Abstract
Background and purpose:
Nebivolol, a selective β1-adrenoceptor antagonist mediating rapid vasodilating effects, is used clinically to treat hypertension. Recently, it was reported that nebivolol also acts as an oestrogen receptor (ER) agonist. To investigate the neuroprotective potential of oestrogens, we assessed the oestrogenic effects of nebivolol in several in vitro neuronal models.
Experimental approach:
Human neuroepithelioma SK-N-MC cells stably transfected with human ER α and β, and mouse N2A neuroblastoma cells expressing human APP695SWE[N2Aswe, stably transfected with the Swedish mutation form of the Alzheimer-associated amyloid precursor protein (APPswe, K670M/N671L)] were incubated with different concentrations of nebivolol and 17β-oestradiol (E2) for 24–48 h. ER activation was detected in a specific reporter assay, and ER-dependent gene expression was measured by quantitative real-time PCR (qRT PCR). Furthermore, cell survival rates were determined, and oxidative stress was induced by hydrogen peroxide and paraquat. Amyloid β protein precursor (APP) processing was investigated, and the cleavage fragments sAPPα and Aβ were quantified via α-, β- and γ-secretase activity assays. Alterations of secretase expression levels were determined by qRT PCR.
Key results:
Nebivolol induces oestrogen-dependent gene transcription, and protects neuronal cells against oxidative stress even at low and physiological concentrations (10−8 M). Moreover, nebivolol modulates processing of APP in mouse neuronal N2Aswe cells by increasing α-secretase activity, ultimately leading to enhanced release of soluble non-amyloidogenic sAPPα.
Conclusions and implications:
We showed that nebivolol acts as ER agonist in neuronal cell lines, and suggest oestrogen-like neuroprotective effects mediated by nebivolol.
Keywords: oestrogen receptors, oxidative stress, neuroprotection, amyloid precursor protein, Alzheimer's disease, selective oestrogen receptor modulator
Introduction
Nebivolol is known to be a selective β1-adrenoceptor antagonist (Van de Water et al., 1988) with endothelium-dependent vasodilating properties mediated through stimulation of endothelial NOS (eNOS) (Broeders et al., 2000; Garban et al., 2004). Interestingly, at high concentrations, nebivolol also has antioxidant actions (Troost et al., 2000; De Groot et al., 2004; Mason et al., 2006; Wagenfeld et al., 2008), although the exact underlying mechanism is not well understood. In addition, it has been reported that nebivolol has oestrogen receptor (ER) agonistic properties (Garban et al., 2004; Grundt et al., 2007). These interesting activities of nebivolol combined with the fact that one central effect of the female sex hormone oestrogen (17β-oestradiol, E2) in endothelial cells is the activation of eNOS (Thomson et al., 1997; Nuedling et al., 1999; Su et al., 2002), suggest that some of the beneficial features of nebivolol as an anti-inflammatory compound (Garbin et al., 2008), reducing atherosclerosis (De Nigris et al., 2008) and osteoporosis (Toker et al., 2008), are based on its oestrogenic properties.
In the past few decades, it has become apparent that, in addition to their effects in sexual differentiation and behaviour, oestrogens modulate a plethora of physiological processes from bone formation, lipid metabolism, cardiovascular function and inflammation to synaptic plasticity (Turgeon et al., 2006). Several molecular mechanisms are known to be involved in the effects of oestrogen. First, a direct genomic mechanism, where nuclear forms of ER, ERα or ERβ activate or repress transcription of oestrogen-responsive genes (McEwen, 2001; Behl, 2002). Second, an indirect genomic effect mediated by oestrogen through an ER-linked second messenger system via ERs, G protein-coupled receptors or insulin-like growth factor-I receptor, which leads to a modulation of cell homeostasis and transcriptional activity (Cardona-Gomez et al., 2001; Mendez et al., 2005). Third, non-genomic effects at high concentrations involve antioxidant effects independently of known ERs and are based on the phenolic structure of oestrogen (Behl et al., 1995; 1997;). Various studies focusing on the neuroprotective capacity of oestrogens have shown that oestrogens provide some protective effects in acute and chronic neurodegenerative diseases like cerebral ischaemia, Alzheimer's disease (AD) and Parkinson's disease (Garcia-Segura et al., 2001; Behl, 2002; Brann et al., 2007).
Here, we describe the oestrogenic effects and potential neuroprotective activities of nebivolol by employing two neuronal in vitro models: the human neuroepithelioma SK-N-MC and mouse N2A neuroblastoma cells expressing human APP695SWE (N2Aswe) (Thinakaran et al., 1996). The stable transfection of the patient-derived so-called Swedish mutation form of the AD-associated APP (APPswe, K670M/N671L) leads to an enhanced neurotoxic amyloid β-protein (Aβ) 40 and 42 production (Pietrzik and Behl, 2005). To determine whether ERs and if so, which subtype, α or β, can be activated by nebivolol, we used SK-N-MC-cell clones stably over-expressing ERα (SK-ERα) or ERβ (SK-ERβ) (Manthey et al., 2001; Zschocke et al., 2002). To analyse the neuroprotective activities that may be relevant to AD, we used N2Aswe cells, which express both ER subtypes endogenously.
Methods
Cells and culture conditions
Experiments were performed with mouse N2A neuroblastoma cell lines expressing human APP695SWE (Thinakaran et al., 1996) and human neuroepithelioma SK-N-MC cells (ATCC no. HTB-10) stably transfected with human ERα and β cDNA (Manthey et al., 2001; Zschocke et al., 2003). Cells were cultured in phenol red-free Dulbecco's modified Eagle's medium (DMEM; Invitrogen Inc., San Diego, CA, USA) supplemented with 10% fetal bovine serum (FBS) under standard culture conditions as described previously (Manthey et al., 2001). For oestrogen-free conditions, the medium was replaced 24 h before experiments with phenol red-free DMEM with 1% charcoal/dextran-treated FBS supplemented with 10−4 M formestane to suppress endogenous oestrogen synthesis.
Experimental procedures
ER binding assay
The specific binding of nebivolol to the ER was analysed by radioligand binding assay as described by Garban et al. (2004). The ER-expressing cell line N2A was cultured 48 h prior to cell harvesting under oestrogen-free conditions using phenol red-free DMEM medium supplemented with 1% dextran-coated charcoal-treated FBS. The cells were harvested by incubating cells at 37°C for 5 min in trypsin EDTA (0.05% trypsin/53 mM EDTA). The cells were washed twice and finally resuspended in ice-cold PBS; 2 × 106 cells were incubated in microcentrifuge assay tubes at RT in the presence of 2 × 10−9 M (KD: 0.1–1 × 10−9 M) [2,4,6,7,16,17-3H]-E2 and different concentrations of nebivolol, from 2 × 10−2 M to 1 × 10−6 M. A 100-fold molar excess of unlabelled E2 was used to determine the specific displaceable binding in a separate tube. After incubation for 1 h, cells were washed twice in 1 mL of ice-cold PBS, and dissolved in 600 µL of 0.1 N NaOH; 10 µL aliquots from each sample were used for determination of total protein concentration by bicinchoninic acid (BCA)-based assay, and 250 µL was used in combination with 4 mL of scintillation fluid and counted in a Packard Tri-Carb LS Counter (PerkinElmer, Waltham, MA, USA). Results are expressed as the mean ± SEM of three independent measurements.
