Abstract
Previous research suggests that the maternal medial prefrontal cortex (mPFC) may play a role in maternal care and that cocaine sensitization before pregnancy can affect neuronal activity within this region. The present work was carried out to test whether the mPFC does actually play a role in the expression of maternal behaviors in the rats and to understand what specific behaviors this cortical area may modulate. In the first experiment, tetrodotoxin (TTX) was used to chemically inactivate the mPFC during tests for maternal behavior latencies. Lactating rats were tested on postpartum day 7–9. The results of this first experiment indicate that there is a large effect of TTX-induced inactivation on retrieval behavior latencies. TTX nearly abolished the expression of maternal retrieval of pups without significantly impairing locomotor activity. In the second experiment, GABA-mediated inhibition was used to test maternal behavior latencies and durations of maternal and other behaviors in postpartum dams. In agreement with experiment 1, it was observed that dams capable of retrieving are rendered incapable by inhibition in the mPFC. GABA-mediated inhibition in the mPFC largely reduced retrieval without altering other indices of maternal care and non-specific behavior such as ambulation time, self-grooming, and inactivity. Moreover, in both experiments dams were able to establish contact with pups within seconds. The overall results indicate that the mPFC may play an active role in modulating maternal care, particularly retrieval behavior. External factors that affect the function of the frontal cortical site may result in significant impairments in maternal goal-directed behavior as reported in our earlier work.
Keywords: Maternal behavior, Medial Prefrontal Cortex, Prelimbic Cortex, Anterior Cingulate Cortex, Pup Retrieval, mPFC, Motivation, Emotion, Reward, Site-specific Injections
1. Introduction
Subcortical neural circuits that mediate the expression of maternal behaviors have been studied in detail across different species of mammals and using various in vivo and ex vivo techniques. In the rat, retrieval, nest building, and pup licking are maternal behaviors that involve the medial preoptic area (mPOA), ventral pallidum (VP), bed nucleus of stria terminalis (BNST), ventral tegmental area (VTA), the shell and core of the nucleus accumbens (NAC), medial and central amygdala and the olfactory system (Fleming and Walsh, 1994; Lee et al., 1999; Li and Fleming, 2003; Numan et al., 1977; Numan and Smith, 1984; Numan et al., 2005b; Numan, 2007; Stack et al., 2002). Kyphotic nursing and nest aggression is associated with neuronal activity in the periaqueductal grey and has also been associated to a diversity of sensory inputs from pups (Kolunie et al., 1994; Lonstein and Stern, 1997a; Lonstein and Stern, 1997b; Lonstein et al., 1998b; Lonstein and Stern, 1998; Stern and Mackinnon, 1978; Stern and Johnson, 1989; Stern and Kolunie, 1993; Stern and Lonstein, 2001). Although less attention has been placed recently on the role of the cerebral cortex in maternal behaviors, there has been considerable historical interest in its participation in multisensory processing and in controlling subcortical outputs leading to maternal care. Beach (1937) and Stone (1938) observed that removal of 1–50% cortical tissue was sufficient to dramatically affect maternal behavior, especially pup retrieval, and this was not merely due to inability to physically move pups. Slotnick and Nigrosh (Slotnick, 1967; Slotnick and Nigrosh, 1975) and Stamm (1955) showed that removal of medial aspects of cerebral cortex, including most of the anterior and posterior cingulate cortex reduces maternal care, observing predominant reductions in retrieval behavior, in rats and mice. A similar impact of midline cortical ablation on retrieval behavior has been observed in other species of rodents (Murphy et al., 1981). Much more recently, Afonso et al. (2007) show that pre-pregnancy excitotoxic lesions of the medial prefrontal cortex (mPFC, including prelimbic and infralimbic areas) significantly alter both sexual and maternal behaviors in female rats. Therefore, the timing of the selective damage to this cortical area before pregnancy can still impair the ability to subsequently express maternal behaviors. It is unclear whether affecting the neuronal activity in mPFC after parturition might also lead to a similar influence on maternal behaviors. If so, then GABA-mediated inhibition or chemical inactivation of the mPFC during the postpartum period should affect maternal behavior expression.
Using functional MRI, Febo and Ferris (2007) and Ferris et al. (2005) previously reported increased neuronal activity in mPFC in response to suckling stimulation from pups. Activity in the maternal prefrontal cortex in response to suckling pups was dramatically reduced by cocaine sensitization before pregnancy. Animals pretreated with cocaine were also observed to display greater levels of retrieval behavior, but a direct causal link between changes in prefrontal cortical activity and retrieval behavior was not provided (Febo and Ferris, 2007). However, the results from this latter study did suggest that limbic regions of the prefrontal cortex may play a role in retrieval and other maternal behaviors and that its role may be adversely affected by cocaine sensitization (Febo and Ferris, 2007). In human functional MRI studies there is also evidence of a role for limbic prefrontal regions in maternal care. Mother’s responding to infant sensory cues showed BOLD activity in areas of the limbic prefrontal cortex such as the anterior cingulate, insula, orbital and ventromedial frontal regions (Bartels and Zeki, 2000; Leibenluft et al., 2004; Lorberbaum et al., 2002; Nitschke et al., 2004; Noriuchi et al., 2008; Ranote et al., 2004; Strathearn et al., 2008; Swain et al., 2008). In the present study, we tested whether neuronal activity in mPFC participates in the expression of maternal care in rat. This was accomplished by localized inactivation with tetrodotoxin or GABA-mediated inhibition of the medial PFC before testing for maternal behaviors. Given the wealth of anatomical data demonstrating connectivity of prefrontal cortical areas with key limbic subcortical sites such as the olfactory cortex, hypothalamus, amygdala and nucleus accumbens (Hoover and Vertes, 2007; Ongur et al., 1998; Ongur and Price, 2000; Ongur et al., 2003; Vertes, 2002; Vertes, 2004), we hypothesized that neuronal activity within mPFC would heavily influence the expression of postpartum maternal behaviors.
2. Results
2.1 Experiment 1: TTX Inactivation of mPFC dramatically reduces drive to retrieve pups
TTX or aCSF was infused into the mPFC of dams 25–30 minutes before testing for latencies to express several maternal behaviors. By chemically inactivating sodium channel conductance on neurons located in mPFC using TTX we anticipated a suppression of neuronal activity within this cortical region that would allow us to test for changes in maternal behaviors.
