Abstract
Early maternal separation and other disruptions of attachment relations are known to increase risk for the later onset of depressive illness in vulnerable individuals. It is suggested here that sensitization involving proinflammatory processes may contribute to this effect. This argument is based on: (1) current notions of the role of proinflammatory cytokines in depressive illness; (2) evidence that proinflammatory cytokines mediate depressive-like behavior during separation in a rodent model of infant attachment; and (3) comparisons of the effects of early proinflammatory activation versus maternal separation on later proinflammatory activity and biobehavioral processes related to depression. The possible interaction of proinflammatory processes and corticotropin-releasing factor in the sensitization process is discussed.
Keywords: depression, separation, attachment, proinflammatory activity, cytokines, hypothalamic-pituitary-adrenal, corticotropin-releasing factor
Introduction
In recent years, there has been a concerted effort to better understand the relationship between stress and depression. Stress promotes depression in vulnerable individuals in at least two ways. First, periods of major stress frequently precipitate the onset of depressive bouts (Bonde et al., 2008; Caspi et al., 2003; Chadda et al., 2007; Gotlib et al., 2008). Second, exposure to stressors during early life—often stressors involving disruption of attachment relations (i.e., separation, abuse, neglect)—increases the risk for the onset of depressive illness in later life (Agid et al., 1999; Bernet & Stein, 1999; Gilman et al., 2003; Reinherz et al., 1999; Takeuchi et al., 2002).
The association between early attachment-figure separation and depression was suggested by the work of Spitz and others in the 1940’s and 50’s, which demonstrated that children often exhibited depressive-like behavior during prolonged separation from their parents in hospitals or other institutional settings (Bowlby et al., 1952; Robertson, 1953; Spitz, 1946). Studies of nonhuman primates that then followed firmly established the importance of the attachment figure in infancy for healthy psychological functioning (Harlow & Harlow, 1969; Mineka & Suomi, 1978) and identified a stage of behavioral response to maternal separation that was marked by passivity and withdrawal. Behavior during this passive or “despair” stage clearly resembled the depressive behavior of separated children (Kaufman & Rosenblum, 1967; Mineka & Suomi, 1978) and soon was established as an early animal model of depressive illness (McArthur & Borsini, 2006). Later studies by Levine and others in both nonhuman primates and rodents demonstrated the variety of essential physiological functions [e.g., cardiovascular, hypothalamic-pituitary-adrenal (HPA)] and behaviors of the infant that were, as a matter of course, controlled and regulated by the presence of the maternal figure (e.g., Hofer, 1987; Mason & Berkson, 1975; Rosenfeld et al., 1992; Schanberg et al., 2003).
In humans, retrospective studies began to document a positive correlation between early attachment-figure separation and increased risk for depression in adulthood (Birtchnell, 1972; Brown et al., 1977). As the association of early attachment disruption and later depression has progressively become more firmly established in the literature (e.g., Kessler et al., 2008; Widom et al., 2007), studies attempting to identify the mechanisms responsible have proliferated as well. Research has implicated the HPA and serotonergic systems among others (Gillespie & Nemeroff, 2007; Spinelli et al., 2007), though the bulk of attention has focused on corticotropin-releasing factor (CRF). A number of lines of evidence suggest that CRF acting at both hypothalamic and extra-hypothalamic sites is a crucial factor determining the effect of early stressors on later depression and anxiety disorders (Bradley et al., 2008; Coplan et al., 2001; Heim et al., 2008; Keen-Rhinehart et al., 2008). Enhancement or sensitization of CRF action on, for instance, HPA, sympathetic, and central monoamine systems as a result of the early trauma provides a unifying mechanism for “stress diathesis” models for the development of depressive illness (Gold et al., 1988; Heim & Nemeroff, 2001), and has helped build a case for current attempts to develop CRF antagonists as anti-depressants (Binneman et al., 2008; Holsboer & Ising, 2008; Valdez, 2006).
This paper will suggest that the association of early attachment disruption with the later onset of depression might also involve a sensitization of proinflammatory pathways; that is, a neuroimmune process. This notion was prompted by ongoing research in our laboratories indicating that proinflammatory activity mediates depressive-like behavior in an animal model of attachment-figure separation. The present paper places these findings in the context of: (1) the now extensive literature documenting that proinflammatory processes can contribute to depressive illness; and, (2) evidence that increased proinflammatory activity in the CNS can exert long-term effects on both behavior and stress-related physiological systems that are relevant to the development of depressive illness. Specifically, the current paper will suggest that stress-induced activation of proinflammatory activity during attachment-figure separation (or possibly other forms of attachment disruption) in early life may increase proinflammatory activity or its effects on other stress-related systems in later life, and thereby increase the chances of depressive illness. Such a process, if confirmed, might be incorporated within the broader framework of the stress-diathesis model of depression and its proposed mediators (e.g., CRF). Others have proposed that stressors and increased proinflammatory activity can cross-sensitize neural circuits underlying depression (Anisman et al., 2003; Tilders & Schmidt, 1999). We build on this idea as it applies to a specific form of an early psychosocial stressor (attachment-figure separation) thought to be particularly potent at promoting the development of depression in later life.
