2.5 | 1.19 | 5 | 61 | 1.3 |
| BxPC-3 | 4 | 13.4 | 1.2 | 6 | 7.55 | 3.7 | 1.77 | 4 | 4.98 | 1.2 |
| Capan-1 | 2 | 5.05 | 1 | 2 | 0.52 | 9.71 | 2 | 12.1 | ||
| Capan-2 | 4 | 2000 | 1 | 3 | 2000 | 1.1 | 1.00 | 4 | 100 | 1 |
| Hs 766T | 4 | 2000 | 1 | 4 | 2000 | 1 | 1.00 | 4 | 100 | 1 |
| Hs 700T | 4 | 27.1 | 3.2 | 4 | 11.5 | 2.2 | 2.36 | 4 | 100 | 1 |
| MIA PaCa-2 | 8 | 19.1 | 2 | 6 | 1.2 | 2.9 | 15.92 | 6 | 60.4 | 2.5 |
| PANC-1 | 6 | 2000 | 1.9 | 6 | 11.5 | 3.7 | 173.91 | 4 | 8.85 | 1.2 |
| Pan02 | 6 | 43 | 14 | 6 | 21.2 | 3.8 | 2.03 | |||
Cell growth assays were performed in 96-well format for five days (n=8/condition/assay). On day 0, cells were plated and on day 1 drugs were added in four fold dilutions. The highest dose of gemcitabine (GEM) was 2000 nM. The highest concentration of JP1201 alone was 100 µM. On day 5, relative cell number was estimated using MTS (Promega, final concentration 333 µg/ml), plates were then incubated for 1 to 3 hours at 37°C, and read at 490 nm. Drug sensitivity curves and IC50s were calculated using in-house software.
The number independent assay performed and the median GEM IC50 +/− SD (nM) for GEM alone and GEM + JP1201, the fold reduction in GEM IC50 in the presence of JP1201 (100nM) and sensitivity to JP1201 alone is displayed.
Shaded rows indicate cell lines that were tested in vivo.
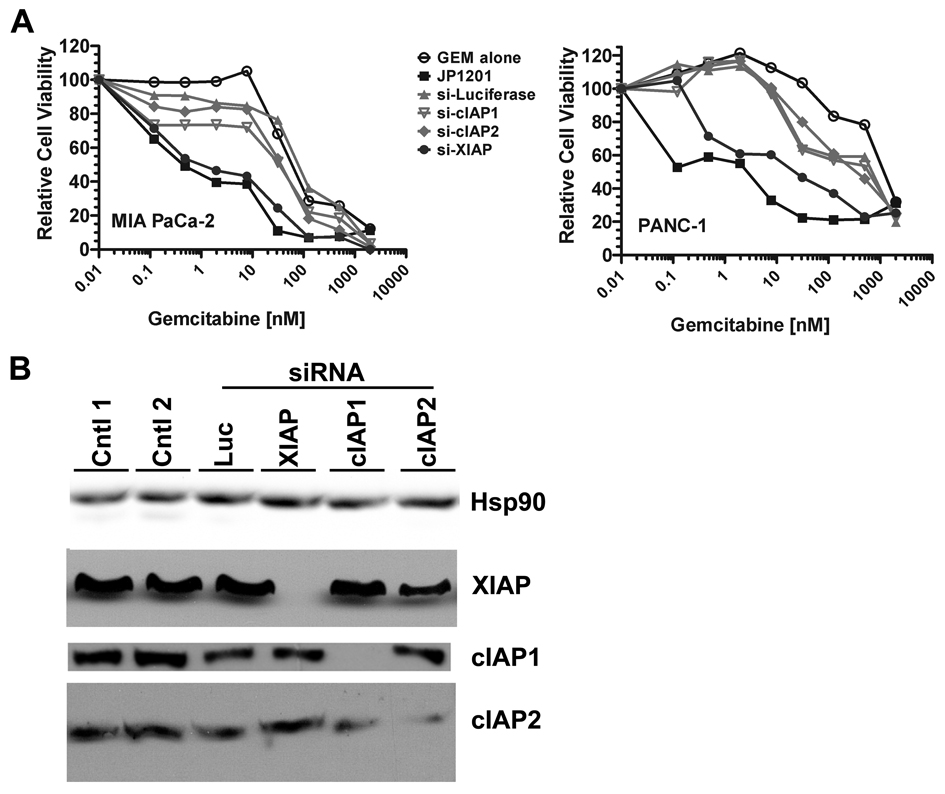
Because JP1201 binds to and inhibits IAPs, we hypothesized that siRNA-mediated knockdown of IAPs would increase sensitivity of pancreatic cancer cells to GEM similar to treatment with JP1201. We found that siRNA-mediated knockdown of XIAP but not cIAP1 or cIAP2 sensitized MIA PaCa-2 and PANC-1 (Fig. 2) cells to treatment with GEM. The increase in sensitivity was similar to treatment with JP1201 (Fig. 2). This data expands work in other pancreas cancer cell lines (8, 17) that show resistance to chemotherapy is mediated in part by XIAP.