Dams were able to notice and attend to the presence of pups within the cage regardless of pretreatment. After returning pups to the home cage, dams would immediately make contact with them and shortly thereafter begin retrieval. Establishing initial contact with pups took on average about 5–30 seconds and no differences were observed between control and TTX dams in the time it took them to initiate contact with pups (t12 = 0.8, p =0.4). It generally took control dams about 50–100 seconds to begin picking pups and moving them to a localized region of the home cage. Completion of retrieval behavior occurred in about 5 minutes. Out of the 9 rats tested, 1 did not retrieve during the 15-minute test. Shortly after completing retrieval behavior, dams would carry out other activities such as continuous grouping of pups that moved away from the litter, moving bedding material around the cage, chewing on food pellets, drinking water, and sniffing around the cage. In some instances dams would also mouth and groom pups and in very few cases nursing postures were taken inside of the initial 15-minute testing period. These latter behaviors generally occurred, if they did, almost at the end of the trial. However, almost all control dams were nursing their pups at 30–60 minutes during the post-test spot checks.
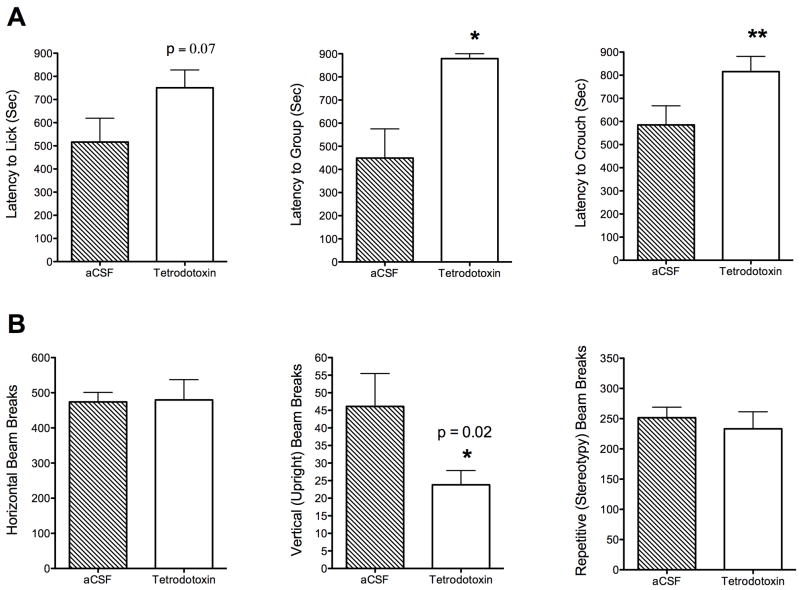
TTX clearly abolished retrieval behavior in rats. Out of the 13 rats tested, only 1 dam retrieved pups (Fig. 1). Latency to retrieve the first and last pups was significantly lower in the TTX treated dams (t20 = 6.4, p < 0.0001 and t20 = 4.1, p =0.0005, respectively). Although grouping and crouching latencies were affected by TTX treatment (t20 = 4.2, p =0.0007 and t20 = 2.2, p =0.04, respectively)(Fig. 2), the latency to groom pups, sleep and nest were not significantly different (data not shown). Dams were very active during the testing session, and even though not recorded in experiment 1, they would frequently and briefly hover over the pups outside the nest area. At 30–60 minutes during the spot checks, TTX injected rats showed significantly less pups in the nest than controls. We observed here that locomotor activity in a novel test cage environment was not affected by TTX treatment, as measured by similarities in horizontal photocell interruptions during 5 minutes using automated activity cages (Fig. 2). We anticipated TTX effects to last the test session since it has been reported to remain in several organs of the body from 30 min to 4 hours (Kao, 1966). However, it is important to note that TTX reduced vertical activity (t17 = 2.5, p =0.02). It is noted that during surgeries, cannula guides were advanced from 2–3 mm below dura mater during. Injector tips extended 1.5 mm beyond the guide tip in order to place injections below 3.5–4.5 mm, resulting in injections that would cover most of mPFC across animals. Gross histological verification of the cannula sites indicated that their location was spread out throughout most of mPFC and was not restricted to a localized region with the prelimbic area (Fig. 1).
Figure 1.
Latency to retrieve 6 pups to nest site during a 15- minute test. Only data for the first and last pup is shown. Groups correspond to PD 7–9 dams microinjected with TTX (n = 13) or aCSF vehicle (n = 9). All data are presented as average seconds ± standard error of the mean. Asterisks denote significant differences at p < 0.05. Above the graphs are Paxinos and Watson atlas coronal maps showing rostral Bregma coordinates 2.70–3.70 mm where microinjections were given.
Figure 2.
A) Latency to express pup licking, grouping into the nest site and crouching over pups during a 15- minute test. Groups correspond to PD 7–9 dams microinjected with TTX (n = 13) or aCSF vehicle (n = 9). All data are presented as average seconds ± standard error of the mean. Asterisks denote significant differences at *p < 0.05 or **p < 0.01. B) Measurement of locomotor activity in response to a novel environment after testing for maternal behaviors. Groups correspond to PD 7–9 dams microinjected with TTX (n = 13) or aCSF vehicle (n = 9). Injections were given into the mPFC approximately 90 minutes before testing. All data are presented as mean ± standard error of the mean. Asterisks denote significant differences at p < 0.05.
2. 2 Experiment 2: GABA receptor stimulation in mPFC controls drive to retrieve pups
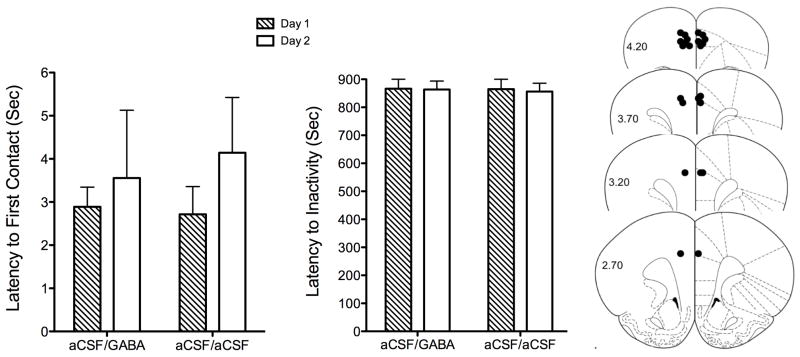
If, as shown in experiment 1, preventing action potential generation with TTX reduces maternal care, then inhibiting neurons locally by stimulating GABA receptors should have a similar effect. For experiment 2, dams were injected with vehicle on the first day of testing. Behavioral observations on this day provided baseline measurements upon which we could compare the effects of GABA-mediated inhibition on the second day. Animals demonstrated a very short latency, around the order of 2–4 seconds, to approach pups on day 1 (Fig. 3). In addition, the latency to become inactive (sleep) was not affected by treatment or day of injection (Fig. 4). This did not vary significantly across test days, regardless of treatment. Out of the total 16 rats tested following vehicle administration on day 1, only 3 did not show any retrieval on this day (the total number includes dams from both control and agonist treatment groups received only vehicle aCSF on day 1). Average retrieval times ranged from 100–500 seconds (Fig. 4). GABA agonist administration to 9 rats on day 2 significantly reduced their retrieval behavior (Fig. 4A; t16 = 4.5, p =0.0004 for the first pup and t16 = 3.6, p =0.002 for the last pup). Retrieval was unaffected in dams that were instead injected with vehicle on day 2 (Fig. 4B). When viewed as percent pups retrieved, GABA receptor-mediated inhibition showed a strong overall effect of reducing retrieval significantly on day 2 (Fig. 5; ANOVA F 3,31 = 5.2, p = 0.005). GABA agonist treated dams also showed significantly lower retrieval as compared with dams injected with vehicle on day 2 instead (F 3,31 = 2.8, p < 0.01).
