Abstract
Background and purpose:
Much interest is currently being focused on the anti-nociceptive effects mediated by nicotinic acetylcholine (nACh) receptors, including their location and mechanism of action. The purpose of this study was to elucidate these issues using 5-iodo-3-(2(S)-azetidinylmethoxy)pyridine (5IA), a nACh receptor agonist, and [125I]5IA.
Experimental approach:
We partially ligated the sciatic nerve of Sprague-Dawley rat to induce neuropathic pain [Seltzer's partial sciatic nerve ligation (PSL) model]. We then examined the changes in nACh receptor density in the CNS using [125I]5IA autoradiography and the involvement of nACh receptors in anti-nociceptive effects in the region where changes occurred.
Key results:
Autoradiographic studies showed that the accumulation of [125I]5IA and the number of nACh receptors in the thalamus of PSL rats were increased about twofold compared with those in the sham-operated rats. No change was observed in other brain regions. Rats injected in the ventral posterolateral thalamic nucleus (VPL) with 5IA demonstrated a significant and dose-dependent anti-allodynic effect and this effect was completely antagonized by mecamylamine, injected with 5IA, into the VPL. The blockade of nACh receptors in the VPL by mecamylamine decreased by 70% the anti-allodynic effect of 5IA, given i.c.v. Moreover, mecamylamine given intra-VPL by itself, induced significant hyperalgesia.
Conclusions and implications:
Our findings suggest that the nACh receptors expressed in the VPL play an important role in the anti-allodynic effects produced by exogenous and endogenous agonists.
Keywords: nicotinic acetylcholine receptor, thalamus, ventral posterolateral thalamic nucleus, 5IA, anti-allodynic effect, neuropathic pain
Introduction
Numerous studies have shown that nicotinic acetylcholine (nACh) receptor agonists possess analgesic effects (Decker et al., 2004; Jain, 2004). Because these effects are not antagonized by opioid receptor antagonists and arise from the activation of nACh receptors (Bannon et al., 1998a; nomenclature follows Alexander et al., 2008), much interest has been focused on nACh receptors as a novel target of anti-nociceptive drugs that do not involve the opioid system. Although morphine is a very well known and effective analgesic, its usefulness for patients with neuropathic pain is limited (Bannon et al., 1998c; Sindrup and Jensen, 1999). In contrast, nACh receptor agonists are able to ameliorate neuropathic pain by affecting systems other than the opioids (Bannon et al., 1998b,c; Decker et al., 1998). Therefore, identification of the sites of action of nACh receptor agonists and the elucidation of their anti-allodynic mechanisms are desirable.
One of the most studied nACh receptor-acting analgesics is ABT-594. This compound is a selective and potent agonist with high affinity for α4β2-nACh receptors (predominant in the brain), low affinity for α7-nACh receptors and no affinity for α1β1γδ-nACh receptors (Donnelly-Roberts et al., 1998; Holladay et al., 1998). By virtue of this selectivity, ABT-594 has analgesic efficacy with an improved therapeutic window compared with non-selective agonists such as epibatidine (Bannon et al., 1998b).
Recently, 5-iodo-3-(2(S)-azetidinylmethoxy)pyridine (5-iodo-A-85380, 5IA), an analogue of ABT-594, was synthesized (Saji et al., 2002). It is also an α4β2-nACh receptor-specific agonist (Ueda et al., 2008) with a relatively good safety profile (Vaupel et al., 2003; Ueda et al., 2004). In addition, radiolabelled 5IA, [123/125I]5IA, was also developed (Saji et al., 2002) and was reported to be a promising ligand for imaging nACh receptors in rodents and in humans (Saji et al., 2002; Mamede et al., 2004; Mamede et al., 2007; Oishi et al., 2007; Brasic et al., 2009). Non-radioactive 5IA and [123/125I]5IA can be considered the same compound with regard to their biodistribution or metabolism. Therefore, the pharmacokinetics, receptor occupancy and binding potential (an index of nACh receptor density) of 5IA are able to be measured readily using [123/125I]5IA. Studies using 5IA have the advantage that pharmacodynamic effects can be compared directly with the pharmacokinetic profile of the compound.
Previous studies have demonstrated the up-regulation of spinal muscarinic acetylcholine receptors (Chen and Pan, 2003) and thalamic cannabinoid CB1 receptors (Siegling et al., 2001) in neuropathic pain models. It has been reported that the up-regulation of muscarinic and CB1 receptors contributes to the increased analgesic efficacy of each agonist. As nACh receptor agonists are also effective against neuropathic pain, up-regulation of nACh receptors may also occur in such models. We hypothesized that brain regions where changes in the density or function of nACh receptors occurred would play an important role in anti-nociceptive effects, provided that such changes were found under neuropathic conditions. In the present study, we used a partial sciatic nerve ligation (PSL) model of neuropathic pain (Seltzer et al., 1990) and examined the changes in nACh receptors in the PSL rats using [125I]5IA. Furthermore, based on the result, we investigated the involvement of the nACh receptors expressed in the region where changes occurred as a result of the model of neuropathic pain.
Methods
Animals
Animal care and experimental procedures were conducted in accordance with our institutional guidelines, and the experimental procedures were approved by the Kyoto University Animal Care Committee.
Male Sprague-Dawley rats weighing 200–250 g were purchased from Japan SLC Co., Ltd. (Hamamatsu, Japan). The rats were kept at a constant ambient temperature under a 12 h light/dark cycle with free access to food and water.
Surgical operation
The PSL neuropathic pain model was established according to a previously published method (Seltzer et al., 1990). Under sodium pentobarbital (50 mg·kg−1, i.p.) anesthesia, the right sciatic nerve was exposed just distal to the branch leading to the posterior biceps femoris/semitendinosus muscles. A 7–0 silk suture was inserted into the nerve and tightly ligated so that the dorsal 1/3–1/2 of the nerve was trapped in the ligature. In the sham-operated rats, the sciatic nerve was exposed, but was left intact.
After recovery from the surgery, the rats were implanted with stainless steel guide cannulae (o.d. 0.7 mm, i.d. 0.38 mm) under sodium pentobarbital (50 mg·kg−1, i.p.) anesthesia for i.c.v. or intra-ventral posterolateral thalamic nucleus (VPL) administration. The rats were placed into a stereotaxic apparatus (SR-5, Narishige Co., Ltd., Tokyo, Japan) and unilaterally implanted with a guide cannula above the lateral ventricle (0.8 mm posterior and 1.5 mm lateral to bregma, 2.0 mm below the outer surface of the skull) for i.c.v. administration or above the left VPL, which was contralateral to the nerve ligation (2.4 mm posterior and 3.3 mm lateral to bregma, 2.0 mm below the outer surface of the skull) for intra-VPL administration. The stereotaxic coordinates were determined following an atlas (Paxinos and Watson, 2005). The guide cannulae were held firmly in place using dental acrylic cement. After surgery, the rats were individually returned to their cages and left to recover for 5 days or more until the experiments.
von Frey filament test
Just before and 2 weeks after the PSL, tactile sensitivity was measured by the up-down method using calibrated von Frey filaments ranging from 0.07 to 26 g (North Coast Medical, Morgan Hill, CA, USA), in a previously described method with slight modifications (Marcil et al., 2006). Briefly, for testing, the rats were individually placed on an elevated wire mesh floor. After a habituation period of 15–30 min, the tactile stimulus was applied to the middle plantar area of the each paw by placing the von Frey filament perpendicular to the surface of the paw. The filament was held in this position with enough force to cause slight bending. Each trial involved 10 applications of filaments every 1–2 s. The threshold was determined as the filament of the lowest stiffness at which the rat responded (quick paw flick) in one or more of the trial. The rats that showed a lower threshold postoperatively than preoperatively in both paws were considered as demonstrating allodynia and were used in the following studies.
