Abstract
Until recently, organized and state-dependent neocortical activity in infant rats was thought to commence with the emergence of delta waves at postnatal day (P)11. This view is changing with the discovery of several forms of cortical activity that are detectible soon after birth, including spindle bursts (SBs) and slow activity transients (SATs). Here we provide further evidence of surprisingly rich cortical activity patterns during early development and document, in P5-P13 rats, the appearance, disappearance, and transient expression of three cortical events and oscillations. EEG activity in frontal, parietal, and occipital cortices was recorded in unanesthetized, head-fixed subjects using 16-channel laminar silicon electrodes and Ag-AgCl electrodes. In addition to SATs, we identified two novel forms of activity: cortical sharp potentials (CSPs) and gamma bursts (GBs). SBs were not observed in these areas. CSPs, defined as discrete, biphasic events with a duration of 250 ms, exhibited an inverted-U developmental trajectory with peak prevalence at P9. In contrast, GBs, defined as brief bursts of 40-Hz activity, increased steadily in prevalence and duration from P5 through P13. The prevalence of SATs decreased steadily across the ages tested here. Furthermore, both CSPs and GBs were more likely to occur during sleep than during wakefulness. Because SATs, CSPs, and GBs exhibit different developmental trajectories and rates of occurrence, and can occur independently of each other, they appear to be distinct patterns of neuronal activity. We hypothesize that these diverse patterns of neurophysiological activity reflect the instantaneous local structure and connectivity of the developing neocortex.
Keywords: sleep, EEG, delta activity, slow activity transient, spindle burst, GABA
1. Introduction
In adult mammals, periods of sleep and wakefulness can be distinguished electrographically using measures of cortical (i.e., EEG) and muscle (i.e., EMG) activity (Rechtschaffen and Kales, 1968). In early infancy, especially in rats and other altricial species at ages before the emergence of highly organized EEG activity, EMG measures coupled with behavior have proven reliable for distinguishing behavioral states (Karlsson and Blumberg, 2002; Seelke and Blumberg, 2005; Seelke et al., 2005). Accordingly, the emergence in rats of slow-wave or delta activity around postnatal day (P)11 (Frank and Heller, 1997; Gramsbergen, 1976; Jouvet-Mounier et al., 1970; Seelke and Blumberg, 2008) has been considered an important indicator of cortical maturation.
Recently, however, the identification of two new forms of cortical activity is changing our view of cortical organization during the early postnatal period. First, slow activity transients (SATs), first identified in premature human infants, are low-frequency (0.1 Hz), high-amplitude events that are difficult to detect using conventional EEG filter settings (Vanhatalo et al., 2005a). Analogous events were observed in infant rats as early as P9; these SATs were biphasic, high-amplitude events with a frequency of 0.1–0.2 Hz (Seelke and Blumberg, 2008). Second, brief bursts of 15-Hz activity, called spindle bursts (SBs), have been identified in primary somatosensory, barrel, and visual cortices (Hanganu et al., 2007; Khazipov et al., 2004; Marcano-Reik and Blumberg, 2008; Minlebaev et al., 2007). SBs are generated in response to spontaneous or evoked sensory stimulation of the limbs, whiskers, and retina. Both SBs and SATs appear to be transient features of early development, decreasing in prevalence across the early developmental period.
As SBs and SATs were being discovered and reported, we were searching for developmental precursors of delta activity in the medial dysgranular cortex within the frontal, parietal, and occipital lobes of infant rats. Although we did not find evidence of SBs along the cortical midline, SATs were apparent in these cortical areas before the emergence of delta waves. In addition, we found evidence of two novel forms of activity that had not previously been reported: a discrete event that we designate a cortical sharp potential (CSP) and a brief 40-Hz oscillation that we designate a gamma burst (GB). Here we describe the developmental trajectories of these events and oscillations and establish their relationship with sleep and wakefulness.
2. Results
Recordings performed using 16-channel laminar Silicon (Si) electrodes revealed a cortex replete with activity, even in subjects as young as P5. The phenomenology and state dependency of three cortical events—CSPs, GBs, and SATs—are described in detail below. Although recordings were performed using Si electrodes in the frontal, parietal, and occipital lobes, only data from the parietal lobe recordings are presented here. Data from all 3 lobes are presented in Supplemental Figure 1.
Cortical Sharp Potentials (CSPs)
Figure 1A (left panel) illustrates a typical Si electrode placement in the parietal lobe of a P9 rat. An enlarged view of the cortex (right panel) shows the electrode track passing through cortical layers I–VI. A representative CSP from this P9 subject is depicted in Figure 1B. As can be seen, CSPs were discrete, biphasic events. A subset of CSPs exhibited phase reversals across cortical layer IV, indicating that the event was generated locally (i.e., within the cortex) and not passively propagated from elsewhere in the brain or generated by movement artifact. A current source density (CSD) analysis (Figure 1C) revealed alternating sources and sinks throughout the depth of the cortex, indicating that the entire cortical column is involved in the generation of CSPs (Kandel and Buzsaki, 1997). Only CSPs exhibiting phase reversals are included in the following analyses.
Figure 1.