Figure 3.
Latency to first contact pups during a 15-minute test on two separate days of testing. Shown on the right is the latency to inactivity (sleep). Rats were tested on day 1 following a single microinjection into the mPFC of aCSF vehicle and then tested on day 2 following administration within the same area of a GABA agonist cocktail mixture (n = 9). Controls received vehicle injections into the mPFC on both days (n = 7). On the right to the graphs are Paxinos and Watson atlas coronal maps showing rostral Bregma coordinates 2.70–4.20 mm where microinjections were given.
Figure 4.
Latency to retrieve 12 pups during 15-minute tests on two separate days of testing. Only data for the first and last pup is shown. A) Rats received aCSF vehicle microinjections on day 1 as a baseline measurement of their retrieval behavior and then received a GABA agonist cocktail mixture on day 2 (n = 9). B) Rats received aCSF vehicle microinjections on both test days (n = 7). All data are presented as average seconds ± standard error of the mean. Asterisks denote significant differences at p < 0.05.
Figure 5.
Percentage of pups retrieved on two separate test days. Groups and number of animals are the same as Fig. 4–5. All data presented as average percent ± standard error of the mean. Asterisks denote significant differences at p < 0.05. *Compared to Day 1 aCSF group, **compared to Day 2 GABA Agonists group.
We also measured duration of specific home cage behaviors during the 15-minute test session. This was accomplished using video analysis using OdLog. Retrieval duration was significantly reduced by muscimol/baclofen treatment on day 2 (ANOVA F 3,31 = 4.1, p = 0.01) (Fig. 6). Duration of grouping also showed a trend towards statistical significance (t16 = 1.9, p =0.06 two-tailed, independent sample t-test). No effect of GABA agonist treatment was observed for the duration of other pup directed behaviors such as licking, hovering over pups, crouching, and burrowing in cage bedding (Fig. 6). Other non-pup directed behaviors, ambulation, inactivity and self-grooming, were also not affected by day of treatment or GABA-mediated inhibition of the mPFC (Fig. 7). Most cannula guide tracks were observed to be located rostrally just above the mPFC (Fig. 4).
Figure 6.
Duration of maternal behaviors on two separate days of testing. Groups and number of animals are the same as Fig. 4–5. All data are presented as average seconds ± standard error of the mean. Asterisk and symbol denotes significant differences at p < 0.05.
Figure 7.
Duration of other home cage behaviors on two separate days of testing. Groups and number of animals are the same as Fig. 4–5. All data are presented as average seconds ± standard error of the mean. Asterisk and symbol denotes significant differences at p < 0.05
3. Discussion
The present work provides significant evidence that neuronal activity in mPFC is important in maternal care. Moreover, it appears that activity of neurons in this cortical structure is important for expression of retrieval behavior. We observed that inactivation of mPFC results in a dramatic reduction in retrieval behavior without a significant loss of locomotor activity in response to a novel environment or the ability to detect and contact pups within the home cage. Inhibition of mPFC through a GABAergic mechanism led to a similar finding. Thus, neuronal activity in mPFC controls the expression of maternal care, as measured by expression of retrieval and grouping pups in a location with the nest.
3.1 Possible subcortical circuitry controlled by mPFC
The neural circuitry underlying many maternal behaviors in rats have been well-studied (Numan, 2007). A long established notion has been that subcortical sites contain the necessary encoding that allows the expression of innate behaviors such as maternal behaviors (Beach, 1937; Stone, 1938). A large body of literature supports a role of mPOA, bed nucleus of the stria terminalis, lateral septum, medial and cortical amygdala, anterior cingulate cortex, NAC, VTA and and substantia nigra, among others (Koch and Ehret, 1991; Li and Fleming, 2003; Lonstein et al., 1998b; Lonstein and Stern, 1998; Numan and Corodimas, 1985; Sheehan et al., 2001; Sheehan et al., 2000; Slotnick and Nigrosh, 1975; Stack et al., 2002). Numan (2007) has hypothesized that the mPOA sends efferent inputs to the VTA, perhaps driving active components of maternal behaviors such as retrieval, grooming, and nesting. Similar circuits may be important for enhancing the reinforcing properties of maternal-pup interactions (Febo and Ferris, 2007; Ferris et al., 2005; Mattson and Morrell, 2005). Work by Numan et al. (2005) has further demonstrated that retrieval behavior is under selective control of D1 receptors in the accumbens shell (Numan et al., 2005a), a region that receives input from the mPFC. Quiescent aspects of maternal behaviors as observed during arched back nursing likely involves an additional set of brain structures such as the periaqueductal grey (Lonstein et al., 1998a; Lonstein and Stern, 1998) and perhaps inhibitory influences upon structures mediating active maternal behaviors (Sheehan et al., 2000). Based on our present data, it is possible that the mPFC participates in the modulation of active maternal behaviors, particularly retrieval, and not the quiescent aspects involved in lactation. Thus, mPFC activity may be closely associated with the known role of the accumbens, VTA and mPOA in the expression of active maternal behaviors and not in maternal arched back nursing. This statement may not be implausible given the connectivity of the mPFC subregions (prelimbic and infralimbic) with subcortical structures that modulate emotional responses and a various goal-oriented behaviors (Hoover and Vertes, 2007). Indeed, there is evidence that both prelimbic and infralimbic areas comprising the mPFC project to specific striatal, amygdala, midbrain and hypothalamic nuclei (Vertes, 2004; Gabbott et al., 2005), and this may in turn play some role in controlling maternal retrieval.
