Abstract
Background and purpose:
Molecular mechanisms underlying the links between dietary intake of flavonoids and reduced cardiovascular disease risk are only partially understood. Key events in the pathogenesis of cardiovascular disease, particularly thrombosis, are inhibited by these polyphenolic compounds via mechanisms such as inhibition of platelet activation and associated signal transduction, attenuation of generation of reactive oxygen species, enhancement of nitric oxide production and binding to thromboxane A2 receptors. In vivo, effects of flavonoids are mediated by their metabolites, but the effects and modes of action of these compounds are not well-characterized. A good understanding of flavonoid structure–activity relationships with regard to platelet function is also lacking.
Experimental approach:
Inhibitory potencies of structurally distinct flavonoids (quercetin, apigenin and catechin) and plasma metabolites (tamarixetin, quercetin-3′-sulphate and quercetin-3-glucuronide) for collagen-stimulated platelet aggregation and 5-hydroxytryptamine secretion were measured in human platelets. Tyrosine phosphorylation of total protein, Syk and PLCγ2 (immunoprecipitation and Western blot analyses), and Fyn kinase activity were also measured in platelets. Internalization of flavonoids and metabolites in a megakaryocytic cell line (MEG-01 cells) was studied by fluorescence confocal microscopy.
Key results:
The inhibitory mechanisms of these compounds included blocking Fyn kinase activity and the tyrosine phosphorylation of Syk and PLCγ2 following internalization. Principal functional groups attributed to potent inhibition were a planar, C-4 carbonyl substituted and C-3 hydroxylated C ring in addition to a B ring catechol moiety.
Conclusions and implications:
The structure–activity relationship for flavonoids on platelet function presented here may be exploited to design selective inhibitors of cell signalling.
Keywords: flavonoids, flavonoid metabolites, flavonoid structure, flavonoid inhibitory mechanisms, platelet signalling
Introduction
Platelets are anucleate discoid-shaped cell fragments derived from the cytoplasm of bone marrow megakaryocytes (Hartwig et al., 1999; Italiano et al., 1999; Patel et al., 2005; Richardson et al., 2005; Patel-Hett et al., 2008). They play an essential role in haemostasis by facilitating the repair of minor vascular injuries through the formation of aggregates over tears within artery walls (Plow and Ginsberg, 2000; Ruggeri, 2002). Inefficient regulation of platelet activation leads to the pathophysiological process, thrombosi,s that can lead to the blocking of cardiac arteries and the blood supply within the brain by thrombi resulting in myocardial infarction (MI) or stroke (Ruggeri, 2002; White and Chew, 2008) respectively.
Subsequent to arterial injury following initial indirect tethering mediated by von Willebrand factor (vWF) and glycoprotein Ib (GPIb), platelets adhere to collagen in the subendothelium, via the receptors glycoprotein VI (GPVI) (Takahashi et al., 1995) and integrin α2β1 (Santoro et al., 1991; Asselin et al., 1997). GPVI receptors cluster (Gibbins et al., 1996; Nieswandt et al., 2000), and the constitutively bound Src-family kinases Fyn and Lyn (Suzuki-Inoue et al., 2002) induce phosphorylation of tyrosine residues in the immunoreceptor tyrosine-based activatory motif (ITAM) present in the cytoplasmic tail of the associated FcRγ-chain (Gibbins et al., 1996; 1997;). The non-receptor tyrosine kinase Syk is recruited, binds to ITAM phosphotyrosine residues through tandem Src-homology 2 (SH2) domains (Benhamou et al., 1993; Gibbins et al., 1997) and becomes tyrosine phosphorylated (Siraganian et al., 2001). This leads to tyrosine phosphorylation of the transmembrane adaptor protein, linker and activator of T-cells (LAT) (Zhang et al., 1998) and the assembly of a complex of proteins which include phosphoinositide-3-kinase (PI3K) (Gibbins et al., 1998) and phospholipase Cγ2 (PLCγ2) (Blake et al., 1994) locating them closer to their substrates at the inner layer of the plasma membrane.
Further signalling events lead to shape change (spreading, formation of filopodia and lamellipodia) (Hartwig et al., 1999; 1992;), aggregation and thrombus formation (Ruggeri, 2002; Gibbins, 2004; Woulfe et al., 2004). Pro-activatory factors including ADP (Jin et al., 1998; Jantzen et al., 1999; Hourani, 2000), 5-HT (Qi et al., 1996; Li et al., 1997), and thromboxane A2 (TxA2) (Fitzgerald, 1991) which are secreted or released, activate and recruit surrounding platelets to the growing haemostatic plug or thrombus (Gibbins, 2004).
The highly abundant dietary flavonoids, quercetin, apigenin and catechin (Hertog et al., 1992; Scalbert and Williamson, 2000) are implicated as negative modulators of cardiovascular disease (CVD) risk, as epidemiological studies have inversely associated the consumption of diets, rich in sources of these compounds, to lowered incidences of stroke (Keli et al., 1996) and MI (Hertog et al., 1993). Key mechanisms proposed for the reduction of CVD risk by flavonoids include anti-oxidant properties (Pignatelli et al., 2000; 2006;), inhibition of platelet aggregation (Beretz et al., 1982; Landolfi et al., 1984; Hubbard et al., 2003; 2004; Guerrero et al., 2005), thrombus formation (Demrow et al., 1995; Osman et al., 1998; Keevil et al., 2000; Briggs et al., 2001; Navarro-Nuñez et al., 2008), 5-HT secretion (Beretz et al., 1982; Landolfi et al., 1984; Guerrero et al., 2005) and TxA2 release (Guerrero et al., 2005), as well as inhibition of tyrosine (Agullo et al., 1997; Gamet-Payrastre et al., 1999; Hubbard et al., 2003), lipid (Matter et al., 1992; Vlahos et al., 1994; Agullo et al., 1997; Gamet-Payrastre et al., 1999; Walker et al., 2000) and serine/threonine (Agullo et al., 1997; Gamet-Payrastre et al., 1999; Holder et al., 2007) kinase activity, binding to the TxA2 receptor (Guerrero et al., 2005), the enhancement of platelet-derived nitric oxide (NO) production (Freedman et al., 2001), and blocking reactive oxygen species (ROS) production (Pignatelli et al., 2000; Pignatelli et al., 2006). A critical consideration for flavonoid mechanisms for inhibition of platelet function is the relationship between variations in key flavonoid functional groups and modes of action elicited external to, and within the platelet cytosol. Furthermore, ex vivo studies showing inhibition of collagen-stimulated platelet function and signalling following the ingestion of quercetin supplements (Hubbard et al., 2004) and flavonoids in dietary sources (Keevil et al., 2000; Hubbard et al., 2006) have suggested the involvement of metabolized flavonoids in their anti-platelet effects.
In this study, we have explored the structural requirements for inhibition of collagen-stimulated platelet function by flavonoids and selected plasma metabolites of quercetin. Inhibitory mechanisms investigated included the attenuation of GPVI signalling pathway proteins following internalization by platelets. We report a multifaceted relationship between flavonoid structure and anti-platelet effects.
Methods
Preparation and stimulation of platelets
Blood was obtained from healthy, aspirin-free, human volunteers with informed consent, following approval from the University of Reading Research Ethics Committee. Platelets were isolated by differential centrifugation as described previously (Gibbins et al., 1996) and suspended in modified Tyrode's-HEPES buffer (134 mM NaCl, 0.34 mM Na2HPO4, 2.9 mM KCl, 12 mM NaHCO3, 20 mM HEPES, 1 mM MgCl2, 5 mM glucose, pH 7.3) to a density of 4 × 108 cells·mL−1 for aggregation and 5-HT secretion experiments. For 5-HT secretion assays, platelets were loaded with [3H]-5-HT as described previously (Cicmil et al., 2002), and released 5-HT was measured by scintillation spectrometry. For experiments involving analyses of protein profiles from whole cell lysates and immunoprecipitated proteins, platelets were suspended to a density of 8 × 108 cells·mL−1 in modified Tyrode's-HEPES buffer containing 1 mM EGTA to prevent aggregation. Platelets (450 µL) were incubated with flavonoids or DMSO [1 µL: 0.2% (v/v)] for 5 min (after 10 s stirring) prior to stimulation with collagen (50 µL) for 90 s in an optical aggregometer at 37°C with continuous stirring.
Immunoprecipitation
Fyn, Syk and PLCγ2 were immunoprecipitated as described previously (Hubbard et al., 2003). Immunoprecipitated proteins were separated by one-dimensional SDS-PAGE using 10% polyacrylamide gels. For the separation of proteins from whole platelet lysates, 10–18% gradient polyacrylamide gels were utilized. Separated proteins within gels were transferred to polyvinylidene difluoride (PVDF) membranes by semi-dry Western blotting.
Immunoblotting
Following the transfer of proteins to PVDF membranes, non-specific binding to membranes was blocked by incubation with 5% (w/v) bovine serum albumin (BSA) dissolved in Tris-buffered saline-Tween (TBS-T) (20 mM Tris-base, 140 mM NaCl, 0.1% Tween®-20; pH 7.6). Membranes were incubated with primary antibodies (1 µg·mL−1) diluted in 2% (w/v) BSA dissolved in TBS-T at 4°C overnight. Tyrosine phosphorylation or activity of immunoprecipitated proteins was detected and blots showing the tyrosine phosphorylation of Syk and PLCγ2 were stripped to remove the anti-phosphotyrosine primary antibody (4G10), before their total levels were measured using anti-Syk, anti-PLCγ2 or anti-Fyn antibodies. Normalization for protein loading was performed by expressing levels of tyrosine phosphorylation or activity of immunoprecipitated proteins relative to total levels of those proteins (also determined by immunoblotting), and expressed as a percentage compared with the untreated control that represented 100% phosphorylation or activity. Blots were washed for 45 min in TBS-T before incubation with a horseradish peroxidase (HRP)-conjugated secondary antibody (1:4000 dilution) for 2 h at room temperature with rotation. Proteins were detected on X-ray film using an enhanced chemiluminescence (ECL) system. Densitometry analysis was performed using a Bio-Rad GS710 densitometer with Quantity One analysis software (Bio-Rad; Hemel Hempstead, UK).
