Abstract
We investigated the temporal and regional profile of blood-brain barrier (BBB) permeability to both large and small molecules after moderate fluid percussion (FP) brain injury in rats and determined the effects of post-traumatic modest hypothermia (33°C/4 h) on these vascular perturbations. The visible tracers biotin-dextrin-amine 3000 (BDA-3K, 3 kDa) and horseradish peroxidase (HRP, 44 kDa) were injected intravenously at 4 h or 3 or 7 days post-TBI. At 30 min after the tracer infusion, both small and large molecular weight tracers were detected in the contusion area as well as remote regions adjacent to the injury epicenter in both cortical and hippocampal structures. In areas adjacent to the contusion site, increased permeability to the small molecular weight tracer (BDA-3K) was evident at 4 h post-TBI and remained visible after 7 days survival. In contrast, the larger tracer molecule (HRP) appeared in these remote areas at acute permeable sites but was not detected at later post-traumatic time periods. A regionally specific relationship was documented at 3 days between the late-occurring permeability changes observed with BDA-3K and the accumulation of CD68-positive macrophages. Mild hypothermia initiated 30 min after TBI reduced permeability to both large and small tracers and the infiltration of CD68-positive cells. These results indicate that moderate brain injury produces temperature-sensitive acute, as well as more long-lasting vascular perturbations associated with secondary injury mechanisms.
Key words: blood-brain barrier, CD68, hypothermia, leukocytes, traumatic brain injury
Introduction
An early consequence of experimental brain trauma is increased vascular permeability leading to brain edema and a complex sequence of inflammatory cascades (Povlishock et al., 1978; Tanno et al., 1992; Dietrich et al., 1994b; Habgood et al., 2007). Alterations in blood-brain barrier (BBB) permeability may contribute to secondary brain injury through the abnormal passage of blood-borne substances into the central nervous system influencing neuronal vulnerability (Stahel et al., 2000; Lucas et al., 2006). Also, recent clinical and experimental studies have implicated BBB leakage in post-traumatic epilepsy and post-concussion syndrome (Korn et al., 2005; Tomkins et al., 2008). Regional patterns of altered vascular permeability have been demonstrated in various models of traumatic brain injury (TBI). Povlishock and colleagues (1978) first showed using a midline fluid percussion (FP) model in cats that extravasation of the large permeability tracer horseradish peroxidase (HRP) could be visualized in brainstem vessels. More recently, permeability alterations have been seen in rodent models of TBI including FP and closed brain injury models (Tanno et al., 1992; Dietrich et al., 1994b; Kinoshita et al., 2002a; Arican et al., 2006; Habgood et al., 2007). For example, Dietrich and colleagues (1994b) reported early extravasation of HRP after moderate FP injury associated with gray and white matter structures undergoing contusion injury and selective neuronal damage. Thus, the importance of early alterations in vascular permeability on neuronal vulnerability and as a therapeutic target has been suggested (Dietrich et al., 1994b; Habgood et al., 2007).
Increased vascular permeability is associated with multiple post-traumatic inflammatory events (Chatzipanteli et al., 2000; Stahel et al., 2000; Morganti-Kossmann et al., 2005). Trauma induced mechanical damage to vascular cell membranes results in endothelial cell necrosis and red blood cell extravasation (Dietrich et al., 1994b; Kinoshita et al., 2002a). Endothelial and leukocyte adhesion molecules are upregulated as a result of primary traumatic events, resulting in recruitment and extravasation of circulating leukocytes into the brain parenchyma (Schoettle et al., 1990). Blood-borne circulating monocytes gain access to the brain parenchyma and may contribute to macrophage-induced secondary injury mechanisms (Dietrich et al., 1994a; Hartl et al., 1997). Together with endogenous astrocyte and microglia activation, these inflammatory cascades represent an important target for neuroprotective interventions (Raivich and Banati, 2004; Lucas et al., 2006; Block et al., 2007). For example, antibodies against proinflammatory cytokines including IL-1β and TNFα have been reported to protect the post-traumatic brain from histopathological damage. Other strategies that target proinflammatory cytokines or specific cellular adhesion molecules (Utagawa et al., 2008) leading to reduced infiltration of blood-borne leukocytes have also been reported (Lucas et al., 2006). Thus, recent evidence has directed therapeutic strategies to reduce systemic sources of proinflammatory cytokines and other toxins that can influence the neurochemical environment of the post-traumatic brain.
Experimental data have also demonstrated that temperature modifications significantly influence neuronal vulnerability and vascular perturbations after brain injury (Dietrich et al., 1994b; Ueda et al., 2003). In the area of brain trauma, two studies have reported that pre- and post-hypothermia reduces blood-brain barrier (BBB) permeability changes to large molecular weight proteins at 60 min after injury. In the study by Jiang and colleagues (1992), induced pre-injury moderate hypothermia (30°C) and maintained until sacrificed (60 min post-trauma) reduced endogenous vascular protein tracer passage into the brain. In the study by Smith and Hall (1996), in animals where brain temperature was not critically controlled and allowed to decrease before and after trauma, mild hypothermia appeared to reduce the degree of BBB leakage to Evans Blue (Smith and Hall, 1996) at 60 min post-trauma. In contrast to cooling, mild hyperthermia aggravates permeability alterations after injury (Dietrich and Bramlett, 2007). Thus, post-traumatic hypothermia may improve traumatic outcome by altering the vascular consequences of brain injury and the subsequent formation of vasogenic edema (Dietrich et al., 1996). Our laboratory, as well as others, has demonstrated that in addition to acute BBB permeability, post-traumatic cooling also reduces the infiltration of circulating inflammatory cells (Chatzipanteli et al., 2000; Sutcliffe et al., 2001). Also decreased levels of proinflammatory cytokines and nitric oxide synthase are reported after clinical (Aibiki et al., 1999) or experimental TBI with modest hypothermia (Goss et al., 1995; Kinoshita et al., 2002b).
In contrast to previous findings where relatively short post-traumatic periods have been evaluated, recent data indicate that the BBB may be disrupted for a post-traumatic period longer than previously appreciated. Habgood and colleagues (2007) recently showed that in contrast to the large tracers such as HRP, smaller molecular weight tracers remain permeable as late as 4 days post-trauma. Because later occurring permeability changes may be signaling the delayed entry of inflammatory cells and toxins into the vulnerable brain tissue (Holmin and Mathiesen, 1999), it would be important to determine whether therapeutic hypothermia would attenuate the later phase of permeability abnormalities after TBI. The major purpose of this study was to assess the effects of a restricted period (4 h) of mild post-traumatic hypothermia (33°C) on both the early- and late-occurring alterations in vascular permeability to large and small permeability tracers. In addition, we investigated temperature sensitive patterns of BBB permeability to the extravasation of CD68 immunoreactive inflammatory cells. In this study, we utilized a TBI model and hypothermic strategy that has previously been reported to reduce histopathological damage and improve neurological outcome (Dietrich et al., 1994a; Matsushita et al., 2001).