The rostral-caudal location of the injection sites varied between experiment 1 (TTX) and 2 (GABA agonists). GABA agonists were injected rostral to the majority of the injection sites for TTX. The depth of the injections was generally similar between the two experiments. Despite the variation in the injection technique, the behavioral results appeared to be similar. Inhibition or inactivation had a negative impact on maternal retrieval behavior. However, a closer observation shows that the TTX injections also affected latency to crouch and produced a non-significant trend towards a reduced latency to lick and a trend towards a longer latency to establish contact. In addition, reduced vertical (upright) activity was observed in response to a novel open field environment. These effects on latencies and on durations of these and other behaviors were not observed in the GABA mediated inhibition study. Instead, we observed that the effect of inhibition through GABA receptor stimulation seemed to be selective for retrieval behavior. These animals received the injections more rostrally. Gabbott et al. (2005) provide flat maps of the subregions of the prefrontal cortex in the rat and they show that projections to subcortical areas can vary across the layers of the cortex and over the rostral-caudal extension. Medial PFC projects to extensive subcortical areas regions such as the mibrain VTA, PAG, lateral hypothalamus, septum, ventral and dorsal striatum in a topographic fashion with outputs varying through the dorsal-ventral extension (Gabbott et al., 2005). However, the overall negative impact of mPFC inactivation or inhibition achieved an overall similar effect, which may have been a consequence of fluid diffusion.
3.2 Role of the cortex in maternal care
The selective role of the mPFC in maternal behaviors was partly demonstrated in classical studies. Removal of the cortical mantle in neonatal cats and hamsters did not affect the development of species typical patterns of maternal and sexual behaviors (Bjursten et al., 1976). However, destruction of the midline cortical regions that include the mPFC rostrally towards the posterior cingulate caudally did have disruptive impact in hamster maternal behaviors (Murphy et al., 1981). This result was further supported in a study published by Stamm (1955), in which only removal of the midline but not lateral regions of the neocortex in rat markedly reduced several maternal behaviors (Stamm, 1955). However, the surgical ablations provided in these studies were extensive, and thus, the disruptive effects on maternal behavior were more widespread. Postpartum rats showing a greater preference for a pup-paired environment also show increased c-fos expression in the ventral striatum and mPFC (Mattson and Morrell, 2005). Fleming and co-workers recently observed that prepregnancy lesions of the medial prefrontal cortex disrupted postpartum retrieval in addition to other maternal behavior parameters measured in Sprague-Dawley rats (Afonso et al., 2007). They report that excitotoxic lesions given before pregnancy to female rats were still able to affect postpartum maternal behaviors. Interestingly, retrieval rates were significantly influenced by mPFC activity, females with mPFC lesions showing longer latencies to retrieve (as reported in the present studies using Long Evans rats). Their study, in addition to ours, supports a role for the mPFC in maternal motivation. They further generalize their findings to female sexual motivation (Afonso et al., 2007). Here, we further demonstrate that not only might the mPFC be involved in the development of maternal behaviors (Afonso et al., 2007) but also play an active part in ongoing maternal behaviors after they have arisen during the early postpartum period.
3.3 GABAergic neurotransmission and maternal behavior
GABA release from neocortical interneurons plays a significant role in intracortical processing and controlling output of excitatory pyramidal cells (Bacci et al., 2005). The mPFC send outputs to subcortical regions that are important for the expression of maternal behavior, particularly retrieval, such as the nucleus accumbens and ventral tegmental area (Numan, 2007; Gabbott et al., 2005). We used to supra-threshold concentrations of GABA agonists that bind and activate GABAA/B receptor subtypes in order to temporally inhibiting the mPFC. However, given that GABA mediated-inhibition reduced retrieval so dramatically, the effects GABA on maternal responding deserve a closer look. There is evidence that both GABAA receptor channel and the GABAB G protein coupled receptors are hormonally modulated. Allosteric modulation of GABAA mediated chloride channel conductance at the barbiturare site by allopregnanolone and progesterone has been well documented (Westerling et al., 1991; Hendersen, 2007). Although there is less evidence for hormonal modulation of the GABAB receptor, Febo and Segarra (2004) reported reductions in GABAB receptor coupled G protein activation in the presence of chronically high estrogen levels. The observed effects were reported for both the entorhinal cortex, which serves important roles in hippocampal dependent learning, and the ventral tegmental area, which contain GABAB receptors that control dopaminergic neuronal firing. More extensive work on estrogen modulated GABA mediated G protein coupling in the guinea pig hypothalamus has been provided by Kelley and associates (Kelly et al., 1992; Qiu et al., 2003). This group has reported similar desensitization effects with estrogen treatment and they have provided significant evidence that the hormonal modulation occurs within minutes, suggesting a novel estrogenic mechanism mediated through the GABAB receptor (Qiu et al., 2008).
GABA-mediated inhibition plays important function in subcortical circuits controlling the expression of maternal behaviors. For example, Numan et al recently administered GABA agonists, muscimol and baclofen, separately into the ventral tegmental area and observed that intra-VTA injections of baclofen disrupted the expression of retrieval behavior without any effect on nursing (Numan et al., 2009). Others have shown that reproductive experience increases the expression of GABAA receptors in the medial preoptic area (Byrnes et al., 2007). Given that estrogen receptors have been identified in neocortical areas (Shughrue et al., 1997), particularly in the mPFC of various species (Montague et al., 2008), it is tempting to speculate that hormonally modulated GABAergic neurotransmission might serve certain cognitive and goal-directed aspects of reproductive behaviors.
3.4 Concluding remarks
Finally, it is important to note caveats in experimental design and the present interpretation of the data. With respect to design, we were unable to discern whether neuronal activity inhibited by TTX or GABA agonists corresponded to that of populations of output neurons within specific layers of the mPFC or to that of passing fibers from other areas of the cortex. Although, we attempted to address this by using GABA agonists (to inhibit local neurons) as opposed to TTX (to inhibit sodium channels on somas, dendrites and axons), this matter is still unclear. Sites that are not involved in maternal retrieval behavior were not chosen as controls in the present study. Thus, cortical area specificity, layer-specific and subregion specificity of injections on maternal behaviors was also not addressed in the present study, but should be accounted for in future work.
With regards to our present interpretation, we argue here that the mPFC might modulate maternal motivation to retrieve pups, as opposed to generalized motor responses. Despite our inability to observe any effects of TTX-inactivation and GABA-mediated inhibition on general activity, arousal and attention to the presence of pups in the home cage, we did find a reduction in upright movements (vertical activity). This was measured over 60 minutes after initial injection; therefore it might be possible that the locomotor reducing effects of TTX may have been present immediately after injections. However, the literature states otherwise. For instance, Corcoran and Quirk (Corcoran and Quirk, 2007) have reported that inactivation in the mPFC with a similar dose regime of TTX does not affect locomotor behavior or lever pressing for food 25 minutes after injections.