Surface Plasmon Resonance
Collagen (100 µg·mL−1) or fibrinogen (100 µg·mL−1) were covalently coupled to a dextran matrix (CM5 Biacore chip) until a response of ≥7000 U was detected, using a Biacore T100 system, following the manufacturer's protocols. Immobilized proteins were activated with N-hydroxysuccinimide (NHS) and 1-ethyl-3-[3-dimethylaminopropyl] carbodiimide (EDC), which form a carbo-diamide linkage between carboxyl moieties on the dextran and primary amide groups on the protein. Unreacted groups were blocked with ethanolamine. HBS-EP buffer (0.1 M HEPES, 1.5 M NaCl, 30 mM EDTA, 0.5% (v/v) surfactant P20; pH 7.4) was used for priming and conditioning the surface of the chip. Flavonoids and metabolites at a range of concentrations (9.375 µM, 18.75 µM, 37.5 µM, 75 µM and 150 µM) were diluted in HBS-EP buffer and maintained in DMSO [5% (v/v)]. The baseline response was determined before the compounds were perfused for 180 s (30 µL·min−1) over the immobilized proteins to allow association to occur. Dissociation was then monitored over a period of 300 s. The immobilized proteins were regenerated with 50 mM NaOH. Solvent correction was performed by subtracting DMSO alone signals and flavonoid or metabolite responses perfused over the dextran matrix, from those of flavonoids and metabolites perfused over immobilized ligands. The response baseline, association and dissociation events were measured in real-time and recorded in a sensorgram. Affinity (KD) was calculated from association (Ka) and dissociation (Kd) constants derived by fitting response units to the 1:1 Langmuir binding model as described in previous studies (O'Shannessy et al., 1996; Myszka, 1997).
In vitro kinase assay of Fyn
Immunoprecipitates suspended in kinase buffer (105 mM NaCl, 20 mM HEPES (pH 7.4), 5 mM MnCl2, 5 mM MgCl2, 2 mM NaF, 1 mM Na3VO4, 10 µM ATP) containing 5 µCi γ-[32P]-ATP per reaction, were incubated at 30°C for 20 min and the kinase reaction terminated through addition of an equal volume of Laemmli reducing sample treatment buffer. Proteins were separated by SDS-PAGE, and transferred to PVDF membranes which were exposed to storage phosphor screens to detect incorporation of 32P (autophosphorylation) into the immobilized kinase. For some experiments, protein was immunoprecipitated from collagen-stimulated platelets before treatment with flavonoids or DMSO [0.2% (v/v)] to assess direct effects of these compounds on kinase activity.
Analysis of flavonoid internalization
MEG-01 cells were isolated from media by centrifugation (1881 ×g), washed and suspended to a density of 4 × 106 cells·mL−1 in modified Tyrode's-HEPES buffer. Prepared cells were incubated with quercetin, tamarixetin or DMSO [0.2% (v/v)] for 30 min at room temperature with gentle agitation. Cells were centrifuged (1881×g), the supernatant was removed and pellets were washed twice with modified Tyrode's-HEPES buffer before fixation on coverslips in 3.7% paraformaldehyde for 20 min and treatment with VectorShield®. The coverslip was sealed to a glass slide prior to confocal analysis. Fluorescence was emitted from treated cells following excitation with an argon laser at 430 nm with detection at 480 nm–500 nm. Three dimensional representations of cells were constructed by the generation of layers in the z dimension which were compiled into z-stacks. A single middle layer from z-stacks was shown.
Statistical analysis
Student's t-tests (unpaired) were performed using Microsoft Excel. Results are presented as the mean of three individual experiments with standard error of mean (SEM) and P-value ≤0.05 considered significant. IC50 values were calculated using non-linear regression analysis within Prism for Windows – version 2.0 (Graphpad Inc., San Diego, CA, USA). Data were presented as a sigmoidal curve with a variable Hill slope. The upper and lower limits of the data range (maximum and minimum % aggregation) for individual compounds were constrained. Affinity (KD) was calculated from association (ka) and dissociation (kd) rates derived from data fitted to the 1:1 Langmuir binding model (O'Shannessy et al., 1996; Myszka, 1997).
Materials
Quercetin, apigenin, catechin, cyanidin, taxifolin and galangin aglyca as well as the metabolite, tamarixetin, were purchased from Extrasynthese (Genay, France). Quercetin-3′-sulphate was prepared as previously described (Day et al., 2001) and queretin-3-glucuronide was purified from French beans by preparative high performance liquid chromatography (HPLC). Flavonoids and metabolites were solubilized in dimethylsulphoxide (DMSO) obtained from Sigma (Poole, UK). Protein A Sepharose (PAS: from Staphylococcus aureus) and protein G Sepharose (PGS: recombinant protein G) were also purchased from Sigma. Collagen [Type I (fibrillar) from equine tendons] was from Nycomed (Munich, Germany), anti-phosphotyrosine monoclonal antibody (4G10) was purchased from Upstate Biotechnology (Millipore; Dundee, Scotland) and anti-Syk (N-19), anti-Syk (LR), anti-PLCγ2 (polyclonal) and anti-Fyn (monoclonal) primary antibodies were obtained from Santa Cruz Biotechnology (Autogen Bioclear UK Ltd; Calne, Wilts, UK). HRP-conjugated secondary antibodies, γ-[32P]-ATP, [3H]-5-HT, N-hydroxysuccinimide (NHS) and 1-ethyl-3-[3-dimethylaminopropyl] carbodiimide (EDC) were from GE Healthcare (Little Chalfont, UK). The ECL detection system was obtained from Pierce (Thermo Fisher Scientific; Rockford, IL, USA) and Vector Shield was from Molecular Probes (Invitrogen Ltd.; Paisley, UK). The human megakaryocytic cell line, MEG-01, was obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). Amphotericin B, fetal calf serum (FCS), penicillin, streptomycin, L-glutamine and culture media were from Invitrogen (Paisley, UK).
Results
Flavonoids and metabolites inhibit collagen-stimulated platelet function with differential potencies
The limited understanding of the link between flavonoid structures and their inhibitory activity on platelet function, as well as the effects of their biologically active metabolites has not been addressed. Therefore, the inhibitory effects of structurally distinct flavonoids (see Figure 1: quercetin, apigenin and catechin) and plasma metabolites of quercetin (tamarixetin, quercetin-3′-sulphate and quercetin-3-glucuronide) on collagen-stimulated (5 µg·mL−1) aggregation and 5-HT secretion were investigated. Flavonoids and metabolites inhibited platelet aggregation and 5-HT secretion with a range of different potencies (Table 1). Despite similar potencies, at certain concentrations [for example, 10 µM (−5 M: log concentration)] apigenin caused a greater level of inhibition than quercetin (compare Figure 2A.ii to Figure 2B.ii). For aggregation, the inhibition caused by 10 µM and 40 µM apigenin were identical (94% inhibition, Figure 2B.ii), whereas 40 µM quercetin completely abolished aggregation (Figure 2A.ii). At the same concentration (40 µM), tamarixetin and quercetin-3′-sulphate also completely inhibited aggregation (Figure 2A.ii).
Figure 1.
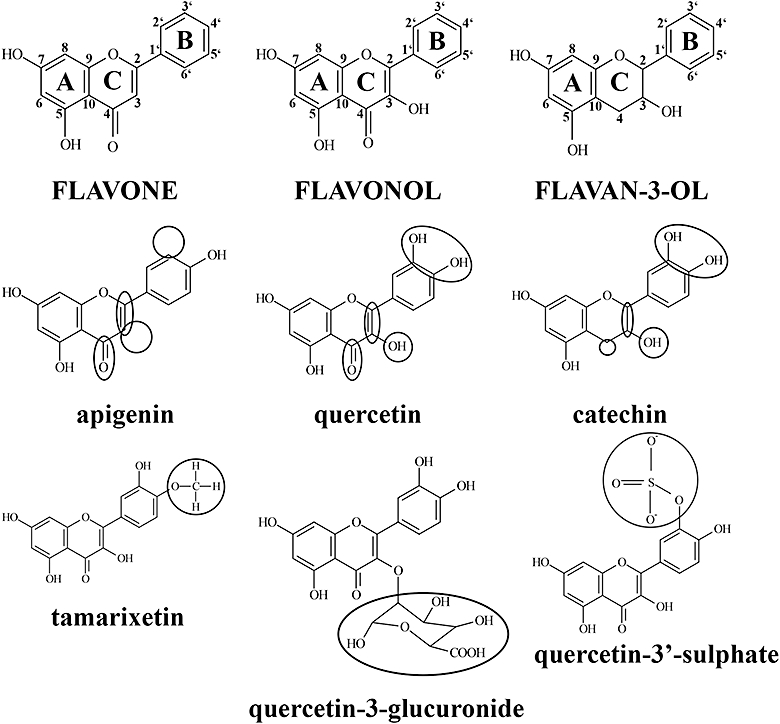
Flavonoid and metabolite structures are defined by variations in key functional groups. The flavonoid structure comprises 15 carbon atoms arranged into a 3 carbon, oxygenated heterocyclic middle ring (C ring) flanked by two aromatic rings (A and B rings). Subgroups of this family of compounds are defined by variations in functional groups integral to the flavonoid nucleus and substituted to the A, C and B rings. Flavones (apigenin) are characterized by a non-hydroxylated C ring, whereas flavonol (quercetin) C rings contain a C-3 hydroxyl group. Flavan-3-ols (catechin) are defined by a non-planar, C-3 hydroxylated C ring that is not substituted with a C-4 carbonyl group. Metabolites generated by intestinal enterocytes and liver hepatocytes are methylated (tamarixetin), sulphated (quercetin-3′-sulphate) or glucuronidated (quercetin-3-glucuronide) counterparts of parent flavonoid aglyca.