In the following sections we first will provide a brief overview of relevant findings regarding the role of proinflammatory activity in depressive illness. Second, we will present evidence primarily from our own comparative work indicating that proinflammatory processes underlie depressive-like behavior during maternal separation. Third, comparisons of the lasting effects of increased proinflammatory activity and maternal separation on relevant biobehavioral measures and proinflammatory activity in later life will be made. Finally, conclusions from these sets of data, as well as limitations and qualifications of the proposal, will be discussed.
Proinflammatory processes in depressive illness
The notion that proinflammatory processes can play an important role in at least some forms of depressive illness has gained widespread acceptance during the last decade. A complete review of the role of proinflammatory activity in depression is beyond the scope of the present paper. A number of more-comprehensive recent reviews of this topic are available (e.g., Dantzer et al., 2008; Dunn et al., 2005; Miura et al., 2008; Schiepers et al., 2005). The purpose here is to provide the basic framework of this idea, to illustrate the kinds of data that support it, and to demonstrate how relevant proinflammatory processes appear to be linked to stress in ways that are amenable to study in laboratory animals.
Proinflammatory cytokines [Interleukin-1 (IL-1), IL-6, tumor necrosis factor-α (TNF-α), and interferon-α (INF- α) among others] are peptides secreted by monocytes/macrophages and microglia upon detection of antigens as part of the innate immune response. The peptide signalers orchestrate a systemic inflammatory response known as the acute phase response, or sickness, that constitutes a first line of defense against pathogen replication (Bauman & Gauldie, 2004). The acute phase response consists of both physiological components such as fever, shifts in the production of proteins by the liver, and HPA activation, as well as behavioral changes. In general, active behaviors are diminished and passive responses predominate. “Sickness behaviors” include reductions in feeding, drinking, socio-sexual activity, and overall interaction with the environment, as well as the seeking of warmth, shivering, piloerection, sleepiness, cognitive impairments, and the assumption of a hunched posture (Hart, 1988; Yirmiya, 1996). The responses largely appear to be motivated behaviors rather than simply the result of debilitation (Aubert, 1999), and seem to be adaptive during times of illness by supporting the production of fever, conserving energy, etc (Hart, 1988). Interestingly, sick animals can also appear “depressed”, i.e., disengaged, asocial, and, in some cases, projecting an impression of sadness.
Evidence that increased proinflammatory cytokine activity contributes to human depression stems from several sources. For instance, levels of circulating cytokines and other markers of the acute phase response have been found to be elevated in depressed patients (Kronfol, 2002). Chemotherapy with proinflammatory cytokines, in particular INF-α, provokes depressive reactions in a substantial proportion of patients (e.g., Miyaoka et al., 1999; Raison et al., 2006), and the depressive effect of cytokines can be reduced with antidepressants (Musselman et al., 2001). Further, exposing laboratory animals to stressors has been found to elicit increased proinflammatory activity and aspects of an acute phase response, including depressive-like, sickness behavior (Maier & Watkins, 1998). Indeed, it has been suggested that cytokine activation in response to stressors may be a major factor accounting for the onset of stress-induced depression (Miura et al., 2008). Thus, study of stress-induced sickness behavior offers a potential framework in which to consider the ability of periods of stress to precipitate depressive episodes. During stressor exposure, increased proinflammatory activity may alter metabolism of tryptophan to reduce central serotonin, enhance HPA activity, and increase central neurotoxic activity (Hayley et al., 2005; Miura et al., 2008). In other words, stress-induced cytokine activity may promote depression by its action on other putative mechanisms of depressive illness, namely: reduced serotonergic activity, HPA hyperactivity, and increased neurotoxic action. Moreover, Koo and Duman (2008) and Goshen et al (2008) recently reported that stressor-induced behavioral signs of depression and associated reductions in neurogenesis in mice were reversed by central antagonism of the IL-1 receptor. These latter findings not only fit well with a growing literature supporting neurogenesis as a key adaptation that may be necessary for the action of typical antidepressants (Balu & Lucki 2008), but they also implicate cytokines in the process.
While such findings suggest involvement of proinflammatory cytokines in stress-induced depression, they say little about a possible role for cytokines in the ability of early attachment disruption to predispose individuals to depression in later life. Most demonstrations that stressors can elicit signs of an acute phase response have employed manipulations (e.g., immobilization) with little psychosocial component and have used older aninals (Deak et al., 2005; Lemay et al., 1990; Maier & Watkins, 1998; Pugh et al., 1999). Though these studies have been valuable for documenting how stressors beget cytokine expression, little remains known about the proinflammatory response of young animals to stressors such as separation from the maternal attachment figure.