One may also interpret the present data as arising from a disruption of well-known functions of the mPFC. The mPFC plays significant roles in attention and working memory processes (Ng et al., 2007; Rossetti and Carboni, 2005) that may have been affected by inhibiton and inactivation. Functions of the mPFC in rats have been tested using other tasks to obtain rewards from the surrounding environment (Walton et al., 2002; Hok et al., 2005;Christakou et al., 2004). Although neuroanatomical work in rats and primates has allowed the recognition of some anatomical similarities of the mPFC across species (Ongur and Price, 2000), the present data in rats might not entirely extend to primates. Human fMRI studies have, however, correlated maternal behavior responses involving positive emotions to be correlated with activity in the orbital prefrontal cortex (Nitschke et al., 2004). An underlying implication of the present work, that is consistent with previous reports (Febo and Ferris, 2007), is that psychiatric illnesses that affect mPFC function may lead to subtle but important changes in the expression of maternal behaviors.
4. Materials and methods
4.1 Subjects
Adult Long-Evans female rats (225–275 g; 70–120 days old) were purchased from Charles River Laboratories (Wilmington, MA). Animals were housed in pairs in a temperature and humidity controlled room and maintained on a 12L:12D light-dark cycle (lights on at 0700 hr–1900 hr). Water and Purina rat chow were provided ad libitum. Home cages consisted of hanging plastic microisolater cages of standard dimensions with woodchip bedding. After mating, dams were housed individually along with their litters. All rats used in the present study are primiparous. Rats were acquired and cared for in accordance with the guidelines published in the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publications No. 85–23, Revised 1985) and adhere to the National Institutes of Health and the American Association for Laboratory Animal Science guidelines. The Institutional Animal Care and Use Committee at Northeastern University approved the protocols used for this study.
4.2 Stereotaxic surgical procedure for microinjections
All drugs and chemicals used for microinjection studies were purchased from Sigma-Aldrich (St Louis, MO). Behavioral testing for experiment 1 was done using 9 control and 13 tetrodotoxin-injected female rats. Behavioral testing in experiment 2 (GABA receptor-mediated inhibition) was done using 7 control and 9 Muscimol/Baclofen injected female rats. Virgin females were housed with sexually experienced male rats for 5 days and then housed individually during pregnancy. Surgical procedures were carried out on postpartum days 2–3 and behavioral testing done 5–6 days later. This time frame was chosen to allow a day of maternal care of newborns prior to surgery and also to avoid extending behavior testing beyond postpartum day 10, in which the motivational properties of pups appear to begin waning (Mattson et al., 2001; Mattson et al., 2003; Mattson and Morrell, 2005). Rats were anesthetized with 2–4% isoflurane gas/air mixture and aligned on the stereotaxic apparatus (Kopf instruments, Tujunga, CA). Body temperature was maintained throughout the surgery with an adjustable warming pad. Bilateral cannula guides (pedestal-mounted 22-gauge stainless steel tubes with 1 mm separation and cut 2–3 mm below the pedestal; Plastics One, Roanoke, VA) were secured on a stereotaxic holder (Model 1766-AP; Kopf Instruments, Tujunga, CA) and lowered into mPFC (Bregma coordinates AP: +3.2 mm, ML: ±0.5 mm and DV: −2 mm for upper mPFC to −3 mm for deeper mPFC; intraraural line was set at −3.4 mm and a flat vertical positioning was verified by sliding the anterior-posterior micromanipulator along the surface of the skull). Cannula guides were anchored to the skull with dental cement and 3 miniature stainless steel self-tapping screws (Thread 0.06 in. 1/8 in. length; J.I. Morris Precision Screws, Southbridge, MA). A bilateral stainless steel obturator (0.35mm diameter, Plastics One, Roanoke, VA) was placed into the guide cannula after surgeries. Both the obturator and stainless steel injectors (28-gauge, bilateral) extended 1.5 mm beyond the tip of the cannula guides (Bregma coordinates for the injection site are DV: −3.5 mm to −4.5 mm into the upper and deeper areas of the prelimbic region, respectively). Rats were given 5–6 days of recovery before behavioral testing. Guide cannula lesion position was examined postmortem in slices covering the medial prefrontal cortex. Frozen fresh tissue slices for experiment 1 were cut inside a −20 °C cryostat at 0.5–1mm thickness just rostral to the cannula placement site and then sectioned at 20–100 μm to locate the cannula’s position in the mPFC (see Supplemental Figure 1 Online). Given the large diameter of the guide cannula’s typically used in microinjection studies, tissue scarring around the cannula site is clearly visible using the present technique. The guiding landmark for locating the cannula guides was the forceps minor of the corpus callosum (see Febo and Ferris, 2007 for example figure). Position data were documented on print outs of an atlas of the rat brain (Paxinos and Watson, 2005). Shown in the Fig.’s 1 and 3 are the locations of the tips of the guide cannulas. Injector and dummy cannula tips extended 1.5 mm beyond the shown sites.
4.3 Maternal Behavior testing following TTX-induced inactivation of mPFC (experiment 1)
Maternal behavior and locomotor activity in response to novelty in experiment 1 were measured following infusion of artificial cerebrospinal fluid (aCSF containing in mM: 145 NaCl, 2.7 KCl, 10 MgCl2, 12 CaCl2, 20 Na2HPO4•7H2O, pH 7.4) or tetrodotoxin (TTX, 5.0 ng per side in aCSF). TTX, which reversibly blocks sodium channel conductance, was used to inhibit action potential generation in the mPFC during behavioral tests. Maternal testing was performed in the home cage in the animal colony during the morning hours (1000–1300 hrs). Litter sizes initially ranged from 10–18 pups, but were standardized to 10–12 pups at the time of surgery on days 2–3. Maternal behavior latencies were tested with 6 pups. All tests in experiment 1 and 2 were conducted with the each dams own pups. Microinjections were given in the laboratory, away from the animal colony, 25–30 minutes before measurements were taken. Injections were made with two 50uL Hamilton syringes connected via PE-50 tubing to a bilateral injector cannula (Plastics One, Roanoke, VA) to give an injection volume of 0.3 μL per side over 1 min. Similar mPFC doses and volumes have been previously used to block cue-induced reinstatement of cocaine self administration (McLaughlin and See, 2003) and extinction of learned fear responses (Corcoran and Quirk, 2007). Infusions were made using a Model R-E Razel pump (St. Albans, VT). The injector was left in place for an additional 2 minutes. Mothers were immediately returned to their cages and transported back to the animal colony. As previously reported (Febo and Ferris, 2007), pups were removed from the home cage for 10–15 minutes and then returned to the cage for testing. A trained observer obtained all of the experimental measures in experiment 1. A nesting area was identified and the pups (6) placed on the side of the cage opposite to the nest. Behaviors measured during a 15 minute testing period included: initial contact with pups, retrieval time for each pup, latency to begin licking pups, latency to hover over pups, latency to crouch, burrow in bedding, inactivity (sleep) and group pups. After returning the pups to the cage, females generally contacted the pups first within seconds to sniff and mouth them and then immediately began retrieving them to the nest. Once all were in the located in a specific area of the home cage, mothers normally would run through a sequence of behaviors that included burrowing in cage bedding, mouthing and sniffing pups, grooming them, grouping pups that move outside the nest, and taking a crouched nursing posture. Animals that did not show any or incomplete retrieval (not retrieving all pups) were given a maximal score of 900 seconds (15-minutes). After completion of the 15-min latency test, mothers were spot checked every 15 minutes for 60 minutes for their position and their pups’ position within the cage. Results were noted as number of pups in cage at 30, 45 and 60mins. Immediately following maternal behavior testing, mothers were placed in automated locomotor activity testing cages (42 cm L × 42 cm W × 30 cm H, with horizontal and vertical infrared beams; Accuscan Instruments, Columbus, OH). Horizontal, vertical and stereotyped activity was measured during a brief 5-minute test, which tested their motor response to the novel environment.