Effect of 5IA and/or mecamylamine on tactile allodynia
For i.c.v. administration, an injection cannula (o.d. 0.35 mm, i.d. 0.18 mm) was inserted 5.0 mm below the surface of the skull along the guide cannula. Then, various concentrations of 5IA (1–10 nmol in 5 µL) or vehicle were infused at 5 µL per rat with a constant rate of 10 µL·min−1 using a microsyringe pump (EP-60; Eicom Corporation, Kyoto, Japan). The cannula was retained in place for an additional 1 min to prevent backflow of the drugs. When required, mecamylamine (5 mg·kg−1) was injected subcutaneously 30 min before i.c.v. administration of 5IA.
For intra-VPL administration, an injection cannula was inserted 6.2 mm below the surface of the skull. Subsequently, vehicle, 5IA (1–50 nmol in 0.5 µL), mecamylamine (1–10 nmol in 0.5 µL) or a mixed solution of 5IA and mecamylamine (10 nmol each in 0.5 µL) was infused at 0.5 µL per rat with a constant rate of 0.4 µL·min−1. The cannula was retained in place for an additional 1 min.
For double injections, the cannula for intra-VPL administration was inserted intra-VPL, and mecamylamine (10 nmol in 0.5 µL) or vehicle was infused in the same way as described above. Five minutes later, the cannula for i.c.v. administration was inserted, and 5IA (10 nmol in 5 µL) or vehicle was infused as described above. Both cannulae were retained in place for an additional 1 min.
Just before and 15, 30, 60, 90 and 120 min after administration, tactile allodynia was evaluated using the von Frey filament test. The results were expressed as a percentage of the maximal possible effect (% MPE) according to the following formula:
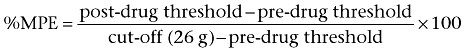 |
Inclined plane test
The inclined plane test was carried out using a sliding apparatus (Medical Agent Co., Ltd., Kyoto, Japan), as described previously (Okada et al., 2002). This procedure was used to evaluate the effects of anti-allodynic drugs on motor function (Fukui et al., 2001; Okada et al., 2002; Yasuda et al., 2005). Intraperitoneal administration of baclofen, which causes muscle relaxation, significantly reduced the slope angle at which rats were no longer able to maintain their position and slid down the table, thus indicating the appropriateness of this procedure (Fukui et al., 2001). Each rat was placed on a stainless steel plate inclined at 30°, and the angle of the plate was increased at a rate of 2°·s−1. The maximum angle of the plane at which the rat maintained its body position without sliding down was determined.
The rats were habituated to the procedure three times per day. After 2 days of habituation, the test was performed three times, and the mean of the last two values was taken as a control. Soon after measuring the control value, drugs or vehicle were administered via each route, as described above, and the maximum angle before sliding down was determined at 15, 30, 60, 90 and 120 min after administration. Drugs were used at the maximum concentration for each administration route. The data are represented as a percentage of the control value.
Ex vivo autoradiography
Ex vivo autoradiography was performed using a previously described method (Kanegawa et al., 2006) with slight modifications. Three groups of rats, a sham-operated group, a 2 week and a 1 month post-PSL group, were used in this experiment. Each group consisted of four to seven animals.
Each rat was injected with 2 MBq of [125I]5IA via its tail vein and killed 60 min after radioligand injection. Their brains were quickly removed, frozen in hexane (−80°C) and cut into 20 µm-thick coronal sections with a cryomicrotome (CM3000, Leica, Germany). The sections were exposed to imaging plates (BAS-UR, Fuji Photo Film Co., Ltd., Tokyo, Japan) with 125I autoradiographic microscale standards (Amersham Biosciences, Buckinghamshire, UK). After 25 h exposure, the [125I]5IA autoradiograms were obtained using a bioimaging analyzer (BAS 3000, Fuji Photo Film Co., Ltd., Tokyo, Japan), and quantitative densitometric analyses were performed with dedicated software (Image Gauge ver. 3.1, Fuji Photo Film Co., Ltd., Tokyo, Japan). Regions of interest were drawn over eight brain regions bilaterally [frontal cortex, striatum, hippocampus, thalamus, pedunculopontine tegmental nucleus (PPTg), nucleus raphe magnus (NRM), locus coeruleus and cerebellum]. Data were represented as the mean of the ligated and contralateral side.
In vitro autoradiography
Three groups of rats were used as described for the ex vivo autoradiography. Each group consisted of five to seven animals. The rats were killed and their brains were removed and frozen immediately. Each frozen brain was cut into 20 µm-thick coronal sections, thaw-mounted onto gelatin-coated glass slides and kept frozen at −80°C until use.
A method for the autoradiographic determination of receptor density was previously published (Tanaka et al., 1993; Suzdak et al., 1994). A binding assay was conducted using a modification of a previously described method (Mukhin et al., 2000). The assay buffer used was 50 mM Tris-HCl (pH 7.0) containing 120 mM NaCl, 5 mM KCl, 2.5 mM CaCl2 and 1 mM MgCl2. The sections were incubated with 23.75–380 pM [125I]5IA (specific activity: 220 Ci·mmol−1) in the same buffer for 1 h at 25°C, then rinsed twice in ice-cold buffer for 5 min each time, and once in distilled water for 1 min, and dried under a cold air stream. Non-specific binding was determined in the presence of 300 µM (–)-nicotine. The sections were exposed to the imaging plates with 125I standards for 20 h. The autoradiograms were obtained and the quantitative analyses were performed in a similar way to that described above. Values for the maximum density of binding sites (Bmax) were gained from saturation binding isotherms (one-site binding) of specific binding by means of nonlinear curve fitting (Prism 5.00, GraphPad Software, San Diego, CA, USA).
Statistical analyses
After the behavioural experiments, the rats were killed, and their brains were removed and frozen immediately. Coronal sections (20 µm) including the VPL were prepared and thaw-mounted onto gelatin-coated glass slides and subjected to Nissl staining. The placements of the tips of the injection cannulae were confirmed by using the atlas (Paxinos and Watson, 2005). Only data from rats with the injection cannulae correctly placed in the VPL were used for the statistical analyses.