Table 1.
Flavonoids and metabolites inhibit collagen-stimulated function with differential potencies
| Flavonoid/metabolite |
logIC50±SEM (M) |
Rmax±SEM (aggregation-% of control) |
Hill slope±SEM |
|||
|---|---|---|---|---|---|---|
| Aggregation | 5-HT secretion | Aggregation | 5-HT secretion | Aggregation | 5-HT secretion | |
| Quercetin | −5.17 ± 0.04 | −5.11 ± 0.02 | 100 ± 0 | 96 ± 0 | −2.79 ± 0.69 | −2.04 ± 0.27 |
| Tamarixetin | −4.75 ± 0.05 | −4.66 ± 0.18 | 96 ± 4 | 83 ± 3 | −2.99 ± 0.80 | −1.69 ± 0.60 |
| Quercetin-3′-sulphate | −4.72 ± 0.01 | −4.73 ± 0.03 | 100 ± 0 | 100 ± 0 | −10.71 ± 2.49 | −2.48 ± 0.41 |
| Apigenin | −5.31 ± 0.04 | −5.36 ± 0.04 | 100 ± 0 | 87 ± 2 | −2.23 ± 0.43 | −2.19 ± 0.32 |
| Catechin | −3.44 ± 0.05 | −3.45 ± 0.04 | 100 ± 0 | 100 ± 0 | −2.91 ± 0.82 | −1.51 ± 0.19 |
Potencies were calculated as the concentration of flavonoids (quercetin, apigenin and catechin) or metabolites (tamarixetin and quercetin-3′-sulphate) that inhibited aggregation or 5-HT secretion by 50% (IC50). LogIC50 values were calculated using non-linear regression and a variable Hill slope with inhibition constants (maximum and minimum % aggregation) individually determined for each compound. The SEM was derived from logIC50 of three individual data sets.
Figure 2.
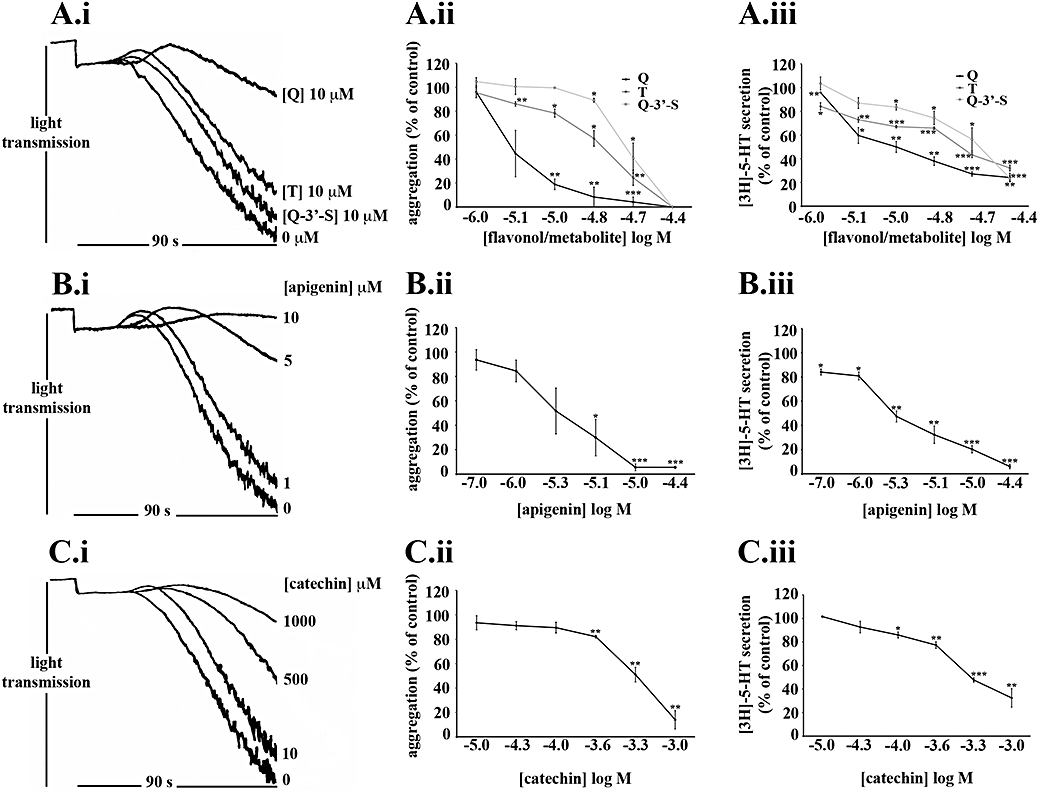
The extent of inhibition of platelet function by flavonoids and metabolites correlate with their potencies. Washed human platelets (4 × 108 cells·mL−1) loaded with [3H]-5-HT were pretreated with increasing concentrations of flavonoids [quercetin (Q): A.i, A.ii and A.iii, apigenin: B.i, B.ii and B.iii and catechin: C.i, C.ii and C.iii], metabolites (tamarixetin (T) and quercetin-3′-sulphate (Q-3′-S): A.i, A.ii and A.iii) or solvent control [DMSO (0.2% v/v)] for 5 min. Platelets were then stimulated with collagen (5 µg·mL−1) for 90 s and aggregation and 5-HT secretion were measured as a percentage of the DMSO-treated, collagen-stimulated control values (100%). The data points represent the mean (n= 3) aggregation or 5-HT secretion for each treatment (±SEM). *P≤ 0.05, **P≤ 0.01 and ***P≤ 0.001 compared with the control (DMSO-treated, collagen-stimulated platelets).
Flavonoids and metabolites inhibited 5-HT secretion with potencies which were not significantly different from those for aggregation. Apigenin and quercetin were the most potent flavonoids tested and there was no significant difference between their logIC50 values (Table 1) for the inhibition of aggregation and 5-HT secretion. Quercetin (10 µM, Figure 2A.ii) inhibited aggregation and apigenin (0.1 µM, Figure 2B.iii) inhibited 5-HT secretion significantly at concentrations known to be achieved in plasma following ingestion of a quercetin-4′-O-β-D-glucoside supplement (Hubbard et al., 2004) and a 2 g bolus of blanched parsley (Meyer et al., 2006) respectively.
By contrast, catechin was the least potent flavonoid examined, with an inhibitory potency two orders of magnitude lower (P≤ 0.01) than that of quercetin (Table 1). As such, in vivo, metabolites of quercetin and apigenin may be more potent inhibitors of platelet function than those of catechin.
Tamarixetin and quercetin-3′-sulphate inhibited with similar potencies but the metabolites were significantly less potent (P≤ 0.01 and P≤ 0.001 respectively) than quercetin (Table 1). Quercetin-3-glucuronide, however, caused low levels of inhibition of collagen-stimulated platelet function (20 µM: approximately 5%, 40 µM: approximately 23%); identical concentrations of quercetin, tamarixetin or quercetin-3′-sulphate either caused substantial (20 µM) or complete (40 µM) inhibition (data not shown).
Therefore, for both aggregation and 5-HT secretion the order of potency established was quercetin = apigenin > tamarixetin = quercetin-3′-sulphate > quercetin-3-glucuronide > catechin (Table 2).
Table 2.
The orders of inhibitory potency established for flavonoids and metabolites
| Action | Orders of inhibitory potency, level of inhibition or binding affinity |
|---|---|
| Inhibition of aggregation and 5-HT secretion | quercetin = apigenin > catechin quercetin > tamarixetin = quercetin-3′-sulphate > quercetin-3-glucuronide |
| Binding to collagen | catechin > apigenin > quercetin = tamarixetin |
| Binding to fibrinogen | tamarixetin > apigenin = catechin > quercetin |
| Inhibition of total protein, Syk and PLCγ2 tyrosine phosphorylation | quercetin > apigenin > catechin quercetin = tamarixetin > quercetin-3′-sulphate = quercetin-3-glucuronide |
| Inhibition of Fyn kinase activity | quercetin > quercetin-3′-sulphate > tamarixetin quercetin > apigenin = catechin |
Flavonoid and metabolite mechanisms may involve binding to collagen and fibrinogen or internalization
The surface plasmon resonance (SPR) assay was used to examine the putative abilities of flavonoids and metabolites to bind to ligands necessary for collagen-stimulated aggregation. Flavonoids (quercetin, apigenin, catechin) and metabolites (tamarixetin) were perfused over immobilized collagen or fibrinogen at a range of concentrations (9.375 µM; 18.75 µM; 37.5 µM; 75 µM; 150 µM). Quercetin, tamarixetin, apigenin and catechin bound collagen and fibrinogen with affinities (KD) ranging over four orders of magnitude (Table 3). The greatest affinities were observed for tamarixetin and catechin. Tamarixetin bound fibrinogen with high picomolar affinity and catechin bound collagen with nanomolar affinity, which raised the possibility of in vivo antagonism by low physiological concentrations of these compounds. Quercetin and apigenin, however, bound to collagen and fibrinogen with low micromolar affinities. So, the order of affinity for collagen was catechin > apigenin > quercetin = tamarixetin and that for fibrinogen was tamarixetin > apigenin = catechin > quercetin (Table 2). The lack of correlation between binding affinities and inhibitory potencies indicated that the disturbance of GPVI-collagen and αIIbβ3-fibrinogen interactions were unlikely to underlie the observed inhibitory effects of these compounds.
