Abstract
Interfacing electronics and recording electrophysiological activity in mechanically active biological tissues is challenging. This challenge extends to recording neural function of brain tissue in the setting of traumatic brain injury (TBI), which is caused by rapid (within hundreds of milliseconds) and large (greater than 5% strain) brain deformation. Interfacing electrodes must be biocompatible on multiple levels and should deform with the tissue to prevent additional mechanical damage. We describe an elastically stretchable microelectrode array (SMEA) that is capable of undergoing large, biaxial, 2-D stretch while remaining functional. The new SMEA consists of elastically stretchable thin metal films on a silicone membrane. It can stimulate and detect electrical activity from cultured brain tissue (hippocampal slices), before, during, and after large biaxial deformation. We have incorporated the SMEA into a well-characterized in vitro TBI research platform, which reproduces the biomechanics of TBI by stretching the SMEA and the adherent brain slice culture. Mechanical injury parameters, such as strain and strain rate, can be precisely controlled to generate specific levels of damage. The SMEA allowed for quantification of neuronal function both before and after injury, without breaking culture sterility or repositioning the electrodes for the injury event, thus enabling serial and long-term measurements. We report tests of the SMEA and an initial application to study the effect of mechanical stimuli on neuron function, which could be employed as a high-content, drug-screening platform for TBI.
Key words: electrophysiology, in vitro studies, outcome measures, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a major public health problem, which is mainly due to motor vehicle accidents, falls, and firearms. Approximately 2% of the U.S. population lives with disabilities caused by TBI, and nearly 15% of returning infantry soldiers from Iraq exhibit symptoms of brain injury (Hoge et al., 2008). TBI, caused by an acceleration or a blow to the head, results in deformation of the brain tissue, and a significant literature on TBI biomechanics concludes that stretch is the primary mechanism of cell and tissue damage (Zhang et al., 2001). The devastating consequences of TBI are ultimately the result of the disruption of normal brain function, including loss of consciousness, coma, epilepsy, motor deficits, and cognitive impairment (Frey, 2003; Schretlen and Shapiro, 2003). At a cellular level, the correlate of these macroscopic changes in behavior and function is the alteration of neuronal electrophysiology.
Microelectrode arrays (MEAs) have been used to evaluate brain function and principles of neural signal processing, both in vitro and in vivo (Beggs and Plenz, 2003; Buzsaki, 2004; Hochberg et al., 2006), increasing our understanding of disease-related dysfunction of brain (Sanchez et al., 2006). Unlike single electrode recordings, the long-range connectivity of neural networks can be studied with these arrays, due to their capability of simultaneous multisite recording, providing additional insight into neuronal information processing and function (Kralik et al., 2001; Diogo et al., 2003). For example, MEAs can record the spatial-temporal pattern of field potentials (Christensen et al., 2000), from which the current source density (CSD) can be calculated (Cheung et al., 2007) to explore the flow of electrical current through the hippocampal circuitry. CSD has been previously applied to interpret changes in synaptic transmission, such as long-term potentiation (LTP) (Taube and Schwartzkroin, 1988), which could be applied to better understand post-traumatic alterations in learning from a spatial-temporal viewpoint (Schwarzbach et al., 2006). With the CSD analysis, it would be possible to identify localized dysfunction after TBI to specific subfields of the hippocampus and even within dendritic, axonal, or cell body layers, depending on the spatial-temporal pattern of current sources and sinks. In addition, higher order behaviors are likely to be dependent on ensemble network behavior rather than on the activity of individual neurons (Deadwyler and Hampson, 1995; Doetsch, 2000), and MEAs can be used to quantify this distributed processing with a level of detail not possible with single electrodes. Current MEAs consist of microfabricated wires, 3-D microstructures, or planar thin-film electrodes on stiff glass or silicon (Nisch et al., 1994; Thiebaud et al., 1999; Yu et al., 2007), or on flexible polyimide foil (Cheung et al., 2007). Neither traditional rigid MEAs nor flexible MEAs withstand deformation above ∼1% strain (Pashley, 1960; Chiu et al., 1994; Spaepen, 2000), which is smaller than the mechanical stimuli required for the study of TBI (Cater et al., 2006; Elkin and Morrison, 2007). Recording from the same sites pre- and post-injury is unlikely if the array must be removed during the injury event. Without pre-injury baseline data, normalization becomes problematic, especially in light of a recent report that existing normalization strategies were not valid (Santhakumar et al., 2003).
We have recently discovered that thin metal films on compliant elastomeric substrates remain electrically conducting even under large and repeated stretching and relaxation (Lacour et al., 2003; Li et al., 2004). Taking advantage of this elastic metallization, we developed an elastically stretchable MEA (SMEA), which is capable of undergoing large, biaxial 2-D stretch. Herein, we demonstrate the utility of this system by stimulating and recording evoked electrical activity in hippocampal slice cultures subjected to biaxial stretch mimicking the biomechanics of TBI. In the current study, the SMEAs were biaxially stretched up to 20% and remained functional, allowing for recording before and after these large stretches. Moreover, it was possible to record signals during less severe biaxial stretch (<8% stretch). Discovering how mechanical stimuli alter brain function is fundamental to understanding post-traumatic brain damage, identifying therapeutic targets, and developing therapies for TBI patients. Because the SMEAs are functional under these rigorous loading conditions, they will also perform under less severe loading conditions applicable to other fields of research.
