Abstract
Survival of altricial infants, including humans and rats, depends on attachment to the caregiver – a process that requires infants to recognize, learn, and remember their attachment figure. The demands of a dynamic environment combined with a maturing organism requires frequent neurobehavioral reorganization. This restructuring of behavior and its supporting neural circuitry can be viewed through the unique lens of attachment learning in rats in which preference learning is enhanced and aversion learning is attenuated. Behavioral restructuring is well adapted to securing the crucial infant-caregiver relationship regardless of the quality of care. With maturation and the end of the infant-caregiver attachment learning period, the complex interplay of neural structures, hormones, and social behavior coordinates the developing rat’ s eventual transition to life outside of the nest. Nevertheless, early-life environmental and physiological stressors can alter the resilient nature of this system, particularly in respect to the amygdala, and these changes may provide important clues to understanding the lasting effects of early stress.
“…development is essentially a dynamic process that promotes reorganization and adaptation across time” (S. Levine, 1982)
The maturing organism has the daunting task of frequently reorganizing behavior to meet not only the demands of a changing environment, but also those of physiological and neural maturation. The myriad processes by which an organism reorganizes its behavior are not well understood, but are thought to include complex interactions between experience, learning, as well as genetic and neural changes. Of course, each reorganization is likely to involve unique processes and can occur either rapidly or gradually. These periods of reorganization, which are also referred to as developmental transitions, are believed to represent periods of vulnerability and have received more experimental attention in recent years (for review see: Adriani and Laviola, 2004; Crews et al. 2007; Hensch, 2004; Hofer and Sullivan, 2008; Rice and Barone, 2000; Sullivan et al., 2009). However, Levine and others have long highlighted the importance of these transitions and the critical role of proper reorganization for normal development (Bell and Denenberg, 1962; Denenberg, 1963; Levine, 1982, 2000).
The infant’s first reorganization occurs with birth as the newborn transitions from an intrauterine to extrauternine life. The familiar rhythmic and warm intrauterine environment is replaced with an environment filled with new sensory experiences. New behaviors – some of which have been practiced in utero – now become critical for survival, including the young animals learning to identify the caregiver, approaching the caregiver and exhibiting the behaviors necessary for survival such as grasping the nipple and nursing. Equally important, however, are the social behaviors the infant must exhibit in order to elicit nurturing from the caregiver and begin the complex interchange with the mother as the process of attachment learning begins. In altricial animals, this survival-dependent learning is typically supported by specialized learning processes early in life, which are referred to as imprinting and are temporally limited to a sensitive period (Bolhuis and Honey, 1998; Hess 1962; Insel and Young, 2001; Moriceau and Sullivan, 2004b; Rajecki et al. 1978; Salzen 1970; Sullivan et al. 2000a). This unique and rapid learning provides an opportunity to question how behavioral and neural transitions occur to support this critical social learning. This attachment learning, which requires a brief learning experience with the specific sensory qualities of the attachment figure (scent, texture, color, sound) during infant-caregiver interactions, is widespread phylogenetically and results in the common behavioral outcome of proximity seeking to the caregiver by the infant (Blass and Teicher, 1980; DeCasper and Fifer, 1980; Hennessy et al. 1980; Hess, 1962; Insel and Young, 2001, Polan and Hofer, 1998). However, this learning also requires the suppression of learning that could interfere with the proximity seeking (Blozovski and Cudennec, 1980; Camp and Rudy, 1988; Collier et al. 1979; Haroutunian and Campbell, 1979; Hess, 1962; Myslivecek, 1997; Stehouwer and Campbell, 1978; Sullivan et al. 2000a, 2009). In this review, we will outline the behavioral neurobiology of attachment learning in the infant rat and the unique role of corticosterone in controlling the termination of this sensitive period, as well as corticosterone’s ability to suppress temporarily attachment learning during the pups’ sensitive period. Research by Levine and his talented students and colleagues is critical for placing this work in our current conceptual framework.
Attachment learning in infant rats
Rat pups must learn the mother’s odor for survival. Since pups do not see or hear until almost the third week of life, this odor provides distal information to enable pups to approach the caregiver. Importantly, the odor also provides proximal information that enables pups to exhibit the behaviors necessary for procuring food and warmth from the mother. Any neutral odor can acquire the properties of the maternal odor simply by placing the odor (i.e. peppermint or citral) on the mother or in the cage during mother-infant interactions (Alberts and May, 1984; Caza and Spear, 1984; Duveau and Godinot, 1988; Galef, 1982; Galef and Kaner, 1980; Sullivan et al. 1990). Since the rat mother’s odor is dependent upon her diet, pups must repeatedly relearn her changing odor (Leon 1975, 1992). This infant rat odor attachment learning is also modeled outside the nest using a classical conditioning paradigm in which a novel odor is paired with either presumably pleasant stimuli (tactile stimulation/stroking, milk, or warmth: Brake 1981; Galef and Sherry, 1973; Johanson and Hall, 1979; Johanson and Teicher, 1980; McLean et al. 1993; Pederson et al. 1982; Sullivan et al. 1986, 1989, 1991; Weldon et al. 1991; Wilson and Sullivan, 1994). The maternal odor, whether naturally or artificially learned through experimentation, elicits proximity seeking/ approach responses in pups (distal cue) but also pups’ contact with the mother and nipple attachment (proximal cues). Without the maternal odor, pups show greatly diminished contact with the mother, fail to nipple attach and exhibit low survival rates (Raineki et al. submitted; Singh and Tobach, 1975). This infant attachment learning is unique in its acquisition and has been characterized as mammalian imprinting, which is similar to the rapid learning that occurs during sensitive period avian imprinting (Bolhuis and Honey, 1998; Hofer and Sullivan, 2008).
While infant sensitive periods are well known for enhanced learning, suppression of learning that could interfere with attachment also occurs. Indeed, attenuation of fear and avoidance was suggested as a critically important characteristic of attachment learning decades ago as imprinting and attachment was first conceptualized (Bowlby, 1969; Hess, 1962). For example, shocking chicks during imprinting for attachment elicits approach learning rather than aversion learning (Hess 1962; Rajecki et al. 1978; Salzen 1970). Even in mammals, such as dogs and nonhuman primates, a caregiver treating the young harshly still supports attachment and elicits attachment learning (Harlow and Harlow, 1965; Maestripieri et al. 1999; Sanchez et al. 2001; Stanley, 1962; Suomi, 2003). Indeed, learning to avoid or fear the source of food and protection (caregiver) would not be ideal for survival and we have suggested that it is better to form a repertoire of proximity-seeking behaviors to the primary caregiver, regardless of the quality of the care received (Hofer and Sullivan, 2008).
This suppression of fear/avoidance learning has also been demonstrated in rat pups still in the sensitive period for attachment learning. Specifically, pairing a novel odor with a painful stimulus, such as 0.5mA shock or tailpinch (rather than the typical milk or stroking), results in pups approaching the odor when it is next encountered (Camp and Rudy, 1988; Haroutunian and Campbell, 1979; Sullivan et al. 1986). Notably, this preferred odor induced through pain pairings also takes on the ability to control the constellation of pup behaviors normally controlled by the natural maternal odor (Figure 1; approach, social behavior with the mother and nipple attachment: Raineki et al. submitted).
Figure 1.
During a sensitive period, pups approach the natural maternal odor or odors paired with stroking or painful 0.5mA shock as demonstrated by a Y- Maze test (A). Pups cannot nipple attach when the maternal odor is removed, although nipple attachment can be reinstated if the maternal odor, or a classically conditioned odor is presented via an air stream into the testing chamber (B). The natural and artificial maternal odors produce enhanced olfactory bulb responding, as shown here with c-Fos immunohistochemistry (C). Note that mere experience with a novel odor or unpaired presentations of the novel odor and reward does not support learning and does not produce the behavioral changes. Asterisk indicates p<0.05.
The inability of pups to exhibit fear/avoidance learning with pain-odor pairings is not due to pups’ developmental differences in pain detection. Aversive stimuli elicit escape responses from pups and pain threshold to shock does not appear to change as shock switches from supporting preference to supporting aversion learning (Barr, 1995; Collier and Bolles, 1980; Emerich, et al. 1985; Fitzgerald, 2005; Stehouwer and Campbell, 1978). The functional significance of pups’ suppressed aversion/fear learning may be due to the rat pup occasionally experiencing some pain from the mother. Specifically, as the mother leaves the nest to tend to her needs, she drags pups still attached to her nipples across the nest and steps on others. She may also retrieve pups by a limb rather than the nape of the neck. All of these interactions induce pain-related vocalizations from the pups (Hofer and Shair, 1978). Learning an aversion to maternal odors associated with this rough treatment would jeopardize pups’ approach responses to warmth, nutrition, safety and ultimately, survival.
While odor approach/preference learning with pain seems paradoxical, this limitation on aversive learning is seen in species other than rats and is important for attachment. For example, during imprinting in chicks, shock enhances following behavior of the mother, although an aversion is learned if the shocks are presented after the sensitive period has ended (Hess, 1962; Salzen, 1970). Moreover, these learning limitations have also been documented in infant dogs that continue to approach a human caregiver that shocks or handles the puppies roughly (Rajecki et al. 1978). The most dramatic demonstration of infant attachment to an abusive caregiver is seen in nonhuman and human primates that continue to approach a caregiver despite rough handling, sometimes resulting in physical injury to the infant (Carlson et al. 1989; Harlow and Harlow, 1965; Maestripieri et al. 1999; Sanchez et al. 2001; Suomi, 2003). In young rat pups, this limitation on learning also extends to inhibitory conditioning and passive avoidance (Blozovski and Cudennec, 1980; Collier et al. 1979; Myslivecek, 1997; Stehouwer and Campbell, 1978).