Luciferase reporter assays
For the functional quantification of nuclear receptor activity, we transiently transfected cells with plasmid DNA containing an oestrogen response element (ERE) (D-MTV-ERE-LUC; Moosmann and Behl, 1999), glucocorticoid response element (GRE) or mouse mammary tumour virus promoter containing four androgen response element (ARE) sequences (Yeh and Chang, 1996) linked to the firefly luciferase gene and cotransfected with a Renilla luciferase expressing plasmid (pRL) for control and normalization of the transfection efficiency. Twelve hours before transfection, cells were seeded at approximately 500 000 cells per well in six-well tissue culture dishes in phenol red-free DMEM supplemented with 10% charcoal/dextran-treated FBS. The transfection was carried out with FuGene HD as described by the supplier; 12 h after transfection, 8000 cells per well were plated on 96-well white microtitre plates, and treated immediately. After 24 h, cells were harvested and analysed with the Dual Luciferase Assay System. The luminescence readings were performed in an automatic counter (Wallac Victor3, PerkinElmer).
RNA extraction and quantification of mRNA expression
Total RNA was extracted 18 h after treatment of the cells using Agilent Total RNA Isolation Mini Kit (Agilent Technologies, Böblingen, Germany) according to the manufacturer's instructions. RNA samples were reversely transcribed by Verso cDNA Kit. Quantitative RT-PCR was performed on an iCycler PCR Thermocycler (Bio-Rad, Munich, Germany) using Absolute SYBRGreen Mix according to the manufacturer's recommendations with primer pairs and conditions listed in Table 1. Relative expression levels were calculated using REST-MCS (relative expression software tool) (Pfaffl et al., 2002), whereas glyceraldehyde-3-phosphate dehydrogenase and RPL19 were used as housekeeping and reference genes.
Table 1.
PCR primers and conditions
| Gene | Symbol | Species | GenBank accession no. | Primer sequence | Annealing temp. (°C)Elongation time (s)Product size (bp) |
|---|---|---|---|---|---|
| Androgen receptor | AR | Human | BC132975 | 5′- gtcaactccaggatgctctac-3′ | 62, 25, 321 |
| Mouse | M37890 | 5′-ccaggagcttggtgagctggta-3′ | |||
| ADAM metallopeptidase domain 9 | ADAM 9 | Human | BC126406 | 5′- cagaatggatatccttgccag-3′ | 62, 40, 496 |
| Mouse | BC047156 | 5′- tcacagtgacaattcttattgc-3′ | |||
| ADAM metallopeptidase domain 10 | ADAM 10 | Human | BC066207 | 5′- ggaacacgagaagctgtgattg-3′ | 62, 40, 229 |
| Mouse | AF009615 | 5′- cagtagtcatcatgattctgctc-3′ | |||
| ADAM metallopeptidase domain 17 | ADAM 17 | Human | BC146658 | 5′- ggattcctttcagcattcttgtc-3′ | 62, 40, 260 |
| Mouse | BC136783 | 5′- gtgagtctgtgctggggtcttc-3′ | |||
| Beta-site APP-cleaving enzyme 1 | BACE 1 | Human | NM_012104 | 5′-cgcagacgctcaacatcc-3′ | 62, 35, 139 |
| 5′- gggcacatacacacccttcc-3′ | |||||
| Mouse | NM_011792 | 5′- cagtgggaccaccaaccttc-3′ | 60, 30, 70 | ||
| 5′-gctgccttgatggacttgac-3′ | |||||
| Baculoviral IAP repeat-containing 3 | Birc3 | Human | AF070674 | 5′-tactacataggacctggaga-3′ | 62, 30, 383 |
| Mouse | NM_007464 | 5′-caagtactcacaccttggaaac-3′ | |||
| Heat shock 27 kDa protein 1 | Hsp B1 | Human | NM_001540 | 5′-ggacgagctgacggtcaag-3′ | 60 30, 245 |
| 5′-cgcgactcgaaggtgactg-3′ | |||||
| Mouse | L27560 | 5′-atcccctgagggcacactta-3′ | 58, 35 137 | ||
| 5′-ccagactgttcagagttcccag-3′ | |||||
| Trefoilfactor 1 | pS2 | Human | NM_003225 | 5′-acaaggtgatctgcg-3′ | 58, 35 190 |
| 5′-gaagcaccaggggac-3′ | |||||
| Mouse | NM_009362 | 5′-cactcgtggtcttcccgtga-3′ | 60, 40, 411 | ||
| 5′-ctgtgtcaccagccagatcca-3′ | |||||
| Presenilin 1 | PSEN1 | Human | NM_000021 | 5′-ctctgcaacagtgtcttgtg-3′ | 62, 30 400 |
| 5′-gttgctgtggactacattac-3′ | |||||
| Mouse | NM_008943 | 5′-acaatggtgtggttggtgaat-3′ | 62, 30 360 | ||
| 5′-agcaggctatggttgtgttcc-3′ | |||||
| Progesterone receptor | PGR | Human | M15716 | 5′-acaccttgcctgaagtttcg-3′ | 62, 20, 195 |
| 5′-ctgtccttttctgggggact-3′ | |||||
| Mouse | M68915 | 5′-gattcagaagccagccagagc-3′ | 62, 20 267 | ||
| 5′-gacctccaaggaccatgccagc-3′ | |||||
| Glyceraldehyde-3-phosphate dehydrogenase | GAPDH | Human | NM_002046 | 5′-cctgcaccaccaactgcttagc-3′ | 62 30 334 |
| Mouse | NM_008084 | 5′-caccaccttcttgatgtcatc-3′ | |||
| Ribosomal protein L19 | RPL19 | Human | NM_000981 | 5′-gaaatcgccaatgccaactc-3′ | 62, 30, 413 |
| Mouse | NM_009078 | 5′-ttccttggtcttagacctgcg-3′ |
Cell survival assay
Survival assays were performed with cells seeded into 96-well microtitre plates at a density of 4000 cells per well in a medium containing 10% FBS, and were allowed to adhere overnight. The following day, the medium was removed and replaced with phenol red-free DMEM containing 1% charcoal/dextran-treated FBS supplemented with 10−4 M formestane; 4 h before treatment of the cells with different concentrations of H2O2 or paraquat (PQ), cells were incubated with oestrogen, nebivolol, the ER antagonist ICI 182780 and l-NMMA, a non-selective NOS inhibitor, at indicated concentrations. After a 12 h incubation period, cell survival was measured using CytoTox-ONE Homogeneous Membrane Integrity assay according to the manufacturer's recommendations, and viable cells were reflected in relative fluorescence units.
Quantification of α, β and γ secretase activity, and sAPPα and Aβswe amounts
For quantification, 150 000 N2Aswe cells per well were seeded on a 48-well plate. The next day, the cell culture medium was replaced with phenol red-free DMEM containing 1% charcoal/dextran-treated FBS supplemented with 10−4 M formestane; after 4 h, cells were treated with several concentrations of E2 and nebivolol in the presence or absence of ICI 182780 for 36 h. All samples were normalized by determining protein concentration by the BCA method. To determine the secretase activity, cells were harvested and assays were performed, according to the manufacturer's instructions, using an α-secretase activity kit and a β-secretase activity assay kit; γ-secretase activity was measured by quantification of a fluorogenic γ-secretase substrate (Farmery et al., 2003). For the determination of the amounts of sAPPα and Aβswe, 100 µL of cell culture supernatants was used in specific and sensitive sAPPα and Aβswe elisas (IBL, Hamburg, Germany).
