Abstract
This study determined the effects of intraperitoneal sodium pyruvate (SP) treatment on the levels of circulating fuels and on cerebral microdialysis levels of glucose (MDglc), lactate (MDlac), and pyruvate (MDpyr), and the effects of SP treatment on neuropathology after left cortical contusion injury (CCI) in rats. SP injection (1000 mg/kg) 5 min after sham injury (Sham-SP) or CCI (CCI-SP) significantly increased arterial pyruvate (p < 0.005) and lactate (p < 0.001) compared to that of saline-treated rats with CCI (CCI-Sal). Serum glucose also increased significantly in CCI-SP compared to that in CCI-Sal rats (p < 0.05), but not in Sham-SP rats. MDpyr was not altered after CCI-Sal, whereas MDlac levels within the cerebral cortex significantly increased bilaterally (p < 0.05) and those for MDglc decreased bilaterally (p < 0.05). MDpyr levels increased significantly in both Sham-SP and CCI-SP rats (p < 0.05 vs. CCI-Sal) and were higher in left/injured cortex of the CCI-SP group (p < 0.05 vs. sham-SP). In CCI-SP rats the contralateral MDlac decreased below CCI-Sal levels (p < 0.05) and the ipsilateral MDglc levels exceeded those of CCI-Sal rats (p < 0.05). Rats with a single low (500 mg/kg) or high dose (1000 mg/kg) SP treatment had fewer damaged cortical cells 6 h post-CCI than did saline-treated rats (p < 0.05), but three hourly injections of SP (1000 mg/kg) were needed to significantly reduce contusion volume 2 weeks after CCI. Thus, a single intraperitoneal SP treatment increases circulating levels of three potential brain fuels, attenuates a CCI-induced reduction in extracellular glucose while increasing extracellular levels of pyruvate, but not lactate, and can attenuate cortical cell damage occurring within 6 h of injury. Enduring (2 week) neuronal protection was achieved only with multiple SP treatments within the first 2 h post-CCI, perhaps reflecting the need for additional fuel throughout the acute period of increased metabolic demands induced by CCI.
Key words: cerebral microdialysis, glucose, lactate, neural injury, pyruvate, serum, traumatic brain injury
Introduction
It is now well established that traumatic brain injury (TBI) results in a dynamic cascade of physiological, biochemical, and cellular alterations that can impact cerebral metabolism and energy production. TBI-induced glutamate release and ionic fluxes (Faden et al., 1989; Katayama et al., 1990, 1995; Yoshino et al., 1992; Fineman et al., 1993; Palmer et al., 1993; Rose et al., 2002) initiate increased metabolic demands, reflected in early, transient increases in cerebral metabolic rates for glucose (Sunami et al., 1989b; Yoshino et al., 1991; Kawamata et al., 1992; Sutton et al., 1994; Lee et al., 1999) and increased anaerobic glycolysis, reflected by an increase in extracellular and tissue lactate levels (Kawamata et al., 1995; Dhillon et al., 1997; Bartnik et al., 2005, 2007b). TBI is also known to induce mitochondrial dysfunction (Vink et al., 1990; Verweij et al., 1997; Xiong et al., 1997), increase free radical production and oxidative stress (Hall et al., 1993, 2004; Marklund et al., 2001; Tavazzi et al., 2005), induce zinc release and accumulation (Suh et al., 2000, 2006; Hellmich et al., 2004, 2007), and activate poly(ADP-ribose) polymerases (PARP) (Laplaca et al., 1999; Besson et al., 2003; Satchell et al., 2003; Clark et al., 2007). All of the latter inter-related effects can have diverse inhibitory effects on multiple components of metabolic pathways and hence impact the injured brain's ability to produce energy sufficient to meet ongoing metabolic demands.
Glucose metabolism in particular will be altered by zinc- or oxidative stress-induced inhibition of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) activity, and/or by zinc- or PARP activation-induced reductions in nicotinamide adenine dinucleotide (NAD+), the co-factor for GAPDH (Sheline et al., 2000; Satchell et al., 2003; Clark et al., 2007). Therefore, although there is a variable period of increased energy demand in the brain after TBI (Sunami et al., 1989a; Nilsson et al., 1994; Bergsneider et al., 1997; Vespa et al., 1999; Kubota et al., 2003; D'Ambrosio et al., 2004), the pathophysiology of TBI can interfere with glucose metabolism, or shunt glucose to pathways involved in combating oxidative damage and nucleotide production (Bartnik et al., 2005, 2007b; Dusick et al., 2007). The alternative fates or uses of glucose post-TBI combined with increased metabolic demands of injured tissue in the face of reduced cerebral blood flow and supply of metabolic substrates may lead to a state of energy crisis and eventual neuronal death.
Recently there has been increasing interest and research into the possibility that provision of exogenous fuels, the monocarboxylates in particular, can be used to augment cerebral metabolism and/or improve outcomes after TBI. For example, it is reported that intravenous lactate accumulates in extracellular fluid and attenuates TBI-induced reductions in extracellular glucose (Chen et al., 2000a, 2000b), reduces TBI-induced cognitive deficits (Rice et al., 2002), attenuates reductions in cortical adenosine triphosphate (ATP) levels (Holloway et al., 2007), and improves oxygen consumption after TBI (Levasseur et al., 2006). Exogenous lactate administered via cerebral microdialysis was also reported to reduce glutamate-induced neuronal damage (Ros et al., 2001), and inhibition of monocarboxylate transporters (MCT) to block lactate uptake was reported to increase neuronal damage after cerebral ischemia (Schurr et al., 2001). Members of our lab have reported that intravenous β-hydroxybutyrate is taken up and oxidized in the adult TBI brain (Prins et al., 2004), and provision of a ketogenic diet after TBI produces an age-dependent reduction in cortical injury volume (Prins et al., 2005). Other exogenous fuels need to be explored as possible therapeutic interventions since conversion of lactate to pyruvate in the lactate dehydrogenase (LDH) reaction requires NAD+ (pyruvate + NADH ↔ lactate + NAD+), the supply of which is already diminished after TBI (Satchell et al., 2003; Clark et al., 2007), and the efficacy of ketone bodies may be limited to younger victims of TBI (Prins et al., 2005; Prins, 2008). Pyruvate is an appealing candidate for such investigations, as it can readily cross the blood-brain barrier (Miller and Oldendorf, 1986) and enter the tricarboxylic acid (TCA) cycle to serve as a cellular energy substrate (Tsacopoulos and Magistretti, 1996; Gonzalez et al., 2005).
Under cell culture conditions exogenous pyruvate appears to protect cells via multiple modes of action. Energy substrates that regenerate NAD+ such as pyruvate and oxaloacetate, but not lactate or β-hydroxybutyrate, are reported to attenuate zinc-induced cell death (Sheline et al., 2000; Lee et al., 2001; Yoo et al., 2004). In vitro studies have shown that pyruvate may reduce cell damage by acting as an efficient fuel source (O'Donnell-Tormey et al., 1987; Maus et al., 1999; Mazzio and Soliman, 2003) via its effects as an anti-oxidant or free radical scavenger (Desagher et al., 1997; Mazzio and Soliman, 2003) and/or its ability to counteract NAD+ reductions and/or glycolytic inhibition after injury-induced activation of PARP (Ying et al., 2003, 2005; Zeng et al., 2007).
In vivo studies in models of hemorrhagic shock have reported that intravenous infusion of solutions containing sodium pyruvate (SP) improved multiple cerebral energetic parameters, prevented hemorrhage-induced reductions in NAD+ and PARP cleavage, attenuated lipid peroxidation, and improved survival rates (Mongan et al., 1999, 2001, 2003; Slovin et al., 2001). The majority of in vivo studies on exogenous SP treatment, however, have been conducted in rodents and administered SP via the intraperitoneal (i.p.) route. In the initial report on this treatment, a single injection of SP (500 or 1000 mg/kg i.p.) after onset of reperfusion significantly decreased mortality, neuronal zinc accumulation, and neuronal cell death in a rat model of transient global ischemia (Lee et al., 2001). Although a subsequent study found no benefits of SP treatment after permanent middle cerebral artery occlusion (Gonzalez-Falcon et al., 2003), significant neurological improvements or neuroprotective effects after one SP treatment have been reported in rodent models of transient or permanent ischemia (Yoo et al., 2004; Yi et al., 2007), quinolinic acid-induced injuries (Ryu et al., 2003, 2004), kainic acid-induced epilepsy (Kim et al., 2007), and hypoglycemia (Suh et al., 2005).
Unfortunately, prior studies of intraperitoneally administered SP have not determined the degree to which this drug is metabolized peripherally (e.g., due to hepatic metabolism or LDH conversion of pyruvate to lactate) or the brain levels of pyruvate that are achieved after SP injection. Therefore, the first aim of the current experiments was to determine the levels of circulating fuels as well as the extracellular levels of glucose, lactate, and pyruvate after a single SP injection in the rat. We then proceeded to test for neuroprotective effects of SP treatment after cortical contusion injury (CCI) in rats, determining both short-term (6 h) and long-term (2 week) histological outcomes in cortical tissue.
Methods
Drug preparation and experimental groups
The SP (P2256, Sigma, St. Louis, MO) was dissolved in sterile water (500 mg/mL, 4.5 M, pH 7.4) immediately prior to scheduled injections. Animals received an injection of SP (500 or 1000 mg/kg i.p.) or an injection of an osmolarity-matched NaCl solution (104.5 mg/kg i.p.) at times indicated below.
Fifteen adult male Sprague Dawley rats (350–460 g) were used to assess the effects of SP treatment (1000 mg/kg) on circulating levels of glucose, lactate, and pyruvate and on the extracellular levels, assessed via cerebral microdialysis (MD), of glucose (MDglc), lactate (MDlac), and pyruvate (MDpyr). These animals were randomly assigned to experimental conditions of sham injury with SP treatment (Sham-SP, n = 6), CCI with SP treatment (CCI-SP, n = 5), or CCI with saline treatment (CCI-Sal, n = 4).
Thirty-six adult male Sprague Dawley rats (350–460 g) were used to assess the effects of SP treatment (500 or 1000 mg/kg) on CCI-induced cortical damage. Fifteen randomly assigned rats with CCI were used to assess neuronal damage in the ipsilateral cortex at 6 h after saline treatment (CCI-Sal, n = 5), a low dose of SP (CCI-500, n = 5), or a high dose of SP (CCI-1000, n = 5). An additional 21 rats were randomized to groups used to assess the volume of cortical necrosis at 2 weeks after unilateral CCI. Three groups received a single post-injury injection of saline (CCI-Sal, n = 5), a low dose of SP (CCI-500, n = 5), or a high dose of SP (CCI-1000, n = 5). Based on data showing short-lived increases in blood levels of pyruvate after a single injection (Fig. 1), a group with three injections of high dose SP (CCI-3 × 1000, n = 6) was also included in the 2 week survival study. All procedures were approved by the UCLA Chancellor's Committee for Animal Research.
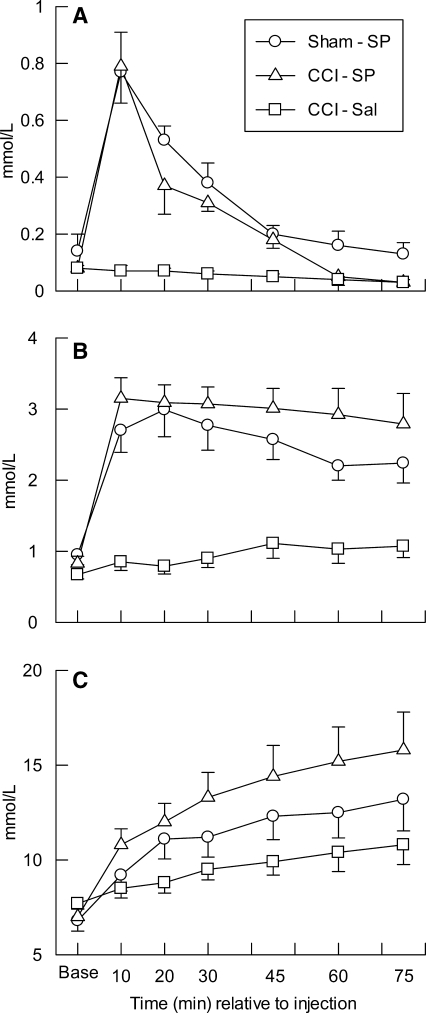
FIG. 1.
Mean (±SEM) arterial serum concentrations of pyruvate (A), lactate (B), and glucose (C) at baseline (Base) and from 10 to 75 min after injection of saline (-Sal) or sodium pyruvate (-SP, 1000 mg/kg) in rats with sham (Sham) or cortical contusion injury (CCI).
In vitro microdialysis probe calibration
Two CMA-10 MD probes (2 mm fiber length, MWCO =20 kDa, O.D. = 0.5 mm; CMA Microdialysis, Stockholm, Sweden) were used for each animal. These probes were infused with sterile 0.9% saline (2 μL/min flow rate) throughout all experimental procedures. All probes were calibrated for in vitro percent recovery (efficiency) prior to and after use in the animal by collecting a minimum of three 15 min dialysis samples as the probe was immersed into a solution containing known concentrations of glucose, lactate, and pyruvate (Calibration A solution, CMA Microdialysis). These in vitro samples and the Calibration A solution, as well as all MD samples collected from left and right hemispheres of each rat, were analyzed (in triplicate) for levels of MDglc (μmol/L), MDlac (μmol/L), and MDpyr (μmol/L) using a CMA-600 Analyzer (CMA Microdialysis). The mean percent recovery for each substrate in each probe was calculated from the ratio of calibration solution-dialysate concentrations. The dead volume of the probe outlet lines (4 μL) was also verified for each probe during the in vitro sampling periods.