Proinflammatory activity during maternal separation
In nonhuman primates, the most well-documented consequence of maternal separation on activity of the immune system is a suppression, particularly of specific immunity (e.g., of T-cell and B-cell activity; Coe, 1993; Laudenslauger, et al., 1982). Yet, these findings do not rule out an enhancement of other immune responses, including those of the innate immune system. Indeed, Coe, Rosenberg, and Levine (1988) found that a 24-hr separation procedure produced a dramatic and prolonged increase in macrophage activity and other signs of an acute phase response in squirrel monkeys. Nevertheless, effects of maternal separation on proinflammatory cytokines and the acute phase response in nonhuman primates have not been studied in any detail, and the relation of proinflammatory activity to behavioral responses during separation in these animals is unknown. Recently, we have examined these questions in a rodent species, the guinea pig.
The guinea pig is a valuable rodent for studies of attachment-figure separation. Using traditional criteria, guinea pigs display better evidence for a specific attachment to the mother than do laboratory rats or mice (Hennessy, 2003; Hennessy & Ritchey, 1987; Jackel & Trillmich, 2003). This difference is probably related to differences in infant development and the nature of maternal care in these species. Unlike rats or mice, newborn guinea pigs are extremely precocial at birth: they are born fully furred with their eyes and ears open. The pups can locomote within minutes of birth and begin to ingest small quantities of solid food and water during the first days of life (Harper, 1976; Schiml & Hennessy, 1990). Lactating guinea pig females have no permanent nest and do not retrieve pups or show substantial amounts of other active maternal behaviors (Konig, 1985). As a result, it is the strong attraction, or attachment, of the pup for the mother that is primarily responsible for maintaining mother-young proximity. Because guinea pigs exhibit better evidence for a true filial attachment process that do laboratory rats or mice, lasting effects of separation can be attributed more definitively to removal of an attachment figure as opposed, for instance, to changes in maternal behavior following return of the pups (e.g., Meaney et al., 1996).
Studies over the last 25 years have shown that there are a large number of similarities in the physiological and behavioral responses that occur during separation in guinea pigs and species of nonhuman primates (Hennessy, 2003). These similarities include activation of stress-related systems (e.g., HPA, sympathetic, central dopamine and norepinephrine) as well as the occurrence of passive behavioral responses during maternal separation. As is the case in nonhuman primate infants, the immediate behavioral response to separation is activation, particularly of vocalizing, which is then followed by a reduction of active behavior and the emergence of a constellation of passive responses, which in the guinea pig include a characteristic crouched stance, prolonged closure of the eyes, and extensive piloerection (Fig. 1; Hennessy et al., 1995). In guinea pigs, however, the response develops much more rapidly than in primates (several hours vs many hours or days). Moreover, we hesitate to refer to the response in guinea pigs as “despair” since it is evoked simply by placing a physically developed pup into a new cage for a few hours. The impression conveyed by the passive response is one of physical illness, an impression that also struck early investigators of the passive response in nonhuman and human primates (Kaufman & Rosenblum, 1967; Spitz, 1946).
Figure 1.
Illustration of the passive response of isolated guinea pig pups. The response is characterized by a crouched stance, closed eyes, and extensive piloerection.
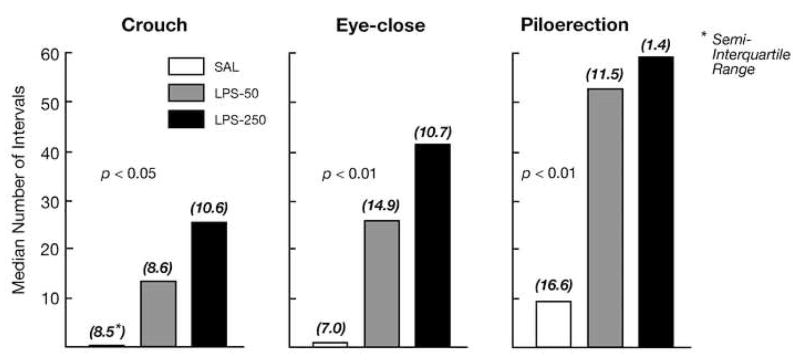
Such observations prompted the hypothesis that passive responses to separation are mediated by proinflammatory cytokines (i.e., represent stress-induced sickness behaviors; Hennessy et al., 2001), which has motivated much of our research over the past several years. This possibility has been addressed experimentally from several different approaches. One tact has been to show that the components of the response seen during separation can be elicited by a proinflammatory cascade. To test this possibility, pups were injected with lipopolysacchride (LPS), a component of the cell wall of gram negative bacteria that elicits a robust acute phase response free of the effects of a replicating pathogen. When these pups were then observed in our test situation for a 1-hour period (during what would be the active response phase under control conditions), we observed a dose-related increase in each of the passive responses relative to pups injected with saline (Fig. 2; Hennessy et al., 2004). Thus, the crouching, eye-closing, and piloerection are indeed expressed as part of the natural repertoire of sickness behaviors in the guinea pig pup.