Neurobiology of attachment learning in infant rat pups
Considerable progress has been made in delineating the neural changes that accompany odor attachment learning in infant rats. The maternal odor, whether natural or artificial (placed on the mother or ingested), as well as odors learned through controlled classical conditioning studies all produce an enhanced response in the olfactory bulb (Figure 1). This has been demonstrated using a variety of neural assessment techniques including 2-deoxyglucose (2-DG) uptake, c-Fos immunohistochemistry, electrophysiology, pCREB, optical imaging (Coopersmith et al. 1986, Johnson et al. 1995; McLean et al. 1999; Roth and Sullivan, 2005; Roth et al. 2006; Sullivan and Leon 1986; Sullivan et al. 1989; Wilson et al. 1987; Yuan et al. 2003a,b). This plasticity relies on high levels of norepinephrine (NE), which prevents habituation of the olfactory bulb’s mitral cells during conditioning and permits plasticity (Langdon et al. 1997; Moriceau and Sullivan 2004b; Okutani et al. 2003; Sullivan et al. 1991, 1992, 2000b; Wilson and Leon 1988; Yuan et al. 2000). The elevated olfactory bulb NE levels are present during the sensitive period due to a hyperfunctioning locus coeruleus (LC), which fails to show recurrent collateral inhibition to stop LC firing and decrease NE output. The LC alpha2 receptors’ autoinhibition becomes functional around 10 days of age (Nakamura and Sakaguchi, 1990; Rangel and Leon, 1995; Winzer-Serhsn and Leslie, 1999), which changes the LC’s responding from 20–30 seconds to just milliseconds and appears to end this period of heightened learning (Moriceau and Sullivan, 2004b; Sullivan et al. 1994; 2000b). Another brain area altered by sensitive period attachment learning is the anterior piriform cortex (Moriceau et al. 2006; Raineki et al. 2009; Roth and Sullivan, 2005), although it is likely other brain areas will emerge with additional exploration.
As we began to explore why pups failed to learn to avoid/fear odors paired with pain, such as 0.5mA shock or being stepped on by the mother, we focused on the amygdala because of its well-documented importance in this type of learning in adults (Fanselow and Gale, 2003; Fanselow and LeDoux, 1999; LeDoux, 2003; Sananes and Campbell, 1989; Schettino and Otto, 2001; Sevelinges et al. 2004). We found that the amygdala does not participate in the infant odor-0.5mA shock learning until pups switch from preference learning to odor avoidance learning (Sullivan et al. 2000a). Specifically, the fear conditioning paradigm of odor-0.5mA shock conditioning produces an odor preference in young rat pups (less than 10 days of age, i.e. sensitive period) but an odor aversion and freezing in older pups, which is similar to that seen in adults. This sensitive period odor-0.5mA shock conditioning supporting odor preference learning is unaltered by suppression of the amygdala via muscimol infusion in sensitive period pups, although this same manipulation blocks fear learning in older pups (Moriceau et al. 2006; Moriceau and Sullivan, 2006). Thus, while this odor-shock procedure activates the same neural circuit as odor learning using stimuli such as milk and stroking (olfactory bulb, anterior piriform cortex, LC), it also requires suppression of the amygdala’s plasticity. Though we originally hypothesized that amygdala immaturity (Berdel and Morys, 2000; Berdel et al. 1997; Bouwmeester et al. 2002; Cunningham et al. 2002; Morys et al. 1999; Nair and Gonzalez-Lima, 1999) was responsible for its lack of plasticity, electrophysiological studies indicate the pain and odor information reach the amygdala during the sensitive period (Thompson et al. 2008), yet the amygdala fails to exhibit the plasticity required for fear learning. As will be noted below, corticosterone has a significant role in suppressing the amygdala’s plasticity in sensitive period pups and suggests the amygdala is sufficiently mature to exhibit plasticity provided corticosterone is slightly elevated.
One of Levine’s enduring legacies is his assertion that “‘the neonate plays by different rules than the adult.’ Often, the assumption is made that the neonate is a miniature adult. When many of the procedures used to study adult organisms are applied to neonates, the results obtained are frequently inaccurate and generate erroneous conclusions” (Levine, 2000). Likewise, the neural circuitry supporting infant learning can be mistakenly viewed simply from the perspective of the adult literature. As highlighted before, though, infant learning is essentially supported by the olfactory bulb, LC and the anterior piriform cortex rather than by the amygdala, which is the crucial structure supporting fear conditioning learning in the adult. With that being said, the infant learning circuitry is not necessarily the result of an immature brain; rather, it has been molded by evolution to support the unique sensitive period attachment learning that allows the infant to attach to the caregiver at all costs. That is, from an adaptive point of view, perhaps it is better for an altricial animal to remain attached to an abusive caregiver than receive no care (Moriceau and Sullivan, 2005; Sullivan et al., 2009)
Natural endogenous increase in the stress hormone corticosterone ends the sensitive period
During the sensitive period, infant rats’ CORT levels are relatively low and fail to show the stress-induced corticosterone release typical of older pups and adults (Butte et al. 1973; Cate and Yasumura, 1975; Dallman, 2000; Grino et al. 1994; Guillet and Michaelson, 1978; Guillet et al. 1980; Henning, 1978; Levine, 1962, 1967, 2001; Walker et al. 1986; Walker and Vrana, 1993). This period of reduced hypothalamic-pituitary-adrenal (HPA) axis responsiveness during neonatal development has been termed the “stress hypo-responsive period” (SHRP). Interestingly, the HPA axis is functional at birth (Martin et al. 1977) and the sensory stimulation provided by the mother during nursing and grooming seems to control the pups’ low CORT levels (Levine, 1962; Stanton and Levine, 1990; van Oers et al. 1998). Indeed, prolonged maternal separation increases pups’ CORT levels, which can be returned to normal low levels with replacement of maternal sensory stimulation or maternal presence. This neonatal reduced corticosterone level is hypothesized to protect the developing brain from the negative influences of stress hormones (Bohn, 1980; Erkine et al. 1979; Sapolsky and Meaney, 1986) as well as to preserve attachment learning, which is described here.
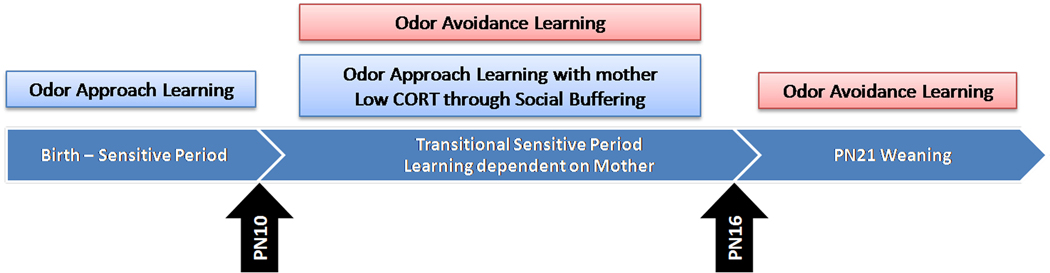
Corticosterone levels gradually increase in infant rats, although a critical level of corticosterone is naturally reached by 10 days of age to prevent attachment learning and permit amygdala-dependent avoidance/fear learning (Moriceau et al. 2004; Moriceau and Sullivan, 2004a, 2006, see Figure 2). A causal link between corticosterone levels, avoidance learning and the amygdala was established through intra-amygdala corticosterone (Moriceau et al. 2004; Moriceau and Sullivan, 2004a, 2006), which was modeled after Takahshi's work on the importance of corticosterone and emergence of fear to predator odor (Moriceau et al. 2004; Takahashi, 1994; Wiedenmayer et al. 2003). Specifically, increasing CORT by systemic (dose 3mg/Kg) injections or by intra-amygdala (dose 50–100ng) infusions during 0.5mA odor-shock conditioning is sufficient to elicit both a fear response and amygdala participation in the sensitive period conditioning (Moriceau et al. 2004; Moriceau and Sullivan, 2004a, 2006). Furthermore, at least during the days following sensitive period termination, decreasing pups’ corticosterone (systemic – adrenalectomy or maternal presence, intra-amygdala - mifepristone) is capable of reinstating the attachment learning and preventing fear learning (Moriceau et al. 2006; Moriceau and Sullivan, 2006).
Figure 2.
Pup attachment learning changes over development. During the earliest days of life, pups have a sensitive period when odor-shock conditioning produces an odor preference. At 10-days of age, pups begin the Transitional Sensitive Period, when pups endogenous corticosterone levels have increased sufficiently to enable amygdala dependent fear/avoidance learning. However, with the mother present at this age pups will revert back to the approach learning of the sensitive period. That is, the mother’s presence will socially buffer pups (i.e. attenuate pups shock-induced corticosterone release) and pups learn a preference. As pups mature, maternal social buffering continues to lower corticosterone but pups only have access to the amygdala dependent fear learning (Moriceau and Sullivan 2006; Raineki et al. 2009; Shionoya et al. 2007; Sullivan et al., 2000a).
Postsensitive period: corticosterone reduction permits attachment learning and suppresses fear learning
As we published the result demonstrating the ability of amygdala corticosterone to terminate pups’ sensitive period for attachment learning, we recalled research from the Levine laboratory indicating that maternal presence could block pups’ stress-induced corticosterone release (Stanton and Levine, 1990; Stanton et al. 1987; Suchecki et al. 1993). The ability of a social stimulus to buffer or attenuate the release of stress-induced corticosterone release is referred to as social buffering. This phenomenon occurs in many species (review: Kikusui et al. 2006). For example, humans’ social affiliation blunts stress-induced CORT release (Kirschbaum et al. 1995; McCloskey et al. 1995; Thorsteinsson and James, 1999) as does maternal presence in adolescent guinea pigs, peers in nonhuman primates, and mate presence in voles (Carter and Keverne, 2002; DeVries et al. 2003; Hennessy et al. 1995, 2002, 2009; Kikusui et al. 2006) and can be heightened or attenuated during different periods of development, such as the postpartum period in rats (Deschamps et al. 2003; Shanks et al. 1999; Toufexis and Walker, 1996; Walker et al. 2004).
Thus, due to the importance of corticosterone in infant rat odor-shock conditioning, we assessed whether the natural attenuation of pups’ stress-induced corticosterone release via social buffering would also alter pups’ learning. We found dramatic effects (Moriceau and Sullivan, 2006). Specifically, pups were given a 45 min odor-0.5mA shock conditioning session with or without an anesthetized mother at one of three ages. Pups were then returned to the nest and the next day given a Y-Maze test (conditioned vs. familiar odor choice) to determine odor preference or aversion learning. Sensitive period pups learned to approach the odor regardless of maternal presence. That is, only the sensitive period pups demonstrated attachment learning, presumably because shock does not increase corticosterone and the pups have no stress response to be socially buffered by the mother. Postsensitive Period pups (12 to 14 days of age for this experiment) learned to avoid the odor if conditioned without the mother, but reverted to the odor preference learning if the mother was present. The oldest pups (weaning age) learned to avoid the odor and maternal presence was without effect. The postsensitive period pups and the weanling pups both show a socially buffered shock-induced corticosterone release, but only the younger pups have their fear learning switched to attachment learning by maternal presence.
