Abstract
OBJECTIVE
The purpose of this study was to assess the diagnostic value of hepatocellular nodule vascularity after microbubble injection for characterization of malignancy in patients with cirrhosis of the liver.
MATERIALS AND METHODS
After sulfur hexafluoride–filled microbubble injection, the vascularity of 236 hepatocellular nodules (1–5 cm in diameter) in 215 patients with cirrhosis (151 men, 64 women; mean age, 62 ± 11 [SD] years) was evaluated by consensus of three reference radiologists. The relation between nodule vascularity in the arterial (10–40 seconds from injection) and portal venous (45 seconds to microbubble disappearance) phases and dimension of malignancy was evaluated by multivariate U statistical analysis. Two blinded independent reviewers using reference criteria classified nodules as benign or malignant after review of unenhanced and contrast-enhanced sonograms.
RESULTS
The final diagnoses were 96 malignant (84 hepatocellular carcinoma, 12 tumors not hepatocellular carcinoma) and 140 benign nodules (57 regenerative and 13 dysplastic nodules, 70 other benign lesions). Nodule hypervascularity during the arterial phase and hypovascularity during the portal venous phase (odds ratio, 27.78) and nodule diameter greater than 2 cm combined with hypervascularity during the arterial phase and isovascularity or hypervascularity during the portal venous phase (odds ratio, 3.3) were related to the presence of malignancy. Contrast-enhanced sonography improved diagnostic accuracy (unenhanced sonography vs contrast-enhanced sonography, 32% vs 71% for reviewer 1 and 22% vs 66% for reviewer 2; p < 0.05, McNemar test) even though hypervascular nodules 2 cm or smaller (malignant, n = 2; benign, n = 40) that appeared isovascular or hypervascular during the portal venous phase were misclassified.
CONCLUSION
Assessment of hepatocellular nodule vascularity after microbubble injection allowed characterization of malignancy, but characterization was limited for hypervascular nodules 2 cm or less in diameter.
Keywords: cirrhosis, hepatocellular nodules, liver, microbubble contrast agents, sonography
Accurate characterization of nodular lesions in the cirrhotic liver is among the most challenging imaging problems [1–3]. Unenhanced gray-scale and color Doppler sonography have limitations in differentiation of malignant from benign hepatocellular nodules, especially in the background of liver cirrhosis, which can substantially change the sonographic appearance of nodules [4–6]. According to the 2001 Barcelona criteria [2, 3], evidence of a characteristic vascular profile consisting of coincidental arterial hypervascularity on contrast-enhanced CT and MRI of nodules larger than 2 cm in patients with liver cirrhosis is considered diagnostic of hepatocellular carcinoma (HCC) [2]. More recently, the value of contrast washout with hypovascularity in the portal venous phase of CT and/or MRI has been recognized [3]. Nodule biopsy is suggested for nodules 2 cm or smaller and for nodules larger than 2 cm if hypervascularity is not detected on contrast-enhanced CT and MRI [2, 3].
Results indicate that because it allows reliable characterization of liver tumors, contrast-enhanced sonography can be used for noninvasive diagnosis of HCC [7–10]. Sulfur hexafluoride–filled microbubbles can be insonated continuously with low transmit power (mechanical index, 0.08–0.21), which allows depiction of lesion vascularity in real time with better temporal resolution and contrast sensitivity than achieved with CT [11]. Although the diagnostic capabilities of contrast-enhanced sonography with real-time insonation for characterization of liver tumors have been analyzed in numerous studies [12–20], no previous study, to our knowledge, has been conducted to extensively analyze the diagnostic capabilities of contrast-enhanced sonography of patients with cirrhosis and biopsy-proven hepatic nodules. The aim of this study was to assess the diagnostic value of the vascularity of hepatocellular nodules after microbubble injection in characterization of malignancy in patients with liver cirrhosis.
Materials and Methods
Patients
Approval with a waiver of informed consent was granted by the ethics committee (equivalent to the institutional review board) for this retrospective study involving three hospitals. From a coordinated and simultaneous computerized search of the databases of the radiologic records of the three hospitals between May 2003 and May 2005 performed by one reference radiologist for each center, 312 patients with cirrhosis and a diagnosis of at least one hepatic nodule detected on sonography or multiphase contrast-enhanced CT or MRI and subsequently imaged with contrast-enhanced sonography were identified. None of the patients had been treated for the nodules before the study, and all patients had a definite diagnosis of liver cirrhosis (Child-Turcotte-Pugh class A or B) related to viral infection (hepatitis B [n = 116], hepatitis C [n = 131], or both [n = 25]), alcohol abuse (n = 38), or autoimmune hepatitis (n = 2). The diagnoses had been obtained with biopsy or unequivocal imaging findings, including irregular liver margins and nodulations.
One to 20 days after identification of the nodules, one or two nodules per patient for a total of 352 hepatocellular nodules were selected for contrast-enhanced sonography after injection of sulfur hexafluoride–filled microbubbles (SonoVue, Bracco). The nodules were selected on the basis of largest diameter and best possible acoustic window. For completion of the diagnostic evaluation, the nodules identified with sonography were imaged with a multiphase cross-sectional technique (CT or MRI) 2–15 days after contrast-enhanced sonography. Nodules highly suspected of being HCC on the basis of clinical (e.g., chronic liver disease related to hepatitis B or C viral infection, increased α-fetoprotein level) and/or imaging criteria (nodule hypervascularity during the arterial phase with or without hypovascularity during the portal venous phase of contrast-enhanced CT and/or MRI according to the Barcelona criteria [2, 3]) and nodules incompletely or not characterized after imaging were biopsied 2–15 days after contrast-enhanced sonography. The histologic specimens were obtained with percutaneous sonographically guided biopsy performed with 18- to 20-gauge modified Menghini needles and were stained with H and E and the Masson trichrome method. A senior pathologist from each center made the diagnosis according to the diagnostic criteria established by the International Working Party on the terminology of nodular hepatocellular lesions [1].
The reference radiologists excluded 116 nodules because of lack of histologic diagnosis (86 nodules) or technical inadequacy of contrast-enhanced sonographic examination due to failure in data storage or incomplete nodule visibility (30 nodules). The final study group consisted of 236 nodules (Table 1) in 215 patients (mean age, 62 ± 11 [SD] years; median, 64 years; range, 29–84 years), including 151 men (mean age, 62 ± 11 years; median, 64 years; range, 29–84 years) and 64 women (mean age, 60 ± 9 years; median, 60 years; range, 30–75 years).
TABLE 1.
