Abstract
Aims
The current study assessed the in vivo antagonist properties of nalmefene using procedures previously used to characterize the opioid antagonists naloxone, naltrexone, 6β-naltrexol and nalbuphine.
Main methods
ICR mice were used to generate antagonist dose-response curves with intraperitoneal (i.p.) nalmefene against fixed A90 doses of morphine in models of morphine-stimulated hyperlocomotion and antinociception. Additional dose-response curves for antagonist precipitated opioid withdrawal were run in mice treated acutely (100 mg/kg, s.c., −4 hours) or chronically (75 mg pellet, s.c., −72 hours) with morphine. Comparisons were made between antagonist potency and degree of precipitated withdrawal.
Key findings
Nalmefene produced dose- and time-related antagonism of morphine-induced increases in locomotor activity with a calculated ID50 (and 95% confidence interval) of 0.014 (0.007–0.027) mg/kg. Nalmefene produced rapid reversal of morphine-induced locomotor activity (5.1 minutes for 50% reduction in morphine effect). A 0.32 mg/kg dose of nalmefene produced blockade of morphine-induced antinociception in the 55°C tail-flick test that lasted approximately 2 hours. Nalmefene was able to potently precipitate withdrawal in mice treated acutely or chronically with morphine.
Significance
These results demonstrate that nalmefene is similar to naloxone and naltrexone with respect to its in vivo pharmacology in mice. Specifically, nalmefene produces potent antagonism of morphine agonist effects while precipitating severe withdrawal. The compound has a slower onset and longer duration of action compared to naloxone and naltrexone. The data allows for a more complete preclinical comparison of nalmefene against other opioid antagonists including the putative opioid neutral antagonist 6β-naltrexol.
Keywords: opioid antagonist, pain, antinociception, mu opioid receptor
Introduction
Nalmefene is one of three CNS penetrating opioid receptor antagonists that have been used clinically. By conventional pharmacological standards all are considered to be pure antagonists. The drug received FDA approval in 1995 for complete or partial reversal of opioid drug effects including respiratory depression and known or suspected opioid overdose (United States Food and Drug Administration, 1999). The compound has also been used off label to reduce pruritus associated with spinal opioids (Pellegrini et al. 2001; Kjellberg and Tramèr 2001) and has been tested in phase II/III clinical trials for management of alcoholism (Karhuvaara et al. 2007; Mason et al. 1999) and pathological gambling (Grant et al. 2006). The effects on gambling have been mixed with higher doses of the drug producing intolerable side effects including nausea and insomnia (Grant et al. 2006). Despite some potential advantages of nalmefene over nalxone including a longer duration of action and better oral bioavailability, the drug has not captured a significant percentage of the opioid antagonist market.
Similar to naloxone and naltrexone, nalmefene may precipitate withdrawal symptoms in patients who are either acutely or chronically treated with opioid agonists (Kaplan et al. 1999; Wang et al. 1998). Withdrawal has also been noted when nalmefene is administered postoperatively to reduce the ventilatory depressant effects of high dose opioid agonists used during surgery (United States Food and Drug Administration, 1999). Withdrawal severity is likely to be related to both the displacement of the agonist from the receptor and the potential inverse agonist effects of the “antagonist” (Bilsky et al. 1996; Sadée et al. 2005; Sirohi et al. 2009). Inverse agonists are thought to stabilize G-protein coupled receptors into an inactive state, suppressing basal or constitutive activity (Bokoch et al. 2010). Evidence has been provided that naloxone and naltrexone act as inverse agonists in opioid exposed systems, thereby increasing the severity of withdrawal compared to that precipitated by a neutral antagonist or weak partial agonist (Raehal et al. 2005; Wang et al. 2001; Wang et al. 2004).
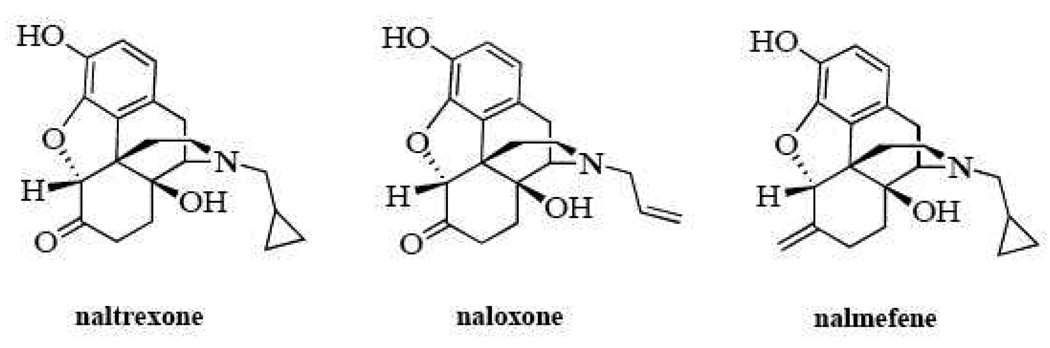
Our laboratory is exploring the structure activity of opioid neutral antagonists as well as inverse agonists, and have tested a number of structurally similar ligands for their activity in opioid naïve and opioid exposed systems. This has led to the identification and characterization of two putative neutral antagonists, 6β-naltrexol and 6β-naloxol. These compounds differ from naltrexone and naloxone by the reduction of the ketone group at the six-position of the parent compound to a hydroxyl moiety (Figure 1). The current study assessed the antagonist properties of nalmefene which substitutes the ketone with a methylene group, maintaining the double bond and its influence on the overall structure of the molecule (Figure 1). The compound was tested in an outbred strain of mice (ICR) using procedures identical to those previously used to characterize naloxone, naltrexone, 6β-naltrexol and nalbuphine (Raehal et al. 2005). This information helps better characterize the preclinical efficacy of nalmefene and will aid in the development of novel opioid antagonists for use in opioid exposed/dependent subjects.