Data analysis and statistical procedures
All experiments were performed independently, and a minimum of three times. Standard deviations and significance were calculated by using GraphPad Prism 4.2 software (GraphPad Software Inc., San Diego, CA, USA). Statistically significant differences between groups were determined by a one-way anova. P values less than 0.05 were considered statistically significant.
Materials
DMEM and FBS were purchased from Invitrogen Inc. Charcoal/dextran-treated FBS (Perbio, Bonn, Germany); formestane (Sigma, St Louis, MO, USA); [2,4,6,7,16,17-3H]-E2 and scintillation fluid were from PerkinElmer LAS (Rodgau, Germany); the BCA-based assay was from Thermo Fisher Scientific (Bonn, Germany); the GRE was from Clontech Laboratories, Inc., (Mountain View, CA, USA); pRL, the Dual Luciferase Assay System and the CytoTox-ONE Homogeneous Membrane Integrity assay were from Promega (Mannheim, Germany); FuGene HD was from Roche (Mannheim, Germany); Agilent Total RNA Isolation Mini Kit was from Agilent Technologies; and Verso cDNA Kit and Absolute SYBRGreen Mix were from Thermo Fischer (Hamburg, Germany). The α-secretase activity kit was from R&D Systems (Minneapolis, MN, USA), the β-secretase activity assay kit was from BioVision (Mountain View, CA, USA) and the fluorogenic γ-secretase substrate was from Merck (Darmstadt, Germany).
17-β Oestradiol (E2) was purchased from Sigma Chemical Co., the ER antagonist ICI 182780 was from Tocris/Biotrend (Cologne, Germany), non-selective NOS inhibitor l-NMMA was purchased from Calbiochem/Merck (Darmstadt, Germany) and d-/l-nebivolol was provided by Berlin Chemie/Menarini AG (Berlin, Germany). Compounds were diluted in ethanol just before treatment of the cells. The drug/molecular target nomenclature conforms to BJP's Guide to Receptors and Channels (Alexander et al., 2008).
Results
Nebivolol binds to ERs and induces a classical genomic ER response
To show the direct interaction of nebivolol with ER, we measured displacement by nebivolol of [3H]-E2 bound to ER in N2A cells. Cells were incubated with 2 × 10−9 M [3H]-E2 in the presence or absence of varying concentrations of nebivolol. A 100-fold molar excess of unlabelled E2 was used to determine the absolute displacement of radiolabelled E2 and as reference for the nebivolol-mediated competition of E2 from ER. The kinetics of [3H]-E2 dissociation by nebivolol showed an IC50 to inhibit the binding of [3H]-E2 at approximately 0.8 × 10−5 M nebivolol (Figure 1), indicating a direct ligand–receptor interaction of nebivolol with ERs.
Figure 1.
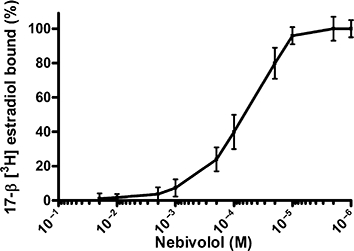
ER binding competition for nebivolol in N2A cells. In this assay, 2 × 10−9 M [3H]-E2 was competing for binding to the ER with increasing concentrations of nebivolol from 1 × 10−6 M to 2 × 10−2 M. A 100-fold molar excess of unlabelled E2 was used to determine the specific displaceable binding. Specific binding was calculated based on the percentage of remaining isotopic activity. Results are expressed as the mean of ±SEM of three independent measurements. The nebivolol concentration at 50% inhibition of [3H]-E2 binding (IC50) = 0.8 × 10−5 M.
Activation of ER-dependent downstream transcriptional activity was investigated in N2A, N2Aswe and SK-N-MC cells stably transfected with ER. For quantification and normalization of transfection efficacy, cells were transiently cotransfected with luciferase reporter plasmids containing ERE, GRE or ARE, and a plasmid constitutively expressing the Renilla luciferase. Stimulation with corticosterone or testosterone induced a positive luciferase response of GRE and ARE luciferase-transfected N2A cells, but no activation by nebivolol in the concentration range 10−6 to 10−9 M (data not shown). A strong response of the ERE induced by nebivolol could be observed in N2A and N2Aswe cells, which endogenously express both ERα and ERβ, and in SK-N-MC cells ectopically expressing either ERα or ERβ. In N2A and N2Aswe cells, nebivolol induced the same response of the ERE, at concentrations of 10−6 to 10−8 M, as the genuine ER agonist E2 (Figure 2A). SK transfectants showed a higher induction of ERE after treatment with E2 (Figure 2B), but nebivolol also induced a significant activation of the ERE. In detail, nebivolol induced the highest response in the SK-ERα expressing cells at a concentration of 10−7 M (4.2-fold), and in SK-ERβ transfectants at 10−8 M (4.3-fold). After co-incubation with the ER antagonist ICI 182780, neither E2 nor nebivolol activated an ERE-dependent response. From these results, we conclude that nebivolol, just like E2, can induce a classical genomic response via the ER subtypes ERα and ERβ.
Figure 2.
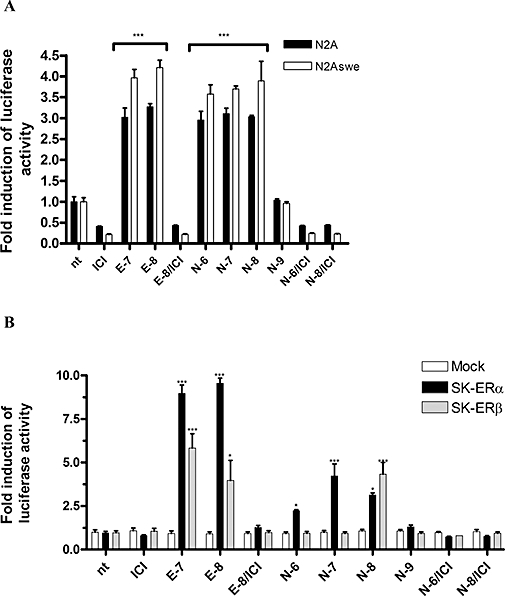
ER reporter assay with N2A and N2Aswe (A) and ER stable transfected SK-N-MC cells (B). Activation of the ER response element was plotted as fold induction of luciferase activity normalized to untreated cells (n.t.). Treatment: n.t., untreated; ICI, ICI 182780 at final concentration (f.c) of 10−6 M; E-7, E2 f.c. 10−7 M; E-8, oestrogen f.c. 10−8 M, N-6, nebivolol f.c. 10−6 M. The results shown represent more than three independent studies. Significance is defined as follows: *P < 0.05; ***P < 0.001 compared with untreated cells (anova).