The ecological significance of the ability of maternal presence to determine pups’ learning may be explained by the changing environment and requisite demands on the pup. During the sensitive period, pups are confined to the nest and depend solely on the mother for nourishment and protection. During this period, the unique neural circuitry of infant pups ensures attachment to the caregiver, despite possible negative stimuli, such as those experienced when the mother moves around the nest, for example stepping on or roughly transporting pups. As the pups mature and become more independent, though, the circuitry continues to change to support the new learning situations the pup will face during the transition to life outside of the nest. Pups are still dependent on the mother for care, though, so they must have the processing capability to differentiate between innocuous negative stimuli and true threats to their survival. As the pup reaches weaning age, it no longer depend on the mother for survival and can begin to leave the nest and explore the outside world. This new independence is facilitated by further changes in the brain that support more adult-like fear learning. Specifically, as pups take on more adult challenges, the underlying adult neural circuitry of these behaviors is functional in the learning of stimuli outside of the nest.
As we explored potential mechanisms for the mother’s ability to socially buffer pups’ stress response, intriguing data on social buffering in postpartum rats was critical in guiding our work. In short, research shows that postpartum mothers exhibit a SHRP corticosterone response when away from their pups, yet a robust corticosterone release to stressor when with pups (Deschamps et al. 2003; Shanks et al. 1999; Toufexis and Walker, 1996; Walker et al. 2004). The Walker lab has shown that pups’ presence controls mothers SHRP through norepinephrine (NE) in the paraventricular nucleus of the hypothalamus (PVN), which is a brain area critical in integrating diverse stressors and enables the organism to have context specific neural and behavioral responses to stressors (review Lupien et al. 2009). Specifically, when the mother is with her pups, the PVN NE exhibits a robust response to stressors to initiate activation of the HPA axis and produce corticosterone release. However, without the pups presence, mothers show an attenuated PVN NE response to stressors and attenuated corticosterone release. As is outlined below, we found a similar control of pup’s PVN NE and corticosterone release by maternal presence.
As we assessed the mechanism for the mother’s ability to socially buffer her pups, we found that during odor-shock conditioning in postsensitive period pups, maternal social buffering of pups’ stress response occurs through attenuation of PVN neural activity (2-DG) and suppression of PVN NE (Table 1; Shionoya et al. 2007). Specifically, using microdialysis, we found that odor shock conditioning induced a large increase in PVN NE in PN12 14 rats, although this NE surge is blocked with maternal presence. Importantly, we could override this social buffering by infusing NE into the PVN, which initiated the activation of the HPA axis and induced fear learning. Thus, while the mother has an active stress system with the pups and pups have an active stress system away from the mother, the neural control appears similar.
Table 1.
Postsensitive period pups learn an aversion from odor-shock conditioning if conditioned away from the mother. The shock produces an increase in PVN NE, which begins activation of the HPA axis and corticosterone release from the adrenal gland. However, if the postsensitive period pups are conditioned with maternal presence (anesthetized mother or the maternal odor), shock fails to increase corticosterone by preventing PVN NE increase (Shionoya et al. 2007).
| Odor-0.5mA shock conditioning in Postsensitive Period pups (10 to 15 days old) | ||
|---|---|---|
| Maternal Presence | Without Maternal Presence | |
| Learning | Odor Preference/Attachment | Odor Aversion/Fear |
| PVN Neural Activity | Attenuated | Elevated |
| PVN NE | Attenuated | Elevated |
| CORT Release | No | Yes |
| Amygdala Plasticity | No | Yes |
Age limits of corticosterone’s ability to control odor preference/avoidance learning
Corticosterone plays a modulatory role in adult fear and avoidance conditioning by increasing or deceasing learning strength (Corodimas et al. 1994; Hui et al. 2004; Pugh et al. 1997; Roozendaal et al. 1996, 2002; Thompson et al. 2004); however, the ability of corticosterone levels to switch between preference and avoidance learning in odor-shock conditioning is unique to pups. Thus, in our next experiments, we defined the age range corticosterone alters sensitive period learning (Upton and Sullivan, submitted). Again, pups were odor-0.5mA shock conditioned with either corticosterone increase at 5 and 6 days of age (injection of corticosterone) or decrease at 15 and 16 days of age (naturally by maternal presence or protein synthesis blocker, Metyrapone). Our results indicate that the age limits for corticosterone’s control of attachment/fear learning are 6 through 15 days of age. We suggest that this age limit protects pups from learning an aversion to the maternal odor while pups are dependent on maternal care and ends when survival without the mother is possible (Greenberg and Ackerman, 1986; Ito et al. 2006; Kikusui and Mori, 2009). We also suggest that pups 5 days of age and younger do not have an amygdala sufficiently mature to support avoidance learning (Berdel and Morys, 2000; Berdel et al. 1997; Bouwmeester et al. 2002; Cunningham et al. 2002; Morys et al. 1999; Nair and Gonzalez-Lima, 1999). Indeed, odor avoidance learning from shock and malaise (1mA and above or LiCl) in this age relies on neural changes within the olfactory bulb and posterior piriform cortex and not the amygdala (Raineki et al. 2009; Shionoya et al. 2006). Together these results suggest that the protective effects of corticosterone on pup learning are finely tuned to the changing demands of pups ecological niche and maturation.
Acute effects of early life stress: Disruption of attachment learning and social behavior
Early life trauma and stress alter pups’ behaviors. For example, rearing pups with a stressed mother increases their corticosterone levels to abnormally high levels. This was elegantly demonstrated in a series of experiments from the Baram lab. Insufficient bedding can stress the mother and elevate her corticosterone level. Baram used this to develop a naturalistic stress paradigm, where mothers are provided with insufficient bedding to build a nest, which results in repeated nest building behaviors and transporting pups to the new nest. This produces an increase in rough handling of pups, but also a decrease in normal maternal behaviors such as grooming and nursing, although pups still gain weight normally (Avishai-Eliner et al. 2001; Gilles et al. 1996; Moriceau et al. submitted; Raineki et al. submitted; Roth and Sullivan, 2005; see Table 2). This procedure increases pups’ corticosterone levels because of elevated release from their adrenal glands, although the mother also delivers her high corticosterone levels to the pups through her milk (Yeh, 1984). This paradigm also changes gene expression throughout the HPA axis and within the frontal cortex (Avishai-Eliner et al. 2001; Gilles et al. 1996; Hatalski et al. 1998). In the short run (less than a day), this procedure does not interfere with pups attachment learning or its supporting neurobiology (Roth and Sullivan, 2005). However, we questioned whether this precocious increase in corticosterone from more extended experience with the mother stressed with low bedding would disrupt attachment learning and the normal social behaviors pups exhibit to the mother. We reared pups using Baram’s insufficient bedding procedure and compared them to normally reared pups from birth to 6 days of age. At 7 days of age, pups were odor-shock conditioned and then tested in a Y-maze to assess whether early life stressful rearing would permit precocious amygdala-dependent fear learning. Indeed, while the normally reared pups showed the age typical odor preference learning, pups reared in the Baram stress paradigm learned an amygdala-dependent odor aversion due to the increased corticosterone levels (Moriceau et al. in press). These results suggested that experience with a stressed mother prematurely ends the SHRP and the sensitive period for attachment learning (Moriceau et al. submitted).
Table 2.
Providing a mother with insufficient bedding to build a nest is stressful and changes maternal behavior compared to mother’s given sufficient bedding. Maternal behavior was observed for 1 hour. While both mothers showed similar levels of typical maternal behaviors, such as licking and nursing, the stressed mother was more likely to engage in behaviors that are painful to pups, such as stepping on the pup and handling the pup roughly. Frequency of behaviors observed during training sessions. From Roth and Sullivan, 2005.
| Percent of observation periods in which behaviors occurred | ||
|---|---|---|
| Stressed-mother | Typical-mother | |
| Behaviors | ||
| Abusive | ||
| Step or jump on | 41.9% | 1.4% |
| Throw | 1.0% | 0% |
| Drop | 6.7% | 0% |
| Drag | 8.6% | 0% |
| Push away/avoid | 14.3% | 0% |
| Rough handling | 24.8% | 5.7% |
| Pup vocalization | 44.8% | 2.9% |
| Normal Maternal | ||
| Retrieve | 36.2% | 37.1% |
| Lick/Anogenital Lick | 28.6% | 71.4% |
| Nurse | 19.0% | 27.1% |
Enduring effects of early life stress
The important role of the early environment for brain and behavioral development and its enduring impact throughout the lifespan has been demonstrated in clinical studies (Bremner, 2003; Gunnar et al., 2009; Kaufman et al. 2000; Nemeroff, 2004; Rutter, 2006; Stein and Kendall, 2004; Teicher et al. 2003), as well as basic research in other species (Bell and Denenberg, 1962; Branchi, 2009; Coe et al., 1983; O’Connor and Cameron 2006; Denenberg, 1963; Harlow and Harlow, 1965; Jordan et al., 1985; Levine, 1952, 1967, 2001; Macri and Wurbel, 2006; Meaney et al. 1988; Rosenzweig et al. 1969; Sackett, 1972; Suchecki and Tufik, 2000: Tang et al., 2006; Weaver et al. 2000). The importance of these early life experiences was initially seen through clinical observation in strong emotional and physical stunting of orphaned and hospitalized infants separated from their mothers (Bowlby 1958, 1969; Spitz 1945), which was mirrored in research on rodents and nonhuman primates and permitted the assessment of a causal relationship between early life stress and altered developmental trajectory. Decades ago, the research by Gig Levine and his students’ and colleagues suggested that early life stress and elevation of the stress hormones are causal mediators of these enduring early life experience effects (Levine, 1962, 1967, 1993, 1994, Rosenfeld et al. 1992; Stanton and Levine, 1990; Suchecki et al. 1993; van Oers et al. 1998). Indeed, a consistent behavioral mark of these early life manipulations was enduring emotional and cognitive problems, with corticosterone and disruption of the HPA axis identified as one potential mediator (Levine, 1993, 1994; Meaney et al. 1993; Meerlo et al. 1999; Sapolsky, 1994). In particular, within a few hours of separation, the stress axis is engaged and shows increases in the stress hormones corticosterone (cortisol in primates) as a result of increases in adrenocorticotropic hormone (ACTH) that control the stress response at the level of the pituitary, although recent research suggests there are ubiquitous effects throughout the brain. This disruption in the HPA axis and its immediate and enduring effects on the brain and behavior has clearly indicated the important role of stress hormones in organizing emotional and cognitive development. More recent research has greatly expanded our understanding of the potential corticosterone related mechanisms and effects using different levels of analysis and has provided a stronger link between clinical and basic research (Andersen et al. 1999; Caldji et al. 2003; Cirulli et al. 2009; Hall et al. 1999; Higley et al. 1991; Kosten et al. 2005; Ladd et al. 2000; Liu et al. 2000; Meaney, 2001; Plotsky and Meaney, 1993; Schore, 2001; Suomi, 1997; Weinberg et al., 1978). The wide phylogenetic representation of these early life experiences on a variety of species, including rodents, humans and nonhuman primates, suggests that a role of the stress system in mediating early life’s enduring effects may have been evolutionarily conserved and supports the use of other species to understand variation in human development in response to stress (Bremner, 2003; Cirulli et al. 2009; Kaufman et al. 2000; Levine, 2001; Lupien et al. 2009; Pryce et al. 2002; Sanchez et al. 2001). Please see other
The importance of the contingency of early life trauma and adult outcome
Repeated pain in early life heightens adult emotionality/anxiety and can attenuate or enhance learning depending on the task. Specifically, infant adverse experiences using shock suggest that unpredictable shock produces greater changes in adult emotionality than predictable shock (Bell and Denenberg, 1962; Denenberg, 1963; Henderson, 1965) and diverges from the adult effects (Shore et al. 1990; Weiss, 1970). Indeed, after early life odor-shock conditioning, we find increased anxiety-like behavior in adulthood, but only in those animals that received unpaired odor-shock in infancy. Using the animal model of anxiety test of dark-light emergence in which the latency to leave a dark box (preferred area for rats) and total time spent in the light compartment (measuring tendency to explore) is measured, we found only the unpaired odor-shock pups differed from animals without any infant conditioning (Tyler et al. 2007). The adult animals that received paired odor-shocks in infancy or no conditioning showed normal behavior. In adulthood, animals that received infant paired odor-shock show a propensity towards depression-like symptoms as assessed by the animal model of depression, the Forced Swim test (Sevelinges et al. submitted). Moreover, following the infant odor-shock conditioning paradigm, adults were given a standard forced swim test, but only those animals that received paired odor-shock in infancy stopped swimming sooner than nonconditioned animals and the animals that received unpaired odor-shock in infancy. The ability of shock experienced at different contingencies to produce divergent adult outcomes strongly suggests that the context of early life trauma is critically important.