The analyses of the data from the von Frey filament test and the inclined plane test were performed using two-way analysis of variance (anova) with repeated measures. If there was a significant difference, a post hoc one-way anova followed by a Tukey-Kramer multiple comparison test was performed using each treatment combination as an independent group. Analyses of the data from the autoradiographic studies were performed using a one-way anova followed by a Tukey-Kramer multiple comparison test. Differences were considered significant when P < 0.05.
Materials
5IA and [125I]5IA were synthesized according to a previously published method (Saji et al., 2002). Mecamylamine hydrochloride, a nACh receptor antagonist, was purchased from Sigma-Aldrich (St. Louis, MO, USA). The sodium pentobarbital for injection was purchased from Dainippon Sumitomo Pharma Co., Ltd. (Osaka, Japan). All drugs were administered in physiological saline solution. All other chemicals used were of reagent grade.
Results
Anti-allodynic effect of i.c.v. administered 5IA
In the present study, the rats presented a bilateral tactile allodynia following the PSL. The paw withdrawal thresholds decreased from 13.4 ± 0.9 g to 3.1 ± 0.3 g and from 13.4 ± 1.0 g to 4.2 ± 0.4 g in the ligated paws and the contralateral paws respectively. First, we evaluated an anti-allodynic effect of i.c.v. administered 5IA, as there has been no previous report regarding whether 5IA is able to palliate neuropathic pain. Figure 1A shows the anti-allodynic effect observed in the ligated paws. Two-way anova demonstrated significant main effects of treatment (F3,90= 5.56, P= 0.007) and time (F4,90= 7.51, P < 0.001) and a significant interaction between treatment and time (F12,90= 2.85, P= 0.003). Injection of 5IA (10 nmol) increased % MPE values significantly at 15 min after administration (Vehicle: −3.4 ± 2, 10 nmol: 38 ± 6). Although the effect peaked at 15 min after administration and decreased gradually, significant increases in % MPE values were also observed in the 3 nmol- and 10 nmol-treated groups 30 min after administration. Similar results were also observed in the contralateral paws (data not shown).
Figure 1.
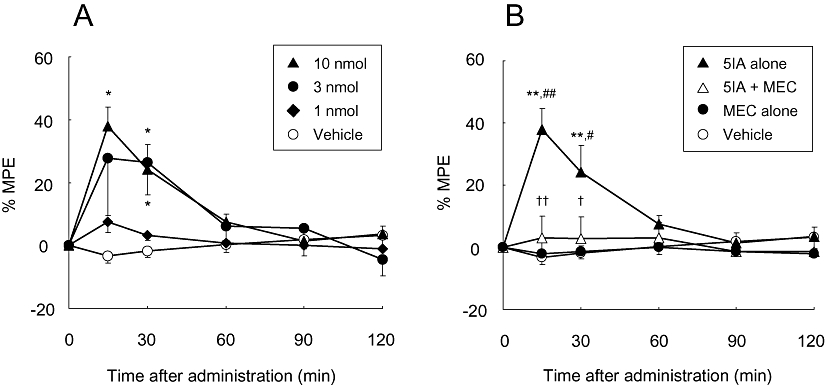
(A) Effect of i.c.v. administered 5IA on neuropathic tactile allodynia. Data are presented as a percentage of the maximum possible effect (% MPE). Each point represents the mean ± SEM of the ligated paws of five to six animals per group. *P < 0.05 versus vehicle at the same time point. (B) Effect of mecamylamine (MEC, 5 mg·kg−1, s.c., 30 min prior) on the anti-allodynic effect induced by 5IA (i.c.v.). Data are presented as a percentage of the maximum possible effect. Each point represents the mean ± SEM of the ligated paws of five to six animals per group. **P < 0.01 versus vehicle, #P < 0.05, ##P < 0.01 versus MEC alone, and †P < 0.05, ††P < 0.01 versus 5IA alone at the same time point. 5IA, 5-iodo-3-(2(S)-azetidinylmethoxy)pyridine; MPE, maximal possible effect.
Next, we examined an effect of mecamylamine, a blood–brain barrier permeable nACh receptor antagonist, on the anti-allodynic effect of 5IA. Significant main effects of treatment (F3,95= 6.75, P= 0.003) and time (F4,95= 5.61, P < 0.001) and a significant interaction between treatment and time (F12,95= 7.44, P < 0.001) were found. Mecamylamine (5 mg·kg−1, s.c.) blocked the effect of 5IA completely and the % MPE values were reduced to levels obtained after treatment with vehicle (Figure 1B).
Changes in the accumulation of [125I]5IA in vivo
We compared [125I]5IA accumulation between the PSL model rats and the sham-operated rats using an ex vivo autoradiographic method. Representative autoradiograms are shown in Figure 2A. The thalamic signals of the PSL groups were stronger than those of the sham-operated group, while the cortical and hippocampal signals were similar among the three groups. There was no difference between the ligated and contralateral sides of the PSL model rats. A significant increase (170%) in the accumulation of [125I]5IA was observed only in the thalamus in both PSL groups and no detectable change was seen in other regions (Figure 2B).
Figure 2.
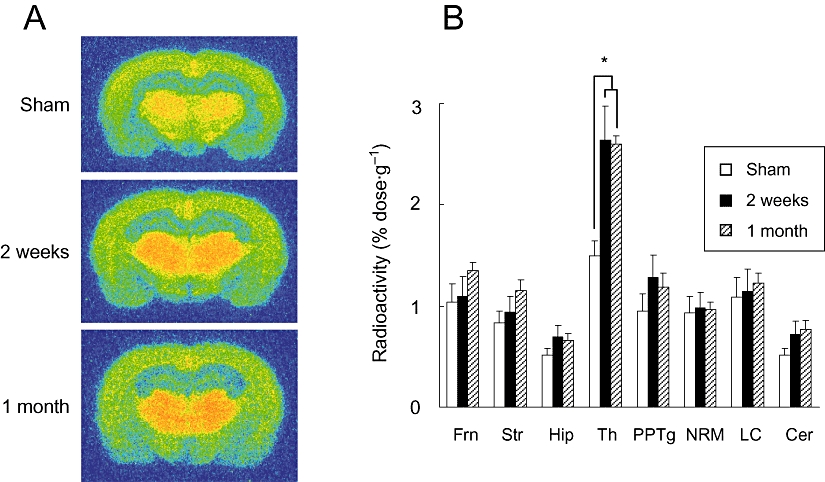
(A) Representative autoradiograms of brain regions from sham-operated and PSL rats given [125I]5IA, i.v. (B) Changes in the regional accumulation of [125I]5IA in Seltzer's PSL model of neuropathic pain. A significant increase was observed only in the thalamus, and no detectable change occurred in other regions. Each column represents the mean ± SEM of four to seven animals per group. *P < 0.05 versus sham. PSL, partial sciatic nerve ligation; 5IA, 5-iodo-3-(2(S)-azetidinylmethoxy)pyridine; Frn, frontal cortex; Str, striatum; Hip, hippocampus; Th, thalamus; PPTg, pedunculopontine tegmental nucleus; NRM, nucleus raphe magnus; LC, locus coeruleus; Cer, cerebellum.