Features of Hepatocellular Nodule Histotypes
| Diameter (cm) |
|||||
|---|---|---|---|---|---|
| Histotype |
n |
Mean ± SD |
Range |
≤ 2 (n) |
> 2 (n) |
| Hepatocellular carcinoma | 84a | 3.1 ± 0.9 | 1–5 | 20 | 64 |
| Other malignant histotype | 12b | 2.5 ± 1.6 | 3–4 | 5 | 7 |
| Dysplastic nodule | 13c | 1.9 ± 0.6 | 1–3 | 7 | 6 |
| Regenerative nodule | 57 | 1.9 ± 0.8 | 1–4 | 46 | 11 |
| Hemangioma | 42d | 1.9 ± 0.7 | 1–3 | 31 | 11 |
| Focal nodular hyperplasia | 2 | 4 ± 0 | 4 | 0 | 2 |
| Hepatocellular adenoma | 1 | 4 ± 0 | 4 | 0 | 1 |
| Pseudotumor |
25e |
1.7 ± 0.6 |
1–3 |
21 |
4 |
| Total | 236 | 2.3 ± 1.1 | 1–5 | 130 | 106 |
Nodules ≤ 2 cm (n = 20) included 16 well-differentiated and four moderately or poorly differentiated hepatocellular carcinomas. Nodules > 2 cm (n = 64) included eight well-differentiated and 56 moderately or poorly differentiated hepatocellular carcinomas.
Other histotypes were intrahepatic cholangiocarcinoma (n = 6), metastatic lesion (n = 4), nodular lymphoma (n = 1), and epithelioid hemangioendothelioma (n = 1).
Dysplastic nodules had a low- (n = 8) or high-grade (n = 5) pattern.
Liver hemangioma had a hypervascular (n = 35) or thrombotic–fibrotic (n = 4) pattern at histologic examination. Other hemangiomas (n = 3) had endothelium-lined vascular channels.
Pseudotumor included focal fibrosis (n = 11), focal fatty changes or focal fat sparing (n = 8), intrahepatic arterioportal nontumorous shunt (n = 5), and focal lymphoid hyperplasia (n = 1).
Contrast-Enhanced Sonographic Examination
The sonographic examinations considered in the present series were performed by board-registered radiologists who had at least 5 years of experience in sonographic imaging of the liver and were affiliated with the three study centers. For consistency, the three centers used the same state-of-the-art sonographic equipment (Acuson Sequoia, Siemens Medical Solutions; convex array 2- to 4-MHz 4C1 transducer) and the same scanning protocol. The protocol consisted of a preliminary gray-scale and color or power Doppler unenhanced sonography followed by contrast-enhanced sonography.
The largest diameter of the nodule was measured in the transverse or longitudinal plane on unenhanced sonography, and the nodule was located in a liver segment according to the Bismuth [21] and Couinaud [22] classification systems. Tumoral vessels were imaged at low flow settings (pulse repetition frequency, 800–1,500 Hz; wall filter, 40–50 Hz; high levels of color vs echo priority and color persistence) and with Doppler spectral analysis of peripheral and intranodular vessels. Sulfur hexafluoride–filled microbubbles were manually injected in a 2.4-mL bolus through an 18- to 20-gauge IV cannula and followed by a 10-mL normal saline flush. Each nodule was scanned with real-time continuous insonation during normal breathing or breath-holding, depending on the best visualization of the tumor. The arterial phase was timed 10–40 seconds after microbubble injection, after which the extended portal venous phase encompassed the time interval from 45 seconds after microbubble injection to microbubble disappearance. No temporal range was assigned to the late phase because microbubble contrast agents are purely intravascular and do not have an interstitial or equilibrium phase.
Technical parameters were Coherent Contrast Imaging (CCI; Acuson Sequoia, Siemens Medical Solutions) or Cadence Contrast Pulse Sequencing (CPS; Acuson Sequoia) for contrast-specific technique; low transmit power (mechanical index, 0.09–0.14); dynamic range, 65 dB; temporal resolution between frames, 75–100 milliseconds (10–13 frames/s); echo-signal gain below noise visibility; signal persistence turned off and one focus below the level of the tumor. Distinct digital cine clips of the unenhanced sonographic scans and of the arterial and portal venous phases of contrast-enhanced sonographic scans were stored on a PC (Pentium 4, Intel) connected to the sonographic equipment through a high-performance hardware-based real-time Moving Picture Experts Group 2 (MPEG-2) encoder (MVR1000, Mediacruise, Canopus) and frame-grabber software (Mediacruise, Canopus). Cine clips were stored on digital video discs (DVDs) after sonography.
Consensus Analysis of Nodule Vascularity
The digital cine clips were reviewed on screen (Pentium 4, Intel; 19-inch [48 cm] thin-film transistor display) by the three reference radiologists during one consensus interpretation session. Discrepant interpretations were resolved by consensus through the involvement of an additional reviewer with experience in contrast-enhanced sonography similar to that of the other reviewers. Nodules of higher, similar, or lower echogenicity compared with the adjacent liver parenchyma after microbubble injection were defined as hypervascular, isovascular, and hypovascular. Hypervascularity could be homogeneously or heterogeneously distributed throughout a nodule. Nodules with dotlike vascularity (tiny separate spots of enhancement) were considered hypovascular. Evidence of peripheral nodular (discontinuous or continuous peripheral nodular appearance) or rimlike vascularity (continuous peripheral ring), followed or not by progressive fill-in, was recorded.
Independent Interpretation of Cine Clips
The digital cine clips were reviewed by two independent radiologists affiliated with one of the three hospitals and with 2 and 7 years of experience in contrast-enhanced sonography of the liver. These reviewers were not involved in the sonographic scanning and were blinded to the patients’ identification, clinical history, biopsy results, and other imaging findings except for the presence of liver cirrhosis. All interpretations were performed on the same computer (Pentium 4, Intel; 19-inch [48 cm] thin-film transistor display; resolution, 2,560 × 1,600 pixels) with DVD player software (PowerDVD, CyberLink).
In each session, a sequence of the cine clips of different nodules was randomly assigned to each reviewer, and any identifying information was masked. Reviewers were asked to express a diagnosis of benign or malignant for each nodule after review of the unenhanced sonographic scans and additional review of the contrast-enhanced sonographic scans. Both reviewers used the same criteria for assessing nodule vascularity, which had been established for the consensus analysis, and were free to perform real-time scrolling of the digital cine clips.
The diagnostic criteria for malignancy on unenhanced sonography were developed from previous studies [4–6]. They included heterogeneous appearance of the nodule and evidence of peripheral hypoechoic halo or satellite nodules on gray-scale sonography and the presence of peripheral arterial vessels with intratumoral branches on color Doppler sonography.
The diagnostic criteria for malignancy on contrast-enhanced sonography were developed from previous studies [11–20] and from vascularity patterns (Table 2) that, combined with nodule dimension, were found significantly related to the presence of malignancy after multivariate U statistical analysis (Table 3; see Statistical Analysis). Nodules considered malignant were hypervascular during the arterial phase and hypovascular during the portal venous phase with or without rimlike peripheral enhancement, were larger than 2 cm and hypervascular during the arterial phase and isovascular or hypervascular during the portal venous phase, or were isovascular during the arterial phase and hypovascular during the portal venous phase. Nodules considered benign were hypovascular during the arterial phase and isovascular during the portal venous phase, were persistently isovascular, or also had peripheral nodular vascularity with centripetal fill-in. Nodules considered indeterminate measured 2 cm or smaller, appeared hypervascular during the arterial phase and isovascular or hypervascular during the portal venous phase, or were persistently hypovascular with or without peripheral rimlike vascularity. Using these criteria, both reviewers expressed diagnostic confidence on a five-grade scale: 1, definitely benign; 2, probably benign; 3, indeterminate; 4, probably malignant; 5, definitely malignant.