Summary
Animal research indicates that both enhanced approach learning and suppressed avoidance learning are important for ensuring the infant learns the attachment to the caregiver to support proximity seeking. The neurobiology for this learning appears to involve a unique neural circuit, involving hyperfunctioning of the locus coeruleus to support approach learning and hypofunctioning of the amygdala to support suppression of avoidance learning. Changing functions of these brain areas, combined with the unique function of corticosterone supporting this learning, enables the rapid and robust modification by the environment through: 1) early life stress prematurely increasing pups’ corticosterone levels and ending the sensitive period and 2) maternal presence socially buffering pups’ corticosterone response to prevent avoidance learning to maintain attachment in older pups.
During this sensitive period, early life trauma and stress have both short-term and long-term effects. We suggest that the enduring effects are mediated, in part, through short-term disruption on attachment learning and social interactions with the mother. Based upon existing basic research in animals, we now understand that elevated levels of the stress hormone corticosterone can impair learning about the mother and attachment, and impaired attachment can result in abnormal social behavior. We suggest that this abnormal social behavior in pups can mediate additional negative effects on pup development and may be partially responsible for the enduring effects of early life stress.
Finally, the results of rat pups’ attachment learning indicate that, while sensitive period learning is usually characterized by enhanced learning, limitations on learning are equally important for ensuring the infant learns to approach the caregiver. This characteristic of attachment learning has been documented in a variety of species ranging from chicks to mammals, including humans.
Implications for understanding attachment in humans
Human infants rapidly attach to their caregivers. As originally suggested by Bowlby (1965), this phenomenon is likely supported by a biological attachment circuit within the brain. The neurobiology of attachment in animals probably most closely aligns with Bowlby’s characterization of the early stages of attachment in infancy, emphasizing proximity seeking and tolerating abuse from the caregiver (Carlson et al. 1989; Harlow and Harlow, 1965; Maestripieri et al. 1999; Sanchez et al. 2001; Sullivan et al. 2009; Suomi, 2003). This is not surprising since Bowlby’s original attachment theory was strongly based on a paradigm-shifting integration of clinical observations (Bowlby, 1958, 1969; Spitz, 1945) and basic research on other animal species concerning attachment (Harlow and Harlow, 1965; Fisher, 1955; Stanley, 1962) and imprinting (Bolhuis and Honey, 1998; Hess, 1962; Lorenz, 1970; Salzen, 1970). During the attachment learning, biologically determined approach and following behaviors become directed toward a specific object, although the object must be learned through experience with the specific sensory qualities of the object, such as its scent, texture, color, and/or sound (Bolhuis and Honey, 1998; Bowlby, 1958, 1969; Cassidy, 1999; Hess, 1962; Hofer, 1987; Hofer and Sullivan, 2008; Nowak et al. 2000; Panksepp, 1998; Reed and Leiderman, 1983).
The attachment circuitry for human infants has yet to be identified. As always, direct translation of research on any species to humans requires caution. For this reason, we do not suggest that attachment in human infant uses the circuitry identified in rats. However, our rat model system can provide greater understanding of the neurobiology of attachment and exploration of unique qualities of neural structures during early life. Additionally, it is a model system that can be used to identify the critical role of social stimuli in infancy and their ability to alter normal processing of sensory stimuli and learning.
Acknowledgment
This work was supported by grants NIH DC003906 and DC009910, NSF IOB0850527, Leon Levy Foundation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adriani W, Laviola G. Windows of vulnerability to psychopathology and therapeutics strategy in the adolescent rodent model. Behav. Pharmacol. 2004;15:341–352. doi: 10.1097/00008877-200409000-00005. [DOI] [PubMed] [Google Scholar]
- Alberts JR, May R. Nonnutritive, thermotactile induction of filial huddling in rat pups. Dev. Psychobiol. 1984;17:161–181. doi: 10.1002/dev.420170207. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Lyss PJ, Dumount NL, Teicher MH. Enduring neurochemical effects of early maternal separation on limbic structures. Ann. N. Y. Acad. Sci. 1999;877:756–759. doi: 10.1111/j.1749-6632.1999.tb09317.x. [DOI] [PubMed] [Google Scholar]
- Avishai-Eliner S, Gilles EE, Eghbal-Ahmadi M, Bar-El Y, Baram TZ. Altered regulation of gene and protein expression of hypothalamic-pituitary-adrenal axis components in an immature rat model of chronic stress. J. Neuroendocrinol. 2001;13:799–807. doi: 10.1046/j.1365-2826.2001.00698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr GA. Ontogeny of nociception and antinociception. NIDA Research Monograph. 1995;158:172–201. [PubMed] [Google Scholar]
- Bell RW, Denenberg VH. The interrelationships of shock and critical periods in infancy as they affect adult learning and activity. Anim. Behav. 1962;11:21–27. [Google Scholar]
- Berdel B, Morys J. Expression of calbindin-D28k and parvalbumin during development of rat's basolateral amygdaloid complex. Inter. J. Dev. Neurosci. 2000;18:501–513. doi: 10.1016/s0736-5748(00)00024-1. [DOI] [PubMed] [Google Scholar]
- Berdel B, Morys J, Maciejewska B. Neuronal changes in the basolateral complex during development of the amygdala of the rat. Inter. J. Dev. Neurosci. 1997;15:755–765. doi: 10.1016/s0736-5748(97)00022-1. [DOI] [PubMed] [Google Scholar]
- Blass EM, Teicher MH. Suckling. Science. 1980;210:15–22. doi: 10.1126/science.6997992. [DOI] [PubMed] [Google Scholar]
- Blozovski D, Cudennec A. Passive avoidance learning in the young rat. Dev. Psychobiol. 1980;13:513–518. doi: 10.1002/dev.420130510. [DOI] [PubMed] [Google Scholar]
- Bohn MC. Granule cell genesis in the hippocampus of rats treated neonatally with hydrocortisone. Neuroscience. 1980;5:2003–2012. doi: 10.1016/0306-4522(80)90045-7. [DOI] [PubMed] [Google Scholar]
- Bolhuis JJ, Honey RC. Imprinting, learning and development: From behaviour to brain and back. Trends Neurosci. 1998;21:306–311. doi: 10.1016/s0166-2236(98)01258-2. [DOI] [PubMed] [Google Scholar]
- Bouwmeester H, Smits K, Van Ree JM. Neonatal development of projections to the basolateral amygdala from prefrontal and thalamic structures in rat. J. Comp. Neurol. 2002;450:241–255. doi: 10.1002/cne.10321. [DOI] [PubMed] [Google Scholar]
- Bowlby J. Attachment. New York: Basic Books; 1965. [Google Scholar]
- Bowlby J. Attachment and Loss. vol. 1. New York: Basic Books; 1969. [Google Scholar]
- Bowlby J. The nature of the child’s tie to the mother. Int. J. Psychoanal. 1958;39:350–373. [PubMed] [Google Scholar]
- Brake SC. Suckling infant rats learn a preference for a novel olfactory stimulus paired with milk delivery. Science. 1981;211:506–508. doi: 10.1126/science.7192882. [DOI] [PubMed] [Google Scholar]
- Branchi I. The mouse communal nest: investigating the epigenetic influences of the early social environment on brain and behavior development. Neurosci Biobehav Review. 2009;33:551–559. doi: 10.1016/j.neubiorev.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Bremner JD. Long-term effects of childhood abuse on brain and neurobiology. Child Adolesc. Psychiat. Clin. N. Am. 2003;12:271–292. doi: 10.1016/s1056-4993(02)00098-6. [DOI] [PubMed] [Google Scholar]
- Butte JC, Kakihana R, Farnham ML, Noble EP. The relationship between brain and plasma corticosterone stress response in developing rats. Endocrinology. 1973;92:1775–1779. doi: 10.1210/endo-92-6-1775. [DOI] [PubMed] [Google Scholar]
- Caldji C, Diorio J, Meaney MJ. Variations in maternal care alter GABA(A) receptor subunit expression in brain regions associated with fear. Neuropsychopharmacology. 2003;28:1950–1959. doi: 10.1038/sj.npp.1300237. [DOI] [PubMed] [Google Scholar]
- Camp LL, Rudy JW. Changes in the characterization of appetitive and aversive events during postnatal development. Dev. Psychobiol. 1988;21:25–42. doi: 10.1002/dev.420210103. [DOI] [PubMed] [Google Scholar]
- Carlson V, Cicchetti D, Barnett D, Braunwald K. Finding order in disorganization: lessons from research on maltreated infant’s attachment to their caregivers. In: Cicchetti D, Carlson V, editors. Child maltreatment: Theory and research on the causes and consequences of child abuse and neglect. New York: Cambridge University Press; 1989. pp. 494–528. [Google Scholar]
- Carter CS, Keverne EB. The neurobiology of social affiliation and pair bonding. In: Pfaff DW, editor. Hormones, brain, and behavior. San Diego: Academic Press; 2002. pp. 299–337. [Google Scholar]
- Cassidy J. The nature of child ties. In: Cassidy J, Shaver PR, editors. Handbook of attachment: Theory, research and clinical applications. New York: Guilford Press; 1999. pp. 3–20. [Google Scholar]
- Cate TE, Yasumura S. Effects of ACTH and histamine stress on serum corticosterone and adrenal cyclic AMP in immature rats. Endocrinology. 1975;96:1044–1047. doi: 10.1210/endo-96-4-1044. [DOI] [PubMed] [Google Scholar]
- Caza PA, Spear NE. Short-term exposure to an odor increases its subsequent preference in preweanling rats: A descriptive profile of the phenomenon. Dev. Psychobiol. 1984;17:407–422. doi: 10.1002/dev.420170407. [DOI] [PubMed] [Google Scholar]