Measurement of the density of nACh receptors in vitro
We performed an autoradiographic saturation assay using [125I]5IA as a radioligand. The saturation binding curves are presented in Figure 3A. As with the ex vivo autoradiography, both PSL groups showed a significant increase in Bmax value that occurred in the thalamus only (Figure 3B). The percentage increase in Bmax values in the thalamus (150%) was similar to those observed in the ex vivo autoradiographic findings.
Figure 3.
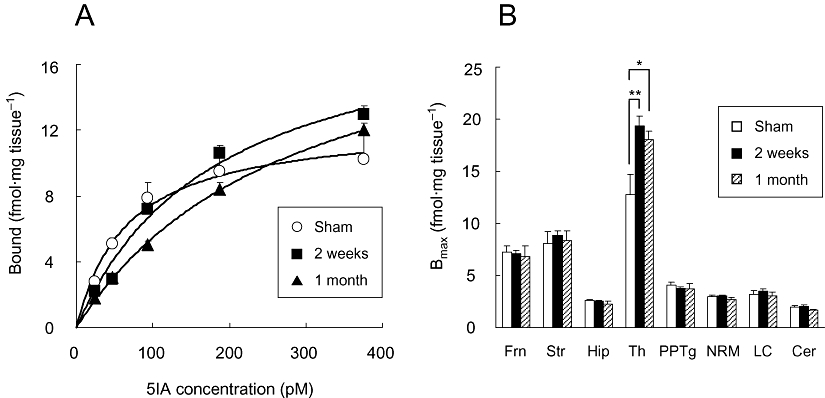
(A) Saturation curves of [125I]5IA binding to sham-operated and PSL rat thalamus determined ex vivo. Each point represents the mean ± SEM of five to seven animals per group. The Bmax values estimated by nonlinear regression analysis of these mean data were 12.5, 19.9 and 21.2 fmol/mg protein in sham-operated, 2 week post-PSL, and 1 month post-PSL group respectively. (B) Changes in nACh receptor density in brain regions of rats with Seltzer's PSL model of neuropathic pain. A significant increase was observed only in the thalamus, and no detectable change occurred in other regions. Each column represents the mean ± SEM of five to seven animals per group. *P < 0.05, **P < 0.01 versus sham. PSL, partial sciatic nerve ligation; 5IA, 5-iodo-3-(2(S)-azetidinylmethoxy)pyridine; Frn, frontal cortex; Str, striatum; Hip, hippocampus; Th, thalamus; PPTg, pedunculopontine tegmental nucleus; NRM, nucleus raphe magnus; LC, locus coeruleus; Cer, cerebellum.
Anti-allodynic effect of 5IA given intra-VPL
Based on previous reports (Kupers and Gybels, 1993; Derbyshire et al., 1997; Gybels, 2001), we predicted that the VPL was involved in the expression of anti-nociceptive effects and investigated the association between nACh receptors expressed in the VPL and the anti-allodynic effect of 5IA. Figure 4A shows the anti-allodynic effects that occurred in the ligated paws of rats that received 5IA intra-VPL. Two-way anova demonstrated significant main effects of treatment (F4,115= 3.97, P= 0.014) and time (F4,115= 13.6, P < 0.001) and a significant interaction between treatment and time (F16,115= 3.11, P < 0.001). There was a significant difference between the 50 nmol-treated group and the vehicle- or 1 nmol-treated groups. Although one-way anova revealed a significant difference between the data at 30 min after administration (P= 0.028), the post hoc Tukey test showed no significant difference.
Figure 4.
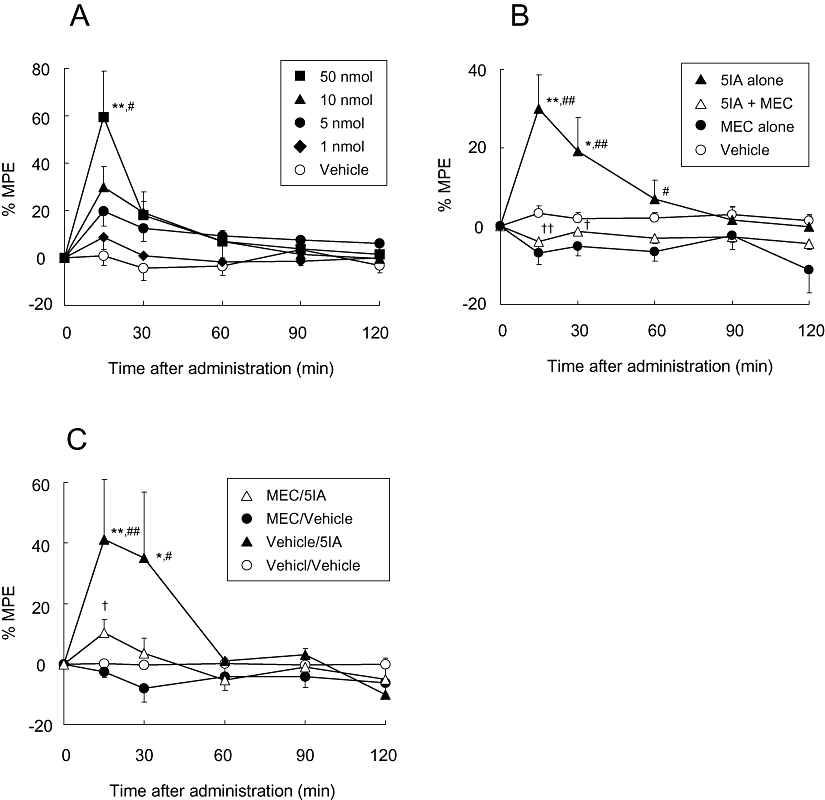
(A) Effect of 5IA injected intra-VPL on neuropathic tactile allodynia. Data are presented as a percentage of the maximum possible effect (% MPE). Each point represents the mean ± SEM of the ligated paws of five to six animals per group. *P < 0.05, **P < 0.01 versus vehicle, and #P < 0.05 versus 1 nmol of 5IA at the same time point. (B) Effect of mecamylamine (MEC, 10 nmol in 0.5 µL) administered concurrently with 5IA in an intra-VPL manner on the anti-allodynic effect produced by 5IA (10 nmol in 0.5 µL, intra-VPL). Data are presented as a percentage of the maximum possible effect. Each point represents the mean ± SEM of the ligated paws of five to six animals per group. *P < 0.05, **P < 0.01 versus vehicle, #P < 0.05, ##P < 0.01 versus MEC alone, and †P < 0.05, ††P < 0.01 versus 5IA alone at the same time point. (C) Effect of mecamylamine (MEC, 10 nmol in 0.5 µL, intra-VPL, 5 min prior) on the anti-allodynic effect produced by 5IA (10 nmol in 5 µL, i.c.v.). Data are presented as a percentage of the maximum possible effect. Each point represents the mean ± SEM of the ligated paws of four to seven animals per group. *P < 0.05, **P < 0.01 versus vehicle/vehicle, #P < 0.05, ##P < 0.01 versus MEC/vehicle, and †P < 0.05 versus vehicle/5IA at the same time point. 5IA, 5-iodo-3-(2(S)-azetidinylmethoxy)pyridine; MPE, maximal possible effect; VPL, ventral posterolateral thalamic nucleus.