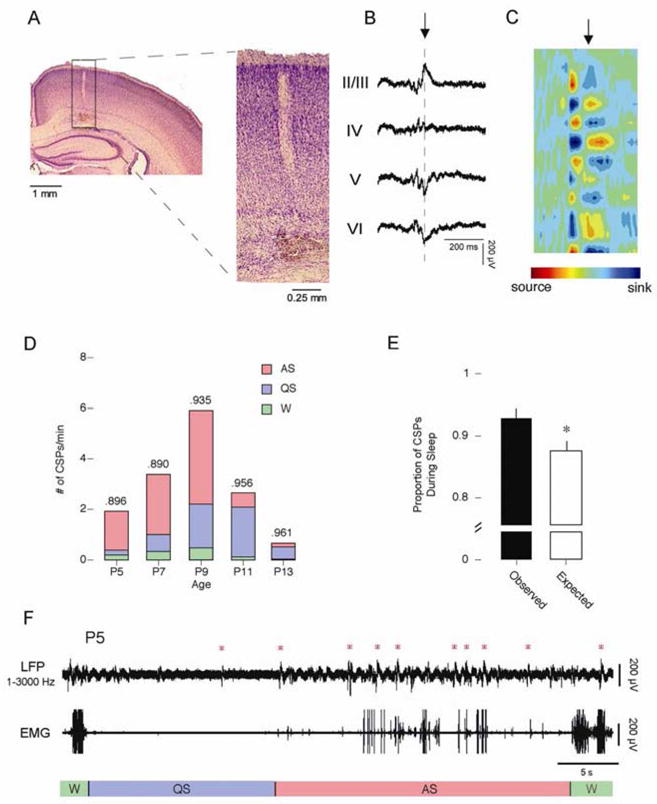
Cortical sharp potentials. A) Location of Si electrode recording site. The right panel shows an expanded view of the rectangle highlighted in A. Cortical layers are identified to the right. B) Representative CSP with embedded GB (Filtered from 1–3000 Hz). The point of phase reversal is identified with a dashed line. C) Current source density (CSD) analysis of averaged CSPs. All 16 channels were used to generate the CSD. Alternating sources (red) and sinks (blue) of ion flows are depicted. The point in the CSD identified by the arrow corresponds to the point in the CSP marked by the arrow in C. D) The number of CSPs per minute across development. The total number of events is broken down to depict the number of events that occurred during AS (red), QS (blue), and wakefulness (green). The proportion of each event that occurred during sleep is given at the top of the column. E) The observed (black) and expected (white) proportion of CSPs that occurred during wakefulness (left) and sleep (right). * significantly different from observed. Mean + s.e. F) Representative sleep-wake cycle from a P5 subject. The top trace depicts cortical activity measured using a Si electrode and filtered from 1–3000 Hz. The bottom trace depicts nuchal EMG activity. Behavioral states are indicated beneath the EMG trace. Red asterisks indicate CSPs.
Mean CSP amplitude increased significantly with age, from 141.0 ± 2.7 μV at P5 to 246.2 ± 11.7 μV at P13 (F4,45 = 25.9, p < .0001). Mean CSP duration ranged from 0.24 ± 0.01 s at P13 to 0.27 ± 0.01 s at P11; ANOVA revealed a small but significant increase across age (F4,45 = 3.5, p < .05).
The mean number of CSPs occurring per minute changed significantly with age (F4,15 = 9.7, p < .0005). As shown in Figure 1D, CSPs exhibited an inverted-U distribution with a peak of 6.7 ± 0.7 per min at P9 and minima of 1.6 ± 0.5 per min and 0.7 ± 0.2 per min at P5 and P13, respectively. Figure 1D also shows that CSPs were most prevalent during AS at P5, P7, and P9. Following the emergence of delta activity at P11, CSPs occurred predominantly during QS. Across all ages, CSPs occurred more frequently during sleep than expected by chance (Figure 1E; t29 = 3.9, p = .0005).
A representative sleep-wake cycle from a P5 rat is shown in Figure 1F. Although CSPs are present during periods of wakefulness and QS-related behavioral quiescence at this age, they cluster predominantly during bouts of AS-related phasic activity.
Gamma Bursts (GBs)
In Figure 2A, a typical Si electrode recording site is again shown, this time alongside an example of a GB (Figure 2B) from a P9 subject. The GB pictured here was embedded in the CSP depicted in Figure 1B. Much like CSPs, a subset of GBs exhibited phase reversals across cortical layer IV which indicates that GBs, like CSPs, can be generated locally within the cortex. Figure 2C shows a CSD analysis from a P9 subject, which confirms that GBs are generated within layer IV. Only GBs exhibiting phase reversals are included in the following analyses.
Figure 2.