Table 3.
Interactions of flavonoids and metabolites with the platelet receptor ligands, collagen and fibrinogen
| Flavonoid/metabolite |
KD (kd/ka)±SEM (M) |
|
|---|---|---|
| Collagen | Fibrinogen | |
| Quercetin | 3.62 ± 3.55 (×10−6) | 9.96 × 10−6± 5.18 (×10−6) |
| Tamarixetin | 1.06 ± 0.52 (×10−6) | 6.15 ± 3.64 (×10−10) |
| Apigenin | 3.38 ± 2.61 (×10−7) | 2.99 ± 1.39 (×10−7) |
| Catechin | 4.81 ± 2.43 (×10−9) | 5.52 ± 1.26 (×10−7) |
Flavonoid and metabolite interactions with collagen and fibrinogen were measured using SPR. Quercetin, apigenin and catechin and tamarixetin, over a range of concentrations (9.375; 18.75; 37.5; 75; 150 × 10−6 M), were perfused for 180 s (30 µL·min−1) over collagen (100 µg·mL−1) or fibrinogen (100 µg·mL−1) immobilized on a dextran matrix. Association of the compounds was monitored over 180 s and dissociation was measured over 300 s in real-time. The baseline represented the response achieved after the subtraction of DMSO alone and flavonoid and metabolite responses following perfusion over the dextran matrix. Affinity (KD) was calculated from association (Ka) and dissociation (Kd) constants derived by fitting response units to the 1:1 Langmuir binding model.
SPR, surface plasmon resonance.
Flavonoids are reported to possess the ability to modulate the function of signalling proteins (Matter et al., 1992; Vlahos et al., 1994; Agullo et al., 1997; Gamet-Payrastre et al., 1999; Walker et al., 2000; Hubbard et al., 2003; Holder et al., 2007). Therefore the ability of platelets and MEG-01 cells (a human megakaryocytic cell line used as a model for platelets) to internalize these compounds was investigated. Both platelets and MEG-01 cells permitted internalization of flavonoids and metabolites, but the substantially larger size of MEG-01 cells enabled more detailed assessment of the access of the compounds to the cytosolic compartment. Quercetin is intrinsically fluorescent (Hollman et al., 1996), with excitation and emission at 430 nm and 480 nm–500 nm, respectively, and the ability of the methylated metabolite isorhamnetin to fluoresce (Hollman et al., 1996) suggested that tamarixetin was also fluorescent.
Confocal microscopy was used to assess the internalization of these compounds. A single middle layer from a series of images in the z dimension is shown with corresponding differential interference contrast (DIC) images and these are overlaid (Figure 3). Low levels of auto-fluorescence were detected in untreated cells (Figure 3A.i–iii), and in treated cells fluorescence was intense and spread across the entire cell (Figure 3B.i–iii,D.i–iii). Higher magnification of treated cells showed that there were higher fluorescence levels in defined regions within the cell, suggesting preferential accumulation of quercetin (Figure 3C.i–iii) and tamarixetin (Figure 3E.i–iii) in sub-cellular compartments. Tamarixetin was localized on the periphery of some cells (Figure 3E.i–iii) indicating possible interactions with the plasma membrane. These data potentially indicate that flavonoids and metabolites may inhibit function by inhibiting signalling events within the platelet cytosol following internalization.
Figure 3.

Internalization of quercetin and tamarixetin by MEG-01 cells. MEG-01 cells (4 × 106 cells·mL−1) were incubated with quercetin (40 µM), tamarixetin (20 µM) or solvent control [DMSO (0.2% v/v)] for 30 min. Fluorescence was detected at 480 nm–500 nm after excitation at 430 nm with an argon laser. Images of a single middle layer from z-stacks are shown (DMSO control: A. i–iii, quercetin: B. i–iii tamarixetin: D. i–iii) and higher magnifications of areas of interest are also shown (quercetin: C. i–iii, tamarixetin: E. i–iii). Images represent results from at least three individual experiments.
Flavonoid and metabolite modulation of signalling in intact platelets
The relative abilities of flavonoids and metabolites to inhibit Fyn kinase activity, total protein tyrosine phosphorylation as well as Syk and PLCγ2 tyrosine phosphorylation was examined to assess the relationship between the potencies of these compounds for inhibiting platelet function with their abilities to modulate GPVI pathway signalling proteins.
Platelets were pretreated with flavonoids or metabolites and stimulated with collagen. They were stimulated under non-aggregating conditions which prevented secondary signalling, so a higher concentration of collagen (25 µg·mL−1) was needed to induce measurable levels of kinase activity and protein tyrosine phosphorylation. Concentrations of flavonoids and metabolites were increased in proportion to collagen levels consistent with previous studies (Hubbard et al., 2003). Fyn kinase activity was measured in vitro, and Syk and PLCγ2 tyrosine phosphorylation levels in addition to total protein tyrosine phosphorylation were assessed by immunoblotting. Levels of inhibition were estimated by densitometry and normalized for protein loading levels (see Methods).
Quercetin was the only flavonoid capable of inhibiting Fyn kinase activity in whole cells (data not shown). Total protein tyrosine phosphorylation (Figure 4), Syk (Figure 5) and PLCγ2 (Figure 6) tyrosine phosphorylation were also inhibited by quercetin. Although quercetin and tamarixetin inhibited similarly, these compounds caused a greater level of inhibition than the other compounds. The flavonol and metabolite at 20 µM inhibited total protein tyrosine phosphorylation (Figure 4A,B), Syk (Figure 5A,B) and PLCγ2 (Figure 6A,B) tyrosine phosphorylation by approximately 20%, 15% and 35% respectively. By contrast, quercetin-3′-sulphate and quercetin-3-glucuronide inhibited total tyrosine phosphorylation (Figure 4C), Syk (Figure 5C) and PLCγ2 (Figure 6C) tyrosine phosphorylation minimally – 40 µM inhibited total protein tyrosine phosphorylation by only 25% of the level of inhibition caused by the same concentration of quercetin or tamarixetin (Figure 4C). So, despite blocking platelet function with a similar potency as tamarixetin, quercetin-3′-sulphate may inhibit other collagen-stimulated signalling pathways including those downstream of P2Y1, P2Y12 and TxA2 receptors (TPα and TPβ) more potently than the GPVI pathway.
Figure 4.
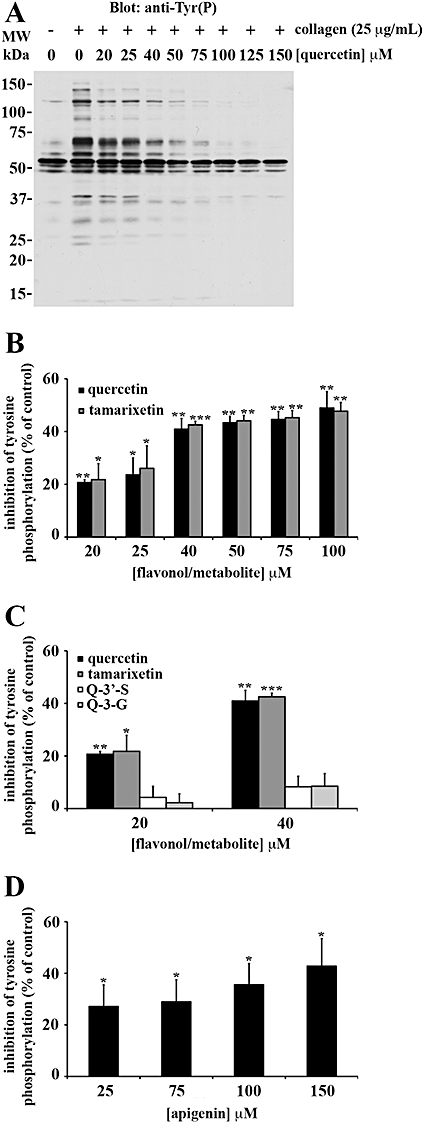
Flavonoids and metabolites block total protein tyrosine phosphorylation in intact platelets: the C ring C-3 hydroxyl group is necessary for potent inhibition. Washed human platelets (8 × 108 cells·mL−1) treated with 1 mM EGTA were pretreated with increasing concentrations of flavonoids (quercetin: A, B and apigenin: D), metabolites [tamarixetin: B, quercetin-3′-sulphate (Q-3′-S): C and quercetin-3-glucuronide (Q-3-G): C] or solvent control [DMSO (0.2% v/v)] for 5 min. Platelets were stimulated with 25 µg·mL−1 collagen for 90 s before the immunodetection of total protein tyrosine phosphorylation, as a percentage of the DMSO-treated, collagen-stimulated control (100%). The bars represent the mean (n= 3) tyrosine phosphorylation for each treatment (±SEM). *P≤ 0.05, **P≤ 0.01 and ***P≤ 0.001 compared with the control (DMSO-treated, collagen-stimulated platelets).
Figure 5.