Figure 2. Inhibition of XIAP improves sensitivity to gemcitabine in pancreatic cancer cell lines.
A, Pancreatic cancer cells, MIA PaCa-2 and PANC-1, were treated with increasing concentrations of gemcitabine alone, or in combination with siRNA (100 nM) specific for the indicated target or JP1201 (100 nM). Cell viability was estimated 4 days after initiation of treatment by a MTS assay in which each condition was replicated 8 times. The data shown is representative of three independent experiments. Transfection with siRNA specific for luciferase (si-Luciferase) was used as a control for cell toxicity mediated by transfection alone. B, Western blot analysis of XIAP, cIAP1, cIAP2 demonstrating specificity of siRNA-mediated knockdown. Controls included no transfection (Cntl 1), transfection reagent alone (Cntl 2), and transfection with a siRNA specific for luciferase (Luc).
JP1201 + gemcitabine is efficacious for established pancreatic tumors
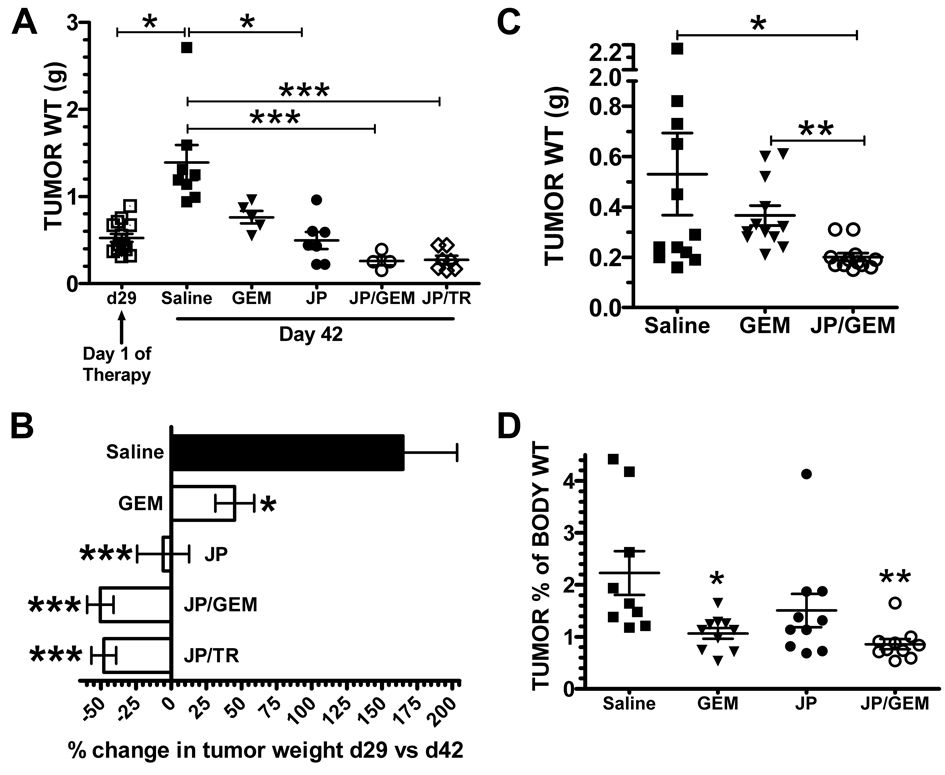
A challenge in treating pancreatic cancer is the fact that patients typically present at an advanced stage. To more closely parallel human disease presentation, we performed a late intervention study. MIA PaCa-2 tumors were grown for 28 days (tumor burden approximately 500 mg) prior to the initiation of therapy. Therapy was given as six doses over 2 weeks. A cohort of animals from each group was sacrificed 24 hours after the first dose of therapy (day 29) to acquire tissue for histological analysis (see below). The remaining animals were sacrificed on day 42, two days following the end of treatment. As demonstrated in figure 3A, treatment with JP1201 + TRAIL or GEM reduced tumor weight significantly (***, p<0.001 vs saline). JP alone also had a significant effect (*, p<0.05, vs. saline) on pancreatic tumor weight, despite having little effect as a single agent in vitro.
Figure 3. JP1201 combined with gemcitabine has potent anti-tumor activity against established pancreatic tumors.