Figure 1.
Chemical structures of naltrexone, naloxone and nalmefene.
Materials and Methods
Animals
Male ICR mice weighing between 25–35 grams were used for all studies. Animals were bred in the vivarium at the University of New England from a breeding stock purchased from Harlan (Indianapolis, Indiana). Mice were housed in groups of five in Plexiglas chambers with food and water available ad libitum. All animals were maintained on a 12 hr light/dark cycle (lights on at 07:00) in a temperature- and humidity-controlled animal colony. All animal experiments were performed under an approved protocol in accordance with institutional guidelines and in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health.
Drug Solutions and Injections
Morphine sulfate was obtained from Spectrum Chemicals (New Brunswick, NJ). Naloxone HCl was obtained through the NIDA Drug Supply program (Bethesda, MD). Mallinckrodt Pharmaceuticals generously donated nalmefene HCL (St. Louis MO). All drugs were dissolved in physiological saline (0.9% NaCl) with injection volume based on the weight of the animal (0.1 mL/10 g bodyweight). Intraperitoneal (i.p.) injections were performed by grasping the mouse by the scruff of the neck and tail and gently arching the spine backward to expose the abdomen. Injections were made by inserting a 30-gauge needle through the skin and abdominal musculature into the peritoneal cavity slightly lateral to the midline. Subcutaneous (s.c.) administration of drug was accomplished by injecting solution directly underneath the skin into the space between the abdominal region and the anterior portion of the hip.
Locomotor Studies
Locomotor activity was quantified using an activity monitoring system (Coulbourn Instruments) and TruScan software. The locomotor chambers consisted of Plexiglas walls and a removable plastic drop pan (dimensions of 10”W × 10”L × 16”H) surrounded by a sensor ring with a 16 × 16 infrared beam array. The TruScan software measured patterns of beam breaks and calculated the position of the mouse with a temporal resolution of 100 milliseconds. Each chamber was wiped down with a dilute cleaning solution between sessions to remove scent. For antagonist studies against morphine-induced hyperlocomotion, mice were habituated to the chambers for 30 minutes. They were then briefly removed from the chambers and administered two injections. Vehicle or doses of naloxone or nalmefene were injected i.p. followed immediately afterwards by a s.c. injection of vehicle or morphine (32 mg/kg). Mice were placed back into the chambers and monitored for an additional 90 minutes. Sessions were divided into twenty-four 5 minute bins (baseline and drug effect time intervals) with the distance traveled calculated for each mouse. To determine if the antagonists alone produced effects on locomotor activity, a modified protocol was used. Mice received an i.p. injection of vehicle or a 1 mg/kg dose of naloxone or nalmefene 30 minutes prior to a 30-minute locomotor session. The lack of habituation to the chambers kept the locomotor activity at a level where increases or decreases in distance traveled could be easily detected. In order to measure the onset of antagonist blockade, a second modified protocol was used. A 120-minute session was used with one minute bins of activity summarized. The first 30 minutes were used as habituation followed by an injection of morphine (32 mg/kg, s.c.) and an additional 30 minutes of monitoring. Mice were then given an i.p. injection of vehicle or nalmefene (0.32 mg/kg) and placed back into the chambers for an additional 60 minutes of monitoring.
Antinociceptive Studies for Determining Duration of Antagonist Blockade
Antinociception was assessed using the 55°C warm water tail-flick test. The latency to the first sign of a rapid tail-flick was taken as the behavioral endpoint (Jannsen et al. 1963). Each mouse was first tested for baseline latency by immersing its tail in the water and recording the time to response. Mice not responding within 5 seconds were excluded from further testing. Mice were then administered a fixed dose of nalmefene (0.32 mg/kg, i.p.) at various times prior to the administration of an approximate A90 dose of morphine (32 mg/kg, s.c.). Mice were tested 20 minutes after the morphine injection, a time corresponding to the peak antinociceptive effect for s.c. morphine. A cutoff latency of 10 seconds was used to prevent tissue damage. Antinociception was calculated using the following formula: % antinociception = 100 × (test latency - control latency) / (10 - control latency). The time course data were plotted to determine the approximate duration of action of nalmefene.