TABLE 2.
Results of Consensus Analysis of Nodule Vascularity
| Arterial Phase |
Portal Venous Phase |
||||
|---|---|---|---|---|---|
| Histotype |
Hypervascular |
Isovascular |
Hypovascular |
Isovascular or Hypervascular |
Hypovascular |
| Hepatocellular carcinoma | 75 | 3 | 6 | 22 | 62a |
| Other malignant histotype | 6 | 1 | 5 | 0 | 12b |
| Dysplastic nodule | 5c | 1 | 7 | 9 | 4 |
| Regenerative nodule | 9d | 41 | 7 | 48 | 9 |
| Hemangioma | 35e | 0 | 7f | 38 | 4g |
| Focal nodular hyperplasia | 2 | 0 | 0 | 1 | 1h |
| Hepatocellular adenoma | 1 | 0 | 0 | 0 | 1 |
| Pseudotumor |
5i |
17 |
3 |
25 |
0 |
| Total | 138 | 63 | 35 | 143 | 93 |
Rimlike vascularity was present in 19 hepatocellular carcinomas.
Rimlike vascularity was present in two metastatic lesions.
All were hypervascular high-grade dysplastic nodules larger than 2 cm (n = 4) or 2 cm or smaller (n = 1) appearing isovascular (n = 3) or hypovascular (n = 2) in portal venous phase.
Hypervascular regenerative nodules were larger than 2 cm and appeared isovascular (n = 5) or hypovascular (n = 4) in portal venous phase.
Hypervascular hemangiomas were larger than 2 cm (n = 2) or 2 cm or smaller (n = 33) and appeared isovascular or hypervascular in portal venous phase.
Peripheral nodular vascularity was present in three hemangiomas with complete (n = 2) or incomplete (n = 1) fill-in in portal venous phase; persistent hypovascularity was present in four hemangiomas.
Hemangiomas had thrombotic–fibrotic pattern at histologic examination.
Focal nodular hyperplasia with telangiectatic pattern was present at histologic examination.
Intrahepatic arterioportal nontumorous shunts had solid echogenic appearance at unenhanced sonography simulating a solid hepatocellular nodule.
TABLE 3.
Results of Multivariate U - Statistical Analysis and Odds Ratio
| Combination |
Arterial Phase |
Portal Venous Phase |
Diameter (cm) |
Malignant/Benign (n) |
Odds Ratio |
95% CI |
|---|---|---|---|---|---|---|
| 1 | Hypervascular | Hypovascular | 63/9 | 27.78 | 12.53–61.59a | |
| > 2 | 49/2 | 71.93 | 16.83–307.33a | |||
| ≤ 2 | 14/7 | 3.24 | 1.25–8.37a | |||
| 2 | Hypervascular | Isovascular or hypervascular | 18/48 | 0.44 | 0.23–0.82 | |
| > 2 | 16/8 | 3.3 | 1.35–8.05a | |||
| ≤ 2 | 2/40 | 0.05 | 0.01–0.22 | |||
| 3 | Isovascular | Hypovascular | — | 4/3 | 1.98 | 0.43–9.07 |
| > 2 | 4/1 | 6.04 | 0.6–54.93a | |||
| ≤ 2 | 0/2 | 0 | ||||
| 4 | Hypovascular | Hypovascular | — | 7/7 | 1.49 | 0.50–4.40 |
| > 2 | 3/3 | 1.47 | 0.29–7.45 | |||
| ≤ 2 | 4/3 | 0.97 | 0.26–3.53 | |||
| 5 | Hypovascular | Isovascular | — | 4/17 | 0.31 | 0.48–4.59 |
| > 2 | 1/1 | 1.46 | 0.09–23.68 | |||
| ≤ 2 | 3/16 | 0.25 | 0.07–0.88 | |||
| 6 | Isovascular | Isovascular | — | 0/53 | 0 | |
| 7 | Peripheral nodular vascularity and fill-in | 0/3 | 0 | |||
Note—Multivariate U statistical analysis was performed on data obtained from consensus analysis of nodule vascularity. The odds ratio expresses the relation with diagnosis of malignancy and is calculated without (—) or with inclusion of the nodule dimension cutoff of 2 cm.
Variable combination selected as criterion for malignancy for independent analysis: significant improvement of malignant classification at multivariate U statistical analysis.
Statistical Analysis
A biostatistician participated in the statistical analysis performed with a computer software package (Analyze-It version 1.63, Analyze-It Software). Multivariate U scores [23] were used to define the diagnostic criteria for malignancy. In this type of multivariate analysis, the degree of indicated malignancy can be assessed without an assumption regarding the relative importance and correlation of the variables of interest. Briefly, the possible values of each variable are scored: nodule diameter (value in centimeters); nodule vascularity in the arterial phase (–1, fill-in; 0, hypovascular; 1, isovascular; 2, hypervascular), and nodule vascularity in the extended portal venous phase (0, hypovascular; 1, isovascular; 2, hypervascular). The profile of each nodule consists of a combination of the variable scores (e.g., the score for nodule diameter, hypervascularity in arterial phase, and hypovascularity in portal phase is 3/2/0), and the U score is computed as the number of profiles unambiguously lower (lower in at least one variable and higher in none) minus the number of profiles unambiguously higher (higher in at least one variable and lower in none). The resulting U scores were analyzed with a Mann-Whitney–type test. Multivariate orderings improving malignant classification of nodules were selected as significant at p <0.05.
Variable profiles that significantly improved malignancy classification (Table 3) were selected as diagnostic criteria for malignancy in the independent analysis. A maximum diameter of 2 cm was selected as the cutoff for malignancy according to receiver operating characteristic (ROC) measurement (area under ROC curve, 0.860; 95% CI, 0.780–0.942).
Retrospective benign or malignant diagnosis was considered true-positive (lesion correctly assessed as malignant, confidence level 4 or 5), false-negative (lesion incorrectly assessed as benign, confidence level 1 or 2, or assessed as indeterminate, confidence level 3), true-negative (lesion correctly assessed as benign, confidence level 1 or 2), or false-positive (lesion incorrectly assessed as malignant, confidence level 4 or 5, or assessed as indeterminate, confidence level 3). The weighted kappa statistic was calculated to assess interobserver agreement in diagnostic confidence both for unenhanced sonography and for contrast-enhanced sonography. The McNemar test was used to compare the sensitivity and specificity of unenhanced sonography with those of contrast-enhanced sonography. Improvement in diagnostic confidence was assessed with ROC curve analysis in a plot of sensitivity (true-positive fraction) against 1 – specificity (false-positive fraction). The area under each ROC curve was calculated with a nonparametric method [24]. The method proposed by Hanley and McNeil [25] was used to compare areas under ROC curves. A value of p < 0.05 was considered to indicate a statistically significant difference.
