Abstract
A survey of the attention literature reveals the prominence of the attentional blink (AB)—a deficit in reporting the second of two targets when presented in close temporal succession. For two decades, this robust attentional phenomenon has been a major topic in attention research because it is informative about the rate at which stimuli can be encoded into consciously accessible representations. The pace of discovery and theoretical advancement concerning the AB has increased rapidly in the past few years with emphasis on new neurophysiological evidence and computational accounts of attentional processes. In this review we extract the central questions and the main lessons learnt from the past, and subsequently provide important directions for future research.
Keywords: Attentional blink, Conscious awareness, Attentional selection, Visual attention, Computational models, Individual differences
1. Introduction
The human mind is severely limited in processing concurrent information at a conscious level of awareness. While reading this text, only a small sub-set of the available information that is coming through the senses is granted access to the brain processes that form the basis of consciously accessible working memory representations. Attention is a cognitive mechanism that helps to select and process important or interesting information (hopefully this text) while irrelevant information (conversations from nearby colleagues, incoming e-mail, etc.) is largely ignored. But when something is selected (e.g. a large billboard), how long does attention dwell on that information before selecting and processing another object or event (a red traffic light)?
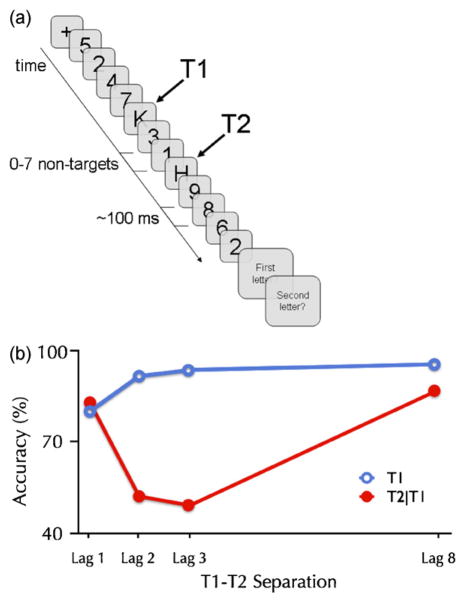
In hundreds of experiments it has been shown that when two targets are to be identified amongst a rapid stream of non-target stimuli (see Fig. 1a), most individuals show a surprisingly long-lasting effect—an attentional blink (AB)—in reporting the second target (Broadbent and Broadbent, 1987; Raymond et al., 1992; Weichselgartner and Sperling, 1987). By systematically varying the serial position of the second target (T2) relative to a prior target (T1), as shown in Fig. 1a, the time course of interference can be measured as a function of the targets’ temporal separation (also referred to as lag, stimulus onset asynchrony (SOA), or target onset asynchrony (TOA)).
Fig. 1. The basic attentional-blink paradigm.
(a) The attentional-blink paradigm as it is most commonly used originates from a classic paper by Chun and Potter (1995). As shown in panel A, a rapid serial visual presentation (RSVP) stream of digits (the non-targets or distractors) is sequentially presented in the middle of the screen, typically at a rate of about 10 items/second. Subjects are instructed to identify two unspecified letters (the targets, referred to as T1 and T2, respectively) embedded within the stream. The paradigm requires subjects to give their responses after presentation of the stream, at their own pace, so that additional interference effects arising from speeded responses are avoided. The primary measure of interest is the percentage of correct T2 reports from trials in which T1 was accurately identified (T2|T1), thereby ensuring that T2s are not missed due to a coincidental lapse of attention. When subjects are instructed to ignore the T1 stimulus, T2 is usually reported accurately regardless of the lag between the two targets (Raymond et al., 1992). This suggests that the AB is the result of attending to T1 and consolidating it into working memory (Chun and Potter, 1995), rather than a perceptual deficit.
(b) Subjects often fail to report T2 when it is presented within 200–500 ms after T1, whereas when the interval is longer, both targets are usually reported in the order in which they were presented. Importantly, when T1 and T2 are presented within about 100 ms SOA, subjects quite often report both targets. This paradoxical finding, referred to as lag-1 sparing (Potter et al., 2002) and discussed later in this paper, has proven to be quite an important aspect of the AB.
There are at least three important reasons why the AB has come to such prominence in the attention literature (Box 1). Firstly, the AB reflects a remarkably long-lasting attentional deficit. For several decades, a central question in attention research was how long an object that must be identified continues to occupy attentional capacity. The AB lasts for several hundred milliseconds, suggesting that the time that attention ‘dwells’ on a target is an order of magnitude longer than previously assumed (Duncan et al., 1994).
Box 1. The impact of the blink.
Whereas the classic attention literature has focused mostly on attention mechanisms that are selective with regard to the location or perceptual characteristics of stimuli, the AB has led to a large body of literature that focuses on temporal aspects of attention. In fact, the AB has become central in the study of attention in the past two decades, judging from the growing number of papers and the number of citations that these papers receive more broadly. Whilst the aim of the current paper is to provide a comprehensive review on the current understanding of the AB by integrating theoretical, behavioral, neuroimaging, and computational data, it may nevertheless be interesting to highlight a number of studies that illustrate the phenomenon’s impact on other research fields.
Within the broad field of attention research, the paradigm has been used effectively in studies on visual search, showing for instance that stimuli with ‘pop-out’ features (unique and salient characteristics in color, size, orientation, or shape, allowing rapid – supposedly pre-attentive – detection when sought amongst other stimuli) do require attention after all (Joseph et al., 1997). The AB has proven to be a useful tool in the study of conscious awareness (Sergent et al., 2005), the interaction between working memory and attention (Nieuwenstein et al., 2007), and mental imagery (Pashler and Shiu, 1999). Pashler and Shiu showed for instance that a previously imagined object (e.g., a tiger) automatically attracts attention when presented in a stream of other items, inducing an AB on a subsequent target (e.g., a digit) that requires identification. Reflected in an attenuated AB, it has been shown that attention is allocated differently in expert meditators (Slagter et al., 2007) and people who often play action video games (Green and Bavelier, 2003). Fundamental knowledge obtained from the AB has even been used to study information processing in bats (Barber et al., 2003), as well as in practical applications such as psychological assessment (Beech et al., 2008), interface design (Spence and Driver, 1997) and warning signals in vehicles (Ho and Spence, 2005). Finally, it has had a large impact in developmental and clinical settings, helping to elucidate cognitive limitations in elderly (Lahar et al., 2001) and people suffering from visual neglect (Husain and Rorden, 2003; Husain et al., 1997), schizophrenia (Li et al., 2002), depression (Rokke et al., 2002), ADHD (Hollingsworth et al., 2001), and dyslexia (Hari and Renvall, 2001).
Secondly, this long-lasting interference effect, which is referred to as the AB in analogy to eye blinks (Raymond et al., 1992), is very robust and can be obtained under a wide variety of task conditions and in the great majority of subjects. Typically, the AB is obtained using a rapid stream of sequentially presented alphanumerical stimuli (Fig. 1a), but it can also be obtained with other types of stimuli such as words (Barnard et al., 2004; Luck et al., 1996), symbols (Chun and Potter, 1995), or pictures (Evans and Treisman, 2005), and with auditory (Duncan et al., 1997) or tactile stimuli (Hillstrom et al., 2002). Consequently, the effect is thought to reflect a very general property of perceptual awareness with broad implications for understanding how the brain perceives any task-relevant stimulus.
Thirdly, not only has the AB proven to be an effective means to study the time-course of attention and memory consolidation, it has also provided researchers with a tool to study one of the most interesting topics in cognitive neuroscience, human consciousness. Investigating how brain processes contribute to conscious information processing is assisted by virtue of the fact that the AB renders a stimulus, which would otherwise be quite easy to see according to its masking and physical characteristics, markedly less visible. Furthermore, the AB does not occur on every trial with a short target interval (see Fig. 1b): for example, within data from a single subject, an AB might occur on half of the trials (so that the subject cannot identify the second target), whereas on the other half of trials no AB occurs (reflected by successful identification of both targets), although identical stimuli are used in the trials. Contrasting these blink and no-blink trials while using identical stimuli and instructions allows one to explore the neural and behavioral consequences of conscious perception (Kim and Blake, 2005; Kranczioch et al., 2005; Marois et al., 2004; Martens et al., 2006b; Sergent et al., 2005; Sergent and Dehaene, 2004).
In recent years the underlying cause of the AB has emerged as a topic of intense debate following a series of important publications that have challenged many of the established theories in the field. This review will discuss the prominent features of the emerging theoretical landscape and the behavioral and neuroscientific data which underlie them. We will then relate these ideas to several computational models that have helped to link the behavioral and neural correlates of the AB into formalized frameworks. Finally, we conclude with a discussion of other interesting developments and new directions in the field.
2. Changing perspectives on the AB
2.1. Central capacity limitations
After discovery of the AB (Broadbent and Broadbent, 1987; Raymond et al., 1992; Weichselgartner and Sperling, 1987), the initial research impetus was to understand at what processing stage a blinked target is lost. Convergent evidence from behavioral (Maki et al., 1997; Martens et al., 2002; Potter et al., 2005; Shapiro et al., 1997a,b; Visser et al., 2005) and neuroimaging studies (Luck et al., 1996; Marois et al., 2004; Nieuwenhuis et al., 2005; Pesciarelli et al., 2007; Rolke et al., 2001; Sergent et al., 2005) suggested that even unreported targets are processed to a late stage in the processing pathway. For instance, it was shown that a blinked target word can facilitate the processing of a subsequently presented word that is semantically related to the blinked target (Martens et al., 2002; Shapiro et al., 1997a). Moreover, blinked words were found to induce electrophysiological activity associated with semantic processing (Luck et al., 1996). This conclusion was buttressed by the finding that the AB is also a fairly central process: it occurs across spatial locations (Duncan et al., 1994), modalities (Arnell and Jenkins, 2004; Arnell and Jolicoeur, 1999; Arnell and Larson, 2002; Jolicoeur, 1999; Jolicœur et al., 2002) (though this is debated (Duncan et al., 1997; Hein et al., 2006; Martens et al., 2009; Potter et al., 1998; Soto-Faraco and Spence, 2002)) and across types of target identification (Raymond et al., 1992). These findings led to theories of central capacity limitations that have dominated the field for a decade (Chun and Potter, 1995; Dehaene et al., 2003; Isaak et al., 1999; Jolicœur and Dell’Acqua, 1999).