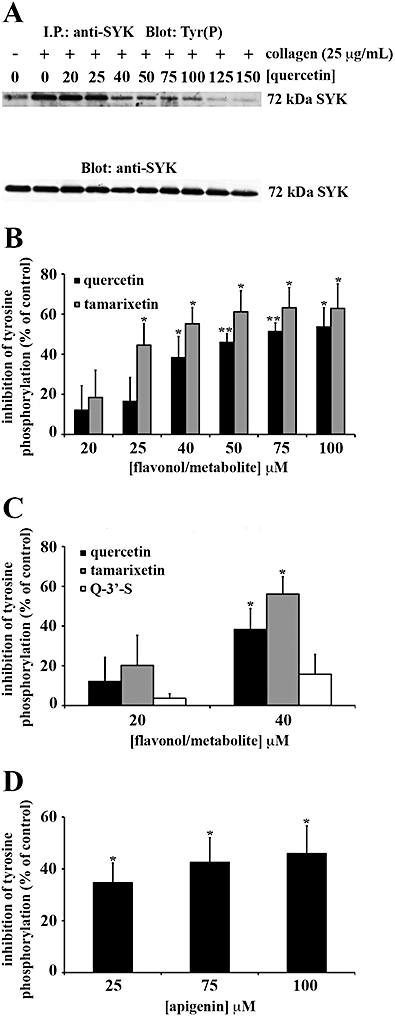
Syk tyrosine phosphorylation in intact platelets is inhibited with greatest potency by flavonoids with planar C rings (quercetin) and metabolites with methylated B rings (tamarixetin). Washed human platelets (8 × 108 cells·mL−1) were treated with 1 mM EGTA prior to incubation for 5 min with flavonoids (quercetin: A, B and apigenin: D), metabolites [tamarixetin: B, quercetin-3′-sulphate (Q-3′-S): C] or solvent control [DMSO (0.2% v/v)], then they were stimulated with 25 µg·mL−1 collagen for 90 s. Syk phosphotyrosine residues were detected and equivalent protein loading was verified by reprobing for Syk. Tyrosine phosphorylation was expressed as a percentage of the DMSO-treated, collagen-stimulated control (100%). The bars represent the mean (n= 3) tyrosine phosphorylation for each treatment (±SEM). *P≤ 0.05, **P≤ 0.01 and ***P≤ 0.001 compared with the control (DMSO-treated, collagen-stimulated platelets).
Figure 6.
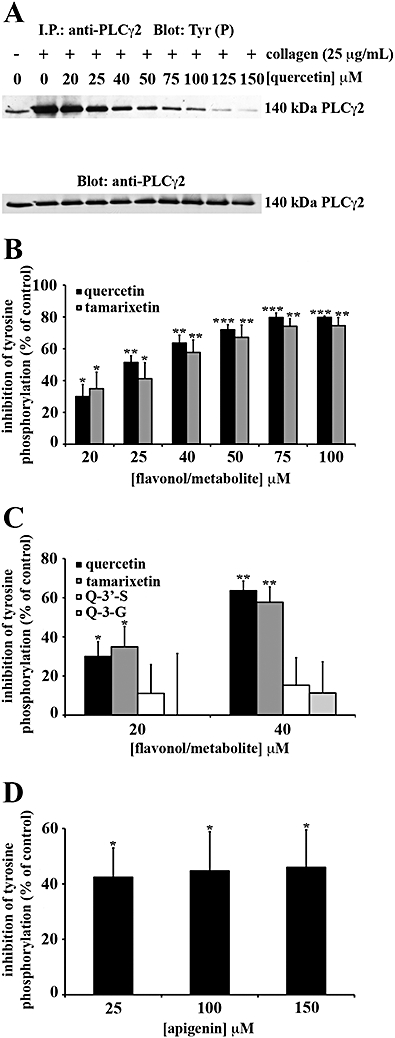
PLCγ2 tyrosine phosphorylation is inhibited with highest potency by flavonoids with planar, hydroxylated C rings (quercetin) and metabolites with methylated B rings (tamarixetin). Washed human platelets (8 × 108 cells·mL−1) treated with 1 mM EGTA were incubated for 5 min with flavonoids (quercetin: A, B and apigenin: D), metabolites [tamarixetin: B, quercetin-3′-sulphate (Q-3′-S): C and quercetin-3-glucuronide (Q-3-G): C] or solvent control (DMSO 0.2% v/v). Platelets were stimulated with 25 µg·mL−1 collagen for 90 s. PLCγ2 phosphotyrosine residues were detected before equivalent protein loading was verified by reprobing for PLCγ2. Tyrosine phosphorylation was expressed as a percentage of the DMSO-treated, collagen-stimulated control (100%). The bars represent the mean (n= 3) tyrosine phosphorylation for each treatment (±SEM). *P≤ 0.05, **P≤ 0.01 and ***P≤ 0.001 compared with the control (DMSO-treated, collagen-stimulated platelets).
Apigenin was a less effective inhibitor of total tyrosine phosphorylation (Figure 4D), Syk (Figure 5D) and PLCγ2 (Figure 6D) tyrosine phosphorylation than quercetin, so the C ring C-3 hydroxyl group and B ring catechol moiety which are modified between the structures of these compounds (see Figure 1) can be attributed to high level inhibition of signalling in intact platelets. At 25 µM, the flavone inhibited total protein and Syk tyrosine phosphorylation by approximately 25% and 35% respectively (Figure 5D). The same concentration of apigenin inhibited PLCγ2 tyrosine phosphorylation by 40 ± 11% (Figure 6D). Therefore, compared with quercetin, the flavone elicited a moderate level of inhibition for signalling.
Catechin remained one of the least potent flavonoids, indicating that the C ring C-2-C-3 double bond and C-4 carbonyl group absent from the structure of this flavonoid, but present within that of quercetin (see Figure 1.), also correlate with high potency inhibition. Although at 25 µM the flavan-3-ol inhibited total protein tyrosine phosphorylation to a significant extent (P≤ 0.05), only 5 ± 1% inhibition was achieved (data not shown). As catechin did not inhibit Syk or PLCγ2 tyrosine phosphorylation, it is possible this compound may target serine/threonine phosphorylation or signalling pathways activated by secondary agonists (for example, ADP, TxA2).
The order for high level inhibition for attenuation of tyrosine phosphorylation in intact platelets was quercetin = tamarixetin > apigenin > quercetin-3′-sulphate = quercetin-3-glucuronide > catechin (Table 2). The clear variations in the extent of inhibition of Fyn, Syk and PLCγ2, suggested that these compounds blocked their activity and tyrosine phosphorylation directly within the platelet cytosolic compartment.
Flavonoids and metabolites inhibit the activity of Fyn in isolation with potencies which correlate with discrete functional groups
Flavonoid and metabolite location within the platelet cytosol would permit direct inhibition of signalling proteins. Examining associations between key flavonoid and metabolite functional groups and inhibition of isolated GPVI signalling pathway proteins may provide a clearer understanding of structural requirements for inhibition of signalling.
Fyn was immunoprecipitated from collagen-stimulated platelets and treated with flavonoids or metabolites before kinase activity was measured. Levels of inhibition were estimated by densitometry, following normalization for protein loading levels.
Quercetin was the most potent inhibitor of Fyn kinase activity, at 20 µM (P≤ 0.01) or 40 µM (P≤ 0.001) (Figure 7C). Equivalent concentrations of tamarixetin were without effect and, although 20 µM and 40 µM quercetin-3′-sulphate were less effective than the same concentrations of quercetin, this metabolite still decreased kinase activity significantly (P≤ 0.05) (Figure 7C).
Figure 7.
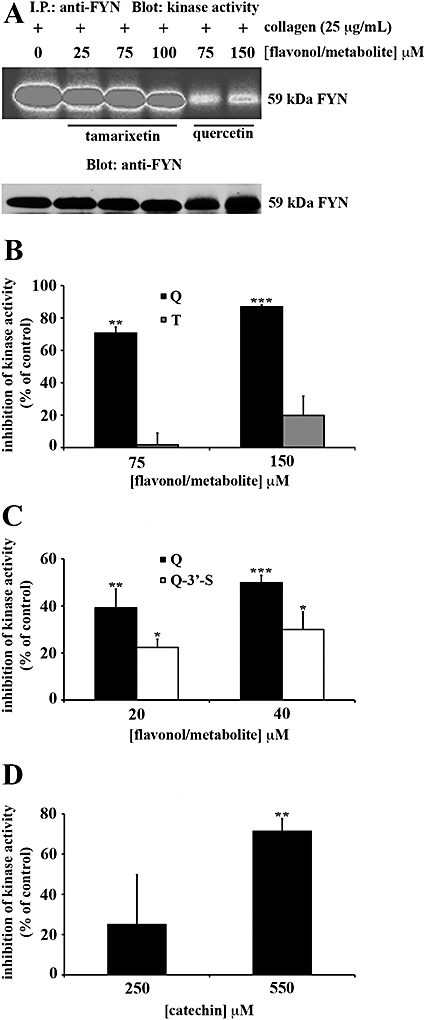
Flavonoids and metabolites block the kinase activity of Fyn in platelet lysates in a manner influenced by their pattern of hydroxylation. Washed human platelets (8 × 108 cells·mL−1) in the presence of EGTA (1 mM) were stimulated with collagen (25 µg·mL−1) for 90 s. Platelets were lysed with ice-cold 1% (v/v) NP40 and Fyn was precipitated. Platelet lysates were incubated with anti-Fyn antibody and protein G Sepharose (PGS) beads for 1 h at 4°C and Fyn immunoprecipitates were pretreated with flavonoids [quercetin (Q): A–C and catechin: D], metabolites [tamarixetin (T): A,B and quercetin-3′-sulphate (Q-3′-S): C] or solvent control [DMSO (0.2% v/v)] for 5 min. Immunoprecipitates were assayed for kinase activity as described in methods. Equivalent protein loading was verified by reprobing for Fyn. Kinase activity was expressed as a percentage of the DMSO-treated, collagen-stimulated control (100%). The bars represent the mean (n= 3) kinase activity for each treatment (±SEM). *P≤ 0.05, **P≤ 0.01 and ***P≤ 0.001 compared with the control (DMSO-treated, collagen-stimulated platelets).
Quercetin, at 75 µM (P≤ 0.01) and 150 µM (P≤ 0.001) almost completely blocked kinase activity (Figure 7A,B). At concentrations equivalent to those of quercetin, apigenin did not inhibit (data not shown) and catechin, at 550 µM, caused a significant inhibition (P≤ 0.01), similar to that with 75 µM quercetin, a concentration sevenfold less (Figure 7D).