A–C, MIA PaCa-2 (1×106) were injected into the pancreas of nude mice on day 0. On day 28 post tumor cell injection (TCI) therapy was initiated and consisted of saline, JP1201 (JP, 6.0 mg/kg), JP + TRAIL (TR, 20 mg/kg) 3x/week via iv injection on the days indicated. Gemcitabine (GEM) alone (n=5) or in combination with JP (A & B, n=4) was given ip at a dose of 175 mg/kg on day 28, and at 100 mg/kg on days 30, 37, and 40 (A, B) and 25 mg/kg 3x/week for two weeks (C, n=12/group). Scatter plots showing mean +/− SEM tumor weight as well as the weights individual tumors for each group are shown (A, C). *, p<0.05; **, p<0.01; ***, p<0.005 by one-way ANOVA. B, Three tumors from each treatment group shown in panel B (n=15 total) were harvested on day 29 post TCI. Final tumor size of each group (day 42) was compared to the mean tumor size on day 29. The percent change in tumor size is shown. *, p<0.05; ***, p<0.0001 vs. saline by one-way ANOVA. D, Pan02 murine pancreatic cancer cells (1×106) were injected orthotopically into C57Bl/6J mice on day 0. Therapy with saline, GEM (25 mg/kg), JP (6.0 mg/kg) or the combination was initiated on day 21 post TCI and given 3x/week for two weeks. The mice were sacrificed on day 35 post TCI and resulting tumor weights are displayed as % of body weight in a scatter plot showing mean +/− SEM (n= 9–10/group). *, p<0.05; **, p<0.01 vs. saline by one-way ANOVA.
Comparison of mean tumor weights on day 42 to the mean weight of tumors harvested on at day 29 (mean of 15 tumors harvested) shows that animals treated with JP1201 in combination with GEM or TRAIL had reductions in mean tumor size of ~50%, while mice treated with saline or GEM had increased mean tumor weight by 150 and 50%, respectively (Fig. 3B). Treatment with JP1201 alone caused a cessation of tumor growth. These data strongly suggest that mice treated with combination therapy underwent tumor regression rather than growth inhibition. To confirm this robust effect we performed histological analysis, which revealed large poorly differentiated tumor tissue in the saline and GEM treated animals with little evidence of remaining normal pancreas tissue. However, treatment with JP1201 alone or in combination with GEM or TRAIL revealed reduced primary tumor burden and an increase the amount of residual normal pancreas (dark purple) as seen by H&E analysis (Fig. S1). A replicate experiment revealed similar results (Fig. 3C). In the replicate experiment the dose of GEM was dropped to 25 mg/kg. We also evaluated the response of orthotopic BxPC-3 tumors using a similar dosing strategy and found that treatment with JP1201 in combination with GEM was effective at controlling tumor size (saline, 0.94 g; combination therapy, 0.35 g).
Impaired drug delivery is proposed as a complicating factor in the poor response of pancreatic tumors to standard therapy. With this in mind, we evaluated the general pharmacokinetic parameters of GEM, its inactive metabolite dFdU, and JP1201 in mice bearing large orthotopic MIA PaCa-2 tumors (Table S1). 54 mice with a mean (+/− SD) pancreas/tumor of 0.47 +/− 0.2 g were used for the study. We found that the plasma half-life and AUC of GEM were elevated by combination with JP1201; however, the contribution of this to the dramatic increase in response in mice treated with combination therapy is unclear. We also identified a surprisingly long plasma and tumor half-life of JP1201, 300–400 min and 900 –1000 min, respectively.
JP1201 maintains effectiveness as a chemosensitizer in a syngenic model
To determine if JP1201 + GEM remained effective in a syngenic model of pancreatic cancer we performed a therapy experiment in C57Bl/6 mice using the murine pancreatic cancer cell line Pan02. Therapy was started three weeks after tumor cell injection and animals were sacrificed on day 35. The combination of JP1201 + GEM was effective at reducing tumor burden in this fully immunocompetent model (Fig. 3D). Of note 5 out of 10 mice in the combination therapy group had no identifiable tumor at the time of necropsy where as 100% of the saline treated mice and mice treated with single agent therapy had significant pancreatic tumor masses. We did find in this model that GEM (25 mg/kg) induced splenomegaly and this was exacerbated by combination with JP1201 (spleen weight: saline, 0.11 g; GEM, 0.19 g; JP 0.11 g; JP/GEM, 0.25 g).
JP1201 reduces metastatic disease
We assessed metastatic burden in the xenograft late interventions studies and found that overall metastatic incidence was 55% in the saline-treated animals and this was reduced to 35, 43, 31, and 14% by treatment with GEM, JP1201, JP1201 + GEM, and JP1201 + TRAIL, respectively.