These theories of central capacity limitations share a high degree of convergence in assuming that all items that are presented in a stream of information are fully processed up to the point of conceptual representations. The AB has thus been described as a deficit in the consolidation of T2 into a reportable working-memory representation arising from a capacity-limited second stage of processing that is tied up with the consolidation of T1.
2.2. Neural correlates
Directly recorded brain related evidence has been an important part of our understanding of the AB, and the excellent temporal resolution of electrophysiological methods (EEG) has been put to good use in disentangling the processing of two separate targets presented in close temporal proximity (Dell’Acqua et al., 2006; Luck et al., 1996; Martens et al., 2006b; Pesciarelli et al., 2007; Rolke et al., 2001; Sergent et al., 2005; Vogel et al., 1998) (Boxes 2 and 3). ERP data from multiple EEG studies have converged on a coherent picture of the time course of cognitive processes involved in the AB. A T2 presented during the AB elicits a normal pattern of neural correlates for at least the first 150 ms of perceptual processing (reflected in the P1 and N1 components) (Vogel et al., 1998), but this processed target fails to elicit the attentional selection response that would normally be observed about 200 ms after stimulus onset (Box 2). Next, this T2 fails to elicit an ERP component that is associated with working memory consolidation (the P3) (Luck et al., 1996; Martens et al., 2006b; Vogel and Luck, 2002; Vogel et al., 1998). Interestingly, despite the fact that a blinked T2 is not selected by attention, it nevertheless activates semantic representations, at least as indexed by the N400 component (Luck et al., 1996; Pesciarelli et al., 2007; Rolke et al., 2001; Vogel et al., 1998) (though more recent evidence suggests that this N400 can be reduced during the AB (Giesbrecht et al., 2007)). The neural data reinforce the behavioral evidence (Maki et al., 1997; Martens et al., 2002; Potter et al., 2005; Shapiro et al., 1997a,b; Visser et al., 2005) that the AB occurs at a late stage of processing, after early perceptual and conceptual representations have been formed.
Box 2. Pinpointing the blink’s onset.
By contrasting trials with either a short or long target lag, electro-encephalography (EEG; a neuroimaging technique that is used to measure electrical activity produced by the brain as recorded by electrodes placed on the scalp) studies have been able to pinpoint the precise moment that brain activity time-locked to T2 shows evidence of the AB occurring (Dell’Acqua et al., 2006; Jolicoeur et al., 2006; Jolicœur et al., 2006). The earliest event-related potential component (ERP; the result of averaging the EEG signal from many trials, time-locked to a specific stimulus, revealing components associated with specific cognitive processes) that is affected seems to be the N2pc component, which is associated with the allocation of attention to a relevant target. Normally, it is evoked in both parietal and occipital areas, roughly 200 ms after the onset of a peripherally presented target. While the N2pc is indeed evoked by a T2 on trials with a long lag between T1 and T2, it is substantially suppressed on trials with a short target lag (during which an AB is likely to occur). Moreover, a more difficult T1 leads to more suppression, whereas a successfully ignored T1 leaves the N2pc induced by T2 intact (Dell’Acqua et al., 2006; Jolicoeur et al., 2006; Jolicœur et al., 2006). A different kind of paradigm, that directly compares brain responses to successfully reported T2s with that of blinked T2s, also finds a difference about 170 ms after T2 onset (Sergent et al., 2005).
What these findings suggest is that the AB is related to stimulus selection processes centered in posterior areas of the brain, and thus relatively early in the anatomical visual processing hierarchy. However, the timing of the N2pc, or the lack thereof, occurs after the perceptual ERP components (N1/P1), and this suggests that the AB is mediated by feedback mechanisms triggered by processing in higher level brain areas that project back to earlier areas. Another implication of the link between the AB and the N2pc, the latter of which has conventionally been studied in visual paradigms involving static displays, is that target selection during the AB is mediated by neural mechanisms that also underlie the rapid deployment of spatial attention (Luck and Hillyard, 1994).
Box 3. Non-blinkers.
One aspect of the AB that has largely been ignored is that the magnitude of the AB effect varies from one individual to another. Some individuals referred to as ‘non-blinkers’ (about 5% of the population) even show little or no AB (see panel A) whatsoever, a finding that questions the fundamental nature of the phenomenon (Martens et al., 2006b).
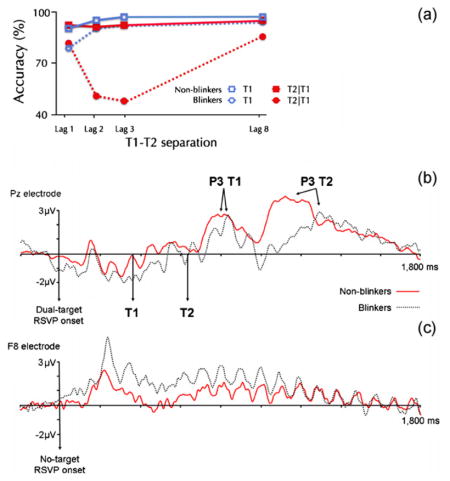
Reproduced from Martens et al., 2006b; Copyright 2006 Massachusetts Institute of Technology. Psychophysiological evidence has shown that target processing differs in ‘blinkers’ (who show a strong AB) and non-blinkers. EEG measurements revealed differences in parietal brain activity, suggesting that non-blinkers are quicker to consolidate the identity of targets than blinkers are. As shown in panel B, these differences are reflected in the latencies of the P3 ERP components induced by targets that are successfully identified despite a T1–T2 interval of 270 ms (lag 3).
In addition, non-blinkers showed more target-related activity over the right ventrolateral prefrontal cortex (assumed to play a role in a wide range of cognitive processes, including the selection of nonspatial information), whereas blinkers showed more distractor-related prefrontal activity (see panel C). This higher level of distractor-related activity in blinkers suggests that they may direct more attention to each non-target than non-blinkers do. Indeed, behavioral evidence confirms that non-blinkers are better in ignoring non-targets than blinkers (Dux and Marois, 2008; Martens and Valchev, 2009).
Intriguingly, no differences have been found in working memory capacity or general intelligence between blinkers and non-blinkers (Martens and Johnson, 2008) (but see Arnell et al., 2010; Colzato et al., 2007, which claim that a correlation between working memory capacity and AB magnitude does exist). It has therefore been suggested that, instead of structural differences, a major source of individual variability in AB magnitude may lie in ‘pre-memory’ processes, which play a crucial role in determining which objects are selected for further processing and memory consolidation (Martens and Johnson, 2008). In other words, the occurrence of an AB may be determined by an allocation policy, which might vary from individual to individual, but also from trial to trial. Taken together with the finding that, within subjects, the AB can be attenuated by added distraction (Arend et al., 2006; Olivers and Nieuwenhuis, 2005, 2006; Taatgen et al., 2009), the possibility that the occurrence of an AB is determined by the way attention is allocated seems plausible.
Research has also focused on understanding what brain regions are involved in the AB and this effort has implicated a network of areas distributed across occipital, parietal, and frontal areas. Evidence comes for instance from fMRI (functional Magnetic Resonance Imaging; a neuroimaging technique that measures changes in blood flow related to neural activity in the brain; Kranczioch et al., 2005; Marcantoni et al., 2003; Marois et al., 2004) and EEG (Sergent et al., 2005) studies showing that activity in the fronto-parietal cortex (anterior cingulate, lateral prefrontal, and parietal regions in particular) is reduced when T2 cannot be reported, even though both targets successfully activate representations in early visual areas, such as V1 (Williams et al., 2008). Furthermore, patients suffering from brain lesions of the right inferior parietal cortex and lateral frontal cortex have shown a stronger and prolonged AB effect (Husain and Rorden, 2003; Husain et al., 1997). In addition, TMS (transcranial magnetic stimulation; a noninvasive method that produces a transient disruption of cortical function by rapidly changing magnetic fields) studies, which can provide causal evidence for the involvement of a brain region, have shown a role of the intraparietal sulcus in the AB (Cooper et al., 2004; Kihara et al., 2007).
Another approach has looked at the synchronization between MEG (magneto-encephalography; a neuroimaging technique that is used to measure the magnetic fields produced by electrical activity in the brain) signals recorded from separate brain areas as a measure of stimulus processing (Gross et al., 2004). This research identifies two distinct networks of brain areas that undergo temporary long-range synchronization; one network consists of brain areas involved in the processing of all stimuli in a rapid serial visual presentation (RSVP) stream, and the other network consists of brain areas that are involved specifically in the processing of targets (see Fig. 2a).
Fig. 2. Neural correlates.
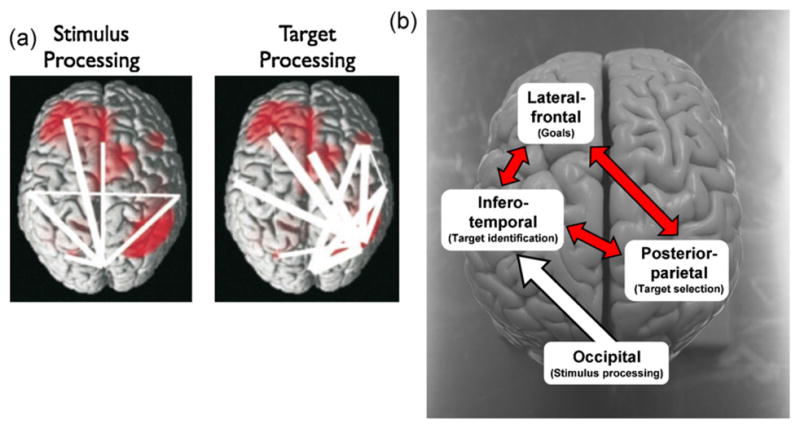
(a) Neuroimaging studies of the AB suggest a divergence of activity between seen and missed T2s in anterior cingulate, lateral prefrontal, and parietal brain regions (Feinstein et al., 2004; Gross et al., 2004; Kranczioch et al., 2005; Marcantoni et al., 2003; Marois et al., 2004; Martens et al., 2006b; Sergent et al., 2005; Shapiro et al., 2007). Panel A shows the stimulus-related (left) and the target-related (right) network as revealed with MEG (Gross et al., 2004, Copyright (2004) National Academy of Sciences, U.S.A.). The thickness of the lines reflects the strength of synchronization between the connected areas. Converging evidence thus suggests that each stimulus activates occipital and infero-temporal regions (associated with perceptual and conceptual processing), but that only reported targets lead to strong synchronization between frontal and parietal areas. Involvement of these latter areas reflects the specific task requirements in an AB paradigm: incoming stimuli have to be compared to an internal target specification (medial prefrontal cortex); if a stimulus matches, it needs to be selected for consolidation and further processing (parietal cortex); the subgoals of identifying two targets have to be maintained while a rapid stream of non-targets is viewed, updating working memory when targets are detected (frontopolar cortex); and decisions have to be made regarding which actions should be taken in response to the perceived targets (anterior cingulate).