Surgery, sampling, and injections for microdialysis procedures
Animals were anesthetized with isoflurane (3% in 100% O2 [1.5–2.0 L/min flow rate] for induction; 1.5–2% for maintenance), and when under a surgical level of anesthesia the animals received an application of ophthalmic ointment to both eyes. During all surgical procedures, body temperature was maintained at 37 ± 0.7°C using a thermostatically controlled heating pad (Harvard Apparatus, Edenbridge, KY). The femoral region was shaved and sterilized by repeated (3x) cleaning with betadine followed by 70% alcohol. A 1.5 cm skin incision was performed over the femoral triangle and the right femoral artery was isolated and catheterized with polyethylene (PE-50) tubing (Becton Dickinson, Franklin Lakes, NJ) for blood sampling (described below). Bupivacaine (0.05–0.07 mg/kg s.c.) was infiltrated into wound margins, and the femoral incision was sutured closed around the arterial line.
After shaving and sterilizing the scalp, the anesthetized animal was placed into a stereotaxic frame, a midline incision was made over the skull, and the skin, fascia, and temporal muscles were reflected bilaterally. Bupivacaine (0.05–0.07mg/kg s.c.) was also infiltrated into this wound margin. A 6 mm diameter circular craniotomy was made over the left parietal cortex, centered at 3 mm posterior and 4 mm lateral to bregma, and 1 mm diameter burr holes were made over the left and right parietal cortex (7.0 mm posterior to bregma ±3.5 mm from midline). After covering the craniotomy site with gel foam and incising the dura mater under each burr hole, two MD probes, mounted to a micromanipulator on the stereotaxic, were slowly lowered to a depth of 2.2 mm below the left and right surface of dura. Four 15 min dialysis samples were collected from each probe after insertion to allow for equilibration and collection of stable baseline measures. Aliquots of arterial blood samples were collected into lithium-heparin coated tubes (Beckman Coulter, Fullerton, CA) at the start and end of the baseline MD sampling and used to measure baseline pH and blood gases (pO2, pCO2, and HCO3, Chiron 238 pH/Blood Gas Analyzer; Ciba Corning Diagnostics, East Walpole, MA). After completion of baseline sampling, the MD probes were retracted from the brain and fibers were maintained in sterile saline during procedures to induce sham injury or CCI.
The CCI was induced using an electronically controlled, dual-stroke, pneumatic piston cylinder (Hydraulics Control, Emeryville, CA) that is mounted onto a stereotaxic micromanipulator, following previously published protocols (Sutton et al., 1993; Lee et al., 1999; Prins et al., 2004; Taylor et al., 2008). A 5 mm diameter flat-tipped impactor, angled 20–22° from vertical to be perpendicular to dura at the craniotomy site, was used to induce CCI to the left parietal cortex (20 psi, 2.37 m/s, 2.0 mm tissue compression for 250 ms). These parameters caused an injury characterized by the disruption/tearing of dura with mild-to-moderate tissue herniation and hematoma formation within the craniotomy site. After ensuring hemostasis, the injury site was again covered with gel foam. Sham injured controls had the gel foam removed and replaced in the craniotomy, but did not experience induction of the CCI.
Immediately after completion of sham injury or CCI, both MD probes were returned to their original locations in the brain and the animals were then injected with either SP (1000 mg/kg) or saline. Beginning at 2 min after this injection (to account for dead volume in outlet lines), dialysis samples were collected every 15 min over the ensuing 75 min. Arterial blood samples were collected at 30 and 75 min to measure post-injection pH and blood gases. Animals were euthanized via sodium pentobarbital (100 mg/kg i.p.) following the final blood and MD sample collection and brains were extracted. Frozen brains were sectioned coronally (20 μm) throughout the regions of probe placements, mounted on gelatinized slides, and stained in cresyl violet to enable determination of the MD probe tracts in left and right hemispheres.
Blood sampling and analyses
In addition to samples used to assess pH and blood gases, arterial blood samples were collected from all animals immediately prior to injury induction and again at 10, 20, 30, 45, 60, and 75 min post-injection. Plasma glucose (mmol/L) and lactate (mmol/L) concentrations in each of these samples were measured using an YSI 2700 Select Biochemistry Analyzer (Yellow Springs, OH). To assess arterial pyruvate levels, an aliquot of each baseline and post-injection arterial blood sample was immediately placed into a microcentrifuge tube containing perchloric acid (4°C), vortexed, centrifuged (3000 rpm for 30 s), and stored overnight at 4°C. The denatured blood sample was then neutralized (pH 7.4) by the addition of Trizma base (1.5 M) and KOH (10 N) and stored at −80°C. Batch analyses were performed, with each arterial sample assayed (in triplicate) for pyruvate in a pyruvate assay kit (BioVision Research Products, Mountain View, CA), following manufacturer instructions. The final concentration of pyruvate (mmol/L) was obtained after correction of values for dilution.
Surgery and injections for neuroprotection procedures
CCI was induced to the left parietal cortex as described above. At 5 min after induction of the CCI the rat was injected with either a 500 mg/kg or 1000 mg/kg dose of SP, or with saline. After ensuring hemostasis the scalp incision was sutured closed, wound margins were infiltrated with bupivacaine (0.1–0.14 mg/kg s.c.), and the animal was recovered from anesthesia. Following a 30 to 45 min period in a warm recovery cage, the animals were returned to their home cages. Animals assigned to the CCI-3 × 1000 group were administered a second and third injection of high-dose (1000 mg/kg) SP at 65 and 125 min after CCI, respectively. Those animals surviving for 2 weeks post-injury were returned to the animal vivarium.
Tissue preparation and histological analyses
At either 6 h or 2 weeks after CCI, animals in the histological studies were deeply anesthetized with sodium pentobarbital (100 mg/kg i.p.) and euthanized by transcardial perfusion with phosphate buffered saline (PBS; 0.9% saline in 0.1 M PB) followed by a paraformaldehyde solution (4%, in 0.1 M PB, pH 7.4). Brains were post-fixed in the same paraformaldehyde solution for 2 h and then placed in a solution of 30% sucrose (in 0.1 M PBS) overnight. Frozen brains were sectioned coronally (40 μm), saving one to three sequential sections every 400 μm, and mounting sections onto gelatinized slides. Three sets of coronal tissue sections were saved for the animals surviving for 6 h post-CCI. These tissue sections were stained for acid fuchsin (AF) and Fluoro-jade B (FJ), markers for neuronal damage (Auer et al., 1984; Schmued and Hopkins, 2000), and for cresyl violet to delineate areas with frank necrosis of tissue. A single set of coronal tissue sections were saved for rats surviving for 2 weeks, and these sections were stained for cresyl violet.
Cell counts 6 h after CCI
Microscopic analyses of tissue from rats surviving for 6 h were performed using a Leica microscope, equipped with brightfield and fluorescent illumination, which was interfaced with a computer using Stereo Investigator software (version 3.0, MicroBrightField Inc., Colchester, VT). After preliminary examinations of cresyl violet-stained tissue, the cortical tissue lying medial to the injury site was determined to be relatively intact (injury “penumbra”) and was thus selected for more systematic analyses. Both FJ- and AF-stained tissue sections at −1.6, −2.4, −3.2, −4.0, and −4.8 mm from bregma (Paxinos and Watson, 1986) were selected for counting of FJ-positive (epifluorescence at 480 nm excitation) or AF-positive (brightfield) neurons in the left/injured medial cortex. Two-dimensional contours encompassing all cortical tissue in the left cortical mantle from 1 to 2 mm left of midline and from cortical surface to the dorsal aspect of the corpus callosum were placed on each selected tissue section. Unbiased cell counts were made for all FJ-positive and AF-positive neurons within the contour areas by an experimenter blind to drug treatment conditions. Counts on each tissue section were expressed as number of cells/mm2, and counts for all sections were summed for each animal prior to calculating group averages.
Cortical tissue loss after CCI
Cresyl violet-stained tissue sections of the animals surviving for 2 weeks after CCI were digitally captured and areas of the contralateral and ipsilateral cortical mantle were measured using the public domain SCION/NIH Image 1.61 program (National Institutes of Health, Bethesda, MD). The areas of non-necrotic cortical tissue in 10 equally spaced sections from AP + 1.6 and −5.6 (Paxinos and Watson, 1986) were obtained, and the cortical tissue volume was calculated as previously described (Sutton et al., 1993). The percent tissue loss in the left/injured cortical mantle was calculated using the formula [100 − (ipsilateral/contralateral) × 100)], as previously described (Taylor et al., 2008).
Statistical analysis
Data for all parameters measured were expressed as group means ± standard error of the mean (SEM). Statistical analyses were conducted using SPSS-15 (SPSS Inc., Chicago, IL) software. Group data was analyzed using univariate or multivariate analysis of variance (ANOVA), with repeated measures when appropriate. Post hoc between groups comparisons were performed using the Tukey-Fisher least significant difference criterion and a paired t test was used for within group comparisons. Statistical significance (α) was set at p < 0.05 for all comparisons.
Results
Arterial pH and blood gases
Data for arterial pH and blood gases at baseline (pre-injury) and at 30 and 75 min after injection of SP or saline are shown in Table 1. ANOVAs on the pO2 and pCO2 data revealed no significant effects of group or time. The ANOVAs on pH and HCO3 data revealed significant between group effects (p < 0.01), significant effects of time (p < 0.001), and significant group × time interactions (p ≤ 0.01). Post hoc analyses revealed no group differences for pre-injection blood pH or HCO3 levels, and no change in pH or HCO3 from baseline to 30 or 75 min post-injection in the CCI-Sal group. Both the Sham-SP and CCI-SP groups had a significant increase in arterial pH and HCO3 levels from baseline to 30 and 75 min post-injection (p < 0.05).
Table 1.
Mean (±SEM) Arterial pH and Blood Gases in Sham or Cortical Contusion Injury Groups with Sodium Pyruvate or Saline Treatment
| |
Baseline |
30 min post-injection |
75 min post-injection |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sham-SP | CCI-SP | CCI-Sal | Sham-SP | CCI-SP | CCI-Sal | Sham-SP | CCI-SP | CCI-Sal | ||
| pH | 7.4 ± 0.02 | 7.4 ± 0.02 | 7.4 ± 0.01 | 7.5 ± 0.01* | 7.5 ± 0.02* | 7.4 ± 0.01 | 7.5 ± 0.01* | 7.5 ± 0.02* | 7.4 ± 0.01 | |
| pO2 | 549 ± 26 | 568 ± 16 | 516 ± 27 | 557 ± 21 | 556 ± 21 | 534 ± 16 | 546 ± 17 | 533 ± 18 | 521 ± 10 | |
| pCO2 | 48 ± 3 | 43 ± 2 | 46 ± 2 | 45 ± 3 | 44 ± 2 | 44 ± 1 | 46 ± 3 | 44 ± 2 | 45 ± 1 | |
| HCO3 | 27.0 ± 0.38 | 27.5 ± 0.73 | 27.4 ± 0.19 | 32.8 ± 1.65* | 34.2 ± 0.99* | 27.2 ± 0.31 | 35.1 ± 2.44* | 34.1 ± 1.15* | 27.0 ± 0.53 | |
p < 0.05 compared to baseline.
Arterial glucose, lactate, and pyruvate
Arterial pyruvate concentrations for the three groups of animals are shown in Figure 1A. One-way ANOVA revealed no significant differences in baseline pyruvate concentrations between groups. Repeated measures ANOVA on post-injection data indicated a significant between group effect (p < 0.001), a significant effect of time (p < 0.001), and a significant group × time interaction (p < 0.001). Post hoc analyses indicated that arterial pyruvate levels were significantly elevated above CCI-Sal concentrations in the Sham-SP (p < 0.001) and CCI-SP (p < 0.005) groups, but the pyruvate levels for Sham-SP and CCI-SP groups did not differ. The blood pyruvate levels were significantly increased above baseline levels through the first 30 min post-injection in the Sham-SP group (p < 0.01) and through the first 45 min post-injection in the CCI-SP group (p < 0.05). Concentrations of circulating pyruvate were significantly decreased below baseline levels at 75 min post-injection in the CCI-SP group (p < 0.05) and at 60 to 75 min post-injection in the CCI-Sal group (p < 0.05).
Arterial lactate concentrations for all animals are illustrated in Figure 1B. A one-way ANOVA indicated no significant group differences for the baseline concentrations of lactate. Repeated measures ANOVA indicated a significant between group effect (p < 0.001), a significant effect of time (p < 0.001), and a significant group × time interaction (p < 0.001). Post hoc analyses indicated that both Sham-SP and CCI-SP groups had significantly (p < 0.001) greater arterial lactate levels than did CCI-Sal animals. The blood lactate levels were significantly increased above baseline from 10 through 75 min post-injection in the Sham-SP group (p < 0.002) as well as in the CCI-SP group (p < 0.003). Post-injection lactate levels for Sham-SP and CCI-SP groups did not differ, indicating that the SP treatment was primarily responsible for the increase in arterial lactate, but a mild contribution of anesthesia or CCI to increasing lactate was suggested by the finding that lactate concentrations in the CCI-Sal group were significantly increased above baseline (p < 0.05) at 30, 45, and 75 min.