4.4 Maternal Behavior testing following GABA receptor stimulation in mPFC (experiment 2)
The purpose of experiment 2 was two-fold. First, TTX might inhibit passing fibers in addition to local neuron populations in the mPFC and as a toxin it may also result in some level of neurotoxicity. GABA agonists, such as muscimol and baclofen, while inhibiting neuronal activity locally, may minimize these effects. Second, to test whether inhibition of mPFC controls maternal behavior expression through a GABA receptor mechanism we administered 2 drugs known to exert agonist effects at GABAA and GABAB receptor subtypes. Five to six days following surgical recovery, females where habituated for 15 minutes to the test environment. Microinjections began on P6–7. The sequence of daily injections was aCSF control on day 1 and muscimol/baclofen injections on day 2. Control rats received aCSF on both days. Microinjections were performed in the laboratory using the same procedures as experiment 1 with the exception that rats were microinjected with either aCSF or a cocktail mixture of the GABAA agonist muscimol (0.1mM) and the GABAB agonist baclofen (1.0mM). Similar doses of these inhibitory GABA agonists have been administered in the PFC to block cocaine and stress induced reinstatement of cocaine self-administration in rats (McFarland and Kalivas, 2001; McFarland et al., 2004). Animals were lightly anesthetized with 1–4% isoflurane to suppress handling stress during the prolonged injection procedure. Behavioral testing was carried out 30 minutes after injections. Animals generally awoke in about 3–5 minutes after turning off the isoflurane gas flow. Maternal behaviors were recorded for 15 minutes in the home cage inside the laboratory using a high-speed digital camera (30 frames per second; Model DFK 21F04, The Imaging Source, Charlotte, NC) for offline quantification of maternal behaviors. The entire home cage was placed inside a custom designed sound attenuation box (30′ H × 27′ L × 23′ W, Med-Associates, St. Albans, VT) that had independently controllable lighting and circulating air. The sound attenuation box ceiling accommodated the digital camera centralized over the test cage. Maternal behavior digital videos were scored offline by a trained observer using ODLog software (http://www.macropodsoftware.com/index.html). For behavioral testing, the nesting site was noted and all pups (12) were reintroduced into the cage with dams and placed opposite to the nest. Twelve pups were used in the 2nd experiment in order to test whether the longer maternal latencies observed in experiment 1 might be due to a reduced number of pups in the cage during testing. Some dams only had 10 pups that were used in the tests. Both latencies to express maternal behaviors and their duration during the 15-minutes sessions were quantified. In addition, other behaviors were noted such as inactivity (sleep) latencies, self-grooming, and ambulation duration. As in experiment 1, rats were assigned a score of 900 seconds if no retrieval was carried out. A trained observer, different from the observer in experiment 1, collected data for experiment 2. Postmortem analysis of guide cannula placement was done using methods similar to those in experiment 1 (see above).
4.5 Statistical analysis
All statistical analysis was performed using Graphpad Prism software (La Jolla, CA). For experiment 1, all maternal behavior latency scores were analyzed using a two-tailed independent samples t-test comparing the means for aCSF and TTX treated groups (p < 0.05). For experiment 2, maternal retrieval latencies were also analyzed by t-test. Unless otherwise specified, all other behavioral measurements were analyzed by a two-way analysis of variance with day (day 1 × day 2) and treatment (aCSF × GABA agonists) as independent variables (p < 0.05).
Supplementary Material
Acknowledgments
This study was supported by a grant from National Institute on Drug Abuse (DA019946) to MF. It’s contents are solely the responsibility of the authors and do not represent the official views of the NIDA. TRJ is recipient of an undergraduate Provost research award at Northeastern University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afonso VM, Sison M, Lovic V, Fleming AS. Medial prefrontal cortex lesions in the female rat affect sexual and maternal behavior and their sequential organization. Behav Neurosci. 2007;121:515–26. doi: 10.1037/0735-7044.121.3.515. [DOI] [PubMed] [Google Scholar]
- Bacci A, Huguenard JR, Prince DA. Modulation of neocortical interneurons: extrinsic influences and exercises in self-control. Trends Neurosci. 2005;28:602–10. doi: 10.1016/j.tins.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural basis of romantic love. Neuroreport. 2000;11:3829–34. doi: 10.1097/00001756-200011270-00046. [DOI] [PubMed] [Google Scholar]
- Beach FA. The neural basis of innate behavior. I. Effects of cortical lesions upon the maternal behavior pattern in the rat. J Comp Psychol. 1937;24:393–440. [Google Scholar]
- Bjursten LM, Norrsell K, Norrsell U. Behavioural repertory of cats without cerebral cortex from infancy. Exp Brain Res. 1976;25:115–30. doi: 10.1007/BF00234897. [DOI] [PubMed] [Google Scholar]
- Byrnes EM, Lee JO, Bridges RS. Alterations in GABA(A) receptor alpha2 subunit mRNA expression following reproductive experience in rats. Neuroendocrinololgy. 2007;85:148–56. doi: 10.1159/000102535. [DOI] [PubMed] [Google Scholar]
- Christakou A, Robbins TW, Everitt BJ. Prefrontal cortical-ventral striatal interactions involved in affective modulation of attentional performance: implications for corticostriatal circuit function. J Neurosci. 2004;24:773–80. doi: 10.1523/JNEUROSCI.0949-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci. 2007;27:840–4. doi: 10.1523/JNEUROSCI.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febo M, Ferris CF. Development of cocaine sensitization before pregnancy affects subsequent maternal retrieval of pups and prefrontal cortical activity during nursing. Neuroscience. 2007;148:400–12. doi: 10.1016/j.neuroscience.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febo M, Segarra AC. Cocaine alters GABAB-mediated G-protein activation in the ventral tegmental area of female rats: modulation by estrogen. Synapse. 2004;54:30–36. doi: 10.1002/syn.20063. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Kulkarni P, Sullivan JM, Jr, Harder JA, Messenger TL, Febo M. Pup suckling is more rewarding than cocaine: evidence from functional magnetic resonance imaging and three-dimensional computational analysis. J Neurosci. 2005;25:149–56. doi: 10.1523/JNEUROSCI.3156-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbott PLA, Warner TA, Jays PRL, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- Hendersen LP. Steroid modulation of GABAA receptor-mediated transmission in the hypothalamus: effects on reproductive function. Neuropharmacology. 2007;52:1439–1453. doi: 10.1016/j.neuropharm.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hok V, Save E, Lenck-Santini PP, Poucet B. Coding for spatial goals in the prelimbic/infralimbic area of the rat frontal cortex. Proc Natl Acad Sci U S A. 2005;102:4602–7. doi: 10.1073/pnas.0407332102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–79. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Kao CY. Tetrodotoxin, saxitoxin and their significance in the study of excitation phenomena. Pharmacol Rev. 1966;18:997–1049. [PubMed] [Google Scholar]