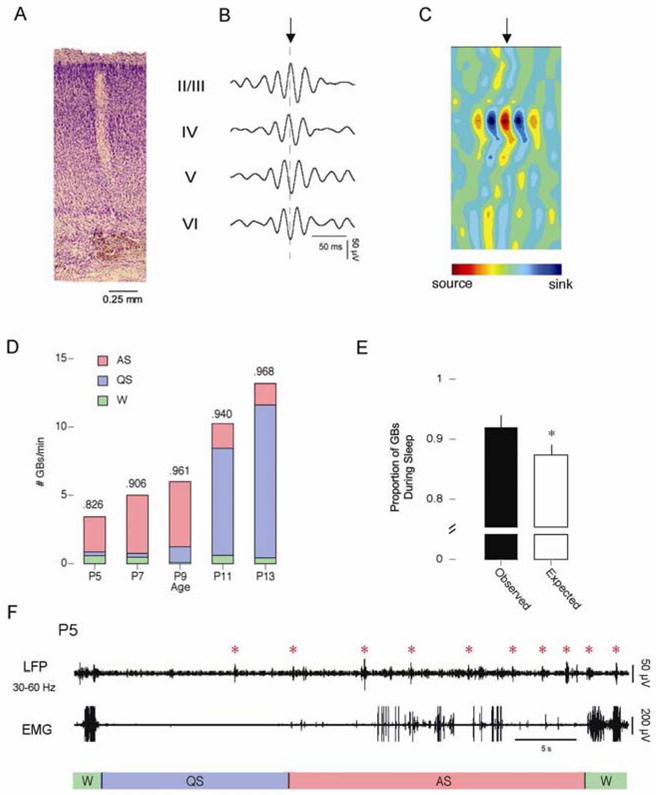
Gamma bursts. A) Expanded view of a Si electrode recording site in a P9 rat. Cortical layers are identified to the right. B) Representative GB (Filtered from 30–60 Hz). The point of phase reversal is identified with a dashed line. C) Current source density (CSD) analysis of averaged GBs. All 16 channels were used to generate the CSD. Alternating sources (red) and sinks (blue) of ion flows are depicted. The point in the waveform identified by the arrow corresponds to the point in the GB marked by the arrow in B. D) The number of GBs per minute across development. The total number of events is broken down to depict the number of events that occurred during AS (red), QS (blue), and wakefulness (green). The proportion of each event that occurred during sleep is given at the top of the column. E) The observed (black) and expected (white) proportion of CSPs that occurred during wakefulness (left) and sleep (right). * significantly different from observed. Mean + s.e. F) Representative sleep-wake cycle from a P5 subject. The top trace depicts cortical activity measured using a Si electrode and filtered from 30–60 Hz. The bottom trace depicts nuchal EMG activity. Behavioral states are indicated beneath the EMG trace. Red asterisks indicate GBs.
Mean GB amplitude increased significantly with age (F4,45 = 11.8, p < .0001), from 33.1 ± 1.4 μV at P5 to 53.5 ± 3.8 μV at P11. Mean GB duration also increased significantly with age (F4,45 = 5.8, p < .001), ranging from 0.18 ± 0.01 s at P7 to 0.26 ± 0.01 s at P13. Mean GB frequency remained stable across age (F4,45 = 1.7), ranging from 36.3 ± 0.4 Hz at P9 to 39.1 ± 0.3 Hz at P7.
The mean number of GBs increased significantly with age from 3.7 ± 2.0 per min at P5 to 18.4 ± 7.8 per min at P13 (F4,15 = 7.3, p < .005; Figure 2D). Much like CSPs, GBs were most prevalent during periods of AS-related phasic activity at P5, P7, and P9. Following the emergence of delta activity at P11, GBs occurred predominantly during periods of QS-related behavioral quiescence. Finally, at all ages, GBs occurred more frequently during sleep than expected by chance (t28 = 4.21, p < .0005) (Figure 2E).
A representative sleep-wake cycle from a P5 rat is shown in Figure 2F. At this age, although GBs are present during periods of wakefulness and QS-related behavioral quiescence, they predominantly cluster during bouts of AS-related phasic activity.
Slow Activity Transients (SATs)
SATs occur in freely moving P9, P11, and P13 rats (Seelke and Blumberg, 2008). To confirm the presence of SATs in head-fixed subjects, as well as to determine their relationship with CSPs and GBs, we examined low-frequency cortical activity using Ag/AgCl electrodes (Tallgren et al., 2005). Due to the small number of subjects tested (n = 2 at each age), only means and trends are reported here.
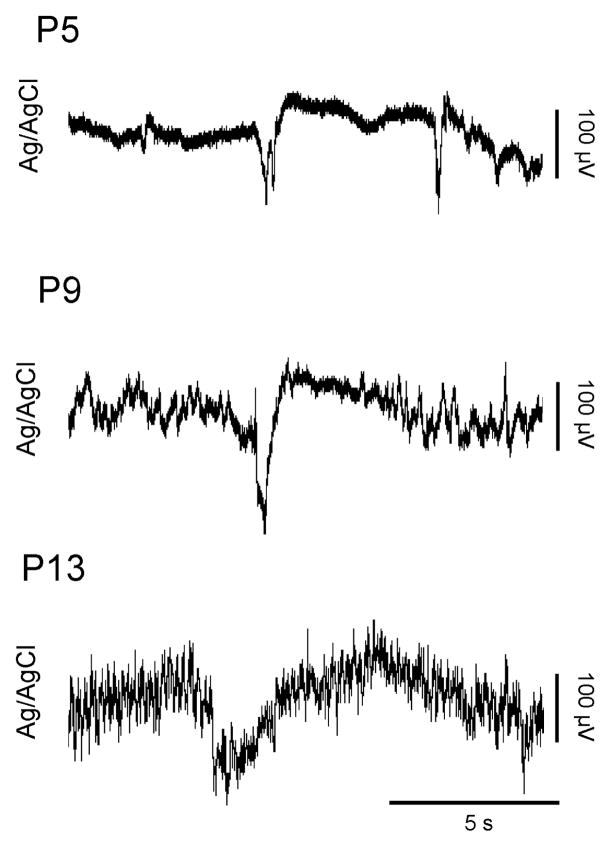
SATs were present as early as P5. Mean SAT duration increased with age from 2.9 ± 0.1 s at P5 to 5.0 ± 0.5 s at P13. The mean amplitude also increased with age, from 165.0 ± 3.5 μV at P5 to 263.5 ± 47.5 μV at P13. Examples of SATs from P5, P9, and P13 subjects are depicted in Figure 3. As shown in Figure 4, and in contrast with CSPs and GBs, the rate of occurrence of SATs was highest at P5 (4.4 ± 1.0 per min) and decreased steadily to a minimum at P13 (0.6 ± 0.1 per min). Although the SATs described here occurred roughly twice as frequently as in the freely moving subjects described previously, the observed developmental trend is similar (Seelke and Blumberg, 2008).