Therefore the order of potency established for inhibition of Fyn kinase activity was quercetin > quercetin-3′-sulphate > tamarixetin = apigenin = catechin (Table 2).
Discussion
Understanding the mechanisms underlying the inhibitory effects of flavonoids and their biologically active metabolites is important to inform the development of strategies to treat thrombosis using these compounds as molecular templates and/or manipulating their dietary levels. Comprehensive dissections of correlations between discrete functional groups within the structures of these compounds and their effects are necessary to consider their modification into more potent and selective small-molecule inhibitors. The present study begins to address the structural basis for the inhibitory mechanisms underlying the effects of flavonoids and quercetin metabolites on platelet function.
A working model (Figure 8) illustrating how flavonoid and metabolite functional groups correlate with inhibition of collagen-stimulated platelet function and plausible mechanisms of action, summarizes the data described in the current study. High potency inhibition of collagen-stimulated function by quercetin, attributed to the planar, C-4 carbonyl substituted C ring and the B ring catechol moiety, may be achieved through attenuation of the activity and tyrosine phosphorylation of GPVI pathway signalling proteins including Fyn, Syk and PLCγ2 within the platelet cytosol. By contrast, apigenin may inhibit function as potently as quercetin in a cumulative manner involving moderate potency inhibition of Syk and PLCγ2 and low potency inhibition of Fyn. As signalling proteins within the same pathway were blocked with different potencies by the flavone, it is likely that apigenin may be internalized by platelets. Greater selectivity for events downstream of Fyn such as Syk and PLCγ2 tyrosine phosphorylation by this flavonoid correlated with the absence of C and B ring hydroxyl groups. This degree of selectivity of the flavone for Syk and PLCγ2 rather than Fyn indicated that manipulation of the hydroxylation pattern of flavonoids may be a viable strategy for designing more selective compounds.
Figure 8.

A working model outlining a structural basis for the actions of flavonoids and their metabolites in inhibition of collagen-stimulated platelet function. Flavonoids (quercetin, apigenin and catechin) and metabolites [tamarixetin, quercetin-3′-sulphate (Q-3′-S) and quercetin-3-glucuronide (Q-3-G)] inhibit platelet aggregation and 5-HT secretion in a differential manner due to variable attenuation of multiple mechanisms. These variations in inhibitory ability are due to distinct structural features including a planar, C-3 hydroxylated and C-4 carbonyl substituted C ring, a B ring catechol moiety and methyl, sulphate and glucuronide groups which are metabolically added. Mechanisms include inhibition of PLCγ2 and Syk tyrosine phosphorylation as well as Fyn kinase activity mediated directly within the cytosol. Although flavonoid and metabolite binding affinities for collagen and fibrinogen did not correlate with their inhibitory potencies, binding to collagen and fibrinogen, disrupting the interactions of these proteins with GPVI and αIIbβ3, respectively, may be a potential mode of action. While potent inhibition of platelet function is more dependent on a planar C ring conjugated to a C-4 carbonyl group and a B ring catechol moiety (quercetin and apigenin), elimination of the C ring C-3 hydroxyl group (apigenin) reduces potency for inhibition of signalling. In vivo, methylated and sulphated metabolites of flavonols may be more active inhibitors of platelet function than glucuronidated metabolites. The differential inhibition of signalling by apigenin suggests internalization and preferential inhibition of Syk and PLCγ2 rather than Fyn by this flavone indicates a degree of selectivity.
Low potency inhibition of platelet function by catechin suggests that the non-planar C ring of the flavan-3-ol prevents high potency inhibition of signalling. Metabolic modification of quercetin with a glucuronide group may, in a similar way, be the underlying cause of low potency inhibition of function by the metabolite. The addition of methyl or sulphate groups to quercetin attributed to moderate potency inhibition of function may be due to high potency inhibition of Syk and PLCγ2 by tamarixetin and of Fyn by quercetin-3′-sulphate respectively.
In the present study, flavonoids with a C ring C-2-C-3 double bond and a C-4 carbonyl group as well as a B ring catechol group inhibited collagen-stimulated platelet function with high potency. The C ring C-2-C-3 double bond responsible for maintaining planarity was demonstrated as considerably more important for high potency inhibition than the C ring C-4 carbonyl group, as removal of the C-4 carbonyl group on a planar C ring (cyanidin) resulted in approximately two- to threefold less potent inhibition than quercetin, whereas with a C-4 carbonyl group on a non-planar C ring (taxifolin), potency was reduced approximately 50- to 60-fold compared with quercetin (data not shown). These data are in accordance with those previously reported describing more potent inhibition of collagen-dependent platelet aggregation, 5-HT secretion and TxA2 release by quercetin and apigenin than by catechin (Beretz et al., 1982; Guerrero et al., 2005). Metabolic addition of a B ring C-4′ methyl or C-3′ sulphate group correlated with reduced potency from a high to moderate level, whereas glucuronidation of the C ring resulted in a dramatic loss in inhibitory potency. Considering the bioavailability profiles of quercetin (100 mg of quercetin from onions or quercetin-4′-glucoside – 7–7.6 µM quercetin in plasma (Graefe et al., 2001)), apigenin (2 g bolus of parsley – 0.1 µM apigenin in plasma (Meyer et al., 2006)) and catechin (35 mg catechin from red wine – 0.077–0.091 µM catechin in plasma (Donovan et al., 1999; Bell et al., 2000)) alongside these findings, in vivo, active flavonoids may include flavonol and flavone metabolites, but not flavan-3-ol metabolites.
Function may be attenuated via mechanisms mediated from the platelet surface and/or the cytosolic compartment. The steep Hill slopes observed alongside the potencies of flavonoids and metabolites (see Table 1), indicated that inhibitory mechanisms of these compounds for platelet function, potentially involving interactions with cell surface proteins or intracellular kinases are possibly complex multistep events rather than 1:1 associations.
One mode of action investigated in the current study considered the sequestration of collagen and fibrinogen from cognate receptors by flavonoids and metabolites. Although the lack of correlation between binding affinities and inhibitory potencies indicated that this was not a mechanism of action, interesting variations in the structural requirements for high affinity binding to collagen and fibrinogen between these compounds were observed. By contrast, a previous report (Guerrero et al., 2005) examining antagonism of the TxA2 receptor within the platelet plasma membrane by flavonoids associated the high potency inhibitors of function, quercetin and apigenin, with higher affinity binding than the low potency inhibitor, catechin. High and low potency inhibition of collagen-stimulated function by quercetin and catechin, respectively, has also been linked to an identical pattern of inhibition for the ROS, hydrogen peroxide (Pignatelli et al., 2000). Furthermore, ex vivo inhibition of the activation of platelets from individuals who had ingested quercetin supplements (Hubbard et al., 2004) or high-level dietary sources (Hubbard et al., 2006) was enhanced over time. Therefore, in vivo, flavonoids may inhibit platelet function through a dynamic structure-dependent mechanistic network.
The differential inhibition of signalling in intact platelets by flavonoids and metabolites observed in the present study suggested that inhibitory mechanisms are mediated from within the platelet cytosol and this evidence was supported by the visualization of quercetin and tamarixetin in platelets (data not shown). The localization of these compounds to the membrane of, and compartments resembling organelles within a megakaryocytic cell line (MEG-01) were not observed in platelets. Saturation of the entire platelet with fluorescence due to the small size of these cells may explain why intracellular regions concentrated with quercetin or tamarixetin were not visualized.
Flavonoid functional groups associated with potent inhibition of signalling in intact platelets included a planar, hydroxylated C ring substituted with a C-4 carbonyl group, a B ring catechol moiety and a metabolically added methyl group on the B ring. These data are in agreement with reported studies demonstrating that poly-hydroxylation of flavonoids containing planar C rings were linked to potent inhibition of PI3K (Agullo et al., 1997; Gamet-Payrastre et al., 1999; Walker et al., 2000), PKC (Agullo et al., 1997; Gamet-Payrastre et al., 1999) and non-specific serine/threonine protein kinase (PIM1) (Holder et al., 2007) activity.
A degree of selectivity may be conferred by the B ring C-4′ methyl group or deletion of the C ring C-3 and B ring C-3′ hydroxyls, as apigenin and tamarixetin inhibited Syk and PLCγ2 tyrosine phosphorylation more potently than Fyn kinase activity. Low potency inhibition of the kinase activity of Fyn in isolation by the flavone and metabolite supported these findings. A sulphate group at the B ring C-3′ position (quercetin-3′-sulphate) was associated with greater inhibitory potency against Fyn kinase activity than a B ring C-4′ methyl group (tamarixetin). Therefore, in vivo, flavonol metabolites modified at the B ring C-3′ position with a sulphate group or containing a catechol moiety on the same ring may be more potent inhibitors of the activity of Src-family kinases than flavone and flavan-3-ol metabolites.
Within this study a flavonol with a non-hydroxylated B ring (galangin) was demonstrated as a very poor inhibitor of Fyn kinase activity (data not shown). The B ring of the high potency inhibitor, quercetin, that contains a catechol group, was found to be involved in van der Waals interactions with the Src-family kinase, Hck (Sicheri et al., 1997) that is highly structurally homologous with Fyn. Therefore, enhanced binding capacity conferred by the involvement of the four sulphate oxygens on the B ring of quercetin-3′-sulphate in van der Waals interactions may underpin the potent inhibitory abilities of this metabolite.
The flavonoid structure has been shown as a viable template for the design of potent and selective small-molecule inhibitors. LY294002, an analogue of quercetin constructed by the omission of hydroxyl groups and substitution of the C-8 position of the A ring with a benzene ring, was more potent than the flavonol and was demonstrated as selective for PI3K (Vlahos et al., 1994).