Acute Physical Dependence
Mice were injected with a high dose of morphine (100 mg/kg, s.c., −4 hours) and returned to their home cage. At t = 0 minutes, withdrawal was precipitated by injecting doses of naloxone (10 mg/kg, i.p.) or nalmefene (0.1–1.0 mg/kg). Immediately after the antagonist injection, mice were placed into clear Plexiglas cylinders with filter paper bottoms and videotaped for 20 minutes. A trained observer blinded to the experimental protocol reviewed the tapes and the number of vertical jumps was recorded as the primary index of withdrawal (Raehal et al. 2005). Among naloxone-precipitated withdrawal signs in mice, frequency of uncontrollable stereotypical jumping (i.e., a hyperactivity response) is widely considered the most sensitive and reliable index of withdrawal intensity and is by far the most commonly used (Klein et al. 2008; Kest et al. 2002). Bodyweight was measured immediately before antagonist injection and immediately after the withdrawal session for each mouse. In addition, an image for each filter paper was digitally captured and later scored for urine output (rough percentage wet versus dry) and number of fecal boli by a blinded observer.
Chronic Physical Dependence
Mice were lightly anesthetized with ether and a small incision was made along the nape of the neck. A 75 mg pellet of morphine was inserted through the incision and pushed caudally so as to rest at the apex of the scapula. The incision was closed using two surgical staples and the mice were returned to their home cages and allowed to recover. Withdrawal was precipitated 72 hours after pellet implantation with an i.p. injection of either naloxone (1 mg/kg) or nalmefene (0.032–1.0 mg/kg). A 20 minute withdrawal period was videotaped with bodyweights being recorded immediately before and after the session. A trained observer blinded to the experimental protocol reviewed the tapes and the number of vertical jumps was recorded as the primary index of withdrawal (Fujimoto et al. 1975). With respect to fecal boli, areas of concentrated diarrhea/smeared feces were counted as discrete entities.
Statistical Analysis
Dose-response curves were analyzed using linear regression with the calculation of ID50 values and 95% confidence intervals (FlashCalc software). Most of the other antinociceptive, locomotor and physical dependence data were analyzed using analysis of variance (ANOVA) followed by appropriate post-hoc analysis. In all cases, significance was established at the p < 0.05 level.
Results
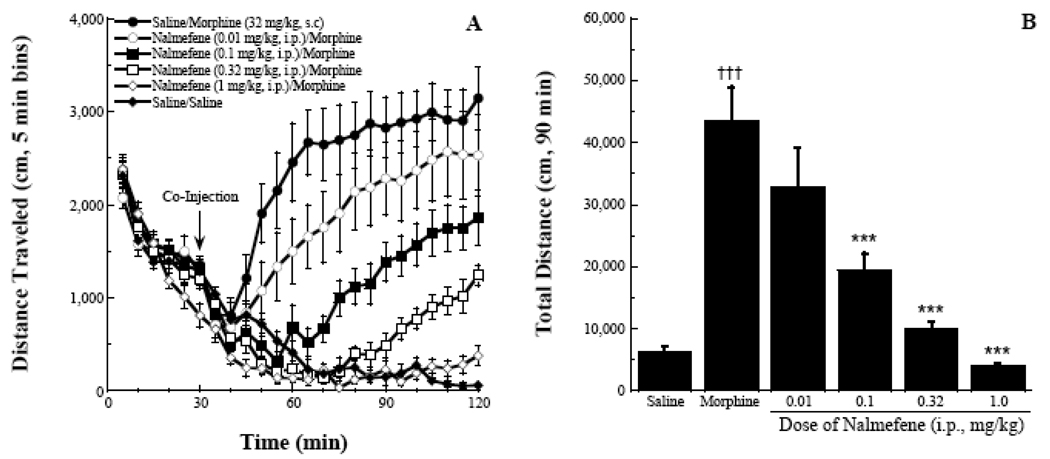
In characterizing the in vivo pharmacology of nalmefene, we took an approach similar to that of earlier work with naltrexone and 6β-naltrexol (Raehal et al. 2005). Figure 2A is the overall summary of a 120-minute activity monitoring session in which locomotor activity was measured following administration of varying doses of nalmefene and an A90 dose of morphine or saline. All groups of mice had a similar habituation profile (decreased distance traveled for each of the successive 5 minute bins, 0–30 minutes). A repeated measures ANOVA of the last 90 minutes of the session yielded significant effects of time, treatment, and the interaction between the two (all p values < 0.001). The saline/saline group continued to habituate to the chambers following injection, with their distance traveled in a five minute bin approaching zero for the last several activity bins. In contrast, saline/morphine produced a robust increase in stereotypical locomotor activity, replicating previous results (Raehal et al. 2005).
Figure 2.
Antagonist effects of nalmefene. Nalmefene produced dose-related blockade of morphine-induced locomotor activity as indexed by distance traveled (panel A) and an area under the curve analysis (panel B). A Tukey’s post-hoc analysis indicated significant differences between the morphine control and higher doses of nalmefene in combination with the morphine dose, *** p < 0.001. A significant difference was also seen between the vehicle and morphine control groups, ††† p < 0.001.