(b) Components, functions, and main interactions within the attentional network that is tentatively assumed to be responsible for the detecting and processing of targets during an AB task (Hommel et al., 2006). Adapted from Hommel et al. (2006), Fig. 1, with kind permission from Springer Science+Business Media, Copyright Springer-Verlag 2006. Photo by first author, courtesy of the Brain Anatomy Department, University Medical Center Groningen, the Netherlands.
The available evidence from these different approaches implies an interactive network consisting of lateral–frontal (for the processing and maintenance of goals and target specifications), infero-temporal (for target identification), posterior-parietal (for target selection), and occipital (for extraction of stimulus characteristics) brain areas, assumed to be involved in the dynamics of attentional selection and processing that result in an AB (Hommel et al., 2006) (see Fig. 2b). The lateral frontal and parietal regions, in particular, have been implicated as likely neural substrates of the AB bottleneck (Marois and Ivanoff, 2005). Interestingly, it has been proposed that it is the feedback activity from the fronto-parietal network down to the primary visual cortex that gives rise to conscious experience (Lamme, 2006), which fits well with the finding that attentional selection, as measured with the N2pc component (Box 2), occurs after a target has evoked early perceptual and semantic representations (Dell’Acqua et al., 2007).
2.3. Breaking the bottleneck
Given that the AB cannot be eliminated even with extensive training (Braun, 1998; Maki and Padmanabhan, 1994; Taatgen et al., 2009), the blink has widely been assumed to reflect an unavoidable limitation in information processing. However, a number of recent studies have questioned the fundamental nature of the phenomenon.
The turning point, it seems, came in the year 2005, after which the pace of discovery and theory increased rapidly. In that year, several papers were published which brought two important lessons: first, that the AB magnitude can be attenuated by reducing the subject’s focus on target identification, and second, that it is possible to identify multiple targets without the occurrence of an AB as long as no intervening non-target is presented.
One of the first of these studies asked subjects to listen to task-irrelevant music or think about their holiday while carrying out an AB task and found strong attenuation of the AB effect (Olivers and Nieuwenhuis, 2005). Although the initial finding could not be fully replicated (Olivers and Nieuwenhuis, 2006), others have shown that task-irrelevant visual motion or flicker (Arend et al., 2006), a change in task instruction (Ferlazzo et al., 2007), or even a concurrent secondary task (Taatgen et al., 2009) can attenuate the AB as well. These findings are incisive because the AB magnitude is being reduced by the addition of extraneous cognitive load, a change in strategy, or irrelevant noise. Although one may expect that distraction from the AB task would deteriorate overall performance, it was suggested that the irrelevant mental activity produced a more distributed attentional state in subjects, leaving the excess of resources that would normally be allocated to T1 available for the processing and consolidation of T2.
A number of studies provided additional evidence that the magnitude of the AB effect can be reduced by manipulating the allocation of attentional resources to the T1 or T2. On the one hand, when the time that is required to process T1 is prolonged, for instance by making the task of identifying T1 more difficult, a stronger AB effect can be observed (Giesbrecht et al., 2009; Martens et al., 2006a; Seiffert and Di Lollo, 1997; Taatgen et al., 2009; Visser, 2007a,b). On the other hand, it has been shown that once an AB is induced by a first target, it can be alleviated substantially if T2 is preceded by a non-target that shares a target defining feature (i.e., both having the same color) (Nieuwenstein et al., 2005). Presumably, pre-cuing the second target leads to a redistribution or accelerated allocation of attention, resulting in a recovery of performance. In a similar way, the AB is postponed when T1 is followed by one or more non-targets that share the color of T1 and T2 (Olivers and Meeter, 2008), presumably by delaying the suppression of attention that is induced when a T1 is followed by regular (uncolored) non-targets. Not only perceptual cues, but also spatial (Nieuwenstein et al., 2005) and temporal cues (Martens and Johnson, 2005) have turned out to be effective in manipulating attention during the AB. For instance, a smaller AB has been observed when, prior to each trial, subjects were informed about the temporal interval between the targets in the upcoming trial (Martens and Johnson, 2005), suggesting that some degree of top-down control (driven by one’s goals or intentions) over the temporal allocation of attention is possible.
The available evidence that questions the fundamental nature of the AB as a hard-wired bottleneck within working memory is perhaps completed by the observation that there are large individual differences in AB magnitude, with some individuals persistently showing a large AB, and others showing little or even no AB at all under identical experimental conditions (Box 3) (Martens and Johnson, 2008; Martens et al., 2009, 2006b; Martens and Valchev, 2009). Large individual differences in working memory capacity are known to exist as well (Conway et al., 2007), but a correlation between working memory capacity and AB magnitude has not consistently been found (Martens and Johnson, 2008).
2.4. Attentional control vs. limited resources
This flurry of recent findings showing that the AB can be attenuated in various ways has prompted renewed discussion of the underlying cause of the blink, exploring in depth the question of whether the blink reflects limited processing resources or is the product of attentional control. It is for instance difficult for limited-capacity models to explain why an improvement in reporting the second target did not negatively affect the accuracy of reporting the first target in both the temporal (Martens and Johnson, 2005) and color (Nieuwenstein et al., 2005; Olivers and Meeter, 2008) cuing studies, as well as in the studies involving distracting mental activity (Arend et al., 2006; Olivers and Nieuwenhuis, 2005, 2006; Taatgen et al., 2009).
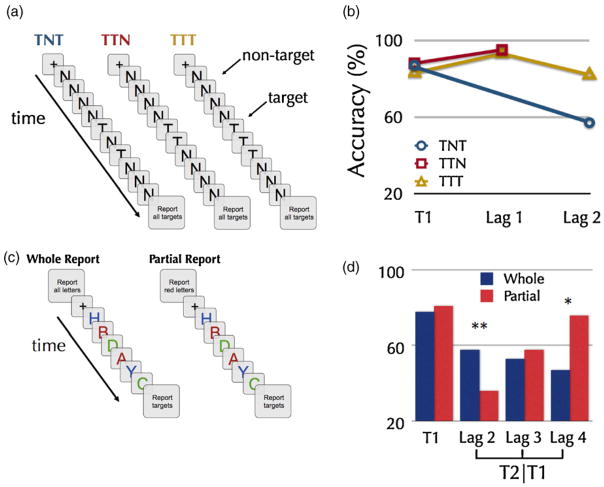
However, changes in the theoretical landscape were perhaps most strongly driven by a number of studies in which it was shown that more than one target can be identified without the occurrence of an AB. Several studies have reported that no AB occurs when up to four targets are to be identified within a stream of non-targets, as long as a target is not followed by a non-target, a finding referred to as ‘spreading the lag-1 sparing effect’ (Di Lollo et al., 2005; Nieuwenstein and Potter, 2006; Olivers et al., 2007; Potter et al., 2008) (see Fig. 3a and b).
Fig. 3. Spreading the sparing and the whole report advantage.
(a) Several studies have reported that no AB occurs when two or more targets are to be identified within a stream of non-targets, as long as a target is not followed by a non-target, a finding referred to as ‘spreading the lag-1 sparing effect’ (Di Lollo et al., 2005; Nieuwenstein and Potter, 2006; Olivers et al., 2007; Potter et al., 2008). Panel A shows a schematic trial representation of three experimental conditions producing an AB (TNT), lag-1 sparing (TTN), and spreading the sparing (TTT), respectively.
(b) Accuracy of reporting the targets in the TNT, TTN, and TTT conditions, presented in an RSVP stream at a rate of 100 ms (Olivers et al., 2007). When T1 is immediately followed by T2, both are often successfully reported (lag-1 sparing). This sparing can be ‘spread’ (spreading the sparing) to additional targets that are presented in immediate succession after T1. Adapted from Olivers et al. (2007), Fig. 1, with kind permission from Springer Science+Business Media, Copyright Springer-Verlag 2007.
(c) These experimental conditions demonstrate how whole report instructions attenuate the AB. Viewing a sequence of six random letters in an RSVP stream at an SOA of 107 ms, subjects were asked to report either all of the letters (whole report) or just letters of a particular color (partial report).
(d) Data from these two letter-report conditions show that the AB is only present for the partial report condition, in which two letters had to be reported (Nieuwenstein and Potter, 2006). Whole report of the entire sequence shows better performance at lag 2 for a letter in exactly the same position as was blinked in the other condition. *p < .05, **p < .01. Copyright (2006) Wiley. Used with permission from Nieuwenstein and Potter, 2006, Psychological Science, Wiley.
In fact, whereas comprehending and remembering an entire sentence (whole report) that is visually presented at 10 words/second is easy, reporting only two uppercase or colored words within the same sentence (partial report) paradoxically induces an AB on the second word when presented within 200–500 ms after the first word (Nieuwenstein and Potter, 2006; Potter et al., 2008) (see Fig. 3c and d). The inability to report the second target in the partial report condition cannot easily be explained as the cost of encoding or remembering the selected first target, because the whole report condition required encoding and remembering the same targets as well as several additional items.