Materials and Methods
Animals
Male Sprague-Dawley rats (n = 5 per group, each weighing 240 − 280 g) obtained from Charles River Breeders were used for all experiments. Animal care was in accordance with the guidelines set by the University of Miami Animal Care and Use Committee, and all animal protocols were approved by the committee prior to study initiation. Animals were kept at a constant temperature controlled room (72°F) for at least 7 days before the study and exposed to a 12 h light/dark cycle. Rats were allowed free access to water, but food was withheld overnight before surgery.
Surgical preparation for fluid percussion injury
The basic surgical preparation for the parasagittal fluid percussion (FP) brain injury was performed according to methods previously described (Dietrich et al., 1994a). Rats were initially anesthetized with 3% halothane and a mixture of 70% nitrous oxide and 30% oxygen 24 h prior to injury, and surgically prepared for FP injury. Briefly, rats were then placed on a stereotaxic frame, and a 4.8 mm craniotomy was made overlying the right parietal cortex (3.8 mm posterior to bregma and 2.5 mm lateral to the midline) (Zilles, 1985). A plastic injury tube was then placed over the exposed dura, was bound by adhesive as well as general acrylic and hardened, and the scalp was closed. After fasting overnight, a FP device was used to produce moderate (1.8–2.2 atm) parasagittal FP brain injury, as previously described (Matsushita et al., 2001). Sham-operated control animals underwent all surgical procedures but were not traumatized. An endotracheal tube was inserted orally, and the rats were mechanically ventilated on a mixture of 70% nitrous oxide, 0.5% to 1.5% halothane with a balance of oxygen. Femoral artery was cannulated with PE-50 tubing. Animals were then immobilized with pancuronium bromide (0.5 mg/kg IV). Following TBI or sham procedures, animals were allowed to survive for 4 h, 3 days, or 7 days after injury. In normothermic animals, rectal and temporalis muscle temperatures were maintained at 37°C for up to 4 h post-injury. In animals undergoing hypothermic therapy, temporalis muscle temperature was reduced to 33°C starting 30 min after the traumatic insult by methods previously described (Kinoshita et al., 2002b). Briefly, mild hypothermia was induced by blowing cooled air over the head to maintain brain temperature between 33.0 and 33.6°C. Hypothermia was maintained at this level for a 4 h period, and then the brain was slowly rewarmed over a 1 h period as previously described (Matsushita et al., 2001).
Blood-brain barrier tracer procedures
Analysis of BBB tracer leakage was performed as previously described (Dietrich et al., 1994b; Habgood et al., 2007). At the time of perfusion, animals were anesthetized and a catheter was inserted into the femoral vein. Each animal received a single intravenous injection of the tracer in the femoral vein (dissolved in 500 μL in saline). The tracers included 5 mg HRP (44 kDa, Sigma-Aldrich, St. Louis, MO) or 3 mg biotin-dextrin-amine 3000 (BDA-3K, 3 kDa; Sigma-Aldrich). Thirty minutes after the tracers had been injected, the animals were transcardially perfused and serially sectioned as described below. For the biotin-labeled tracers, sections containing the injury site were bleached with hydrogen peroxide (0.02% in 50% methanol in 0.1 M phosphate buffer, pH 7.2) for 30 min to block endogenous peroxidase activity and then washed three times in phosphate buffers and developed using the DAB method as previously described (Habgood et al., 2007).
Images were obtained by montaging wide field FB-fluorescent 40× magnification images using Neurolucida 7.5.1 software (MicroBrightField, Inc., Colchester, VT). Higher magnification optical imaging was performed using an LSM 510 laser scanning confocal microscope (Carl Zeiss, Wetzlar, Germany) using 25 × 0.8 ma and 63 × 1.2 ma water-immersion lenses. To minimize experimental variability, sections from each experimental group were processed in parallel and imaged identically. For imaging, 10 sections were selected from each animal. To quantify the areas of tracer leakage, unbiased stereological methods were used. Areas of tracer leakage were traced with Neurolucida software in sections spaced 200 μm apart.
Perfusion fixation and immunohistochemistry
Animals were reanesthetized with 2% halothane and perfused with cold saline (2 min, 75 mL) followed by 4% paraformaldehyde (300 mL) solution, and the brains were removed and placed in 4% paraformaldehyde at 4°C for 20 h. Brains were transferred to 20% sucrose in 0.1 M phosphate-buffered solution and kept in sucrose solution at 4°C until they were ready to be vibratome sectioned (Leica Microsystems, Chantilly, VA). Sections (70 μm) were blocked by treatment with appropriate pre-immune serum. Tissue sections were rinsed with 0.1 M phosphate-buffered saline (pH 7.4). Sections were incubated overnight at 4°C with the mouse anti-rat monocytes/macrophages (CD68) monoclonal antibody (Chemicon International, Temecula, CA). Primary antibody binding was detected either with Alexa-Fluor secondary antibody conjugates (1:200; Molecular Probes, Eugene, OR) (confocal analysis) or with biotinylated secondary antibodies (1:200; Vector Laboratories) followed by diaminobenzidine (DAB) staining. Controls lacking the primary antibody were run in parallel. Sections were coverslipped with Vectashield mounting medium (Vector Laboratories) for confocal analysis and with Permount (Fisher Scientific, Pittsburgh, PA) for DAB-stained sections.
3-D solid model
The set of random systematic sections throughout the region of interest (BBB leakage area) were traced using Neurolucida software. Consecutive sections were logged into the serial section manager and z-position of each section was indicated.
Stereology
The number of CD68 positive cells was quantified in areas showing abnormal vascular permeability. Serial vibratome sections (70 μm) of the rat brain at the level of injury were divided into four groups; each group contained 10 sections representing the traumatized area. For estimation of the number of CD68-immunopositive cells, DAB was used as the chromagen. Both cortex and hippocampus regions in normothermic and hypothermic animals at 3 and 7 days after TBI were analyzed using an Axiophot (Zeiss, Thornwood, NY) research microscope, furnished with a fully motorized 3-D LEP stage, Optronix cooled video camera, and MicroBrightField Stereo-Investigator software package. To perform cell number estimation in the structure volume, the optical fractionator method and optical dissector probe were used. Dimension of the optical dissector was designed based upon the cell distribution on the section, and optical fractionator grid size was determined based upon the results of the preliminary count of the naïve brain sample to allow 200 counts per brain region. Cortex and hippocampus were analyzed separately. Immunoreactive cells were those that had degrees of cell body stain greater than controls lacking primary antibody.