Materials and Methods
Fabricating and packaging SMEAs
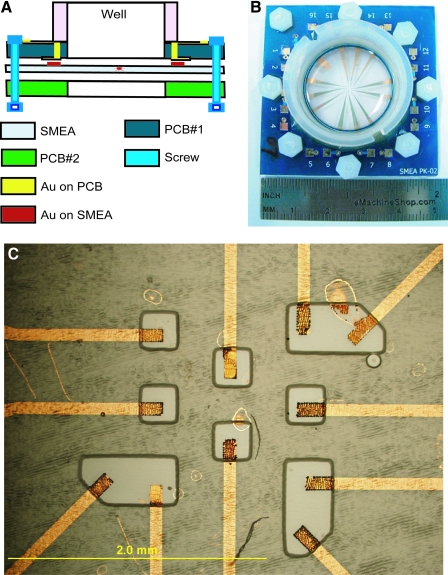
The elastically stretchable MEA was based on the large elastic stretchability of gold thin-film conductors fabricated on an elastomeric membrane (Lacour et al., 2003). Our SMEA was a patterned metal film (3 nm Cr/75 nm Au/3 nm Cr) sandwiched between a silicone membrane with Young's modulus (E) ∼ 1 MPa and a silicone layer with E ∼ 160 MPa. The membrane was a 280 μm thick layer of polydimethylsiloxane (PDMS, Sylgard 184, Dow Corning). The Cr/Au/Cr layers were sequentially deposited by electron beam evaporation and patterned by microfabrication techniques into twelve conductors. The conductors were encapsulated with a 15μm thick layer of photo-patternable silicone (WL-5150, Dow Corning) that functioned as an elastomeric, electrical insulator. Vias were opened in the insulator to expose 11 recording electrodes (100 μm × 200 μm; Fig. 1), a reference electrode, and 12 peripheral contacts. Platinum black was electroplated on the surfaces of the recording electrodes. Each SMEA was packaged between two printed circuit boards with circular openings in their centers (Fig. 1). The membrane exposed in the circle formed a culture well. The circuit boards interfaced with a commercial MEA amplifier and data acquisition system (MultiChannel Systems, Germany). The packaged SMEA was incorporated in our in vitro TBI research platform (Morrison et al., 2006). It was stretched biaxially by pushing the exposed membrane over a tubular indenter; imposed strain and strain rate were controlled by computer (Fig. 2). High-speed video recorded the imposed deformation.
FIG. 1.
Schematic and image of the SMEA package. (A) After fabrication, the SMEA was clamped between two printed circuit boards (PCB) to form an interface between it and the amplifier. A plastic well was cemented onto the top PCB to form a tissue culture chamber. (B) Image of a packaged SMEA. The 49 mm × 49 mm sandwich of PCB-SMEA-PCB was used as an interface with the MCS multichannel amplifier for recording and stimulation. (C) Image of the 11-electrode array in the center of the SMEA. The tips (100 μm × 200 μm) of the patterned conductors were exposed by photopatterned vias in the encapsulation layer to form 11 independent recording electrodes and were modified by platinum black to reduce electrode impedance. (Color image may be found on our website at www.liebertpub.com/jon)
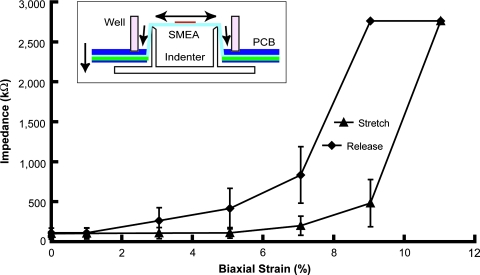
FIG. 2.
Average impedance at 1 kHz of SMEA electrodes during stretch (n = 10, ± standard error). The SMEA was biaxially stretched up to 11% strain and then released incrementally. The impedance of each electrode at each static stretch was measured in aCSF. During SMEA stretch, the impedance gradually increased up to approximately 2.8 MΩ and recovered to about 100 kΩ after release. (Inset) the packaged SMEA was mounted on our injury device, which precisely displaced the SMEA over a hollow tubular indenter, resulting in a biaxial strain in the SMEA that was verified optically. (Color image may be found on our website at www.liebertpub.com/jon)
Organotypic hippocampal slice cultures on Millicell-CM membranes
All animal procedures were approved by the Columbia University IACUC. The hippocampus of a post-natal (day 8–11) rat pup was removed aseptically and cut into sections 400 μm thick with a McIlwain tissue chopper (Brinkmann Instruments). Slices were transferred to Millicell-CM membranes (Millipore) and fed through the membrane with nutrient medium (50% minimum essential medium with Earle's salts, 25% heat inactivated horse serum, 25% Hank's balanced salt solution, 1 mM glutamine, and 4.5% glucose; Invitrogen). Cultures were maintained in an incubator (5% CO2, 37°C) before use. Prior to recording, individual cultures were cut from Millicell-CM membranes, inverted onto SMEAs, and held in place with a nylon mesh. Cultures were perfused with artificial cerebrospinal fluid (aCSF in mM: 125 NaCl, 3.5 KCl, 26 NaHCO3, 1.2 KH2PO4, 1.3 MgCl2, 2.4 CaCl2, 10 glucose; pH 7.40), which was bubbled with 95% O2/5% CO2 and pre-warmed to 37°C.
Organotypic hippocampal slice cultures on SMEAs
Before being used for culture, SMEAs were cleaned by air plasma treatment (Harrick PDC-32G, Harrick Scientific) for 30 s, coated overnight with a mixture of poly-L-lysine (320 μg/mL; Sigma) and laminin (80 μg/mL; Invitrogen), and then incubated with culture medium overnight. Hippocampal slices were cultured on SMEAs for 2 weeks, and their neural activity was recorded every 2–3 days.
Verification of induced strain
The induced strain within the SMEA electrodes and the cultured hippocampal slices was analyzed from images of the stretch event. Adhesion of the cultured tissue to the SMEA during stretch was verified with high-speed video (1280 × 1280 pixels at 50 frames/s; Motion Pro 2000, DEL Imaging, Cheshire, CT). Total area of the hippocampal tissue before stretch (A0) and during stretch (Amax, at the maximum strain) was measured with ImageJ software. Deformation was quantified by calculating Lagrangian strain:
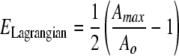 |
(1) |
Electrophysiological recording
The SMEAs were mounted in the multichannel amplifier (MEA1060-Inv-BC, MultiChannel Systems), and signals were recorded with a sampling rate of 20 kHz and a 5 kHz analog, anti-aliasing filter. An integrated heater maintained the temperature of the cultures at 37°C. Spontaneous firing rate was calculated from 10 s bins. A programmable stimulator (STG2004, MultiChannel Systems) was used to generate constant current, bi-phasic stimuli (a positive phase first for 100 μs followed by a negative phase for 100 μs) to evoke responses. A sync pulse was simultaneously generated by the stimulator to provide a stimulus trigger for the recording system. Stimulus magnitude is indicated in the discussion below.
Stimulus-response curves
From evoked responses, S/R curves were constructed by increasing the intensity of the stimulus in 10 μA increments from 0 μA to 200 μA and recording the response at the other electrodes. The response magnitude was plotted against the stimulus magnitude and fit to a sigmoidal function of the form:
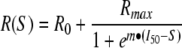 |
(2) |
where R was the evoked response, R0 was the background response, Rmax was the maximum amplitude of the response, I50 was the current that produced a half maximal response, S was the intensity of stimulus, and m was proportional to the slope of the linear region of the sigmoid.