The baseline and post-injection arterial glucose concentrations for all animals are illustrated in Figure 1C, where it can be seen that serum glucose levels increased gradually over time in the CCI-Sal group and the glucose levels increased more rapidly and to a higher level in the Sham-SP and CCI-SP groups. One-way ANOVA indicated no significant group differences in glucose levels prior to induction of injury and SP or saline injection. Repeated measures ANOVA indicated no significant between group effect (p = 0.109), a significant effect of time (p < 0.001), and a significant group × time interaction (p < 0.005). Post hoc analyses indicated that the CCI-SP glucose levels were significantly increased relative to the CCI-Sal group (p < 0.05), but that the glucose levels in the Sham-SP group were not significantly different from either the CCI-Sal or the CCI-SP groups. The blood glucose levels were significantly increased above baseline from 10 through 75 min post-injection in the Sham-SP group (p < 0.004), the CCI-SP group (p < 0.005), and the CCI-Sal group (p < 0.05).
Cerebral microdialysis measures of glucose, lactate, and pyruvate
The in vitro percent recoveries (efficiency) for glucose in the MD probes were 13.7 ± 0.9% and 13.2 ± 0.8% prior to and after in vivo procedures, respectively. Corresponding values for lactate were 21.6 ± 1.2% (pre) and 21.4 ± 1.3% (post), while probes had 20.6 ± 1.3% (pre) and 21.1 ± 1.4% (post) recovery rates for pyruvate. Data for MDpyr were lost for one Sham-SP rat, due to unacceptable values (>25%) for the coefficient of variation calculated from mean ± standard deviation values on repeat analyses of the dialysis samples, but MDglc and MDlac in this subject were retained in the final data analyses. The pre-injury baseline dialysate concentrations, calculated as the average of the two 15 min samples immediately preceding MD probe removals, were 354.1 ± 21.8 μmol/L for glucose, 227.1 ± 15.2 μmol/L for lactate, and 26.9 ± 2.7 μmol/L for pyruvate. ANOVAs revealed no significant differences between groups or hemispheres and no significant group × hemisphere interactions for the baseline levels (μmol/L) of MDglc, MDlac, or MDpyr. All group data for MDglc, MDlac, and MDpyr measures, not corrected for percent recovery, were thereafter expressed as the percent change (increase or decrease) from mean baseline values to control for different recovery rates (range of 10 to 33%) in different probes.
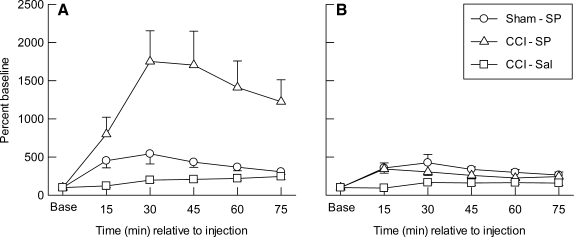
The percent baseline data for MDpyr in the ipsilateral (A) and contralateral (B) cortical samples are illustrated in Figure 2. It can be seen that mean MDpyr levels were at or above baseline values in both hemispheres, in all groups, and at all time points after sham injury or CCI. Repeated measures ANOVA indicated significant main effects for group (p <0.002), hemisphere (p < 0.002), and time (p < 0.001); significant effects for the group × hemisphere (p < 0.002), hemisphere ×time (p < 0.001) and group × time (p < 0.001) interactions; and a significant group × hemisphere × time interaction (p <0.001). In the left/injured cortex (Fig. 2A), there was a significant effect of group (p < 0.002), time (p < 0.001), and the group × time interaction (p < 0.001). Post hoc tests indicated that ipsilateral MDpyr was significantly higher in the CCI-SP group than in either the Sham-SP (p < 0.003) or CCI-Sal (p < 0.001) groups, but the post-injection increases in MDpyr seen in the Sham-SP group (Fig. 2A) were not significant compared to the CCI-Sal levels. The post-injection MDpyr values in the ipsilateral cortex were significantly increased above baseline at all sampling time points in the Sham-SP (p < 0.05) and CCI-SP groups (p < 0.05), but did not increase above baseline values in the CCI-Sal group. In the contralateral cortex (Fig. 2B) there was a significant effect of group (p < 0.004), time (p < 0.001), and the group × time interaction (p < 0.014), with higher levels of MDpyr occurring in the Sham-SP (p < 0.001) and CCI-SP (p < 0.015) groups compared to CCI-Sal animals. The contralateral MDpyr levels did not differ between Sham-SP and CCI-SP treatment groups. The post-injection MDpyr values in the contralateral cortex were also significantly increased above baseline at all sampling time points in the Sham-SP (p < 0.05) and CCI-SP groups (p < 0.02), but did not increase above baseline values in the CCI-Sal group.
FIG. 2.
Mean (±SEM) extracellular changes in concentrations of pyruvate, expressed as percent of baseline (Base), measured in microdialysis samples collected every 15 min from the ipsilateral (A) or contralateral (B) cerebral cortex after injection of saline (-Sal) or sodium pyruvate (-SP, 1000 mg/kg) in rats with sham (Sham) or left cortical contusion injury (CCI).
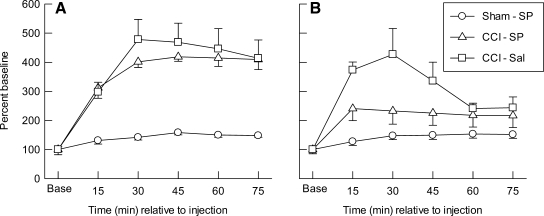
The percent baseline data for MDlac in the ipsilateral (A) and contralateral (B) cortical samples are illustrated in Figure 3. It can be seen that mean MDlac levels were above baseline values in both hemispheres, in all groups, and at all time points after sham injury or CCI. Repeated measures ANOVA indicated significant main effects for group (p < 0.001), hemisphere (p < 0.002), and time (p < 0.001); significant effects for the group × hemisphere (p < 0.02), hemisphere × time (p < 0.001), and group × time (p < 0.001) interactions; and a significant group × hemisphere × time interaction (p < 0.001). For the left/injured cortex (Fig. 3A), there was a significant effect of group (p < 0.001), time (p < 0.001), and the group ×time interaction (p < 0.001). Post hoc tests indicated that ipsilateral MDlac was significantly higher in the CCI-SP (p < 0.001) and CCI-Sal (p < 0.001) groups than it was in the Sham-SP animals, but MDlac did not differ between the two CCI conditions. The post-injection MDlac values in the ipsilateral cortex were significantly increased above baseline at all sampling time points in the CCI-Sal (p < 0.01) and CCI-SP groups (p < 0.005), and were also increased significantly (p < 0.05) above baseline at 45 and 75 min in the Sham-SP group. For MDlac in the contralateral cortex (Fig. 3B), there was a significant effect of group (p < 0.003), time (p < 0.001), and the group × time interaction (p < 0.001). Post hoc tests indicated that the cortical MDlac contralateral to injury was significantly higher in the CCI-SP (p < 0.05) and CCI-Sal (p < 0.001) groups than in the Sham-SP animals, and that MDlac was significantly lower in the CCI-SP group compared to CCI-Sal (p < 0.05). The post-injection MDlac values in the contralateral cortex were significantly increased above baseline at all sampling time points in the CCI-Sal group (p < 0.02), from 30 through 75 min sampling epochs in the Sham-SP group (p < 0.05), but only in the first 15 min for the CCI-SP animals (p < 0.05).
FIG. 3.
Mean (±SEM) extracellular changes in concentrations of lactate, expressed as percent of baseline (Base), measured in microdialysis samples collected every 15 min from the ipsilateral (A) or contralateral (B) cerebral cortex after injection of saline (-Sal) or sodium pyruvate (-SP, 1000 mg/kg) in rats with sham (Sham) or left cortical contusion injury (CCI).
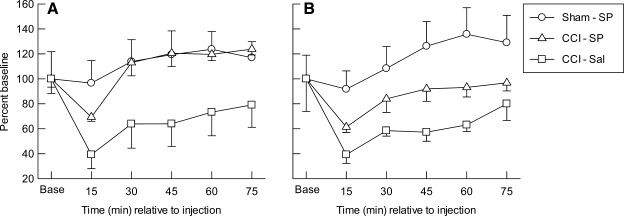
The percent baseline data for MDglc in the ipsilateral (A) and contralateral (B) cortical samples are illustrated in Figure 4. Repeated measures ANOVA indicated significant main effects for group (p < 0.003) and time (p < 0.002), but not for hemisphere or any of the two- or three-way interactions. For the left/injured cortex (Fig. 4A), there was a significant effect of group (p < 0.007) and time (p < 0.003), but not for the group × time interaction. Post hoc analyses indicated that ipsilateral MDglc was significantly higher in the Sham-SP (p < 0.003) and CCI-SP (p < 0.007) groups compared to CCI-Sal, but the overall levels for ipsilateral MDglc did not differ between the CCI-SP and Sham-SP groups. The post-injection MDglc values in the ipsilateral cortex were not altered from baseline in Sham-SP animals. In both of the CCI groups, the left/injured cortex MDglc levels were significantly (p < 0.05) decreased below baseline only at the first 15 min sampling epoch, while the post-injection levels were significantly increased over baseline levels in the CCI-SP group during the 60 (p < 0.05) and 75 min (p < 0.002) sampling epochs. For the contralateral cortex (Fig. 4B), there was also a significant effect of group (p < 0.006) and time (p < 0.003), but not for the group × time interaction. Post hoc analyses indicated that MDglc was significantly higher in the contralateral cortex of the Sham-SP group compared to that in the CCI-Sal animals (p = 0.002) as well as the CCI-SP group (p < 0.05). The overall levels for contralateral MDglc in the CCI-SP and CCI-Sal groups did not differ significantly. Compared to baseline, the post-injection MDglc levels were not significantly altered at any time point in the Sham-SP group but were significantly (p < 0.05) decreased below baseline during the first 15 min sampling period in CCI-SP (p < 0.001) and CCI-Sal (p < 0.05) groups.
FIG. 4.
Mean (±SEM) extracellular changes in concentrations of glucose, expressed as percent of baseline (Base), measured in microdialysis samples collected every 15 min from the ipsilateral (A) or contralateral (B) cerebral cortex after injection of saline (-Sal) or sodium pyruvate (-SP, 1000 mg/kg) in rats with sham (Sham) or left cortical contusion injury (CCI).
Cortical cell counts at 6 h after CCI
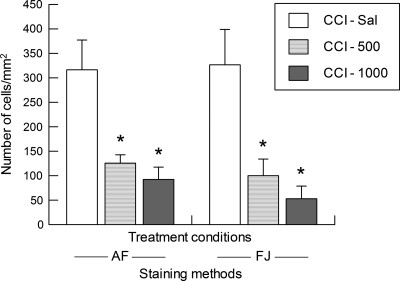
The data for counts of dead/dying neurons in the cortex ipsilateral and medial to the site of CCI, for both the FJ and AF staining methods, are illustrated in Figure 5. It can be seen that, regardless of staining method used, the CCI-Sal rats surviving for 6 h after injury had greater numbers of dead/dying cells in the injured cortex than did either the low (500 mg/kg) or high (1000 mg/kg) dose SP groups. One-way ANOVAs on the AF and FJ cell count data revealed a significant effect of treatment (p < 0.002), with post hoc tests confirming that both the CCI-500 and CCI-1000 groups had significantly fewer AF-positive (p < 0.05) and FJ-positive (p < 0.05) cells per mm2 of cortical tissue than did the CCI-Sal group. No differences were found between the CCI-500 and CCI-1000 groups or between the two methods used to stain damaged neurons.
FIG. 5.
Mean (±SEM) number of acid fuchsin (AF) or Fluoro-jade B (FJ) positive cells in the cortex ipsilateral and medial to cortical contusion injury (CCI) at 6 h after injection of saline (-Sal) or sodium pyruvate at a dose of 500 mg/kg (-500) or 1000 mg/kg (-1000). *p < 0.05 vs. CCI-Sal.
Cortical tissue loss at 2 weeks
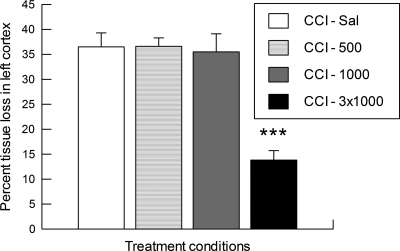
One-way ANOVA on the volume of cortical tissue between AP + 1.6 and −5.6 in the contralateral hemisphere revealed no significant effects of saline or SP treatment. Therefore, we proceeded to express the volume of cortical tissue damage in the left/injured hemisphere as a percentage tissue loss compared to the contralateral cortex. As is shown in Figure 6, rats in the CCI-Sal, CCI-500, and CCI-1000 groups had nearly equivalent percent tissue loss in the ipsilateral cortex by 2 weeks post-injury, indicating that there was no enduring neuroprotection after a single injection of either the low (500 mg/kg) or high (1000 mg/kg) dose of SP. However, those rats receiving three spaced injections of the high-dose SP (CCI-3x1000) had significantly reduced tissue loss in the ipsilateral cortex at the 2 week survival time compared to each of the single-injection groups (one-way ANOVA group effect and post hoc comparisons, all p < 0.001).