Statistics
Tracer leakage and total CD68-positive cell counts were analyzed using two-way analysis of variance (ANOVA) followed by Student-Newman-Keuls post hoc comparison. Data were expressed as mean ± SEM.
Results
Physiological variables
Physiological measurements of rectal and brain temperature, PCO2, PO2, pH, mean arterial blood pressure (MAP), and pH are given in Table 1. Physiological variables were taken 15 min prior to TBI (Table 1), and 15 min, 1, 2, 3 (data not shown), and 4 h (Table 1) after injury. All physiological variables were within normal ranges. The only significant difference between the groups was seen in the temporalis muscle temperature where the hypothermic group had significantly reduced temporalis temperature compared to that of the normothermic TBI animals as predicted.
Table 1.
Physiologic Variables (mean ± SEM)
| |
TBI, 3 days |
TBI, 7 days |
||
|---|---|---|---|---|
| Normothermia | Hypothermia | Normothermia | Hypothermia | |
| Pre-trauma | ||||
| pH | 7.43 ± 0.01 | 7.44 ± 0.01 | 7.44 ± 0.01 | 7.40 ± 0.03 |
| pCO2 | 41.7 ± 1.1 | 39.8 ± 1.1 | 39.1 ± 0.9 | 41.4 ± 0.8 |
| pO2 | 130.8 ± 8.9 | 149.7 ± 8.5 | 147.0 ± 13.5 | 152.4 ± 10.7 |
| MABP | 126.2 ± 2.8 | 125.4 ± 5.9 | 117.0 ± 2.1 | 120.2 ± 4.3 |
| Brain temperature | 36.8 ± 0.1 | 36.6 ± 0.1 | 36.7 ± 0.1 | 36.7 ± 0.1 |
| Rectal temperature | 37.0 ± 0.0 | 37.0 ± 0.0 | 36.9 ± 0.1 | 39.9 ± 0.1 |
| Post-trauma, 4 h | ||||
| pH | 7.42 ± 0.02 | 7.42 ± 0.02 | 7.43 ± 0.01 | 7.41 ± 0.01 |
| pCO2 | 38.9 ± 1.5 | 36.1 ± 1.9 | 36.6 ± 0.6 | 40.0 ± 1.7 |
| pO2 | 151.8 ± 9.2 | 144.6 ± 7.6 | 121.4 ± 11.8 | 126.92 ± 12.7 |
| MABP | 119.5 ± 3.1 | 124.7 ± 3.6 | 108.8 ± 2.5 | 121.8 ± 4.1 |
| Brain temperature | 36.7 ± 0.1 | 33.0 ± 0.1* | 36.8 ± 0.1 | 33.0 ± 0.1* |
| Rectal temperature | 36.7 ± 0.0 | 34.7 ± 0.3 | 37.0 ± 0.0 | 35.1 ± 0.1 |
All physiological variables were within normal range through the experiments.
TBI, traumatic brain injury; MABP, mean arterial blood pressure.
p < 0.05 compared with normothermia.
Permeability alterations
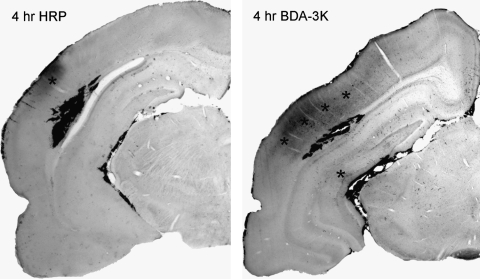
At 4 h and 3 days following moderate FP injury, local areas of increased vascular permeability to HRP (44 kDa) and biotin-labeled tracer BDA-3K (3 kDa) were clearly observed within the contused area (Figs. 1 and 2). Within overlying cortical and subcortical hippocampal areas within the ipsilateral traumatized hemisphere, leakage was also apparent. At 4 h, strong leakage was observed within the contusion site with both tracers (Fig. 1). However, extravasation of BDA-3K was more apparent within pericontusional cortical and subcortical areas than seen with HRP. At 3 days, mild HRP extravasation was noted in the overlying cerebral cortex with little leakage seen in the underlying hippocampus except for large vessels running close to the lateral cistern. In contrast, with the small molecular weight tracer, there was more profound evidence of tracer diffusion into areas immediately surrounding the primary injury site, overlying cerebral cortex, hippocampus, and thalamic regions (Fig. 2).
FIG. 1.
Micrograph of horseradish peroxidase (HRP, 44 kDa) and biotin-dextran-amine 3000 (BDA-3K) permeability at 4 h post-injury. BDA-3K and HRP extravasation are shown (asterisk) in the cortical contusion space, along with pericontusional areas of the ipsilateral cerebral cortex and subcortical structures. At this early time point, there appears to be more leakage of the small molecular tracer, BDA-3K, compared to that of HRP in vulnerable brain regions.
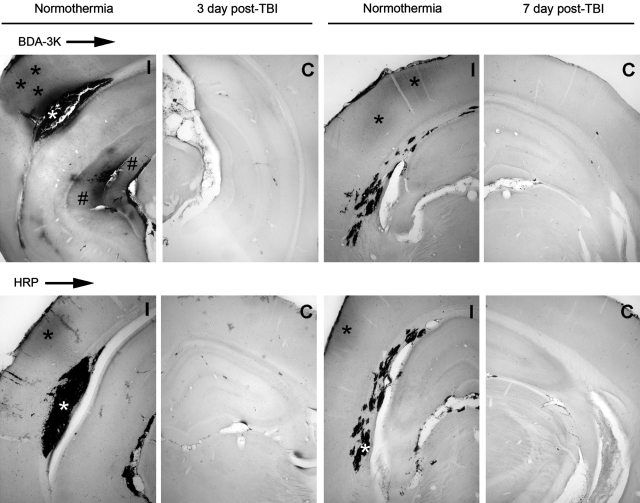
FIG. 2.