Whereas one might still claim that this whole-report performance ‘superiority’ is due to a benefit of not having to use a specification or template to detect the targets (also referred to as an ‘attentional set’), the ‘spreading the sparing effect’ happens under the same attentional settings that usually lead to an AB. Though it must be noted that there certainly is a limit to the number of discrete items that can be stored in working memory (Di Lollo et al., 2005; Luck and Vogel, 1997; Nieuwenstein and Potter, 2006; Olivers et al., 2007; Potter et al., 2008), the observation that, within the AB period, mean performance improves rather than deteriorates with an increased number of targets poses a real problem for any resource-depletion account of the AB. Furthermore, the findings that changing task instruction (Nieuwenstein and Potter, 2006) or adding an extraneous cognitive load (Taatgen et al., 2009) can attenuate the AB stand as convincing demonstrations that the AB is strongly affected by task set.
On the other hand, it is certainly true that there are tradeoffs which can be observed between identification of T1 and the blinked target, for example by emphasizing T1 salience with a color change (Dux et al., 2009) or using a strict conditional analysis (Dell’Acqua et al., 2009). These studies are incisive in emphasizing that there is some competition between identifying closely spaced targets and a central bottleneck may be a contributing factor to the AB (Dux and Marois, 2009).
So what conclusions can be drawn from these experiments? It is now clear that the AB effect is not as easily described as a processing capacity limitation as has been previously assumed. What these studies suggest is that the configuration of our attentional circuitry to the task of detecting targets sets the stage for an AB to occur. The AB is evoked when one or more targets are followed by either a non-target item in an RSVP stream, or a blank screen of sufficient duration (Nieuwenstein et al., 2009). Cuing effects (Nieuwenstein et al., 2005) as well as ERP studies (Martens et al., 2006b; Vogel and Luck, 2002) suggest that the AB itself results from a delay in the allocation of attention to a second target. When a target cannot be attended rapidly, it becomes particularly vulnerable to backward masking, and thereby is ‘blinked’ in an RSVP stream (Nieuwenstein, 2006; Vul et al., 2008). These findings have paved the way for new efforts to simulate the time course of attentional dynamics in RSVP tasks which give rise to the AB.
3. Promising new avenues: modeling
There has been a surge of interest in computational models of possible neural mechanisms underlying the AB (Bowman and Wyble, 2007; Dehaene et al., 2003; Fragopanagos et al., 2005; Nieuwenhuis et al., 2005; Olivers and Meeter, 2008; Shih, 2008; Su et al., in press; Taatgen et al., 2009; Wyble et al., 2009). Modelers have been able to frame questions that have motivated new experimental work ranging from the pharmacological underpinnings of the AB to an understanding of the neural correlates of consciousness. Furthermore, in attempting to explain the data involving distraction, spreading the sparing, and whole report as means of eliminating the AB, recent models have converged on the idea that the AB is the result of attentional control, and this fits nicely with neural data showing how attentional deployment is suppressed during the AB (Box 2). Perhaps most importantly, computational models are a promising method of closing the gap between high level theories of the AB, and our understanding of its neural correlates. In the following section, we summarize questions that models have posed and the answers that have so far been discovered.
3.1. Is the AB caused by attentional mechanisms in the brainstem?
There is an intriguing similarity between the time course of the AB and the activation dynamics of the locus coeruleus (LC), a small nucleus in the brainstem that is thought to direct the central deployment of attention via a potent noradrenergic projection to much of the cortical mantle (Aston-Jones et al., 2000). In monkeys, the neurons of the LC fire a powerful burst in response to a visually presented target, and this burst is followed by a refractory period of several hundred milliseconds induced by rising levels of noradrenaline/norepinephrine (a chemical compound with dual roles as a hormone and a neurotransmitter, affecting large parts of the brain including areas where attention and responding actions are controlled).
A computational model of the temporal dynamics of LC activation (Nieuwenhuis et al., 2005) proposes that lag-1 sparing is a direct behavioral manifestation of the duration of this burst of firing, and that the AB reflects the subsequent refractory period. The model demonstrates how presentation of a T1 in an RSVP stream might trigger neurons in the LC to fire. The following burst of noradrenergic modulation produces an attentional enhancement that allows the target to be encoded into working memory. The T2 can benefit from this attention but only if it arrives immediately after the T1. By about 200 ms after the onset of T1, the LC refractory period has begun and for several hundred milliseconds afterwards, the T2 is incapable of triggering a second attentional response.
The LC model predicts that the refractory period which induces the AB is mediated by noradrenergic mechanisms, and thus, the application of an adrenergic agent should directly influence the neural mechanisms underlying the AB. However, an experiment designed to test this hypothesis with the central administration of the adrenergic agonist clonidine has failed to affect the time course of the AB, despite producing deleterious effects on T1 accuracy, increased array search times, and a reduction in blood pressure (Nieuwenhuis et al., 2007). This failure to confirm the prediction suggests that the temporal alerting response induced by a salient cue (see Nieuwenhuis et al., 2007) is most likely distinct from the mechanism underlying the AB.
Correct or not, the LC model has emphasized the role of time in visual attention. It successfully predicted that it is the temporal interval between the T1 and the T2 which determines whether sparing will occur. This means that a non-target between T1 and T2 need not cause an AB if the temporal gap between the targets is on the order of about 100 ms. This prediction has been confirmed in several contexts (Bowman and Wyble, 2007; Martens et al., 2006b; Potter et al., 2002) and is a central tenet of several contemporary AB models (Bowman and Wyble, 2007; Olivers and Meeter, 2008; Shih, 2008; Taatgen et al., 2009; Wyble et al., 2009).
3.2. Is consciousness mediated by an ‘all-or-none’ dynamic?
Another example of a computational model that explicitly connects with neurobiological data is the Global Workspace model. Developed by Dehaene and colleagues (Dehaene et al., 2003), this model is a neural network (an interconnected network of simple processing elements, often used as a computational analogy to neurally implemented processes within the brain) simulation which details the processing of stimuli from early sensory representations, to full-blown conscious events. Entry into conscious awareness requires coordinated activation of a large population of neurons in both sensory and high-level areas that are interconnected by long distance connections to form a “global neuronal workspace”. The model contains several nodes, each of which corresponds to a thalamocortical column (a group of cortical and thalamic neurons that are highly interconnected), and these nodes are interconnected via a pattern of feedforward and feedback excitatory connections climbing up and down the processing hierarchy, as well as inter-columnar inhibitory projections. The AB is simulated as the result of a widespread and self propagating pattern of activation induced by the T1 that blocks the entry of T2 into the global workspace for a window of about 200 ms.
An important contribution of the global workspace account is that it provided an interesting framework for the exploration of the phenomenal and neural correlates of consciousness during the AB. The model predicts that entry of an item into the arena of the global workspace proceeds with a nonlinear ‘ignition’ of recurrent processing (back and forth processing between two different parts of a neural network, for instance between higher-order processing areas (fronto-parietal cortex) and lower visual processing areas (occipital cortex) of the brain, thus mutually influencing each other) when a stimulus successfully recruits a wide ranging population of neurons throughout the brain to induce a conscious percept. This dynamic should produce an all-or-none activation dynamic within the brain, in which a target either successfully makes the transition to a full blown conscious representation or disappears entirely under the influence of a backward mask.
The AB allows for a test of this prediction by providing a way to study a mixture of ‘seen’ and ‘absent’ responses to above-threshold targets with identical perceptual characteristics. During the AB, when subjects are asked to report their subjective awareness of the T2, responses are clustered into two peaks at opposite ends of a scale (Sergent and Dehaene, 2004). The all-or-none nature of subjective awareness of T2 is accompanied by a similar all-or-none dichotomy between the neural correlates of T2s that are either seen or missed during the AB as found in a parallel EEG study (Sergent et al., 2005).
While the global workspace account is a compelling simulation of conscious awareness, its simulation of the AB fails to account for the fact that subjects can reliably encode two successive targets presented at lag 1. This shortcoming highlights the need for a more complex explanation, and later models have addressed ‘lag-1 sparing’ as a finding of primary importance by the inclusion of explicitly simulated attentional mechanisms.
3.3. Is there a functional role of the AB?
Recent models (Bowman and Wyble, 2007; Olivers and Meeter, 2008; Taatgen et al., 2009; Wyble et al., 2009) have succeeded in reproducing quite a broad range of behavioral data pertaining to the AB, and these data include some of the recent results that have troubled the classical bottleneck theory. These more recent models propose that the AB results not from a capacity limitation, but from a suppression of attention during T1 processing, an idea that is highly congruent with the finding of a suppressed N2pc component during the AB (Box 2). Here we compare three of these models. The central question that arises from this comparison is whether the AB plays a useful role in perception and what that role might be.
In the Threaded Cognition model (Taatgen et al., 2009), the AB is proposed to arise from an overzealous attentional control mechanism that suspends target detection during ongoing processing of a target. This hypothesis allows the model to explain why the AB can be attenuated by distraction; the brain is too busy doing something else and thereby neglects to shut off attention to protect the encoding and consolidation of T1 as it normally would. The authors support this idea with new behavioral data illustrating how the AB can be attenuated with a secondary task. Finally, the model provides the first explicit computational account of how non-blinkers may differ from blinkers (see Box 3). The claim of this model is that the attentional control that underlies the AB may be useful in other circumstances, but is unnecessary in an RSVP context.
In the Boost and Bounce model (Olivers and Meeter, 2008), the AB is described as a way of keeping non-targets from intruding into working memory. The idea is that detection of a target triggers an attentional ‘boost’, presumably by a prefrontal cortex/basal ganglia network. This boost helps a target to enter working memory, but can inadvertently carry over to affect a following non-target. To prevent this ‘boosted’ non-target from entering working memory, a separate mechanism, called the ‘bounce’, is triggered to suppress attention and block further processing. If a T2 occurs during this bounce, it will be unattended and its representation is likely to decay, leading to an AB for that target. A major virtue of this model is its relative simplicity, but it remains an open issue whether it can successfully explain the ability of distraction to eliminate the AB without affecting T1 accuracy (Olivers and Meeter, 2008; Taatgen et al., 2009).