Nebivolol protected N2Aswe cells against reactive oxygen species (ROS)-induced stress
Oestrogens and oestrogenic compounds have been shown to have different neuroprotective abilities under oxidative stress conditions (Behl, 2002). At pharmacological concentrations (10−6M and higher) they have been demonstrated to be based on the antioxidative radical scavenging phenolic structure of 17β-oestradiol (Behl et al., 1995; 1997;). At physiological concentrations, oestrogens also acquire ER-mediated genomic activities (Manthey and Behl, 2006). Interestingly, antioxidative properties have been also described for nebivolol (Wagenfeld et al., 2008). To investigate the protective properties of nebivolol in neuronal cells against oxidative stress induced by ROS, we exposed N2Aswe cells to H2O2 (0–1000 µM) and the herbicide PQ (0–1000 µM). While H2O2 causes a short acute burst of oxygen radicals, PQ induces a delayed accumulation of radicals (Yang and Sun, 1998). After both treatments, we observed a protective effect of E2 and nebivolol (Figure 3). At a nebivolol concentration of 10−8 M, a significantly increased cell survival could be detected: approximately 30% protection at 100 µM H2O2, 16% at 75 µM and 18% at 125 µM PQ compared to untreated controls (Figure 3). Interestingly, we found that nebivolol at a concentration range of 10−6 to 10−8 M promoted cell survival comparable to protection mediated by E2 at 10−8 M. The increased survival in this standard cell-based protection assays strongly indicates a direct neuroprotective effect of nebivolol. This effect was inhibited by the ER antagonist, ICI 182780, clearly suggesting that the mechanism of neuroprotection induced by nebivolol is ER-dependent genomic and not structurally based antioxidant. Furthermore, cotreatment with the non-selective NOS inhibitor l-NMMA did not affect the survival rates; this would seem to exclude the involvement of an NOS-dependent action in the neuroprotection provided by nebivolol.
Figure 3.
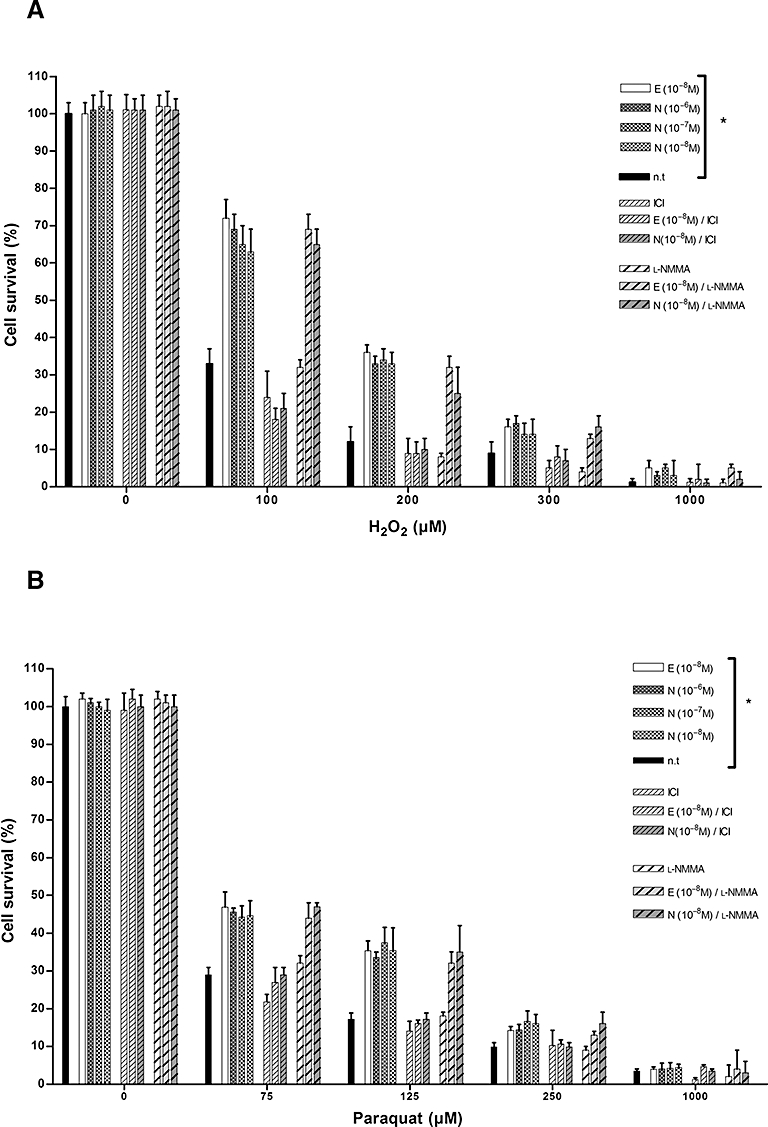
Nebivolol and E2 increased the survival of N2Aswe cells after H2O2 or PQ-induced oxidative stress. Cells were pretreated with oestrogen (f.c. 10−8 M), different nebivolol concentrations as indicated, ICI 182780 (ICI, f.c. 10−6 M), l-NMMA (f.c. 10−6 M) and oestrogen and nebivolol together with ICI 182780 and l-NMMA, followed by exposure to H2O2 (A) and PQ (0–1000 µM) (B). Data show mean cell survival from a representative experiment (n = 9). *P < 0.05, compared with untreated cells (n.t) (anova).
Nebivolol displayed an oestrogenic gene expression profile
To determine further the properties of nebivolol as an ER agonist, we compared nebivolol-induced gene expression with oestrogen-induced gene expression in N2Aswe and SK-ERα and SK-ERβ cells, focusing on a battery of well-known oestrogen-regulated genes. It is well known that ER-driven gene expression is largely modulated by and dependent on the activity of several cofactors. The presence of these cofactors is tissue and cell type specific, suggesting that common or general ER-marker genes do not exist (O'Lone et al., 2004). We tested a group of known neuronal ER-responsive genes, and found that few of them were controlled by nebivolol and E2 (Table 2 and Figure 4). The mRNA expression of the trefoilfactor 1 (PS2), a commonly known ERα-responsive gene (Brown et al., 1984), was increased by E2 and nebivolol in SK-ERα cells (Figure 4A), as well as the mRNA of Birc3 (Figure 4B) and Hsp 1B (Figure 4C). In N2Aswe cells, pS2 mRNA or expression changes through nebivolol or oestrogen were not detectable. Interestingly, the increased expression of BIRC3 and Hsp 1B mRNA by nebivolol was only seen at 10−8 M. Expression analysis of the progesterone receptor (Figure 4D) showed a general up-regulation in all cells after treatment with E2 and nebivolol. But exclusively in the controls (mock-transfected SK cells and cotreatment with ICI 182780), an up-regulation was not found. In addition, in N2Aswe cells, androgen receptor mRNA was down-regulated by E2 (−2.2-fold) and nebivolol (−3.3-fold at 10−6 M, −7-fold at 10−8 M), whereas SK transfectants showed no significant regulation of androgen receptor (AR) expression except the up-regulation in SK-ERβ cells after E2 (+1.8-fold) (Figure 4E).
Table 2.