Figure 3.
Representative SATs from rats at P5 (top), P9 (middle) and P13 (bottom). Note how the duration of the SATs and their complexity increase with age.
Figure 4.
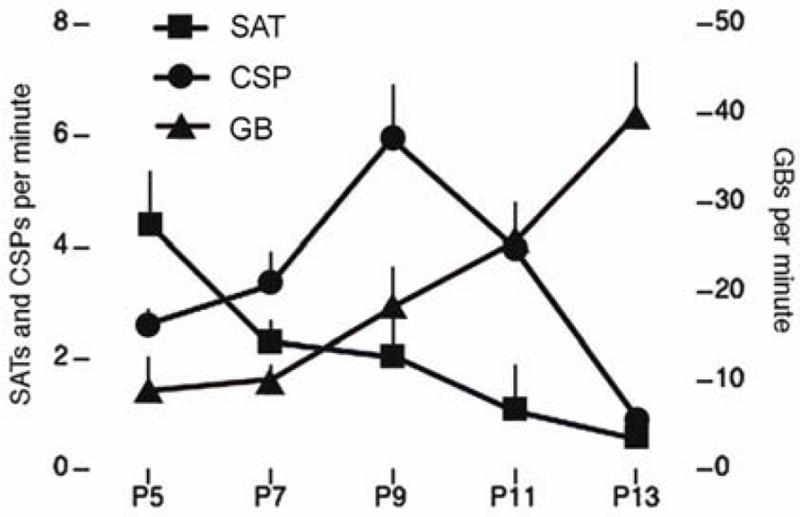
Distinct developmental trajectories of SATs (squares), CSPs (circles), and GBs (triangles) between P5 and P13. Mean + s.e.
SATs were detected during periods of wakefulness, QS, and AS, as described previously (Seelke and Blumberg, 2008). Furthermore, at P9, P11, and P13, SATs most frequently occurred during periods of QS-related behavioral quiescence. At P5 and P7, however, SATs were most commonly associated with periods of AS-related phasic activity. Thus, much like CSPs and GBs, SATs exhibited a pattern of state-dependency that changed over the course of development.
3. Discussion
Recording from medial dysgranular cortex, we report here the expression of two spontaneous cortical events – designated CSPs and GBs – as well as SATs, which have been described previously in rats (Seelke and Blumberg, 2008). (A recent paper also reports oscillations similar to the GBs reported here in infant rats (Yang et al., 2009).) All three cortical events exhibited state-dependent activity as early as P5, nearly a week before the developmental emergence of delta activity. Furthermore, two of these events, SATs and CSPs, were expressed transiently, exhibiting peak prevalence before the emergence of delta activity. It should be noted that the occurrence of one cortical event does not preclude the occurrence of another, but neither does it necessitate their occurrence. For example, Figure 5A depicts the co-occurrence of an SAT, CSP, and GB, whereas Figure 5B depicts an SAT that occurs in isolation. Because SATs, CSPs, and GBs exhibit different developmental trajectories and rates of occurrence, and can occur independently of each other, we conclude that they represent distinct patterns of neuronal activity.
Figure 5.
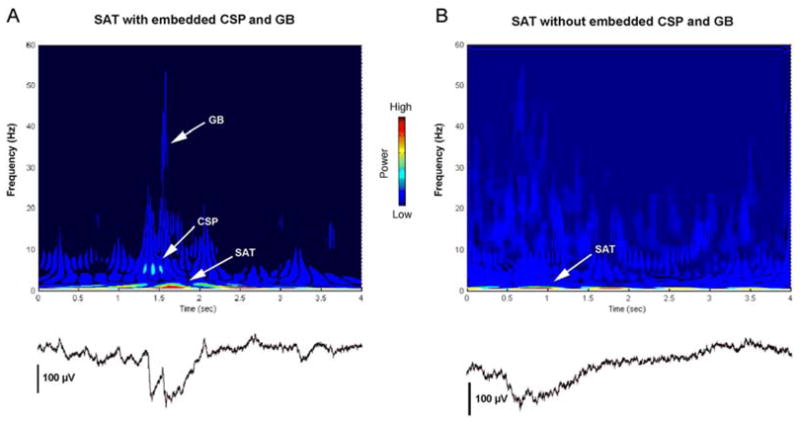
(A) Wavelet analysis of an SAT co-occurring with a CSP and a GB. The CSP and GB are both embedded within the SAT, and can be clearly seen in the corresponding local field potential (below). The SAT has dominant frequency of <1 Hz, the CSP has a dominant frequency of 5–15 Hz, and the GB has a dominant frequency of 30–55 Hz. (B) Wavelet analysis of an SAT occurring in isolation, with the corresponding local field potential (below). The SAT depicted here has a dominant frequency of <1 Hz.
These events and oscillations are only now coming to light for a variety of reasons. First, SATs and CSPs are expressed transiently and are most prevalent at ages when the cortex was only recently thought to exhibit little organized activity. Second, SATs have a dominant frequency of 0.1 Hz, and are therefore only detectable using electrodes and recording equipment that are sensitive to such low frequencies (Vanhatalo et al., 2005b). Finally, the likelihood of detecting these events and oscillations may have also been improved by using silicon depth electrodes and maintaining brain temperature at 37°C (Karlsson and Blumberg, 2004; Seelke and Blumberg, 2005).