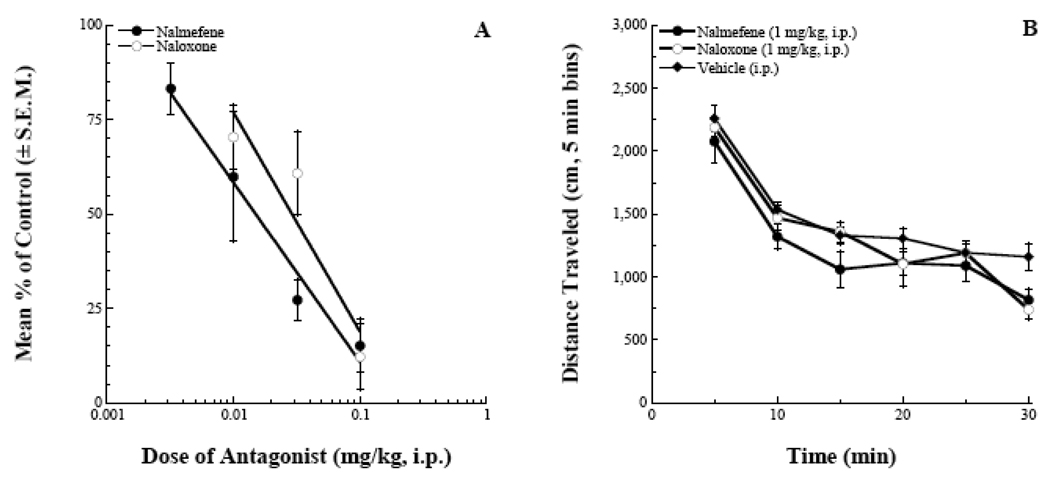
Nalmefene reduced the morphine effect in a dose-dependent manner as indexed by the magnitude of the 5-minute bins of activity (Figure 2A) and the area under the curve (AUC) analysis (Figure 2B). The ANOVA for the AUC analysis yielded an F(7,63) = 23.5, p < 0.001 and the post-hoc analysis indicated that the four highest doses of nalmefene tested all significantly blocked the morphine effect. Figure 3 estimates the potency of nalmefene to antagonize the morphine locomotor effect. In this analysis, data from the 20–60 minute bins following agonist/antagonist injections were used. This represented a time window where the morphine effect was becoming manifest and the effects of the antagonist were still capable of fully blocking the opioid agonist activity (Raehal et al. 2005). Fitting the data using linear regression software (FlashCalc, Dr. Michael Ossipov, University of Arizona) indicated an ID50 value for nalmefene (95% confidence interval) of 0.014 (0.007–0.027) mg/kg. This antagonist potency was approximately equal to that of naloxone when tested under similar conditions: 0.029 (0.018–0.046) mg/kg (Figure 3A). A modified locomotor assay was run to assess the potential non-specific effects of the two antagonists when given at supramaximal doses (1 mg/kg, i.p.). Analysis of the data indicated that there was no main effect of treatment, F(2,145) = 2.50, p > 0.05 (Figure 3B).
Figure 3.
Summary graphs of antagonist effects in the locomotor assay. Nalmefene was approximately equipotent to naloxone in blocking the locomotor stimulating effects of a fixed dose of morphine (panel A, 95% confidence intervals overlap). When given at supramaximal doses (1 mg/kg, i.p.), neither antagonist produced a significant effect on locomotor activity compared to vehicle treated mice (panel B, p > 0.05).
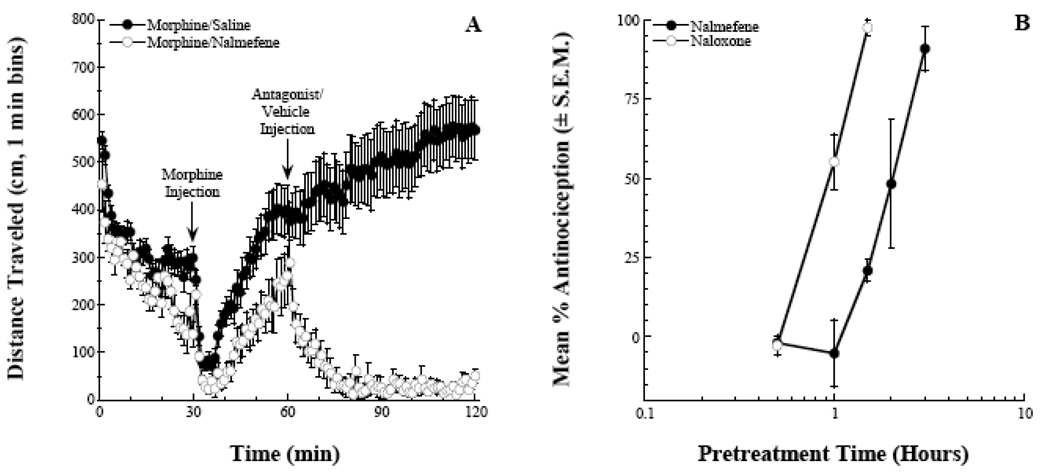
In order to further quantify the antagonist effects of nalmefene in vivo, two additional antagonist experiments were conducted. Figure 4A represents a modified locomotor assay in which the antagonist is administered after the morphine effect is already established (e.g., reversal rather than prevention). A vehicle injection had no effect on the morphine-induced hyperlocomotion (the effect continued to strengthen through the remaining 60 minutes of monitoring). In contrast, a 0.32 mg/kg dose of nalmefene quickly and completely reversed the morphine effect. When this was expressed as a percentage of pre-injection activity, the initial reductions in distance traveled were approximately linear. The calculated time to 50% reduction (95% confidence interval) of the morphine effect was 5.4 (4.2–6.9) minutes for the group. This time of onset is slightly longer compared to naloxone, 3.3 (3.0–3.6) minutes, and comparable to 6β-naltrexol, 5.1 (4.5–5.9) minutes (Raehal et al. 2005).