- Cirulli F, Francia N, Berry A, Aloe L, Alleva E, Suomi JS. Early life stress as a risk for mental health: Role of neurotrophins from rodents to non-human primates. Neurosci. Biobehav. Rev. 2009;33:573–585. doi: 10.1016/j.neubiorev.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe CL, Glass JC, Wiener SG, Levine S. Behavioral, but not physiological, adaptation to repeated separation in mother and infant primates. Psychoneuroendocrinology. 1983;8:401–409. doi: 10.1016/0306-4530(83)90019-7. [DOI] [PubMed] [Google Scholar]
- Collier AC, Bolles RC. The ontogenesis of defensive reactions to shock in preweanling rats. Dev. Psychobiol. 1980;13:141–150. doi: 10.1002/dev.420130206. [DOI] [PubMed] [Google Scholar]
- Collier A, Mast J, Meyer D, Jacobs C. Approach-avoidance conflict in preweanling rats: A developmental study. Anim. Learn. Behav. 1979;7:514–520. [Google Scholar]
- Coopersmith R, Lee S, Leon M. Olfactory bulb responses after odor aversion learning by young rats. Brain Res. 1986;389:271–277. doi: 10.1016/0165-3806(86)90195-1. [DOI] [PubMed] [Google Scholar]
- Corodimas KP, LeDoux JE, Gold PW, Schulkin J. Corticosterone potentiation of conditioned fear in rats. Ann. N. Y. Acad. Sci. 1994;746:392–393. doi: 10.1111/j.1749-6632.1994.tb39264.x. [DOI] [PubMed] [Google Scholar]
- Crews F, He J, Hodge C. Adolescent cortical development: A critical period of vulnerability for addiction. Pharmacol. Biochem. Behav. 2007;86:189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J. Comp. Neurol. 2002;453:116–130. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- DeCasper AJ, Fifer WP. Of human bonding: Newborns prefer their mother’s voices. Science. 1980;208:1174–1176. doi: 10.1126/science.7375928. [DOI] [PubMed] [Google Scholar]
- Dallman M. Editorial: Moments in Time—The Neonatal Rat Hypothalamo-Pituitary-Adrenal Axis. Endocrinology. 2000;141:1590–1592. doi: 10.1210/endo.141.5.7527. [DOI] [PubMed] [Google Scholar]
- Denenberg VH. Early experience and emotional development. Scientific America. 1963;208:138–146. doi: 10.1038/scientificamerican0663-138. [DOI] [PubMed] [Google Scholar]
- Deschamps S, Woodside B, Walker CD. Pups presence eliminates the stress hyporesponsiveness of early lactating females to a psychological stress representing a threat to the pups. J. Neuroendorinol. 2003;15:486–497. doi: 10.1046/j.1365-2826.2003.01022.x. [DOI] [PubMed] [Google Scholar]
- DeVries AC, Glasper ER, Detillion CE. Social modulation of stress responses. Physiol. Behav. 2003;79:399–407. doi: 10.1016/s0031-9384(03)00152-5. [DOI] [PubMed] [Google Scholar]
- Duveau A, Godinot F. Influence of the odorization of the rearing environment on the development of odor-guided behavior in rat pups. Physiol. Behav. 1988;42:265–270. doi: 10.1016/0031-9384(88)90080-7. [DOI] [PubMed] [Google Scholar]
- Emerich DF, Scalzo FM, Enters EK, Spear NE, Spear LP. Effects of 6-hydroxydopamine-induced catecholamine depletion on shock-precipitated wall climbing of infant rat pups. Dev. Psychobiol. 1985;18:215–227. doi: 10.1002/dev.420180303. [DOI] [PubMed] [Google Scholar]
- Erkine MS, Geller E, Yuwiler A. Effects of neonatal hydrocortisone treatment on pituitary and adrenocortical responses to stress in young rats. Neuroendocrinology. 1979;29:191–199. doi: 10.1159/000122922. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Gale GD. The amygdala, fear, and memory. Ann. N. Y. Acd. Sci. 2003;985:125–134. doi: 10.1111/j.1749-6632.2003.tb07077.x. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, LeDoux JE. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23:229–232. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- Fisher AE. Doctoral Dissertation. Pennsylvania State University; 1955. The effects of differential early treatment on the social and exploratory behavior of puppies. [Google Scholar]
- Fitzgerald M. The development of nociceptive circuits. Nat. Rev. Neurosci. 2005;6:507–520. doi: 10.1038/nrn1701. [DOI] [PubMed] [Google Scholar]
- Galef BG., Jr Acquisition and waning of exposure-induced attraction to a nonnatural odor in rat pups. Dev. Psychobiol. 1982;15:470–490. doi: 10.1002/dev.420150510. [DOI] [PubMed] [Google Scholar]
- Galef BG, Jr, Kaner HC. Establishment and maintenance of preference for natural and artificial olfactory stimuli in juvenile rats. J. Comp. Physiol. Psychol. 1980;94:588–595. doi: 10.1037/h0077693. [DOI] [PubMed] [Google Scholar]
- Galef BG, Jr, Sherry DF. Mother's milk: A medium for transmission of cues reflecting the flavor of mother's diet. J. Comp. Physiol. Psychol. 1973;83:374–378. doi: 10.1037/h0034665. [DOI] [PubMed] [Google Scholar]
- Gilles E, Schultz L, Baram T. Abnormal corticosterone regulation in an immature rat model of continuous chronic stress. Pediatr. Neurol. 1996;15:114–119. doi: 10.1016/0887-8994(96)00153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg D, Ackerman SH. Genetically obese mice are predisposed to gastric stress ulcers. Behav. Neurosci. 1986;98:435–440. doi: 10.1037//0735-7044.98.3.435. [DOI] [PubMed] [Google Scholar]
- Grino M, Paulmyer-Lacroix O, Faudon M, Renard M, Anglade G. Blockade of alpha 2-adrenoceptors stimulates basal and stress-induced adrenocorticotropin secretion in the developing rat through a central mechanism independent from corticotropin-releasing factor and arginine vasopressin. Endocrinology. 1994;135:2549–2557. doi: 10.1210/endo.135.6.7988443. [DOI] [PubMed] [Google Scholar]
- Guillet R, Michaelson SM. Corticotropin responsiveness in the neonatal rat. Neuroendocrinology. 1978;27:119–125. doi: 10.1159/000122804. [DOI] [PubMed] [Google Scholar]
- Guillet R, Saffran M, Michaelson SM. Pituitary-adrenal response in neonatal rats. Endocrinology. 1980;106:991–994. doi: 10.1210/endo-106-3-991. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Frenn K, Wewerka SS, Van Ryzin MJ. Moderate versus severe early life stress: associations with stress reactivity and regulation in 10–12-year-old children. Psychoneuroendocrinology. 2009;34:62–75. doi: 10.1016/j.psyneuen.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall FS, Wilkinson LS, Humby T, Robbins TW. Maternal deprivation of neonatal rats produces enduring changes in dopamine function. Synapse. 1999;32:37–43. doi: 10.1002/(SICI)1098-2396(199904)32:1<37::AID-SYN5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Harlow H, Harlow M. The affectional system. In: Schrier A, Harlow H, Stollnitz F, editors. Behavior of nonhuman primates. vol. 2. New York: Academic Press; 1965. pp. 287–334. [Google Scholar]
- Haroutunian V, Campbell BA. Emergence of interoceptive and exteroceptive control of behavior in rats. Science. 1979;205:927–929. doi: 10.1126/science.472715. [DOI] [PubMed] [Google Scholar]
- Hatalski CG, Guirquis C, Baram TZ. Corticotropin releasing factor mRNA expression in the hypothalamic paraventricular nucleus and the central nucleus of the amygdala is modulated by repeated acute stress in the immature rat. J. Neuroendocrinol. 1998;10:663–669. doi: 10.1046/j.1365-2826.1998.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson ND. Acquisition and retention of conditioned fear during different stages in the development of mice. J. Comp. Physiol. Psychol. 1965;59:430–442. doi: 10.1037/h0022039. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Kaiser S, Sachser N. Social buffering of stress response: Diversity, mechanisms, and functions. Front. Neuroendocrinol. 2009;30:470–482. doi: 10.1016/j.yfrne.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Li j, Levine S. Infant responsiveness to maternal cues in mice of 2 inbred lines. Dev. Psychobiol. 1980;13:77–84. doi: 10.1002/dev.420130111. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Maken DS, Graves FC. Presence of mother and unfamiliar female alters levels of testosterone, progesterone, cortisol, adrenocorticotropin, and behavior in maturing guinea pigs. Horm. Behav. 2002;42:42–52. doi: 10.1006/hbeh.2002.1794. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Nigh CK, Sims ML, Long SJ. Plasma cortisol and vocalization responses of postweaning age guinea pigs to maternal and sibling separation: evidence for filial attachment after weaning. Dev. Psychobiol. 1995;28:103–115. doi: 10.1002/dev.420280204. [DOI] [PubMed] [Google Scholar]
- Henning SJ. Plasma concentrations of total and free corticosterone during development in the rat. Am. J. Physiol. 1978;235:E451–E456. doi: 10.1152/ajpendo.1978.235.5.E451. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period regulation. Annu. Rev. Neurosci. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- Hess E. Ethology: An approach to the complete analysis of behavior. In: Brown R, Galanter E, Hess E, Mendler G, editors. New directions in psychology. New York: Holt, Rinehart and Winston; 1962. pp. 159–199. [Google Scholar]
- Higley JD, Hasert MF, Suomi SJ, Linnoila M. Nonhuman primate model of alcohol abuse: effects of early experience, personality, and stress on alcohol consumption. Proc. Nat. Acad. Sci. USA. 1991;88:7261–7265. doi: 10.1073/pnas.88.16.7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer MA, Shair HN. Ultrasonic vocalization during social interaction and isolation in 2-weeek-old rats. Dev. Psychobiol. 1978;11:495–504. doi: 10.1002/dev.420110513. [DOI] [PubMed] [Google Scholar]
- Hofer MA. Shaping forces within early social relationships. In: Krasnegor NA, Blass EM, Hofer MA, Smotherman VP, editors. Perinatal development. New York: Academic Press; 1987. pp. 251–274. [Google Scholar]
- Hofer MA, Sullivan RM. Toward a neurobiology of attachment. In: Nelson CA, Luciana M, editors. Handbook of developmental cognitive neuroscience. Cambridge: MIT Press; 2008. pp. 787–806. [Google Scholar]