Results
Consensus Analysis of Nodule Vascularity
Table 1 shows the histologic diagnoses and the diameter distribution for each histologic category. Table 2 shows the nodule vascularity patterns in the arterial and portal venous phases. The grade of relation between nodule vascularity combined with diameter and malignancy diagnosis and the number of nodules in each category are shown in Table 3.
Malignant lesions appeared prevalently (81/96 nodules) hypervascular during the arterial phase and hypovascular (74/96 nodules) during the portal venous phase (Figs. 1–3). Well-differentiated HCCs appeared prevalently (14/24 nodules) isovascular in the portal venous phase except for 10 nodules that appeared hypovascular. Moderately or poorly differentiated HCCs appeared prevalently (52/60 nodules) hypovascular in the portal venous phase except for eight nodules that appeared isovascular. Benign lesions exhibited variable vascularity (Figs. 4–6) and were prevalently (121/140 nodules) isovascular or hypervascular during the portal venous phase.
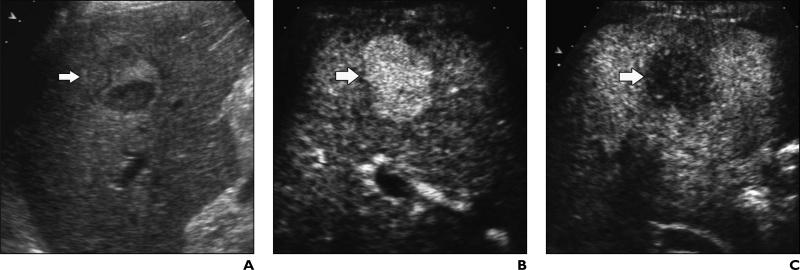
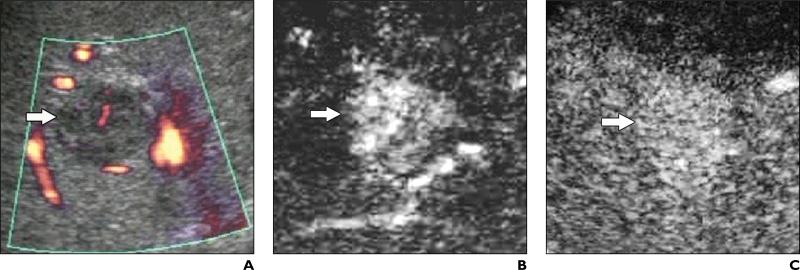
Fig. 1.
53-year-old man with liver cirrhosis related to hepatitis C virus infection and with poorly differentiated hepatocellular carcinoma.
A, Unenhanced longitudinal sonogram shows heterogeneous nodule (arrow) with diameter of 2.5 cm.
B and C, Contrast-enhanced Cadence Contrast Pulse Sequencing (CPS; Acuson Sequoia, Siemens Medical Solutions) longitudinal sonograms show nodule (arrow) is homogeneously hypervascular 25 seconds after microbubble injection during arterial phase (B) and hypovascular in comparison with adjacent liver parenchyma during portal venous phase (C).
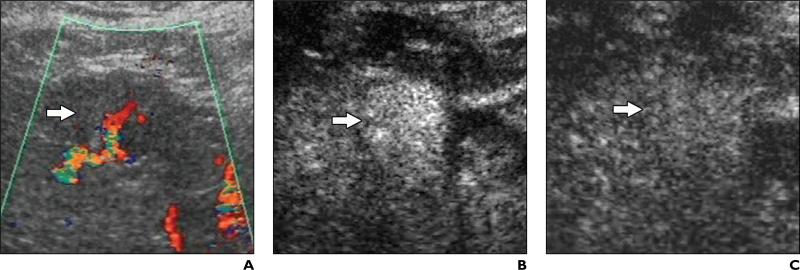
Fig. 3.
42-year-old man with liver cirrhosis related to hepatitis B virus infection and with poorly differentiated hepatocellular carcinoma.
A, Unenhanced longitudinal sonogram shows nodule (arrow) has diameter of 3 cm and appears hyperechoic.
B and C, Contrast-enhanced Coherent Contrast Imaging (CCI; Acuson Sequoia, Siemens Medical Solutions) longitudinal sonograms show nodule (arrow) appears persistently hypovascular during arterial phase (B) 35 seconds after microbubble injection and during portal venous phase (C) 130 seconds after microbubble injection, also with evidence of peripheral rimlike enhancement.
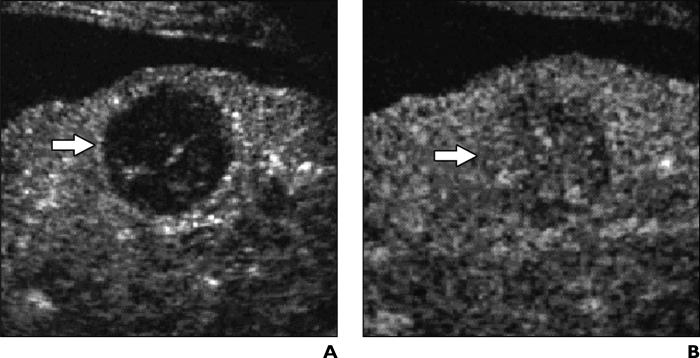
Fig. 4.
75-year-old man with liver cirrhosis related to hepatitis B virus infection and with high-grade dysplastic nodule.
A, Unenhanced oblique color Doppler sonogram shows nodule (arrow) has diameter of 2.3 cm and contains intranodular vessel.
B and C, Contrast-enhanced Coherent Contrast Imaging (CCI; Acuson Sequoia, Siemens Medical Solutions) oblique sonograms show nodule (arrow) has homogeneous hypervascular appearance during arterial phase (B) and appears isovascular during portal venous phase (C) 130 seconds after microbubble injection.
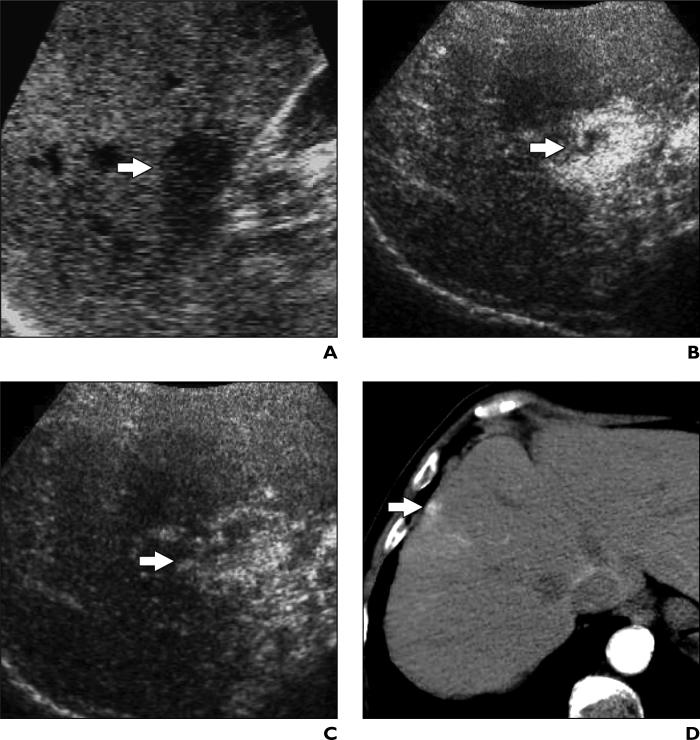
Fig. 6.