Figure 4.
Nalmefene antagonism of morphine effects. Panel A depicts the reversal of established morphine-induced hyperlocomotion (32 mg/kg, s.c.) by the administration of nalmefene (0.32 mg/kg, i.p.). Panel B represents a time-course of nalmefene-pretreatment blockade (0.32 mg/kg, i.p.) of morphine-induced antinociception (32 mg/kg, s.c.) in the 55°C tail-flick assay. Naloxone is included as a comparator (1 mg/kg, i.p.).
To further address the duration of action of the antagonist, nalmefene was administered to mice at a fixed dose (0.32 mg/kg, i.p.) at various time intervals prior to a morphine injection (32 mg/kg, s.c.). Antinociception in the 55°C tail-flick assay was then assessed at t = 20 minutes, a time corresponding to peak morphine effects in this assay. Pretreatment times up to 1 hour prior to morphine completely blocked the morphine effect (Figure 4B). The antagonist effects of nalmefene were absent when the antagonist was administered 3 hours prior to morphine. Based on our previous work with naloxone (Raehal et al. 2005), the nalmefene duration of action was approximately twice that of naloxone in ICR mice.
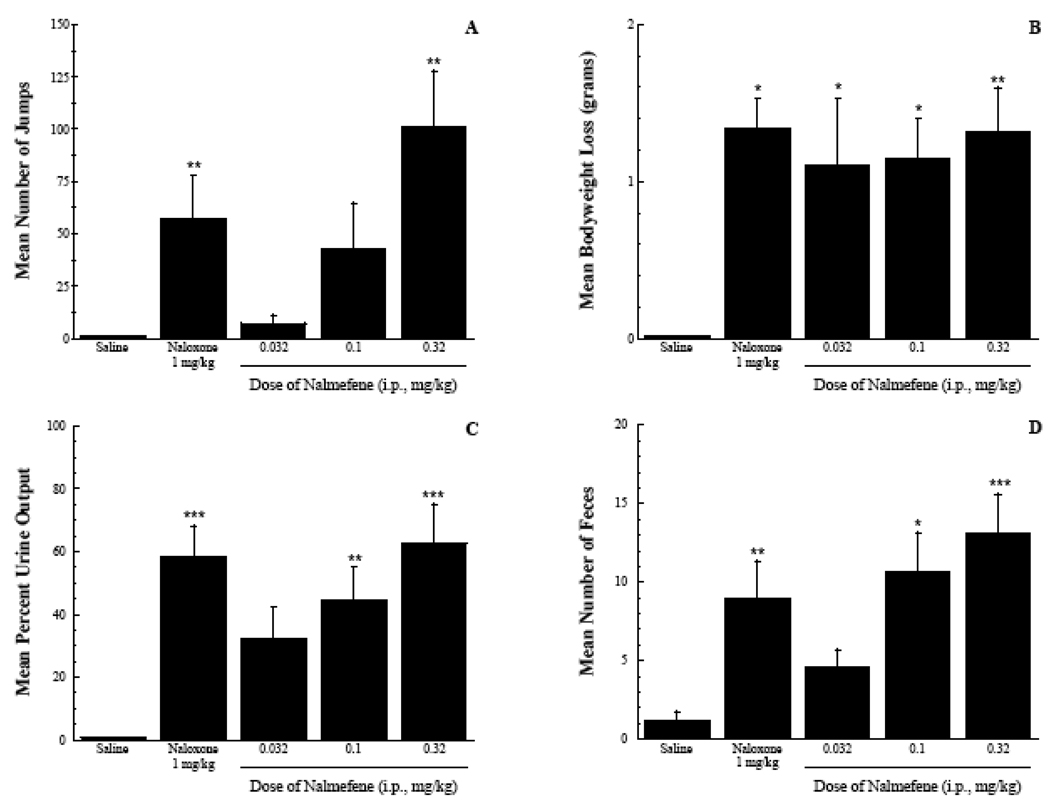
The last set of experiments assessed the ability of nalmefene to precipitate withdrawal in opioid dependent mice. Different doses of nalmefene were administered to mice treated with either acute high dose morphine (Figure 5) or with a morphine pellet for 72 hours (Figure 6). Several different parameters were measured including vertical jumps, bodyweight loss, urine output and mean number of feces. In general, nalmefene precipitated dose-related withdrawal with a severity that was equivalent to that of naloxone in both the acute and chronic dependence assays. The vertical jumping measure was the most sensitive for detecting dose escalation of the antagonist effect. An ANOVA of the acute dependence data yielded an F(4,38) = 5.14, p < 0.01, with a 1 mg/kg dose of nalmefene producing equivalent withdrawal to a 10 mg/kg dose of naloxone. Measurements of bodyweight loss, urine output and mean number of feces were all elevated compared to saline controls, but there was no obvious dose-related effect of nalmefene (Figure 5).
Figure 5.
Precipitation of opioid withdrawal in an acute dependence assay. ANOVA analysis is presented in the results section. Tukey’s post-hoc analyses indicated significant differences between the saline control group and antagonist treatment groups, * p < 0.05, ** p < 0.01.
Figure 6.