Finally, in the eSTST model (Wyble et al., 2009) the AB is proposed to play an important role in parsing the continual stream of visual input into attentional episodes. Targets presented together, as in the case of lag-1 sparing, are encoded within an ongoing window of attention. Targets presented separately, are stored in distinct working memory representations, and the AB reflects the suppression of attention which provides this separation. During sparing or whole report, a sequence of targets is combined into a single attentional episode (see also Bowman and Wyble, 2007; Hommel and Akyürek, 2005). Importantly, this model also makes predictions about the drawbacks of encoding information without an AB. For example, during an ongoing attentional episode, as occurs during sparing or whole report, enhanced encoding of identity comes at the expense of episodic information. As a behavioral consequence, the temporal order of the targets is poorly encoded, and repetition blindness (a phenomenon that is related to but distinct from the AB, showing that subjects are less likely to detect the repetition of a target than they are to detect a second, different target) is enhanced and temporal order information is lost because, according to the model, an entire sequence of targets is combined into a single episode.
These models represent the cutting edge of theoretical work in identifying the role that the AB plays in perception, and empirical work has only just begun to make headway in testing their predictions (see Nieuwenstein et al., 2009). In future work, it will be particularly important to carefully explore the consequences of attenuating the AB because such manipulations may come at a cost. We already know that attenuating the AB does not markedly impair T1 report (Olivers and Meeter, 2008; Taatgen et al., 2009). However, information concerning temporal order, repetitions, and the correct binding of features into targets may be compromised when the occurrence of an AB is somehow avoided (Wyble et al., 2009). Such evidence would be an important clue that the AB plays a functional role in visual perception.
3.4. How to bridge the neuro-cognitive gap?
A challenge for current computational and theoretical accounts of the AB lies in connecting more directly with neurobiological data. One promising way to accomplish this is by not only simulating behavioral performance, but by predicting human EEG or fMRI brain activity as well, for instance by summating the activation value of simulated neurons as a function of time (Craston et al., 2009). By comparing for example simulated EEG traces to real EEG traces, a model can be constrained to adhere to real-time neural correlates in addition to reproducing behavioral data, thereby providing tremendous leverage in confirming the plausibility of simulated neural mechanisms. While such models are too simple to explain the neurobiological underpinnings of an ERP, they benefit from connecting simultaneously with both the behavioral and the electrophysiological correlates of information processing.
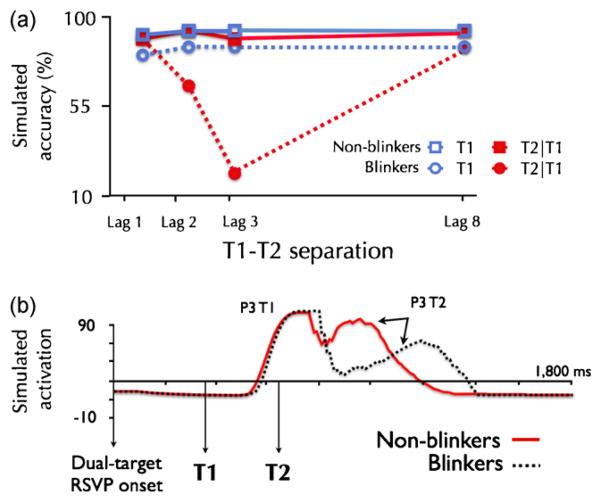
To provide a concrete example: The eSTST model simulates the encoding of a target into working memory as a sustained period of recurrent processing lasting several hundred milliseconds. The simulated P3 from this processing is similar in latency and duration to the P3 recorded from subjects in a similar task (Craston et al., 2009; Martens et al., 2006b; Vogel and Luck, 2002). In the model, the AB is produced by suppressing the deployment of attention, which both reduces T2 accuracy in simulated behavior, and delays the P3 evoked by the T2 in simulated EEG. Using this model, non-blinkers can be simulated by removing the suppression of attention, which alleviates the behavioral AB effect (Fig. 4a) and removes the delay of the simulated P3 component (Fig. 4b) as is observed in human EEG data (see Box 3). Thus, by simulating the neural correlates of the difference between blinkers and non-blinkers, modeling work supports the theory that non-blinkers do not use the suppressive mechanism that produces the AB in normal subjects (Martens et al., 2006b; Taatgen et al., 2009).
Fig. 4. Non-blinker simulations.
(a) Simulated behavioral data for blinkers and non-blinkers by the eSTST model. Non-blinkers are simulated by reducing the suppression of attention during T1 consolidation.
(b) Simulated ERPs produced by the model for blinkers and non-blinkers with a T1-T2 interval of 270 ms (lag 3). These artificial waveforms were generated by averaging together activation values of nodes in the model responsible for encoding targets into working memory. Notably, the model dynamics have a similar delay in the P3 induced by T2 for blinkers relative to non-blinkers as observed in real ERP recordings (Box 3).
4. Future directions and conclusions
As the empirical and theoretical landscapes of the AB have rapidly shifted, several promising research directions have emerged. Next to the virtual ERP approach discussed in the previous section, the following three topics seem especially promising areas of research to elucidate the AB phenomenon.
As mentioned before, several neuroimaging studies have revealed a fronto-temporo-parietal attentional network to be involved during the AB, which fits with the idea that the AB reflects a relatively late, post-perceptual processing effect. Indeed no modulations of early perceptual components had previously been found during the AB (Vogel et al., 1998). However, three studies recently reported attenuation of neural responses in V1 (a posterior part of the occipital cortex and the first cortical area to receive inputs from the eyes; also referred to as the primary visual cortex, area 17, or striate cortex) during the AB (Hein et al., 2009; Stein et al., 2008; Williams et al., 2008). It has been suggested that such modulations do not take place in the initial feed-forward sweep (the initial and rapid processing of a visual stimulus by a sequence of stages that follow one another without input from recurrent feedback loops) of visual processing but, instead, occur later in processing as iterative loops (a sequence of feedback and feedforward connections between later and earlier visual processing stages) are established between the frontal-parietal network and early processing areas such as V1 (Williams et al., 2008). In other words, these findings suggest that in Fig. 2b an additional red arrow should be drawn from the frontal-parietal areas back to the occipital area, which fits with the target-related synchronization between these areas that is shown in Fig. 2a. This recurrent activity may actually be crucial for the conscious perception of selected targets (Lamme, 2006; Martens et al., 2002; Stein et al., 2008). Integrated computational models of attention, memory, and conscious perception will be especially useful in understanding the recurrent dynamics that produce low-level visual effects of late stage processing.
Another promising approach that may shed new light on the underlying causes of the AB is to study and compare specific groups of subjects that tend to show varying degrees of AB magnitude. Examples of such groups of interest include patients (Husain and Rorden, 2003; Husain et al., 1997), elderly (Lahar et al., 2001), and bilinguals (Colzato et al., 2008), who all show a stronger and/or longer AB effect, as opposed to action-video gamers (Green and Bavelier, 2003), expert meditators (Slagter et al., 2007), and non-blinkers (Box 3) who have either reduced or entirely absent ABs.
A final area of growing interest concerns interactions between the emotional valence of stimuli and the AB. In an early study, the magnitude of the AB was found to be smaller when emotionally negative target words were used than when neutral words were used. However, this breakthrough effect was not present in patients with damage to the left amygdala (a group of neurons located deep within the medial temporal lobe of the brain, important for the expression and processing of emotions; Anderson and Phelps, 2001). Replications of this effect suggest that it is the arousing quality rather than the positive or negative emotional value of stimuli that permits such a breakthrough (de Jong et al., 2009; Keil and Ihssen, 2004). It has also been found that an emotionally charged non-target can induce an AB on a subsequent target (Arnell et al., 2007; Most et al., 2005). Such effects also apply to the emotional quality that is attached to neutral stimuli via classical conditioning (a form of associative learning, inducing a reflexive response to a neutral stimulus by linking the neutral stimulus to a significant (i.e. pleasant or painful) stimulus; also referred to as Pavlovian conditioning; Smith et al., 2006). The AB paradigm also provides a path to investigate emotional processing in subjects that score either high or low on types of concern such as spider phobia (Trippe et al., 2007).
In this review, we have tried to consolidate the theoretical landscape of the AB, which has undergone a remarkable change in the course of the past few years as a result of striking new behavioral evidence. Limited processing resources of some sort clearly play an important role in the AB, but the picture that has recently emerged seems to be more complex, suggesting that attentional regulation and a tradeoff between identity and episodic forms of information is involved. Although far from having settled on an explanation the field now stands with a perspective that this apparent cognitive limitation may in fact reflect a mechanism of attentional control that can be reduced by distraction, and in some subjects is absent altogether.
The continuing investigation, which features a population of computationally explicit accounts, pursues a number of questions that remain unanswered. Does the AB fulfill a useful purpose? Do non-blinkers suffer a cost in some other sort of task? What determines whether a target will be seen or missed on a particular trial? Finally and perhaps most importantly, questions that exploration of the AB seems well suited to answer are how a stimulus can break into the arena of conscious awareness, and under which circumstances will that stimulus leave either a trace of its presence or a blind spot within working memory.
