Abstract
The forkhead box transcription factor, FOXD3, is a stemness factor that prevents the production of melanocyte progenitors from the developing neural crest; however, its role in human cancers is not known. Transformation of melanocytes gives rise to melanoma. In two-thirds of melanomas, the serine/threonine kinase B-RAF is mutated to a constitutively active form. Here, we demonstrate that FOXD3 levels are up-regulated following attenuation of B-RAF and MEK signaling in mutant B-RAF harboring human melanoma cells. This effect was selective since FOXD3 was not up-regulated following MEK inhibition in wild-type B-RAF melanoma cells and mutant B-RAF thyroid carcinoma cells. Ectopic FOXD3 expression potently inhibited melanoma cell growth without altering mutant B-RAF activation of ERK1/2. Inhibition of cell growth was due to a potent G1 cell cycle arrest and was associated with p53-dependent up-regulation of p21Cip1. FOXD3-induced cell cycle arrest was prevented by p53 depletion and, to a lesser extent, p21Cip1 depletion. These studies demonstrate that FOXD3 is suppressed by B-RAF, uncover a novel role and mechanism for FOXD3 as a negative cell cycle regulator and have implications for the repression of melanocytic lineage cells.
Keywords: B-RAF, FOXD3, melanoma, p21Cip1, p53
Introduction
Forkhead box (FOX) transcription factors represent a closely related family of proteins that mediate cell-cycle progression, survival, and differentiation (1, 2). The defining feature of FOX transcription factors is a conserved DNA-binding region known as the forkhead box or winged helix domain. FoxD3(formerly Genesis/HFH2) was originally identified due to its expression in embryonic stem (ES) cells (3) and it serves numerous indispensable roles during development (4). Foxd3 is required for maintainingpluripotent cells in the early mouse embryo and Foxd3 knockout mice die early during mouse development since it functions to maintain cells of the inner cell mass (5), trophoblast progenitors (6) and neural crest cell precursors (7). Use of a conditional mouse knockout model shows a requirement for Foxd3 in the establishment of murine ES cell lines (8). FoxD3 regulates specification of the neural crest lineage. Its expression is temporally down-regulated in neural crest cells during the wave of cell emigration that gives rise to melanocytes (9). In vitro and in vivo inhibition of FoxD3 in early-migrating neural crest cells results in an expansion of the melanoblasts at the expense of other lineages. Conversely, over-expression of FoxD3 in late-migrating neural crest cells prevents melanoblast formation in favor of the glial and neuronal lineages (9). Re-expression of FoxD3, as well as other early neural crest markers, has been reported in quail pigment cells that have undergone dedifferentiation in culture (10). However, the exact mechanism by which FoxD3 directs neural crest development remains unclear.
Melanoma, the deadliest form of skin cancer, arises from the transformation of melanocytes. Because treatment options for advanced melanoma remain limited, identifying regulators of aberrant melanoma growth is critical. Recent developments have led to the identification of B-RAF mutations, which hyperactivate the ERK1/2 pathway, in approximately two-thirds of melanomas (11). The ERK1/2 pathway may also be activated through mutations in N-Ras, autocrine growth factor action or up-regulation of G-protein coupled receptors (12, 13) underscoring the importance of ERK1/2 pathway to melanoma progression. Additionally, B-RAF mutations have been identified in approximately 30% of thyroid carcinomas and 14% of colorectal cancer (11). Inhibitors to RAF and MEK are currently being investigated in clinical trials (14).
FOX transcription factors are known to both promote and inhibit the onset and progression of tumors; however, their role in human melanoma is poorly understood. In zebrafish, FoxD3 represses expression of microphthalmia-associated transcription factor (MITF) (15, 16), a regulator of melanocyte development. MITF is amplified in a subset of human melanomas, is regulated by B-RAF signaling (17), and in some contexts co-operates with B-RAFV600E to promote the transformation of human melanocytes (18). Together, with evidence of FoxD3 functioning as a potent antagonist of expansion of the melanoblast lineage, these studies led us to examine the regulation and actions of FOXD3 in human melanoma cells. Here, we show that FOXD3 expression is enhanced following depletion of B-RAF or inhibition of MEK in human mutant B-RAF melanoma cells. Expression of Foxd3 results in a potent inhibition of proliferation in multiple mutant B-RAF melanoma lines by inducing a G1 arrest. Mechanistically, Foxd3/FOXD3 acts by causing up-regulation of the cyclin-dependent kinase inhibitor, p21Cip1 and repression of cyclin A. Regulation of p21Cip1 isp53-dependent and FOXD3-dependent cell cycle arrest is prevent by p53 depletion and partially by p21Cip1 depletion. These findings identify FOXD3 as a B-RAF target and novel cell-cycle repressor in melanoma and may give insight to the mechanism by which Foxd3 prevents melanoblast differentiation during development.
Materials and Methods
Cell culture
Human melanoma cell lines, WM793, WM115 and WM3211, were kindly donated by Dr. Meenhard Herlyn (Wistar Institute, Philadelphia). SK-MEL-28 and A375 cells were purchased from ATCC (Manassas, VA). WM793, WM115, WM3211 and SK-MEL-28 cells were cultured in MCDB 153 medium containing 20% Leibovitz L-15 medium, 2% fetal bovine serum, 0.2% sodium bicarbonate, and 5 μg/ml insulin. A375 cells were cultured in DMEM with 10% FBS and non-essential amino acids. Neonatal foreskins were isolated and cultured, as previously described (19). Thyroid cancer cell lines, WRO, NPA and ARO, were maintained in RPMI 1640 supplemented with 10% FBS. NPA and ARO cell harbor mutant B-RAF, WRO cells are wild-type for B-RAF (20).
Inhibitors
U0126 was purchased from Cell Signaling Technology (Danvers MA). AZD6244/ARRY-142866 was kindly provided by Dr. Paul Smith (AstraZeneca, UK Limited) (21).
Short-interfering RNA (siRNA)
WM793 and WM115 cells were transfected for 4 hours with chemically synthesized siRNAs (Dharmacon, Lafayette, CO) at a final concentration of 25 nM using OligofectAMINE (Invitrogen, Carlsbad CA). The siRNA sequences for B-RAF #1, p21 #1, cyclin D1 #10 and p53 #5 were ACAGAGACCUCAAGAGUAAUU, GAUGGAACUUCGACUUUGU, ACAACUUCCUGUCCUACUAUU and GAGGUUGGCUCUGACUGUAUU, respectively. The non-targeting siRNA, UAGCGACUAAACACAUCAAUU, was used as a control.