Results
Measuring impedance of SMEAs during deformation
A 0.1 μA peak-to-peak 1 kHz sine wave was applied to the electrodes of SMEA with aCSF inside the well. The potential difference was recorded at different strain levels. The impedance of the medium plus the reference electrode (platinum wire) was negligible compared to the impedance of the microelectrodes. The impedance at 1 kHz of individual SMEA electrodes in aCSF was 50–150 kΩ before stretch, increased with stretch, and recovered during relaxation to its original level when completely relaxed (Fig. 2). At biaxial strains below 8%, the impedance (at 1 kHz) was between 0.1 MΩ and 1.0 MΩ, which was adequate for extracellular electrical recording. The increase in electrode impedance during deformation was reversible, and the metal traces remained conductive. The gradual increase of impedance during stretch indicated that the SMEA electrodes were electrically robust during large biaxial deformation.
Recording neural activity after multiple cycles of stretch
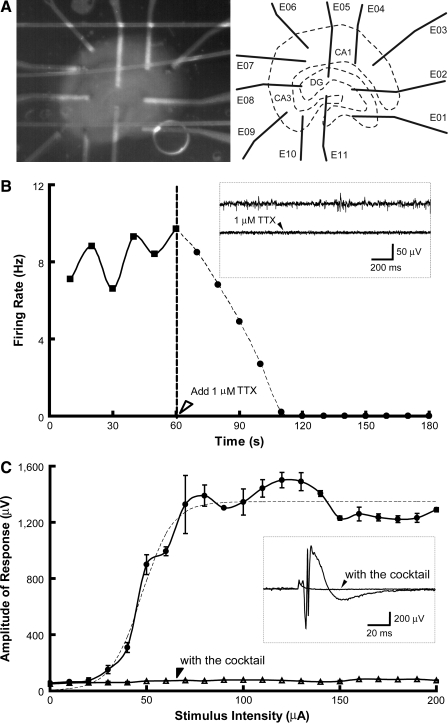
An SMEA was stretched and relaxed for 30 cycles (∼0.5 s for each cycle)––10 times to 10% strain, 10 times to 15% strain, and 10 times to 20% strain––before organotypic hippocampal slice cultures were placed on it, and neuronal activity was recorded. The CA1, CA3, and dentate gyrus (DG) regions of a hippocampal slice culture were positioned over electrodes of the SMEA (Fig. 3A) while being perfused with oxygenated aCSF. The low electrical noise of 1.86 μVrms (rms, root mean square) was comparable to commercial MEAs (MEA-PARRAY 200/30iR-Ti-gr; MultiChannel Systems). Constant current stimuli were applied through two SMEA electrodes beneath the DG region (Fig. 3A). Spontaneous activity and evoked field potentials were recorded (Fig. 3B and C). Suppression of spontaneous activity by 1 μM tetrodotoxin (TTX, voltage sensitive sodium channel antagonist; Sigma) confirmed its biological origin (one channel shown in Fig. 3B). Stimulus-response data from one electrode in the CA3 region are shown in Figure 3C, together with the stimulus-response (S/R) curve fitted to a sigmoidal function (Fig. 3C, dashed line). A cocktail of 1 μM TTX, 50 μM bicuculline (BIC, competitive antagonist of GABAA receptors; Sigma), 100 μM D-2-amino-5-phosphonovaleric acid (APV, NMDA receptor antagonist; Sigma), and 100 μM 6-cyano-7-nitroquinoxaline-2,3-dione disodium salt (CNQX, non-NMDA glutamate receptor antagonist; Tocris) blocked evoked activity, leaving only stimulus artifacts (Fig. 3C). These results show that the SMEA can be stretched biaxially many times and remain capable of neural recording and stimulation.
FIG. 3.
Spontaneous activity and evoked field potentials were recorded from a hippocampal slice using the SMEA. Before recording, an SMEA was subjected to 30 cycles of stretching to demonstrate its mechanical robustness. (A) A light micrograph of a hippocampal slice (5 DIV) on the SMEA (a nylon mesh holds the slice in place) and a schematic of the hippocampal anatomy. (B) Spontaneous activity of spikes was recorded using the SMEA, and the activity was diminished by 1 μM TTX. (Inset) Spontaneous activity before and after TTX from one electrode (E10). The peak-to-peak amplitude of spontaneous spikes ranged from 15 μV to more than 60 μV over a noise level of 1.86 μVrms. After 1 μM TTX was applied to the recording chamber, the firing rate monotonically decreased from approximately 8 Hz to 0 in 2 min. (C) Evoked field potentials were recorded from a hippocampal slice using the SMEA. A series of constant current, bipolar, biphasic stimuli (with increasing amplitude) was applied through two SMEA electrodes (E05 and E11) located within the DG region, and evoked field potentials were recorded from the other nine electrodes. The amplitude of evoked response in one channel (E10) located in the CA3 region was plotted versus intensity of applied stimuli (n = 3 each). The S/R curve was fitted with a sigmoidal function (dashed line). (Inset) An evoked field potential (at a stimulus intensity of 150 μA) recorded from one SMEA electrode (E10) before and after the cocktail was given. The response was essentially eliminated with a pharmacological cocktail of neuronal channel and receptor antagonists (1 μM TTX, 50 μM BIC, 100 μM APV, and 100 μM CNQX), and the remaining signal was attributed to stimulus artifact. The artifact was generated, in part, by the stimulator sync pulse and was present even when the stimulus intensity was set to 0.