References
- Anderson AK, Phelps EA. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411:305–307. doi: 10.1038/35077083. [DOI] [PubMed] [Google Scholar]
- Arend I, Johnston S, Shapiro K. Task-irrelevant visual motion and flicker attenuate the attentional blink. Psychonomic Bulletin & Review. 2006;13 (4):600–607. doi: 10.3758/bf03193969. [DOI] [PubMed] [Google Scholar]
- Arnell KM, Jenkins R. Revisiting within-modality and cross-modality attentional blinks: effects of target-distractor similarity. Perception & Psychophysics. 2004;66 (7):1147–1161. doi: 10.3758/bf03196842. [DOI] [PubMed] [Google Scholar]
- Arnell KM, Jolicoeur P. The attentional blink across stimulus modalities: evidence for central processing limitations. Journal of Experimental Psychology: Human Perception and Performance. 1999;25 (3):630–648. [Google Scholar]
- Arnell KM, Killman KV, Fijavz D. Blinded by emotion: target misses follow attention capture by arousing distractors in RSVP. Emotion. 2007;7 (3):465–477. doi: 10.1037/1528-3542.7.3.465. [DOI] [PubMed] [Google Scholar]
- Arnell KM, Larson JM. Cross-modality attentional blinks without preparatory task-set switching. Psychonomic Bulletin & Review. 2002;9 (3):497–506. doi: 10.3758/bf03196305. [DOI] [PubMed] [Google Scholar]
- Arnell KM, Stokes KA, Maclean MH, Gicante C. Executive control processes of working memory predict attentional blink magnitude over and above storage capacity. Psychological Research. 2010;74 (1):1–11. doi: 10.1007/s00426-008-0200-4. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Cohen J. Locus coeruleus and regulation of behavioral flexibility and attention. Progress in Brain Research. 2000;126:165–182. doi: 10.1016/S0079-6123(00)26013-5. [DOI] [PubMed] [Google Scholar]
- Barber JR, Razak KA, Fuzessery ZM. Can two streams of auditory information be processed simultaneously? Evidence from the gleaning bat Antrozous pallidus. Journal of Comparative Physiology. A, Neuroethology, Sensory, Neural, and Behavioral Physiology. 2003;189 (11):843–855. doi: 10.1007/s00359-003-0463-6. [DOI] [PubMed] [Google Scholar]
- Barnard PJ, Scott S, Taylor J, May J, Knightley W. Paying attention to meaning. Psychological Science. 2004;15 (3):179–186. doi: 10.1111/j.0956-7976.2004.01503006.x. [DOI] [PubMed] [Google Scholar]
- Beech AR, Kalmus E, Tipper SP, Baudouin JY, Flak V, Humphreys GW. Children induce an enhanced attentional blink in child molesters. Psychological Assessment. 2008;20 (4):397. doi: 10.1037/a0013587. [DOI] [PubMed] [Google Scholar]
- Bowman H, Wyble B. The simultaneous type, serial token model of temporal attention and working memory. Psychological Review. 2007;114 (1):38. doi: 10.1037/0033-295X.114.1.38. [DOI] [PubMed] [Google Scholar]
- Braun J. Vision and attention: the role of training. Nature. 1998;393:424–425. doi: 10.1038/30875. [DOI] [PubMed] [Google Scholar]
- Broadbent DE, Broadbent MHP. From detection to identification: response to multiple targets in rapid serial visual presentation. Perception & Psychophysics. 1987;42 (2):105–113. doi: 10.3758/bf03210498. [DOI] [PubMed] [Google Scholar]
- Chun MM, Potter MC. A two-stage model for multiple target detection in rapid serial visual presentation. Journal of Experimental Psychology: Human Perception and Performance. 1995;21 (1):109–127. doi: 10.1037//0096-1523.21.1.109. [DOI] [PubMed] [Google Scholar]
- Colzato LS, Bajo MT, van den Wildenberg W, Paolieri D, Nieuwenhuis S, La Heij W, et al. How does bilingualism improve executive control? A comparison of active and reactive inhibition mechanisms. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2008;34 (2):302. doi: 10.1037/0278-7393.34.2.302. [DOI] [PubMed] [Google Scholar]
- Colzato LS, Spapè M, Pannebakker MM, Hommel B. Working memory and the attentional blink: blink size is predicted by individual differences in operation span. Psychonomic Bulletin & Review. 2007;14:1051–1057. doi: 10.3758/bf03193090. [DOI] [PubMed] [Google Scholar]
- Conway ARA, Jarrold C, Kane MJ, Towse JN, editors. Variation in Working Memory. Oxford University Press; New York: 2007. [Google Scholar]
- Cooper ACG, Humphreys GW, Hulleman J, Praamstra P, Georgeson M. Transcranial magnetic stimulation to right parietal cortex modifies the attentional blink. Experimental Brain Research. 2004;155 (1):24–29. doi: 10.1007/s00221-003-1697-9. [DOI] [PubMed] [Google Scholar]
- Craston P, Wyble B, Chennu S, Bowman H. The attentional blink reveals serial working memory encoding: evidence from virtual and human event-related potentials. Journal of Cognitive Neuroscience. 2009;21 (3):550–566. doi: 10.1162/jocn.2009.21036. [DOI] [PubMed] [Google Scholar]
- de Jong PJ, Koster EHW, Cieraad R, Martens S. Emotional facial expressions and the attentional blink: attenuated blink for angry and happy faces irrespective of social anxiety. Cognition and Emotion. 2009;23 (8):1640–1652. [Google Scholar]
- Dehaene S, Sergent C, Changeux JP. A neuronal network model linking subjective reports and objective physiological data during conscious perception. Proceedings of the National Academy of Sciences. 2003;100 (14):8520–8525. doi: 10.1073/pnas.1332574100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell’Acqua R, Pesciarelli F, Jolicoeur P, Eimer M, Peressotti F. The interdependence of spatial attention and lexical access as revealed by early asymmetries in occipito-parietal ERP activity. Psychophysiology. 2007;44 (3):436–443. doi: 10.1111/j.1469-8986.2007.00514.x. [DOI] [PubMed] [Google Scholar]
- Dell’Acqua R, Sessa P, Jolicoeur P, Robitaille N. Spatial attention freezes during the attention blink. Psychophysiology. 2006;43 (4):394–400. doi: 10.1111/j.1469-8986.2006.00411.x. [DOI] [PubMed] [Google Scholar]
- Dell’Acqua R, Jolicoeur P, Luria R, Pluchino P. Re-evaluating encoding-capacity limitations as a cause of the attentional blink. Journal of Experimental Psychology: Human Perception and Performance. 2009;35 (2):338–351. doi: 10.1037/a0013555. [DOI] [PubMed] [Google Scholar]
- Di Lollo V, Kawahara J, Shahab Ghorashi SM, Enns JT. The attentional blink: resource depletion or temporary loss of control? Psychological Research. 2005;69 (3):191–200. doi: 10.1007/s00426-004-0173-x. [DOI] [PubMed] [Google Scholar]
- Duncan J, Martens S, Ward R. Restricted attentional capacity within but not between sensory modalities. Nature. 1997;387:808–810. doi: 10.1038/42947. [DOI] [PubMed] [Google Scholar]
- Duncan J, Ward R, Shapiro K. Direct measurement of attentional dwell time in human vision. Nature. 1994;369 (6478):313–315. doi: 10.1038/369313a0. [DOI] [PubMed] [Google Scholar]
- Dux PE, Asplund CL, Marois R. Both exogenous and endogenous target salience manipulations support resource depletion accounts of the attentional blink: a reply to Olivers, Spalek, Kawahara, and Di Lollo (2009) Psychonomic Bulletin & Review. 2009;16 (1):219–224. doi: 10.3758/PBR.16.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dux PE, Marois R. Distractor inhibition predicts individual differences in the attentional blink. PLoS ONE. 2008;3(10) doi: 10.1371/journal.pone.0003330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dux PE, Marois R. The attentional blink: A review of data and theory. Attention, Perception & Psychophysics. 2009;71:1683–1700. doi: 10.3758/APP.71.8.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans KK, Treisman A. Perception of objects in natural scenes: is it really attention free? Journal of Experimental Psychology: Human Perception and Performance. 2005;31 (6):1476–1492. doi: 10.1037/0096-1523.31.6.1476. [DOI] [PubMed] [Google Scholar]
- Feinstein JS, Stein MB, Castillo GN, Paulus MP. From sensory processes to conscious perception. Consciousness and Cognition. 2004;13 (2):323–335. doi: 10.1016/j.concog.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Ferlazzo F, Lucido S, Di Nocera F, Fagioli S, Sdoia S. Switching between goals mediates the attentional blink effect. Experimental Psychology. 2007;54 (2):89–98. doi: 10.1027/1618-3169.54.2.89. [DOI] [PubMed] [Google Scholar]
- Fragopanagos N, Kockelkoren S, Taylor JG. A neurodynamic model of the attentional blink. Cognitive Brain Research. 2005;24 (3):568–586. doi: 10.1016/j.cogbrainres.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Giesbrecht B, Sy JL, Elliott JC. Electrophysiological evidence for both perceptual and postperceptual selection during the attentional blink. Journal of Cognitive Neuroscience. 2007;19 (12):2005–2018. doi: 10.1162/jocn.2007.19.12.2005. [DOI] [PubMed] [Google Scholar]
- Giesbrecht B, Sy JL, Lewis MK. Personal names do not always survive the attentional blink: behavioral evidence for a flexible locus of selection. Vision Research. 2009;49 (10):1378–1388. doi: 10.1016/j.visres.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CS, Bavelier D. Action video game modifies visual selective attention. Nature. 2003;423 (6939):534–537. doi: 10.1038/nature01647. [DOI] [PubMed] [Google Scholar]
- Gross J, Schmitz F, Schnitzler I, Kessler K, Shapiro K, Hommel B, et al. Modulation of long-range neural synchrony reflects temporal limitations of visual attention in humans. Proceedings of the national Academy of Sciences. 2004;101 (35):13050–13055. doi: 10.1073/pnas.0404944101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari R, Renvall H. Impaired processing of rapid stimulus sequences in dyslexia. Trends in Cognitive Sciences. 2001;5 (12):525–532. doi: 10.1016/s1364-6613(00)01801-5. [DOI] [PubMed] [Google Scholar]