41-year-old woman with liver cirrhosis and intrahepatic arterioportal shunt.
A, Unenhanced oblique sonogram shows one nodule (arrow) with diameter of 1.5 cm.
B and C, Contrast-enhanced Coherent Contrast Imaging (CCI; Acuson Sequoia, Siemens Medical Solutions) oblique sonograms after microbubble show injection nodule (arrow) is encompassed by diffuse hypervascular appearance persisting during arterial (B) and portal venous (C) phases.
D, Contrast-enhanced CT scan of nodule (arrow) shows clear hypervascularity in arterial phase. Because it appeared solid on unenhanced sonography, nodule was biopsied and later surgically resected.
Independent Interpretation of Cine Clips
Nodules misclassified by both reviewers included malignant (n = 2; both HCC) and benign (n = 40; hemangioma, high-grade dysplastic nodule, intrahepatic arterioportal shunt) hypervascular nodules 2 cm in diameter or smaller that appeared isovascular or hypervascular in the portal venous phase. These nodules were considered indeterminate according to the diagnostic criteria used in the study. Moreover, the isovascular or hypervascular benign nodules that appeared hypovascular in the portal venous phase (n = 12; regenerative and dysplastic nodules, hepatocellular adenoma, atypical telangiectatic focal nodular hyperplasia) were misclassified as malignant, whereas the HCCs (n = 3) and thrombotic–fibrotic hemangiomas (n = 4) with persistent hypovascularity during both the arterial and portal venous phases were classified as indeterminate.
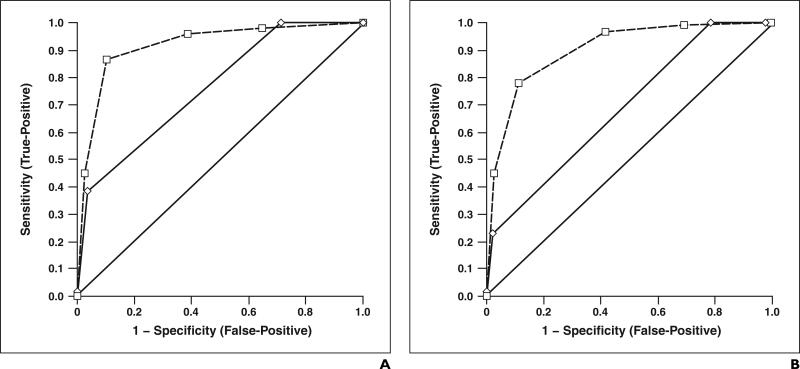
Additional review of contrast-enhanced sonograms versus unenhanced sonograms significantly improved (p < 0.05) both diagnostic confidence (Table 4) and interobserver agreement (weighted κ = 0.66 vs 0.92). After additional review of contrast-enhanced sonograms, reviewer 1 (Fig. 7A) changed the diagnostic confidence score for 170 of 236 nodules. In the cases of 108 (50 malignant, 58 benign) of the 170 nodules, contrast-enhanced sonography aided reviewer 1 in making a correct diagnosis. In the cases of 35 of the nodules (20 malignant, 15 benign), reviewer 1 became more confident in the correct characterization, shifting the diagnostic score from 4 to 5 for malignant lesions and from 2 to 1 for benign lesions. In the cases of 27 of 170 nodules, an incorrect diagnosis was made (seven malignant, 20 benign). After unenhanced sonography, reviewer 1 assessed 154 lesions (59 malignant, 95 benign) as indeterminate. This number was reduced to 49 (nine malignant, 40 benign) after additional review of contrast-enhanced sonograms.
TABLE 4.
Independent Interpretation of Cine Clips: Diagnostic Performance and Confidence
| Reviewer 1 |
Reviewer 2 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Unenhanced Sonography |
Contrast-Enhanced Sonography |
Unenhanced Sonography |
Contrast-Enhanced Sonography |
|||||
| Performance Characteristic |
% |
n |
% |
n |
% |
n |
% |
n |
| Sensitivity | 38 | 37/96 | 86 | 83/96 | 23 | 22/96 | 78 | 75/96 |
| Specificity | 28 | 40/140 | 61 | 86/140 | 21 | 30/140 | 58 | 82/140 |
| Positive predictive value | 27 | 37/137 | 60 | 83/137 | 16 | 22/133 | 56 | 75/133 |
| Negative predictive value | 40 | 40/99 | 86 | 86/99 | 29 | 30/103 | 79 | 82/103 |
| Accuracy |
32 |
77/236 |
71 |
169/236 |
22 |
52/236 |
66 |
157/236 |
| Confidencea | 0.763 | 0.703–0.823 | 0.916 | 0.878–0.955 | 0.687 | 0.619–0.754 | 0.903 | 0.864–0.942 |
Note—Values are results of off-site retrospective analysis after review of unenhanced sonograms and after additional review of contrast-enhanced sonograms. All differences are statistically significant (p < 0.05).
Diagnostic confidence is expressed as area under the receiver operating characteristic curve; numbers are real value and 95% CI.
Fig. 7.
Results of receiver operating characteristic analysis show diagnostic confidence in diagnosis of malignancy for unenhanced (solid curve) and contrast-enhanced (dashed curve) sonography. Diagonal line from 0 to 1 represents hypothetical technique with which malignant nodules cannot be differentiated from benign nodules.
A, Graph shows results for reviewer 1.
B, Graph shows results for reviewer 2.
After additional review of contrast-enhanced sonograms, reviewer 2 (Fig. 7B) changed the diagnostic confidence score for 163 of 236 nodules. In the cases of 114 (52 malignant, 62 benign) of the 163 nodules, contrast-enhanced sonography aided reviewer 2 in making a correct diagnosis. In the cases of 29 nodules (19 malignant, 10 benign) reviewer 2 became more confident in the correct characterization, shifting the diagnostic score from 4 to 5 for malignant lesions and from 2 to 1 for benign lesions. In the cases of 20 of 163 nodules, an incorrect diagnosis was made (two malignant, 18 benign). After unenhanced sonography, reviewer 2 assessed 181 lesions (74 malignant, 107 benign) as indeterminate. This number was reduced to 60 (18 malignant, 42 benign) after additional review of contrast-enhanced sonograms.