Recording neural activity during a cycle of stretch and relaxation
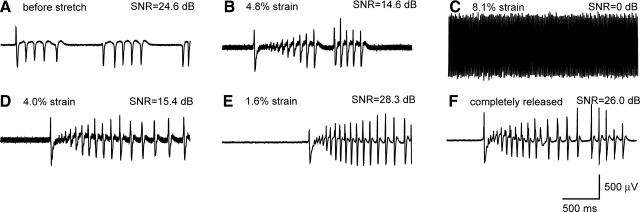
In this subset of studies, organotypic hippocampal slice cultures were placed on the SMEAs and held in place with a nylon mesh using standard electrophysiological procedures (Heuschkel et al., 2002). Neuronal activity was measured while the SMEA underneath the tissue was stretched biaxially. In this way, the SMEA was stretched but not the tissue such that the recording performance of the SMEA could be evaluated without the confound of tissue injury caused by its deformation. Bursting activity was induced with 100 μM BIC to reduce tonic inhibition. The signals' biological origin was confirmed pharmacologically, as it was blocked by a combination of 1 μM TTX, 100 μM APV, and 100 μM CNQX (data not shown). Figure 4A shows BIC-induced activity when the SMEA was in the resting state. The SMEA was then biaxially stretched up to 8.1% strain (Fig. 4B and C) and relaxed gradually (Fig. 4D–F). Neural activity was detected at all stages except at 8.1% strain, where the neural signals were submerged in the noise from increased SMEA resistance (Fig. 4C). Signal-to-noise ratio (SNR) was calculated as the power ratio between a signal (meaningful information) and the background noise. As shown in Figure 4, the SNR decreased as the SMEA was stretched and then increased back as the SMEA was released. During relaxation the noise receded, neural activity was detected again, and the recording performance of the SMEA recovered. These results show that the SMEA remained functional during and after sustained mechanical deformation.
FIG. 4.
Recording of bicuculline-induced bursting activity from a hippocampal slice culture (24 DIV) during stretch of the SMEA. 50 μM BIC was applied to the culture to induce robust electrical activity. The SMEA was stretched and released incrementally while activity was continuously recorded at each step. The signal recorded from one electrode is presented: (A) before stretch, (B) 4.8% biaxial strain, (C) 8.1% biaxial strain, (D) 4.0% biaxial strain, (E) 1.6% biaxial strain, and (F) completely released. The noise level increased along with stretch and then recovered after the SMEA was completely released. The initial signal to noise ratio (SNR = 24.6 dB) decreased as the SMEA was stretched, but recovered when it was completely released (SNR = 26.0 dB).
Stretching and monitoring organotypic hippocampal slices directly cultured on SMEAs
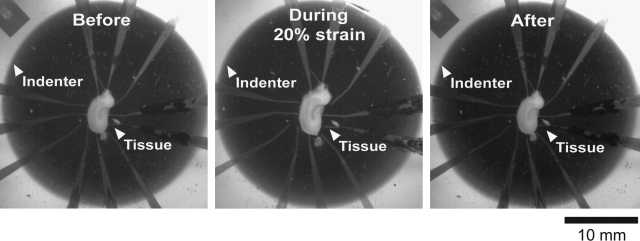
In this subset of studies, organotypic hippocampal slice cultures were directly grown on SMEAs to mechanically bond culture and SMEA, as previously shown (Morrison et al., 2006). The SMEAs were then biaxially stretched by precisely displacing them over a hollow, tubular indenter (Fig. 2, inset). In these studies, the adherent cultures stretched with the substrate SMEA (Fig. 5). The cultures were maintained in sterile conditions at all times. The actual deformation of the tissue was verified by image analysis for calculation of Lagrangian strain. As shown in Figure 5, the tissue was stretched up to 20% together with the SMEA (a cycle duration of ∼0.5 s), indicating that the adhesion force between the tissue and the SMEA was sufficient to impart the substrate deformation for strains as large as 20%. This result was consistent with our previous data, which demonstrated that organotypic hippocampal slices cultured on silicone membranes could be stretched up to 35%, and then were injured due to the induced mechanical deformation (Morrison et al., 2000, 2003). During relaxation, both the tissue and the SMEA electrodes recovered to their original shape, and no relative displacement between them was observed (Fig. 5). This result demonstrated that the substrate-embedded SMEA electrodes deformed with the tissue during stretch, such that the electrodes would monitor neuronal activity from the same anatomic locations before, during, and after deformation.
FIG. 5.
Images of an SMEA with an adherent hippocampal slice culture before, during, and after stretch. The hippocampal slice culture (5DIV) underwent deformation with the embedded SMEA electrodes. Confirmed by image analysis, a maximum deformation of 20% biaxial strain was imposed on the slice culture. Further analysis determined that the relative positions of the electrodes within the tissue slice remained fixed.
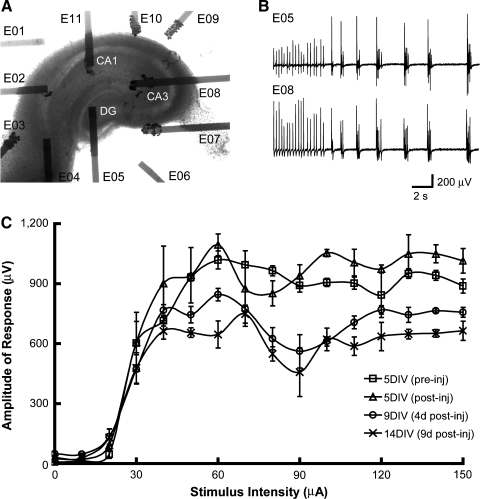
In a follow-up experiment, organotypic hippocampal slice cultures were directly grown on SMEAs (Fig. 6A) and dynamically deformed. On the fifth day in vitro (5 DIV), a culture was stretched to 8.0% biaxial strain and relaxed (a cycle duration of ∼0.5 s). Image analysis of high-speed video confirmed the extent of deformation of both the SMEA and adherent tissue. Pre- and post-injury neuronal activity was recorded from the same culture, which is a significant advantage of the SMEA over other electrode designs. Immediately post-injury, we observed spontaneous and continuous bursting (Fig. 6B), which was not present prior to injury. This intense synchronized bursting lasted for 20 s and then attenuated to sparse, synchronized, single spikes for at least an additional 180 s. Because the cultures were injured sterilely, serial recordings were conducted in the same culture at 4 and 9 days post-injury. At these later time points, these sparse synchronized spikes were not evident. Stimulus-response curves were also constructed pre- and post-injury (Fig. 6C). The Rmax in control cultures changed little during the 2 week culture period on the SMEA (∼5.4% decrease from 5 to 14 DIV), indicating good biocompatibility of the SMEAs up to 14 DIV. However, an 8.0% biaxial strain markedly decreased Rmax (∼36% decrease by day 9 post-injury), indicating that the number of excitable neurons significantly decreased after injury.
FIG. 6.