- Kelly MJ, Loose MD, Ronnekleiv OK. Estrogen suppresses μ-opioid and GABAB-mediated hyperpolarization of hypothalamic arcuate neurons. J Neurosci. 1992;12:2745–2750. doi: 10.1523/JNEUROSCI.12-07-02745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M, Ehret G. Parental behavior in the mouse: effects of lesions in the entorhinal/piriform cortex. Behav Brain Res. 1991;42:99–105. doi: 10.1016/s0166-4328(05)80044-0. [DOI] [PubMed] [Google Scholar]
- Kolunie JM, Stern JM, Barfield RJ. Maternal aggression in rats: effects of visual or auditory deprivation of the mother and dyadic pattern of ultrasonic vocalizations. Behav Neural Biol. 1994;62:41–9. doi: 10.1016/s0163-1047(05)80057-3. [DOI] [PubMed] [Google Scholar]
- Lee A, Li M, Watchus J, Fleming AS. Neuroanatomical basis of maternal memory in postpartum rats: selective role for the nucleus accumbens. Behav Neurosci. 1999;113:523–38. doi: 10.1037//0735-7044.113.3.523. [DOI] [PubMed] [Google Scholar]
- Leibenluft E, Gobbini MI, Harrison T, Haxby JV. Mothers’ neural activation in response to pictures of their children and other children. Biol Psychiatry. 2004;56:225–32. doi: 10.1016/j.biopsych.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Li M, Fleming AS. The nucleus accumbens shell is critical for normal expression of pup-retrieval in postpartum female rats. Behav Brain Res. 2003;145:99–111. doi: 10.1016/s0166-4328(03)00135-9. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Stern JM. Role of the midbrain periaqueductal gray in maternal nurturance and aggression: c-fos and electrolytic lesion studies in lactating rats. J Neurosci. 1997a;17:3364–78. doi: 10.1523/JNEUROSCI.17-09-03364.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonstein JS, Stern JM. Somatosensory contributions to c-fos activation within the caudal periaqueductal gray of lactating rats: effects of perioral, rooting, and suckling stimuli from pups. Horm Behav. 1997b;32:155–66. doi: 10.1006/hbeh.1997.1416. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Simmons DA, Stern JM. Functions of the caudal periaqueductal gray in lactating rats: kyphosis, lordosis, maternal aggression, and fearfulness. Behav Neurosci. 1998a;112:1502–18. doi: 10.1037//0735-7044.112.6.1502. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Simmons DA, Swann JM, Stern JM. Forebrain expression of c-fos due to active maternal behaviour in lactating rats. Neuroscience. 1998b;82:267–81. doi: 10.1016/s0306-4522(97)00283-2. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Stern JM. Site and behavioral specificity of periaqueductal gray lesions on postpartum sexual, maternal, and aggressive behaviors in rats. Brain Res. 1998;804:21–35. doi: 10.1016/s0006-8993(98)00642-8. [DOI] [PubMed] [Google Scholar]
- Lorberbaum JP, Newman JD, Horwitz AR, Dubno JR, Lydiard RB, Hamner MB, Bohning DE, George MS. A potential role for thalamocingulate circuitry in human maternal behavior. Biol Psychiatry. 2002;51:431–45. doi: 10.1016/s0006-3223(01)01284-7. [DOI] [PubMed] [Google Scholar]
- Mattson BJ, Williams S, Rosenblatt JS, Morrell JI. Comparison of two positive reinforcing stimuli: pups and cocaine throughout the postpartum period. Behav Neurosci. 2001;115:683–94. doi: 10.1037//0735-7044.115.3.683. [DOI] [PubMed] [Google Scholar]
- Mattson BJ, Williams SE, Rosenblatt JS, Morrell JI. Preferences for cocaine- or pup-associated chambers differentiates otherwise behaviorally identical postpartum maternal rats. Psychopharmacology (Berl) 2003;167:1–8. doi: 10.1007/s00213-002-1351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson BJ, Morrell JI. Preference for cocaine- versus pup-associated cues differentially activates neurons expressing either Fos or cocaine- and amphetamine-regulated transcript in lactating, maternal rodents. Neuroscience. 2005;135:315–28. doi: 10.1016/j.neuroscience.2005.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–63. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24:1551–60. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- Montague D, Weickert CS, Tomaskovic-Crook E, Rothmond DA, Kleinman JE, Rubinow DR. Oestrogen receptor α localisation in the prefrontal cortex of three mammalian species. J Neuroendocrinol. 2008;20:893–903. doi: 10.1111/j.1365-2826.2008.01743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng CW, Noblejas MI, Rodefer JS, Smith CB, Poremba A. Double dissociation of attentional resources: prefrontal versus cingulate cortices. J Neurosci. 2007;27:12123–31. doi: 10.1523/JNEUROSCI.2745-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschke JB, Nelson EE, Rusch BD, Fox AS, Oakes TR, Davidson RJ. Orbitofrontal cortex tracks positive mood in mothers viewing pictures of their newborn infants. Neuroimage. 2004;21:583–92. doi: 10.1016/j.neuroimage.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Noriuchi M, Kikuchi Y, Senoo A. The functional neuroanatomy of maternal love: mother’s response to infant’s attachment behaviors. Biol Psychiatry. 2008;63:415–23. doi: 10.1016/j.biopsych.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Numan M, Rosenblatt JS, Komisaruk BR. Medial preoptic area and onset of maternal behavior in the rat. J Comp Physiol Psychol. 1977;91:146–64. doi: 10.1037/h0077304. [DOI] [PubMed] [Google Scholar]
- Numan M, Smith HG. Maternal behavior in rats: evidence for the involvement of preoptic projections to the ventral tegmental area. Behav Neurosci. 1984;98:712–27. doi: 10.1037//0735-7044.98.4.712. [DOI] [PubMed] [Google Scholar]