Discussion
The diagnostic capabilities of contrast-enhanced sonography in characterization of liver tumors have been analyzed extensively in previous studies [12–20]. Those studies included patients with and those without cirrhosis and were general evaluations of the diagnostic accuracy of contrast-enhanced sonography. The presence of a background of liver cirrhosis, however, can substantially change the sonographic appearance of hepatocellular nodules and makes the differential diagnosis of malignant and benign lesions much more difficult than in normal liver. Because, to our knowledge, no previous study has been conducted to analyze the diagnostic capabilities and clinical utility of contrast-enhanced sonography in characterization of hepatocellular nodules in a large series of patients with cirrhosis, we performed this retrospective study.
Owing to the prevalent arterial blood supply of HCC, evidence of nodule hypervascularity during the arterial phase and of hypovascularity during the portal venous phase with or without peripheral rimlike vascularity was closely related to the presence of malignancy. This vascular pattern was known from studies conducted with contrast-enhanced sonography [11–20], CT [26–29], and MRI [30–32]. Evidence of peripheral rimlike vascularity was previously related to the HCC pseudocapsule [11–15], although it could simply have been caused by peripheral vessels. In our study, other vascular patterns were found to be related to the presence of malignancy, provided nodule diameter was considered. In particular, evidence of isovascularity in the arterial phase with hypovascularity in the portal venous phase or of hypervascularity in the arterial phase with isovascularity or hypervascularity in the portal venous phase was related to the presence of malignant growth in nodules larger than 2 cm.
We found that evidence of hypervascularity in the arterial phase followed by isovascularity in the portal venous phase in hepatocellular nodules 2 cm in diameter or smaller was equivocal because it was seen both in well-differentiated HCCs and in hypervascular benign nodules. This finding was due to the particularly high percentage of hypervascular small hemangiomas in patients with liver cirrhosis [1–3] and the frequent presence of hypervascular high-grade dysplastic nodules and intrahepatic arterioportal shunts. The persistent microbubble uptake in malignant nodules, which mostly corresponded to an isovascular or hypervascular appearance of well-differentiated HCC in the portal venous phase, was probably due to the similarity of microbubble pooling in well-differentiated HCC and adjacent liver parenchyma, as previously reported [12, 13]. A small number of HCCs had a persistent hypovascular appearance, as previously described [11], resembling fibrotic or thrombosed hemangiomas.
Early results of CT and MRI studies suggested that almost all lesions becoming enhanced in the arterial phase in patients with cirrhosis were HCC [33]. The results of our study performed with contrast-enhanced sonography, as of other studies performed with CT or MRI [34–38], are different from those early results. We found that a large portion of false-positive diagnoses of HCC are due to the presence of enhancing benign lesions. As many as 25% of lesions 2 cm or smaller with arterial enhancement but without venous washout in cirrhotic liver remain stable or regress over time and thus are not HCC [3]. Consequently, differentiation between benign and malignant nodules 2 cm or smaller that are hypervascular in the arterial phase and isovascular or hypervascular in the portal venous phase cannot be achieved with either contrast-enhanced sonography or cross-sectional imaging techniques, including CT and MRI. Thus contrast-enhanced sonography has the same limitations as contrast-enhanced CT and MRI in the noninvasive characterization of hepatocellular nodules 2 cm or smaller, which are often misclassified [2]. Differentiation often is not possible even at histologic examination because pathologists disagree about the dividing line between high-grade dysplastic nodules and well-differentiated HCC, stromal invasion being the most helpful sign for the differential diagnosis [3, 35].
Most of the regenerative and dysplastic nodules appeared isovascular or hypovascular in the arterial phase and isovascular in the portal venous phase owing to the prevalent portal blood supply and were correctly interpreted as benign. However, benign nodules appearing isovascular or hypervascular in the arterial phase and hypovascular in the portal venous phase were misclassified as malignant. Peripheral nodular vascularity followed by progressive fill-in was found to be strongly related to a benign diagnosis because it was typically found in hemangiomas, as described in previous studies [11–20]. Pseudotumors appearing isovascular or hypovascular during the arterial phase did not lead to nodule mis-diagnosis because they had sustained contrast enhancement in the portal phase.
The diagnostic criteria developed from the present series improved the accuracy of sonography in the diagnosis of malignancy. A limited number of misclassified nodules, mostly 2 cm in diameter or smaller, were found at independent interpretation of cine clips. The number was much lower than could be expected from the results of the consensus analysis, in which there was an evident overlap in vascularity between malignant and benign nodules. The difference between the independent and consensual analysis was due to combined analysis of unenhanced sonograms and contrast-enhanced sonograms, which provided both morphologic and vascular criteria for the characterization of hepatocellular nodules. The diagnostic performance and confidence of independent analysis revealed high interobserver agreement, even though the reviewers had different levels of experience. This result is explained by the characteristic vascular patterns observed in most nodules. Inevitable interobserver variability, however, accounts for the differences in diagnostic performance and confidence, which were consistently higher for contrast-enhanced sonography than for unenhanced sonography.
The sensitivity, negative predictive value, and diagnostic confidence for contrast-enhanced sonography in our study were similar to the values previously reported for CT [34, 37, 38] and MRI [32, 37, 39]. We found limited specificity, positive predictive value, and accuracy for contrast-enhanced sonography in comparison with results of previous studies of contrast-enhanced sonography involving patients with normal livers [11–20] and those of previous studies of CT and MRI involving patients with liver cirrhosis [32, 34–39]. This difference was probably due to the frequent presence of coincidental small hypervascular benign nodules, including hemangiomas and dysplastic nodules, in our series and in general in cirrhotic liver that appeared isovascular in the portal venous phase, as do most well-differentiated HCCs. Contrast-enhanced sonography may potentially further increase the percentage of instances in which benign nodules appear hypervascular in the arterial phase owing to the high sensitivity of contrast-specific sonographic techniques to the harmonic signals produced by microbubble insonation.
According to the results of this study, contrast-enhanced sonography should be considered a preliminary examination after unenhanced sonography to exclude malignancy. It is a reliable alternative to CT and MRI for characterizing hepatocellular nodules detected during sonographic surveillance. The diagnostic criteria developed for contrast-enhanced sonography are similar to the corresponding criteria proposed by the Barcelona committee for CT and MRI [2, 3]. These diagnostic criteria allow characterization of most malignant hepatocellular nodules provided that a typical pattern is identified (hypervascularity in the arterial phase and hypovascularity in the portal venous phase; for nodules larger than 2 cm, hypervascularity or isovascularity in the arterial phase and isovascularity in the portal venous phase). If an equivocal pattern is identified (e.g., hypervascularity not followed by hypovascularity of nodules 2 cm or smaller, persistent hypovascularity, or isovascularity or hypervascularity followed by hypovascularity in the portal venous phase of images of benign nodules larger than 2 cm), hepatocellular nodules are often misclassified, and either follow-up imaging for rapid progression or biopsy for confirmation should be performed.