Spontaneous activity and evoked field potentials were recorded from an injured hippocampal slice using the SMEA. (A) A light micrograph of a hippocampal slice (2DIV) cultured on an SMEA with electrodes contacting the CA1, CA3, and DG regions. At 5DIV, the tissue and SMEA were biaxially stretched to 8.0% strain, and neuronal activity was monitored with the SMEA for an additional 9 days post-injury. (B) Immediately post-injury, spontaneous and continuous bursting was simultaneously recorded in multiple regions, which was not present before deformation. This bursting activity continued for 20 s and was then followed for 3 min by synchronized single bursts recorded on multiple electrodes simultaneously. Recordings from two electrodes (E05 in DG and E08 in CA3) are depicted. (C) Stimulus-response curves (stimulated at E05, n = 3) were generated immediately pre-injury (5DIV, pre-inj), immediately post-injury (5DIV, post-inj), 4 days post-injury (9DIV), and 9 days post-injury (14DIV) with the response recorded from E08 shown. The S/R curves were fit with sigmoidal functions. The maximum response amplitude (Rmax) decreased 36% at 9 days post-injury, indicating a loss of excitable neurons.
Discussion
We have developed a novel, elastically stretchable microelectrode array with fundamental differences to existing MEAs. Our SMEAs were elastically stretchable and capable of undergoing large, biaxial 2-D stretch while simultaneously recording neuronal activity. Our results showed that the SMEAs were capable of chronic and simultaneous multisite recording of extracellular signals from brain slice cultures before and after controlled deformation when integrated with our in vitro TBI research platform (Kim et al., 2004). By culturing cells or tissue directly on the SMEA, serial analysis of neuronal network function over extended periods was possible. Because the substrate-embedded electrodes deform with the substrate and adherent tissue, the electrodes remained in the same relative position to the neural units pre- and post-deformation, allowing for normalization of post-traumatic changes to pre-injury values.
Electrophysiological studies in animal models of TBI have yielded mechanistic insights into the causes of post-injury neuropathology including an increased hyperexcitability of the hippocampus and a reduced threshold to seizure-like discharges (Reeves et al., 2000; Golarai et al., 2001; Santhakumar et al., 2001). Understanding the mechano-transduction mechanisms that produce pathological changes in cellular physiology is facilitated by precise control over the inducing mechanical stimulus, as well as the extracellular environment. The difficulty of control over the transfer of mechanical forces to cells in vivo and the complex extracellular environment have motivated the development of in vitro models to mechanically stimulate neurons (Morrison et al., 1998). Not meant to replace, but to complement in vivo models, in vitro models allow for the precise mechanical stimulation of cultured neurons by the deformation of their underlying substrate, which in turn deforms the adherent cells (Ellis et al., 1995). The induced pathology has been shown to recapitulate multiple aspects of the in vivo pathology (Kao et al., 2004).
The long-range connectivity of neural networks can be studied with MEAs, which allow for the simultaneous recording from up to 100 discreet locations with recording rates up to 30 kHz (Warren et al., 2001). These arrays provide additional insight into neuronal information processing and function compared to recordings from single electrodes (Kralik et al., 2001; Diogo et al., 2003). Techniques such as patch clamping provide detailed information about a single cell, and field potential recordings provide information within a restricted spatial region (Santhakumar et al., 2003). However, given the distributed processing and storage of information in the brain, recording from an extended network of interacting neurons may be more appropriate since this ensemble network behavior of large populations of neurons probably encodes for higher order behaviors (Engel et al., 2001; Harris et al., 2003).
Traditional microelectrode array (MEA) technologies, based on integrated circuit (IC) and microelectro-mechanical system (MEMS) fabrication processes using materials with no or limited deformability (Madou, 2002; Cao et al., 2008), are simply not compatible with existing in vitro models of TBI, which rely on deformation of the underlying culture substrate (Egert et al., 1998; Warren et al., 2001). PDMS as a soft, stretchable substrate has been used for fabricating elastic and foldable semiconductor circuits (Sun et al., 2006; Kim et al., 2008) and multielectrode arrays for surface stimulation (Meacham et al., 2008). However, no previous work has developed stretchable microelectrode arrays that have the capability of detecting weak bioelectrical signals from nerve tissues. An alternative method could use an in vivo style MEA consisting of pin-like electrodes approaching the tissue from above (Williams et al., 1999; Diogo et al., 2003). However, if the MEA were left in place during deformation, the rigid electrodes would act like micro-knives and slice the tissue during deformation, severing connections and tearing cells. Less rigid arrays for in vivo implantation have been developed on the substrates with limited deformability, e.g. polyimide substrates (Rousche et al., 2001). However, they cannot endure large biaxial tensile strain and are not compatible with deformations necessary for TBI research (Chiu et al., 1994; Spaepen, 2000). Moreover, compared to brain, these electrodes are stiff and would have a similar cutting effect. Taking a baseline recording, and then reimplanting such an array post-injury can be considered, but recording from the same neurons would be unlikely, thereby complicating analyses. As an alternative, one could injure cultures or tissue by crush; however, these biomechanics are not representative of the most common TBIs such as falls, car accidents, or blunt trauma in which the major injury mechanism is stretch (Zhang et al., 2001; Ommaya et al., 2002). Another alternative deformable MEA technology uses conductive rubbers—silicones filled with carbon or silver particles—as the electrode material. However, they cannot be patterned with submillimeter feature sizes and present high electrical resistivity that may change significantly under stretching, leading to poor electrode performance and reliability (Tamai, 1982; Liu et al., 2001).
In contrast, our approach combines an elastomeric substrate with photolithographically patterned metal conductors to produce submillimeter elastic structures that remain electrically conductive while being stretched up to 90% uniaxially (Lacour et al., 2004). We have leveraged this technological advance to produce SMEAs that combine the utility of MEAs for recording of ensemble electrical activity with the ability to induce mechanical deformation in cultured tissue. We have demonstrated simultaneous mechanical stimulation and recording, making possible recording from the same set of neurons pre-, during, and post-mechanical stimulus. Furthermore, because the culture's sterility was maintained, long-term in vitro recordings were possible.