Figure 2.
Median number of 60-s intervals (and semi-interquartile range of scores) in which guinea pig pups injected with saline or one of two doses of LPS exhibited a crouched stance, closed eyes, and extensive piloerection during 60 min of isolation in a novel environment, a duration of separation in which levels of passive behavior typically are low.
If proinflammatory cytokines contribute to the behavior of separated guinea pig pups, then it should be possible to demonstrate increased cytokine activity, and direct physiological effects of increased cytokine activity, during separation. In agreement with these expectations, gene expression of TNF-α in spleen was found to be elevated over baseline rates at one and three hours with our isolation procedure (Hennessy et al., 2007a), and an increase in core temperature attributable to the absence of the mother—and not correlated with physical activity—has been observed (Hennessy et al., 2008).
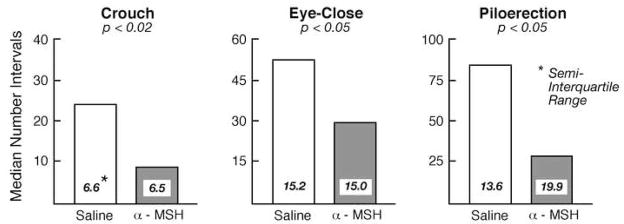
Perhaps the most direct means of documenting that passive behaviors of separated pups are mediated by proinflammatory activity is by showing that anti-inflammatory compounds reduce passive behavior during separation. In a first study, the peptide α-melanocyte-stimulating hormone (α-MSH) was administered to separated pups. α-MSH is a naturally occurring peptide with a variety of physiological and behavioral effects. We chose it for our initial study because is has a very potent and broad spectrum of anti-inflammatory activity. We found that intracerebroventricular (ICV) infusion of α-MSH significantly reduced each of the three passive responses during a separation of 3 hr, a duration of separation that is sufficient to induce a robust passive response (Fig. 3; Schiml-Webb et al., 2006). Because the same dose and administration procedures also decreased passive behavior induced by LPS injection (Hennessy et al., 2007b), it appeared that it was the anti-inflammatory property of α-MSH that was responsible for the behavioral effect.
Figure 3.
Median number of 60-s intervals (and semi-interquartile range of scores) in which guinea pig pups infused ICV with either saline or 25 μg of α-MSH exhibited a crouched stance, closed eyes, and extensive piloerection during 3 hr of isolation in a novel environment. High levels of passive behavior typically emerge by 3 hr of separation. Shown are values pooled across observations made during Min 0–30, 60–90, and 150–180.
Prostaglandins are inflammatory products induced by cytokines that contribute to sickness behaviors (Johnson et al., 1993; Yirmiya et al., 1997). Therefore, we also assessed the effect of indomethacin, which inhibits the activity of cyclo-oxygenase-2, a rate-limiting enzyme for the production of prostaglandin E2 during inflammatory challenges. Peripheral injection of indomethacin (which readily crosses the blood-brain barrier) significantly reduced passive responses of guinea pig pups during 3 hours of separation (Hennessy et al., 2007b). Finally, we examined IL-10, a cytokine that has a variety of anti-inflammatory actions, including reversal of LPS-induced behavioral effects (Bluthe et al., 1999; Nava et al., 1997; Smith et al., 1999). An association of this peptide with depression is suggested by the finding that administration of antidepressants increases levels of IL-10 (Kubera et al., 2000). We found that ICV IL-10 across doses ranging from 12.5 to 250 ng significantly reduced all measures of passive responding (Perkeybile et al., in press). In summary, three quite different anti-inflammatory agents have now been observed to reduce the passive responding of guinea pig pups during a several-hour period of separation from the mother. These results strongly suggest proinflammatory processes contribute to the passive responses observed during attachment-figure separation. The results are consistent with the somewhat limited findings in monkeys (Coe et al., 1988) and raise the possibility that proinflammatory activity may underlie depressive-like behavior during the “despair” stage of separation in primates as well.
Lasting effects of enhanced proinflammatory activity during early life
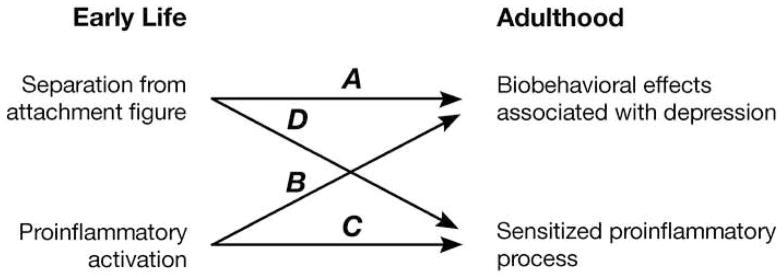
If depressive-like behavior during infancy is mediated by increased proinflammatory activity, and if proinflammatory activity in adulthood contributes to the onset of depression, then perhaps the association of early attachment-figure separation and other forms of attachment disruption (e.g., neglect, abuse) with an increased likelihood of developing depressive illness might be due in part to lasting effects on, or of, proinflammatory activity. That is, proinflammatory processes that contribute to depression (either the proinflammatory activity itself or its effects on other relevant systems) may be sensitized by the proinflammatory response occurring during the early insult. If this were the case, one would expect to find similarities in the effects of early attachment-figure separation and early proinflammatory activation. Specifically, Arrows A and B in Figure 4 indicate that one would predict that both separation from the attachment figure and enhanced proinflammatory activity during early life should produce physiological and behavioral outcomes associated with depression. Arrows C and D illustrate it should be possible to show that the same two early interventions also increase proinflammatory activity, or sensitize its effects on other relevant systems, in later life. Here we will review extant findings that bear on these predictions.