FIG. 6.
Tissue loss in the left/injured cortical mantle, expressed as percentage of tissue volume in the contralateral cortical mantle (mean ± SEM), at 2 weeks after cortical contusion injury (CCI) in rats with a single (5 min post-CCI) injection of saline (-Sal) or sodium pyruvate at a dose of 500 mg/kg (-500) or 1000 mg/kg (-1000), and rats with three injections (5, 65, and 125 min post-CCI) of the 1000 mg/kg dose of sodium pyruvate (-3x1000). ***p < 0.001 vs. CCI-Sal, CCI-500, or CCI-1000.
Discussion
One key finding of the current study is that a single intraperitoneal injection of SP can significantly increase arterial levels of pyruvate, lactate, and glucose in both uninjured and TBI rats. Furthermore, under conditions of CCI a single SP treatment increases cerebral extracellular levels of pyruvate, but not lactate, and attenuates a CCI-induced reduction of extracellular glucose. Another key finding is that systemically administered SP can markedly reduce the neuronal cell death within the cerebral cortex ipsilateral to a moderately severe CCI. TBI can therefore be added to the growing list of neurological conditions for which exogenous pyruvate treatment has been shown to reduce brain damage, including hemorrhagic shock, transient global ischemia, transient or permanent focal ischemia, kainic or quinolinic acid-induced excitotoxicity, amyotrophic lateral sclerosis, and hypoglycemia (Mongan et al., 1999, 2001, 2002, 2003; Lee et al., 2001; Ryu et al., 2003, 2004, 2006; Yoo et al., 2004; Suh et al., 2005; Yi et al., 2007; Kim et al., 2007; Park et al., 2007).
Physiological and metabolic effects of CCI and SP treatment
The current results indicate that a single administration of SP (1000 mg/kg i.p.) in rats with sham injury or CCI increased arterial pH at 30 and 75 min post-injection, an effect likely related to the concomitant increase in bicarbonate levels, as blood gases (pO2 and pCO2) were not altered by SP treatment. Our data also indicate that this dose of SP increases arterial pyruvate levels for only 30 to 45 min, with concomitant rapid increases in arterial lactate (from 10 to 75 min post-injection) and a slow but steady increase in arterial glucose concentrations occurring over a 75 min period after SP injection. These findings contrast with a report indicating that intraperitoneally administered SP did not alter blood pH or plasma glucose (Gonzalez-Falcon et al., 2003), but are consistent with the finding that intravenously administered pyruvate increases arterial pH and serum bicarbonate (Mongan et al., 1999, 2001) and that elevations in serum pyruvate lead to rapidly increasing lactate concentrations, with more delayed increases in serum glucose concentrations ( Mongan et al., 1999, 2001; Slovin et al., 2001; Gonzalez et al., 2005). The increased arterial lactate levels after SP treatment in sham and CCI conditions likely reflect peripheral metabolism (circulating LDH activity or hepatic metabolism), with conversion of injected pyruvate to lactate. Likewise, the eventual (60–75 min) reductions in arterial pyruvate and increasing arterial lactate levels (by 30 min) in CCI-Sal conditions may reflect either anesthetic effects or an injury-induced increase in peripheral LDH activity or hepatic metabolism. For all experimental conditions, the increase in arterial lactate appears to have led to lactate-induced hepatic gluconeogenesis. In addition, the gradual and significant increase in glucose concentration in the CCI-Sal group suggests that some component of the glucose increase seen in all groups may be related to prolonged anesthesia. In the CCI groups this increase in arterial glucose may be due to injury-induced alterations in hypothalamic centers regulating plasma glucose concentrations (Smythe et al., 1984; Dunn-Meynell et al., 1994; Roe et al., 1998; Grundy et al., 2001) and peripheral corticosterone release (Gottesfeld et al., 2002; McCullers et al., 2002). The additive nature of both an SP- and CCI-induced increase in circulating glucose is reflected by the fact that arterial glucose levels in CCI-SP rats approximate the sum of levels seen in Sham-SP and CCI-Sal groups, with post-injection glucose levels in CCI-SP being significantly elevated above those after CCI-Sal.
The in vitro percent recoveries (probe efficiency) and the in vivo basal extracellular levels of MDglc, MDlac, and MDpyr obtained in the current study were in the range of those previously reported in rodent studies of experimental TBI (Kawamata et al., 1995; Krishnappa et al., 1999; Bentzer et al., 2000; Ip et al., 2003; Stover et al., 2003; Alves et al., 2005; Geeraerts et al., 2006). As shown in the CCI-Sal controls, unilateral CCI resulted in a three- to five-fold increase in MDlac and an initial ∼60% reduction of MDglc in the ipsilateral peri-contusional cortex as well as in the contralateral cortex, with MDpyr levels remaining within baseline range after injury. These acute effects of CCI on MDglc and MDlac, likely reflective of increased glutamatergic activity with post-injury anaerobic hyperglycolysis (Alessandri et al., 1996, 1999; Nilsson et al., 1990), are very similar to those previously reported after CCI in rats (Krishnappa et al., 1999), and are more substantial than the acute cerebral metabolic alterations reported for rodent models of fluid percussion (Kawamata et al., 1995; Dhillon et al., 1997) or impact acceleration brain injury (Geeraerts et al., 2006, 2008).
The microdialysis data in the current study clearly show that a single intraperitoneal injection of SP is capable of altering the extracellular concentrations of metabolic fuels. After sham injury the SP treatment did not significantly alter MDglc levels, but significantly increased MDpyr by three- to five-fold above baseline for 60–75 min and increased MDlac to a lesser degree (147–158% of baseline) within both the left and right cortex. These data in sham operates suggest that: (1) pyruvate may be preferentially transported from blood to the brain after SP treatment or (2) transported lactate may be taken up by cells to a greater extent than is pyruvate. The former alternative seems most plausible, because when both pyruvate and lactate levels increase in serum they will compete for access to proton-coupled MCT proteins (Oldendorf, 1973). Furthermore, the transport kinetics of MCT1 located in the vascular endothelium show that transporter affinity for pyruvate (Km of ∼1 mM) is approximately three-fold greater than that for lactate (Pierre and Pellerin, 2005). Rapid transport and utilization of pyruvate in the brain have been previously reported (Cremer et al., 1982; Hara et al., 1986; Miller and Oldendorf, 1986; Mongan et al., 1999, 2001). In addition, 13C nuclear magnetic resonance spectrometry has demonstrated that [3–13]C-pyruvate is equivalent to [1–13]C-glucose as a TCA substrate and that exogenous pyruvate was predominantly metabolized in the neuronal compartment (Gonzalez et al., 2005).
In rats with CCI and SP treatment, the MDpyr levels were increased to a level comparable with that seen in Sham-SP rats in the contralateral cortex, but were increased to a far greater degree (12- to 17-fold above baseline) in the ipsilateral peri-contusional cortex. It is possible that this large increase in ipsilateral MDpyr levels in the CCI-SP group is simply due to a breach of the blood-brain barrier. However, the MDlac increases in cortex ipsilateral to CCI did not differ significantly between the CCI-SP and the CCI-Sal groups, even though the CCI-SP group had much higher levels of serum lactate. To attribute these findings to a disrupted blood-brain barrier, the concentration or diffusion gradients between blood and brain would need to be altered in a way that would prohibit lactate flux but would enable pyruvate flux. As discussed above for sham injury conditions, alternative explanations are that: (1) diffusion gradients and MCT activity favor influx of pyruvate over lactate in the CCI-SP treatment condition, or (2) lactate flux from serum to brain interstitial spaces in the CCI-SP condition was matched by cerebral metabolic rates for lactate such that MDlac levels did not differ between CCI-SP and CCI-Sal treatments. Since MCT1 transporters facilitate both influx as well as efflux of monocarboxylates at the blood-brain barrier, the current results could also reflect an increased influx of pyruvate combined with an increased transporter-mediated efflux of lactate from the brain after TBI. Each of the above interpretations can also be supported by data in the contralateral cortex, where levels of MDpyr increased 356–424% while those of MDlac increased only 230–240% above baseline in the CCI-SP group.
The SP treatment-induced reduction of CCI-induced MDlac increases in the contralateral cortex and bilateral attenuation of CCI-induced decreases in MDglc indicates there was a pyruvate-induced reduction of contralateral hyperglycolysis and a bilateral “glucose sparing” effect in the CCI-SP group. This latter effect could be due to metabolic utilization of pyruvate, lactate, or both, in order to meet increased energy demands after CCI (discussed below). The higher levels of MDglc in the SP treatment group after CCI could also reflect increased flux of serum glucose, since the arterial glucose levels were significantly higher in CCI-SP vs. CCI-Sal treatment conditions. This latter explanation may be unlikely in the face of injury-induced cerebral hyperglycolysis (demand exceeding supply), when glucose transport can be markedly decreased or repressed (Pardridge, 1983). However, reductions of serum glucose with insulin treatment can significantly reduce MDglc in TBI patients as well as increase the microdialysis lactate − pyruvate ratio and glutamate levels (Vespa et al., 2006). Thus, the overall pattern of changes seen in circulating fuels as well as in the levels of MDglc, MDlac, and MDpyr after SP treatment appears to favorable alter the post-TBI metabolic environment.
As proposed by Pellerin and Magistretti, during periods of increased cerebral activation the astrocyte-neuronal lactate shuttle (ANLS) will be operative in normal brain tissue to enable uptake and glycolytic processing of glucose by astroglial cells and the lactate produced can be transferred to neurons to support their metabolic requirements (Pellerin and Magistretti, 1994; Pellerin et al., 2007). To the extent that MCT transporter kinetics, simple diffusion, and the ANLS can support glial-derived lactate use in neurons (Oldendorf, 1973; Dienel and Cruz, 2004; Simpson et al., 2007), there is ample support for the cerebral uptake and use of lactate to support neuronal energy production and viability in conditions of normal brain activation (Schurr, 2006). As is illustrated in the current study, during the acute phase of cerebral metabolic hyperglycolysis after TBI, there is a massive increase in the extracellular concentrations of lactate, which may reflect an increase in the ANLS, but also reflects a rate of lactate production that is either much higher than necessary to support neuronal energy metabolism or an inability of neurons to utilize this energy source. Since lactate metabolism for production of energy requires NAD+ (for the LDH reaction, converting lactate to pyruvate), it seems most likely that a substantial proportion of the lactate accumulating after TBI is not being used as a neuronal energy source due to a TBI-induced activation of PARP and a reduction in cytosolic NAD+ (Laplaca et al., 2001; Satchell et al., 2003; Besson et al., 2003; Clark et al., 2007). However, due to the abilities of pyruvate to replenish cytosolic NAD+ and stimulate the pyruvate dehydrogenase complex (PDH) (Mongan et al., 2003; Sharma et al., 2003) it seems feasible that an increase in pyruvate after SP treatment, or after exogenous lactate treatment (LaManna et al., 1993), could also facilitate or enable neuronal use of lactate in the post-TBI microenvironment.
Histologic effects of SP treatment after CCI
The present results demonstrate that a single intraperitoneal administration of SP at 500–1000 mg/kg given within 5 min after a moderately severe CCI significantly reduced the number of damaged neurons within the ipsilateral cerebral cortex at 6 h post-CCI. This neuroprotection was equally apparent in AF-stained tissue and in the more recently developed FJ marker for neuronal degeneration; results with both methods revealed only slightly improved neuroprotection with the 1000 mg/kg compared to the 500 mg/kg dose. In the SP-treated rats surviving for 2 weeks after CCI, there was no enduring neuroprotection with a single injection of either a 500 or 1000 mg/kg dose, based on percent tissue loss in the injured cortex. When three injections of the 1000 mg/kg dose of SP were administered, however, the cortical tissue loss induced by moderate CCI was significantly reduced at the 2 week time point. These findings are generally compatible with neuroprotection reported for these doses of SP after transient forebrain ischemia (Lee et al., 2001) and excitotoxic lesions (Ryu et al., 2003, 2004) at 3 or 7 days, respectively, where similar degrees of neuroprotection for SP at either 500 or 1000 mg/kg occurred regardless of whether treatment was initiated immediately or at 1 h post-injury.