Subsequently, we injected a mixed solution of 5IA and mecamylamine (10 nmol each) intra-VPL and performed the von Frey filament test. Two-way anova demonstrated significant main effects of treatment (F3,85= 11.34, P < 0.001) and time (F4,85= 5.22, P= 0.001) and a significant interaction between treatment and time (F12,85= 4.05, P < 0.001). Mecamylamine antagonized the 5IA-induced anti-allodynic effect completely, and furthermore, the % MPE values of mecamylamine-treated rats tended to decrease compared with those of the vehicle-treated rats, although the difference was not significant (Figure 4B).
Involvement of nACh receptors in the VPL in the anti-allodynic effect induced by i.c.v. 5IA administration
To explore to what extent nACh receptors in the VPL were involved in the anti-allodynic effects arising from central nACh receptor activation, we evaluated the effect of intra-VPL administered mecamylamine on the anti-allodynic effects produced by i.c.v. administered 5IA. The results from the ligated paws are illustrated in Figure 4C. Two-way anova demonstrated significant main effects of treatment (F3,105= 7.91, P= 0.001) and time (F4,105= 5.65, P < 0.001) and a significant interaction between treatment and time (F12,105= 2.49, P= 0.008). The rats administered vehicle intra-VPL followed by 10 nmol of 5IA i.c.v. (Vehicle/5IA group) demonstrated significantly elevated % MPE values at 15 and 30 min after administration. This result was consistent with the anti-allodynic effect produced by i.c.v. administered 5IA (Figure 1). Pretreatment with intra-VPL mecamylamine (10 nmol) significantly inhibited the effect of i.c.v. administered 5IA (10 nmol). The % MPE values for rats treated with mecamylamine alone (MEC/Vehicle group) also tended to decrease compared with those of the vehicle treated rats, although the difference was not significant.
Hyperalgesic effect of intra-VPL administered mecamylamine
To examine the effects of mecamylamine on tactile allodynia in more detail, we injected multiple doses of mecamylamine intra-VPL and performed the von Frey filament test. Data from the ligated paws are shown in Figure 5A. There was a significant main effect of treatment (F3,95= 6.77, P= 0.003) but no effect of time nor interaction between time and treatment. The post hoc Tukey test demonstrated a significant difference between vehicle-treated group and 5 nmol- or 10 nmol-treated groups. The calculated area under the curves of each group clearly demonstrated the dose-dependent hyperalgesic effects of mecamylamine (Figure 5B).
Figure 5.
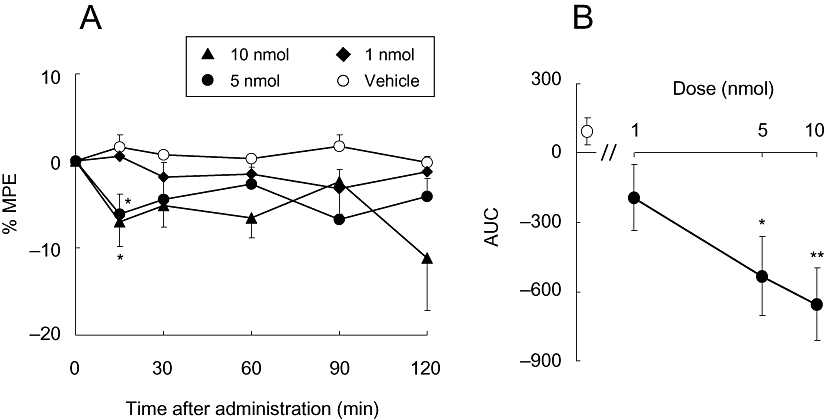
(A) Effect of mecamylamine injected intra-VPL on neuropathic tactile allodynia. Data are presented as a percentage of the maximum possible effect (% MPE). Each point represents the mean ± SEM of the ligated paws of five to six animals per group. *P < 0.05 versus vehicle at the same time point. (B) Dose-response curve of mecamylamine-induced hyperalgesia. The open symbol represents the AUC of the vehicle-treated group, and the closed symbols represent that of each concentration of the mecamylamine-treated group. *P < 0.05, **P < 0.01 versus vehicle. AUC, area under the curves; MPE, maximal possible effect; VPL, ventral posterolateral thalamic nucleus.
Effects of 5IA and mecamylamine on motor function
We conducted an inclined plane test to evaluate the effects of 5IA and mecamylamine on general motor functions. The maximum angle that the rats were able to endure before slipping down the plane was not significantly different between the vehicle and drug-treated groups at any time point (Table 1, data not shown for mecamylamine).
Table 1.
Effects of 5IA on motor functions
| Drug |
Time after administration |
||||
|---|---|---|---|---|---|
| 15 min | 30 min | 60 min | 90 min | 120 min | |
| i.c.v. | |||||
| Vehicle | 98 ± 1 | 102 ± 4 | 98 ± 2 | 92 ± 3 | 94 ± 3 |
| 5IA | 93 ± 2 | 97 ± 3 | 88 ± 4 | 96 ± 3 | 93 ± 1 |
| Intra-VPL | |||||
| Vehicle | 99 ± 3 | 99 ± 2 | 97 ± 4 | 100 ± 2 | 97 ± 3 |
| 5IA | 100 ± 4 | 97 ± 2 | 99 ± 2 | 97 ± 1 | 102 ± 2 |
Data are expressed as a percentage of the control level (‘angle of sliding’), which was determined before drug administration in each animal, and represent the mean ± SEM of five to six animals per group. No significant difference was observed between the two groups (two-way anova with repeated measures).
5IA, 5-iodo-3-(2(S)-azetidinylmethoxy)pyridine; VPL, ventral posterolateral thalamic nucleus.
Discussion and Conclusions
In the present study, we examined the changes in central nACh receptor density in a rat model of neuropathic pain and the involvement of nACh receptors in anti-nociceptive effects in the region where changes occurred. First, we evaluated the anti-allodynic effect of 5IA after i.c.v. administration and found that the anti-allodynic effect was dose-dependent. Because the effect was completely antagonized by mecamylamine, the anti-allodynic effect of 5IA must occur via nACh receptors. No significant difference was observed between the vehicle and 5IA treated groups in the inclined plane test suggesting that 5IA exhibited anti-allodynic effects without affecting motor functions.