JP1201 induces apoptosis and TNFα expression in orthotopic tumors
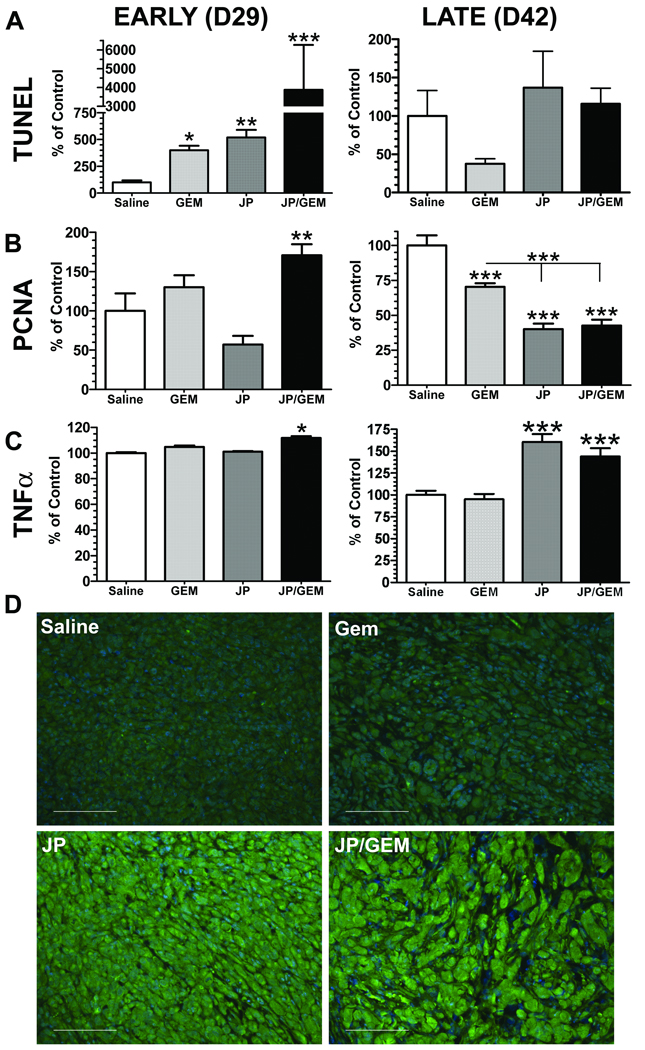
To assess induction of apoptosis after therapy, MIA PaCa-2 tumors from animals sacrificed on day 29 and day 42 (Fig. 3A) were analyzed. TUNEL immunofluorescence revealed that there was an acute induction of apoptosis in the tumor following administration of JP1201 combined with GEM. However after the full course of therapy there was no difference in the TUNEL signal between any of the treatment groups (Fig. 4A). We also evaluated cell proliferation by immunohistochemistry for PCNA. PCNA reactivity was elevated in the combination (JP/GEM) at the early time point (d29); however single agent and combination therapy significantly reduced PCNA reactivity at the late time point (Fig. 4B). Furthermore, cell proliferation in tumors treated with JP1201 and the combination of (JP/GEM) were reduced significantly compared to treatment with GEM alone (Fig. 4B).
Figure 4. Induction of apoptosis using combination therapy.
A–D, Tumors from animals sacrificed one day after initiation of therapy (EARLY (D29)) or after the cessation of therapy (LATE (D42)) were formalin fixed and paraffin embedded. A–C, Data from a minimum of 4 tumors from each group are normalized to the saline group and are representative of at least two independent assays on the same tumor tissue. A, TUNEL analysis was performed and analyzed using immunofluorescence. *, p<0.05; **, p<0.01; ***, p<0.001 vs. saline by one-way ANOVA. B, Cell proliferation was measured by immunofluorescence for PCNA. **, p<0.01; ***, p<0.001 by one way ANOVA. C & D, The expression level of TNFα was determined by immunofluorescence. *p<0.05; ***, p<0.001 by one way ANOVA. D, Representative images of TNFα (green) immunofluorescence, scale bar, 50 µm.
TNFα has been implicated in the response to Smac mimetics (18). Therefore we evaluated the level of TNFα protein in tumors from each group at early (d29) and late (d42) time points. TNFα levels were increased modestly by combination therapy at the early time point but were elevated dramatically by JP1201 alone or in combination with GEM at the late time point (Fig. 4C,D). We believe that the TNFα detected is of human tumor cell origin. Pan02 tumor tissue from controls and animals treated with JP1201 alone or in combination with GEM revealed sparse reactivity with the rabbit anti- TNFα antibody used in figure 4 (Fig. S2) and a rat anti-mouse TNFα antibody (data not shown). These results are consistent with PCR-based analysis of Pan02 tumor tissue, which did not show induction of TNFα (data not shown). Taken together our observations suggest that Pan02 cells do not produce TNFα upon treatment with JP1201 and that the majority of TNFα staining observed in MIA PaCa-2 xenografts is of tumor cell origin.