- Hui GK, Figueroa IR, Poytress BS, Roozendaal B, McGaugh JL, Weinberger NM. Memory enhancement of classical fear conditioning by post-training injections of corticosterone in rats. Neurobiol. Learn. Mem. 2004;81:67–74. doi: 10.1016/j.nlm.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Insel TR, Young LJ. The neurobiology of attachment. Nat. Rev. Neurosci. 2001;2:129–136. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- Ito A, Kikusui T, Mori Y. Effects of early weaning on anxiety and autonomic responses to stress in rats. Behav. Brain Res. 2006;171:87–93. doi: 10.1016/j.bbr.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Woo CC, Duong H, Nguyen V, Leon M. A learned odor evokes an enhanced Fos-like glomerular response in the olfactory bulb of young rats. Brain Res. 1995;699:192–200. doi: 10.1016/0006-8993(95)00896-x. [DOI] [PubMed] [Google Scholar]
- Johanson IB, Hall WG. Appetitive learning in 1-day-old rat pups. Science. 1979;205:419–421. doi: 10.1126/science.451612. [DOI] [PubMed] [Google Scholar]
- Johanson IB, Teicher MH. Classical conditioning of an odor preference in 3-day-old rats. Behav. Neural. Biol. 1980;29:132–136. doi: 10.1016/s0163-1047(80)92596-0. [DOI] [PubMed] [Google Scholar]
- Jordan TC, Hennessy MB, Gonzalez CA, Levine S. Social and environmental factors influencing mother–infant separation-reunion in squirrel monkeys. Physiol. Behav. 1985;34:489–493. doi: 10.1016/0031-9384(85)90038-1. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Plotsky PM, Nemeroff CB, Charney DS. Effects of early adverse experiences on brain structure and function: clinical implications. Biol. Psychiatry. 2000;48:778–790. doi: 10.1016/s0006-3223(00)00998-7. [DOI] [PubMed] [Google Scholar]
- Kikusui T, Mori Y. Behavioral and neurochemical consequences of early weaning in rodents. J. Neuroendocrinol. 2009;21:427–431. doi: 10.1111/j.1365-2826.2009.01837.x. [DOI] [PubMed] [Google Scholar]
- Kikusui T, Winslow JT, Mori Y. Social buffering: Relief from stress and anxiety. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2006;361:2215–2228. doi: 10.1098/rstb.2006.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Klauer T, Filipp SH, Hellhammer DH. Sex-specific effects of social support on cortisol and subjective responses to acute psychological stress. Psychosom. Med. 1995;57:23–31. doi: 10.1097/00006842-199501000-00004. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJD, Bombace JC, Lee HJ, Kim JJ. Sex-selective effects of neonatal isolation on fear conditioning and foot shock sensitivity. Behav. Brain Res. 2005;157:235–244. doi: 10.1016/j.bbr.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Meaney MJ, Plotsky PM. Long-term behavioral and neuroendocrine adaptations to adverse early experience. Prog. Brain Res. 2000;122:81–103. doi: 10.1016/s0079-6123(08)62132-9. [DOI] [PubMed] [Google Scholar]
- Langdon PE, Harley CW, McLean JH. Increased β adrenoceptor activation overcomes conditioned olfactory learning induced by serotonin depletion. Dev. Brain Res. 1997;114:261–264. doi: 10.1016/s0165-3806(97)00090-4. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. The emotional brain, fear, and the amygdala. Cell. Mol. Neurobiol. 2003;23:727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon M. Dietary control maternal pheromone in the lactating rat. Physiol. Behav. 1975;14:311–319. doi: 10.1016/0031-9384(75)90039-6. [DOI] [PubMed] [Google Scholar]
- Leon M. Neuroethology of olfactory preference development. J. Neurobiol. 1992;23:1557–1573. doi: 10.1002/neu.480231012. [DOI] [PubMed] [Google Scholar]
- Levine S. A further study of infantile handling and adult avoidance learning. J. Pres. 1952;25:70–80. doi: 10.1111/j.1467-6494.1956.tb01289.x. [DOI] [PubMed] [Google Scholar]
- Levine S. Plasma-free corticosteroid response to electric shock in rats stimulated in infancy. Science. 1962;135:795–796. doi: 10.1126/science.135.3506.795-a. [DOI] [PubMed] [Google Scholar]
- Levine S. Maternal and environmental influences on the adrenocortical response to stress in weanling rats. Science. 1967;156:258–260. doi: 10.1126/science.156.3772.258. [DOI] [PubMed] [Google Scholar]
- Levine S. Comparative and psychological perspectives on development. In: Collins WA, editor. The concept of development. Hillsdale: Lawrence Erlbaum Associates, Inc; 1982. pp. 29–54. [Google Scholar]
- Levine S. The psychoendocrinology of stress. Ann. N. Y. Acad. Sci. 1993;697:61–67. doi: 10.1111/j.1749-6632.1993.tb49923.x. [DOI] [PubMed] [Google Scholar]
- Levine S. The ontogeny of the hypothalamic-pituitary-adrenal axis. The influence of maternal factors. Ann. N. Y. Acad. Sci. 1994;746:275–288. doi: 10.1111/j.1749-6632.1994.tb39245.x. [DOI] [PubMed] [Google Scholar]
- Levineq S. Influence of psychological variables on the activity of the hypothalamic-pituitary-adrenal axis. Eur. J. Pharmacol. 2000;405:149–160. doi: 10.1016/s0014-2999(00)00548-3. [DOI] [PubMed] [Google Scholar]
- Levine S. Primary social relationships influence the development of the hypothalamic-pituitary-adrenal axis in the rat. Physiol. Behav. 2001;73:255–260. doi: 10.1016/s0031-9384(01)00496-6. [DOI] [PubMed] [Google Scholar]
- Liu D, Caldji C, Sharma S, Plotsky P, Meaney M. Influence of neonatal rearing conditions on stress-induced adrenocorticotropin responses and norepinephrine release in the hypothalamic paraventricular nucleus. J. Neuroendocrinol. 2000;12:5–12. doi: 10.1046/j.1365-2826.2000.00422.x. [DOI] [PubMed] [Google Scholar]
- Lorenz KZ. Companions as factors in the bird’s environment. In: Lorenz KZ, editor. Studies in animal and human behavior. Cambridge: Harvard University Press; 1970. [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain behaviour and cognition. Nature Rev. Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Tomaszycki M, Carroll KA. Consistency and change in the behavior of rhesus macaque abusive mothers with successive infants. Dev. Psychobiol. 1999;34:29–35. [PubMed] [Google Scholar]
- Macri S, Wurbel H. Developmental plasticity of HPA and fear responses in rats: a critical review of the maternal mediation hypothesis. Horm. Behav. 2006;50:667–680. doi: 10.1016/j.yhbeh.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Martin CE, Cake MH, Hartmann PE, Cook IF. Relationship between foetal corticosterone, maternal progesterone and parturition in the rat. Acta Endocrinol. 1977;84:167–176. doi: 10.1530/acta.0.0840167. [DOI] [PubMed] [Google Scholar]
- McCloskey LA, Figueredo AJ, Koss MP. The effects of systemic family violence in children’s mental health. Child Dev. 1995;66:1239–1261. [PubMed] [Google Scholar]
- McLean JH, Darby-King A, Sullivan RM, King SR. Serotonergic influence on olfactory learning in the neonate rat. Behav. Neural. Biol. 1993;60:152–162. doi: 10.1016/0163-1047(93)90257-i. [DOI] [PubMed] [Google Scholar]
- McLean JH, Harley CW, Darby-King A, Yuan Q. pCREB in the neonate rat olfactory bulb is selectively and transiently increased by odor preference-conditioned training. Learn. Mem. 1999;6:608–618. doi: 10.1101/lm.6.6.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Ann. Rev. Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH, van Berkel C, Bhatnagar S, Sapolsky RM. Effects of neonatal handling on age-related impairments associated with the hippocampus. Science. 1988;239:766–768. doi: 10.1126/science.3340858. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Bhatnagar S, Larocque S, McCormick C, Shanks M, Sharama S, Smythe J, Viau V, Plotsky P. Individual differences in the hypothalamic-pituitary-adrenal stress response and hypothalamic CRF system. Ann. N. Y. Acad. Sci. 1993;697:70–85. doi: 10.1111/j.1749-6632.1993.tb49924.x. [DOI] [PubMed] [Google Scholar]
- Meerlo P, Horvath KM, Nagy GM, Bolus B, Koolhaas JM. The influence of postnatal handling on adult neuroendocrine and behavioral stress reactivity. J. Neuroendocrinology. 1999;11:925–933. doi: 10.1046/j.1365-2826.1999.00409.x. [DOI] [PubMed] [Google Scholar]
- Moncek F, Duncko R, Johansson BB, Jezova D. Effect of environmental enrichment on stress related systems in rats. J. Neuroendocrinol. 2004;16:423–431. doi: 10.1111/j.1365-2826.2004.01173.x. [DOI] [PubMed] [Google Scholar]
- Moriceau S, Roth TL, Okotoghaide T, Sullivan RM. Corticosterone controls the developmental emergence of fear and amygdala function to predator odors in infant rat pups. Int. J. Dev. Neurosci. 2004;22:415–422. doi: 10.1016/j.ijdevneu.2004.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Shionoya K, Jakubs K, Sullivan RM. Early life stress disrupts attachment learning: The role of amygdala corticosterone, locus coeruleus CRH and olfactory bulb NE. J Neurosci. doi: 10.1523/JNEUROSCI.4106-09.2009. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Corticosterone influences on mammalian neonatal sensitive-period learning. Behav. Neurosci. 2004a;118:274–281. doi: 10.1037/0735-7044.118.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Unique neural circuitry for neonatal olfactory learning. J. Neurosci. 2004b;24:1182–1189. doi: 10.1523/JNEUROSCI.4578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Neurobiology of infant attachment. Dev. Psychobiol. 2005;47:230–242. doi: 10.1002/dev.20093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Maternal presence serves as a switch between learning fear and attraction in infancy. Nat. Neurosci. 2006;9:1004–1006. doi: 10.1038/nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Wilson DA, Levine S, Sullivan RM. Dual circuitry for odor-shock conditioning during infancy: Corticosterone switches between fear and attraction via amygdala. J. Neurosci. 2006;26:6737–6748. doi: 10.1523/JNEUROSCI.0499-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morys J, Berdel B, Jagalska-Majewska H, Luczynska A. The basolateral amygdaloid complex - its development, morphology and functions. Folia Morphol. (Warszawa) 1999;58:29–46. [PubMed] [Google Scholar]