The experiments described here were performed in unanesthetized subjects that were head-fixed within a stereotaxic apparatus. The question can be raised as to whether the cortical activity described in these subjects differs from cortical activity observed in unrestrained subjects. Comparing the present results with those from a previous study in unrestrained infant rats (Seelke and Blumberg, 2008), in both we observed a decrease in the number of SATs across development as well as the emergence of delta activity at P11. Also, in both head-fixed and unrestrained subjects we observed sleep-wake cycles that typically proceed from wakefulness to QS to AS. However, head-fixed infants, in comparison to unrestrained infants, typically exhibit relatively longer and shorter durations of sleep and wake bouts, respectively. Thus, we do not believe that head restraint can account for the developmental changes in cortical activity reported here.
There are several mechanisms that might account for the developmental trajectories exhibited by SATs, CSPs, and GBs. For example, whereas in the adult brain gamma-aminobutyric acid (GABA) typically hyperpolarizes postsynaptic cells (Krnjevic and Schwartz, 1967; Owens and Kriegstein, 2002; Owens et al., 1999), during early infancy it can have depolarizing effects (Ben-Ari, 2002; Represa and Ben-Ari, 2005; Rivera et al., 1999). The shift in GABAergic functioning in cortex occurs around the end of the first postnatal week (Payne et al., 2003) upon the developmental upregulation of the potassium-chloride co-transporter, KCC2 (Rivera et al., 1999). The developmental decrease in SAT prevalence has been linked to this “GABA switch” (Vanhatalo et al., 2005a). Likewise, the possible developmental continuity between infant GBs and adult gamma oscillations (specifically their production in cortical layer IV (Kandel and Buzsaki, 1997; Sukov and Barth, 2001), and the adult gamma oscillation’s dependence on inhibitory GABAergic activity (Beierlein et al., 2000; Ritz and Sejnowski, 1997; Whittington et al., 2000)), suggest that the developmental increase in GBs may also be linked to the shift in GABA’s functional effects.
It also seems likely that changes in cortical anatomy affect the expression of cortical activity. During the first two postnatal weeks, the structure of the cortex undergoes significant changes, including the migration of neurons into cortical layers II and III and the formation of short- and long-range connections (Miller, 1988). The peak of CSP activity at P9 coincides with the formation of long-range corticocortical networks in layers II and III, which provide the majority of connections between the ipsilateral and contralateral cortex, respectively (Hendry et al., 2003). We hypothesize that the predominance of CSPs early in development reflects the activity of a cortex that lacks long-range corticocortical connections. As cortical layers differentiate and as long-range networks are formed, cortical activity would be expected to change accordingly.
In contrast to mammals, the reptilian pallium consists of three layers but lacks the equivalent of mammalian layers II and III, and thus long-range palliopallial connections do not exist within this structure. It has been hypothesized that the absence of palliopallial connections in reptiles is associated with their lack of slow wave activity, such as delta waves (Rattenborg, 2006). However, several decades ago, researchers discovered a “high-voltage, fast spike potential” within the reptilian pallium that predominantly occurs during periods of behaviorally defined sleep. These events were mono-, bi-, or polyphasic with a mean duration of 150 ms and an amplitude of 200–300 μV (Hartse, 1994; Hartse and Rechtschaffen, 1974).
We hypothesize that CSPs are the mammalian analog of reptilian “high-voltage, fast spike potentials,” and their transience is a result of the changing structure of the infant neocortex. The similarity between infant cortical activity and reptilian pallial activity highlights how cortical activity (i.e., its amplitude, duration, profile, and prevalence) mirrors the instantaneous anatomical structure and interconnectivity of the cortex. As the cortex undergoes developmental changes—such as the formation of long-range networks—cortical activity can be expected to change accordingly. From this perspective, and as suggested by the results presented here, there may be greater similarities in cortical activity among different species with comparable neural structures than within members of the same species at different ages.
Spindle bursts have been identified in primary somatosensory and visual cortex, and are generated in response to sensory stimulation, such as stroking or manipulation of the limbs and whiskers, and retinal waves (Hanganu et al., 2006; Hanganu et al., 2007; Khazipov et al., 2004; Marcano-Reik and Blumberg, 2008; Minlebaev et al., 2007). Based upon these findings, it has been stated that SBs are the dominant form of activity within the infant cortex (Minlebaev et al., 2007). However, SBs have only been identified within primary sensory areas, and in developing rats these areas comprise less than 40% of the cortical sheet (A. M. H. Seelke and L. A. Krubitzer, unpublished observations). Very little is currently known about the development of cortical activity outside primary sensory areas.
Here we recorded from the medial dysgranular cortex and found evidence that, contrary to what has previously been thought, cortical activity in infant rats is both plentiful and diverse. However, at the sites recorded from here, we found no evidence of SBs. Accordingly, SBs may be the dominant form of activity only in the infant primary sensory cortex. Thus, we conclude that different cortical areas, at each moment of development, can generate distinctly different types of neural activity that, we believe, reflect local cortical structure as well as short- and long-range connectivity.
4. Experimental Procedure
All experiments were performed under National Institutes for Health guidelines for the care of animals in research and were approved by the Institutional Animal Care and Use Committee of the University of Iowa.