Additionally, we evaluated if TNFα induction was detectable in vitro after treatment with JP1201 or siRNA-mediated XIAP knockdown (Table S2). Cells (MIA PaCa-2, PANC-1, AsPC-1, and BxPC3) were treated with JP1201 (100 nM) or subjected to XIAP knockdown. Conditioned media was harvested 12, 48, and 96 hours post treatment and analyzed for TNFα levels by ELISA. These results demonstrate that during this time frame a single treatment with JP1201 or knockdown of XIAP does not consistently or robustly induce TNFα expression in these cell lines.
JP1201 + gemcitabine prolongs survival in mice with orthotopic pancreatic tumors
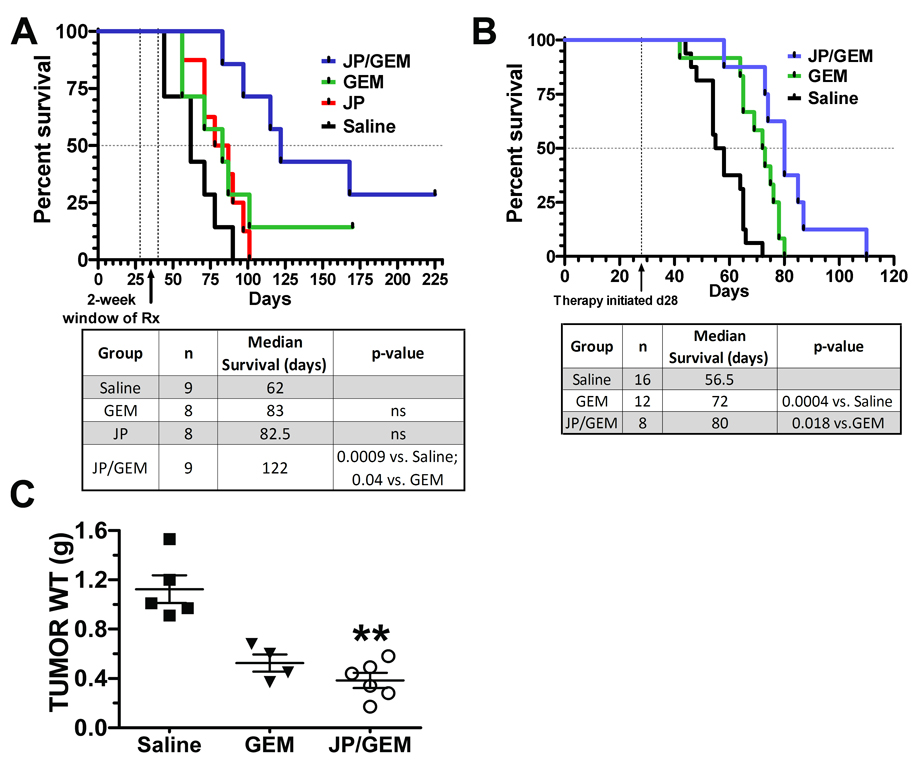
Animal models frequently show therapy-induced tumor growth inhibition, but these drugs often fail to transition to the clinical setting (19). Although, we show tumor regression in a model of advanced cancer, we investigated if the addition of JP1201 to GEM would improve survival. Use of objective endpoints such as survival may help to improve the clinical success of agents validated in animal models (19). Tumor establishment and dosing schedule was identical to that in figure 3A. Following therapy, animals were monitored until they met institutional criteria necessitating human sacrifice. Figure 5A shows that animals bearing orthotopic MIA PaCa-2 tumors treated with JP1201 and GEM had improved survival compared to saline (p<0.001) or treatment with GEM alone (p<0.05). This supports our hypothesis that addition of JP1201 to standard GEM therapy induces tumor regression, leading to improvement in outcomes, such as survival.
Figure 5. Combination of JP1201 with gemcitabine enhances survival of mice with pancreatic cancer.
A, Mice bearing established MIA PaCa-2 tumors were treated using the late intervention protocol described in Fig. 3A. Mice were treated with saline (black line), gemctiabine (GEM, 25 mg/kg, green line), JP1201 (6.0 mg/kg, red line), or the combination of JP/GEM (blue line) 3x/week for two weeks. Animals were monitored and sacrificed when they demonstrated objective signs of tumor burden or when they appeared moribund (as determined by a blinded observer). B, p48-Cre:KRasG12D:Ink4a/Arflox/lox PDAC animals were treated with saline, GEM or combination of JP/GEM starting at 4 weeks of age. Therapy was delivered ip continuously 3x/week until sacrifice. C, A second cohort of PDAC animals underwent 4 weeks of therapy (Saline, n=5; GEM, n=4; JP/GEM, n=6) and were sacrificed at 8 weeks of age. Tumors were excised and weighed and a scatter plot showing mean +/− SEM tumor weight is displayed. Compared to control (1.124 ± 0.1135 g), JP/GEM significantly reduced tumor growth (0.3833 ± 0.1492 g) **, p<0.01. Each panel represents a single animal experiment.