Western blotting and immunoprecipitation
Cells were lysed and analyzed by Western blotting as previously described (22). Primary antibodies used were: ERK1/2 (K-23), B-RAF (F-7), p53 (DO-1), and cyclin A (H-432) from Santa Cruz Biotech. Inc. (Santa Cruz, CA); cyclin D1 (DCS-6) from BD Biosciences (San Jose, CA); p21 (DCS60) and phospho-ERK1/2 (E10) from Cell Signaling Tech.; β-galactosidase (Z378A) from Promega (Madison, WI); FOXD3 (Poly6317) from BioLegend; (San Diego CA) and V5 epitope (46-0705) from Invitrogen. Chemiluminescence was visualized on Fluor-S and Versadoc MultiImagers and quantitated using Quantity-One software (Bio-Rad Hercules, CA).
Lentiviral construction and transduction
pLenti6/TR and pLenti4/TO/V5-GW/LacZ were purchased from Invitrogen. Murine Foxd3 (generously provided by Robert Hromas, Indiana University Medical Center, Indianapolis) and human FOXD3 were cloned into pENTR™/D-TOPO (Invitrogen) and LR-recombined into pLenti4/TO/V5-DEST. Expression constructs and packaging plasmids pLP1, pLP2, and pLP/VSVG were co-transfected into HEK293FT cells to generate viral particles. Cells were transduced with particles for 72 hours, and then selected with blasticidin (pLenti6/TR) or zeocin (pLenti4 constructs), as described previously (22, 23). WM793TR, WM115TR, SK-MEL-28TR and A375TR cells are clonal isolates selected for high expression of the Tet-repressor (TR) and used for sequential transductions. Transgene expression was induced with either tetracycline or doxycycline (0.1 μg/ml). Similar results were obtained with either inducing agent.
Lentiviral shRNA constructs
DNA oligonucleotides (listed in Supplemental Table 1) were annealed and ligated into pENTR™/H1/TO using the manufacturer’s kit and protocol. shRNAs cassettes were recombined into pLenti4/BLOCK-iT™-DEST. Lentivirus particles were packaged and used as above.
Quantitative RT-PCR
Total cellular RNA was extracted using the PerfectPure RNA Cultured Cell Kit (5 Prime, Gaithersburg, MD). cDNA was made using the iScript™ cDNA Synthesis Kit (Bio-Rad). Quantitative PCR was performed using iQ™ SYBR green supermix (Bio-Rad), 0.8 μM oligonucleotide primers and 0.1 μg cDNA. The primers used are listed in Supplemental Table 2. Primer specificity was confirmed by melt curve analysis and TAE gel electrophoresis. Reaction conditions were as follows: denaturation at 94°C for 30 sec; annealing at 50°C for 30 sec and elongation at 72°C for 30 sec; 50 cycles in total. PCR was performed on an iCycler with MyiQ™ version 1.0 software (Bio-Rad). Relative mRNA levels were calculated using the Comparative Ct method (ΔCt) (24).
Growth assays
For cell growth, 2 × 105 cells were plated in complete medium +/− inducing agent. Cells were trypsinized, counted, and then replated every 3 days. For growth in soft agar, 2% agar was overlayed with bottom agar (0.3%), which in turn was overlaid with top agar (0.3%) containing 6 × 103 cells/ml. Polymerized soft agar was overlaid with another layer of acellular soft agar and finally by medium. Doxycycline was added to a final concentration of 0.2 μg/ml. Cells were grown for 14 days replacing the medium every three days. Photos were taken on an Olympus IX70 microscope with Image Pro Plus software.
S phase entry assays
Cells were cultured in complete medium containing 10 μM EdU (5-ethynyl-2′-deoxyuridine) for 8 hours. Cells were trypsinized and processed using the Click-iT™ EdU Flow Cytometry Assay Kit and protocol (Invitrogen). Incorporated EdU was stained with Alexa Fluor® 647 azide. Cells were analyzed by flow cytometry on a BD FacsCanto™.
Immunofluorescence
Cells on coverslips were fixed in 3.7% formaldehyde, permealized with 0.5% Triton X-100, and stained with Hoechst 33342 (Molecular Probes Inc. Eugene, OR), anti-RhoGDI (K-21) from Santa Cruz, and anti-V5 epitope from Invitrogen. Goat anti-rabbit IgG-Alexa Fluor 594 and anti-mouse-Alexa Fluor 488 (Molecular Probes) were used as secondary antibodies. Photos were taken on an Olympus BX61 microscope with IPLab software.
Statistics
Analysis was performed using Minitab software. For ANOVA, Tukey’s and Dunnett’s post hoc tests were performed.