Subjects
Thirty P5, P7, P9, P11, and P13 rats (n = 6 at each age) from 30 litters were used (body weight: 11.2–42.7 g across all ages). All subjects were born to Harlan Sprague-Dawley rats housed in the animal colony at the University of Iowa. The rats were raised in litters that were culled to 8 pups within 3 days of birth (day of birth = P0). Litters and mothers were raised in standard laboratory cages (48 × 20 × 26 cm) in which food and water were available ad libitum. All rats were maintained on a 12-h light-dark schedule with lights on at 7 a.m.
Procedure
On the day of testing, a subject with a visible milk band was removed from the litter, weighed, and anesthetized using isoflurane. An incision was made in the scalp, which was retracted to expose the skull. The skull was bleached and Vetbond (3M, St. Paul, MN) was applied to strengthen the skull. Holes were drilled for later insertion of electrodes. Bipolar stainless steel EMG electrodes (50 μm diameter; California Fine Wire, Grover Beach, CA) were bilaterally implanted into the nuchal muscle and a ground wire was looped through the skin of the back; both were secured with collodion. The subject was securely wrapped in gauze and allowed to recover for 1 h in an incubator maintained at thermoneutrality. Following this recovery period, a custom-made device, which allowed the subject’s head to be securely fixed and stabilized, was attached to the skull using cyanoacrylate gel. The device was then secured to the stereotaxic apparatus and the subject was allowed to acclimate for at least 1 h, or until the subject exhibited normal cycling between behavioral states.
In all subjects, local field potentials (LFPs) were recorded throughout the depth of the frontal, parietal, and occipital lobes. Recordings were performed using 16-channel laminar silicon (Si) electrodes with 100-μm separation between recording sites (NeuroNexus Technologies, Ann Arbor, MI). The electrode was lowered into medial dysgranular cortex, 1–2 mm off midline (at its most medial point, primary somatosensory cortex is 2–3 mm off midline in subjects at these ages), to a depth of 1.60–1.70 mm. A ground wire was placed in the contralateral hemisphere.
Silicon electrodes were used to record from cortical layers II–VI. However, these electrodes are not capable of detecting activity with a frequency below 1 Hz. Thus, in 2 subjects at each age, we also recorded cortical activity using custom-made Ag/AgCl electrodes composed of 3 Teflon-insulated silver wires with exposed chlorinated tips. One wire served as a recording electrode, another as a reference electrode, and the third served as a ground (the ground electrode was placed in cerebellum contralateral to the recording and reference electrodes). Chlorination allowed for more efficient exchange of ions between tissue and electrode, resulting in more accurate recording of low-frequency cortical activity (Tallgren et al., 2005). Ag/AgCl surface electrodes record the summed activity of a large area of tissue, and do not provide a depth profile of the activity being recorded. In those subjects where Ag/AgCl electrodes were used, the recording and reference electrodes were placed 1–2 mm rostral and caudal to the Si electrode.
The Si electrode was connected to a unity gain headstage and digital amplifier (Tucker-Davis Technologies, Alachua, FL). The signals from the electrode were amplified (×10,000), filtered (1–3000 Hz), and sampled at 12.5 kHz. Ag/AgCl electrodes were connected to a differential amplifier (A-M Systems, Carlsborg, WA), filtered using either AC (0.1–300 Hz) or DC settings, amplified (AC: ×10,000; DC: ×500–10,000), and sampled at 3 kHz. EMG electrodes were connected to a differential amplifier (A-M Systems, Carlsborg, WA) and the signals were filtered (300–5000 Hz) and amplified (×10,000). All data were acquired using Spike2 software (Cambridge Electronic Design, Cambridge, United Kingdom) and saved to hard disk for off-line analysis.
When only Si electrodes were used, 15–30 min of data were recorded. When both Si and Ag/AgCl electrodes were used, 2 h of data (1 h each of AC and DC filtered Ag/AgCl electrode data) were recorded from each subject. Si electrode settings were identical for both recording sessions.
At the conclusion of each recording session, the most dorsal and ventral Si electrode recording sites were marked with a small electrolytic lesion. The subject was then deeply anesthetized using Nembutal (200 mg/kg) and transcardially perfused using a solution of phosphate buffered saline followed by a 10% formalin solution. The brain was removed and placed in a solution of 10% formalin and 30% sucrose. The brain was then sliced into 50 μm coronal sections using a freezing microtome. The sections were stained using a Nissl stain (cresyl violet) and the resulting slides were examined to verify the location of recording sites.
Data Analysis and Statistical Tests
CSPs, which were clearly visible in the raw Si electrode trace, were defined as discrete, biphasic events with an amplitude of at least 2× baseline. They were seen throughout the depth of the cortex (i.e., in every channel of the Si electrode recording). GBs were also visible in the raw Si electrode trace, but were filtered (30–60 Hz) before further analysis. GBs were defined as discrete bouts of 40-Hz activity with an amplitude of at least 2x baseline and consisting of at least 3 complete cycles.
A representative sample consisting of the first 300 s of each Si electrode recording (including frontal, parietal, and occipital lobe recordings) was scored, and the total number of CSPs and GBs was determined for each recording site in each subject. A subset of both CSPs and GBs exhibited phase reversals, that is, transitions from positive to negative polarity across one or more cortical layers. The number of phase-reversing events was determined within each recording site.
The first 10 CSPs and GBs in each record were selected for further analysis. The peak-to-trough amplitude and duration of each event was determined. For GBs, the number of cycles per burst was also determined, and the mean waveform frequency was calculated. All values were averaged within subjects and analysis of variance (ANOVA) was performed using Statview and JMP statistical packages (SAS, Cary, NC). Planned comparisons were performed here and elsewhere to assess within-age differences between cortical lobes. For all analyses α < 0.05.