Compared to traditional MEAs, our SMEAs have some limitations in terms of materials and dimensions. The recording area of our SMEA electrodes is larger than current rigid or flexible MEAs with electrodes between 10 and 50 μm in diameter. The result is that recordings cannot be assigned as precisely to anatomical structures as is possible with rigid arrays. Our electrode dimensions are constrained by the dimensions of the smallest features that can be reliably patterned on the PDMS substrate and within the encapsulating silicone. For example, smaller vias in the encapsulating silicone are currently not feasible because of residual encapsulation, which is not fully developed in smaller features. A second limitation of the SMEA electrode size is the number of electrodes that can be patterned in an array the size of a hippocampal slice. The reported SMEAs consisted of 12 electrodes, compared to the 60 electrodes of the commercially available MCS arrays. A denser electrode array would improve spatial resolution of the recorded signals and subsequent analyses such as CSD. Currently we are advancing our fabrication techniques to produce a new generation of SMEAs with increased electrode density (i.e., reduced electrode size and increased number per area) to address these limitations. A higher electrode density will also allow for stimulation of more specific anatomic locations and circuitry. Moreover, we continue to improve the robustness of the stretchable gold layer with the goal of recording during larger biaxial deformations. However, we have shown that the current SMEAs were functional after repeated 20% biaxial strain, which is sufficient to induce a moderate-to-severe injury corresponding to 20–40% cell death in the pyramidal cell layers (Cater et al., 2007).
In the area of TBI research, the significance of our proposed technology is several-fold. An in vitro model using SMEAs could form the basis of a rapid screening tool for the discovery of novel neuroprotective compounds. Because cultures adhere to the integral electrodes on the substrate, recordings will be made from the same neurons pre- and post-injury, whereby post-injury response can be normalized to pre-injury data. Analysis of electrophysiology can be automated by computer and multiplexed to record from multiple cultures simultaneously (van Bergen et al., 2003). This increased throughput and reduced cost of in vitro models compared to cost with animal models would make such a system attractive for screening and discovery of therapeutic compounds (Shimono et al., 2002; Ray et al., 2003). Moreover, electrophysiological measures are likely to be the most relevant in terms of behavior, cognition, or consciousness and are likely to be a sensitive measure of the function and health of neurons to complement other experimental measures, such as cell death. Significantly, therapeutic strategies directed at repair and restoration of function could be assessed with this system. Use of the SMEA in long-term recording of long-distance connectivity in hippocampal slice cultures could be used in the assessment of new repair strategies that hold the promise to restore function for the 5.3 million Americans currently living with a long-term disability due to TBI.
Research into the mechanisms that are responsible for post-traumatic neuronal dysfunction has the potential to reduce the associated mortality, morbidity, and health-care costs associated with TBI. The technological innovation of SMEAs could enable new studies to understand these mechanisms in greater detail with the long-term goal of developing new therapies for head injured patients. In addition, the ability to fabricate micro-electromechanical systems onto substrates that are flexible and even conformable to the human body promises breakthroughs in biological and biomedical fields beyond the study of TBI. Retina-shaped implants, skull-conformal sensor arrays, deformable electrodes for medical devices, such as endotracheal tubes or smart pacemakers, and even artificial electronic skin are a few examples of potential future applications.
Acknowledgments
The authors would like to acknowledge the assistance of J. Jones. This work was supported by NIH (NINDS R21 052794), the New Jersey Commission on Science and Technology, and a University Research Fellowship from the Royal Society (S.P.L.).
Author Disclosure Statement
No competing financial interests exist.
References
- Beggs J.M. Plenz D. Neuronal avalanches in neocortical circuits. J. Neurosci. 2003;23:11167–11177. doi: 10.1523/JNEUROSCI.23-35-11167.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G. Large-scale recording of neuronal ensembles. Nat. Neurosci. 2004;7:446–451. doi: 10.1038/nn1233. [DOI] [PubMed] [Google Scholar]
- Cao Q. Kim H.S. Pimparkar N. Kulkarni J.P. Wang C. Shim M. Roy K. Alam M.A. Rogers J.A. Medium-scale carbon nanotube thin-film integrated circuits on flexible plastic substrates. Nature. 2008;454:495–500. doi: 10.1038/nature07110. [DOI] [PubMed] [Google Scholar]
- Cater H.L. Gitterman D. Davis S.M. Benham C.D. Morrison B., III Sundstrom L.E. Stretch-induced injury in organotypic hippocampal slice cultures reproduces in vivo post-traumatic neurodegeneration: role of glutamate receptors and voltage-dependent calcium channels. J. Neurochem. 2007;101:434–447. doi: 10.1111/j.1471-4159.2006.04379.x. [DOI] [PubMed] [Google Scholar]
- Cater H.L. Sundstrom L.E. Morrison B., III Temporal development of hippocampal cell death is dependent on tissue strain but not strain rate. J Biomech. 2006;39:2810–2818. doi: 10.1016/j.jbiomech.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Cheung K.C. Renaud P. Tanila H. Djupsund K. Flexible polyimide microelectrode array for in vivo recordings and current source density analysis. Biosens. Bioelectron. 2007;22:1783–1790. doi: 10.1016/j.bios.2006.08.035. [DOI] [PubMed] [Google Scholar]