Results
B-RAFV600E regulates FOXD3 levels in human melanoma cells
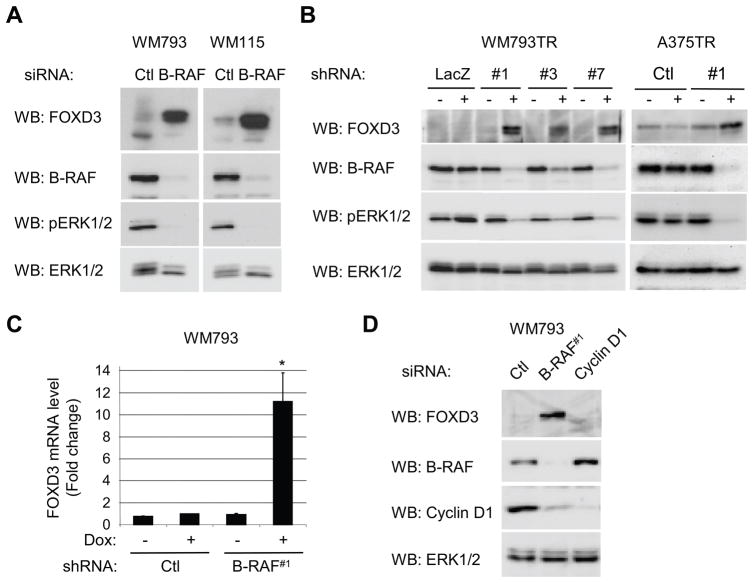
We examined whether B-RAF regulates FOXD3 levels in vertical growth phase (WM793 and WM115) and metastatic melanoma (A375) cells that harbor activating B-RAF mutations (11, 12). In WM793 and WM115 cells, transient siRNA-mediated knockdown of B-RAF led to an inhibition of ERK1/2 activation but increased protein expression of FOXD3 (Fig. 1A). To exclude concerns regarding off-target effects of RNA interference, we engineered three independent shRNA sequences targeting different regions of the B-RAF mRNA in an inducible system. Efficient B-RAF knockdown with all three shRNAs was associated with up-regulation of FOXD3 and inhibition of ERK1/2 (Fig. 1B). We have previously reported that C-RAF and A-RAF expression levels are not altered by B-RAF siRNAs (25) and similarly no effect on C-RAF and A-RAF was observed in the B-RAF shRNA systems (Supplemental Fig. 1). No effect on FOXD3 expression was observed with non-targeting control siRNA or LacZ shRNA. FOXD3 levels were also enhanced following inducible expression of B-RAF shRNA but not control shRNA in A375 cells (Fig. 1B). Up-regulation of FOXD3 occurred at the mRNA level since inducible knockdown of B-RAF led to an 11-fold increase in FOXD3 mRNA levels, as measured by quantitative RT-PCR (Fig. 1C). FOXD3 up-regulation could be un-coupled from cell cycle progression since cyclin D1 knockdown, which induces a potent G1 arrest (26), did not lead to up-regulation of FOXD3 (Fig. 1D). These data show that mutant B-RAF signaling represses the expression of FOXD3 in human melanoma cells.
Figure 1.
Inhibition of B-RAF up-regulates FOXD3 levels in melanoma cells. A, WM793 and WM115 cells were transfected with non-targeting control (Ctl) or B-RAF (duplex #1) siRNA. Seventy-two hours post-transfection, cells were lysed and analyzed by Western blotting for FOXD3, B-RAF, phospho-ERK1/2 (pERK1/2) and ERK1/2. B, WM793TR cells expressing either control (LacZ2.1) or B-RAF (hairpins #1, #3, and #7) shRNA were induced (+) with tetracycline or not induced (−) for 6 days. Cells were lysed and samples analyzed by Western blotting as in A. The right-hand panels show A375TR cells expressing either Ctl or B-RAF #1 shRNA −/+ doxycycline for 6 days. C, WM793TR cells expressing either control or B-RAF shRNA were induced (+) with doxycycline or not induced (−) for 5 days. FOXD3 and actin mRNA levels were analyzed by quantitative RT-PCR. Graphed is the average and standard deviation from three independent experiments. The asterisk indicates statistical significance comparing B-RAF shRNA + doxycycline to other conditions (p<0.05). D, WM793 cells were transfected with Ctl, B-RAF#1 or cyclin D1 siRNA. After 72 hours, cell lysates were analyzed by Western blotting for FOXD3, B-RAF, cyclin D1 and ERK1/2.
MEK activity is required to down-regulate FOXD3 in mutant B-RAF cells
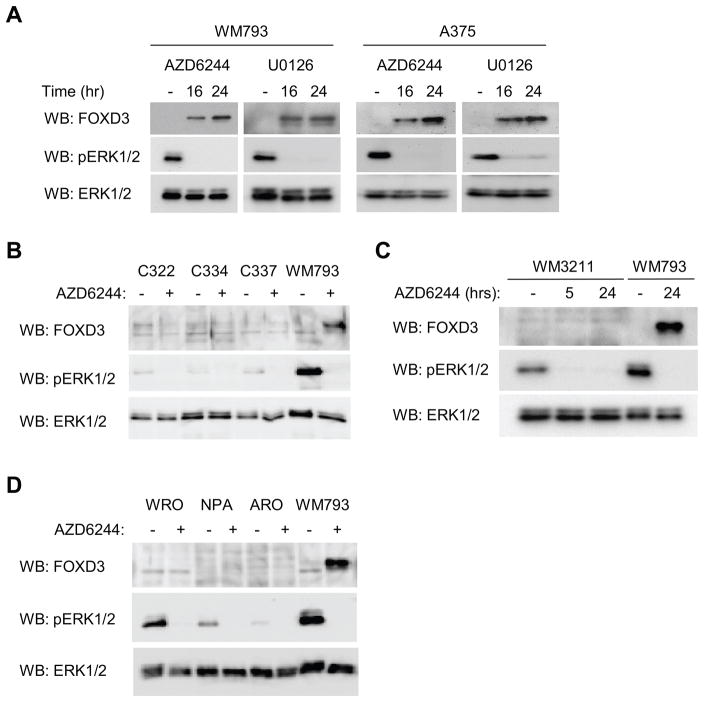
Inhibitors to the B-RAF-MEK pathway are in phase I/II trials for melanoma (27). We utilized the clinical grade MEK inhibitor, AZD6244 (21) and, in parallel, the commonly used non-clinical grade MEK inhibitor, U0126 (28). Inhibition of MEK-ERK1/2 signaling with either AZD6244 or U0126 up-regulated FOXD3 levels in WM793 and A375 cells (Fig. 2A). FOXD3 levels were low/undetectable in human melanocytes. Furthermore, we did not observe up-regulation of FOXD3 following AZD6244 treatment of melanocytes (Fig. 2B) and WM3211 cells (Fig. 2C) that are wild-type for B-RAF. Finally, inhibition of MEK in thyroid cancer lines, two of which harbor B-RAF mutations, also failed to up-regulate FOXD3 (Fig. 2D). Thus, MEK activity is required to suppress FOXD3 expression selectively in mutant B-RAF melanoma cells.
Figure 2.
MEK signaling is required to suppress FOXD3 expression. A, WM793 and A375 cells were treated with 3.3 μM AZD6244 or 5 μM U0126 for 16 and 24 hours. Cell lysates were analyzed by Western blotting for the indicated proteins. B, Three independent melanocytes cultures (C322, C334 and C337) and WM793 (as a positive control) were treated −/+ AZD6244 for 24 hours. Cell lysates were analyzed by Western blot as indicated. C, Wild-type B-RAF WM3211 melanoma cells were treated with AZD6244 for 5 and 24 hours. WM793 represents a positive control. Cell lysates were analyzed by Western blot as indicated. D, Wild-type and mutant B-RAF thyroid cancer cell lines were analyzed, as in A–C.