JP1201 + gemcitabine is effective in an aggressive transgenic model of pancreatic cancer
Improvements in mouse modeling may increase the rate of successful transition of preclinical compounds into clinical use. Genetically engineered mouse models represent one such approach to improve mouse models of human disease. We employed a transgenic mouse model of pancreatic cancer based on activating mutations of the oncogene Kras and deletion of the tumor suppressor gene Ink4a (14, 20). Animals with pancreas selective activation of Kras and homozygous deletion of Ink4a develop aggressive disease at approximately 6 weeks of age and die between 8–11 weeks post birth. Animals were genotyped, placed randomly into treatment cohorts (saline, GEM or JP1201 + GEM) and therapy was initiated at approximately 4 weeks of age. We performed a survival (Fig. 5B) and an endpoint study (Fig. 5C). The results demonstrate that the combination of JP1201 + GEM is superior to GEM alone (p<0.05) in prolonging survival and that combination therapy significantly reduces tumor burden (p<0.01) after 4 weeks of therapy.
Discussion
The prognosis for patients with pancreatic cancer is poor (1). Chemotherapeutic options are limited, and the standard of care, GEM, improves survival only minimally (21). Resistance to apoptosis is a critical event in tumorigenesis as it allows for the development of mutations, survival in harsh conditions, and proliferation in an anchorage-independent fashion (22). Efforts to increase therapy-induced apoptosis are appropriate and attractive for cancer chemotherapy (5, 22). We demonstrate that JP1201, a novel Smac mimetic, robustly improves the efficacy of standard chemotherapy in pancreatic cancer xenografts, syngenic tumors, and spontaneous transgenic tumors in mice. In vitro mechanistic studies demonstrated that inhibition of XIAP is critical for sensitization of MIA PaCa-2 and PANC-1 cells. While in vivo treatment with JP1201 resulted in induction of TNFα, these data suggest that JP1201 promotes apoptosis via two potential mechanisms that result in enhanced activity of GEM.
TRAIL is known to induce apoptosis in a variety of cell lines and is currently being pursued as a potential cancer therapy. We show here a moderate effect of TRAIL monotherapy in vivo and potent anti-tumor activity in animals that were treated with the combination of JP1201 and TRAIL. This mirrors the in vitro data with MIA PaCa-2 cells. Other studies in glioma (23) and pancreatic cancer (10) models show TRAIL alone is ineffective but TRAIL combined with inhibition of XIAP can induce apoptosis and inhibit tumor growth. These studies showed a reduction in tumor burden and/or an increase in survival, consistent with the results presented here. Thus, our data support that Smac mimetics in combination with TRAIL is a promising anticancer strategy.
Since GEM is the standard of care for pancreatic cancer, we determined if inhibition of XIAP would sensitize tumors to GEM therapy. We found that knockdown of XIAP but not related IAPs using siRNA increases sensitivity to GEM in pancreatic cancer cell lines. This data expands on work in other pancreatic cancer cell lines that shows resistance to chemotherapy is mediated in part by XIAP (8, 17). We also show that the sensitization to GEM with XIAP knockdown is strikingly similar to the sensitization seen with JP1201 in vitro; supporting the concept that JP1201 sensitizes pancreatic cancer to GEM through inhibition of XIAP.
We also explored whether JP1201 would sensitize tumors to GEM treatment. We demonstrated a significant decrease in tumor weights in animals treated with the combination of JP1201 and GEM compared to saline in a late intervention model in both immune-compromised and immune-competent mice. The JP1201 sensitization was also evident at lower doses of GEM. In a separate experiment, this effect translated into increased survival in tumor-bearing animals. These data alone are very suggestive that Smac mimetics combined with standard therapy could greatly improve pancreatic cancer patient response to therapy. This study adds to the growing body of literature that demonstrate the potency of Smac mimetics in combination with various standards of care, such as a study done in a glioblastoma model where Smac mimetics were shown to enhance tumor cell death after irradiation treatment (24). We further explored the effect of combination therapy in an aggressive transgenic model of pancreatic cancer and again saw increased survival.