Nebivolol-induced gene expression in neuronal cells
| Function | Gene |
Cell |
||
|---|---|---|---|---|
| N2Aswe | SK-ERα | SK-ERβ | ||
| ER responsive | PS2 (TFF1) | → | ↑ | → |
| BIRC3 | ↑ | ↑ | → | |
| HSP 1B | ↑ | ↑ | → | |
| PR | ↑ | ↑ | ↑ | |
| AR | ↓ | → | → | |
| APP processing | ||||
| α-Secretase | ||||
| ADAM 9 | ↑ | → | ↑ | |
| ADAM 10 | → | → | → | |
| ADAM 17 | → | → | → | |
| β-Secretase | → | → | → | |
| BACE1 | → | → | → | |
| γ-Secretase | ||||
| Presenillin 1 | → | → | → | |
Comparison of gene expression between N2Aswe and SK-N-MC ERα- and ERβ-transfected cells after nebivolol treatment. Cells were treated with nebivolol (f.c. 10−8 M) for 18 h. Genes were considered differentially expressed when the difference in expression level between untreated and treated cells was more than 1.5-fold and a P value <0.05. (↑, significantly up-regulated; ↓, significantly down-regulated, →, genes were not significantly affected).
Figure 4.
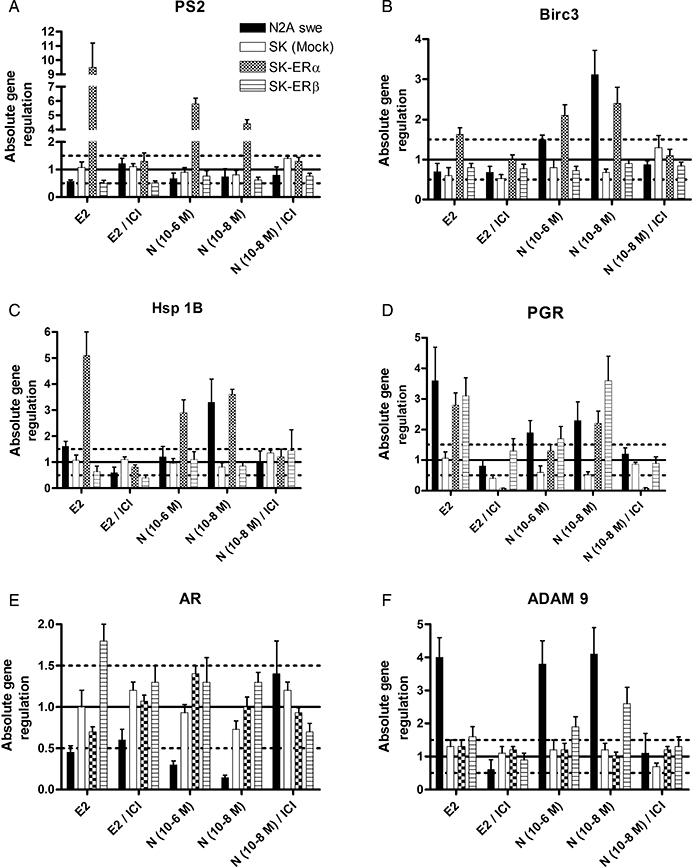
Relative mRNA expression ratios of E2 and nebivolol-regulated genes in N2Aswe and SK-N-MC ERα and ERβ over-expressing cells. Cells were treated with E2 (f.c. 10−8 M), nebivolol (10−6 M and 10−8M) and oestrogen and nebivolol (10−8M) together with ICI 182780 for 18 h. Genes were considered differentially expressed when the expression levels between untreated and treated cells (expressed as a ratio) were more than 1.5-fold difference (dotted lines) and a P value <0.05. Fold change of gene expression levels was based on the ΔΔCt method with normalization of the raw data to housekeeping genes by using the relative expression software tool (REST-MCS V2.0).
Nebivolol and E2 lowered Aβ generation by activating α-secretase activity in N2Aswe cells
Oestrogens modulate the biochemical processing of the AD-associated APP (Xu et al., 1998; Manthey et al., 2001). To investigate a potential effect of nebivolol on APP processing, the activities of APP cleavage by the three known secretases (α, β, γ) and selected cleavage products of APP were determined. Interestingly, we found treatment with nebivolol or E2 enhanced the activity of the α-secretase. The significant stimulation of α-secretase activity with E2 or nebivolol was approximately 1.5- to 1.8-fold at 10−7 and 10−8 M E2 compared to untreated controls, while E2 caused a slightly higher increase in α-secretase (Figure 5A). This effect could be completely suppressed by cotreatment with the ER antagonist ICI 182780 (Figure 5A). In addition, neither the activity of γ-secretase nor the activity of the Aβ-generating β-secretase was influenced by oestrogen or nebivolol (data not shown). As cleavage products of the APP processing, we found an accumulation of the potentially neurotrophic sAPPα peptide in N2Aswe cells after treatment with E2 and nebivolol and, consistently, a decline of the neurotoxic Aβ40/42 peptide amount. Cotreatment with the ER antagonist ICI 182780 repressed these processes, indicating an ER-mediated mechanism (Figure 5A–C). For further analysis of the enhanced α-secretase activity related to a possible ER-mediated genomic effect, we analysed mRNA expression of α-, β- and γ-secretases (Table 2). Indeed, we found that nebivolol and E2 induced an up-regulation of the potential α-secretase ADAM 9 in N2Aswe (fourfold) and SK-ERβ (2.6- and 1.6-fold, respectively) cells (Figure 4F). Expression levels of ADAM10 and 17, the other putative α-secretases, and presenillin1 (PSEN1) as part of the β-secretase complex and β-site APP-cleaving enzyme 1 (BACE1, γ-secretase) were not affected by nebivolol and E2 (data not shown).
Figure 5.
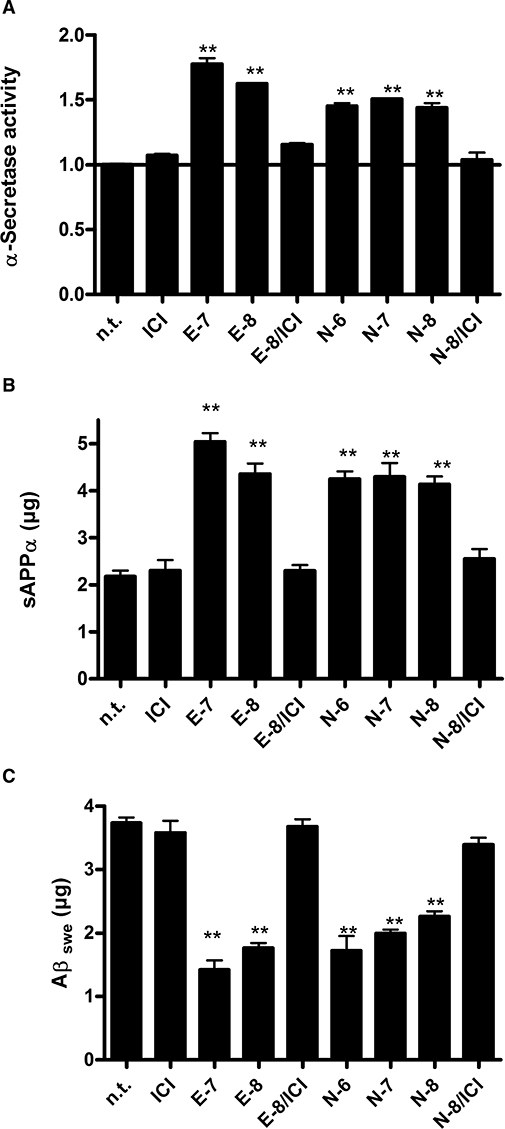
Modulation of the generation of sAPPα and Aβswe induced by the enhanced α-secretase activity observed after nebivolol and E2 treatment. (A) Quantification of sAPPα (elisa). (B) Quantification of Aβ swe (elisa). (C) α-Secretase activity assay; activity was plotted as fold induction normalized to untreated cells (n.t.). Treatments: n.t., untreated; ICI, ICI 182780 at final concentration (f.c) of 10−6 M; E-7, oestrogen f.c. 10−7 M; E-8, oestrogen f.c. 10−8 M, N-6, nebivolol f.c. 10−6 M, N-7, nebivolol f.c. 10−7 M, N-8, nebivolol f.c. 10−8 M. The histograms represent results from more than three independent studies. **P < 0.005, compared with untreated cells of the same kind (anova).