We found that bilateral up-regulation of thalamic nACh receptors occurred in the PSL model of neuropathic pain. This is the first report of up-regulation of thalamic nACh receptors under chronic painful conditions. Because the up-regulation of both muscarinic and cannabinoid CB1 receptors have been reported to contribute to the increased analgesic efficacy of each agonist (Siegling et al., 2001; Chen and Pan, 2003), up-regulated nACh receptors may also contribute to the potentiation of anti-allodynic effects produced by nACh receptor agonists and attenuate neuropathic pain. Consistent with Seltzer's report (Seltzer et al., 1990), the unilateral PSL caused bilateral tactile allodynia in the present study; and thus, the up-regulation must occur bilaterally. In the present study, we performed an autoradiographic saturation assay to determine the regional Bmax of nACh receptors. As no report concerning autoradiographic saturation assays using [125I]5IA has been published, it is important to validate the method used. Doura et al. (2008) performed quantitative autoradiography using [125I]5IA, but, in their study, they incubated brain slices with only a single concentration of [125I]5IA and did not perform a saturation assay. Thus, a direct comparison between our present data and theirs is not possible. Nevertheless, the ratios of the thalamus to the striatum or the cortex of our present data were consistent in the data reported by Doura et al. (2008), suggesting that our method was valid.
A previous PET study showed that the increase in cerebral blood flow in the VPL positively correlated with pain intensity, suggesting involvement of the VPL in pain transmission (Derbyshire et al., 1997). Furthermore, electrical stimulation of the VPL has produced pain alleviation in both rat models and patients with neuropathic pain (Kupers and Gybels, 1993; Gybels, 2001). These findings suggest that the VPL is involved in the expression of anti-nociceptive effects. Indeed, we demonstrated that 5IA administered locally into the VPL significantly and dose-dependently reversed tactile allodynia. This effect was antagonized by coadministered mecamylamine. As no significant difference was observed between the vehicle and 5IA treated groups in the results of an inclined plane test, the changes in % MPE values observed in the 5IA treated groups were considered to reflect the analgesic effect, not motor dysfunction. These findings suggest that the nACh receptors expressed in the VPL were involved in the anti-allodynic effect that occurred after nACh receptor agonist administration. This was consistent with the finding that blockade of nACh receptors in the VPL caused a decrease in the anti-allodynic effect of i.c.v.-administered 5IA. Moreover, the intra-VPL injection of mecamylamine alone induced significant decreases in % MPE values. Mecamylamine is not an inverse agonist, but a non-competitive antagonist (Jensen et al., 2005). Thus, during neuropathic pain, an intrinsic anti-allodynic mechanism by which ACh activates nACh receptors expressed in the VPL may be present and antagonism of these receptors by mecamylamine causes the hyperalgesic effect. That is to say, the nACh receptors expressed in the VPL may participate in anti-allodynic effects produced not only by exogenous but also endogenous agonists.
The present findings do not negate the involvement of nACh receptors in anti-allodynic effects outside of the VPL. Previous pharmacological studies reported that the central sites involved in nACh receptor-mediated antinociception were the NRM and the PPTg. The antinociception produced by nicotinic stimulation of the PPTg or the NRM depended upon muscarinic cholinergic, 5-hydroxytryptaminergic and adrenergic systems at the level of the lumbar spinal cord (Iwamoto and Marion, 1993). Curzon et al. (1998) showed that microinjection of nACh receptor agonists (epibatidine and A-85380) into the NRM produced antinociception against heat stimuli, and these effects were prevented by coadministration of mecamylamine into the NRM. Indeed, we demonstrated that VPL blockade by mecamylamine before i.c.v. administration of 5IA decreased the anti-allodynic effect by up to approximately 70%, not 100%. Thus, the remainder of the anti-allodynic effect is possibly caused by the binding of 5IA to the NRM and/or the PPTg.
Recently, Mogg et al. (2004) have reported that 5IA can functionally activate the α4β2-nACh and α6β2-nACh receptors. However, Perry et al. have reported that the nACh receptors expressed in the thalamus are mainly of the α4β2 sub-type (Perry et al., 2002) and that the α6 subunit is present in less than 4% of the thalamic nACh receptors (Perry et al., 2007). Therefore, the anti-allodynic effect we reported was probably mediated via the α4β2-nACh receptors, at least when 5IA was administered into the VPL. Whereas many results have shown that α4 subunit-containing nACh receptors play an important role in nACh receptor-mediated antinociception (Bitner et al., 1998; Marubio et al., 1999; Bitner et al., 2000), the contribution of the α6β2-nACh receptors to the anti-nociceptive effect is unknown. As A-186253 has been developed as a specific antagonist of the α4β2-nACh receptors (Itier et al., 2004), it may be possible to investigate α6β2-nACh receptor-mediated antinociception using both 5IA and A-186253.
In summary, we have demonstrated for the first time that up-regulation of thalamic nACh receptors occurs in a model of chronic pain. Moreover, we found that intra-VPL administration of 5IA attenuated tactile allodynia, dose-dependently. This effect was completely antagonized by co-administered mecamylamine. The blockade of nACh receptors in the VPL by mecamylamine caused a decrease in the anti-allodynic effect of i.c.v.-administered 5IA. These findings indicate that the nACh receptors expressed in the VPL are a potential site of the anti-nociceptive action produced by 5IA. Furthermore, mecamylamine, given intra-VPL, induced a hyperalgesic effect. This effect is likely to be responsible for the mecamylamine antagonism of the intrinsic anti-allodynic mechanism induced by endogenous acetylcholine. These findings suggest that the nACh receptors expressed in the VPL play an important role in the anti-allodynic effects produced by exogenous and endogenous agonists.
Acknowledgments
We thank Dr Takayuki Nakagawa (Department of Molecular Pharmacology, Graduate School of Pharmaceutical Sciences, Kyoto University) for his technical support and invaluable discussion regarding the behavioural tests. This work was supported in part by a Grant-in-Aid for Scientific Research (A) (19209041) and a Grant-in-Aid for Young Scientists (B) (19790869) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and a grant from the Smoking Research Foundation.