In contrast to the transient cortical protection seen after a single injection in CCI rats, a 500 mg/kg SP injection 30 min after reperfusion was reported to provide near complete neuroprotection in hippocampus at 3, 15, or 30 days after transient global ischemia (Lee et al., 2001). Subsequent to initiation of the current study, this same group reported that doses of SP between 62.5 and 250 mg/kg reduced infarct volume 24 h after either transient or permanent focal ischemia, when administered 30 min post-injury and regardless of whether SP was given intravenously or intraperitoneally. In addition, they showed that SP (125 mg/kg i.p.) given 30 min after permanent focal cerebral ischemia significantly reduced infarct volume at 14 days post-injury (Yi et al., 2007). However, the inability of a single SP injection to provide enduring neuroprotection after CCI is compatible with the reported failures of SP (500 or 1000 mg/kg) to reduce infarct volume after transient or permanent focal ischemia (Yi et al., 2007) and for single doses of SP (250, 500, or 1000 mg/kg) to reduce cortical infarct volume when studied 24 h after permanent ischemia (Gonzalez-Falcon et al., 2003). These latter authors also failed to find any benefits of repeated injections (at 0.5, 6, 13, and 18 h) of the 500 or 1000 mg/kg doses of SP, which contrasts with our finding of reduced cortical contusion volume if repeated injections of 1000 mg/kg were administered after CCI. These failures of single or multiple SP treatments to reduce cerebral damage in stroke may be due to inability to supply infarcted areas with pyruvate.
Using magnetic resonance spectroscopy, an SP dose (250 mg/kg) found to reduce infarct volume was also shown to significantly lower the lactate-creatine ratio in ischemic brain regions, while this ratio remained at saline control levels after an ineffective SP dose (1000 mg/kg). This finding led the authors to suggest that the loss of protection seen with higher doses of pyruvate may be related to increased lactate production (Yi et al., 2007). This hypothesis seems unlikely in the case of TBI, as our microdialysis data indicate that MDlac in cortex ipsilateral to CCI was similar in saline- and SP-treated groups and the high dose of SP (1000 mg/kg) also reduced MDlac within the cerebral cortex contralateral to CCI.
Our rationale for giving three injections, spaced at 1 h intervals, in the current study was based on our data showing only brief elevations of circulating pyruvate after a single SP treatment and our prior study showing that CCI induced an increase in metabolic demands and a reduction in ATP that persisted for at least 2 h post-injury (Lee et al., 1999). The success of this repeated treatment protocol after CCI leads us to suggest that supplemental fuel(s) must be available to the injured brain throughout the period(s) of increased neuronal activity and metabolic demands in order to protect neurons from energy crisis leading to cell death. Residual cortical necrosis apparent at 2 weeks in our rats with multiple SP treatments, and the larger volumes of injury seen after a single injection, may be due to insufficient duration of exogenous fuel delivery, or delivery may have been reduced or impaired at later times due to CCI-induced reductions in cerebral blood flow (Cherian et al., 1994; Sutton et al., 1994; Kochanek et al., 1995). We did not assess arterial or cerebral concentrations of fuels in these CCI rats with three SP treatments. Future studies will be needed to determine these factors and the optimal number, dosage level, and spacing intervals for multiple treatments.
Monocarboxylate use and effects after brain injury
The metabolic results of the current study clearly show that administration of SP constitutes not only a “pyruvate supplementation study” but also a “lactate supplementation study” and, with lactate-induced gluconeogenesis, a potential “glucose supplementation study.” While the SP treatment effect of increasing circulating supplies of all three metabolic substrates may represent an optimal scenario for an injured brain in need of additional fuel, the interconversion of these metabolic substrates also prohibit firm conclusions regarding the particular substrate used by injury-compromised cells in order to support neuronal survival. This limitation applies not only to the current study demonstrating an SP treatment-induced reduction in cortical neuronal injury after experimental TBI but to the most, if not all, of the prior studies of SP treatment efficacy after other acquired neurological injuries in which circulating fuel concentrations have not been assessed. Since serum pyruvate levels will also increase after lactate infusion (LaManna et al., 1993), this limitation applies as well to those studies reporting neurological benefits in TBI models after lactate administration where pyruvate, and frequently glucose, levels have not been assessed (Chen et al., 2000a; Rice et al., 2002; Levasseur et al., 2006; Holloway et al., 2007). Future work on supplemental monocarboxylate administration after brain injury will no doubt benefit by including measures of the administered substrate on serum and extracellular levels and/or the metabolic rates of pyruvate, lactate, and glucose as well as by directly comparing effects of separate administration of each of these substrates in the injury model of interest. Empirical manipulations of proportional levels of each substrate may eventually reveal the relative benefits or contributions of the individual fuels on neuroprotection after brain injury.
Despite the preceding limitations, inferences regarding the potential mechanisms underlying SP-induced neuroprotection observed after TBI can be made based on prior research. It is most likely that some benefits of exogenous pyruvate treatment after brain injury are not simply due to monocarboxylate metabolism and are not lactate-mediated effects (Ryu et al., 2004). SP treatment-induced reductions in cell death may be related to effects on TBI-induced increases in zinc, activation of the DNA repair enzyme PARP, and the latter's consumption and depletion of NAD+ (Laplaca et al., 1999; Suh et al., 2000, 2006; Besson et al., 2003; Satchell et al., 2003; Hellmich et al., 2004, 2007; Clark et al., 2007). Multiple in vitro studies have demonstrated that exogenous pyruvate can directly chelate zinc or attenuate zinc- or H2O2-induced activation of PARP and the reductions in cytosolic NAD+ and ATP production, and pyruvate can block or attenuate induction of pro-apoptotic pathways in neuronal, glial, or endothelial cells ( Sheline et al., 2000; Lee et al., 2001, 2003, 2004; Ying et al., 2002, 2005; Chen and Liao, 2003; Yoo et al., 2004; Zeng et al., 2007). The ability of SP treatment to block or greatly attenuate the accumulation of zinc in neurons is one primary mechanism by which exogenous pyruvate provides neuroprotection after transient forebrain ischemia (Lee et al., 2001), retinal ischemia (Yoo et al., 2004), and epileptic seizures (Kim et al., 2007).
Exogenous pyruvate administration has been shown to prevent reductions of NAD+ and ATP and the cleavage of PARP in brain tissue after hemorrhagic shock (Mongan et al., 2001, 2003). The ability of pyruvate to replenish NAD+ and reduce PARP activation indicates that this treatment option may be preferable to provision of PARP inhibitors after TBI (Laplaca et al., 2001; Satchell et al., 2003; Besson et al., 2003, 2005; Clark et al., 2007), since the latter treatment would also impair the beneficial actions of PARP for repair of DNA strand breaks. The preceding actions of exogenous pyruvate on NAD+ repletion are congruent with mechanisms thought to underlie the benefits of nicotinamide (vitamin B3) in models of experimental TBI (Hoane et al., 2003, 2006a, 2006b; Holland et al., 2008), although additional actions of pyruvate are likely since nicotinamide did not reduce infarct volume in a model of permanent focal ischemia where SP treatment reduced both infarct volume and NAD+ reductions in the ischemic tissue (Yi et al., 2007).
In addition to the preceding factors, SP treatment-induced reductions in cell death may be related to effects on TBI-induced oxidative and nitrosative stress (Hall et al., 1993, 2004; Marklund et al., 2001; Tavazzi et al., 2005), which can alter activity of multiple enzymes crucial for glycolysis and respiratory oxidation, including GAPDH and PDH (Tabatabaie et al., 1996; Humphries and Szweda, 1998; Ralser et al., 2007). An inhibition of PDH after TBI (Kochanek et al., 2006; Opii et al., 2007) will reduce oxidative decarboxylation and entry of pyruvate into the TCA cycle, with concomitant decreases in reducing equivalents needed for NADH production, oxidative phosphorylation, and production of ATP (Mallet and Sun, 1999; Mongan et al., 2001). SP treatment-induced elevations of pyruvate may ameliorate these factors due to the ability of pyruvate to stimulate PDH activity and its own oxidation (Sharma et al., 2003). A TBI-induced increase in pyruvate carboxylase mediated anaplerosis (Bartnik et al., 2007a) would, in the presence of increased pyruvate levels, also contribute to an increase in mitochondrial NADH. A relative decrease in cytosolic NADH and associated increase in the H+ gradient across the mitochondrial membrane, reflected in the reduced extracellular lactate-pyruvate ratio after SP treatment, would facilitate oxidative phosphorylation. Utilization of exogenously provided pyruvate in the TCA cycle could also facilitate, or reduce the bioenergetic consequences of, GAPDH inhibition and shunting of glucose to the pentose phosphate pathway where glucose can be utilized to regenerate reducing equivalents (NADPH) needed to replenish glutathione, and hence glutathione peroxidase to aid in antioxidant defense (Bartnik et al., 2005, 2007b; Dusick et al., 2007; Ralser et al., 2007).
These likely metabolic and antioxidant properties of SP treatment after TBI are consistent with prior in vitro studies on pyruvate actions (O'Donnell-Tormey et al., 1987; Desagher et al., 1997; Kashiwagi et al., 1997; Maus et al., 1999; Mazzio and Soliman, 2003; Chung et al., 2004; Wang et al., 2007) and in vivo studies where SP treatment reduced markers of oxidative stress after quinolinic acid (iNOS, peroxynitrite, and lipid peroxidation) (Ryu et al., 2004) or increased extracellular pyruvate while decreasing glutamate, improving brain redox state and ATP, creatine and phosphocreatine levels, and reducing lipid peroxidation and PDH inhibition in models of hemorrhagic shock (Mongan et al., 1999, 2001, 2003). Several of these benefits conferred by pyruvate supplementation that are not seen for lactate are probably due to the now well recognized ability of pyruvic acid and esters of pyruvate to act as anti-oxidant as well as anti-inflammatory agents (Das, 2006; Fink, 2007).
Conclusion
In conclusion, the neuroprotective effects observed after SP treatment in the current study are likely due to multiple and interrelated effects including the ability of pyruvate to be utilized as a neuronal energy substrate, to serve as an antioxidant, to reduce zinc accumulation or PARP activation, and to replenish cytosolic NAD+. Via the latter actions exogenous pyruvate could facilitate PARP-mediated DNA repair, enable the use of glucose in pathways that increase antioxidant capacity, and enable conversion of lactate to pyruvate for energy production. Redox state alterations after exogenous pyruvate administration may also impact signaling pathways to down-regulate cell death pathways and up-regulate cell survival pathways (Lee et al., 2003, 2004; Sharma et al., 2003). Further research will be needed to determine which of these mechanisms prevail for SP-induced neuroprotection after TBI, as well as to determine the optimal dosage, treatment window, and duration required for pyruvate supplementation to reduce cell damage and improve functional outcomes after TBI.
Acknowledgments
The authors thank Monica Wong, Sima Ghavim, Joyce Wei, and Daniel Hirt for their superb technical assistance. This research was supported by the UCLA Brain Injury Research Center, and awards (nos. R01NS27544, R01NS37363, and P01NS058489) from the National Institute of Neurological Disorders and Stroke (NINDS). The content is the sole responsibility of the authors and does not necessarily represent official views of the NINDS or the National Institutes of Health.
Author Disclosure Statement
No conflicting financial interests exist.