- Chiu S.L. Leu J. Hop S. Fracture of metal-polymer line structures. I. Semiflexible polyimide. J. Appl. Phys. 1994;76:5136–5142. [Google Scholar]
- Christensen T.A. Pawlowski V.M. Lei H. Hildebrand J.G. Multi-unit recordings reveal context-dependent modulation of synchrony in odor-specific neural ensembles. Nat. Neurosci. 2000;3:927–931. doi: 10.1038/78840. [DOI] [PubMed] [Google Scholar]
- Deadwyler S.A. Hampson R.E. Ensemble activity and behavior: what's the code? Science. 1995;270:1316–1318. doi: 10.1126/science.270.5240.1316. [DOI] [PubMed] [Google Scholar]
- Diogo A.C. Soares J.G. Koulakov A. Albright T.D. Gattass R. Electrophysiological imaging of functional architecture in the cortical middle temporal visual area of Cebus apella monkey. J. Neurosci. 2003;23:3881–3898. doi: 10.1523/JNEUROSCI.23-09-03881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch G.S. Patterns in the brain. Neuronal population coding in the somatosensory system. Physiol. Behav. 2000;69:187–201. doi: 10.1016/s0031-9384(00)00201-8. [DOI] [PubMed] [Google Scholar]
- Egert U. Schlosshauer B. Fennrich S. Nisch W. Fejtl M. Knott T. Muller T. Hammerle H. A novel organotypic long-term culture of the rat hippocampus on substrate-integrated multielectrode arrays. Brain Res. Brain Res. Protoc. 1998;2:229–242. doi: 10.1016/s1385-299x(98)00013-0. [DOI] [PubMed] [Google Scholar]
- Elkin B.S. Morrison B., III Region-specific tolerance criteria for the living brain. Stapp. Car. Crash. J. 2007;51:127–138. doi: 10.4271/2007-22-0005. [DOI] [PubMed] [Google Scholar]
- Ellis E.F. McKinney J.S. Willoughby K.A. Liang S. Povlishock J.T. A new model for rapid stretch-induced injury of cells in culture: characterization of the model using astrocytes. J. Neurotrauma. 1995;12:325–339. doi: 10.1089/neu.1995.12.325. [DOI] [PubMed] [Google Scholar]
- Engel A.K. Fries P. Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat. Rev. Neurosci. 2001;2:704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- Frey L.C. Epidemiology of posttraumatic epilepsy: a critical review. Epilepsia. 2003;44(Suppl 10):11–17. doi: 10.1046/j.1528-1157.44.s10.4.x. [DOI] [PubMed] [Google Scholar]
- Golarai G. Greenwood A.C. Feeney D.M. Connor J.A. Physiological and structural evidence for hippocampal involvement in persistent seizure susceptibility after traumatic brain injury. J. Neurosci. 2001;21:8523–8537. doi: 10.1523/JNEUROSCI.21-21-08523.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris K.D. Csicsvari J. Hirase H. Dragoi G. Buzsaki G. Organization of cell assemblies in the hippocampus. Nature. 2003;424:552–556. doi: 10.1038/nature01834. [DOI] [PubMed] [Google Scholar]
- Heuschkel M.O. Fejtl M. Raggenbass M. Bertrand D. Renaud P. A three-dimensional multi-electrode array for multi-site stimulation and recording in acute brain slices. J. Neurosci. Methods. 2002;114:135–148. doi: 10.1016/s0165-0270(01)00514-3. [DOI] [PubMed] [Google Scholar]
- Hochberg L.R. Serruya M.D. Friehs G.M. Mukand J.A. Saleh M. Caplan A.H. Branner A. Chen D. Penn R.D. Donoghue J.P. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006;442:164–171. doi: 10.1038/nature04970. [DOI] [PubMed] [Google Scholar]
- Hoge C.W. McGurk D. Thomas J.L. Cox A.L. Engel C.C. Castro C.A. Mild traumatic brain injury in U.S. soldiers returning from Iraq. N. Engl. J. Med. 2008;358:453, 463. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- Kao C.Q. Goforth P.B. Ellis E.F. Satin L.S. Potentiation of GABA(A) currents after mechanical injury of cortical neurons. J. Neurotrauma. 2004;21:259–270. doi: 10.1089/089771504322972059. [DOI] [PubMed] [Google Scholar]
- Kim D.H. Ahn J.H. Choi W.M. Kim H.S. Kim T.H. Song J. Huang Y.Y. Liu Z. Lu C. Rogers J.A. Stretchable and foldable silicon integrated circuits. Science. 2008;320:507–511. doi: 10.1126/science.1154367. [DOI] [PubMed] [Google Scholar]
- Kim Y.T. Hitchcock R.W. Bridge M.J. Tresco P.A. Chronic response of adult rat brain tissue to implants anchored to the skull. Biomaterials. 2004;25:2229–2237. doi: 10.1016/j.biomaterials.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Kralik J.D. Dimitrov D.F. Krupa D.J. Katz D.B. Cohen D. Nicolelis M.A. Techniques for long-term multisite neuronal ensemble recordings in behaving animals. Methods. 2001;25:121–150. doi: 10.1006/meth.2001.1231. [DOI] [PubMed] [Google Scholar]
- Lacour S.P. Jones J. Wagner S. Suo Z. Design and performance of thin metal film interconnects for skin-like electronic circuit. IEEE Electron Device Lett. 2004;25:179–181. [Google Scholar]
- Lacour S.P. Wagner S. Huang Z. Suo Z. Stretchable gold conductors on elastomeric substrates. Appl. Phys. Lett. 2003;82:2404–2406. [Google Scholar]
- Li T. Huang Z. Suo Z. Lacour S.P. Wagner S. Stretchability of thin metal films on elastomer substrates. Appl. Phys. Lett. 2004;85:3435–3437. [Google Scholar]
- Liu W. Pecht M.G. Xie J. Fundamental reliability issues associated with commercial particle-in-elastomer interconnection systems. IEEE Trans. Compon. Packag. Technol. 2001;24:520–525. [Google Scholar]
- Madou M. Fundamentals of Microfabrication. CRC Press; Boca Raton, FL: 2002. [Google Scholar]
- Meacham K.W. Giuly R.J. Guo L. Hochman S. DeWeerth S.P. A lithographically-patterned, elastic multi-electrode array for surface stimulation of the spinal cord. Biomed. Microdevices. 2008;10:259–269. doi: 10.1007/s10544-007-9132-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison B., III Cater H.L. Benham C.D. Sundstrom L.E. An in vitro model of traumatic brain injury utilising two-dimensional stretch of organotypic hippocampal slice cultures. J. Neurosci. Methods. 2006;150:192–201. doi: 10.1016/j.jneumeth.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Morrison B., III Cater H.L. Wang C.C. Thomas F.C. Hung C.T. Ateshian G.A. Sundstrom L.E. A tissue level tolerance criterion for living brain developed with an in vitro model of traumatic mechanical loading. Stapp. Car. Crash. J. 2003;47:93–105. doi: 10.4271/2003-22-0006. [DOI] [PubMed] [Google Scholar]