We conclude that in vivo, flavonoid metabolites may inhibit function through multiple mechanisms which are influenced by functional groups within and on the periphery of the core flavonoid skeleton, including (i) the chromenone (2H-1-benzopyran) C ring C-2-C-3 double bond that maintains planarity; (ii) variations in C and B ring hydroxyl group substitutions; and (iii) metabolically added sulphate and methyl groups.
Cardiovascular disease may be modulated in a limited manner through manipulation of the diet, but this study highlights key features within the structures of flavonoids and plasma metabolites of quercetin which could inform the development of more selective, and therefore safer, therapeutic agents, which may be an effective strategy to utilize these polyphenolic compounds to reduce incidences of thrombotic disorders.
Acknowledgments
The work was supported by the Biotechnology and Biological Sciences Research Council and the British Heart Foundation.
Glossary
Abbreviations:
- 5-HT
5-hydroxytryptamine
- CVD
cardiovascular disease
- DIC
differential interference contrast
- GPIb
glycoprotein Ib
- GPVI
glycoprotein VI
- ITAM
immunoreceptor tyrosine-based activatory motif
- LAT
linker and activator of T-cells; MI, myocardial infarction
- PI3K
phosphoinositide-3-kinase
- PLCγ2
phospholipase Cγ2
- ROS
reactive oxygen species
- SH2
Src-homology 2
- SPR
surface plasmon resonance
- TxA2
thromboxane A2
- vWF
von Willebrand factor
Conflicts of interest
We have no conflicts of interest.
References
- Agullo G, Gamet-Payrastre L, Manenti S, Viala C, Remesy C, Chap H, et al. Relationship between flavonoids structure and inhibition of phosphatidylinositol 3-kinase: a comparison with tyrosine kinase and protein kinase C inhibition. Biochem Pharmacol. 1997;53:1649–1657. doi: 10.1016/s0006-2952(97)82453-7. [DOI] [PubMed] [Google Scholar]
- Asselin J, Gibbins JM, Achison M, Lee YH, Morton LF, Farndale RW, et al. A collagen-like peptide stimulates tyrosine phosphorylation of syk and phospholipase Cγ2 in platelets independent of the integrin α2β1. Blood. 1997;89:1235–1242. [PubMed] [Google Scholar]
- Bell JRC, Donovan JL, Wong R, Waterhouse AL, German JB, Walzem RL, et al. (+)-catechin in human plasma after ingestion of a single serving of reconstituted red wine. Am J Clin Nutr. 2000;71:103–108. doi: 10.1093/ajcn/71.1.103. [DOI] [PubMed] [Google Scholar]
- Benhamou M, Ryba NJ, Kihara H, Nishikata H, Siraganian RP. Protein-tyrosine kinase p72syk in high affinity IgE receptor signaling. Identification as a component of pp72 and association with the receptor gamma chain after receptor aggregation. J Biol Chem. 1993;268:23318–23324. [PubMed] [Google Scholar]
- Beretz A, Cazenave J-P, Anton R. Inhibition of aggregation and secretion of human platelets by quercetin and other flavonoids: structure-activity relationships. Agents Actions. 1982;12:382–387. doi: 10.1007/BF01965408. [DOI] [PubMed] [Google Scholar]
- Blake RA, Schieven GL, Watson SP. Collagen stimulates tyrosine phosphorylation of phospholipase C-gamma 2 but not phospholipase C-gamma 1 in human platelets. FEBS Lett. 1994;353:212–216. doi: 10.1016/0014-5793(94)01037-4. [DOI] [PubMed] [Google Scholar]
- Briggs WH, Folts JD, Osman HE, Goldman IL. Administration of raw onion inhibits platelet-mediated thrombosis in dogs. J Nutr. 2001;131:2619–2622. doi: 10.1093/jn/131.10.2619. [DOI] [PubMed] [Google Scholar]
- Cicmil M, Thomas JM, Leduc M, Bon C, Gibbins JM. Platelet endothelial cell adhesion molecule-1 signaling inhibits the activation of human platelets. Blood. 2002;99:137–144. doi: 10.1182/blood.v99.1.137. [DOI] [PubMed] [Google Scholar]
- Day AJ, Mellon F, Barron D, Sarrazin G, Morgan MRA, Williamson G. Human metabolism of dietary flavonoids: identification of plasma metabolites of quercetin. Free Radic Res. 2001;35:941–952. doi: 10.1080/10715760100301441. [DOI] [PubMed] [Google Scholar]
- Demrow HS, Slane PR, Folts JD. Administration of wine and grape juice inhibits in vivo platelet activity and thrombosis in stenosed canine coronary arteries. Circulation. 1995;91:1182–1188. doi: 10.1161/01.cir.91.4.1182. [DOI] [PubMed] [Google Scholar]
- Donovan JL, Bell JR, Kasim-Karakas S, German JB, Walzem RL, Hansen RJ, et al. Catechin is present as metabolites in human plasma after consumption of red wine. J Nutr. 1999;129:1662–1668. doi: 10.1093/jn/129.9.1662. [DOI] [PubMed] [Google Scholar]
- Fitzgerald GA. Mechanisms of platelet activation: thromboxane A2 as an amplifying signal for other agonists. Am J Cardiol. 1991;68:11B–15B. doi: 10.1016/0002-9149(91)90379-y. [DOI] [PubMed] [Google Scholar]
- Freedman JE, Parker C, Li L, Perlman JA, Frei B, Ivanov V, et al. Select flavonoids and whole juice from purple grapes inhibit platelet function and enhance nitric oxide release. Circulation. 2001;103:2792–2798. doi: 10.1161/01.cir.103.23.2792. [DOI] [PubMed] [Google Scholar]
- Gamet-Payrastre L, Manenti S, Gratacap M-P, Tulliez J, Chap H, Payrastre B. Flavonoids and the inhibition of PKC and PI 3-kinase. Gen Pharmacol. 1999;32:279–286. doi: 10.1016/s0306-3623(98)00220-1. [DOI] [PubMed] [Google Scholar]
- Gibbins JM. Platelet adhesion signalling and the regulation of thrombus formation. J Cell Sci. 2004;117:3415–3425. doi: 10.1242/jcs.01325. [DOI] [PubMed] [Google Scholar]
- Gibbins J, Asselin J, Farndale R, Barnes M, Law CL, Watson SP. Tyrosine phosphorylation of the Fc receptor gamma-chain in collagen-stimulated platelets. J Biol Chem. 1996;271:18095–18099. doi: 10.1074/jbc.271.30.18095. [DOI] [PubMed] [Google Scholar]
- Gibbins JM, Okuma M, Farndale R, Barnes M, Watson SP. Glycoprotein VI is the collagen receptor in platelets which underlies tyrosine phosphorylation of the Fc receptor γ-chain. FEBS Lett. 1997;413:255–259. doi: 10.1016/s0014-5793(97)00926-5. [DOI] [PubMed] [Google Scholar]
- Gibbins JM, Briddon S, Shutes A, van Vugt MJ, van de Winkel JG, Saito T, et al. The p85 subunit of phosphatidylinositol 3-kinase associates with the Fc receptor gamma-chain and linker for activator of T cells (LAT) in platelets stimulated by collagen and convulxin. J Biol Chem. 1998;273:34437–34443. doi: 10.1074/jbc.273.51.34437. [DOI] [PubMed] [Google Scholar]
- Graefe EU, Wittig J, Mueller S, Riethling AK, Uehleke B, Drewelow B, et al. Pharmacokinetics and Bioavailability of quercetin glycosides in humans. J Clin Pharmacol. 2001;41:492–499. doi: 10.1177/00912700122010366. [DOI] [PubMed] [Google Scholar]
- Guerrero JA, Lozano ML, Castillo J, Benavente-Garcia O, Vicente V, Rivera J. Flavonoids inhibit platelet function through binding to the thromboxane A2 receptor. J Thromb Haemost. 2005;3:369–376. doi: 10.1111/j.1538-7836.2004.01099.x. [DOI] [PubMed] [Google Scholar]
- Hartwig JH. Mechanisms of actin rearrangements mediating platelet activation. J Cell Biol. 1992;118:1421–1442. doi: 10.1083/jcb.118.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig JH, Barkalow K, Azim A, Italiano J. The elegant platelet: signals controlling actin assembly. Thromb Haemost. 1999;82:392–398. [PubMed] [Google Scholar]
- Hertog MGL, Hollman PCH, Katan MB. Content of potentially anticarcinogenic flavonoids of 28 vegetables and 9 fruits commonly consumed in the Netherlands. J Agric Food Chem. 1992;40:2379–2383. [Google Scholar]
- Hertog MGL, Feskens EJM, Hollman PCH, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993;342:1007–1011. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- Holder S, Zemskova M, Zhang C, Tabrizizad M, Bremer R, Neidigh JW, Lilly MB. Characterisation of a potent and selective small-molecule inhibitor of the PIM1 kinase. Mol Cancer Ther. 2007;6:163–172. doi: 10.1158/1535-7163.MCT-06-0397. [DOI] [PubMed] [Google Scholar]
- Hollman PCH, Van Trijp JMP, Buysman MNCP. Fluorescence detection of flavonols in HPLC by postcolumn chelation with aluminium. Anal Chem. 1996;68:3511–3515. doi: 10.1021/ac960461w. [DOI] [PubMed] [Google Scholar]
- Hourani SMO. Pharmacology of the platelet ADP receptors: agonists and antagonists. Haematology. 2000;85:58–65. [Google Scholar]
- Hubbard GP, Stevens JM, Cicmil M, Sage T, Jordan PA, Williams CM, et al. Quercetin inhibits collagen-stimulated platelet activation through inhibition of multiple components of the glycoprotein VI signalling pathway. J Thromb Haemost. 2003;1:1079–1088. doi: 10.1046/j.1538-7836.2003.00212.x. [DOI] [PubMed] [Google Scholar]