We next analyzed data collected using both Si and Ag/AgCl electrodes. CSPs and GBs were identified in Si electrode recordings as described above. SATs were identified in Ag/AgCl recordings and comprised high-amplitude, low-frequency, discrete cortical events (Vanhatalo and Kaila, 2006; Vanhatalo et al., 2005a). SATs exhibited similar characteristics in both AC-filtered and DC-coupled records. Here we present data from AC-filtered records. The number of SATs, CSPs, and GBs in the first 1800 s of each record was determined, and the number of events per minute was calculated for each subject.
A follow-up analysis examined the first 10 min of each recording. In order to ensure that we were examining only locally generated events and oscillations, for this and all the following analyses we applied a conservative definition of CSPs and GBs, that is, we only analyzed those events and oscillations that exhibited phase reversals. The mean number of CSPs and GBs per minute was then determined. Periods of sleep and wakefulness were identified using nuchal EMG, as described elsewhere (Blumberg et al., 2005; Karlsson and Blumberg, 2002; Seelke and Blumberg, 2005). The state dependency of each event was determined by comparing the proportion of events that occurred during sleep and wakefulness to the proportion of time spent in sleep and wakefulness. Groups were compared using paired t tests.
Sleep periods were further divided into quiet sleep (QS) and active sleep (AS) bouts. QS durations were characterized by a period of nuchal muscle atonia and, from P11 on, by the appearance of delta activity in the neocortical EEG (Seelke and Blumberg, 2008). AS durations were characterized by a period of nuchal atonia coupled with twitches of the nuchal muscle and, after P11, the absence of cortical delta activity.
Current source density (CSD) analyses were performed on selected EEG data. CSD analyses were performed on a waveform produced by averaging at least 30 phase-reversing CSPs or GBs within a single record. All 16 channels were used to generate the CSD (Khazipov et al., 2004). For these analyses, custom-written code was implemented using the Matlab wavelet toolbox (The MathWorks, Natick, MA).
Supplementary Material
Acknowledgments
This research was supported by a research grant (MH50701) and an Independent Scientist Award (MH66424) from the National Institute of Mental Health (to M.S.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beierlein M, Gibson JR, Connors BW. A network of electrically coupled interneurons drives synchronized inhibition in neocortex. Nature Neuroscience. 2000;3:904–10. doi: 10.1038/78809. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of GABA during development: The nature of the nurture. Nature Reviews Neuroscience. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Blumberg MS, Seelke AMH, Lowen SB, Karlsson KÆ. Dynamics of sleep-wake cyclicity in developing rats. Proceedings of the National Academy of Science. 2005;102:14860–14864. doi: 10.1073/pnas.0506340102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Heller HC. Development of REM and slow wave sleep in the rat. American Journal of Physiology. 1997;272:R1792–R1799. doi: 10.1152/ajpregu.1997.272.6.R1792. [DOI] [PubMed] [Google Scholar]
- Gramsbergen A. The development of the EEG in the rat. Developmental Psychobiology. 1976;9:501–515. doi: 10.1002/dev.420090604. [DOI] [PubMed] [Google Scholar]
- Hanganu IL, Ben-Ari Y, Khazipov R. Retinal waves trigger spindle bursts in the neonatal rat visual cortex. The Journal of Neuroscience. 2006;26:6728–6736. doi: 10.1523/JNEUROSCI.0752-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanganu IL, Staiger JF, Ben-Ari Y, Khazipov R. Cholinergic modulation of spindle bursts in the neonatal rat visual cortex in vivo. The Journal of Neuroscience. 2007;27:5694–705. doi: 10.1523/JNEUROSCI.5233-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartse KM. Sleep in insects and nonmammalian vertebrates. In: Roth T, et al., editors. Principles and Practice of Sleep Medicine. Saunders; Philadelphia: 1994. pp. 95–104. [Google Scholar]
- Hartse KM, Rechtschaffen A. Effect of atropine sulfate on the sleep-related EEG spike activity of the tortoise, Geochelone carbonaria. Brain, Behavior and Evolution. 1974;9:81–94. doi: 10.1159/000123657. [DOI] [PubMed] [Google Scholar]
- Hendry SH, Hsiao SS, Brown MC. Fundamentals of sensory systems. In: Squire LR, et al., editors. Fundamental Neuroscience. Academic Press; 2003. [Google Scholar]
- Jouvet-Mounier D, Astic L, Lacote D. Ontogenesis of the states of sleep in rat, cat, and guinea pig during the first postnatal month. Developmental Psychobiology. 1970;2:216–239. doi: 10.1002/dev.420020407. [DOI] [PubMed] [Google Scholar]
- Kandel A, Buzsaki G. Cellular-synaptic generation of sleep spindles, spike-and-wake discharges, and evoked thalamocortical responses in the neocortex of the rat. The Journal of Neuroscience. 1997;17:6783–6797. doi: 10.1523/JNEUROSCI.17-17-06783.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson KÆ, Blumberg MS. The union of the state: Myoclonic twitching is coupled with nuchal muscle atonia in infant rats. Behavioral Neuroscience. 2002;116:912–917. doi: 10.1037//0735-7044.116.5.912. [DOI] [PubMed] [Google Scholar]