Precipitation of opioid withdrawal in a chronic dependence assay. ANOVA analysis is presented in the results section. Tukey’s post-hoc analyses indicated significant differences between the saline control group and antagonist treatment groups, * p < 0.05, ** p < 0.01, *** p < 0.001.
The chronic dependence assay resulted in a higher level of dependence and withdrawal (as indexed by higher frequency of the vertical jumping behavior and a leftward shift in the antagonist dose-response curve). The ANOVA for the jumping data yielded an F(4,61) = 7.15, p < 0.001. The 0.32 mg/kg dose of nalmefene produced significantly greater jumping than the vehicle control, and an equivalent withdrawal compared to a 1 mg/kg doss of naloxone (p > 0.05). Nalmefene also produced significant effects on bodyweight loss, urine output and mean number of feces compared to vehicle control. The ANOVAs yielded F(4,61) = 3.95, 7.88 and 6.82, respectively (all p values < 0.01). The effects of nalmefene were generally dose-related with the exception of bodyweight loss (Figure 6).
Discussion
Nalmefene was first approved for human use in 1995 as a reversal agent for treatment of opioid overdose. Nalmefene has a demonstrated longer duration of antagonist action compared to naloxone (Glass et al. 1994). This effect provides a theoretical advantage over naloxone when treating life-threatening respiratory depression associated with sustained released (Oxycontin) or long-duration (methadone) opioid agonists (Glass et al. 1994). Baxter Health Care discontinued U.S. sales of the drug in 2008 (www.fda.gov) though interest remains in it use as indexed by continued clinical trials with nalmefene as an adjunct for alcoholism and other addictions (www.clinicaltrials.gov).
Our interest in the compound stems from previous work characterizing the opioid antagonists naloxone, naltrexone, and the various derivatives of the parent compounds. These ligands can act as inverse agonists or neutral antagonists depending on the state of the receptor system (e.g., opioid-naïve versus opioid-exposed test systems)(Sadée et al. 2005). Nalmefene shares a number of structural similarities to naloxone and naltrexone (e.g., a phenol domain and complex ring structure) but also differs from them (i.e., replacement of a cabonyl at the 6-position of the molecule with a methylene group). In contrast, the putative neutral antagonists 6β-naltrexol and 6α- and 6β-naloxol carry a hydroxyl group at the 6-position. The in vivo pharmacology of nalmefene in rodent models has not been extensively described in the peer-reviewed literature relative to naloxone and naltrexone. The aims of the current study were to more fully characterize the opioid antagonist properties of the drug under conditions similar to those that we previously used to investigate naloxone, naltrexone and 6β-naltrexol (Raehal et al. 2005).
The potency of nalmefene to block acute effects of morphine was determined in assays of antinociception and locomotor activity. Nalmefene dose-dependently decreased the effects of morphine with approximately equal potency to naloxone. This is consistent with clinical data demonstrating roughly equal potency of naloxone and nalmefene (Glass et al. 1994). Nalmefene did not stimulate locomotor activity by itself even at the highest dosing levels tested. Nalmefene also did not produce antinociception in the 55°C degree tail-flick assay at doses up to 10 mg/kg (data not shown). These data confirm a pure antagonist profile of nalmefene under opioid-naïve conditions. In the locomotor assay, there was some indication that the nalmefene blockade waned in the final 10–15 minutes of the observation period (75–90 minutes post-dosing). This may be due to the antagonist effects wearing off or continued increases in CNS levels of morphine following s.c. administration of the compound. We attempted to address this issue by completing a time course study using various pretreatment times for nalmefene injection (0.32 mg/kg, i.p.) with a fixed dose of morphine (32 mg/kg, s.c.) given at t = 0 minutes. The 55°C tail-flick assay was used as an endpoint as this allowed for a single determination of agonist activity (%-antinociception) at the time of agonist peak effect. Nalmefene was fully effective as an antagonist of morphine antinociception for a minimum of 60 minutes under the conditions examined. There was a gradual wearing off of the antagonist effect over the subsequent two hours of testing. This duration of action was approximately twice as long naloxone and naltrexone in rodents, but significantly shorter than the antagonist actions of 6β-naltrexol (Raehal et al. 2005).
In the dependence assays, vertical jumping was used as the primary withdrawal measure, as it is one of the most reliable measures of withdrawal severity (Klein et al. 2008; Kest et al. 2002). These data correlated with both dose and duration of opioid exposure and the dose of naloxone or nalmefene injected (Blasig et al. 1973; Kest et al. 2002, Raehal et al. 2005). In the acute dependence assay, naloxone and nalmefene precipitated significant withdrawal jumping at doses that blocked acute effects of morphine. As expected, higher doses of nalmefene elicited further increases in withdrawal jumping, and these behaviors were not observed in opioid-naïve or saline treated animals. The other indices of withdrawal were not robust enough in this acute opioid exposure paradigm to allow for discrimination between doses. In the more severe chronic exposure assay, the nalmefene dose-response curve displayed the characteristic leftward shift in potency observed with other opioid inverse agonists (e.g., naloxone and naltrexone). Significantly lower doses of nalmefene were required to precipitate moderate to severe withdrawal. The higher level of dependence/withdrawal also allowed for discrimination of dose effects on some of the other behavioral indices (bodyweight loss, number of feces, etc.).