Glossary
Abbreviations:
- 5IA
5-iodo-3-(2(S)-azetidinylmethoxy)pyridine
- MPE
maximal possible effect
- nACh
nicotinic acetylcholine
- NRM
nucleus raphe magnus
- PPTg
pedunculopontine tegmental nucleus
- VPL
ventral posterolateral thalamic nucleus
Conflicts of interest
The authors state that they have no conflicts of interest.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edn. Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannon AW, Decker MW, Curzon P, Buckley MJ, Kim DJ, Radek RJ, et al. ABT-594 [(R)-5-(2-azetidinylmethoxy)-2-chloropyridine]: a novel, orally effective antinociceptive agent acting via neuronal nicotinic acetylcholine receptors: II. In vivo characterization. J Pharmacol Exp Ther. 1998a;285:787–794. [PubMed] [Google Scholar]
- Bannon AW, Decker MW, Holladay MW, Curzon P, Donnelly-Roberts D, Puttfarcken PS, et al. Broad-spectrum, non-opioid analgesic activity by selective modulation of neuronal nicotinic acetylcholine receptors. Science. 1998b;279:77–81. doi: 10.1126/science.279.5347.77. [DOI] [PubMed] [Google Scholar]
- Bannon AW, Decker MW, Kim DJ, Campbell JE, Arneric SP. ABT-594, a novel cholinergic channel modulator, is efficacious in nerve ligation and diabetic neuropathy models of neuropathic pain. Brain Res. 1998c;801:158–163. doi: 10.1016/s0006-8993(98)00596-4. [DOI] [PubMed] [Google Scholar]
- Bitner RS, Nikkel AL, Curzon P, Arneric SP, Bannon AW, Decker MW. Role of the nucleus raphe magnus in antinociception produced by ABT-594: immediate early gene responses possibly linked to neuronal nicotinic acetylcholine receptors on serotonergic neurons. J Neurosci. 1998;18:5426–5432. doi: 10.1523/JNEUROSCI.18-14-05426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitner RS, Nikkel AL, Curzon P, Donnelly-Roberts DL, Puttfarcken PS, Namovic M, et al. Reduced nicotinic receptor-mediated antinociception following in vivo antisense knock-down in rat. Brain Res. 2000;871:66–74. doi: 10.1016/s0006-8993(00)02442-2. [DOI] [PubMed] [Google Scholar]
- Brasic JR, Zhou Y, Musachio JL, Hilton J, Fan H, Crabb A, et al. Single photon emission computed tomography experience with (S)-5-[(123)I]iodo-3-(2-azetidinylmethoxy)pyridine in the living human brain of smokers and nonsmokers. Synapse. 2009;63:339–358. doi: 10.1002/syn.20611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SR, Pan HL. Up-regulation of spinal muscarinic receptors and increased antinociceptive effect of intrathecal muscarine in diabetic rats. J Pharmacol Exp Ther. 2003;307:676–681. doi: 10.1124/jpet.103.055905. [DOI] [PubMed] [Google Scholar]
- Curzon P, Nikkel AL, Bannon AW, Arneric SP, Decker MW. Differences between the antinociceptive effects of the cholinergic channel activators A-85380 and (+/−)-epibatidine in rats. J Pharmacol Exp Ther. 1998;287:847–853. [PubMed] [Google Scholar]
- Decker MW, Curzon P, Holladay MW, Nikkel AL, Bitner RS, Bannon AW, et al. The role of neuronal nicotinic acetylcholine receptors in antinociception: effects of ABT-594. J Physiol Paris. 1998;92:221–224. doi: 10.1016/s0928-4257(98)80014-4. [DOI] [PubMed] [Google Scholar]
- Decker MW, Rueter LE, Bitner RS. Nicotinic acetylcholine receptor agonists: a potential new class of analgesics. Curr Top Med Chem. 2004;4:369–384. doi: 10.2174/1568026043451447. [DOI] [PubMed] [Google Scholar]
- Derbyshire SW, Jones AK, Gyulai F, Clark S, Townsend D, Firestone LL. Pain processing during three levels of noxious stimulation produces differential patterns of central activity. Pain. 1997;73:431–445. doi: 10.1016/S0304-3959(97)00138-3. [DOI] [PubMed] [Google Scholar]
- Donnelly-Roberts DL, Puttfarcken PS, Kuntzweiler TA, Briggs CA, Anderson DJ, Campbell JE, et al. ABT-594 [(R)-5-(2-azetidinylmethoxy)-2-chloropyridine]: a novel, orally effective analgesic acting via neuronal nicotinic acetylcholine receptors: I. In vitro characterization. J Pharmacol Exp Ther. 1998;285:777–786. [PubMed] [Google Scholar]
- Doura MB, Gold AB, Keller AB, Perry DC. Adult and periadolescent rats differ in expression of nicotinic cholinergic receptor subtypes and in the response of these subtypes to chronic nicotine exposure. Brain Res. 2008;1215:40–52. doi: 10.1016/j.brainres.2008.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui M, Nakagawa T, Minami M, Satoh M. Antinociceptive effects of intracerebroventricularly administered P2 purinoceptor agonists in the rat. Eur J Pharmacol. 2001;419:25–31. doi: 10.1016/s0014-2999(01)00947-5. [DOI] [PubMed] [Google Scholar]
- Gybels J. Thalamic stimulation in neuropathic pain: 27 years later. Acta Neurol Belg. 2001;101:65–71. [PubMed] [Google Scholar]
- Holladay MW, Wasicak JT, Lin NH, He Y, Ryther KB, Bannon AW, et al. Identification and initial structure-activity relationships of (R)-5-(2-azetidinylmethoxy)-2-chloropyridine (ABT-594), a potent, orally active, non-opiate analgesic agent acting via neuronal nicotinic acetylcholine receptors. J Med Chem. 1998;41:407–412. doi: 10.1021/jm9706224. [DOI] [PubMed] [Google Scholar]
- Itier V, Schonbachler R, Tribollet E, Honer M, Prinz K, Marguerat A, et al. A-186253, a specific antagonist of the alpha 4 beta 2 nAChRs: its properties and potential to study brain nicotinic acetylcholine receptors. Neuropharmacology. 2004;47:538–557. doi: 10.1016/j.neuropharm.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Iwamoto ET, Marion L. Adrenergic, serotonergic and cholinergic components of nicotinic antinociception in rats. J Pharmacol Exp Ther. 1993;265:777–789. [PubMed] [Google Scholar]
- Jain KK. Modulators of nicotinic acetylcholine receptors as analgesics. Curr Opin Investig Drugs. 2004;5:76–81. [PubMed] [Google Scholar]
- Jensen AA, Frolund B, Liljefors T, Krogsgaard-Larsen P. Neuronal nicotinic acetylcholine receptors: structural revelations, target identifications, and therapeutic inspirations. J Med Chem. 2005;48:4705–4745. doi: 10.1021/jm040219e. [DOI] [PubMed] [Google Scholar]