- Hein G, Alink A, Kleinschmidt A, Muller NG. The attentional blink modulates activity in the early visual cortex. Journal of Cognitive Neuroscience. 2009;21 (1):197–206. doi: 10.1162/jocn.2008.21026. [DOI] [PubMed] [Google Scholar]
- Hein G, Parr A, Duncan J. Within-modality and cross-modality attentional blinks in a simple discrimination task. Perception & Psychophysics. 2006;68 (1):54–61. doi: 10.3758/bf03193655. [DOI] [PubMed] [Google Scholar]
- Hillstrom AP, Shapiro KL, Spence C. Attentional limitations in processing sequentially presented vibrotactile targets. Perception & Psychophysics. 2002;64 (7):1068–1082. doi: 10.3758/bf03194757. [DOI] [PubMed] [Google Scholar]
- Ho C, Spence C. Assessing the effectiveness of various auditory cues in capturing a driver’s visual attention. Journal of Experimental Psychology: Applied. 2005;11 (3):157–174. doi: 10.1037/1076-898X.11.3.157. [DOI] [PubMed] [Google Scholar]
- Hollingsworth DE, McAuliffe SP, Knowlton BJ. Temporal allocation of visual attention in adult attention deficit hyperactivity disorder. Journal of Cognitive Neuroscience. 2001;13 (3):298–305. doi: 10.1162/08989290151137359. [DOI] [PubMed] [Google Scholar]
- Hommel B, Akyürek EG. Lag-1 sparing in the attentional blink: benefits and costs of integrating two events into a single episode. The Quarterly Journal of Experimental Psychology Section A. 2005;1 (1):1–11. doi: 10.1080/02724980443000647. [DOI] [PubMed] [Google Scholar]
- Hommel B, Kessler K, Schmitz F, Gross J, Akyürek E, Shapiro K, et al. How the brain blinks: towards a neurocognitive model of the attentional blink. Psychological Research. 2006;70 (6):425–435. doi: 10.1007/s00426-005-0009-3. [DOI] [PubMed] [Google Scholar]
- Husain M, Rorden C. Non-spatially lateralized mechanisms in hemispatial neglect. Nature Reviews Neuroscience. 2003;4 (1):26–36. doi: 10.1038/nrn1005. [DOI] [PubMed] [Google Scholar]
- Husain M, Shapiro K, Martin J, Kennard C. Abnormal temporal dynamics of visual attention in spatial neglect patients. Nature. 1997;385 (6612):154–156. doi: 10.1038/385154a0. [DOI] [PubMed] [Google Scholar]
- Isaak MI, Shapiro KL, Martin J. The attentional blink reflects retrieval competition among multiple RSVP items: tests of the interference model. Journal of Experimental Psychology: Human Perception and Performance. 1999;25 (6):1774–1792. doi: 10.1037//0096-1523.25.6.1774. [DOI] [PubMed] [Google Scholar]
- Jolicoeur P. Restricted attentional capacity between sensory modalities. Psychonomic Bulletin & Review. 1999;6 (1):87–92. doi: 10.3758/bf03210813. [DOI] [PubMed] [Google Scholar]
- Jolicœur P, Dell’Acqua R. Attentional and structural constraints on visual encoding. Psychological Research. 1999;62 (2):154–164. [Google Scholar]
- Jolicoeur P, Sessa P, Dell’Acqua R, Robitaille N. Attentional control and capture in the attentional blink paradigm: evidence from human electrophysiology. European Journal of Cognitive Psychology. 2006;18 (4):560–578. [Google Scholar]
- Jolicœur P, Sessa P, Dell’Acqua R, Robitaille N. On the control of visual spatial attention: evidence from human electrophysiology. Psychological Research. 2006;70 (6):414–424. doi: 10.1007/s00426-005-0008-4. [DOI] [PubMed] [Google Scholar]
- Jolicœur P, Tombu M, Oriet C, Stevanovski B. Common Mechanisms in Perception and Action: Attention & Performance. XIX. 2002. From perception to action: making the connection; pp. 558–586. [Google Scholar]
- Joseph JS, Chun MM, Nakayama K. Attentional requirements in a ‘pre-attentive’ feature search task. Nature. 1997;387:805. doi: 10.1038/42940. [DOI] [PubMed] [Google Scholar]
- Keil A, Ihssen N. Identification facilitation for emotionally arousing verbs during the attentional blink. Emotion. 2004;4 (1):23–35. doi: 10.1037/1528-3542.4.1.23. [DOI] [PubMed] [Google Scholar]
- Kihara K, Hirose N, Mima T, Abe M, Fukuyama H, Osaka N. The role of left and right intraparietal sulcus in the attentional blink: a transcranial magnetic stimulation study. Experimental Brain Research. 2007;178 (1):135–140. doi: 10.1007/s00221-007-0896-1. [DOI] [PubMed] [Google Scholar]
- Kim CY, Blake R. Psychophysical magic: rendering the visible ‘invisible’. Trends in Cognitive Sciences. 2005;9 (8):381–388. doi: 10.1016/j.tics.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Kranczioch C, Debener S, Schwarzbach J, Goebel R, Engel AK. Neural correlates of conscious perception in the attentional blink. Neuroimage. 2005;24 (3):704–714. doi: 10.1016/j.neuroimage.2004.09.024. [DOI] [PubMed] [Google Scholar]
- Lahar CJ, Isaak MI, McArthur AD. Age differences in the magnitude of the attentional blink. Aging, Neuropsychology, and Cognition. 2001;8 (2):149–159. [Google Scholar]
- Lamme VAF. Towards a true neural stance on consciousness. Trends in Cognitive Sciences. 2006;10 (11):494–501. doi: 10.1016/j.tics.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Li CR, Lin W, Yang Y, Huang C, Chen T, Chen Y. Impairment of temporal attention in patients with schizophrenia. Neuroreport. 2002;13 (11):1427. doi: 10.1097/00001756-200208070-00016. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA. Spatial filtering during visual search: evidence from human electrophysiology. Journal of Experimental Psychology: Human Perception and Performance. 1994;20 (5):1000–1014. doi: 10.1037//0096-1523.20.5.1000. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Vogel EK. The capacity of visual working memory for features and conjunctions. Nature. 1997:279–280. doi: 10.1038/36846. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Vogel EK, Shapiro KL. Word meanings can be accessed but not reported during the attentional blink. Nature. 1996;383 (6601):616–618. doi: 10.1038/383616a0. [DOI] [PubMed] [Google Scholar]
- Maki WS, Frigen K, Paulson K. Associative priming by targets and distractors during rapid serial visual presentation: does word meaning survive the attentional blink? Journal of Experimental Psychology: Human Perception and Performance. 1997;23 (4):1014–1034. doi: 10.1037//0096-1523.23.4.1014. [DOI] [PubMed] [Google Scholar]
- Maki WS, Padmanabhan G. Transient suppression of processing during rapid serial visual presentation: acquired distinctiveness of probes modulates the attentional blink. Psychonomic Bulletin & Review. 1994;1 (4):499–504. doi: 10.3758/BF03210954. [DOI] [PubMed] [Google Scholar]
- Marcantoni WS, Lepage M, Beaudoin G, Bourgouin P, Richer F. Neural correlates of dual task interference in rapid visual streams: an fMRI study. Brain and Cognition. 2003;53 (2):318–321. doi: 10.1016/s0278-2626(03)00134-9. [DOI] [PubMed] [Google Scholar]
- Marois R, Ivanoff J. Capacity limits of information processing in the brain. Trends in Cognitive Sciences. 2005;9 (6):296–305. doi: 10.1016/j.tics.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Marois R, Yi DJ, Chun MM. The neural fate of consciously perceived and missed events in the attentional blink. Neuron. 2004;41 (3):465–472. doi: 10.1016/s0896-6273(04)00012-1. [DOI] [PubMed] [Google Scholar]
- Martens S, Elmallah K, London R, Johnson A. Cuing and stimulus probability effects on the P3 and the AB. Acta Psychologica. 2006a;123 (3):204–218. doi: 10.1016/j.actpsy.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Martens S, Johnson A. Timing attention: cuing target onset interval attenuates the attentional blink. Memory and Cognition. 2005;33 (2):234–240. doi: 10.3758/bf03195312. [DOI] [PubMed] [Google Scholar]
- Martens S, Johnson A. Working memory capacity, intelligence, and the magnitude of the attentional blink revisited. Experimental Brain Research. 2008;129:43–52. doi: 10.1007/s00221-008-1551-1. [DOI] [PubMed] [Google Scholar]
- Martens S, Johnson A, Bolle M, Borst J. A quick visual mind can be a slow auditory mind: individual differences in attentional selection across modalities. Experimental Psychology. 2009;56 (1):33–40. doi: 10.1027/1618-3169.56.1.33. [DOI] [PubMed] [Google Scholar]
- Martens S, Munneke J, Smid H, Johnson A. Quick minds don’t blink: electrophysiological correlates of individual differences in attentional selection. Journal of Cognitive Neuroscience. 2006b;18 (9):1423–1438. doi: 10.1162/jocn.2006.18.9.1423. [DOI] [PubMed] [Google Scholar]
- Martens S, Valchev N. Individual differences in the attentional blink: the important role of irrelevant information. Experimental Psychology. 2009;56 (1):18–26. doi: 10.1027/1618-3169.56.1.18. [DOI] [PubMed] [Google Scholar]
- Martens S, Wolters G, van Raamsdonk M. Blinks of the mind: memory effects of attentional processes. Journal of Experimental Psychology: Human Perception and Performance. 2002;28 (6):1275–1287. [PubMed] [Google Scholar]
- Most SB, Chun MM, Widders DM, Zald DH. Attentional rubbernecking: cognitive control and personality in emotion-induced blindness. Psychonomic Bulletin & Review. 2005;12 (4):654. doi: 10.3758/bf03196754. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Gilzenrat MS, Holmes BD, Cohen JD. The role of the locus coeruleus in mediating the attentional blink: a neurocomputational theory. Journal of Experimental Psychology: General. 2005;134 (3):291. doi: 10.1037/0096-3445.134.3.291. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, van Nieuwpoort IC, Veltman DJ, Drent ML. Effects of the noradrenergic agonist clonidine on temporal and spatial attention. Psychopharmacology (Berl) 2007;193 (2):261–269. doi: 10.1007/s00213-007-0770-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenstein MR. Top-down controlled, delayed selection in the attentional blink. Journal of Experimental Psychology: Human Perception and Performance. 2006;32 (4):973–985. doi: 10.1037/0096-1523.32.4.973. [DOI] [PubMed] [Google Scholar]