Patterns of horseradish peroxidase (HRP, 44 kDa) and biotin-dextran-ammine-3000 (BDA-3K) permeability. At 3 days after normothermic TBI, BDA-3K and HRP extravasation is clearly demarcated (asterisk) in the cerebral cortex along with the area of the evolving cortical contusion at the gray-white interface (white asterisk). BDA-3K leakage is also observed within the hippocampus and fimbria of the ipsilateral (I) hemisphere (#). In contrast, less BDA-3K and HRP leakage is observed at 7 days post-TBI. In contrast to HRP, significantly greater leakage of the small molecular weight tracer BDA-3K is seen in similar vulnerable brain regions. No leakage of HRP and BDA-3K was detected in the contralateral hemisphere (C).
At 7 days post-injury, the BDA-3K marker was observed mostly in ipsilateral cortical and hippocampal areas. Although patterns of vascular permeability were somewhat similar to that seen in the 3 day group, the intensities of tracer were less. In contrast to earlier periods, there was little evidence of HRP cortical permeability seen at earlier time points (i.e., 4 h and 3 days). More focal patterns of vascular permeability were observed in hippocampal regions including the hippocampal fissure (Fig. 2).
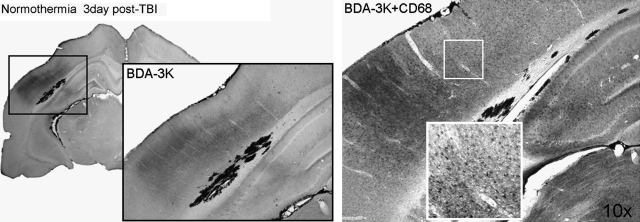
Areas of BDA-3K leakage also corresponded to increased numbers of CD68-positive cells. Figure 3 shows an area of small tracer leakage in a normothermic animal 3 days post-injury with double staining of CD68-positive cells. This spatial relationship may indicate that the infiltration of these cells into the damaged parenchyma correspond to areas of BBB breakdown at this time point.
FIG. 3.
Assessment of BDA-3K permeability corresponds to sites of increased CD68 immunoreactivity. CD68 immunoreactive cells are shown at 3 days after TBI. CD68 immunoreactive macrophages are associated with areas of vascular permeability. Higher magnification demonstrating the morphology of immunoreactive cells in cortical (insets) and hippocampal regions.
Cellular immunohistochemistry
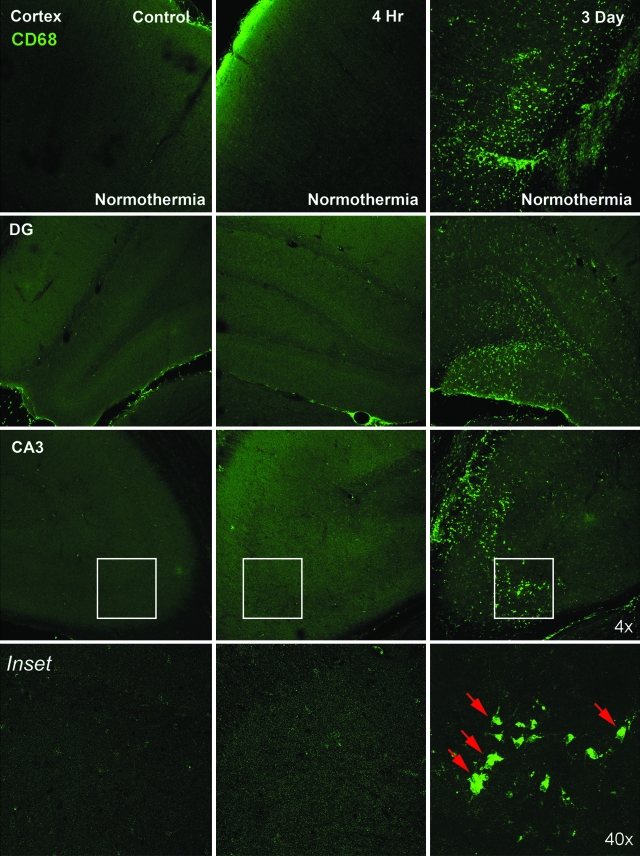
Increased CD68 immunoreactivity (green) was observed 3 days after TBI in normothermic animals (Fig. 4). Immunoreactive cells were most frequently observed in the area of cortical contusion, CA3 pyramidal cell region, dentate gyrus of the hippocampus, and thalamus. At 3 days after TBI, analysis of sections showed a spatial relationship between increased vascular permeability to the small molecular weight tracer and the appearance of CD68 immunoreactive cells. This was commonly seen in the cortical contusion area, CA1 hippocampus, and fimbria.
FIG. 4.
Macrophage extravasation is seen at 3 days post-injury. CD68 positive cells (green) were detected in the cortex, CA3, and dentate gyrus (DG) regions of hippocampus at 3 days after TBI. However, at 4 h, no CD68-positive cells were present in these regions, indicating that macrophage extravasation is a delayed event after trauma. Red arrows point to macrophages.
Quantitative assessment
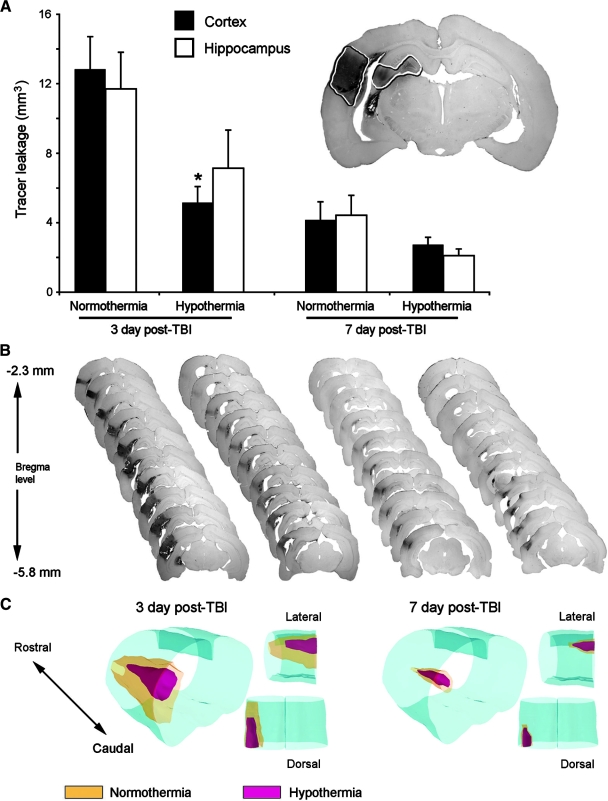
Volumetric analysis of brain sections corresponding to animals subjected to post-traumatic hypothermia showed decreased BDA-3K leakage at 3 and 7 days post-injury in the cortex and hippocampus compared to normothermic TBI rats (Fig. 5A). Two-way ANOVA was significant for group (p < 0.002), time (p < 0.001), and group × time (p < 0.02) in the cortex. Further post hoc analysis of the cortical region showed significant differences (p < 0.05) between the normothermia and hypothermia treated animals at 3 days post-TBI. Hypothermia appeared to blunt the leakage of the small molecular tracer compared to that in normothermic animals. There was a trend for this to occur at 7 days as well, but this was not significant. Analysis of the BBB leakage in the hippocampus was also significant for time (p < 0.001) but not for group (p = 0.06). However, there was a trend for the hypothermia treated animals to show a decrease in the amount of BBB leakage compared to that of normothermia animals within the hippocampus.