We also found that treatment with JP1201 induced TNFα in vivo. Previous reports (12, 25, 26) have shown that Smac mimetic sensitive cell lines produce TNFα, can be induced to produce more TNFα after Smac mimetic treatment, and are dependent on TNFα for Smac mimetic-induced cell death. However, there is currently no published data indicating Smac mimetics induce TNFα in resistant cell lines, such as MIA PaCa-2. Possible explanations include prolonged treatment with JP1201 may induce TNFα production. Alternatively, the in vivo tumor microenvironment may be required to facilitate TNFα production after treatment with JP1201 or other Smac mimetics. In that regard, it is worth noting that while the pancreatic cell lines showed in vitro resistance to JP1201, xenografts did show reduction of tumor burden with JP1201 as a monotherapy, which might be linked to the production of TNFα. These observations suggest that alternative pathways may be engaged that promote cell death. TNFα expression has been linked to non-canonical activation of NFκB as a result of cIAP1/2 degradation (25, 27). NFκB can in turn stimulate autocrine expression of TNFα (25, 26, 28, 29), which activates receptor interacting protein kinase 1 (RIPK1) via the TNF receptor and subsequently induces caspase activation. In support of this, preliminary studies suggest that NFκB actively particpates in JP1201-mediated sensitization of pancreatic tumor cells, as treatment of cells with GEM, JP1201 and an IKK inhibitor (SC-514) increased the IC50 for GEM 3–13 fold compared to JP1201 + GEM (data not shown). Thus JP1201 could promote apoptosis by inducing the degradation of cIAP1/2, resulting in NFκB activation, and inhibiting the activity of XIAP both of which could potentiate the efficacy of GEM (30).
In summary resistance to therapy-induced apoptosis is a major road block in the treatment of patients with pancreatic cancer. Another significant challenge is the lack of predictive power of pre-clinical models of pancreatic cancer. In an effort to address both of these challenges, we have demonstrated potent anti-tumor activity of a unique Smac mimetic in animal models of pancreatic cancer that are robust and clinically relevant. We believe our data support the development of agents designed to sensitize pancreatic tumors to apoptosis and suggest JP1201 deserves serious consideration for clinical investigation.
Supplementary Material
Acknowledgments
Grant Support: This work was supported in part by a sponsored research agreement from Joyant Pharmaceuticals (to. R. Brekken), the Effie Marie Cain Scholarship in Angiogenesis Research (to R. Brekken), and postdoctoral fellowship from Susan G. Komen for the Cure (to S. Dineen).
We thank Drs. Xiaodong Wang, Lai Wang, and Lin Li for advice and technical support and members of the Brekken lab and Drs. Niranjan Awasthi and Roderich Schwarz for support and thoughtful discussion.
Footnotes
Disclosure of Potential Conflicts of Interest
R.A. Brekken: commercial research grant from Joyant Pharmaceuticals
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA: a cancer journal for clinicians. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Westphal S, Kalthoff H. Apoptosis: targets in pancreatic cancer. Molecular cancer. 2003;2:6. doi: 10.1186/1476-4598-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fesik SW. Promoting apoptosis as a strategy for cancer drug discovery. Nature reviews. 2005;5:876–885. doi: 10.1038/nrc1736. [DOI] [PubMed] [Google Scholar]
- 4.Salvesen GS, Duckett CS. IAP proteins: blocking the road to death's door. Nat Rev Mol Cell Biol. 2002;3:401–410. doi: 10.1038/nrm830. [DOI] [PubMed] [Google Scholar]
- 5.Reed JC. Apoptosis-targeted therapies for cancer. Cancer cell. 2003;3:17–22. doi: 10.1016/s1535-6108(02)00241-6. [DOI] [PubMed] [Google Scholar]
- 6.Lopes RB, Gangeswaran R, McNeish IA, Wang Y, Lemoine NR. Expression of the IAP protein family is dysregulated in pancreatic cancer cells and is important for resistance to chemotherapy. International journal of cancer. 2007;120:2344–2352. doi: 10.1002/ijc.22554. [DOI] [PubMed] [Google Scholar]
- 7.Karikari CA, Roy I, Tryggestad E, et al. Targeting the apoptotic machinery in pancreatic cancers using small-molecule antagonists of the X-linked inhibitor of apoptosis protein. Molecular cancer therapeutics. 2007;6:957–966. doi: 10.1158/1535-7163.MCT-06-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shrikhande SV, Kleeff J, Kayed H, et al. Silencing of X-linked inhibitor of apoptosis (XIAP) decreases gemcitabine resistance of pancreatic cancer cells. Anticancer research. 2006;26:3265–3273. [PubMed] [Google Scholar]