Discussion
Nebivolol is an established, highly selective β1-adrenoceptor antagonist used for the treatment of hypertension with unique vasodilator effects based upon its ability to activate eNOS via ER-mediated pathways (Grundt et al., 2007; Van Bortel et al., 2008). Based on the initial finding that nebivolol can display oestrogenic properties in endothelial preparations and human MCF-7 cells (Garban et al., 2004), together with the well-known activities of oestrogens in neuronal cells, we were interested in the possible effects of nebivolol in neuronal cells and any potential overlap of the actions of nebivolol and E2-mediated neuroprotective activities (Behl and Manthey, 2000; Maggi et al., 2004; Brann et al., 2007).
We found that nebivolol induces an ER-mediated response in established neuronal in vitro systems. Nebivolol activated both known ER subtypes even at low concentrations of 10−8 M, while an induction of other steroid receptors as androgen receptor or glucocorticoid receptor does not occur. In addition, the analysis of oestrogen-responsive gene expression revealed that nebivolol displayed similar effects to E2, but differences occurred when studying different cell lines. For instance, nebivolol but not E2 induced an up-regulation of the Birc3 mRNA, an anti-apoptotic protein, in N2Aswe cells. In SK-ERα cells, this up-regulation of Birc3 was only seen after treatment with E2, not with nebivolol. With regard to expression levels of the AR mRNA, in N2Aswe cells we only observed minor changes in the expression level with E2, but a pronounced down-regulation with nebivolol. Whereas in SK cells transfected with either ERα or ERβ, no changes were observed with either E2 or nebivolol. This could be due to a different cofactor composition, which triggers gene expression or requires the presence of both ERs (Chang et al., 2006). The ability of nebivolol to act as an oestrogen agonist and in part as an antagonist means that nebivolol has been designated to be a selective ER modulator (SERM).
To investigate the potential neuroprotective activity of nebivolol further, we exposed N2Aswe to oxidative stress, a condition frequently observed in acute brain injuries (e.g. cerebral ischaemia, stroke) or during chronic neurodegeneration (e.g. AD). It is well known that E2 can protect against oxidative stress in vitro and in vivo (Singer et al., 1996; Harms et al., 2001; Behl, 2002; Miller et al., 2005), and several groups have described the antioxidant protective effects of nebivolol (Janssen et al., 2001; De Groot et al., 2004; Wagenfeld et al., 2008). Here, we found that N2Aswe cells pretreated with nebivolol or E2 showed an increased cell survival rate of more than 20% against H2O2- or PQ-induced ROS stress compared to untreated controls or cells co-incubated with the ER antagonist ICI 182780. Metoprolol, another β1-selective antagonist neither led to an activation of the ER nor did it show any significant protective effect in the cell survival assays (data not shown). In addition, in both neuronal in vitro models, the expression of β1-adrenoceptors was not detectable (data not shown). When taken together, these results clearly indicate that the protective activity of nebivolol is dependent on ERs as reported for E2's ER-dependent neuroprotection (Garcia-Segura et al., 2001; Harms et al., 2001; Gelinas et al., 2004). Oestrogenic up-regulation and activation of eNOS via E2 or nebivolol, which mediates the beneficial vasorelaxing effects in endothelial cells (Darblade et al., 2002; Grundt et al., 2007), appeared not to be involved in the neuronal cells. In fact, we did not detect any changes in the expression levels of eNOS, iNOS and nNOS in N2A and SK-N-MC cells following E2 and nebivolol treatment (10−6–10−9 M) (data not shown). Furthermore, cotreatment of E2 or nebivolol with the non-selective NOS inhibitor l-NMMA had no effect on the cell survival after ROS treatment.
To study a pathogenic mechanism relevant for neurodegenerative conditions, we analysed the AD-associated processing of APP in N2Aswe cells after administration of E2 and nebivolol. The cerebral extracellular protein deposition, the ‘senile plaques’, which are a major neuropathological hallmark of AD, consists mainly of the extracellularly aggregated 40–42 residue peptide called Aβ. Amyloid β derived from APP after consecutive cleavage by β- and γ-secretases and is called amyloidogenic processing. An alternative proteolysis of APP with α- (instead of β-) secretase followed by γ-secretase cleavage leads to generation and release of other non-amyloidogenic fragments and the neurotrophic sAPPα peptide (non-amyloidogenic processing; De Strooper and Annaert, 2000). A beneficial effect of E2 in in vitro and in vivo models of AD has been frequently reported (Brann et al., 2007), and genomic (direct and indirect genomic) as well as non-genomic modes of action of E2 have been proposed (Behl, 2002). We examined the secretase activities and the amount of Aβ and sAPPα after treatment of N2Aswe cells with E2 and nebivolol. Indeed, we detected an enhanced generation of sAPPα and a decreased production of Aβ after E2 and nebivolol treatment, which could be inhibited by cotreatment with ICI 182780 indicating again an ER-mediated effect. Furthermore we found that α-secretase activity was increased, but β- and γ-secretase activities were unchanged. We demonstrated that E2 and nebivolol induced an increased expression of the putative α-secretase ADAM 9, and this could be a potential molecular mechanism for the promotion of the non-amyloidogenic APP processing. Interestingly, the expression of other putative α-secretases, such as ADAM 10 and 17 (Asai et al., 2003; Deuss et al., 2008), as well as β-secretase (presenilin 1) and γ-secretase (BACE1), was not affected by nebivolol. In conclusion, α-secretase cleavage of APP both precludes the deposition of the Aβ and releases the neuroprotective sAPPα; pharmacological up-regulation of α-secretase may provide an alternative therapeutic approach for AD (Pietrzik and Behl, 2005).
In summary, based on our findings we propose that nebivolol: (i) displays oestrogenic activity in neuronal cells; (ii) may potentially act as a SERM; and (iii) has antioxidative protective features and modulates APP processing in a beneficial neuroprotective manner. The potential of nebivolol to provide these neuroprotective effects should be studied further in in vivo models.
Acknowledgments
This work has been supported in part by a grant of the Menarini/Berlin Chemie AG to C.B. The authors wish to thank Bernd Moosmann for fruitful discussion and his input for the binding assay.