- Hubbard GP, Wolffram S, Lovegrove JA, Gibbins JM. Ingestion of quercetin inhibits platelet aggregation and essential components of the collagen-stimulated platelet activation pathway in humans. J Thromb Haemost. 2004;2:2138–2145. doi: 10.1111/j.1538-7836.2004.01067.x. [DOI] [PubMed] [Google Scholar]
- Hubbard GP, Wolffram S, Gibbins JM, Lovegrove JA. Ingestion of onion soup high in quercetin inhibits platelet aggregation and essential components of the collagen-stimulated platelet activation pathways in man: a pilot study. Br J Nutr. 2006;96:482–488. [PubMed] [Google Scholar]
- Italiano JE, Jr, Lecine P, Shivdasani RA, Hartwig JH. Blood platelets are assembled principally at the ends of proplatelet processes produced by differentiated megakaryocyte. J Cell Biol. 1999;147:1299–1312. doi: 10.1083/jcb.147.6.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzen HM, Gousset L, Bhaskar V, Vincent D, Tai A, Reynolds EE, Conley PB. Evidence for two distinct G-protein-coupled ADP receptors mediating platelet activation. Thromb Haemost. 1999;81:111–117. [PubMed] [Google Scholar]
- Jin J, Daniel JL, Kunapuli SP. Molecular basis for ADP-induced platelet activation. II. The P2Y1 receptor mediates ADP-induced intracellular calcium mobilization and shape change in platelets. J Biol Chem. 1998;273:2030–2034. doi: 10.1074/jbc.273.4.2030. [DOI] [PubMed] [Google Scholar]
- Keevil JG, Osman HE, Reed JD, Folts JD. Grape juice but not orange juice or grapefruit juice inhibits human platelet aggregation. J Nutr. 2000;130:53–56. doi: 10.1093/jn/130.1.53. [DOI] [PubMed] [Google Scholar]
- Keli SO, Hertog MGL, Feskens EJM, Kromhout D. Dietary flavonoids, antioxidant vitamins, and the incidence of stroke. Arch Intern Med. 1996;154:637–642. [PubMed] [Google Scholar]
- Landolfi R, Mower RL, Steiner M. Modification of platelet function and arachidonic acid metabolism by bioflavonoids: structure-activity relations. Biochem Pharmacol. 1984;33:1525–1530. doi: 10.1016/0006-2952(84)90423-4. [DOI] [PubMed] [Google Scholar]
- Li N, Wallén NH, Ladjevardi M, Hjemdahl P. Effects of serotonin on platelet activation in whole blood. Blood, Coagul Fibrinolysis. 1997;8:517–523. doi: 10.1097/00001721-199711000-00006. [DOI] [PubMed] [Google Scholar]
- Matter WF, Brown RF, Vlahos CJ. The inhibition of phosphatidylinositol 3-kinase by quercetin and analogs. Biochem Biophys Res Commun. 1992;186:624–631. doi: 10.1016/0006-291x(92)90792-j. [DOI] [PubMed] [Google Scholar]
- Meyer H, Bolarinwa A, Wolfram G, Linseisen J. Bioavailability of apigenin from apiin-rich parsley in humans. Ann Nutr Metab. 2006;50:167–172. doi: 10.1159/000090736. [DOI] [PubMed] [Google Scholar]
- Myszka DG. Kinetic analysis of macromolecular using surface Plasmon resonance biosensors. Curr Opin Biotechnol. 1997;8:50–57. doi: 10.1016/s0958-1669(97)80157-7. [DOI] [PubMed] [Google Scholar]
- Navarro-Nuñez L, Lozano ML, Palomo M, Martinez C, Vicente V, Castillo J, et al. Apigenin inhibits platelet adhesion and thrombus formation and synergises with aspirin in the suppression of the arachidonic acid pathway. J Agric Food Chem. 2008;56:2970–2976. doi: 10.1021/jf0723209. [DOI] [PubMed] [Google Scholar]
- Nieswandt B, Bergmeier W, Schulte V, Rackebrandt K, Gessner JE, Zirngibl H. Expression and function of the mouse collagen receptor glycoprotein VI is strictly dependent on its association with the FcR gamma chain. J Biol Chem. 2000;275:23998–24002. doi: 10.1074/jbc.M003803200. [DOI] [PubMed] [Google Scholar]
- O'Shannessy DJ, Winzor DJ. Interpretation of deviations from pseudo-first-order kinetic behavior in the characterization of ligand binding by biosensor technology. Anal Biochem. 1996;236:275–283. doi: 10.1006/abio.1996.0167. [DOI] [PubMed] [Google Scholar]
- Osman HE, Maalej N, Shanmuganayagam D, Folts JD. Grape juice but not orange or grapefruit juice inhibits platelet activity in dogs and monkeys (Macaca fasciularis) J Nutr. 1998;128:2307–2312. doi: 10.1093/jn/128.12.2307. [DOI] [PubMed] [Google Scholar]
- Patel SR, Hartwig JH, Italiano JE., Jr The biogenesis of platelets from megakaryocyte proplatelets. J Clin Investig. 2005;115:3348–3354. doi: 10.1172/JCI26891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel-Hett S, Richardson JL, Schulze H, Drabek K, Isaac NA, Hoffmeister K, et al. Visualization of microtubule growth in living platelets reveals a dynamic marginal band with multiple microtubules. Blood. 2008;111:4605–4616. doi: 10.1182/blood-2007-10-118844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatelli P, Pulcinelli FM, Celestini A, Lenti L, Ghiselli A, Gazzaniga PP, Violi F. The flavonoids quercetin and catechin synergistically inhibit platelet function by antagonizing the intracellular production of hydrogen peroxide. Am J Clin Nutr. 2000;72:1150–1155. doi: 10.1093/ajcn/72.5.1150. [DOI] [PubMed] [Google Scholar]
- Pignatelli P, Ghiselli A, Buchetti B, Carnevale R, Natella F, Germano G, et al. Polyphenols synergistically inhibit oxidative stress in subjects given red and white wine. Atherosclerosis. 2006;188:77–83. doi: 10.1016/j.atherosclerosis.2005.10.025. [DOI] [PubMed] [Google Scholar]
- Plow EF, Ginsberg MH. The molecular basis for platelet function. In: Hoffman R, Benz EJ, Shattil SJ, Furie B, Cohen HJ, Silberstein LE, et al., editors. Haematology: Basic Principles and Practice. 3rd edn. New York: Churchill Livingstone; 2000. p. 1741. [Google Scholar]
- Qi R, Ozaki Y, Satoh K, Kurota K, Asazuma N, Yatomi Y, et al. Quantitative measurement of various 5-HT receptor antagonists on platelet activation induced by serotonin. Thromb Res. 1996;81:43–54. doi: 10.1016/0049-3848(95)00212-x. [DOI] [PubMed] [Google Scholar]
- Richardson JL, Shivdasani RA, Boers C, Hartwig JH, Italiano JE., Jr Mechanisms of organelle transport and capture along proplatelets during platelet production. Blood. 2005;106:4066–4075. doi: 10.1182/blood-2005-06-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri ZM. Platelets in artherothrombosis. Nat Med. 2002;8:1227–1234. doi: 10.1038/nm1102-1227. [DOI] [PubMed] [Google Scholar]
- Santoro SA, Walsh JJ, Staatz WD, Baranski KJ. Distinct determinants on collagen support α2β1 integrin-mediated platelet adhesion and platelet activation. Cell Regul. 1991;2:905–913. doi: 10.1091/mbc.2.11.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. J Nutr. 2000;130:2073S–2085S. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- Sicheri F, Moarefi I, Kuriyan J. Crystal structure of the Src family tyrosine kinase Hck. Nature. 1997;385:602–609. doi: 10.1038/385602a0. [DOI] [PubMed] [Google Scholar]
- Siraganian RP, Zhang J, Suzuki K, Sada K. Protein tyrosine kinase Syk in mast cell signaling. Mol Immunol. 2001;38:1229–1233. doi: 10.1016/s0161-5890(02)00068-8. [DOI] [PubMed] [Google Scholar]
- Suzuki-Inoue K, Tulasne D, Shen Y, Bori-Sanz T, Inoue O, Jung SM, et al. Association of Fyn and Lyn with the proline-rich domain of glycoprotein VI regulates intracellular signaling. J Biol Chem. 2002;277:21561–21566. doi: 10.1074/jbc.M201012200. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Hanano M, Moroi M, Shibata A. Substitution of Val for Met at residue 239 of platelet glycoprotein Ib alpha in Japanese patients with platelet-type von Willebrand disease. Thromb Haemost. 1995;73:1197. [PubMed] [Google Scholar]
- Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- Walker EH, Pacold ME, Perisic O, Stephens L, Hawkins PT, Wymann MP, et al. Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin and staurosporine. Mol Cell. 2000;6:909–919. doi: 10.1016/s1097-2765(05)00089-4. [DOI] [PubMed] [Google Scholar]
- White HD, Chew DP. Acute myocardial infarction. Lancet. 2008;372:570–584. doi: 10.1016/S0140-6736(08)61237-4. [DOI] [PubMed] [Google Scholar]
- Woulfe D, Yang J, Prevost N, O'Brien P, Fortna R, Tognolini M. Signalling receptors on platelets and megakaryocytes. In: Gibbins JM, Mahaut-Smith MP, et al., editors. Methods in Molecular Biology-Platelets and Megakaryocytes, Volume 2: Perspectives and Techniques. Totowa, NJ: Humana Press; 2004. pp. 3–31. Vol. 273. [DOI] [PubMed] [Google Scholar]
- Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]