FIG. 5.
Post-traumatic hypothermia reduces BBB permeability changes seen after moderate TBI. (A) At 3 and 7 days after TBI, post-traumatic hypothermia significantly reduces the volume of BDA-3K protein extravasation in the ipsilateral rat cortex and hippocampus. (B) Representative serial brain sections stained with DAB showing BBB permeability alterations at 3 days (first and second columns) and 7 days (third and fourth columns) in normothermia (left) and hypothermia (right) animals. (C) 3-D reconstruction of serial sections shown in B. Red, hypothermia; yellow, normothermia. Data are presented as mean ± SEM; *p < 0.05 compared to normothermia for the respective time point and brain region; n = 5 per group.
Figure 5B shows representative serial sections analyzed for tracer leakage from bregma levels −2.3 to −5.8 mm. In normothermic animals 3 days post-trauma, there is a significant tracer leakage compared to that in hypothermia-treated animals. Figure 5C shows a three-dimensional comparison of the effects of hypothermia on BBB permeability in the cortex, where hypothermia (red) decreases BBB permeability compared to normothermia (yellow). Assessment of the hemisphere contralateral to injury did not show any alterations in BBB permeability, similar to that in sham-operated animals (data not shown).
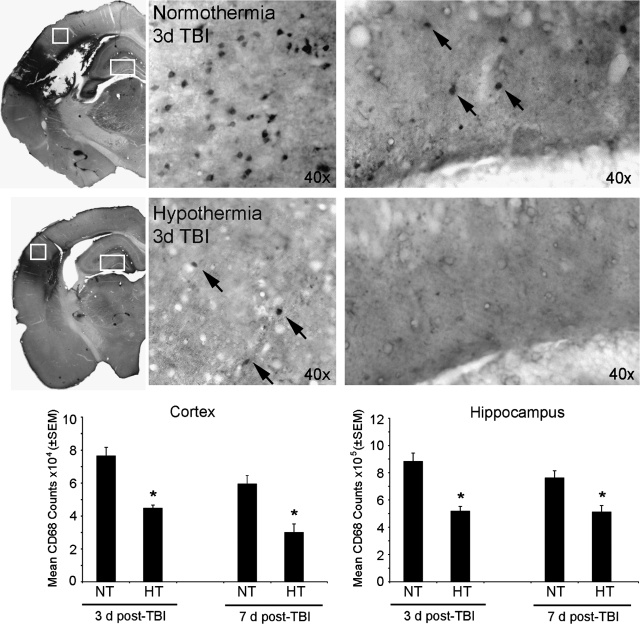
Quantitative cell counts in the cerebral cortex and hippocampus for CD68 immunoreactive cells also showed a significant decrease in the number of infiltrating macrophages in the cortex and hippocampus compared to that in normothermic TBI animals (Fig. 6). Two-way ANOVA was significant for group (p < 0.001) and time (p < 0.04) in the cortical CD68 cell counts. Post hoc analysis demonstrated a temporal decline in the number of infiltrating cells along with an overall effect of hypothermia reducing this infiltration as well within each time point. Two-way ANOVA was also significant for group (p < 0.001) in the hippocampus, with further post hoc analysis showing a similar decrease in the number of CD68-positive cells due to hypothermia treatment within each time point.
FIG. 6.
Post-traumatic hypothermia decreases the number of infiltrating CD68-positive cells in the cortex and hippocampus. Stereological analyses of cortical and hippocampal sections stained with anti-CD68 antibody indicate that hypothermia significantly reduces the number of macrophages compared to that in normothermic controls at 3 and 7 days post-TBI. Data are presented as mean ± SEM; *p < 0.05 compared to normothermia; n = 5 per group. Arrows indicate CD68-positive cells. NT, normothermia; HT, hypothermia.
Discussion
In the present study, evidence for increased vascular permeability was seen at both early (4 h and 3 days) and later (7 days) time periods after moderate FP brain injury. At early post-traumatic time periods, both large and small molecular weight tracers could be seen leaking into the brain parenchyma at sites of primary vascular injury, including the cortical contusion, CA3 region of hippocampus, and fimbria. However, at later post-traumatic periods, only the small molecular weight tracer could be seen gaining access to extravascular spaces. Thus, in this TBI model that produces both focal and diffuse brain pathology (Dietrich et al., 1994a), acute as well as long-lasting alterations in vascular permeability may contribute to the pathogenesis of this traumatic insult. Associated with later-occurring areas of increased vascular permeability was the accumulation of CD68 immunoreactive inflammatory cells. This finding is in contrast to previous data showing that early BBB leakage is not related to white blood cell adherence (Hartl et al., 1997). Because inflammatory cells can be a significant source of proinflammatory cytokines and other potentially toxic molecules (Schoettle et al., 1990; Allan and Rothwell, 2005), this is an important secondary injury mechanism that may be targeted for therapeutic interventions. Indeed, therapeutic hypothermia reduced late-occurring (3 days) vascular permeability changes and the infiltration of perivascular CD68 immunoreactive macrophages (3 and 7 days). The lack of a significant difference in BBB damage at 7 days (Fig. 5) between normothermic and hypothermic groups may be due to the methods of permeability quantification. We did not measure amounts of tracer in the blood but did appreciate that the intensity of the leakage is not uniform in the area being evaluated. Nevertheless, taken together, these findings support and extend previous studies that have assessed the effects of post-traumatic hypothermia on vascular permeability and inflammatory processes after brain injury. In contrast to previous studies, the current BBB study evaluated the consequences of mild hypothermia initiated in the post-injury setting on the permeability changes to both large and small tracers at early and late time points.