The onset of CNS antagonist blockade for the compounds being characterized can be measured and compared if the testing conditions remain consistent across assessments. We have used a modified locomotor assay that increases the temporal resolution of blockade by measuring one-versus 5-minute bins of activity. To compare compounds we have determined equivalent antagonist potencies to reverse morphine-induced locomotion. Using these equivalent doses, we then looked at how quickly a fixed dose of morphine could be reversed (time to half-maximal morphine effect). Nalmefene produced reversal of morphine-induced hyperlocomotion with an onset that was slightly slower than naltrexone and naloxone (approximately 5 versus 3 minutes) and equivalent to 6β-naltrexol. Taken together with the dependence/precipitated withdrawal data (see below), the time to half-maximal reversal does not appear to explain the propensity of opioid antagonists to precipitate withdrawal (e.g., a slower onset antagonist such as nalmefene produced a similar level of withdrawal compared to equivalent antagonist doses of naloxone and naltrexone). This would argue against the possibility that the observed differential effects of 6β-naltrexol and naloxone/naltrexone precipitation of withdrawal is due to its differential onset of receptor antagonist action (Divin et al. 2008).
A limitation of the current methodology for assessing the rate of CNS antagonism of opioid effects is that it relied on just the locomotor assay. The locomotor assay can be set with a temporal resolution (one minute or less) that is not feasible with the tail-flick assay. Nevertheless, the estimates we have been able to obtain with the antagonists tested (current studies and Raehal et al. 2005) have been consistent between in vitro and in vivo assays, as well as with some measures of human potency (Glass et al. 1994). The behaviors measured (opioid-induced locomotor activity and antinociception in the 55°C tail-flick) are CNS mediated and likely involve systems relevant to addiction liability, tolerance and physical dependence. Furthermore, we examined the antagonist properties of nalmefene under a variety of conditions including (a) pre-treatment to prevent morphine antinociception, (b) co-administration to block morphine-induced locomotion, and (c) reversal of an already established morphine effect (locomotor activity). Additional studies are clearly needed to fully understand the complex agonist/antagonist interactions that occur in vivo in opioid naïve and dependent states, but the current studies add to our understanding of the pharmacology of a structurally similar yet distinct set of opioid antagonists under a set of standardized conditions.
Conclusion
In summary, we have provided a more complete characterization of the in vivo pharmacology of nalmefene with respect to its antagonist properties and propensity to precipitate withdrawal. The studies were designed to extend previous work with naloxone, naltrexone and 6β-naltrexol, allowing us to compare between these compounds in an effort to better understand the structure-activity relationships of opioid inverse agonists and neutral antagonists. The reversal of morphine activity was significantly slower than with naloxone or naltrexone and equivalent to 6β-naltrexol. The severity of withdrawal with nalmefene was, however, more in line with that of the inverse agonists, arguing that time of antagonist entry into the CNS is unlikely to explain differences observed between 6β-naltrexol and the other compounds.
Acknowledgements
The authors acknowledge the generous support of the NIDA Drug Supply program for providing naloxone HCl and to Alan Benson and Mallinckrodt Inc. for supplying nalmefene HCl. The authors also thank Dr. Glenn Stevenson for help with the preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bilsky EJ, Bernstein RN, Wang Z, Sadée W, Porreca F. Effects of naloxone and D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2 and the protein kinase inhibitors H7 and H8 on acute morphine dependence and antinociceptive tolerance in mice. Journal of Pharmacology and Experimental Therapeutics. 1996;277(1):484–490. [PubMed] [Google Scholar]
- Blasig J, Herz A, Reinhold K, Zieglgansberger S. Development of physical dependence on morphine in respect to time and dosage and quantification of the precipitated withdrawal syndrome in rats. Psychopharmacologia. 1973;33(1):19–38. doi: 10.1007/BF00428791. [DOI] [PubMed] [Google Scholar]
- Bokoch MP, Zou Y, Rasmussen SG, Liu CW, Nygaard R, Rosenbaum DM, Fung JJ, Choi HJ, Thian FS, Kobilka TS, Puglisi JD, Weis WI, Pardo L, Prosser RS, Mueller L, Kobilka BK. Ligand-specific regulation of the extracellular surface of a G-protein-coupled receptor. Nature. 2010;463(7277):108–112. doi: 10.1038/nature08650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divin MF, Bradbury FA, Carroll FI, Traynor JR. Neutral antagonist activity of naltrexone and 6beta-naltrexol in naïve and opioid-dependent C6 cells expressing a mu-opioid receptor. British Journal of Pharmacology. 2009;156(7):1044–1053. doi: 10.1111/j.1476-5381.2008.00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto JM, Roerig S, Wang RI, Chatterjie N, Inturrisi CE. Narcotic antagonist activity of several metabolites of naloxone and naltrexone tested in morphine dependent mice. Proceedings for the Society of Experimental Biology and Medicine. 1975;148(2):443–448. doi: 10.3181/00379727-148-38558. [DOI] [PubMed] [Google Scholar]