Expression of Foxd3 inhibits melanoma cell growth
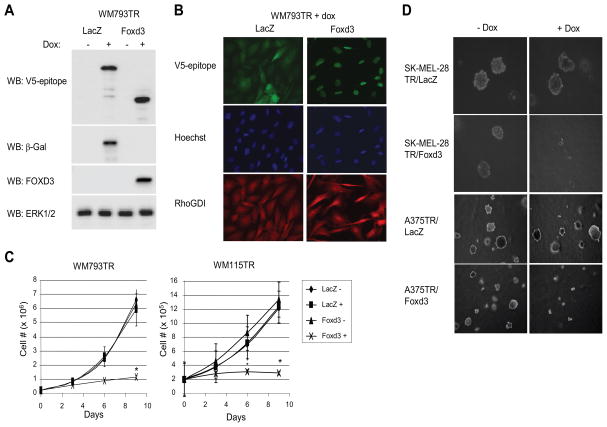
To determine the role of Foxd3, we engineered inducible expression of V5-tagged mouse Foxd3 in multiple mutant B-RAF lines. As a control, we generated V5-tagged β-galactosidase expressing cells. Expression of Foxd3 in WM793 cells was induced in response to addition of doxycycline (Fig. 3A) and localized to the nucleus (Fig. 3B). Similarly, inducible expression of Foxd3 in WM115, SK-MEL-28, and A375 cells was confirmed (Supplemental Fig. 2). Non-induced WM793TR/Foxd3 cells grew at a similar rate to non-induced and induced LacZ cells (Fig. 3C, left graph). However, expression of Foxd3 dramatically inhibited cell growth. Expression of Foxd3 also effectively inhibited growth of WM115 cells (Fig. 3C, right graph). Growth in soft agar is a well-established assay for determining the malignant behavior of tumor cell lines. Expression of Foxd3 potently inhibited growth in soft agar of metastatic melanoma cell lines, SK-MEL-28 and A375 (Fig. 3D). FOXD3 expression in SK-MEL-28 cells decreased the number of colonies by 93%; whereas LacZ expression caused a 15% decrease. In A375 cells, FOXD3 expression did not alter the number of colonies but rather reduced colony size by an average of 56%. Expression of LacZ in A375 did not affect the size of colonies. These data represent a novel finding that Foxd3 is an inhibitor of melanoma cell growth.
Figure 3.
Inducible expression of Foxd3 blocks melanoma growth in vitro. A, WM793TR cells expressing doxycycline-inducible β-galactosidase (LacZ) or murine Foxd3 were induced with 0.1 μg/ml doxycycline for 5 days. Lysates were immunoblotted for the respective transgenes, ERK1/2 and the C-terminal V5-epitope tag found on both transgenes. B, Induced WM793TR cells were stained with anti-V5-epitope (green) to detect LacZ or Foxd3, as well as Hoechst 33342 (blue) and anti-RhoGDI (red) to label nuclei and cytoplasm, respectively. C, WM793TR and WM115TR cells expressing LacZ or Foxd3 were treated −/+ doxycycline, trypsinized, counted and replated every 3 days for nine days. Average cell numbers and standard error bars are given for 3 independent experiments. * p<0.05. D, SK-MEL-28TR and A375TR cells expressing LacZ or Foxd3 were embedded in 0.3% agar and allowed to grow for approximately 2 weeks. SK-MEL-28TR colonies were photographed at 20X magnification and A375TR colonies were photographed at 10X magnification.
Foxd3 inhibits G1/S cell cycle progression
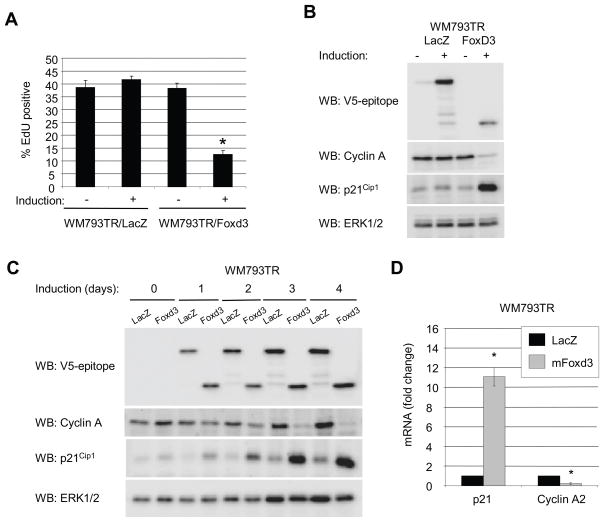
To determine whether Foxd3 expression inhibited cell cycle progression, we performed propidium iodide/flow cytometry experiments. Expression of Foxd3 caused a reduction in the percent of S phase cells and increased the G0/G1 population, although not as dramatically as MEK inhibition with AZD6244 (Supplemental Fig. 3A and 3B). We did not observe an increase in a sub-G0/G1 peak or annexin V staining (data not shown) indicating that expression of Foxd3 does not induce apoptosis. In DNA synthesis labeling experiments, WM793 cell entry into S phase was inhibited by expression of Foxd3 (Fig. 4A). Additionally, expression of cyclin A was reduced in Foxd3-expressing cells (Fig. 4B). We further defined the effects of Foxd3 expression by analyzing the levels of G1/S cell cycle regulators. Notably, expression of a negative cell cycle regulator, p21Cip1, was enhanced following expression of Foxd3 (Fig. 4B). Since it is difficult to distinguish cause versus effect from a single time-point (72 hrs), we performed time-course experiments. Up-regulation of p21Cip1 occurred rapidly (24–48 hrs) following induction of Foxd3 and preceded the efficient inhibition of cyclin A expression (Fig. 4C). Up-regulated p21Cip1 in FOXD3 expressing cells co-immunoprecipitated with CDK4 and cyclin D1/cyclin D3, in which p21Cip1 is thought to stabilize complexes (29) but also with CDK2, consistent with it playing an inhibitory role in G1/S progression (data not shown). Up-regulation of p21Cip1 and down-regulation of cyclin A occurred at the mRNA level, as demonstrated by quantitative RT-PCR (Fig. 4D). Together, these data show that expression of Foxd3 inhibits G1/S progression, which is associated with up-regulation of p21Cip1.