Although previous studies have used relatively large tracers to assess patterns of vascular permeability (Dietrich et al., 1994b; Smith and Hall, 1996), few studies have determined whether these vascular permeabilities remain at longer post-traumatic periods. In a recent BBB study, Habgood and colleagues (2007) emphasized that smaller tracer molecules were still able to enter the brain as late as 4 days in a model of local cortical damage. This finding supports a period of potential secondary damage from barrier disruption extending for longer periods than previously thought. Indeed, in the present study, we found that up to 7 days post-injury, permeability changes to the small molecular weight BDA-3K were observed within several regions of the traumatized hemisphere. Importantly, post-traumatic hypothermia while reducing early leakage, also reduced later-occurring permeability abnormalities as demonstrated by the use of BDA-3K. Thus, a relatively restricted period of post-traumatic hypothermia (4 h), in addition to targeting early vascular perturbations, may also be effective in reducing later-occurring vascular changes that could also contribute to secondary injury mechanisms.
Post-traumatic inflammatory processes are felt to participate in the pathophysiology of TBI (Toulmond and Rothwell, 1995). Activation of microglia and astrocytes and the infiltration of circulating monocytes can be important sources of toxic substances including proinflammatory cytokines (Kumar and Evans, 1997; Holmin and Mathiesen, 1999; Block et al., 2007). Increased vascular permeability can also lead to increased edema and breakdown of the extracellular matrix leading to progressive vascular injury. Previous studies have demonstrated a temporal pattern of inflammatory processes including the accumulation of neutrophils and macrophages and microglial activation in this TBI model (Soares et al., 1995; Whalen et al., 1997; Stahel et al., 2000). Our present findings show that post-traumatic hypothermia decreases the number of infiltrating CD68 immunoreactive leukocytes at sites of altered vascular permeability at both 3 and 7 days post-injury. Because post-traumatic hypothermia only significantly reduced BBB permeability at the 3 day period, these results may emphasize a role of leukocytes and proteases in vascular damage at specific post-injury time periods. Alternatively, a longer cooling period than used in the present study may be required to target these later-occurring vascular perturbations. Although, a temporal and spatial relationship between BBB leakage and CD68-positive macrophage infiltration was shown in this study, additional experiments will be required to directly evaluate cause-and-effect relationships between these two vascular perturbations after trauma.
Early alterations in vascular permeability result from mechanical damage to endothelial cell membranes, resulting in bleeding and the leakage of large tracers such as Evans Blue and HRP (Dietrich et al, 1994b). Alterations in vascular permeability can also result from the hyperactivation of matrix metalloproteinases (MMP), a family of extracellular zinc- and calcium-dependent proteases that degrade the extracellular matrix and other extracellular proteins (Romanic et al., 1998; Gidday et al., 2005). Activation of MMPs is essential for remodeling of the extracellular tissue space, morphogenesis, and wound healing (Page-McCaw et al., 2007). However, excessive proteolytic activity with MMPs may also be detrimental in degrading components of the extracellular matrix, leading to progressive microvascular damage including hemorrhage (Rosenberg and Navratil, 1997; Gidday et al., 2005). Because altered BBB integrity can lead to activation of inflammatory processes and subsequent edema formation, pharmacological approaches including inhibition of MMPs as well as the testing of MMP-9 knockout mice (Asahi et al., 2001) decrease the incidence of BBB permeability, cerebral infarction, and incidences of hemorrhage. Because MMPs play a dominant role in the transmigration of neutrophils and other circulating leukocytes, strategies targeting the vascular consequences of TBI, including the use of therapeutic hypothermia, have been investigated (Dietrich et al., 1994a; Chatzipanteli et al., 2000). In this regard, previous studies using the same trauma model have shown that post-traumatic hypothermia reduces the expression of MMP-9 (Truettner et al., 2005). In the present study, post-traumatic hypothermia may therefore be reducing both the early- and the late-occurring BBB changes and thereby inflammatory cell recruitment by MMP-9 dependent mechanisms, as well. Whether the late accumulation of invading macrophages is responsible or a consequence of the permeability changes remains to be determined.
In addition to reducing the infiltration of blood-borne monocytes, hypothermia may also affect the endogenous inflammatory response in models of trauma. Previous studies have shown that following injury, microglial activation, for example, can be documented both biochemically and immunocytochemically (Lu et al., 2001; Koshinaga et al., 2007). These endogenous cells are an important component of the immune response to injury and a source of a variety of chemokines and cytokines (Raivich and Banati, 2004; Block et al., 2007). In a previous study, post-traumatic hypothermia-reduced microglia activation after brain injury (Kumar and Evans, 1997). In regards to BBB changes, some studies have suggested that factors released from extravasated plasma constituents can lead to microglial activation (Lu et al., 2001). Thus, the benefits of post-traumatic hypothermia on BBB integrity may be targeting both endogenous, as well as exogenous inflammatory processes.
In conclusion, this study provides novel information regarding both the acute as well as long-term cerebrovascular consequences of TBI. This study used both large and small molecular tracers to demonstrate that permeability alterations were present days after moderate FP injury. Also, post-traumatic hypothermia reduced late-occurring permeability changes associated with the infiltration of CD68 immunoreactive macrophages at specific post-injury periods. These studies support the concept that early hypothermia treatment may be protecting the post-traumatic brain by targeting both early and more delayed vascular and inflammatory processes.
Acknowledgments
This work was supported by NIH grants (nos. NS030291 and NS42133). We wish to thank Coleen Atkins for scientific discussions, Beata Frydel for helping with 3-D reconstructions, and Jeremy Lytle for editorial assistance in manuscript preparation.
Author Disclosure Statement
The authors declare no financial interest related to the materials and methods presented in this manuscript.