- Glass PS, Jhaveri RM, Smith LR. Comparison of potency and duration of action of nalmefene and naloxone. Anesthesia and Analgesia. 1994;78(3):536–541. doi: 10.1213/00000539-199403000-00021. [DOI] [PubMed] [Google Scholar]
- Grant JE, Potenza MN, Hollander E, Cunningham-Williams R, Nurminen T, Smits G, Kallio A. Multicenter investigation of the opioid antagonist nalmefene in the treatment of pathological gambling. American Journal of Psychiatry. 2006;163(2):303–312. doi: 10.1176/appi.ajp.163.2.303. [DOI] [PubMed] [Google Scholar]
- Jannsen PAJ, Niemegeers CJE, Dorg JGH. The inhibitory effects of fentanyl and other morphine-like analgesics on the warm water-induced tail withdrawal reflex in rats. ArzneimForsch. 1963;13:502–505. [PubMed] [Google Scholar]
- Kaplan JL, Marx JA, Calabro JJ, Gin-Shaw SL, Spiller JD, Spivey WL, Gaddis GM, Zhao N, Harchelroad FP., Jr Double-blind, randomized study of nalmefene and naloxone in emergency department patients with suspected narcotic overdose. Annals of Emergency Medicine. 1999;34(1):42–50. doi: 10.1016/s0196-0644(99)70270-2. [DOI] [PubMed] [Google Scholar]
- Karhuvaara S, Simojoki K, Virta A, Rosberg M, Löyttyniemi E, Nurminen T, Kallio A, Mäkelä R. Targeted nalmefene with simple medical management in the treatment of heavy drinkers: a randomized double-blind placebo-controlled multicenter study. Alcoholism: Clinical and Experimental Research. 2007;31(7):1179–1187. doi: 10.1111/j.1530-0277.2007.00401.x. [DOI] [PubMed] [Google Scholar]
- Kest B, Palmese CA, Hopkins E, Adler M, Juni A, Mogil JS. Naloxone-precipitated withdrawal jumping in 11 inbred mouse strains: evidence for common genetic mechanisms in acute and chronic morphine physical dependence. Neuroscience. 2002;115(2):463–469. doi: 10.1016/s0306-4522(02)00458-x. [DOI] [PubMed] [Google Scholar]
- Kjellberg F, Tramèr MR. Pharmacological control of opioid-induced pruritus: a quantitative systematic review of randomized trials. European Journal of Anaesthesiology. 2001;18(6):346–357. doi: 10.1046/j.0265-0215.2000.00826.x. [DOI] [PubMed] [Google Scholar]
- Klein G, Juni A, Arout CA, Waxman AR, Inturrisi CE, Kest B. Acute and chronic heroin dependence in mice: contribution of opioid and excitatory amino acid receptors. European Journal of Pharmacology. 2008;586(1–3):179–188. doi: 10.1016/j.ejphar.2008.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason BJ, Salvato FR, Williams LD, Ritvo EC, Cutler RB. A double-blind, placebo-controlled study of oral nalmefene for alcohol dependence. Archives of General Psychiatry. 1999;56(8):719–724. doi: 10.1001/archpsyc.56.8.719. [DOI] [PubMed] [Google Scholar]
- Pellegrini JE, Bailey SL, Graves J, Paice JA, Shott S, Faut-Callahan M. The impact of nalmefene on side effects due to intrathecal morphine at cesarean section. AANA Journal. 2001;69(3):199–205. [PubMed] [Google Scholar]
- Raehal KM, Lowery JJ, Bhamidipati CM, Paolino RM, Blair JR, Wang D, Sadée W, Bilsky EJ. In vivo characterization of 6β-naltrexol, an opioid ligand with less inverse agonist activity compared with naltrexone and naloxone in opioid-dependent mice. Journal of Pharmacology and Experimental Therapeutics. 2005;313(3):1150–1162. doi: 10.1124/jpet.104.082966. [DOI] [PubMed] [Google Scholar]
- Sadée W, Wang D, Bilsky EJ. Basal opioid receptor activity, neutral antagonists, and therapeutic opportunities. Life Sciences. 2005;76(13):1427–1437. doi: 10.1016/j.lfs.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Sirohi S, Dighe SV, Madia PA, Yoburn BC. The relative potency of inverse opioid agonists and a neutral opioid antagonist in precipitated withdrawal and antagonism of analgesia and toxicity. Journal of Pharmacology and Experimental Therapeutics. 2009;330(3):513–519. doi: 10.1124/jpet.109.152678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Raehal KM, Bilsky EJ, Sadée W. Inverse agonists and neutral antagonists at mu opioid receptor (MOR): possible role of basal receptor signaling in narcotic dependence. Journal of Neurochemistry. 2001;77(6):1590–1600. doi: 10.1046/j.1471-4159.2001.00362.x. [DOI] [PubMed] [Google Scholar]
- Wang D, Raehal KM, Lin ET, Lowery JJ, Kieffer BL, Bilsky EJ, Sadée W. Basal signaling activity of mu opioid receptor in mouse brain: role in narcotic dependence. Journal of Pharmacology and Experimental Therapeutics. 2004;308(2):512–520. doi: 10.1124/jpet.103.054049. [DOI] [PubMed] [Google Scholar]
- Wang DS, Sternbach G, Varon J. Nalmefene: a long-acting opioid antagonist. Clinical applications in emergency medicine. Journal of Emergency Medicine. 1998;16(3):471–475. doi: 10.1016/s0736-4679(98)00019-5. [DOI] [PubMed] [Google Scholar]