Glossary
Abbreviations:
- AD
Alzheimer's disease
- APP
amyloid β protein precursor
- E2
17-β oestradiol
- eNOS
endothelial NOS
- ER
oestrogen receptor
- ERE
oestrogen response element
- GRE
glucocorticoid response element
Conflict of interest
Authors declare no conflict of interest.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edition (2008 revision) Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai M, Hattori C, Szabo B, Sasagawa N, Maruyama K, Tanuma S-I, et al. Putative function of ADAM9, ADAM10, and ADAM17 as APP [alpha]-secretase. Biochem Biophys Res Commun. 2003;301:231–235. doi: 10.1016/s0006-291x(02)02999-6. [DOI] [PubMed] [Google Scholar]
- Behl C. Ooestrogen as a neuroprotective hormone. Nat Rev Neurosci. 2002;3:433–442. doi: 10.1038/nrn846. [DOI] [PubMed] [Google Scholar]
- Behl C, Manthey D. Neuroprotective activities of oestrogen: an update. J Neurocytol. 2000;29:351–358. doi: 10.1023/a:1007109222673. [DOI] [PubMed] [Google Scholar]
- Behl C, Widmann M, Trapp T, Holsboer F. 17-Beta estradiol protects neurons from oxidative stress-induced cell death in vitro. Biochem Biophys Res Commun. 1995;216:473–482. doi: 10.1006/bbrc.1995.2647. [DOI] [PubMed] [Google Scholar]
- Behl C, Skutella T, Lezoualc'h F, Post A, Widmann M, Newton CJ, et al. Neuroprotection against oxidative stress by estrogens: structure-activity relationships. Mol Pharm. 1997;51:535–541. [PubMed] [Google Scholar]
- Brann DW, Dhandapani K, Wakade C, Mahesh VB, Khan MM. Neurotrophic and neuroprotective actions of oestrogen: basic mechanisms and clinical implications. Steroids. 2007;72:381–405. doi: 10.1016/j.steroids.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broeders MA, Doevendans PA, Bekkers BC, Bronsaer R, Van Gorsel E, Heemskerk JW, et al. Nebivolol: a third-generation beta-blocker that augments vascular nitric oxide release: endothelial beta(2)-adrenergic receptor-mediated nitric oxide production. Circulation. 2000;102:677–684. doi: 10.1161/01.cir.102.6.677. [DOI] [PubMed] [Google Scholar]
- Brown AM, Jeltsch JM, Roberts M, Chambon P. Activation of pS2 gene transcription is a primary response to oestrogen in the human breast cancer cell line MCF-7. Proc Natl Acad Sci USA. 1984;81:6344–6348. doi: 10.1073/pnas.81.20.6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona-Gomez GP, Mendez P, Doncarlos LL, Azcoitia I, Garcia-Segura LM. Interactions of oestrogens and insulin-like growth factor-I in the brain: implications for neuroprotection. Brain Res Brain Res Rev. 2001;37:320–334. doi: 10.1016/s0165-0173(01)00137-0. [DOI] [PubMed] [Google Scholar]
- Chang EC, Frasor J, Komm B, Katzenellenbogen BS. Impact of oestrogen receptor beta on gene networks regulated by oestrogen receptor alpha in breast cancer cells. Endocrinology. 2006;147:4831–4842. doi: 10.1210/en.2006-0563. [DOI] [PubMed] [Google Scholar]
- Darblade B, Pendaries C, Krust A, Dupont S, Fouque MJ, Rami J, et al. Estradiol alters nitric oxide production in the mouse aorta through the alpha-, but not beta-, oestrogen receptor. Circ Res. 2002;90:413–419. doi: 10.1161/hh0402.105096. [DOI] [PubMed] [Google Scholar]
- De Groot AA, Mathy MJ, Van Zwieten PA, Peters SL. Antioxidant activity of nebivolol in the rat aorta. J Cardiovasc Pharmacol. 2004;43:148–153. doi: 10.1097/00005344-200401000-00022. [DOI] [PubMed] [Google Scholar]
- De Nigris F, Mancini FP, Balestrieri ML, Byrns R, Fiorito C, Williams-Ignarro S, et al. Therapeutic dose of nebivolol, a nitric oxide-releasing [beta]-blocker, reduces atherosclerosis in cholesterol-fed rabbits. Nitric Oxide. 2008;19:57–63. doi: 10.1016/j.niox.2008.03.004. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Annaert W. Proteolytic processing and cell biological functions of the amyloid precursor protein. J Cell Sci. 2000;113(11):1857–1870. doi: 10.1242/jcs.113.11.1857. Pt. [DOI] [PubMed] [Google Scholar]
- Deuss M, Reiss K, Hartmann D. Part-time alpha-secretases: the functional biology of ADAM 9, 10 and 17. Curr Alzheimer Res. 2008;5:187–201. doi: 10.2174/156720508783954686. [DOI] [PubMed] [Google Scholar]
- Farmery MR, Tjernberg LO, Pursglove SE, Bergman A, Winblad B, Naslund J. Partial purification and characterization of gamma-secretase from post-mortem human brain. J Biol Chem. 2003;278:24277–24284. doi: 10.1074/jbc.M211992200. [DOI] [PubMed] [Google Scholar]
- Garban HJ, Buga GM, Ignarro LJ. Oestrogen receptor-mediated vascular responsiveness to nebivolol: a novel endothelium-related mechanism of therapeutic vasorelaxation. J Cardiovasc Pharmacol. 2004;43:638–644. doi: 10.1097/00005344-200405000-00005. [DOI] [PubMed] [Google Scholar]
- Garbin U, Fratta Pasini A, Stranieri C, Manfro S, Mozzini C, Boccioletti V, et al. Effects of nebivolol on endothelial gene expression during oxidative stress in human umbilical vein endothelial cells. Mediators Inflamm. 2008;2008:367590. doi: 10.1155/2008/367590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Segura LM, Azcoitia I, Doncarlos LL. Neuroprotection by estradiol. Prog Neurobiol. 2001;63:29–60. doi: 10.1016/s0301-0082(00)00025-3. [DOI] [PubMed] [Google Scholar]
- Gelinas S, Bureau G, Valastro B, Massicotte G, Cicchetti F, Chiasson K, et al. Alpha and beta estradiol protect neuronal but not native PC12 cells from paraquat-induced oxidative stress. Neurotox Res. 2004;6:141–148. doi: 10.1007/BF03033216. [DOI] [PubMed] [Google Scholar]
- Grundt C, Meier K, Grundt A, Lemmer B. Evidence for an estradiol-agonistic action of nebivolol in spontaneously hypertensive rats. J Hypertens. 2007;25:1001–1007. doi: 10.1097/HJH.0b013e3280987710. [DOI] [PubMed] [Google Scholar]
- Harms C, Lautenschlager M, Bergk A, Katchanov J, Freyer D, Kapinya K, et al. Differential mechanisms of neuroprotection by 17 beta-estradiol in apoptotic versus necrotic neurodegeneration. J Neurosci. 2001;21:2600–2609. doi: 10.1523/JNEUROSCI.21-08-02600.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen PM, Zeitz O, Rahman A, Hasenfuss G. Protective role of nebivolol in hydroxyl radical induced injury. J Cardiovasc Pharmacol. 2001;38(Suppl. 3):S17–23. doi: 10.1097/00005344-200112003-00004. [DOI] [PubMed] [Google Scholar]
- Maggi A, Ciana P, Belcredito S, Vegeto E. Oestrogens in the nervous system: mechanisms and nonreproductive functions. Annu Rev Physiol. 2004;66:291–313. doi: 10.1146/annurev.physiol.66.032802.154945. [DOI] [PubMed] [Google Scholar]