References
- Aibiki M. Maekawa S. Ogura S. Kinoshita Y. Kawai N. Yokono S. Effect of moderate hypothermia on systemic and internal jugular plasma IL-6 levels after traumatic brain injury in humans. J. Neurotrauma. 1999;16:225–232. doi: 10.1089/neu.1999.16.225. [DOI] [PubMed] [Google Scholar]
- Allan S.M. Rothwell N.J. Inflammation in central nervous system injury. Philos. Trans. R. Soc. Lond. Biol. Sci. 2005;358:1669–1677. doi: 10.1098/rstb.2003.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arican N. Kaya M. Yorulman C. Kalayci R. Ince H. Kucuk M. Fincanci S.K. Elmas I. Effect of hypothermia on blood-brain barrier permeability following traumatic brain injury in chronically ethanol-treated rats. Int. J. Neurosci. 2006;116:1249–1261. doi: 10.1080/00207450600550303. [DOI] [PubMed] [Google Scholar]
- Asahi M. Wang X. Mori T. Sumii T. Jung J.C. Moskowitz M.A. Fini M.E. Lo E.H. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J. Neurosci. 2001;21:7724–7732. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block M.L. Zecca L. Hong J.S. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Neuroscience. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Chatzipanteli K. Alonso O.F. Kraydieh S. Dietrich W.D. Importance of post-traumatic hypothermia and hypothermia on the inflammatory response after fluid-percussion brain injury: biochemical and immunocytochemical studies. J. Cereb. Blood Flow Metab. 2000;20:531–542. doi: 10.1097/00004647-200003000-00012. [DOI] [PubMed] [Google Scholar]
- Dietrich W.D. Alonso O. Busto R. Globus M.Y. Ginsberg M.D. Post-traumatic brain hypothermia reduces histopathological damage following concussive brain injury in the rat. Acta Neuropathol. (Berlin) 1994a;87:250–258. doi: 10.1007/BF00296740. [DOI] [PubMed] [Google Scholar]
- Dietrich W.D. Alonso O. Halley M. Early microvascular and neuronal consequences of traumatic brain injury: a light and electron microscopic study in rats. J. Neurotrauma. 1994b;11:289–301. doi: 10.1089/neu.1994.11.289. [DOI] [PubMed] [Google Scholar]
- Dietrich W.D. Bramlett H.M. Hyperthermia and central nervous system injury. In: H.S. Sharma., editor. Progress in Brain Research: Neurobiology of Hyperthermia. Vol. 162. Academic/Elsevier; New York: 2007. pp. 201–217. [DOI] [PubMed] [Google Scholar]
- Dietrich W.D. Busto R. Globus M.Y. Ginsberg M.D. Brain damage and temperature: cellular and molecular mechanisms. In: B. Siesjo., editor; T. Wieloch., editor. Advances in Neurology, Cellular and Molecular Mechanisms of Ischemic Brain Damage. Vol. 71. Lippincott-Raven; Philadelphia: 1996. pp. 177–198. [PubMed] [Google Scholar]
- Gidday J.M. Gasche Y.G. Copin J.C. Shah A.R. Perez R.S. Shapiro S.D. Chan P.H. Park T.S. Leukocyte-derived matrix metalloproteinase-9 mediates blood-brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. Am. J. Physiol. Heart Circ. Physiol. 2005;289:H558–568. doi: 10.1152/ajpheart.01275.2004. [DOI] [PubMed] [Google Scholar]
- Goss J.R. Styren S.D. Miller P.D. Kochanek P.M. Palmer A.M. Marion D.W. DeKosky S.T. Hypothermia attenuates the normal increase in interleukin 1β RNA and nerve growth factor following traumatic brain injury in the rat. J. Neurotrauma. 1995;12:159–167. doi: 10.1089/neu.1995.12.159. [DOI] [PubMed] [Google Scholar]
- Habgood M.D. Bye N. Dziegielewska K.M. Ek C.J. Lane M.A. Potter A. Morganti-Kossmann C.M. Saunders N.R. Changes in blood-brain barrier permeability to large and small molecules following traumatic brain injury in mice. Eur. J. Neurosci. 2007;25:231–238. doi: 10.1111/j.1460-9568.2006.05275.x. [DOI] [PubMed] [Google Scholar]
- Hartl R. Medary M. Ruge M. Arfors K.E. Ghajar J. Blood-brain barrier breakdown occurs early after traumatic brain injury and is not related to white blood cell adherence. Acta Neurochir. 1997;Suppl. 70:240–242. doi: 10.1007/978-3-7091-6837-0_74. [DOI] [PubMed] [Google Scholar]
- Holmin S. Mathiesen T. Long-term intracerebral inflammatory response after experimental focal brain injury in rat. Neuroreport. 1999;10:1889–1891. doi: 10.1097/00001756-199906230-00017. [DOI] [PubMed] [Google Scholar]
- Jiang J.Y. Lyeth B.G. Kapasi M.Z. Jenkins L.W. Povlishock J.T. Moderate hypothermia reduces blood-brain barrier disruption following traumatic brain injury in the rat. Acta Neuropathol. 1992;84:495–500. doi: 10.1007/BF00304468. [DOI] [PubMed] [Google Scholar]
- Kinoshita K. Chatzipanteli K. Alonso O.F. Howard M. Dietrich W.D. The effect of brain temperature on hemoglobin extravasation after traumatic brain injury. J. Neurosurg. 2002a;97:945–953. doi: 10.3171/jns.2002.97.4.0945. [DOI] [PubMed] [Google Scholar]
- Kinoshita K. Chatzipanteli K. Vitarbo E. Truettner J.S. Alonso O.F. Dietrich W.D. Interleukin-1β messenger ribonucleic acid and protein levels after fluid percussion brain injury in rats: the importance of injury severity and brain temperature. Neurosurgery. 2002b;51:195–203. doi: 10.1097/00006123-200207000-00027. [DOI] [PubMed] [Google Scholar]
- Korn A. Golan H. Melamed I. Pascual-Marqui R. Friedman A. Focal cortical dysfunction and blood-brain barrier disruption in patients with postconcussion syndrome. J. Clin. Neurophysiol. 2005;22:1–9. doi: 10.1097/01.wnp.0000150973.24324.a7. [DOI] [PubMed] [Google Scholar]
- Koshinaga M. Suma T. Fukushima M. Tsubol I. Aizawa S. Katayama Y. Rapid microglial activation induced by traumatic brain injury is independent of blood brain barrier disruption. Histol. Histopathol. 2007;22:129–135. doi: 10.14670/HH-22.129. [DOI] [PubMed] [Google Scholar]
- Kumar K. Evans A.T. Effect of hypothermia on microglial reaction in ischemic brain. Clin. Neurosci. Neuropath. 1997;8:947–950. doi: 10.1097/00001756-199703030-00026. [DOI] [PubMed] [Google Scholar]
- Lu J. Moochhala S. Kaur C. Ling E.A. Cellular inflammatory response associated with breakdown of the blood-brain barrier after closed head injury in rats. J. Neurotrauma. 2001;18:399–408. doi: 10.1089/089771501750170976. [DOI] [PubMed] [Google Scholar]