- Myslivecek J. Inhibitory learning and memory in newborn rats. Prog. Neurobiol. 1997;53:399–430. doi: 10.1016/s0301-0082(97)00036-1. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Sakaguchi T. Development and plasticity of the locus coeruleus. A review of recent physiological and pharmacological experimentation. Prog. Neurobiol. 1990;34:505–526. doi: 10.1016/0301-0082(90)90018-c. [DOI] [PubMed] [Google Scholar]
- Nair H, Gonzalez-Lima F. Extinction of behavior in infant rats: development of functional coupling between septal, hippocampal, and ventral tegmental regions. J. Neurosci. 1999;19:8646–8655. doi: 10.1523/JNEUROSCI.19-19-08646.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB. Neurobiological consequences of childhood trauma. J. Clin. Psychiatry. 2004;65:18–28. [PubMed] [Google Scholar]
- Nowak R, Porter RH, Levy F, Orgeur P, Schaal B. Role of mother-young interactions in the survival of offspring in domestic mammals. Rev. Reprod. 2000;5:153–163. doi: 10.1530/ror.0.0050153. [DOI] [PubMed] [Google Scholar]
- O’Connor J, Cameron J. Translating Research Findings on Early Experience to Prevention: Animal and Human Evidence on Early Attachment Relationships. Amer. J. Preventive Med. 2006;31:175–181. doi: 10.1016/j.amepre.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Okutani F, Zhang JJ, Otsuka T, Yagi F, Kaba H. Modulation of olfactory learning in young rats through intrabulbar GABA(A) receptor. Eur. J. Neurosci. 2003;18:2031–2036. doi: 10.1046/j.1460-9568.2003.02894.x. [DOI] [PubMed] [Google Scholar]
- Panksepp J. Affective neuroscience: The foundations of human and animal emotions. New York: Oxford University Press; 1998. [Google Scholar]
- Pedersen PE, Williams CL, Blass EM. Activation and odor conditioning of suckling behavior in 3-day-old albino rats. J. Exp. Psychol. Anim. Behav. Process. 1982;8:329–341. [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ. Early postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Mol. Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Polan HJ, Hofer MA. Olfactory preference for mother over home nest shaving by newborn rats. Dev. Psychobiol. 1998;33:5–20. [PubMed] [Google Scholar]
- Pryce CR, Ruedi-Bettschen D, Dettling AC, Feldon J. Early life stress: Long-term physiological impact in rodents and primates. News Physiol. Sci. 2002;17:150–155. doi: 10.1152/nips.01367.2001. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Tremblay D, Fleshner M, Rudy JW. A selective role for corticosterone in contextual-fear conditioning. Behav. Neurosci. 1997;111:503–511. [PubMed] [Google Scholar]
- Raineki C, Moriceau S, Sullivan RM. Developing a neurobehavioral animal model of infant attachment to an abusive caregiver. doi: 10.1016/j.biopsych.2009.12.019. (Submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Shionoya K, Sander K, Sullivan RM. Ontogeny of odor-LiCl vs. odor-shock learning: Similar behaviors but divergent ages of functional amygdala emergence. Learn. Mem. 2009;16:114–121. doi: 10.1101/lm.977909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajecki DW, Lamb ME, Obmascher P. Toward a general theory of infantile attachment: A comparative review of aspects of the social bond. Behav. Brain Sci. 1978;3:417–464. [Google Scholar]
- Rangel S, Leon M. Early odor preference training increases olfactory bulb norepinephrine. Dev. Brain Res. 1995;85:187–191. doi: 10.1016/0165-3806(94)00211-h. [DOI] [PubMed] [Google Scholar]
- Reed GL, Leiderman PH. Is imprinting appropriate model for human attachment? Int. J. Behav. Dev. 1983;6:51–69. [Google Scholar]
- Rice D, Barone S., Jr Critical period of vulnerability for the developing nervous system: Evidence from humans and animal models. Environ. Health Perspect. 2000;108:511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Carmi O, McGaugh JL. Adrenocortical suppression blocks the memory-enhancing effects of amphetamine and epinephrine. Proc. Natl. Acad. Sci. USA. 1996;93:1429–1433. doi: 10.1073/pnas.93.4.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Quirarte GL, McGaugh JL. Glucocorticoids interact with the basolateral amygdala beta-adrenoceptor-cAMP/cAMP/PKA system in influencing memory consolidation. Eur. J. Neurosci. 2002;15:553–560. doi: 10.1046/j.0953-816x.2001.01876.x. [DOI] [PubMed] [Google Scholar]
- Rosenfeld P, Suchecki D, Levine S. Multifactorial regulation of the hypothalamic-pituitary-adrenal axis during development. Neurosci. Biobehav. Rev. 1992;16:553–568. doi: 10.1016/s0149-7634(05)80196-4. [DOI] [PubMed] [Google Scholar]
- Rosenzweig MR, Bennett EL, Diamond MC, Wu SY, Slagle RW, Saffran E. Influences of environmental complexity and visual stimulation on development of occipital cortex in rat. Brain Res. 1969;14:427–445. doi: 10.1016/0006-8993(69)90120-6. [DOI] [PubMed] [Google Scholar]
- Roth T, Moriceau S, Sullivan RM. Opioid modulation of Fos protein expression and olfactory circuitry plays a pivotal role in what neonates remember. Learn. Mem. 2006;13:590–598. doi: 10.1101/lm.301206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth T, Sullivan RM. Memory of early maltreatment: Neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biol. Psychiatry. 2005;57:823–831. doi: 10.1016/j.biopsych.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Rutter M. Implications of resilience concepts for scientific understanding. Ann. N. Y. Acad. Sci. 2006;1094:1–12. doi: 10.1196/annals.1376.002. [DOI] [PubMed] [Google Scholar]
- Sackett GP. Exploratory behavior of rhesus monkeys as a function of rearing condition and sex. Dev. Psychol. 1972;6:260–270. [Google Scholar]
- Salzen E. Imprinting and environmental learning. In: Aronson LR, Tobach E, Lehrman DS, Rosenblatt JS, editors. Development and Evolution of Behavior. San Francisco: W.H. Freeman; 1970. pp. 158–178. [Google Scholar]
- Sananes CB, Campbell BA. Role of the central nucleus of the amygdala in olfactory heart rate conditioning. Behav Neurosci. 1989;103:519–525. [PubMed] [Google Scholar]
- Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: Evidence from rodent and primate models. Dev. Psychopathol. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. The physiological relevance of glucocorticoid endangerment of hippocampus. Ann N. Y. Acad. Sci. 1994;746:294–307. doi: 10.1111/j.1749-6632.1994.tb39247.x. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res. 1986;396:64–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- Schettino LF, Otto T. Patterns of Fos expression in amygdala and ventral perirhinal cortex induced by training in an olfactory fear-conditioning paradigm. Behav. Neurosci. 2001;115:1257–1272. doi: 10.1037//0735-7044.115.6.1257. [DOI] [PubMed] [Google Scholar]
- Schore AN. The effects of early relational trauma on right brain development, affect regulation, and infant mental health. Inf. Ment. Health J. 2001;22:201–269. [Google Scholar]
- Sevelinges Y, Gervais R, Messaoudi B, Granjon L, Mouly AM. Olfactory fear conditioning induces field potential potentiation in rat olfactory cortex and amygdala. Learn. Mem. 2004;11:761–769. doi: 10.1101/lm.83604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevelinges Y, Moriceau S, Holman P, Miner C, Muzny K, Gervais R, Mouly AM, Sullivan RM. Enduring effects of infant memories: Infant odor-shock conditioning attenuates amygdala activity and adult fear conditioning. Biol. Psychiatry. 2007;62:1070–1079. doi: 10.1016/j.biopsych.2007.04.025. [DOI] [PubMed] [Google Scholar]
- Sevelinges Y, Mouly AM, Raineki C, Moriceau S, Forest C, Sullivan RM. Adult depression-like behavior and amygdala dysfunction rescued by odor previously paired with shock in infancy. doi: 10.1016/j.dcn.2010.07.005. (Submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks N, Windle RJ, Perks P, Wood S, Ingram CD, Lightman SL. The hypothalamic-pituitary-adrenal axis response to endotoxin is attenuated during lactation. J. Neuroendocrinology. 1999;11:587–596. doi: 10.1046/j.1365-2826.1999.00400.x. [DOI] [PubMed] [Google Scholar]
- Shionoya K, Moriceau S, Bradstock P, Sullivan RM. Maternal modulation of hypothalamic paraventricular nucleus norepinephrine switches avoidance learning to preference learning in preweanling rat pups. Horm. Behav. 2007;52:391–400. doi: 10.1016/j.yhbeh.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shionoya K, Moriceau S, Lunday L, Miner C, Roth TL, Sullivan RM. Development switch in neural circuitry underlying odor-malaise learning. Learn. Mem. 2006;13:801–808. doi: 10.1101/lm.316006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore TJ, Foy MR, Levine S, Thompson RF. Unpredictable and uncontrollable stress impairs neuronal plasticity in the rat hippocampus. Brain Res. Bull. 1990;24:663–667. doi: 10.1016/0361-9230(90)90005-k. [DOI] [PubMed] [Google Scholar]
- Singh PJ, Tobach E. Olfactory bulbectomy and nursing behavior in rat pups (Wistar DAB) Devel Psychobiol. 1975;8:151–164. doi: 10.1002/dev.420080207. [DOI] [PubMed] [Google Scholar]
- Spitz R. Hospitalism: An inquiry into the genesis of psychiatric conditions in early childhood. Psychoanal. Stud. Child. 1945;1:53–74. [PubMed] [Google Scholar]
- Stanley W. Differential human handling as reinforcing events and as treatments influencing later social behavior in basenji puppies. Psychol. Rep. 1962;10:775–788. [Google Scholar]