Figure 4.
Foxd3 induces a G0/G1 arrest in melanoma. A, WM793TR cells were induced for 5 days. For the final 8 hours, 10 μM EdU was included. Cells were processed for flow cytometry and analyzed on a BD FacsCanto™. The graph represents the average percent of EdU-positive cells and standard error of 4 independent experiments (* p<0.05). B, Lysates from WM793TR cells were immunoblotted for the V5-epitope, cyclin A, p21 and ERK1/2. C, WM793TR cells were induced for 0, 1, 2, 3, and 4 days. Lysates were immunoblotted for the V5-epitope, cyclin A, p21Cip1 and ERK1/2. D, WM793TR/LacZ and WM793TR/Foxd3 cells were induced (+) with doxycycline or not induced (−) for 5 days. p21Cip1, and cyclin A mRNA levels were analyzed by quantitative RT-PCR. Graphed is the average fold change relative to actin and standard deviation from three independent experiments (* p<0.05).
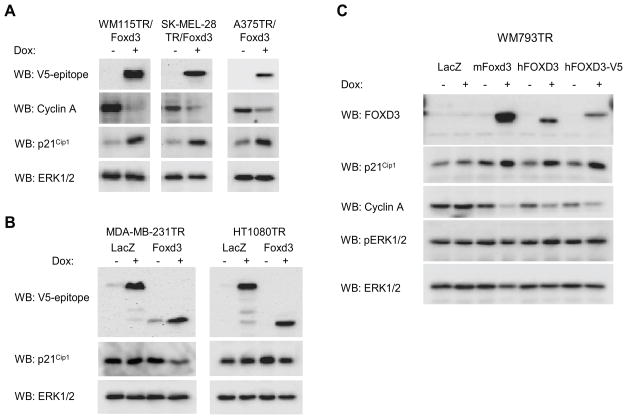
Foxd3 expression leads to up-regulation of p21Cip1 selectively in melanoma cells
We expanded our analysis on cell cycle regulators to additional melanoma cell lines and to non-melanoma cell types. Inducible expression of Foxd3 resulted in down-regulation of cyclin A and up-regulation of p21Cip1 in WM115, SK-MEL-28, and A375 cells (Fig. 5A). Foxd3 expression did not lead to up-regulation of other CDK inhibitors, namely p27Kip1 and p57Kip2 (Supplemental Fig. 4). To determine whether Foxd3 elicited similar effects in non-melanoma tumor types, we generated inducible Foxd3 lines for MDA-MB-231, a human breast cancer line, and HT-1080, derived from a human fibrosarcoma. Expression of Foxd3 did not up-regulate p21Cip1 levels in either MDA-MB-231 or HT-1080 cells (Fig. 5B). Thus, we observe effects of Foxd3 on p21Cip1 in melanoma cells but not in two other cell types.
Figure 5.
Expression of Foxd3 up-regulates p21Cip1 in melanoma cell lines but not in a breast or a fibrosarcoma cell line. A, Foxd3-inducible WM115, SK-MEL-28, and A375 cells were treated with 0.1 μg/ml doxycycline for 5 days. Cell lysates were analyzed by Western blotting for the V5-epitope, cyclin A, p21Cip1 and ERK1/2. B, Foxd3 and LacZ inducible forms of the human breast cancer line, MDA-MB-231, and human fibrosarcoma line, HT-1080, were generated. Cell lines were treated −/+ 0.1 μg/ml doxycycline for 5 days. Cell lysates were analyzed by Western blotting for the V5-epitope, p21Cip1 and ERK1/2. C, WM793 expressing inducible LacZ, mouse Foxd3-V5, human FOXD3 and human FOXD3-V5 were treated −/+ doxycycline for 5 days. Lysates were analyzed by Western blotting for FOXD3, p21Cip1, cyclin A, phospho-ERK1/2 (pERK1/2) and total ERK1/2.
Human FOXD3 regulates p21Cip1 levels
The above experiments were performed using mouse Foxd3. Although the mouse and human forms of this protein are >88% identical, we wanted to confirm our findings with human FOXD3. To this end, we cloned human FOXD3 and expressed it using the inducible lentiviral system as either a non-tagged or a V5 tagged protein. Western blotting showed expression of FOXD3 and FOXD3-V5 in WM793 cells in response to doxycycline (Fig. 5C). Importantly, FOXD3 or FOXD3-V5 expression led to up-regulation of p21Cip1 and down-regulation of cyclin A. These results are consistent with our findings with mouse Foxd3, although more dramatic effects were seen with the latter likely due to enhanced transgene expression. Levels of cell cycle proteins are regulated by B-RAF-MEK-ERK1/2 signaling in melanoma cells (30, 31). Phospho-ERK1/2 levels were elevated and unaltered following FOXD3 expression in WM793 cells (Fig. 5C).
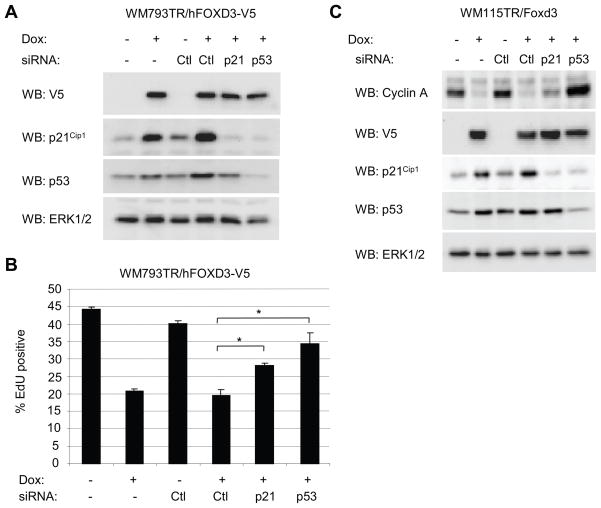
FOXD3-dependent inhibition of S phase entry is prevented by p21Cip1 and p53 knockdown
Since p21Cip1 is a known cell cycle inhibitor, we tested its role in the FOXD3-induced cell cycle arrest. We also analyzed a role for p53 since it is known to regulate p21Cip1 expression, and is wild-type in the majority of melanoma cells including WM793 and WM115 (32–34). We utilized siRNA to knockdown either p21Cip1 or p53 in FOXD3 expressing cells. Notably, FOXD3-induced expression of p21Cip1 was dependent on p53 (Fig. 6A). Similar to mouse Foxd3, expression of human FOXD3 in WM793 inhibited entry into S phase (Fig. 6B). Transfection with non-targeting siRNA did not affect S phase entry in either non-expressing cells or FOXD3-expressing cells. By contrast, knockdown of p21Cip1 resulted in a partial restoration of S phase entry in FOXD3-expressing cells. Interestingly, knockdown of p53 restored S phase entry to a level that was not statistically different from the non-induced control knockdown. Western blot analysis confirmed that p21Cip1 or p53 knockdowns were persistent through the course of the experiment and cyclin A levels in FOXD3-expressing cells were enhanced following p21Cip1 or p53 knockdown correlating with S phase entry (data not shown). Additionally in WM115 cells, knockdown of p53 prevented FOXD3-induced inhibition of cyclin A expression and p21Cip1 knockdown elicited a partial reversal of cyclin A levels (Fig. 6C). These results suggest a critical role for p53 in FOXD3-dependent p21Cip1 induction and cell cycle arrest.