- Lucas S.M. Rothwell N.J. Gibson R.M. The role of inflammation in CNS injury and disease. Brit. J. Pharmacol. 2006;147:S232–240. doi: 10.1038/sj.bjp.0706400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita Y. Bramlett H.M. Alonso O.F. Dietrich W.D. Posttraumatic hypothermia is neuroprotective in a model of traumatic brain injury complicated by a secondary hypoxic insult. Crit. Care Med. 2001;29:2060–2066. doi: 10.1097/00003246-200111000-00004. [DOI] [PubMed] [Google Scholar]
- Morganti-Kossmann M.C. Bye N. Nguyen P. Kossmann T. Influence of brain trauma on blood-brain barrier properties. In: E. De Vries., editor; A. Prat., editor. The Blood-Brain Barrier and Its Microenvironment. Taylor & Francis; New York: 2005. pp. 457–480. [Google Scholar]
- Page-McCaw A. Ewald A.J. Werb Z. Matrix metalloproteinases and the regulation of tissue remodeling. Nat. Rev. Mol. Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povlishock J.T. Becker D.P. Sullivan H.G. Miller J.D. Vascular permeability alterations to horseradish peroxidase in experimental brain injury. Brain Res. 1978;22:223–239. doi: 10.1016/0006-8993(78)90404-3. [DOI] [PubMed] [Google Scholar]
- Raivich G. Banati R. Brain microglia and blood-derived macrophages: molecular profiles and functional roles in multiple sclerosis and animal models of autoimmune demyelinating disease. Brain Res. Brain. Res. Rev. 2004;46:261–281. doi: 10.1016/j.brainresrev.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Romanic A.M. White R.F. Arleth A.J. Ohlstein E.H. Barone F.C. Matrix metalloproteinase expression increases after cerebral focal ischemia in rats: inhibition of matrix metalloproteinase-9 reduces infarct size. Stroke. 1998;29:1020–1030. doi: 10.1161/01.str.29.5.1020. [DOI] [PubMed] [Google Scholar]
- Rosenberg G.A. Navratil M. Metalloproteinase inhibition blocks edema in intracerebral hemorrhage in the rat. Neurology. 1997;48:921–926. doi: 10.1212/wnl.48.4.921. [DOI] [PubMed] [Google Scholar]
- Schoettle R.J. Kochanek P.M. Magargee M.J. Uhl M.W. Nemoto E.M. Early polymorphonuclear leukocyte accumulation correlates with the development of posttraumatic cerebral edema in rats. J. Neurotrauma. 1990;7:207–217. doi: 10.1089/neu.1990.7.207. [DOI] [PubMed] [Google Scholar]
- Smith J.L. Hall E.D. Mild pre- and posttraumatic hypothermia attenuates blood-brain barrier damage following controlled cortical impact injury in the rat. J. Neurotrauma. 1996;13:1–9. doi: 10.1089/neu.1996.13.1. [DOI] [PubMed] [Google Scholar]
- Soares H.D. Hicks R.R. Smith D. McIntosh T.K. Inflammatory leukocyte recruitment and diffuse neuronal degradation are separate pathological processes resulting from traumatic brain injury. J. Neurosci. 1995;15:8223–8233. doi: 10.1523/JNEUROSCI.15-12-08223.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahel P.F. Shohami E. Younis F.M. Kariya K. Otto V.I. Lenzlinger P.M. Grosjean M.B. Eugster H.P. Trentz O. Kossmann T. Morganti-Kossmann M.C. Experimental closed head injury: analysis of neurological outcome, blood-brain barrier dysfunction, intra-cranial neutrophil infiltration, and neuronal cell death in mice deficient in genes for pro-inflammatory cytokines. J. Cereb. Blood Flow Metab. 2000;20:369–380. doi: 10.1097/00004647-200002000-00019. [DOI] [PubMed] [Google Scholar]
- Sutcliffe I.T. Smith H.A. Stanimirovic D. Hutchison J.S. Effects of moderate hypothermia on IL-1β induced leukocyte rolling and adhesion in pial micro-circulation of mice and on proinflammatory gene expression in human cerebral endothelial cells. J. Cereb. Blood Flow Metab. 2001;21:1310–1319. doi: 10.1097/00004647-200111000-00007. [DOI] [PubMed] [Google Scholar]
- Tanno H. Nockels R.P. Pitts L.H. Noble L.J. Breakdown of the blood-brain barrier after fluid percussive brain injury in the rat. Part 1: Distribution and time course of protein extravasation. J. Neurotrauma. 1992;9:21–32. doi: 10.1089/neu.1992.9.21. [DOI] [PubMed] [Google Scholar]
- Tomkins O. Shelef I. Kaizerman I. Misk A. Afawi Z. Eliushin A. Gidon M. Cohen A. Zumsteg D. Friedman A. Blood-brain barrier disruption in post-traumatic epilepsy. J. Neurol. Neurosurg. Psychiatry. 2008;79:774–777. doi: 10.1136/jnnp.2007.126425. [DOI] [PubMed] [Google Scholar]
- Toulmond S. Rothwell N.J. Interleukin-1 receptor antagonist inhibits neuronal damage caused by fluid percussion injury in the rat. Brain Res. 1995;671:261–266. doi: 10.1016/0006-8993(94)01343-g. [DOI] [PubMed] [Google Scholar]
- Truettner J.S. Alonso O.F. Dietrich W.D. Influence of therapeutic hypothermia on matrix metalloproteinase activity after traumatic brain injury in rats. J. Cereb. Blood Flow Metab. 2005;25:1505–1516. doi: 10.1038/sj.jcbfm.9600150. [DOI] [PubMed] [Google Scholar]
- Ueda Y. Wei E.P. Kontos H.A. Suehiro E. Povlishock J.T. Effects of delayed, prolonged hypothermia on the pial vascular response after traumatic brain injury in rats. J. Neurosurg. 2003;99:899–906. doi: 10.3171/jns.2003.99.5.0899. [DOI] [PubMed] [Google Scholar]
- Utagawa A. Bramlett H.M. Daniels L. Lotocki G. Dekaban G.A. Weaver L.C. Dietrich W.D. Transient blockage of the CD11d/CD18 integrin reduces contusion volume and macrophage infiltration after traumatic brain injury in rats. Brain Res. 2008;1207:155–163. doi: 10.1016/j.brainres.2008.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen M.J. Carlos T.M. Clark R.S. Marion D.W. DeKosky S.T. Heineman S. Schiding J.K. Memarzadeh F. Kochanek P.M. The effect of brain temperature on acute inflammation after traumatic brain injury in rats. J. Neurotrauma. 1997;14:561–572. doi: 10.1089/neu.1997.14.561. [DOI] [PubMed] [Google Scholar]
- Zilles L. The Cortex of the Rat. A Stereotaxic Atlas. Springer; New York: 1985. [Google Scholar]