- Numan M, Corodimas KP. The effects of paraventricular hypothalamic lesions on maternal behavior in rats. Physiol Behav. 1985;35:417–25. doi: 10.1016/0031-9384(85)90318-x. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ, Pliakou N, Stolzenberg DS, Mullins OJ, Murphy JM, Smith CD. The effects of D1 or D2 dopamine receptor antagonism in the medial preoptic area, ventral pallidum, or nucleus accumbens on the maternal retrieval response and other aspects of maternal behavior in rats. Behav Neurosci. 2005a;119:1588–604. doi: 10.1037/0735-7044.119.6.1588. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ, Schwarz JM, Neuner CM, Flood TF, Smith CD. Medial preoptic area interactions with the nucleus accumbens-ventral pallidum circuit and maternal behavior in rats. Behav Brain Res. 2005b;158:53–68. doi: 10.1016/j.bbr.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Numan M. Motivational systems and the neural circuitry of maternal behavior in the rat. Dev Psychobiol. 2007;49:12–21. doi: 10.1002/dev.20198. [DOI] [PubMed] [Google Scholar]
- Numan M, Stolzenberg DS, Dellevigne AA, Correnti CM, Numan MJ. Temporary inactivation of ventral tegmental area neurons with either muscimol or baclofen reversibly disrupts maternal behavior in rats through different underlying mechanisms. Behav Neurosci. 2009;123:740–51. doi: 10.1037/a0016204. [DOI] [PubMed] [Google Scholar]
- Ongur D, An X, Price JL. Prefrontal cortical projections to the hypothalamus in macaque monkeys. J Comp Neurol. 1998;401:480–505. [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–19. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Ongur D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol. 2003;460:425–49. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- Peters YM, O’Donnell P, Carelli RM. Prefrontal cortical cell firing during maintenance, extinction, and reinstatement of goal-directed behavior for natural reward. Synapse. 2005;56:74–83. doi: 10.1002/syn.20129. [DOI] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Ronnekleiv OK, Kelly MJ. Rapid signalling of estrogen in hypothalamic neurons involves a novel G-protein coupled estrogen receptor that activates protein kinase C. J Neurosci. 2003;23:9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Ronnekleiv OK, Kelly MJ. Modulation of hypothalamic neuronal activity through a novel G-protein coupled estrogen membrane receptor. Steroids. 2008;73:985–991. doi: 10.1016/j.steroids.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranote S, Elliott R, Abel KM, Mitchell R, Deakin JF, Appleby L. The neural basis of maternal responsiveness to infants: an fMRI study. Neuroreport. 2004;15:1825–9. doi: 10.1097/01.wnr.0000137078.64128.6a. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JS. Nonhormonal basis of maternal behavior in the rat. Science. 1967;156:1512–4. doi: 10.1126/science.156.3781.1512. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Carboni S. Noradrenaline and dopamine elevations in the rat prefrontal cortex in spatial working memory. J Neurosci. 2005;25:2322–9. doi: 10.1523/JNEUROSCI.3038-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan T, Paul M, Amaral E, Numan MJ, Numan M. Evidence that the medial amygdala projects to the anterior/ventromedial hypothalamic nuclei to inhibit maternal behavior in rats. Neuroscience. 2001;106:341–56. doi: 10.1016/s0306-4522(01)00286-x. [DOI] [PubMed] [Google Scholar]
- Sheehan TP, Cirrito J, Numan MJ, Numan M. Using c-Fos immunocytochemistry to identify forebrain regions that may inhibit maternal behavior in rats. Behav Neurosci. 2000;114:337–52. doi: 10.1037//0735-7044.114.2.337. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor α- and β- mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Slotnick BM. Disturbances of maternal behavior in the rat following lesions of the cingulate cortex. Behaviour. 1967;29:204–36. doi: 10.1163/156853967x00127. [DOI] [PubMed] [Google Scholar]
- Slotnick BM, Nigrosh BJ. Maternal behavior of mice with cingulate cortical, amygdala, or septal lesions. J Comp Physiol Psychol. 1975;88:118–27. doi: 10.1037/h0076200. [DOI] [PubMed] [Google Scholar]
- Stack EC, Balakrishnan R, Numan MJ, Numan M. A functional neuroanatomical investigation of the role of the medial preoptic area in neural circuits regulating maternal behavior. Behav Brain Res. 2002;131:17–36. doi: 10.1016/s0166-4328(01)00370-9. [DOI] [PubMed] [Google Scholar]
- Stamm JS. The function of the median cerebral cortex in maternal behavior of rats. J Comp Physiol Psychol. 1955;48:347–56. doi: 10.1037/h0042977. [DOI] [PubMed] [Google Scholar]
- Stern JM, Mackinnon DA. Sensory regulation of maternal behavior in rats: effects of pup age. Dev Psychobiol. 1978;11:579–86. doi: 10.1002/dev.420110607. [DOI] [PubMed] [Google Scholar]
- Stern JM, Kolunie JM. Maternal aggression of rats is impaired by cutaneous anesthesia of the ventral trunk, but not by nipple removal. Physiol Behav. 1993;54:861–8. doi: 10.1016/0031-9384(93)90293-o. [DOI] [PubMed] [Google Scholar]
- Stern JM, Lonstein JS. Neural mediation of nursing and related maternal behaviors. Prog Brain Res. 2001;133:263–78. doi: 10.1016/s0079-6123(01)33020-0. [DOI] [PubMed] [Google Scholar]
- Stone CP. Effects of cortical destruction on reproductive behavior and maze learning in albino rats. J Comp Psychol. 1938;26:217–236. [Google Scholar]
- Strathearn L, Li J, Fonagy P, Montague PR. What’s in a smile? Maternal brain responses to infant facial cues. Pediatrics. 2008;122:40–51. doi: 10.1542/peds.2007-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Tasgin E, Mayes LC, Feldman R, Constable RT, Leckman JF. Maternal brain response to own baby-cry is affected by cesarean section delivery. J Child Psychol Psychiatry. 2008;49:1042–52. doi: 10.1111/j.1469-7610.2008.01963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP. Analysis of projections from the medial prefrontal cortex to the thalamus in the rat, with emphasis on nucleus reuniens. J Comp Neurol. 2002;442:163–87. doi: 10.1002/cne.10083. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Walton ME, Bannerman DM, Rushworth MF. The role of rat medial frontal cortex in effort-based decision making. J Neurosci. 2002;22:10996–1003. doi: 10.1523/JNEUROSCI.22-24-10996.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerling P, Lindgren S, Meyerson B. Functional changes in GABAA receptor stimulation during the oestrous cycle of the rat. Br J Pharmacol. 1991;103:1580–1584. doi: 10.1111/j.1476-5381.1991.tb09830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.