The principal limitations of this study were its retrospective nature and the strict inclusion criteria, which led to exclusion of a large number of nodules. In particular, because a reliable reference standard was necessary for calculation of diagnostic performance and confidence, approximately one third of hepatocellular nodules were excluded because of lack of a histologic diagnosis.
In conclusion, assessment of the vascularity of hepatocellular nodules after microbubble injection allowed nodule characterization in patients with liver cirrhosis. Characterization, however, was limited in hypervascular nodules 2 cm or smaller appearing isovascular or hypervascular in the portal venous phase of imaging.
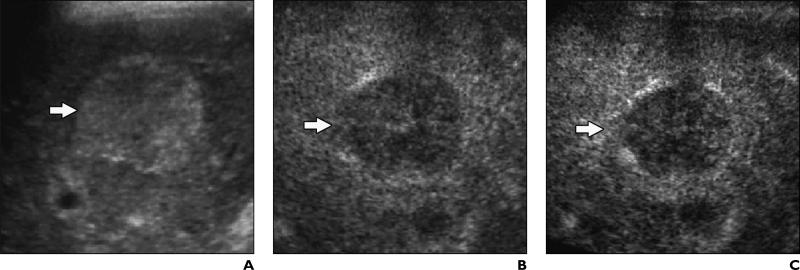
Fig. 2.
46-year-old woman with liver cirrhosis related to hepatitis C virus infection and with well-differentiated hepatocellular carcinoma.
A, Unenhanced transverse color Doppler sonogram shows nodule (arrow) has diameter of 3 cm and contains intranodular vessels.
B and C, Contrast-enhanced Coherent Contrast Imaging (CCI; Acuson Sequoia, Siemens Medical Solutions) transverse sonograms show nodule (arrow) appears homogeneously hypervascular 35 seconds after microbubble injection during arterial phase (B) and isovascular to adjacent liver during portal venous phase (C) 160 seconds after microbubble injection because of persistent microbubble uptake in nodule.
Fig. 5.
75-year-old man with liver cirrhosis related to hepatitis B virus infection and with regenerative nodule.
A and B, Contrast-enhanced Coherent Contrast Imaging (CCI; Acuson Sequoia, Siemens Medical Solutions) longitudinal sonograms show nodule (arrow) is 2.5 cm in diameter and appears hypovascular during arterial phase (A) 25 seconds after microbubble injection. Lesion became isovascular in portal venous phase (B) 140 seconds after microbubble injection and is isovascular in portal venous phase 150 seconds after injection.
Acknowledgments
Supported in part by grant number UL1RR024143 from the National Institutes of Health.
References
- 1.International Working Party Terminology of nodular hepatocellular lesions. Hepatology. 1995;22:983–993. doi: 10.1016/0270-9139(95)90324-0. [DOI] [PubMed] [Google Scholar]
- 2.Bruix J, Sherman M, Llovet JM, et al. EASL Panel of Experts on HCC. Clinical management of hepatocellular carcinoma: conclusions of the Barcelona-2000 EASL Conference—European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 3.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 4.Nino-Murcia M, Ralls PW, Jeffrey RB, Jr, Johnson M. Color flow Doppler characterization of focal hepatic lesions. AJR. 1992;159:1195–1197. doi: 10.2214/ajr.159.6.1332456. [DOI] [PubMed] [Google Scholar]
- 5.Numata K, Tanaka K, Mitsui K, Morimoto M, Inoue S, Yonezawa H. Flow characteristics of hepatic tumors at color Doppler sonography: correlation with arteriographic findings. AJR. 1993;160:515–521. doi: 10.2214/ajr.160.3.8381573. [DOI] [PubMed] [Google Scholar]
- 6.Lee MG, Auh YH, Cho KS, Chung YH, Lee IC, Kang EM. Color Doppler flow imaging of hepatocellular carcinomas: comparison with metastatic tumors and hemangiomas by three-step for grading color hues. Clin Imaging. 1996;20:199–203. doi: 10.1016/0899-7071(95)00017-8. [DOI] [PubMed] [Google Scholar]
- 7.Albrecht T, Blomley M, Bolondi L, et al. EFSUMB Study Group Guidelines for the use of contrast agents in ultrasound. Ultraschall Med. 2004 January;25:249–256. doi: 10.1055/s-2004-813245. [DOI] [PubMed] [Google Scholar]
- 8.Kim TK, Choi BI, Han JK, Hong HS, Park SH, Moon SG. Hepatic tumors: contrast agent–enhancement patterns with pulse inversion harmonic US. Radiology. 2000;216:411–417. doi: 10.1148/radiology.216.2.r00jl21411. [DOI] [PubMed] [Google Scholar]
- 9.Dill-Macky M, Burns P, Khalili K, Wilson S. Focal hepatic masses: enhancement patterns with SH U 508 A and pulse inversion US. Radiology. 2002;222:95–102. doi: 10.1148/radiol.2221010092. [DOI] [PubMed] [Google Scholar]
- 10.Bryant T, Blomley MJ, Albrecht T, et al. Improved characterization of liver lesions with liver-phase uptake of liver specific microbubbles: prospective multicenter trials. Radiology. 2004;232:799–809. doi: 10.1148/radiol.2323030596. [DOI] [PubMed] [Google Scholar]
- 11.Bolondi L, Gaiani S, Celli N, et al. Characterization of small nodules in cirrhosis by assessment of vascularity: the problem of hypovascular hepatocellular carcinoma. Hepatology. 2005;42:27–34. doi: 10.1002/hep.20728. [DOI] [PubMed] [Google Scholar]
- 12.Quaia E, Calliada F, Bertolotto M, et al. Characterization of focal liver lesions by contrast-specific US modes and a sulfur hexafluoride–filled microbubble contrast agent: diagnostic performance and confidence. Radiology. 2004;232:420–430. doi: 10.1148/radiol.2322031401. [DOI] [PubMed] [Google Scholar]
- 13.Nicolau C, Catalá V, Vilana R, et al. Evaluation of hepatocellular carcinoma using SonoVue, a second generation ultrasound contrast agent: correlation with cellular differentiation. Eur Radiol. 2004;14:1092–1099. doi: 10.1007/s00330-004-2298-0. [DOI] [PubMed] [Google Scholar]
- 14.Gaiani S, Celli N, Piscaglia F, et al. Usefulness of contrast-enhanced perfusional sonography in the assessment of hepatocellular carcinoma hypervascular at spiral computed tomography. J Hepatol. 2004;41:421–426. doi: 10.1016/j.jhep.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 15.Giorgio A, Ferraioli G, Tarantino L, et al. Contrast-enhanced sonographic appearance of hepatocellular carcinoma in patients with cirrhosis: comparison with contrast-enhanced helical CT appearance. AJR. 2004;183:1319–1326. doi: 10.2214/ajr.183.5.1831319. [DOI] [PubMed] [Google Scholar]