Figure 6.
p21Cip1 and p53 are required for in FOXD3-induced cell cycle arrest. A, WM793TR/FOXD3-V5 were transfected with either control, p21Cip1 or p53 siRNA. After 24 hours for recovery, cells were treated −/+ 0.1 μg/ml doxycycline for 3 days. Lysates were analyzed by Western blotting for V5, p21Cip1, p53 and total ERK1/2. B, Cells were processed as above except that cells were treated for 5 days with doxycycline and for the final 8 hours, 10 μM EdU was included. Cells were processed for flow cytometry and analyzed on a BD FacsCanto™. The graph represents the average percent of EdU-positive cells and standard error of 3 independent experiments. Asterisks indicate statistical significance (p<0.05). C, WM115TR/FOXD3-V5 were transfected with either control, p21Cip1 or p53 siRNA. After 24 hours for recovery, cells were treated −/+ 0.1 μg/ml doxycycline for a further 5 days. Lysates were analyzed by Western blotting for cyclin A, V5, p21Cip1, p53 and total ERK1/2.
Discussion
In this study, we report novel findings on a stem cell transcription factor, FOXD3, in melanoma cells. We demonstrate that expression of FOXD3 is regulated by mutant B-RAF and that FOXD3 is a potent melanoma cell cycle inhibitor. Mechanistically, we show that FOXD3 up-regulates the cyclin-dependent kinase inhibitor p21Cip1 in a p53-dependent manner and that FOXD3-mediated cell cycle arrest shows dependence on p21Cip1 and p53.
B-RAF is mutated in approximately two-thirds of melanoma. Mutant B-RAF and MEK signaling is required for aberrant cell proliferation (26, 31, 35, 36) but the mechanisms remain poorly understood. Here, we show that depletion of B-RAF expression and inhibition of MEK up-regulates FOXD3 expression. ERK1/2 regulation of other forkhead transcription factors has been reported. For example, ERK phosphorylation promotes FOXO3a proteasomal degradation (37) and FOXM1c nuclear translocation (38). We demonstrate that mutant B-RAF represses FOXD3 at the mRNA level; thus, the mechanism is distinct from the aforementioned post-translational modification of forkhead proteins. The promoter region of FOXD3 contains multiple potential SP-1, AP-1, CRE, E-box and Ets-family binding sites, any of which could serve as a portal for ERK1/2-dependent actions. Regulation may also occur via activation of the transcription factor E2F4, which has been shown to bind to the FOXD3 promoter region (39). While FOXD3 is suppressed by B-RAF and its expression is sufficient to induce a cell cycle arrest, initial experiments show that shRNA-mediated depletion of FOXD3 in melanoma cells does not reverse AZD6244-mediated inhibition of S phase entry (Supplemental Fig. 5). These data suggest that additional factors likely contribute to cell cycle arrest following B-RAF inhibition. This is not surprising since B-RAF-MEK signaling regulates multiple cell cycle events including levels of cyclin D1, Cks1/SKP2, and the cyclin-dependent kinase inhibitor, p27Kip1 (26, 30), as well as the Brn2 and MITF transcription factors, which are required for melanoma proliferation (17, 40). The interplay between FOXD3 and these other cell cycle regulators in melanoma requires further analysis.
Most reports on Foxd3 focus on its role in development since Foxd3(−/−) mouse embryos die shortly after implantation due to the loss progenitor cells needed to form the epiblast and trophoblast (5, 6). We are the first to show a role for FOXD3 as a regulator of aberrant tumor cell proliferation. Such findings are consistent with FOXD3 acting as a suppressor of melanoblasts during development (4). FOXD3-induced cell cycle arrest was associated with up-regulation of p21Cip1 in a manner dependent on p53 in melanoma cells but not in either of the two non-melanoma cells that we examined. FOXD3 may alter post-translational modification of p53, which occurs at multiple sites via several distinct mechanisms (41), and/or the recruitment of co-activators and co-repressors to p53. Interesting recent findings suggest that FoxD3 may act indirectly by sequestering the transcription factor, PAX3, from binding its DNA targets (16) and PAX3 has been shown to control p53-dependent functions in the developing neural tube and in lung carcinoma and mouse fibroblast lines (42, 43). FOXD3-dependent inhibition of S phase entry and cyclin A expression was prevented by p53 and p21Cip1 depletion. With regard to p21Cip1, the effect was incomplete indicating that additional p53-dependent mechanisms may play a role or that residual p21Cip1 following the knockdown elicits effects. An additional consequence of FOXD3 expression was up-regulation of cyclin D1 (data not shown), although this may represent an effect rather than a cause since cyclin D1 is required for S phase entry of melanoma cells (30) and in some experiments was reversed following p21Cip1 and p53 knockdown.