- Karlsson KÆ, Blumberg MS. Temperature-induced reciprocal activation of infant hippocampal field activity. Journal of Neurophysiology. 2004;91:583–588. doi: 10.1152/jn.00953.2003. [DOI] [PubMed] [Google Scholar]
- Khazipov R, Sirota A, Leinekugel X, Holmes GL, Ben-Ari Y, Buzsaki G. Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature. 2004;432:758–61. doi: 10.1038/nature03132. [DOI] [PubMed] [Google Scholar]
- Krnjevic K, Schwartz S. The action of gamma-aminobutyric acid on cortical neurones. Experimental Brain Research. 1967;3:320–36. doi: 10.1007/BF00237558. [DOI] [PubMed] [Google Scholar]
- Marcano-Reik AJ, Blumberg MS. The corpus callosum modulates spindle-burst activity within homotopic regions of somatosensory cortex in newborn rats. European Journal of Neuroscience. 2008;28:1457–66. doi: 10.1111/j.1460-9568.2008.06461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW. Development of projection and local circuit neurons in neocortex. In: Peters A, Jones EG, editors. Cerebral Cortex. Vol. 7. Plenum; New York: 1988. pp. 133–175. [Google Scholar]
- Minlebaev M, Ben-Ari Y, Khazipov R. Network mechanisms of spindle-burst oscillations in the neonatal rat barrel cortex in vivo. Journal of Neurophysiology. 2007;97:692–700. doi: 10.1152/jn.00759.2006. [DOI] [PubMed] [Google Scholar]
- Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition? Nature Reviews Neuroscience. 2002;3:715–27. doi: 10.1038/nrn919. [DOI] [PubMed] [Google Scholar]
- Owens DF, Liu X, Kriegstein AR. Changing properties of GABA(A) receptor-mediated signaling during early neocortical development. Journal of Neurophysiology. 1999;82:570–83. doi: 10.1152/jn.1999.82.2.570. [DOI] [PubMed] [Google Scholar]
- Payne JA, Rivera C, Voipio J, Kaila K. Cation-chloride co-transporters in neuronal communication, development and trauma. Trends in Neuroscience. 2003;26:199–206. doi: 10.1016/S0166-2236(03)00068-7. [DOI] [PubMed] [Google Scholar]
- Rattenborg NC. Evolution of slow-wave sleep and palliopallial connectivity in mammals and birds: A hypothesis. Brain Research Bulletin. 2006;69:20–29. doi: 10.1016/j.brainresbull.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A, editors. A manual of standardized terminology, techniques, and scoring system for sleep stages of human subjects. UCLA Brain Information Service/Brain Research Institute; Los Angeles: 1968. [Google Scholar]
- Represa A, Ben-Ari Y. Trophic actions of GABA on neuronal development. Trends in Neuroscience. 2005;28:278–283. doi: 10.1016/j.tins.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ritz R, Sejnowski TJ. Synchronous oscillatory activity in sensory systems: new vistas on mechanisms. Current Opinions in Neurobiology. 1997;7:536–46. doi: 10.1016/s0959-4388(97)80034-7. [DOI] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl- co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Seelke AMH, Blumberg MS. Thermal and nutritional modulation of sleep in infant rats. Behavioral Neuroscience. 2005;119:603–611. doi: 10.1037/0735-7044.119.2.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelke AMH, Blumberg MS. The microstructure of active and quiet sleep as cortical delta activity emerges in infant rats. Sleep. 2008;31:691–699. doi: 10.1093/sleep/31.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelke AMH, Karlsson KÆ, Gall AJ, Blumberg MS. Extraocular muscle activity, rapid eye movements, and the development of active and quiet sleep. European Journal of Neuroscience. 2005;22:911–920. doi: 10.1111/j.1460-9568.2005.04322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukov W, Barth DS. Cellular mechanisms of thalamically evoked gamma oscillations in auditory cortex. Journal of Neurophysiology. 2001;85:1235–45. doi: 10.1152/jn.2001.85.3.1235. [DOI] [PubMed] [Google Scholar]
- Tallgren P, Vanhatalo S, Kaila K, Voipio J. Evaluation of commercially available electrodes and gels for recording of slow EEG potentials. Clinical Neurophysiology. 2005;116:799–806. doi: 10.1016/j.clinph.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Vanhatalo S, Kaila K. Development of neonatal EEG activity: From phenomenology to physiology. Seminars in Fetal and Neonatal Medicine. 2006;11:471–478. doi: 10.1016/j.siny.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Vanhatalo S, Palva JM, Andersson S, Rivera C, Voipio J, Kaila K. Slow endogenous activity transients and developmental expression of KCC2 in the immature human cortex. European Journal of Neuroscience. 2005a;22:199–206. doi: 10.1111/j.1460-9568.2005.04459.x. [DOI] [PubMed] [Google Scholar]
- Vanhatalo S, Voipio J, Kaila K. Full-band EEG (FbEEG): an emerging standard in electroencephalography. Clinical Neurophysiology. 2005b;116:1–8. doi: 10.1016/j.clinph.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Kopell N, Ermentrout B, Buhl EH. Inhibition-based rhythms: experimental and mathematical observations on network dynamics. International Journal of Psychophysiology. 2000;38:315–36. doi: 10.1016/s0167-8760(00)00173-2. [DOI] [PubMed] [Google Scholar]
- Yang JW, Hanganu-Opatz IL, Sun JJ, Luhmann HJ. Three patterns of oscillatory activity differentially synchronize developing neocortical networks in vivo. J Neurosci. 2009;29:9011–25. doi: 10.1523/JNEUROSCI.5646-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.