References
- Alessandri B. Doppenberg E. Zauner A. Woodward J. Choi S. Bullock R. Evidence for time-dependent glutamate-mediated glycolysis in head-injured patients: a microdialysis study. Acta Neurochir. 1999;Suppl. 75:25–28. doi: 10.1007/978-3-7091-6415-0_6. [DOI] [PubMed] [Google Scholar]
- Alessandri B. Landolt H. Langemann H. Gregorin J. Hall J. Gratzl O. Application of glutamate in the cortex of rats: a microdialysis study. Acta Neurochir. 1996;Suppl. 67:6–12. doi: 10.1007/978-3-7091-6894-3_2. [DOI] [PubMed] [Google Scholar]
- Alves O.L. Bullock R. Clausen T. Reinert M. Reeves T.M. Concurrent monitoring of cerebral electrophysiology and metabolism after traumatic brain injury: an experimental and clinical study. J. Neurotrauma. 2005;22:733–749. doi: 10.1089/neu.2005.22.733. [DOI] [PubMed] [Google Scholar]
- Auer R.N. Wieloch T. Olsson Y. Siesjo B.K. The distribution of hypoglycemic brain damage. Acta Neuropathol. (Berlin) 1984;64:177–191. doi: 10.1007/BF00688108. [DOI] [PubMed] [Google Scholar]
- Bartnik B.L. Hovda D.A. Lee P.W. Glucose metabolism after traumatic brain injury: estimation of pyruvate carboxylase and pyruvate dehydrogenase flux by mass isotopomer analysis. J. Neurotrauma. 2007a;24:181–194. doi: 10.1089/neu.2006.0038. [DOI] [PubMed] [Google Scholar]
- Bartnik B.L. Lee S.M. Hovda D.A. Sutton R.L. The fate of glucose during the period of decreased metabolism after fluid percussion injury: a 13C NMR study. J. Neurotrauma. 2007b;24:1079–1092. doi: 10.1089/neu.2006.0210. [DOI] [PubMed] [Google Scholar]
- Bartnik B.L. Sutton R.L. Fukushima M. Harris N.G. Hovda D.A. Lee S.M. Upregulation of pentose phosphate pathway and preservation of tricarboxylic acid cycle flux after experimental brain injury. J. Neurotrauma. 2005;22:1052–1065. doi: 10.1089/neu.2005.22.1052. [DOI] [PubMed] [Google Scholar]
- Bentzer P. Davidsson H. Grande P.-O. Microdialysis-based long-term measurements of energy-related metabolites in the rat brain following a fluid percussion trauma. J. Neurotrauma. 2000;17:441–447. doi: 10.1089/neu.2000.17.441. [DOI] [PubMed] [Google Scholar]
- Bergsneider M. Hovda D.A. Shalmon E. Kelly D.F. Vespa P. Martin N.A. Phelps M. McArthur D.L. Caron M.J. Kraus J.F. Becker D.P. Cerebral hyperglycolysis following severe traumatic brain injury in humans: a positron emission tomography study. J. Neurosurgery. 1997;86:241–251. doi: 10.3171/jns.1997.86.2.0241. [DOI] [PubMed] [Google Scholar]
- Besson V.C. Croci N. Boulu R.G. Plotkine M. Marchand- Verrecchia C. Deleterious poly(ADP-ribose)polymerase-1 pathway activation in traumatic brain injury in rat. Brain Res. 2003;989:58–66. doi: 10.1016/s0006-8993(03)03362-6. [DOI] [PubMed] [Google Scholar]
- Besson V.C. Zsengeller Z. Plotkine M. Szabo C. Marchand-Verrecchia C. Beneficial effects of PJ34 and INO-1001, two novel water-soluble poly(ADP-ribose) polymerase inhibitors, on the consequences of traumatic brain injury in rat. Brain Res. 2005;1041:149–156. doi: 10.1016/j.brainres.2005.01.096. [DOI] [PubMed] [Google Scholar]
- Chen C.J. Liao S.L. Zinc toxicity on neonatal cortical neurons: involvement of glutathione chelation. J. Neurochem. 2003;85:443–453. doi: 10.1046/j.1471-4159.2003.01691.x. [DOI] [PubMed] [Google Scholar]
- Chen T. Qian Y.Z. Di X. Rice A. Zhu J.P. Bullock R. Lactate/glucose dynamics after rat fluid percussion brain injury. J. Neurotrauma. 2000a;17:135–142. doi: 10.1089/neu.2000.17.135. [DOI] [PubMed] [Google Scholar]
- Chen T. Qian Y.Z. Rice A. Zhu J.P. Di X. Bullock R. Brain lactate uptake increases at the site of impact after traumatic brain injury. Brain Res. 2000b;861:281–287. doi: 10.1016/s0006-8993(00)01992-2. [DOI] [PubMed] [Google Scholar]
- Cherian L. Robertson C.S. Contant C.F. Bryan R.M. Lateral cortical impact injury in rats: cerebrovascular effects of varying depth of cortical deformation and impact velocity. J. Neurotrauma. 1994;11:573–585. doi: 10.1089/neu.1994.11.573. [DOI] [PubMed] [Google Scholar]
- Chung S.J. Lee S.H. Lee Y.J. Park H.S. Bunger R. Kang Y.H. Pyruvate protection against endothelial cytotoxicity induced by blockade of glucose uptake. J. Biochem. Mol. Biol. 2004;37:239–245. doi: 10.5483/bmbrep.2004.37.2.239. [DOI] [PubMed] [Google Scholar]
- Clark R.S. Vagni V.A. Nathaniel P.D. Jenkins L.W. Dixon C.E. Szabo C. Local administration of the poly(ADP-ribose) polymerase inhibitor INO-1001 prevents NAD + depletion and improves water maze performance after traumatic brain injury in mice. J. Neurotrauma. 2007;24:1399–1405. doi: 10.1089/neu.2007.0305. [DOI] [PubMed] [Google Scholar]
- Cremer J.E. Teal H.M. Cunningham V.J. Inhibition, by 2-oxo acids that accumulate in maple-syrup-urine disease, of lactate, pyruvate, and 3-hydroxybutyrate transport across the blood-brain barrier. J. Neurochem. 1982;39:674–677. doi: 10.1111/j.1471-4159.1982.tb07945.x. [DOI] [PubMed] [Google Scholar]
- D'Ambrosio R. Fairbanks J.P. Fender J.S. Born D.E. Doyle D.L. Miller J.W. Post-traumatic epilepsy following fluid percussion injury in the rat. Brain. 2004;127:1–11. doi: 10.1093/brain/awh038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das U.N. Pyruvate is an endogenous anti-inflammatory and anti-oxidant molecule. Med. Sci. Monit. 2006;12:RA79–84. [PubMed] [Google Scholar]
- Desagher S. Glowinski J. Premont J. Pyruvate protects neurons against hydrogen peroxide-induced toxicity. J. Neurosci. 1997;17:9060–9067. doi: 10.1523/JNEUROSCI.17-23-09060.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon H.S. Dose J.M. Scheff S.W. Prasad M.R. Time course of changes in lactate and free fatty acids after experimental brain injury and relationship to morphologic damage. Exp. Neurol. 1997;146:240–249. doi: 10.1006/exnr.1997.6524. [DOI] [PubMed] [Google Scholar]
- Dienel G.A. Cruz N.F. Nutrition during brain activation: does cell-to-cell lactate shuttling contribute significantly to sweet and sour food for thought? Neurochem. Int. 2004;45:321–351. doi: 10.1016/j.neuint.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Dunn-Meynell A. Pan S. Levin B.E. Focal traumatic brain injury causes widespread reductions in rat brain norepinephrine turnover from 6 to 24 h. Brain Res. 1994;660:88–95. doi: 10.1016/0006-8993(94)90842-7. [DOI] [PubMed] [Google Scholar]
- Dusick J.R. Glenn T.C. Lee W.N. Vespa P.M. Kelly D.F. Lee S.M. Hovda D.A. Martin N.A. Increased pentose phosphate pathway flux after clinical traumatic brain injury: a [1,2-(13)C(2)]glucose labeling study in humans. J. Cereb. Blood Flow Metab. 2007;27:1593–1602. doi: 10.1038/sj.jcbfm.9600458. [DOI] [PubMed] [Google Scholar]
- Faden A.I. Demediuk P. Panter S.S. Vink R. The role of excitatory amino acids and NMDA receptors in traumatic brain injury. Science. 1989;244:798–800. doi: 10.1126/science.2567056. [DOI] [PubMed] [Google Scholar]
- Fineman I. Hovda D.A. Smith M. Yoshino A. Becker D.P. Concussive brain injury is associated with a prolonged accumulation of calcium: a 45Ca autoradiographic study. Brain Res. 1993;624:94–102. doi: 10.1016/0006-8993(93)90064-t. [DOI] [PubMed] [Google Scholar]
- Fink M.P. Ethyl pyruvate: a novel anti-inflammatory agent. J. Intern. Med. 2007;261:349–362. doi: 10.1111/j.1365-2796.2007.01789.x. [DOI] [PubMed] [Google Scholar]
- Geeraerts T. Friggeri A. Mazoit J.X. Benhamou D. Duranteau J. Vigue B. Posttraumatic brain vulnerability to hypoxia-hypotension: the importance of the delay between brain trauma and secondary insult. Intensive Care Med. 2008;34:551–560. doi: 10.1007/s00134-007-0863-0. [DOI] [PubMed] [Google Scholar]
- Geeraerts T. Ract C. Tardieu M. Fourcade O. Mazoit J.X. Benhamou D. Duranteau J. Vigue B. Changes in cerebral energy metabolites induced by impact-acceleration brain trauma and hypoxic-hypotensive injury in rats. J. Neurotrauma. 2006;23:1059–1071. doi: 10.1089/neu.2006.23.1059. [DOI] [PubMed] [Google Scholar]
- Gonzalez S.V. Nguyen N.H. Rise F. Hassel B. Brain metabolism of exogenous pyruvate. J. Neurochem. 2005;95:284–293. doi: 10.1111/j.1471-4159.2005.03365.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Falcon A. Candelario-Jalil E. Garcia-Cabrera M. Leon O.S. Effects of pyruvate administration on infarct volume and neurological deficits following permanent focal cerebral ischemia in rats. Brain Res. 2003;990:1–7. doi: 10.1016/s0006-8993(03)03378-x. [DOI] [PubMed] [Google Scholar]
- Gottesfeld Z. Moore A.N. Dash P.K. Acute ethanol intake attenuates inflammatory cytokines after brain injury in rats: a possible role for corticosterone. J. Neurotrauma. 2002;19:317–326. doi: 10.1089/089771502753594882. [DOI] [PubMed] [Google Scholar]
- Grundy P.L. Harbuz M.S. Jessop D.S. Lightman S.L. Sharples P.M. The hypothalamo-pituitary-adrenal axis response to experimental traumatic brain injury. J. Neurotrauma. 2001;18:1373–1381. doi: 10.1089/08977150152725669. [DOI] [PubMed] [Google Scholar]
- Hall E.D. Andrus P.K. Yonkers P.A. Brain hydroxyl radical generation in acute experimental head injury. J. Neurochem. 1993;60:588–594. doi: 10.1111/j.1471-4159.1993.tb03189.x. [DOI] [PubMed] [Google Scholar]
- Hall E.D. Detloff M.R. Johnson K. Kupina N.C. Peroxynitrite-mediated protein nitration and lipid peroxidation in a mouse model of traumatic brain injury. J. Neurotrauma. 2004;21:9–20. doi: 10.1089/089771504772695904. [DOI] [PubMed] [Google Scholar]
- Hara T. Yokoi F. Iio M. Brain ischemia and infarction positively visualized by pyruvate-1-11C using positron-emission tomography. Eur. J. Nucl. Med. 1986;12:21–26. doi: 10.1007/BF00638790. [DOI] [PubMed] [Google Scholar]
- Hellmich H.L. Eidson K.A. Capra B.A. Garcia J.M. Boone D.R. Hawkins B.E. Uchida T. Dewitt D.S. Prough D.S. Injured Fluoro-Jade-positive hippocampal neurons contain high levels of zinc after traumatic brain injury. Brain Res. 2007;1127:119–126. doi: 10.1016/j.brainres.2006.09.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmich H.L. Frederickson C.J. Dewitt D.S. Saban R. Parsley M.O. Stephenson R. Velasco M. Uchida T. Shimamura M. Prough D.S. Protective effects of zinc chelation in traumatic brain injury correlate with upregulation of neuroprotective genes in rat brain. Neurosci. Lett. 2004;355:221–225. doi: 10.1016/j.neulet.2003.10.074. [DOI] [PubMed] [Google Scholar]
- Hoane M.R. Akstulewicz S.L. Toppen J. Treatment with vitamin B3 improves functional recovery and reduces GFAP expression following traumatic brain injury in rats. J. Neurotrauma. 2003;20:1189–1199. doi: 10.1089/089771503770802871. [DOI] [PubMed] [Google Scholar]
- Hoane M.R. Gilbert D.R. Holland M.A. Pierce J.L. Nicotinamide reduces acute cortical neuronal death and edema in the traumatically injured brain. Neurosci. Lett. 2006a;408:35–39. doi: 10.1016/j.neulet.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Hoane M.R. Kaplan S.A. Ellis A.L. The effects of nicotinamide on apoptosis and blood-brain barrier breakdown following traumatic brain injury. Brain Res. 2006b;1125:185–193. doi: 10.1016/j.brainres.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Holland M.A. Tan A.A. Smith D.C. Hoane M.R. Nicotinamide treatment provides acute neuroprotection and GFAP regulation following fluid percussion injury. J. Neurotrauma. 2008;25:140–152. doi: 10.1089/neu.2007.0312. [DOI] [PubMed] [Google Scholar]
- Holloway R. Zhou Z. Harvey H.B. Levasseur J.E. Rice A.C. Sun D. Hamm R.J. Bullock M.R. Effect of lactate therapy upon cognitive deficits after traumatic brain injury in the rat. Acta Neurochir. (Wien) 2007;149:919–927. doi: 10.1007/s00701-007-1241-y. [DOI] [PubMed] [Google Scholar]