There is increasing interest in the role of tumor initiating cells with stem cell-like properties in melanoma and other malignancies. Recent studies suggest that the ability of individual cells to form tumors may be more widespread in melanoma compared to other malignancies (44). Foxd3 was initially identified as a transcriptional-repressor expressed in normal and malignant embryonic stem cells (3) and embryonic stem cells or teratocarcinoma cells can not be established from Foxd3 knockout embryos (5). Furthermore, FoxD3 is believed to contribute to the pluripotency and self-renewal of embryonic stem cells through a complex negative-feedback loop with the transcription factors, Oct4 and Nanog (45). An interesting possibility raised from up-regulation of FOXD3 following B-RAF-MEK targeting is that melanoma cells are re-programmed towards a less well differentiated state following B-RAF inhibition. Since treatment options for melanoma are extremely limited in part due to the high resistance of melanoma to chemotherapy, elucidation of FOXD3 function in subgroups of tumor cells as well as the general tumor mass population may be extremely important.
Supplementary Material
Acknowledgments
Grant Support: American Cancer Society (RSG-08-03-01-CSM) and National Institutes of Health (R01-GM067893 and R01-CA125103) to A.E. Aplin. Ethan Abel was supported, in part, by T32-HL-07194.
We are grateful to Dr. Paul Smith (AstraZeneca UK Limited) for providing AZD6244/ARRY-142866, Dr. Meenhard Herlyn (Wistar Institute) for WM melanoma cell lines, Dr Jeffrey Knauf (Memorial Sloan Kettering Cancer Center) and Dr. James Fagin (University of Cincinnati) for the thyroid cancer cell lines, and Dr. Robert Hromas (Indiana University Medical Center, Indianapolis) for providing the Foxd3 cDNA. We acknowledge Diane Colello (Albany Medical College) for the HT1080TR and MDA-MB-231TR cells. Diane Colello was supported by NIH grant, GM51540, to Dr. Susan LaFlamme (Albany Medical College). We thank Whitney Longmate for technical assistance.
Footnotes
Note: Throughout this manuscript we use the nomenclature FOXD3, Foxd3 and FoxD3 to refer to the human, mouse and all other chordate species form of this protein, respectively.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Wijchers PJ, Burbach JP, Smidt MP. In control of biology: of mice, men and Foxes. Biochem J. 2006;397:233–46. doi: 10.1042/BJ20060387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 2007;7:847–59. doi: 10.1038/nrc2223. [DOI] [PubMed] [Google Scholar]
- 3.Sutton J, Costa R, Klug M, et al. Genesis, a winged helix transcriptional repressor with expression restricted to embryonic stem cells. J Biol Chem. 1996;271:23126–33. doi: 10.1074/jbc.271.38.23126. [DOI] [PubMed] [Google Scholar]
- 4.Thomas AJ, Erickson CA. The making of a melanocyte: the specification of melanoblasts from the neural crest. Pigment Cell Melanoma Res. 2008;21:598–610. doi: 10.1111/j.1755-148X.2008.00506.x. [DOI] [PubMed] [Google Scholar]
- 5.Hanna LA, Foreman RK, Tarasenko IA, Kessler DS, Labosky PA. Requirement for Foxd3 in maintaining pluripotent cells of the early mouse embryo. Genes Dev. 2002;16:2650–61. doi: 10.1101/gad.1020502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tompers DM, Foreman RK, Wang Q, Kumanova M, Labosky PA. Foxd3 is required in the trophoblast progenitor cell lineage of the mouse embryo. Dev Biol. 2005;285:126–37. doi: 10.1016/j.ydbio.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Teng L, Mundell NA, Frist AY, Wang Q, Labosky PA. Requirement for Foxd3 in the maintenance of neural crest progenitors. Development. 2008;135:1615–24. doi: 10.1242/dev.012179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, Labosky PA. Regulation of embryonic stem cell self-renewal and pluripotency by Foxd3. Stem Cells. 2008;26:2475–84. doi: 10.1634/stemcells.2008-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kos R, Reedy MV, Johnson RL, Erickson CA. The winged-helix transcription factor FoxD3 is important for establishing the neural crest lineage and repressing melanogenesis in avian embryos. Development. 2001;128:1467–79. doi: 10.1242/dev.128.8.1467. [DOI] [PubMed] [Google Scholar]
- 10.Real C, Glavieux-Pardanaud C, Le Douarin NM, Dupin E. Clonally cultured differentiated pigment cells can dedifferentiate and generate multipotent progenitors with self-renewing potential. Dev Biol. 2006;300:656–69. doi: 10.1016/j.ydbio.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 11.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 12.Satyamoorthy K, Li G, Gerrero MR, et al. Constitutive mitogen-activated protein kinase activation in melanoma is mediated by both BRAF mutations and autocrine growth factor stimulation. Cancer Res. 2003;63:756–9. [PubMed] [Google Scholar]
- 13.Marin YE, Namkoong J, Cohen-Solal K, et al. Stimulation of oncogenic metabotropic glutamate receptor 1 in melanoma cells activates ERK1/2 via PKCepsilon. Cell Signal. 2005;18:1279–86. doi: 10.1016/j.cellsig.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Fecher LA, Amaravadi R, Schuchter LM. Effectively targeting BRAF in melanoma: a formidable challenge. Pigment Cell Melanoma Res. 2008;21:410–1. doi: 10.1111/j.1755-148X.2008.00485.x. [DOI] [PubMed] [Google Scholar]
- 15.Ignatius MS, Moose HE, El-Hodiri HM, Henion PD. Colgate/hdac1 repression of foxd3 expression is required to permit mitfa-dependent melanogenesis. Dev Biol. 2008;313:568–83. doi: 10.1016/j.ydbio.2007.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas AJ, Erickson CA. FOXD3 regulates the lineage switch between neural crest-derived glial cells and pigment cells by repressing MITF through a non-canonical mechanism. Development. 2009;136:1849–58. doi: 10.1242/dev.031989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wellbrock C, Rana S, Paterson H, Pickersgill H, Brummelkamp T, Marais R. Oncogenic BRAF regulates melanoma proliferation through the lineage specific factor MITF. PLoS ONE. 2008;3:e2734. doi: 10.1371/journal.pone.0002734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garraway LA, Widlund HR, Rubin MA, et al. Integrative genomic analyses identify MITF as a lineage survival oncogene amplified in malignant melanoma. Nature. 2005;436:117–22. doi: 10.1038/nature03664. [DOI] [PubMed] [Google Scholar]