- 16.D'Onofrio M, Rozzanigo U, Caffarri S, Zogno A, Procacci C. Contrast-enhanced US of hepatocellular carcinoma. Radiol Med (Torino) 2004;107:293–303. [PubMed] [Google Scholar]
- 17.Kim SH, Lee JM, Lee JY, et al. Value of contrast-enhanced sonography for the characterization of focal hepatic lesions in patients with diffuse liver disease: receiver operating characteristic analysis. AJR. 2005;184:1077–1084. doi: 10.2214/ajr.184.4.01841077. [DOI] [PubMed] [Google Scholar]
- 18.Nicolau C, Vilana R, Catala V, et al. Importance of evaluating all vascular phases on contrast-enhanced sonography in the differentiation of benign from malignant focal liver lesions. AJR. 2006;186:158–167. doi: 10.2214/AJR.04.1009. [DOI] [PubMed] [Google Scholar]
- 19.Fan ZH, Chen MH, Dai Y, et al. Evaluation of primary malignancies of the liver using contrast-enhanced sonography: correlation with pathology. AJR. 2006;186:1512–1519. doi: 10.2214/AJR.05.0943. [DOI] [PubMed] [Google Scholar]
- 20.Leen E, Ceccotti P, Kalogeropoulou C, et al. Prospective multicenter trial evaluating a novel method of characterizing focal liver lesions using contrast-enhanced sonography. AJR. 2006;186:1551–1559. doi: 10.2214/AJR.05.0138. [DOI] [PubMed] [Google Scholar]
- 21.Bismuth H. Surgical anatomy and anatomical surgery of the liver. World J Surg. 1982;6:3–8. doi: 10.1007/BF01656368. [DOI] [PubMed] [Google Scholar]
- 22.Couinaud C. Le foie: études anatomiques et chirurgicales. Masson; Paris, France: 1957. pp. 9–12. [Google Scholar]
- 23.Wittkowski KM, Lee E, Nussbaum R, Chamian FN, Krueger JG. Combining several ordinal measures in clinical studies. Stat Med. 2004;23:1579–1592. doi: 10.1002/sim.1778. [DOI] [PubMed] [Google Scholar]
- 24.Beck JR, Shultz EK. The use of relative operating characteristic (ROC) curves in test performance evaluation. Arch Pathol Lab Med. 1986;110:13–20. [PubMed] [Google Scholar]
- 25.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–843. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 26.Matsui O, Kadoya M, Kameyama T, et al. Benign and malignant nodules in cirrhotic livers: distinction based on blood supply. Radiology. 1991;178:493–497. doi: 10.1148/radiology.178.2.1846240. [DOI] [PubMed] [Google Scholar]
- 27.Lim JH, Kim EY, Lee WJ, et al. Regenerative nodules in liver cirrhosis: findings at CT during arterial portography and CT hepatic arteriography with histopathologic correlation. Radiology. 1999;210:451–458. doi: 10.1148/radiology.210.2.r99fe04451. [DOI] [PubMed] [Google Scholar]
- 28.Lim JH, Cho JM, Kim EY, Park CK. Dysplastic nodules in liver cirrhosis: evaluation of hemodynamics with CT during arterial portography and CT hepatic arteriography. Radiology. 2000;214:869–874. doi: 10.1148/radiology.214.3.r00mr12869. [DOI] [PubMed] [Google Scholar]
- 29.Hayashi M, Matsui O, Ueda K, Kawamori Y, Gabata T, Kadoya M. Progression to hypervascular hepatocellular carcinoma: correlation with intranodular blood supply evaluated with CT during intraarterial injection of contrast material. Radiology. 2002;225:143–149. doi: 10.1148/radiol.2251011298. [DOI] [PubMed] [Google Scholar]
- 30.Earls JP, Theise ND, Weinreb JC, et al. Dysplastic nodules and hepatocellular carcinoma: thin-section MR imaging of explanted cirrhotic livers with pathologic correlation. Radiology. 1996;201:207–214. doi: 10.1148/radiology.201.1.8816545. [DOI] [PubMed] [Google Scholar]
- 31.Hussain SM, Zondervan PE, IJzermans JN, Schalm SW, de Man RA, Krestin GP. Benign versus malignant hepatic nodules: MR imaging findings with pathologic correlation. RadioGraphics. 2002;22:1023–1036. doi: 10.1148/radiographics.22.5.g02se061023. [DOI] [PubMed] [Google Scholar]
- 32.Krinsky GA, Lee VS, Theise ND, et al. Hepatocellular carcinoma and dysplastic nodules in patients with cirrhosis: prospective diagnosis with MR imaging and explantation correlation. Radiology. 2001;219:445–454. doi: 10.1148/radiology.219.2.r01ma40445. [DOI] [PubMed] [Google Scholar]
- 33.Lee HM, Lu DS, Krasny RM, Busuttil R, Kadell B, Lucas J. Hepatic lesion characterization in cirrhosis: significance of arterial hypervascularity on dual-phase helical CT. AJR. 1997;169:125–130. doi: 10.2214/ajr.169.1.9207511. [DOI] [PubMed] [Google Scholar]
- 34.Baron RL, Peterson MS. Screening the cirrhotic liver for hepatocellular carcinoma with CT and MR imaging: opportunities and pitfalls. RadioGraphics. 2001;21:S117–S132. doi: 10.1148/radiographics.21.suppl_1.g01oc14s117. [DOI] [PubMed] [Google Scholar]
- 35.Kojiro M. Focus on dysplastic nodules and early hepatocellular carcinoma: an Eastern point of view. Liver Transpl. 2004;10(2 suppl 1):S3–S8. doi: 10.1002/lt.20042. [DOI] [PubMed] [Google Scholar]
- 36.Lim JH, Kim CK, Lee WJ, et al. Detection of hepatocellular carcinomas and dysplastic nodules in cirrhotic livers: accuracy of helical CT in transplant patients. AJR. 2000;175:693–698. doi: 10.2214/ajr.175.3.1750693. [DOI] [PubMed] [Google Scholar]
- 37.Rode A, Bancel B, Douek P, et al. Small nodule detection in cirrhotic livers: evaluation with US, spiral CT, and MRI and correlation with pathologic examination of explanted liver. J Comput Assist Tomogr. 2001;25:327–336. doi: 10.1097/00004728-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Valls C, Cos M, Figueras J, et al. Pretransplantation diagnosis and staging of hepatocellular carcinoma in patients with cirrhosis: value of dual-phase helical CT. AJR. 2004;182:1011–1017. doi: 10.2214/ajr.182.4.1821011. [DOI] [PubMed] [Google Scholar]
- 39.Bhartia B, Ward J, Guthrie JA, Robinson PJ. Hepatocellular carcinoma in cirrhotic livers: double-contrast thin-section MR imaging with pathologic correlation of explanted tissue. AJR. 2003;180:577–584. doi: 10.2214/ajr.180.3.1800577. [DOI] [PubMed] [Google Scholar]