- 9.Li L, Thomas RM, Suzuki H, De Brabander JK, Wang X, Harran PG. A small molecule Smac mimic potentiates TRAIL- and TNFalpha-mediated cell death. Science (New York, NY. 2004;305:1471–1474. doi: 10.1126/science.1098231. [DOI] [PubMed] [Google Scholar]
- 10.Vogler M, Walczak H, Stadel D, et al. Small molecule XIAP inhibitors enhance TRAIL-induced apoptosis and antitumor activity in preclinical models of pancreatic carcinoma. Cancer Res. 2009;69:2425–2434. doi: 10.1158/0008-5472.CAN-08-2436. [DOI] [PubMed] [Google Scholar]
- 11.Vogler M, Walczak H, Stadel D, et al. Targeting XIAP bypasses Bcl-2-mediated resistance to TRAIL and cooperates with TRAIL to suppress pancreatic cancer growth in vitro and in vivo. Cancer Res. 2008;68:7956–7965. doi: 10.1158/0008-5472.CAN-08-1296. [DOI] [PubMed] [Google Scholar]
- 12.Petersen SL, Wang L, Yalcin-Chin A, et al. Autocrine TNFalpha signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer cell. 2007;12:445–456. doi: 10.1016/j.ccr.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dineen SP, Lynn KD, Holloway SE, et al. Vascular endothelial growth factor receptor 2 mediates macrophage infiltration into orthotopic pancreatic tumors in mice. Cancer research. 2008;68:4340–4346. doi: 10.1158/0008-5472.CAN-07-6705. [DOI] [PubMed] [Google Scholar]
- 14.Aguirre AJ, Bardeesy N, Sinha M, et al. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 2003;17:3112–3126. doi: 10.1101/gad.1158703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nature reviews. 2002;2:897–909. doi: 10.1038/nrc949. [DOI] [PubMed] [Google Scholar]
- 16.Hamacher R, Schmid RM, Saur D, Schneider G. Apoptotic pathways in pancreatic ductal adenocarcinoma. Molecular cancer. 2008;7:64. doi: 10.1186/1476-4598-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Jian Z, Xia K, et al. XIAP is related to the chemoresistance and inhibited its expression by RNA interference sensitize pancreatic carcinoma cells to chemotherapeutics. Pancreas. 2006;32:288–296. doi: 10.1097/01.mpa.0000218314.67111.fb. [DOI] [PubMed] [Google Scholar]
- 18.He S, Wang L, Miao L, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 19.Man S, Munoz R, Kerbel RS. On the development of models in mice of advanced visceral metastatic disease for anti-cancer drug testing. Cancer metastasis reviews. 2007;26:737–747. doi: 10.1007/s10555-007-9087-6. [DOI] [PubMed] [Google Scholar]
- 20.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science (New York, NY. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujioka S, Niu J, Schmidt C, et al. NF-kappaB and AP-1 connection: mechanism of NF-kappaB-dependent regulation of AP-1 activity. Molecular and cellular biology. 2004;24:7806–7819. doi: 10.1128/MCB.24.17.7806-7819.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 23.Fulda S, Wick W, Weller M, Debatin KM. Smac agonists sensitize for Apo2L/TRAIL- or anticancer drug-induced apoptosis and induce regression of malignant glioma in vivo. Nature medicine. 2002;8:808–815. doi: 10.1038/nm735. [DOI] [PubMed] [Google Scholar]
- 24.Vellanki SH, Grabrucker A, Liebau S, et al. Small-molecule XIAP inhibitors enhance gamma-irradiation-induced apoptosis in glioblastoma. Neoplasia (New York, NY. 2009;11:743–752. doi: 10.1593/neo.09436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vince JE, Wong WW, Khan N, et al. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 2007;131:682–693. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 26.Varfolomeev E, Blankenship JW, Wayson SM, et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131:669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 27.Bai L, Chen W, Wang X, Tang H, Lin Y. IKKbeta-mediated nuclear factor-kappaB activation attenuates smac mimetic-induced apoptosis in cancer cells. Molecular cancer therapeutics. 2009;8:1636–1645. doi: 10.1158/1535-7163.MCT-09-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaither A, Porter D, Yao Y, et al. A Smac mimetic rescue screen reveals roles for inhibitor of apoptosis proteins in tumor necrosis factor-alpha signaling. Cancer Res. 2007;67:11493–11498. doi: 10.1158/0008-5472.CAN-07-5173. [DOI] [PubMed] [Google Scholar]
- 29.Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 30.Lu J, Bai L, Sun H, et al. SM-164: a novel, bivalent Smac mimetic that induces apoptosis and tumor regression by concurrent removal of the blockade of cIAP-1/2 and XIAP. Cancer Res. 2008;68:9384–9393. doi: 10.1158/0008-5472.CAN-08-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.