Figure 4.
Illustration of predictions. Both separation from the attachment figure and early proinflammatory activation are predicted to result in biobehavioral effects associated with depression (Arrows A and B) and a sensitization of the proinflammatory response or its effects (Arrrows C and D) in adulthood.
Immunological challenge in early postnatal life has lasting effects on HPA activity in laboratory animals. Whereas neonatal immune activation with, for instance, LPS has been observed to produce no effect or variable effects on basal HPA hormone levels (del Rey et al., 1996; Ellis et al., 2005, 2006; Hodgson et al., 2001; Shanks et al., 1995, 2000; Walker et al., 2006), a more-consistent finding has been an exaggerated response to stressors, such as restraint, in adults exposed to LPS in the neonatal period (Hodgson et al., 2001; Shanks et al., 1995; Shanks et al., 2000). This effect appears to be achieved at least in part through a disruption of negative feedback. Specifically, LPS administration to rats in the first week of life was found to reduce ACTH suppression in response to the synthetic glucocorticoid dexamethasone, reduce glucocorticoid receptor density in hippocampus, hypothalamus, and frontal cortex, and to enhance CRF and arginine vasopressin expression in the median eminence (Shanks et al., 1995). These findings are relevant to depression because enhanced HPA activity and suppression of negative feedback have long been known to frequently accompany major depressive episodes (Carroll et al.,1968; Sachar et al., 1970) and may play a causative role in the disorder (Shelton, 2007). And as noted above, an increase in HPA activity is one means by which stress-driven increases in cytokine activity have been proposed to induce the onset of a depressive episode (Miura et al, 2008). In contrast to the effects of early LPS, exposure of infant rats to an actual bacterial infection typically has been found to have no effect on later HPA responses (Bilbo et al., 2005a , b; 2006), or even to reduce later responses (Bilbo et al., 2008b ). It seems that the presence of a replicating pathogen in addition to just cytokine release in some way negates or alters the later response. Alternatively, the procedures used in the different sets of studies may have resulted in differences in the degree or nature of the cytokine response.
How do these effects of early immunological challenge compare to those of attachment-figure separation? First, it should be said that relevant studies examined rats and mice almost exclusively, and effects of maternal separation in these species—particularly effects on HPA activity—may be due in large part to changes in treatment of the pup by the mother, rather than to absence of the attachment-figure per se (Lehmann & Feldon, 2000; Meaney et al., 1996). For that reason, similarities in the outcome of early immunological challenge and of early maternal separation in rats and mice might be due to similarities in the treatment the pups receive as a result of these manipulations (Hennessy et al., 2007a). With that caution in mind, there are similarities in the effects of early immunological challenge and maternal separation, though only with more-prolonged periods of separation.
Brief periods of maternal separation, the so-called “handling” procedure typically reduces HPA responsiveness to mild and moderate stressors at later ages (Levine, 1967; Meaney et al., 1996). When separations are extended to several hours, later HPA responsiveness increases from handled levels and can exceed that of controls (Plotsky et al., 2005). Moreover, this increase in HPA activity appears to be achieved by the same means as observed following early immunological challenge: a reduction of glucocorticoid receptor expression in areas (e.g., hippocampus) mediating negative feedback on CRF and arginine vasopression production, resulting in enhanced HPA response to stressors (Meaney et al., 1996). In sum, prolonged separation from the mother and early proinflammatory activation with LPS both have been found to increase measures of HPA activity, and to do so at least in part through a reduction of glucocorticoid negative feedback. The lasting consequences of early LPS administration and of prolonged maternal separation on HPA activity are similar to each other and to changes in HPA activity that accompany, and may promote, depressive behavior.