- Kanegawa N, Kiyono Y, Kimura H, Sugita T, Kajiyama S, Kawashima H, et al. Synthesis and evaluation of radioiodinated (S,S)-2-(alpha-(2-iodophenoxy)benzyl)morpholine for imaging brain norepinephrine transporter. Eur J Nucl Med Mol Imaging. 2006;33:639–647. doi: 10.1007/s00259-005-0017-y. [DOI] [PubMed] [Google Scholar]
- Kupers RC, Gybels JM. Electrical stimulation of the ventroposterolateral thalamic nucleus (VPL) reduces mechanical allodynia in a rat model of neuropathic pain. Neurosci Lett. 1993;150:95–98. doi: 10.1016/0304-3940(93)90116-3. [DOI] [PubMed] [Google Scholar]
- Mamede M, Ishizu K, Ueda M, Mukai T, Iida Y, Fukuyama H, et al. Quantification of human nicotinic acetylcholine receptors with 123I-5IA SPECT. J Nucl Med. 2004;45:1458–1470. [PubMed] [Google Scholar]
- Mamede M, Ishizu K, Ueda M, Mukai T, Iida Y, Kawashima H, et al. Temporal change in human nicotinic acetylcholine receptor after smoking cessation: 5IA SPECT study. J Nucl Med. 2007;48:1829–1835. doi: 10.2967/jnumed.107.043471. [DOI] [PubMed] [Google Scholar]
- Marcil J, Walczak JS, Guindon J, Ngoc AH, Lu S, Beaulieu P. Antinociceptive effects of tetrodotoxin (TTX) in rodents. Br J Anaesth. 2006;96:761–768. doi: 10.1093/bja/ael096. [DOI] [PubMed] [Google Scholar]
- Marubio LM, del Mar Arroyo-Jimenez M, Cordero-Erausquin M, Lena C, Le Novere N, de Kerchove d'Exaerde A, et al. Reduced antinociception in mice lacking neuronal nicotinic receptor subunits. Nature. 1999;398:805–810. doi: 10.1038/19756. [DOI] [PubMed] [Google Scholar]
- Mogg AJ, Jones FA, Pullar IA, Sharples CG, Wonnacott S. Functional responses and subunit composition of presynaptic nicotinic receptor subtypes explored using the novel agonist 5-iodo-A-85380. Neuropharmacology. 2004;47:848–859. doi: 10.1016/j.neuropharm.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Mukhin AG, Gundisch D, Horti AG, Koren AO, Tamagnan G, Kimes AS, et al. 5-Iodo-A-85380, an alpha4beta2 subtype-selective ligand for nicotinic acetylcholine receptors. Mol Pharmacol. 2000;57:642–669. doi: 10.1124/mol.57.3.642. [DOI] [PubMed] [Google Scholar]
- Oishi N, Hashikawa K, Yoshida H, Ishizu K, Ueda M, Kawashima H, et al. Quantification of nicotinic acetylcholine receptors in Parkinson's disease with (123)I-5IA SPECT. J Neurol Sci. 2007;256:52–60. doi: 10.1016/j.jns.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Okada M, Nakagawa T, Minami M, Satoh M. Analgesic effects of intrathecal administration of P2Y nucleotide receptor agonists UTP and UDP in normal and neuropathic pain model rats. J Pharmacol Exp Ther. 2002;303:66–73. doi: 10.1124/jpet.102.036079. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 5th edn. San Diego, CA: Elsevier Academic Press; 2005. [Google Scholar]
- Perry DC, Xiao Y, Nguyen HN, Musachio JL, Davila-Garcia MI, Kellar KJ. Measuring nicotinic receptors with characteristics of alpha4beta2, alpha3beta2 and alpha3beta4 subtypes in rat tissues by autoradiography. J Neurochem. 2002;82:468–481. doi: 10.1046/j.1471-4159.2002.00951.x. [DOI] [PubMed] [Google Scholar]
- Perry DC, Mao D, Gold AB, McIntosh JM, Pezzullo JC, Kellar KJ. Chronic nicotine differentially regulates alpha6- and beta3-containing nicotinic cholinergic receptors in rat brain. J Pharmacol Exp Ther. 2007;322:306–315. doi: 10.1124/jpet.107.121228. [DOI] [PubMed] [Google Scholar]
- Saji H, Ogawa M, Ueda M, Iida Y, Magata Y, Tominaga A, et al. Evaluation of radioiodinated 5-iodo-3-(2(S)-azetidinylmethoxy)pyridine as a ligand for SPECT investigations of brain nicotinic acetylcholine receptors. Ann Nucl Med. 2002;16:189–200. doi: 10.1007/BF02996300. [DOI] [PubMed] [Google Scholar]
- Seltzer Z, Dubner R, Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990;43:205–218. doi: 10.1016/0304-3959(90)91074-S. [DOI] [PubMed] [Google Scholar]
- Siegling A, Hofmann HA, Denzer D, Mauler F, De Vry J. Cannabinoid CB(1) receptor upregulation in a rat model of chronic neuropathic pain. Eur J Pharmacol. 2001;415:R5–R7. doi: 10.1016/s0014-2999(01)00798-1. [DOI] [PubMed] [Google Scholar]
- Sindrup SH, Jensen TS. Efficacy of pharmacological treatments of neuropathic pain: an update and effect related to mechanism of drug action. Pain. 1999;83:389–400. doi: 10.1016/S0304-3959(99)00154-2. [DOI] [PubMed] [Google Scholar]
- Suzdak PD, Foged C, Andersen KE. Quantitative autoradiographic characterization of the binding of [3H]tiagabine (NNC 05-328) to the GABA uptake carrier. Brain Res. 1994;647:231–241. doi: 10.1016/0006-8993(94)91322-6. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Fukuuchi Y, Gomi S, Takashima S, Mihara B, Shirai T, et al. Reduction in second-messenger ligand binding sites after brain ischemia – autoradiographic Bmax and Kd determinations using digital image analysis. Brain Res Bull. 1993;32:49–56. doi: 10.1016/0361-9230(93)90318-6. [DOI] [PubMed] [Google Scholar]
- Ueda M, Iida Y, Kitamura Y, Kawashima H, Ogawa M, Magata Y, et al. 5-Iodo-A-85380, a specific ligand for alpha4beta2 nicotinic acetylcholine receptors, prevents glutamate neurotoxicity in rat cortical cultured neurons. Brain Res. 2008;1199:46–52. doi: 10.1016/j.brainres.2007.10.107. [DOI] [PubMed] [Google Scholar]
- Ueda M, Iida Y, Mukai T, Mamede M, Ishizu K, Ogawa M, et al. 5-[123I]Iodo-A-85380: assessment of pharmacological safety, radiation dosimetry and SPECT imaging of brain nicotinic receptors in healthy human subjects. Ann Nucl Med. 2004;18:337–344. doi: 10.1007/BF02984473. [DOI] [PubMed] [Google Scholar]
- Vaupel DB, Tella SR, Huso DL, Mukhin AG, Baum I, Wagner VO, et al. Pharmacology, toxicology, and radiation dosimetry evaluation of [I-123]5-I-a-85380, a radioligand for in vivo imaging of cerebral neuronal nicotinic acetylcholine receptors in humans. Drug Dev Res. 2003;58:149–168. [Google Scholar]
- Yasuda T, Miki S, Yoshinaga N, Senba E. Effects of amitriptyline and gabapentin on bilateral hyperalgesia observed in an animal model of unilateral axotomy. Pain. 2005;115:161–170. doi: 10.1016/j.pain.2005.02.026. [DOI] [PubMed] [Google Scholar]