- Humphries K.M. Szweda L.I. Selective inactivation of alpha-ketoglutarate dehydrogenase and pyruvate dehydrogenase: reaction of lipoic acid with 4-hydroxy-2-nonenal. Biochemistry. 1998;37:15835–15841. doi: 10.1021/bi981512h. [DOI] [PubMed] [Google Scholar]
- Ip E.Y. Zanier E.R. Moore A.H. Lee S.M. Hovda D.A. Metabolic, neurochemical, and histologic responses to vibrissa motor cortex stimulation after traumatic brain injury. J. Cereb. Blood Flow Metab. 2003;23:900–910. doi: 10.1097/01.WCB.0000076702.71231.F2. [DOI] [PubMed] [Google Scholar]
- Kashiwagi A. Nishio Y. Asahina T. Ikebuchi M. Harada N. Tanaka Y. Takahara N. Taki H. Obata T. Hidaka H. Saeki Y. Kikkawa R. Pyruvate improves deleterious effects of high glucose on activation of pentose phosphate pathway and glutathione redox cycle in endothelial cells. Diabetes. 1997;46:2088–2095. doi: 10.2337/diab.46.12.2088. [DOI] [PubMed] [Google Scholar]
- Katayama Y. Becker D.P. Tamura T. Hovda D.A. Massive increases in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. J. Neurosurg. 1990;73:889–900. doi: 10.3171/jns.1990.73.6.0889. [DOI] [PubMed] [Google Scholar]
- Katayama Y. Maeda T. Koshinaga M. Kawamata T. Tsubokawa T. Role of excitatory amino acid-mediated ionic fluxes in traumatic brain injury. Brain Pathol. 1995;5:427–435. doi: 10.1111/j.1750-3639.1995.tb00621.x. [DOI] [PubMed] [Google Scholar]
- Kawamata T. Katayama Y. Hovda D.A. Yoshino A. Becker D.P. Administration of excitatory amino acid antagonists via microdialysis attenuates the increase in glucose utilization seen following concussive brain injury. J. Cereb. Blood Flow Metab. 1992;12:12–24. doi: 10.1038/jcbfm.1992.3. [DOI] [PubMed] [Google Scholar]
- Kawamata T. Katayama Y. Hovda D.A. Yoshino A. Becker D.P. Lactate accumulation following concussive brain injury: the role of ionic fluxs induced by excitatory amino acids. Brain Res. 1995;674:196–204. doi: 10.1016/0006-8993(94)01444-m. [DOI] [PubMed] [Google Scholar]
- Kim T.Y. Yi J.S. Chung S.J. Kim D.K. Byun H.R. Lee J.Y. Koh J.Y. Pyruvate protects against kainate-induced epileptic brain damage in rats. Exp. Neurol. 2007;208:159–167. doi: 10.1016/j.expneurol.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Kochanek A.R. Kline A.E. Gao W.M. Chadha M. Lai Y. Clark R.S. Dixon C.E. Jenkins L.W. Gel-based hippocampal proteomic analysis 2 weeks following traumatic brain injury to immature rats using controlled cortical impact. Dev. Neurosci. 2006;28:410–419. doi: 10.1159/000094167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanek P.M. Marion D.W. Zhang W. Schiding J.K. White M. Palmer A.M. Clark R.S.B. O'Malley M.E. Styren S.D. Ho C. Dekosky S.T. Severe controlled cortical impact in rats: assessment of cerebral edema, blood blow, and contusion volume. J. Neurotrauma. 1995;12:1015–1025. doi: 10.1089/neu.1995.12.1015. [DOI] [PubMed] [Google Scholar]
- Krishnappa I.K. Contant C.F. Robertson C.S. Regional changes in cerebral extracellular glucose and lactate concentrations following severe cortical impact injury and secondary ischemia in rats. J. Neurotrauma. 1999;16:213–224. doi: 10.1089/neu.1999.16.213. [DOI] [PubMed] [Google Scholar]
- Kubota M. Nakamura T. Sunami K. Ozawa Y. Yamaura A. Makino H. Changes in local cerebral glucose utilization, DC potential and extracellular potassium in various degrees of experimental cerebral contusion. No To Shinkei. 2003;41:799–805. [PubMed] [Google Scholar]
- LaManna J.C. Harrington J.F. Vendel L.M. Abi-Saleh K. Lust W.D. Harik S.I. Regional blood-brain lactate influx. Brain Res. 1993;614:164–170. doi: 10.1016/0006-8993(93)91030-v. [DOI] [PubMed] [Google Scholar]
- Laplaca M.C. Raghupathi R. Verma A. Pieper A.A. Saatman K.E. Snyder S.H. McIntosh T.K. Temporal patterns of poly(ADP-ribose) polymerase activation in the cortex following experimental brain injury in the rat. J. Neurochem. 1999;73:205–213. doi: 10.1046/j.1471-4159.1999.0730205.x. [DOI] [PubMed] [Google Scholar]
- Laplaca M.C. Zhang J. Raghupathi R. Li J.H. Smith F. Bareyre F.M. Snyder S.H. Graham D.I. McIntosh T.K. Pharmacologic inhibition of poly(ADP-ribose) polymerase is neuroprotective following traumatic brain injury in rats. J. Neurotrauma. 2001;18:369–376. doi: 10.1089/089771501750170912. [DOI] [PubMed] [Google Scholar]
- Lee J.Y. Kim Y.H. Koh J.Y. Protection by pyruvate against transient forebrain ischemia in rats. J. Neurosci. 2001;21:RC171. doi: 10.1523/JNEUROSCI.21-20-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.M. Wong D.A. Samii A. Hovda D.A. Evidence for energy failure following irreversible traumatic brain injury. Ann. NY Acad. Sci. 1999;893:337–340. doi: 10.1111/j.1749-6632.1999.tb07849.x. [DOI] [PubMed] [Google Scholar]
- Lee Y.J. Kang I.J. Bunger R. Kang Y.H. Mechanisms of pyruvate inhibition of oxidant-induced apoptosis in human endothelial cells. Microvasc. Res. 2003;66:91–101. doi: 10.1016/s0026-2862(03)00052-9. [DOI] [PubMed] [Google Scholar]
- Lee Y.J. Kang I.J. Bunger R. Kang Y.H. Enhanced survival effect of pyruvate correlates MAPK and NF-kappaB activation in hydrogen peroxide-treated human endothelial cells. J. Appl. Physiol. 2004;96:793–801. doi: 10.1152/japplphysiol.00797.2003. [DOI] [PubMed] [Google Scholar]
- Levasseur J.E. Alessandri B. Reinert M. Clausen T. Zhou Z. Altememi N. Bullock M.R. Lactate, not glucose, up-regulates mitochondrial oxygen consumption both in sham and lateral fluid percussed rat brains. Neurosurgery. 2006;59:1122–1130. doi: 10.1227/01.NEU.0000245581.00908.AF. [DOI] [PubMed] [Google Scholar]
- Mallet R.T. Sun J. Mitochondrial metabolism of pyruvate is required for its enhancement of cardiac function and energetics. Cardiovasc. Res. 1999;42:149–161. doi: 10.1016/s0008-6363(98)00300-9. [DOI] [PubMed] [Google Scholar]
- Marklund N. Clausen F. Ander T.L. Hillered L. Monitoring of reactive oxygen species production after traumatic brain injury in rats with microdialysis and the 4-hydroxybenzoic acid trapping method. J. Neurotrauma. 2001;18:1217–1227. doi: 10.1089/089771501317095250. [DOI] [PubMed] [Google Scholar]
- Maus M. Marin P. Israel M. Glowinski J. Premont J. Pyruvate and lactate protect striatal neurons against N-methyl-D-aspartate-induced neurotoxicity. Eur. J. Neurosci. 1999;11:3215–3224. doi: 10.1046/j.1460-9568.1999.00745.x. [DOI] [PubMed] [Google Scholar]
- Mazzio E. Soliman K.F. Pyruvic acid cytoprotection against 1-methyl-4-phenylpyridinium, 6-hydroxydopamine and hydrogen peroxide toxicities in vitro. Neurosci. Lett. 2003;337:77–80. doi: 10.1016/s0304-3940(02)01327-7. [DOI] [PubMed] [Google Scholar]
- McCullers D.L. Sullivan P.G. Scheff S.W. Herman J.P. Traumatic brain injury regulates adrenocorticosteroid receptor mRNA levels in rat hippocampus. Brain Res. 2002;947:41–49. doi: 10.1016/s0006-8993(02)02904-9. [DOI] [PubMed] [Google Scholar]
- Miller L.P. Oldendorf W.H. Regional kinetic constants for blood-brain barrier pyruvic acid transport in conscious rats by the monocarboxylic acid carrier. J. Neurochem. 1986;46:1412–1416. doi: 10.1111/j.1471-4159.1986.tb01756.x. [DOI] [PubMed] [Google Scholar]
- Mongan P.D. Capacchione J. Fontana J.L. West S. Bunger R. Pyruvate improves cerebral metabolism during hemorrhagic shock. Am. J. Physiol. Heart Circ. Physiol. 2001;281:H854–H864. doi: 10.1152/ajpheart.2001.281.2.H854. [DOI] [PubMed] [Google Scholar]
- Mongan P.D. Capacchione J. West S. Karaian J. Dubois D. Keneally R. Sharma P. Pyruvate improves redox status and decreases indicators of hepatic apoptosis during hemorrhagic shock in swine. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H1634–1644. doi: 10.1152/ajpheart.01073.2001. [DOI] [PubMed] [Google Scholar]
- Mongan P.D. Fontana J.L. Chen R. Bunger R. Intravenous pyruvate prolongs survival during hemorrhagic shock in swine. Am. J. Physiol. 1999;277:H2253–2263. doi: 10.1152/ajpheart.1999.277.6.H2253. [DOI] [PubMed] [Google Scholar]
- Mongan P.D. Karaian J. Van Der Schuur B.M. Via D.K. Sharma P. Pyruvate prevents poly-ADP ribose polymerase (PARP) activation, oxidative damage, and pyruvate dehydrogenase deactivation during hemorrhagic shock in swine. J. Surg. Res. 2003;112:180–188. doi: 10.1016/s0022-4804(03)00148-3. [DOI] [PubMed] [Google Scholar]
- Nilsson P. Hillered L. Ponten U. Ungerstedt U. Changes in cortical extracellular levels of energy-related metabolites and amino acids following concussive brain injury in rats. J. Cereb. Blood Flow Metab. 1990;10:631–637. doi: 10.1038/jcbfm.1990.115. [DOI] [PubMed] [Google Scholar]
- Nilsson P. Ronne-Engström E. Fink R. Ungerstedt U. Carlson H. Hillered L. Epileptic seizure activity in the acute phase following cortical impact trauma in rat. Brain Res. 1994;637:227–232. doi: 10.1016/0006-8993(94)91237-8. [DOI] [PubMed] [Google Scholar]
- O'Donnell-Tormey J. Nathan C.F. Lanks K. DeBoer C.J. de la Harpe J. Secretion of pyruvate. An antioxidant defense of mammalian cells. J. Exp. Med. 1987;165:500–514. doi: 10.1084/jem.165.2.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldendorf W.H. Carrier-mediated blood-brain barrier transport of short-chain monocarboxylic organic acids. Am. J. Physiol. 1973;224:1450–1453. doi: 10.1152/ajplegacy.1973.224.6.1450. [DOI] [PubMed] [Google Scholar]
- Opii W.O. Nukala V.N. Sultana R. Pandya J.D. Day K.M. Merchant M.L. Klein J.B. Sullivan P.G. Butterfield D.A. Proteomic identification of oxidized mitochondrial proteins following experimental traumatic brain injury. J. Neurotrauma. 2007;24:772–789. doi: 10.1089/neu.2006.0229. [DOI] [PubMed] [Google Scholar]
- Palmer A.M. Marion D.W. Botscheller M.L. Swedlow P.E. Styren S.D. Dekosky S.T. Traumatic brain injury-induced excitotoxicity assessed in a controlled cortical impact model. J. Neurochem. 1993;61:2015–2024. doi: 10.1111/j.1471-4159.1993.tb07437.x. [DOI] [PubMed] [Google Scholar]
- Pardridge W.M. Brain metabolism: a perspective from the blood-brain barrier. Physiol. Rev. 1983;63:1481–1535. doi: 10.1152/physrev.1983.63.4.1481. [DOI] [PubMed] [Google Scholar]
- Park J.H. Hong Y.H. Kim H.J. Kim S.M. Kim M.J. Park K.S. Sung J.J. Lee K.W. Pyruvate slows disease progression in a G93A SOD1 mutant transgenic mouse model. Neurosci. Lett. 2007;413:265–269. doi: 10.1016/j.neulet.2006.11.058. [DOI] [PubMed] [Google Scholar]
- Paxinos G. Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; Orlando, FL: 1986. [Google Scholar]
- Pellerin L. Bouzier-Sore A.K. Aubert A. Serres S. Merle M. Costalat R. Magistretti P.J. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia. 2007;55:1251–1262. doi: 10.1002/glia.20528. [DOI] [PubMed] [Google Scholar]
- Pellerin L. Magistretti P.J. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci. USA. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre K. Pellerin L. Monocarboxylate transporters in the central nervous system: distribution, regulation and function. J. Neurochem. 2005;94:1–14. doi: 10.1111/j.1471-4159.2005.03168.x. [DOI] [PubMed] [Google Scholar]
- Prins M.L. Cerebral metabolic adaptation and ketone metabolism after brain injury. J. Cereb. Blood Flow Metab. 2008;28:1–16. doi: 10.1038/sj.jcbfm.9600543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins M.L. Fujima L.S. Hovda D.A. Age-dependent reduction of cortical contusion volume by ketones after traumatic brain injury. J. Neurosci. Res. 2005;82:413–420. doi: 10.1002/jnr.20633. [DOI] [PubMed] [Google Scholar]
- Prins M.L. Lee S.M. Fujima L.S. Hovda D.A. Increased cerebral uptake and oxidation of exogenous betaHB improves ATP following traumatic brain injury in adult rats. J. Neurochem. 2004;90:666–672. doi: 10.1111/j.1471-4159.2004.02542.x. [DOI] [PubMed] [Google Scholar]