- Nieuwenstein MR, Chun MM, van der Lubbe RHJ, Hooge ITC. Delayed attentional engagement in the attentional blink. Journal of Experimental Psychology: Human Perception and Performance. 2005;31 (6):1463–1475. doi: 10.1037/0096-1523.31.6.1463. [DOI] [PubMed] [Google Scholar]
- Nieuwenstein MR, Johnson A, Kanai R, Martens S. Cross-task repetition amnesia: impaired recall of RSVP targets held in memory for a secondary task. Acta Psychologica. 2007;125 (3):319–333. doi: 10.1016/j.actpsy.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Nieuwenstein MR, Potter MC. A comparison of whole versus partial report in rapid serial visual presentation. Psychological Science. 2006;17 (6):471–475. doi: 10.1111/j.1467-9280.2006.01730.x. [DOI] [PubMed] [Google Scholar]
- Nieuwenstein MR, Potter MC, Theeuwes J. Unmasking the attentional blink. Journal of Experimental Psychology: Human Perception and Performance. 2009;35 (1):159–169. doi: 10.1037/0096-1523.35.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivers CNL, Meeter M. A boost and bounce theory of temporal attention. Psychological Review. 2008;115 (4):836–863. doi: 10.1037/a0013395. [DOI] [PubMed] [Google Scholar]
- Olivers CNL, Nieuwenhuis S. The beneficial effect of concurrent task-irrelevant mental activity on temporal attention. Psychological Science. 2005;16 (4):265–269. doi: 10.1111/j.0956-7976.2005.01526.x. [DOI] [PubMed] [Google Scholar]
- Olivers CNL, Nieuwenhuis S. The beneficial effects of additional task load, positive affect, and instruction on the attentional blink. Journal of Experimental Psychology: Human Perception and Performance. 2006;32 (2):364–379. doi: 10.1037/0096-1523.32.2.364. [DOI] [PubMed] [Google Scholar]
- Olivers CNL, van der Stigchel S, Hulleman J. Spreading the sparing: against a limited-capacity account of the attentional blink. Psychological Research. 2007;71 (2):126–139. doi: 10.1007/s00426-005-0029-z. [DOI] [PubMed] [Google Scholar]
- Pashler H, Shiu LP. Do images involuntarily trigger search? A test of Pillsbury’s hypothesis. Psychonomic Bulletin & Review. 1999;6 (3):445–448. doi: 10.3758/bf03210833. [DOI] [PubMed] [Google Scholar]
- Pesciarelli F, Kutas M, Dell’Acqua R, Peressotti F, Job R, Urbach TP. Semantic and repetition priming within the attentional blink: an event-related brain potential (ERP) investigation study. Biological Psychology. 2007;76 (1–2):21–30. doi: 10.1016/j.biopsycho.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Potter MC, Chun MM, Banks BS, Muckenhoupt M. Two attentional deficits in serial target search: the visual attentional blink and an amodal task-switch deficit. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1998;24 (4):979–992. doi: 10.1037//0278-7393.24.4.979. [DOI] [PubMed] [Google Scholar]
- Potter MC, Dell Acqua R, Pesciarelli F, Job R, Peressotti F, OConnor DH. Bidirectional semantic priming in the attentional blink. Psychonomic Bulletin & Review. 2005;12 (3):460. doi: 10.3758/bf03193788. [DOI] [PubMed] [Google Scholar]
- Potter MC, Nieuwenstein M, Strohminger N. Whole report versus partial report in RSVP sentences. Journal of Memory and Language. 2008;58 (4):907–915. doi: 10.1016/j.jml.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter MC, Staub A, OConnor DH. The time course of competition for attention: attention is initially labile. Journal of Experimental Psychology: Human Perception and Performance. 2002;28 (5):1149–1162. doi: 10.1037//0096-1523.28.5.1149. [DOI] [PubMed] [Google Scholar]
- Raymond JE, Shapiro KL, Arnell KM. Temporary suppression of visual processing in an RSVP task: an attentional blink. Journal of Experimental Psychology: Human Perception and Performance. 1992;18 (3):849–860. doi: 10.1037//0096-1523.18.3.849. [DOI] [PubMed] [Google Scholar]
- Rokke PD, Arnell KM, Koch MD, Andrews JT. Dual-task attention deficits in dysphoric mood. Journal of Abnormal Psychology. 2002;111 (2):370–379. [PubMed] [Google Scholar]
- Rolke B, Heil M, Streb J, Hennighausen E. Missed prime words within the attentional blink evoke an N400 semantic priming effect. Psychophysiology. 2001;38 (2):165–174. [PubMed] [Google Scholar]
- Seiffert AE, Di Lollo V. Low-level masking in the attentional blink. Journal of Experimental Psychology: Human Perception and Performance. 1997;23 (4):1061–1073. [Google Scholar]
- Sergent C, Baillet S, Dehaene S. Timing of the brain events underlying access to consciousness during the attentional blink. Nature Neuroscience. 2005;8 (10):1391–1400. doi: 10.1038/nn1549. [DOI] [PubMed] [Google Scholar]
- Sergent C, Dehaene S. Evidence for an all-or-none bifurcation during the attentional blink. Psychological Science. 2004;15 (11):720–728. doi: 10.1111/j.0956-7976.2004.00748.x. [DOI] [PubMed] [Google Scholar]
- Shapiro K, Driver J, Ward R, Sorensen RB. A failure to extract visual tokens but not visual types. Psychological Science. 1997a;8 (2):95–100. [Google Scholar]
- Shapiro K, Johnston S, Vogels W, Zaman A, Roberts N. Increased functional magnetic resonance imaging activity during nonconscious perception in the attentional blink. Neuroreport. 2007;18 (4):341. doi: 10.1097/WNR.0b013e32801299e2. [DOI] [PubMed] [Google Scholar]
- Shapiro KL, Caldwell J, Sorensen RE. Personal names and the attentional blink: a visual “cocktail party” effect. Journal of Experimental Psychology: Human Perception and Performance. 1997b;23 (2):504–514. doi: 10.1037//0096-1523.23.2.504. [DOI] [PubMed] [Google Scholar]
- Shih SI. The attention cascade model and attentional blink. Cognitive Psychology. 2008;56 (3):210–236. doi: 10.1016/j.cogpsych.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Slagter HA, Lutz A, Greischar LL, Francis AD, Nieuwenhuis S, Davis JM, et al. Mental training affects distribution of limited brain resources. PLoS Biology. 2007;5 (6):e138. doi: 10.1371/journal.pbio.0050138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SD, Most SB, Newsome LA, Zald DH. An emotion-induced attentional blink elicited by aversively conditioned stimuli. Emotion. 2006;6 (3):523–527. doi: 10.1037/1528-3542.6.3.523. [DOI] [PubMed] [Google Scholar]
- Soto-Faraco S, Spence C. Modality-specific auditory and visual temporal processing deficits. The Quarterly Journal of Experimental Psychology Section A. 2002;55 (1):23–40. doi: 10.1080/02724980143000136. [DOI] [PubMed] [Google Scholar]
- Spence C, Driver J. Cross-modal links in attention between audition, vision, and touch: implications for interface design. International Journal of Cognitive Ergonomics. 1997;1 (4):351–373. [Google Scholar]
- Stein T, Vallines I, Schneider WX. Primary visual cortex reflects behavioral performance in the attentional blink. Neuroreport. 2008;19 (13):1277. doi: 10.1097/WNR.0b013e32830bab02. [DOI] [PubMed] [Google Scholar]
- Su L, Bowman H, Barnard PJ, Wyble B. Process algebraic modelling of attentional capture and human electrophysiology in interactive systems. Formal Aspects of Computing. in press. [Google Scholar]
- Taatgen NA, Juvina I, Schipper M, Borst J, Martens S. Too much control can hurt: a threaded cognition model of the attentional blink. Cognitive Psychology. 2009;59:1–29. doi: 10.1016/j.cogpsych.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Trippe RH, Hewig J, Heydel C, Hecht H, Miltner WHR. Attentional blink to emotional and threatening pictures in spider phobics: electrophysiology and behavior. Brain Research. 2007;1148:149–160. doi: 10.1016/j.brainres.2007.02.035. [DOI] [PubMed] [Google Scholar]
- Visser TA. Masking T1 difficulty: processing time and the attentional blink. Journal of Experimental Psychology: Human Perception and Performance. 2007a;33 (2):285. doi: 10.1037/0096-1523.33.2.285. [DOI] [PubMed] [Google Scholar]
- Visser TA. T1 difficulty and the attentional blink: expectancy versus backward masking. The Quarterly Journal of Experimental Psychology Section A. 2007b;60 (7):936–951. doi: 10.1080/17470210600847727. [DOI] [PubMed] [Google Scholar]
- Visser TAW, Merikle PM, Di Lollo V. Priming in the attentional blink: perception without awareness? Visual Cognition. 2005;12 (7):1362–1372. [Google Scholar]
- Vogel EK, Luck SJ. Delayed working memory consolidation during the attentional blink. Psychonomic Bulletin & Review. 2002;9 (4):739–743. doi: 10.3758/bf03196329. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Luck SJ, Shapiro KL. Electrophysiological evidence for a post-perceptual locus of suppression during the attentional blink. Journal of Experimental Psychology: Human Perception and Performance. 1998;24:1656–1674. doi: 10.1037//0096-1523.24.6.1656. [DOI] [PubMed] [Google Scholar]
- Vul E, Nieuwenstein M, Kanwisher N. Temporal selection is suppressed, delayed, and diffused during the attentional blink. Psychological Science. 2008;19 (1):55–61. doi: 10.1111/j.1467-9280.2008.02046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichselgartner E, Sperling G. Dynamics of automatic and controlled visual attention. Science. 1987;238 (4828):778–780. doi: 10.1126/science.3672124. [DOI] [PubMed] [Google Scholar]
- Williams MA, Visser TAW, Cunnington R, Mattingley JB. Attenuation of neural responses in primary visual cortex during the attentional blink. The Journal of Neuroscience. 2008;28 (39):9890–9894. doi: 10.1523/JNEUROSCI.3057-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyble B, Bowman H, Nieuwenstein M. The attentional blink and episodic distinctiveness: sparing at a cost. Journal of Experimental Psychology: Human Perception and Performance. 2009;35 (3):787–807. doi: 10.1037/a0013902. [DOI] [PMC free article] [PubMed] [Google Scholar]