- Stanton ME, Levine S. Inhibition of infant glucocorticoid stress response: specific role of maternal cues. Dev. Psychobiol. 1990;23:411–426. doi: 10.1002/dev.420230504. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Wallstrom J, Levine S. Maternal contact inhibits pituitary-adrenal stress responses in preweanling rats. Dev. Psychobiol. 1987;20:131–145. doi: 10.1002/dev.420200204. [DOI] [PubMed] [Google Scholar]
- Stehouwer DJ, Campbell BA. Habituation of the forelimb-withdrawal response in neonatal rats. J. Exp. Psychol. Anim. Behav. Process. 1978;4:104–119. doi: 10.1037//0097-7403.4.2.104. [DOI] [PubMed] [Google Scholar]
- Stien P, Kendall J. Psychological trauma and the developing brain: Neurologically based interventions for troubled children. London: Haworth Press Inc; 2004. [Google Scholar]
- Suchecki D, Rosenfeld P, Levine S. Maternal regulation of the hypothalamic-pituitary-adrenal axis in the infant rat: the roles of feeding and stroking. Brain Res. 1993;75:185–192. doi: 10.1016/0165-3806(93)90022-3. [DOI] [PubMed] [Google Scholar]
- Suchecki D, Tufik S. Long-term effects of maternal deprivation on the corticosterone response to stress in rats. Behav Brain Res. 2000;111:99–106. doi: 10.1152/ajpregu.1997.273.4.R1332. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Hofer MA, Brake SC. Olfactory-guided orientation in neonatal rats is enhanced by a conditioned change in behavioral state. Dev. Psychobiol. 1986;19:615–623. doi: 10.1002/dev.420190612. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Landers M, Yeaman B, Wilson DA. Good memories of bad events in infancy. Nature. 2000a;407:38–39. doi: 10.1038/35024156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Leon M. Early olfactory learning induces an enhanced olfactory bulb response in young rats. Dev. Brain Res. 1986;27:278–282. doi: 10.1016/0165-3806(86)90256-7. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Moriceau S, Raineki C, Roth T. Ontogeny of infant fear learning and amygdala. In: Gazzaniga MS, editor. Cognitive Neuroscience. Cambridge: MIT Press; 2009. pp. 889–904. [Google Scholar]
- Sullivan RM, Stackenwalt G, Nasr F, Lemon C, Wilson DA. Association of an odor with activation of olfactory bulb noradrenergic beta-receptors or locus coeruleus stimulation is sufficient to produce learned approach responses to that odor in neonatal rats. Behav. Neurosci. 2000b;114:957–962. doi: 10.1037/0735-7044.114.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Taborsky-Barba S, Mendoza R, Itano A, Leon M, Cotman CW, Payne TF, Lott I. Olfactory classical conditioning in neonates. Pediatrics. 1991;87:511–518. [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA, Lemon C, Gerhardt G. Bilateral 6-OHDA lesions of the locus coeruleus impair associative olfactory learning in newborn rats. Brain Res. 1994;643:306–309. doi: 10.1016/0006-8993(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA, Leon M. Norepinephrine and learning-induced plasticity in infant rat olfactory system. J. Neurosci. 1989;9:3998–4006. doi: 10.1523/JNEUROSCI.09-11-03998.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA, Wong R, Correa A, Leon M. Modified behavioral and olfactory bulb responses to maternal odors in preweanling rats. Dev. Brain Res. 1990;53:243–247. doi: 10.1016/0165-3806(90)90013-o. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Zyzak DR, Skierkowski P, Wilson DA. The role of olfactory bulb norepinephrine in early olfactory learning. Dev. Brain Res. 1992;70:279–282. doi: 10.1016/0165-3806(92)90207-d. [DOI] [PubMed] [Google Scholar]
- Suomi SJ. Early determinants of behaviour: evidence from primate studies. Br. Med. Bull. 1997;53:170–184. doi: 10.1093/oxfordjournals.bmb.a011598. [DOI] [PubMed] [Google Scholar]
- Suomi SJ. Gene-environment interactions and the neurobiology of social conflict. Ann. N. Y. Acad. Sci. 2003;1008:132–139. doi: 10.1196/annals.1301.014. [DOI] [PubMed] [Google Scholar]
- Tang AC, Akers KG, Reeb BC, Romeo RD, McEwen BS. Programming social, cognitive, and neuroendocrine development by early exposure to novelty. Proc. Natl. Acad. Sci. U.S.A. 2006;103:15716–15721. doi: 10.1073/pnas.0607374103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi LK. Organizing action of corticosterone on the development of behavioral inhibition in the preweanling rat. Dev. Brain Res. 1994;81:121–127. doi: 10.1016/0165-3806(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neurosci. Biobehav. Rev. 2003;27:33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- Thompson BL, Erickson K, Schulkin J, Rosen JB. Corticosterone facilitates retention of contextually conditioned fear and increases CRH mRNA expression in the amygdala. Behav. Brain Res. 2004;149:209–215. doi: 10.1016/s0166-4328(03)00216-x. [DOI] [PubMed] [Google Scholar]
- Thompson JV, Sullivan RM, Wilson DA. Developmental emergence of fear learning corresponds with changes in amygdala synaptic plasticity. Brain Res. 2008;1200:58–65. doi: 10.1016/j.brainres.2008.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsteinsson EB, James JE. A meta-analysis of the effects of experimental manipulations of social support during laboratory stress. Psychol. Health. 1999;14:869–886. [Google Scholar]
- Toufexis DJ, Walker CD. Noradrenergic facilitation of the adrenocorticotropin response to stress is absent during lactation in the rat. Brain Res. 1996;737:71–77. doi: 10.1016/0006-8993(96)00627-0. [DOI] [PubMed] [Google Scholar]
- Tyler K, Sullivan RM, Moriceau S, Greenwood-Van Meerveld B. A new model of long-term colonic hypersensitivity in adult rats induced by neonatal unpredictable vs. predictable shock. Neurogastro. Motility. 2007;19:761–768. doi: 10.1111/j.1365-2982.2007.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oers HJ, de Kloet ER, Li C, Levine S. The ontogeny of glucocorticoid negative feedback: influence of maternal deprivation. Endocrinology. 1998;139:2838–2846. doi: 10.1210/endo.139.6.6037. [DOI] [PubMed] [Google Scholar]
- Walker CD, Deschamps S, Proulx K, Tu M, Salzman C, Woodside B, Lupien S, Gallo-Payet N, Richard D. Mother to infant or infant to mother? Reciprocal regulation of responsiveness to stress in rodents and the implications for humans. J. Psychiatry Neurosci. 2004;29:364–382. [PMC free article] [PubMed] [Google Scholar]
- Walker C, Sapolsky R, Meaney M, Vale W, Rivier C. Increased pituitary sensitivity to glucocorticoid feedback during the stress nonresponsive period in the neonatal rat. Endocrinology. 1986;119:1816–1821. doi: 10.1210/endo-119-4-1816. [DOI] [PubMed] [Google Scholar]
- Walker SJ, Vrana KE. Pituitary corticotrophin function during the stress hyporesponsive period in neonatal rats. Neuroendocrinology. 1993;57:1003–1010. doi: 10.1159/000126464. [DOI] [PubMed] [Google Scholar]
- Weaver SA, Aherne FX, Meaney MJ, Schaefer AL, Dixon WT. Neonatal handling permanently alters hypothalamic-pituitary-adrenal axis function, behaviour, and body weight in boars. J. Endocrinol. 2000;164:349–359. doi: 10.1677/joe.0.1640349. [DOI] [PubMed] [Google Scholar]
- Weinberg J, Smotherman WP, Levine S. Early handling effects on neophobia and conditioned taste aversion. Physiol. Behav. 1978;20:589–596. doi: 10.1016/0031-9384(78)90251-2. [DOI] [PubMed] [Google Scholar]
- Weiss JM. Somatic effects of predicable and unpredictable shock. Psychosom. Med. 1970;32:397–408. doi: 10.1097/00006842-197007000-00008. [DOI] [PubMed] [Google Scholar]
- Weldon DA, Travis ML, Kennedy DA. Posttraining D1 receptor blockade impairs odor conditioning in neonatal rats. Behav. Neurosci. 1991;105:450–458. doi: 10.1037//0735-7044.105.3.450. [DOI] [PubMed] [Google Scholar]
- Wiedenmayer CP, Magarinos AM, McEwen BS, Barr GA. Mother lowers glucocorticoid levels of preweaning rats after acute threat. Ann. N. Y. Acad. Sci. 2003;1008:304–307. doi: 10.1196/annals.1301.038. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Leon M. Spatial patterns of olfactory single-unit responses to learned olfactory cues in young rats. J. Neurophysiol. 1988;59:1770–1782. doi: 10.1152/jn.1988.59.6.1770. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM. Neurobiology of associative learning in the neonate: Early olfactory learning. Behav. Neural. Biol. 1994;61:1–18. doi: 10.1016/s0163-1047(05)80039-1. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM, Leon M. Single-unit analysis of postnatal olfactory learning: Modified olfactory bulb output response patterns to learned attractive odors. J. Neurosci. 1987;7:3154–3162. doi: 10.1523/JNEUROSCI.07-10-03154.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzer-Serhan UH, Leslie FM. Expression of alpha2A adrenoceptors during rat neocortical development. J. Neurobiol. 1999;38:259–269. doi: 10.1002/(sici)1097-4695(19990205)38:2<259::aid-neu8>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Yeh KY. Corticosterone concentrations in the serum and milk of lactating rats: Parallel changes after induced stress. Endocrinology. 1984;115:1364–1370. doi: 10.1210/endo-115-4-1364. [DOI] [PubMed] [Google Scholar]
- Yuan Q, Harley CW, Bruce JC, Darby-King A, McLean JH. Isoproterenol increases CREB phosphorylation and olfactory bulb nerve-evoked potentials in normal and 5-HT-depleted olfactory bulb rat pups only at doses that produce odor preference learning. Learn. Mem. 2000;7:413–421. doi: 10.1101/lm.35900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q, Harley CW, McLean JH. Mitral cell β 5-HT2A receptor co-localization and cAMP co-regulation: A new model of norepinephrine-induced learning in the olfactory bulb. Learn. Mem. 2003a;10:5–15. doi: 10.1101/lm.54803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q, Harley CW, McLean JH, Knopfel T. Optical imaging of odor preference memory in the rat olfactory bulb. J. Neurophysiol. 2003b;87:3156–3159. doi: 10.1152/jn.00917.2001. [DOI] [PubMed] [Google Scholar]