There also are similarities in the behavioral consequences of early cytokine activation and maternal separation. Administration of LPS shortly after birth has been reported to produce several lasting effects that collectively could be characterized as reflecting increased emotionality or anxiety. These include reduced time in, and number of entries into, the open arms of an elevated plus maze (Breivik et al., 2002; Walker et al., 2004b ), more-prolonged behavioral activation in response to noise exposure (Shanks et al., 2000), reduced exploration in open field and hole-board apparatuses (Breivik et al., 2002; Spencer et al., 2005), and more reactivity (e.g., startle) and immobility during an encounter with a conspecific in an unfamiliar environment (Granger et al., 1996; Granger et al., 2001). As with HPA effects, these behavioral effects of cytokine activation are most consistent with those of prolonged maternal separation, rather than of infant “handling”. While it should be noted that behavioral effects of prolonged separation have varied widely—largely it seems because of differences in the procedures used (Lehmann & Feldon, 2000)—several studies have reported evidence for increased emotionality (e.g., decreased open-field exploration, tendency to avoid open arms of elevated plus maze; Francis et al., 2002; Huot et al., 2001; Patchev et al., 1997; Wigger & Neumann, 1999) as well as increased ingestion of alcohol (Huot et al., 2001). Thus, both early immune challenge and prolonged maternal separation appear capable of producing outcomes suggestive of increased anxiety, which frequently is comorbid with depression. In one study in which rats were infected with actual bacteria in early life, depressive-like behavior following inescapable shock in adulthood was actually reduced relative to controls (Bilbo et al., 2008b ). As with effects on HPA activity, actual infection with a replicating pathogen has effects on behavior that sharply differ from those seen when cytokine activation is achieved in the absence of a replicating pathogen by administering LPS.
If the early proinflammatory response to separation sensitizes later proinflammatory processes (proinflammatory activity itself or its effects on other systems), which then promotes depression, one might also expect both direct activation of proinflammatory cytokines in early life and attachment-figure separation to enhance proinflammatory processes in adulthood (Arrows C and D in Fig. 4). Effects of induction of a cytokine cascade in early life on adult cytokine-related measures (Arrow C) have been examined, and, once again, the results are mixed. Shanks et al (2000) found early LPS reduced an inflammatory condition (arthritis model) in rats, while Boisse et al (2004) observed adult rats treated with LPS in infancy to have a reduced febrile and COX-2 response to LPS but increased hypothalamic levels of COX-2. Others have observed reductions in fever and peripheral cytokine responses when exposed to the same bacterial (LPS) or viral mimetic as experienced during the preweaning period, but not when exposed to the heterotypic mimetic (Ellis et al., 2005, 2006). Some of the reduction in effects of a second administration of the same mimetic probably reflects the commonly observed tolerance to repeated exposure (e.g., Soszynski, 2002) or a tolerance-like effect that depends on initial exposure during a sensitive early period (Ellis et al., 2006). Nonetheless, several studies have now found that neonatal bacterial infection enhances proinflammatory responses following LPS exposure in adulthood. These effects include an enhanced febrile response, elevated central and peripheral cytokine activity, and increased central COX-2 levels and glia activity, and appear in large part to reflect reduced tolerance to later immunological challenges (Bilbo et al., 2005a, 2007a, 2008a). However, reduced cytokine responses to LPS in early infected rats have also been observed (Bilbo et al., 2005b ). Clearly more work is needed to better sort out the precise conditions that lead to the different outcomes, but it is certain that early cytokine activation can alter later cytokine activity. At least under some conditions, rats exposed to bacterial infection in infancy, and the cytokine cascade that it induces, exhibit enhanced proinflammatory activity in adulthood.
It is also known that cytokine activation can sometimes sensitize the effects of cytokines, as well as effects of stressors, on HPA activity in adult animals. Cytokine activation was observed to enhance later HPA responsiveness to immune challenge or stressor (e.g., novelty) exposure, and also increased later expression of hypothalamic CRF, AVP, and the CRF1 receptor under resting conditions (Anisman et al, 2003; Schmidt et al., 2003). Though these studies were performed in adult animals, they suggest that the enhanced HPA activity seen in rats or mice exposed as infants to a cytokine cascade (e.g., Shanks et al., 2000) may be a direct result of the cross-sensitization of cytokines on hypothalamic activity driving ACTH release. We are not aware of studies of such sensitization effects on measures of negative feedback of the HPA axis.
Proinflammatory effects on behavior also can be sensitized by prior cytokine activation. That is, previous injection with cytokines or LPS in adulthood increased the sickness behavior response to later cytokine or LPS injection (Anisman et al., 2003; Hayley et al., 2001); and bacterial infection in infancy enhanced the adult behavioral response to cytokine exposure (Bilbo et al., 2008a ).
We recently obtained preliminary evidence for sensitization of separation behavior in infant guinea pigs. When guinea pig pups were separated from the mother for 3 hours on consecutive days, there was a robust increase from the first day to the second in levels of those passive responses that appear to be mediated by proinflammatory processes (Table 1; Hennessy et al., 2008). Further, this increase in passive behavior was associated with a more-rapidly occurring febrile response to the absence of the mother during the second separation. These results parallel those in adult rats in which injection of LPS enhanced the sickness response to LPS or TNF-α 24 hr later (Hayley et al., 2001). Finally, memory impairment induced by early bacterial infection was found to be abolished in rats following administration of an IL-1 synthesis inhibitor (Bilbo et al., 2005a ). In summary, there is evidence that increased proinflammatory activity can sensitize physiological systems thought to underlie depressive illness as well as depressive-like (sickness) behavior. This process provides a potential means by which enhanced proinflammatory activity during early separation or other forms of attachment disruption might facilitate the development of depressive conditions at a later life stage (Anisman et al., 2003).