- 19.Conner SR, Scott G, Aplin AE. Adhesion-dependent activation of the ERK1/2 cascade is by-passed in melanoma cells. J Biol Chem. 2003;278:34548–54. doi: 10.1074/jbc.M305797200. [DOI] [PubMed] [Google Scholar]
- 20.Kimura ET, Nikiforova MN, Zhu Z, Knauf JA, Nikiforov YE, Fagin JA. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. 2003;63:1454–7. [PubMed] [Google Scholar]
- 21.Davies BR, Logie A, McKay JS, et al. AZD6244 (ARRY-142886), a potent inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase 1/2 kinases: mechanism of action in vivo, pharmacokinetic/pharmacodynamic relationship, and potential for combination in preclinical models. Mol Cancer Ther. 2007;6:2209–19. doi: 10.1158/1535-7163.MCT-07-0231. [DOI] [PubMed] [Google Scholar]
- 22.Spofford LS, Abel EV, Boisvert-Adamo K, Aplin AE. Cyclin D3 expression in melanoma cells is regulated by adhesion-dependent PI-3 kinase signaling and contributes to G1-S progression. J Biol Chem. 2006;281:25644–51. doi: 10.1074/jbc.M600197200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boisvert-Adamo K, Aplin AE. Mutant B-RAF mediates resistance to anoikis via Bad and Bim. Oncogene. 2008;27:3301–12. doi: 10.1038/sj.onc.1211003. [DOI] [PubMed] [Google Scholar]
- 24.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucl Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boisvert-Adamo K, Aplin AE. B-RAF and PI-3 kinase signaling protect melanoma cells from anoikis. Oncogene. 2006;25:4848–56. doi: 10.1038/sj.onc.1209493. [DOI] [PubMed] [Google Scholar]
- 26.Bhatt KV, Hu R, Spofford LS, Aplin AE. Mutant B-RAF signaling and cyclin D1 regulate Cks1/S-phase kinase-associated protein 2-mediated degradation of p27(Kip1) in human melanoma cells. Oncogene. 2007;26:1056–66. doi: 10.1038/sj.onc.1209861. [DOI] [PubMed] [Google Scholar]
- 27.Montagut C, Settleman J. Targeting the RAF-MEK-ERK pathway in cancer therapy. Cancer Lett. 2009;283:125–34. doi: 10.1016/j.canlet.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 28.Favata MF, Horiuchi KY, Manos EJ, et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–32. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- 29.Cheng M, Olivier P, Diehl JA, et al. The p21(Cip1) and p27(Kip1) CDK ‘inhibitors’ are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J. 1999;18:1571–83. doi: 10.1093/emboj/18.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhatt KV, Spofford LS, Aram G, McMullen M, Pumiglia K, Aplin AE. Adhesion control of cyclin D1 and p27Kip1 levels is deregulated in melanoma cells through BRAF-MEK-ERK signaling. Oncogene. 2005;12:3459–71. doi: 10.1038/sj.onc.1208544. [DOI] [PubMed] [Google Scholar]
- 31.Solit DB, Garraway LA, Pratilas CA, et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 2006;439:358–62. doi: 10.1038/nature04304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.el-Deiry WS, Tokino T, Velculescu VE, et al. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–25. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 33.Volkenandt M, Schlegel U, Nanus DM, Albino AP. Mutational analysis of the human p53 gene in malignant melanoma. Pigment Cell Res. 1991;4:35–40. doi: 10.1111/j.1600-0749.1991.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 34.Smalley KS, Contractor RS, Haass NK, et al. An organometallic protein kinase inhibitor pharmacologically activates p53 and induces apoptosis in human melanoma cells. Cancer Res. 2007;67:1–9. doi: 10.1158/0008-5472.CAN-06-1538. [DOI] [PubMed] [Google Scholar]
- 35.Hingorani SR, Jacobetz MA, Robertson GP, Herlyn M, Tuveson DA. Suppression of BRAF(V599E) in human melanoma abrogates transformation. Cancer Res. 2003;63:5198–202. [PubMed] [Google Scholar]
- 36.Michaloglou C, Vredeveld LC, Mooi WJ, Peeper DS. BRAF(E600) in benign and malignant human tumours. Oncogene. 2008;27:877–95. doi: 10.1038/sj.onc.1210704. [DOI] [PubMed] [Google Scholar]
- 37.Yang JY, Zong CS, Xia W, et al. ERK promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation. Nat Cell Biol. 2008;10:138–48. doi: 10.1038/ncb1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma RYM, Tong THK, Cheung AMS, Tsang ACC, Leung WY, Yao K-M. Raf/MEK/MAPK signaling stimulates the nuclear translocation and transactivating activity of FOXM1c. J Cell Sci. 2005;118:795–806. doi: 10.1242/jcs.01657. [DOI] [PubMed] [Google Scholar]
- 39.Weinmann AS, Yan PS, Oberley MJ, Huang TH, Farnham PJ. Isolating human transcription factor targets by coupling chromatin immunoprecipitation and CpG island microarray analysis. Genes Dev. 2002;16:235–44. doi: 10.1101/gad.943102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goodall J, Wellbrock C, Dexter TJ, Roberts K, Marais R, Goding CR. The Brn-2 transcription factor links activated BRAF to melanoma proliferation. Mol Cell Biol. 2004;24:2923–31. doi: 10.1128/MCB.24.7.2923-2931.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kruse JP, Gu W. SnapShot: p53 posttranslational modifications. Cell. 2008;133:930–30.e1. doi: 10.1016/j.cell.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pani L, Horal M, Loeken MR. Rescue of neural tube defects in Pax-3-deficient embryos by p53 loss of function: implications for Pax-3- dependent development and tumorigenesis. Genes Dev. 2002;16:676–80. doi: 10.1101/gad.969302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Underwood TJ, Amin J, Lillycrop KA, Blaydes JP. Dissection of the functional interaction between p53 and the embryonic proto-oncoprotein PAX3. FEBS Lett. 2007;581:5831–5. doi: 10.1016/j.febslet.2007.11.056. [DOI] [PubMed] [Google Scholar]
- 44.Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–8. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pan G, Li J, Zhou Y, Zheng H, Pei D. A negative feedback loop of transcription factors that controls stem cell pluripotency and self-renewal. Faseb J. 2006;20:1730–2. doi: 10.1096/fj.05-5543fje. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.