- Ralser M. Wamelink M.M. Kowald A. Gerisch B. Heeren G. Struys E.A. Klipp E. Jakobs C. Breitenbach M. Lehrach H. Krobitsch S. Dynamic rerouting of the carbohydrate flux is key to counteracting oxidative stress. J. Biol. 2007;6:10. doi: 10.1186/jbiol61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice A.C. Zsoldos R. Chen T. Wilson M.S. Alessandri B. Hamm R.J. Bullock M.R. Lactate administration attenuates cognitive deficits following traumatic brain injury. Brain Res. 2002;928:156–159. doi: 10.1016/s0006-8993(01)03299-1. [DOI] [PubMed] [Google Scholar]
- Roe S.Y. McGowan E.M. Rothwell N.J. Evidence for the involvement of corticotrophin-releasing hormone in the pathogenesis of traumatic brain injury. Eur. J. Neurosci. 1998;10:553–559. doi: 10.1046/j.1460-9568.1998.00064.x. [DOI] [PubMed] [Google Scholar]
- Ros J. Pecinska N. Alessandri B. Landolt H. Fillenz M. Lactate reduces glutamate-induced neurotoxicity in rat cortex. J. Neurosci. Res. 2001;66:790–794. doi: 10.1002/jnr.10043. [DOI] [PubMed] [Google Scholar]
- Rose M.E. Huerbin M.B. Melick J. Marion D.W. Palmer A.M. Schiding J.K. Kochanek P.M. Graham S.H. Regulation of interstitial excitatory amino acid concentrations after cortical contusion injury. Brain Res. 2002;935:40–46. doi: 10.1016/s0006-8993(02)02445-9. [DOI] [PubMed] [Google Scholar]
- Ryu J.K. Choi H.B. McLarnon J.G. Combined minocycline plus pyruvate treatment enhances effects of each agent to inhibit inflammation, oxidative damage, and neuronal loss in an excitotoxic animal model of Huntington's disease. Neuroscience. 2006;141:1835–1848. doi: 10.1016/j.neuroscience.2006.05.043. [DOI] [PubMed] [Google Scholar]
- Ryu J.K. Kim S.U. McLarnon J.G. Neuroprotective effects of pyruvate in the quinolinic acid rat model of Huntington's disease. Exp. Neurol. 2003;183:700–704. doi: 10.1016/s0014-4886(03)00214-0. [DOI] [PubMed] [Google Scholar]
- Ryu J.K. Kim S.U. McLarnon J.G. Blockade of quinolinic acid-induced neurotoxicity by pyruvate is associated with inhibition of glial activation in a model of Huntington's disease. Exp. Neurol. 2004;187:150–159. doi: 10.1016/j.expneurol.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Satchell M.A. Zhang X. Kochanek P.M. Dixon C.E. Jenkins L.W. Melick J. Szabo C. Clark R.S. A dual role for poly-ADP-ribosylation in spatial memory acquisition after traumatic brain injury in mice involving NAD + depletion and ribosylation of 14-3-3gamma. J. Neurochem. 2003;85:697–708. doi: 10.1046/j.1471-4159.2003.01707.x. [DOI] [PubMed] [Google Scholar]
- Schmued L.C. Hopkins K.J. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874:123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- Schurr A. Lactate: the ultimate cerebral oxidative energy substrate? J. Cereb. Blood Flow Metab. 2006;26:142–152. doi: 10.1038/sj.jcbfm.9600174. [DOI] [PubMed] [Google Scholar]
- Schurr A. Payne R.S. Miller J.J. Tseng M.T. Rigor B.M. Blockade of lactate transport exacerbates delayed neuronal damage in a rat model of cerebral ischemia. Brain Res. 2001;895:268–272. doi: 10.1016/s0006-8993(01)02082-0. [DOI] [PubMed] [Google Scholar]
- Sharma P. Karian J. Sharma S. Liu S. Mongan P.D. Pyruvate ameliorates post ischemic injury of rat astrocytes and protects them against PARP mediated cell death. Brain Res. 2003;992:104–113. doi: 10.1016/j.brainres.2003.08.043. [DOI] [PubMed] [Google Scholar]
- Sheline C.T. Behrens M.M. Choi D.W. Zinc-induced cortical neuronal death: contribution of energy failure attributable to loss of NAD(+) and inhibition of glycolysis. J. Neurosci. 2000;20:3139–3146. doi: 10.1523/JNEUROSCI.20-09-03139.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson I.A. Carruthers A. Vannucci S.J. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J. Cereb. Blood Flow Metab. 2007;27:1766–1791. doi: 10.1038/sj.jcbfm.9600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slovin P.N. Huang C.J. Cade J.R. Wood C.E. Nasiroglu O. Privette M. Orbach P. Skimming J.W. Sodium pyruvate is better than sodium chloride as a resuscitation solution in a rodent model of profound hemorrhagic shock. Resuscitation. 2001;50:109–115. doi: 10.1016/s0300-9572(01)00325-2. [DOI] [PubMed] [Google Scholar]
- Smythe G.A. Grunstein H.S. Bradshaw J.E. Nicholson M.V. Compton P.J. Relationships between brain noradrenergic activity and blood glucose. Nature. 1984;308:65–67. doi: 10.1038/308065a0. [DOI] [PubMed] [Google Scholar]
- Stover J.F. Sakowitz O.W. Beyer T.F. Dohse N.K. Kroppenstedt S.N. Thomale U.W. Schaser K.D. Unterberg A.W. Effects of LY379268, a selective group II metabotropic glutamate receptor agonist on EEG activity, cortical perfusion, tissue damage, and cortical glutamate, glucose, and lactate levels in brain-injured rats. J. Neurotrauma. 2003;20:315–326. doi: 10.1089/089771503765172273. [DOI] [PubMed] [Google Scholar]
- Suh S.W. Aoyama K. Matsumori Y. Liu J. Swanson R.A. Pyruvate administered after severe hypoglycemia reduces neuronal death and cognitive impairment. Diabetes. 2005;54:1452–1458. doi: 10.2337/diabetes.54.5.1452. [DOI] [PubMed] [Google Scholar]
- Suh S.W. Chen J.W. Motamedi M. Bell B. Listiak K. Pons N.F. Danscher G. Frederickson C.J. Evidence that synaptically-released zinc contributes to neuronal injury after traumatic brain injury. Brain Res. 2000;852:268–273. doi: 10.1016/s0006-8993(99)02095-8. [DOI] [PubMed] [Google Scholar]
- Suh S.W. Frederickson C.J. Danscher G. Neurotoxic zinc translocation into hippocampal neurons is inhibited by hypothermia and is aggravated by hyperthermia after traumatic brain injury in rats. J. Cereb. Blood Flow Metab. 2006;26:161–169. doi: 10.1038/sj.jcbfm.9600176. [DOI] [PubMed] [Google Scholar]
- Sunami K. Nakamura T. Kubota M. Ozawa Y. Namba H. Yamaura A. Makino H. Spreading depression following experimental head injury in the rat. Neurol. Med. Chir. (Tokyo) 1989a;29:975–980. doi: 10.2176/nmc.29.975. [DOI] [PubMed] [Google Scholar]
- Sunami K. Nakamura T. Ozawa Y. Kubota M. Namba H. Yamaura A. Hypermetabolic state following experimental head injury. Neurosurg. Rev. 1989b;12:400–411. doi: 10.1007/BF01790682. [DOI] [PubMed] [Google Scholar]
- Sutton R.L. Hovda D.A. Adelson P.D. Benzel E.C. Becker D.P. Metabolic changes following cortical contusion: relationships to edema and morphological changes. Acta Neurochir. (Wien) 1994;60:446–448. doi: 10.1007/978-3-7091-9334-1_122. Suppl. [DOI] [PubMed] [Google Scholar]
- Sutton R.L. Lescaudron L. Stein D.G. Unilateral cortical contusion injury in the rat: vascular disruption and temporal development of cortical necrosis. J. Neurotrauma. 1993;10:135–149. doi: 10.1089/neu.1993.10.135. [DOI] [PubMed] [Google Scholar]
- Tabatabaie T. Potts J.D. Floyd R.A. Reactive oxygen species-mediated inactivation of pyruvate dehydrogenase. Arch. Biochem. Biophys. 1996;336:290–296. doi: 10.1006/abbi.1996.0560. [DOI] [PubMed] [Google Scholar]
- Tavazzi B. Signoretti S. Lazzarino G. Amorini A.M. Delfini R. Cimatti M. Marmarou A. Vagnozzi R. Cerebral oxidative stress and depression of energy metabolism correlate with severity of diffuse brain injury in rats. Neurosurgery. 2005;56:582–589. doi: 10.1227/01.neu.0000156715.04900.e6. [DOI] [PubMed] [Google Scholar]
- Taylor A.N. Rahman S.U. Sanders N.C. Tio D.L. Prolo P. Sutton R.L. Injury severity differentially affects short- and long-term neuroendocrine outcomes of traumatic brain injury. J. Neurotrauma. 2008;25:311–323. doi: 10.1089/neu.2007.0486. [DOI] [PubMed] [Google Scholar]
- Tsacopoulos M. Magistretti P.J. Metabolic coupling between glia and neurons. J. Neurosci. 1996;16:877–885. doi: 10.1523/JNEUROSCI.16-03-00877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij B.H. Muizelaar J.P. Vinas F.C. Peterson P.L. Xiong Y. Lee C.P. Mitochondrial dysfunction after experimental and human brain injury and its possible reversal with a selective N-type calcium channel antagonist (SNX-111) Neurol. Res. 1997;19:337–339. doi: 10.1080/01616412.1997.11740821. [DOI] [PubMed] [Google Scholar]
- Vespa P. Boonyaputthikul R. McArthur D.L. Miller C. Etchepare M. Bergsneider M. Glenn T. Martin N. Hovda D. Intensive insulin therapy reduces microdialysis glucose values without altering glucose utilization or improving the lactate/pyruvate ratio after traumatic brain injury. Crit. Care. Med. 2006;34:850–856. doi: 10.1097/01.CCM.0000201875.12245.6F. [DOI] [PubMed] [Google Scholar]
- Vespa P.M. Nuwer M. Ronne-Engstrom E. Hovda D.A. Martin N.A. Becker D.P. Increased incidence and impact of nonconvulsive and convulsive seizures after traumatic brain injury as detected by continuous electroencephalographic monitoring. J. Neurosurg. 1999;91:750–760. doi: 10.3171/jns.1999.91.5.0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink R. Head V.A. Rogers P.J. McIntosh T.K. Faden A.I. Mitochondrial metabolism following traumatic brain injury in rats. J. Neurotrauma. 1990;7:21–27. doi: 10.1089/neu.1990.7.21. [DOI] [PubMed] [Google Scholar]
- Wang X. Perez E. Liu R. Yan L.J. Mallet R.T. Yang S.H. Pyruvate protects mitochondria from oxidative stress in human neuroblastoma SK-N-SH cells. Brain Res. 2007;1132:1–9. doi: 10.1016/j.brainres.2006.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y. Gu Q. Peterson P.L. Muizelaar J.P. Lee C.P. Mitochondrial dysfunction and calcium perturbation induced by traumatic brain injury. J. Neurotrauma. 1997;14:23–34. doi: 10.1089/neu.1997.14.23. [DOI] [PubMed] [Google Scholar]
- Yi J.S. Kim T.Y. Kyu K.D. Koh J.Y. Systemic pyruvate administration markedly reduces infarcts and motor deficits in rat models of transient and permanent focal cerebral ischemia. Neurobiol. Dis. 2007;26:94–104. doi: 10.1016/j.nbd.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Ying W. Alano C.C. Garnier P. Swanson R.A. NAD + as a metabolic link between DNA damage and cell death. J. Neurosci. Res. 2005;79:216–223. doi: 10.1002/jnr.20289. [DOI] [PubMed] [Google Scholar]
- Ying W. Chen Y. Alano C.C. Swanson R.A. Tricarboxylic acid cycle substrates prevent PARP-mediated death of neurons and astrocytes. J. Cereb. Blood Flow Metab. 2002;22:774–779. doi: 10.1097/00004647-200207000-00002. [DOI] [PubMed] [Google Scholar]
- Ying W. Garnier P. Swanson R.A. NAD +repletion prevents PARP-1-induced glycolytic blockade and cell death in cultured mouse astrocytes. Biochem. Biophys. Res. Commun. 2003;308:809–813. doi: 10.1016/s0006-291x(03)01483-9. [DOI] [PubMed] [Google Scholar]
- Yoo M.H. Lee J.Y. Lee S.E. Koh J.Y. Yoon Y.H. Protection by pyruvate of rat retinal cells against zinc toxicity in vitro, and pressure-induced ischemia in vivo. Invest. Ophthalmol. Vis. Sci. 2004;45:1523–1530. doi: 10.1167/iovs.03-1315. [DOI] [PubMed] [Google Scholar]
- Yoshino A. Hovda D.A. Katayama Y. Kawamata T. Becker D.P. Hippocampal CA3 lesion prevents postconcussive metabolic dysfunction in CA1. J. Cereb. Blood Flow Metab. 1992;12:996–1006. doi: 10.1038/jcbfm.1992.137. [DOI] [PubMed] [Google Scholar]
- Yoshino A. Hovda D.A. Kawamata T. Katayama Y. Becker D.P. Dynamic changes in local cerebral glucose utilization following cerebral concussion in rats: evidence of a hyper- and subsequent hypometabolic state. Brain Res. 1991;561:106–119. doi: 10.1016/0006-8993(91)90755-k. [DOI] [PubMed] [Google Scholar]
- Zeng J. Yang G.Y. Ying W. Kelly M. Hirai K. James T.L. Swanson R.A. Litt L. Pyruvate improves recovery after PARP-1-associated energy failure induced by oxidative stress in neonatal rat cerebrocortical slices. J. Cereb. Blood Flow Metab. 2007;27:304–315. doi: 10.1038/sj.jcbfm.9600335. [DOI] [PubMed] [Google Scholar]