Table 1.
Median values (semi-interquartile range) of passive behaviors of pups tested alone on 2 consecutive days
| Behavior | Day 1 | Day 2 |
|---|---|---|
| Crouch | 6.0 (8.6) | 31.0 (11.9) |
| Eye-close | 6.0 (5.1) | 27.5 (6.6) |
| Piloerection | 9.0 (15.1) | 37.5 (7.4) |
| Full passive (all 3 together) | 0.5 (2.1) | 16.0 (8.1) |
As for Arrow D in Figure 4 (early separation leading to a sensitized proinflammatory process), a study in which preweaning mice were subjected to prolonged maternal separation and adulthood cytokine responses were observed showed that separation enhanced cytokine expression to viral infection (Avitsur et al., 2006). Of particular relevance in the present context, the authors note in the Discussion that the results of a preliminary study suggested that early separation also enhances sickness behavior in response to later viral infection. Further, although the focus of the present paper is on the postnatal stressor of maternal separation, it warrants mention that exposing pregnant rats to a stressor (restraint) can increase proinflammatory activity in the adult offspring (Vanbesien-Mailliot et al., 2007). Finally, two human experiments suggest a relation between childhood stressors and increased proinflammatory activity in adulthood. In a longitudinal study, measures of childhood maltreatment (e.g., abuse, maternal rejection) predicted higher levels of proinflammatory markers at 32 years of age (Danese et al., 2007). Further, Pace et al (2006) found that a group of depressed men who reported high levels of childhood trauma exhibited a greater proinflammatory response to a laboratory psychosocial stressor than did a comparison group of non-depressed men reporting less childhood trauma.
Conclusions, Qualifications, and Limitations
It has long been known that disruption of an infant’s attachment to its mother produces maladaptive, depressive-like responses during the time of the separation itself, and is associated with increased risk of depression and other psychopathologies (e.g., anxiety) in adulthood. Further, separation from an attachment figure ranks high among psychosocial stressors in its potency at stimulating stress responses, particularly of the HPA axis (Hennessy, 1997). It appears that one of the responses that can be elicited by the stressor of attachment-figure separation is an activation of proinflammatory processes characteristic of an acute phase response. Results from our laboratories indicate that such processes underlie depressive-like behavior exhibited by separated guinea pig pups. And though results have often been conflicting, data reviewed here provide reason to suspect that sensitization of proinflammatory responses or their effects may contribute to depressive behavior in adulthood. If this were to prove to be the case, it would seem that the processes involved could readily be incorporated in current stress-diathesis models for the development of depressive illness, which focus in large part on sensitization of CRF and its effects on the HPA axis and other relevant physiological systems (e.g., central monoamine, sympathetic; Gold et al., 1988. Heim et al., 2008).
The proposal presented here might fit within such schemes in at least two general ways. First, as suggested by the cross-sensitization data presented above, proinflammatory activity may sensitize CRF responses. That is, part of the impact of early cytokine activation may be through CRF-dependent pathways. Second, the separation-induced cytokine response might itself be mediated by CRF. Although CRF increases levels of glucocorticoids, which are well known for their anti-inflammatory actions, CRF itself has proinflammatory effects (Ilias & Mastorakos, 2003). It is possible that exposure to the stressor of separation increases CRF activity, which in turn activates proinflammatory processes. In line with this reasoning, we have found that injection of CRF induces passive separation behaviors during the initial (typically active) phase of separation in guinea pig pups in the same fashion as does injection of LPS (Hennessy et al., 1995). Furthermore, the passive behavior elicited by CRF, as well as by LPS, is reduced if the pups are also administered α-MSH, which, as indicated above, has widespread anti-inflammatory action (Schiml-Webb et al., submitted). Injection of just a CRF-receptor antagonist delays the onset of passive behavior (McInturf & Hennessy, 1996), suggesting that the effects of CRF injection reflect actions of endogenous CRF. In sum, CRF could conceivably stimulate proinflammatory activity that is then subject to sensitization; and/or CRF systems could be sensitized by the increased proinflammatory activity in early life.