- Manthey D, Behl C. From structural biochemistry to expression profiling: neuroprotective activities of oestrogen. Neuroscience. 2006;138:845–850. doi: 10.1016/j.neuroscience.2005.10.058. [DOI] [PubMed] [Google Scholar]
- Manthey D, Heck S, Engert S, Behl C. Oestrogen induces a rapid secretion of amyloid beta precursor protein via the mitogen-activated protein kinase pathway. Eur J Biochem. 2001;268:4285–4291. doi: 10.1046/j.1432-1327.2001.02346.x. [DOI] [PubMed] [Google Scholar]
- Mason RP, Kubant R, Jacob RF, Walter MF, Boychuk B, Malinski T. Effect of nebivolol on endothelial nitric oxide and peroxynitrite release in hypertensive animals: role of antioxidant activity. J Cardiovasc Pharmacol. 2006;48:862–869. doi: 10.1097/01.fjc.0000238593.67191.e2. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Invited review: oestrogens effects on the brain: multiple sites and molecular mechanisms. J Appl Physiol. 2001;91:2785–2801. doi: 10.1152/jappl.2001.91.6.2785. [DOI] [PubMed] [Google Scholar]
- Mendez P, Azcoitia I, Garcia-Segura LM. Interdependence of ooestrogen and insulin-like growth factor-I in the brain: potential for analysing neuroprotective mechanisms. J Endocrinol. 2005;185:11–17. doi: 10.1677/joe.1.06058. [DOI] [PubMed] [Google Scholar]
- Miller NR, Jover T, Cohen HW, Zukin RS, Etgen AM. Oestrogen can act via oestrogen receptor alpha and beta to protect hippocampal neurons against global ischemia-induced cell death. Endocrinology. 2005;146:3070–3079. doi: 10.1210/en.2004-1515. [DOI] [PubMed] [Google Scholar]
- Moosmann B, Behl C. The antioxidant neuroprotective effects of oestrogens and phenolic compounds are independent from their oestrogenic properties. Proc Natl Acad Sci USA. 1999;96:8867–8872. doi: 10.1073/pnas.96.16.8867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuedling S, Kahlert S, Loebbert K, Doevendans PA, Meyer R, Vetter H, et al. 17 Beta-estradiol stimulates expression of endothelial and inducible NO synthase in rat myocardium in-vitro and in-vivo. Cardiovasc Res. 1999;43:666–674. doi: 10.1016/s0008-6363(99)00093-0. [DOI] [PubMed] [Google Scholar]
- O'Lone R, Frith MC, Karlsson EK, Hansen U. Genomic targets of nuclear oestrogen receptors. Mol Endocrinol. 2004;18:1859–1875. doi: 10.1210/me.2003-0044. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzik C, Behl C. Concepts for the treatment of Alzheimer's disease: molecular mechanisms and clinical application. Int J Exp Pathol. 2005;86:173–185. doi: 10.1111/j.0959-9673.2005.00435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer CA, Rogers KL, Strickland TM, Dorsa DM. Oestrogen protects primary cortical neurons from glutamate toxicity. Neurosci Lett. 1996;212:13–16. doi: 10.1016/0304-3940(96)12760-9. [DOI] [PubMed] [Google Scholar]
- Su TT, Guo B, Kawakami Y, Sommer K, Chae K, Humphries LA, et al. PKC-beta controls I kappa B kinase lipid raft recruitment and activation in response to BCR signaling. Nat Immunol. 2002;3:780–786. doi: 10.1038/ni823. [DOI] [PubMed] [Google Scholar]
- Thinakaran G, Teplow DB, Siman R, Greenberg B, Sisodia SS. Metabolism of the ‘Swedish’ amyloid precursor protein variant in neuro2a (N2a) cells. Evidence that cleavage at the ‘beta-secretase’ site occurs in the golgi apparatus. J Biol Chem. 1996;271:9390–9397. doi: 10.1074/jbc.271.16.9390. [DOI] [PubMed] [Google Scholar]
- Thomson AJ, Telfer JF, Kohnen G, Young A, Cameron IT, Greer IA, et al. Nitric oxide synthase activity and localization do not change in uterus and placenta during human parturition. Hum Reprod. 1997;12:2546–2552. doi: 10.1093/humrep/12.11.2546. [DOI] [PubMed] [Google Scholar]
- Toker S, Toker A, Gulcan E, Gulcan A. Nebivolol may be beneficial via nitric oxide on osteoporosis treatment. Bone. 2008;42:S89. [Google Scholar]
- Troost R, Schwedhelm E, Rojczyk S, Tsikas D, Frolich JC. Nebivolol decreases systemic oxidative stress in healthy volunteers. Br J Clin Pharmacol. 2000;50:377–379. doi: 10.1046/j.1365-2125.2000.00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon JL, Carr MC, Maki PM, Mendelsohn ME, Wise PM. Complex actions of sex steroids in adipose tissue, the cardiovascular system, and brain: insights from basic science and clinical studies. 10.1210/er.2005-0020. Endocr Rev. 2006;27:575–605. doi: 10.1210/er.2005-0020. [DOI] [PubMed] [Google Scholar]
- Van Bortel LM, Fici F, Mascagni F. Efficacy and tolerability of nebivolol compared with other antihypertensive drugs: a meta-analysis. Am J Cardiovasc Drugs. 2008;8:35–44. doi: 10.2165/00129784-200808010-00005. [DOI] [PubMed] [Google Scholar]
- Van De Water A, Janssens W, Van Neuten J, Xhonneux R, De Cree J, Verhaegen H, et al. Pharmacological and hemodynamic profile of nebivolol, a chemically novel, potent, and selective beta 1-adrenergic antagonist. J Cardiovasc Pharmacol. 1988;11:552–563. doi: 10.1097/00005344-198805000-00007. [DOI] [PubMed] [Google Scholar]
- Wagenfeld L, Himpel O, Galambos P, Matthiesen N, Wiermann A, Richard G, et al. Protective effects of nebivolol on oxygen free radical-induced vasoconstrictions in vitro. Med Sci Monit. 2008;14:BR109–BR112. [PubMed] [Google Scholar]
- Xu H, Gouras GK, Greenfield JP, Vincent B, Naslund J, Mazzarelli L, et al. Oestrogen reduces neuronal generation of Alzheimer beta-amyloid peptides. Nat Med. 1998;4:447–451. doi: 10.1038/nm0498-447. [DOI] [PubMed] [Google Scholar]
- Yang W-L, Sun AY. Paraquat-induced cell death in PC12 cells. Neurochem Res. 1998;23:1387–1394. doi: 10.1023/a:1020750706762. [DOI] [PubMed] [Google Scholar]
- Yeh S, Chang C. Cloning and characterization of a specific coactivator, ARA70, for the androgen receptor in human prostate cells. Proc Natl Acad Sci USA. 1996;93:5517–5521. doi: 10.1073/pnas.93.11.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zschocke J, Manthey D, Bayatti N, Behl C. Functional interaction of oestrogen receptor alpha and caveolin isoforms in neuronal SK-N-MC cells. J Steroid Biochem Mol Biol. 2003;84:167–170. doi: 10.1016/s0960-0760(03)00026-8. [DOI] [PubMed] [Google Scholar]
- Zschocke J, Manthey D, Bayatti N, Van Der Burg B, Goodenough S, Behl C. Oestrogen receptor alpha-mediated silencing of caveolin gene expression in neuronal cells. J Biol Chem. 2002;277:38772–38780. doi: 10.1074/jbc.M205664200. [DOI] [PubMed] [Google Scholar]