- Morrison B., III Meaney D.F. Margulies S.S. McIntosh T.K. Dynamic mechanical stretch of organotypic brain slice cultures induces differential genomic expression: relationship to mechanical parameters. J. Biomech. Eng. 2000;122:224–230. doi: 10.1115/1.429650. [DOI] [PubMed] [Google Scholar]
- Morrison B., III Saatman K.E. Meaney D.F. McIntosh T.K. In vitro central nervous system models of mechanically induced trauma: a review. J. Neurotrauma. 1998;15:911–928. doi: 10.1089/neu.1998.15.911. [DOI] [PubMed] [Google Scholar]
- Nisch W. Bock J. Egert U. Hammerle H. Mohr A. A thin film microelectrode array for monitoring extracellular neuronal activity in vitro. Biosens. Bioelectron. 1994;9:737–741. doi: 10.1016/0956-5663(94)80072-3. [DOI] [PubMed] [Google Scholar]
- Ommaya A.K. Goldsmith W. Thibault L. Biomechanics and neuropathology of adult and paediatric head injury. Br. J. Neurosurg. 2002;16:220–242. doi: 10.1080/02688690220148824. [DOI] [PubMed] [Google Scholar]
- Pashley D. A study of the deformation and fracture of single-crystal gold films of high strength inside an electron microscope. Proc. Roy. Soc. Lond. 1960;255:218–231. [Google Scholar]
- Ray A.M. Benham C.D. Roberts J.C. Gill C.H. Lanneau C. Gitterman D.P. Harries M. Davis J.B. Davies C.H. Capsazepine protects against neuronal injury caused by oxygen glucose deprivation by inhibiting I(h) J. Neurosci. 2003;23:10146–10153. doi: 10.1523/JNEUROSCI.23-31-10146.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves T.M. Kao C.Q. Phillips L.L. Bullock M.R. Povlishock J.T. Presynaptic excitability changes following traumatic brain injury in the rat. J. Neurosci. Res. 2000;60:370–379. doi: 10.1002/(SICI)1097-4547(20000501)60:3<370::AID-JNR12>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Rousche P.J. Pellinen D.S. Pivin D.P., Jr. Williams J.C. Vetter R.J. Kipke D.R. Flexible polyimide-based intracortical electrode arrays with bioactive capability. IEEE Trans. Biomed. Eng. 2001;48:361–371. doi: 10.1109/10.914800. [DOI] [PubMed] [Google Scholar]
- Sanchez J.C. Mareci T.H. Norman W.M. Principe J.C. Ditto W.L. Carney P.R. Evolving into epilepsy: multiscale electrophysiological analysis and imaging in an animal model. Exp. Neurol. 2006;198:31–47. doi: 10.1016/j.expneurol.2005.10.031. [DOI] [PubMed] [Google Scholar]
- Santhakumar V. Ratzliff A.D. Jeng J. Toth Z. Soltesz I. Long-term hyperexcitability in the hippocampus after experimental head trauma. Ann. Neurol. 2001;50:708–717. doi: 10.1002/ana.1230. [DOI] [PubMed] [Google Scholar]
- Santhakumar V. Voipio J. Kaila K. Soltesz I. Post-traumatic hyperexcitability is not caused by impaired buffering of extracellular potassium. J. Neurosci. 2003;23:5865–5876. doi: 10.1523/JNEUROSCI.23-13-05865.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schretlen D.J. Shapiro A.M. A quantitative review of the effects of traumatic brain injury on cognitive functioning. Int. Rev. Psychiatry. 2003;15:341–349. doi: 10.1080/09540260310001606728. [DOI] [PubMed] [Google Scholar]
- Schwarzbach E. Bonislawski D.P. Xiong G. Cohen A.S. Mechanisms underlying the inability to induce area CA1 LTP in the mouse after traumatic brain injury. Hippocampus. 2006;16:541–550. doi: 10.1002/hipo.20183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimono K. Baudry M. Panchenko V. Taketani M. Chronic multichannel recordings from organotypic hippocampal slice cultures: protection from excitotoxic effects of NMDA by non-competitive NMDA antagonists. J. Neurosci. Methods. 2002;120:193–202. doi: 10.1016/s0165-0270(02)00202-9. [DOI] [PubMed] [Google Scholar]
- Spaepen F. Interfaces and stresses in thin films. Acta Materialia. 2000;48:31–42. [Google Scholar]
- Sun Y. Choi W.M. Jiang H. Huang Y.Y. Rogers J.A. Controlled buckling of semiconductor nanoribbons for stretchable electronics. Nat. Nanotechnol. 2006;1:201–207. doi: 10.1038/nnano.2006.131. [DOI] [PubMed] [Google Scholar]
- Tamai T. Electrical properties of conductive elastomer as electrical contact material. IEEE Trans. Compon. Packag. Technol. 1982;5:56–61. [Google Scholar]
- Taube J.S. Schwartzkroin P.A. Mechanisms of long-term potentiation: a current-source density analysis. J. Neurosci. 1988;8:1645–1655. doi: 10.1523/JNEUROSCI.08-05-01645.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaud P. Beuret C. Koudelka-Hep M. Bove M. Martinoia S. Grattarola M. Jahnsen H. Rebaudo R. Balestrino M. Zimmer J. Dupont Y. An array of Pt-tip microelectrodes for extracellular monitoring of activity of brain slices. Biosens. Bioelectron. 1999;14:61–65. doi: 10.1016/s0956-5663(98)00098-0. [DOI] [PubMed] [Google Scholar]
- van Bergen A. Papanikolaou T. Schuker A. Moller A. Schlosshauer B. Long-term stimulation of mouse hippocampal slice culture on microelectrode array. Brain Res. Brain Res. Protoc. 2003;11:123–133. doi: 10.1016/s1385-299x(03)00024-2. [DOI] [PubMed] [Google Scholar]
- Warren D.J. Fernandez E. Normann R.A. High-resolution two-dimensional spatial mapping of cat striate cortex using a 100-microelectrode array. Neuroscience. 2001;105:19–31. doi: 10.1016/s0306-4522(01)00174-9. [DOI] [PubMed] [Google Scholar]
- Williams J.C. Rennaker R.L. Kipke D.R. Long-term neural recording characteristics of wire microelectrode arrays implanted in cerebral cortex. Brain Res. Brain Res. Protoc. 1999;4:303–313. doi: 10.1016/s1385-299x(99)00034-3. [DOI] [PubMed] [Google Scholar]
- Yu Z. McKnight T.E. Ericson M.N. Melechko A.V. Simpson M.L. Morrison B., III Vertically aligned carbon nanofiber arrays record electrophysiological signals from hippocampal slices. Nano Lett. 2007;7:2188–2195. doi: 10.1021/nl070291a. [DOI] [PubMed] [Google Scholar]
- Zhang L. Yang K.H. King A.I. Biomechanics of neurotrauma. Neurol. Res. 2001;23:144–156. doi: 10.1179/016164101101198488. [DOI] [PubMed] [Google Scholar]