Some qualifications and limitations of the present proposal should also be noted. The above discussion should not be interpreted as a claim that maternal separation produces a complete acute phase response, that all separation behaviors are mediated by cytokine activity, or that cytokine activity is the only mediator of those behaviors that are affected by cytokine action. Rather, we are suggesting that attachment-figure separation evokes some elements of the acute phase response, that some separation behaviors (e.g., active behaviors) are likely not affected by cytokine activity, and that the behaviors that are affected by cytokines may be affected by various other mediators as well. One significant limitation of the present proposal is the absence of direct evidence for central cytokine activation underlying separation behavior. Sickness behavior is thought to result from the effect of cytokines on central neural structures (Maier & Watkins, 1998). In the guinea pig pup, we have found that separation increases peripheral cytokine activity (Hennessy et al., 2007a), and that centrally administered anti-inflammatory agents attenuate passive behavioral responses (Perkeybile et al., in press; Schiml-Webb et al., 2006). While these findings strongly suggest proinflammatory involvement, the nature and site of the putative central proinflammatory activity that induces behavioral change remains to be determined. Further, long-lasting effects of early experience on depressive-like responses in the guinea pig model have yet to be identified. Whether proinflammatory activity mediates behavior during maternal separation in primate infants also is unknown. However, the recent finding that INF-α administration produces sickness behavior in rhesus monkeys, which includes a hunched posture resembling the posture seen in the despair stage of maternal separation, certainly is consistent with this possibility (Felger et al., 2007).
Finally, since this volume is dedicated to the late Seymour (Gig) Levine and his pervasive influence on the field of early experience, it seems fitting to note that the general notion of a link between one’s early experience and later immunological functioning and health was recognized by Gig long ago (Levine & Cohen, 1959; Solomon et al., 1968). While a fuller recognition of the implications of such early findings had to await the interpretative context provided by more-recent results and theory from the field of psychoneuroimmunology, Gig’s ideas and findings here, as in so many other areas, presaged modern trends in developmental and neuroscience research.
Acknowledgments
Preparation of this paper was supported by grant MH068228 from the National Institute of Mental Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agid O, Shapira B, Zislin J, Ritsner M, Hanin B, Murad H, Troudart T, Bloch M, Heresco-Levy U, Lerer B. Environment and vulnerability to major psychiatric illness: a case study of early parental loss in major depression, bipolar disorder and schizophrenia. Molecular Psychiat. 1999;4:163–172. doi: 10.1038/sj.mp.4000473. [DOI] [PubMed] [Google Scholar]
- Anisman H, Merali Z, Hayley S. Sensitization associated with stressors and cytokine treatments. Brain, Behav Immun. 2003:86–93. doi: 10.1016/s0889-1591(02)00100-9. [DOI] [PubMed] [Google Scholar]
- Aubert A. Sickness and behaviour in animals: a motivational perspective. Neurosci Biobeh Rev. 1999;23:1029–1036. doi: 10.1016/s0149-7634(99)00034-2. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Hunzeker J, Sheridan JF. Role of early stress in the individual differences in host response to viral infection. Brain Behav Immun. 2006;20:339–348. doi: 10.1016/j.bbi.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Balu DT, Lucki I. Adult hippocampal neurogenesis: regulation, functional implications, and contribution to disease pathology. Neurosci Biobehav Rev. 2008:19. doi: 10.1016/j.neubiorev.2008.08.007. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann H, Gauldie J. The acute phase response. Immunol Today. 1994;15:74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- Bernet CZ, Stein MB. Relationship of childhood maltreatment to the onset and course of major depression in adulthood. Depression and Anxiety. 1999;9:169–174. [PubMed] [Google Scholar]
- Bilbo SD, Biedenkapp JC, Der-Avakian A, Watkins LR, Rudy JW, Maier SF. Neonatal infection-induced memory impairment after lipopolysacchride in adulthood is prevented via caspase-1 inhibition. J Neurosci. 2005a;25:8000–8009. doi: 10.1523/JNEUROSCI.1748-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Levkoff LH, Mahoney JH, Watkins LR, Rudy JW, Maier SF. Neonatal infection induces memory impairments following an immune challenge in adulthood. Behav Neurosci. 2005b;119:293–301. doi: 10.1037/0735-7044.119.1.293. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Newsum NJ, Sprunger DB, Watkins LR, Rudy JW, Maier SF. Differential effects of neonatal handling on early life infection-induced alterations in cognition in adulthood. Brain, Behav Immun. 2007;21:332–342. doi: 10.1016/j.bbi.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Rudy JW, Watkins LR, Maier SF. A behavioural characterization of neonatal infection-facilitated memory impairment in adult rats. Behav Brain Res. 2006;169:39–47. doi: 10.1016/j.bbr.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Wieseler J, Barrientos RM, Watkins LR, Maier SF. Rats infected early in life with bacteria exhibit exaggerated fever, sickness beavior, and lack of endotoxin tolerance in adulthood. Brain Behav Immun. 2008a;22 (Supplement):3–4. [Google Scholar]
- Bilbo SD, Yirmiya R, Amat J, Paul ED, Watkins LR, Maier SF. Bacterial infection early in life protects against stressor-induced depressive-like symptoms in adult rats. Psychoneuroendocrinol. 2008b;33:261–269. doi: 10.1016/j.psyneuen.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binneman B, Feltner D, Kolluri S, Shi Y, Qiu R, Stiger T. A 6-week randomized, placebo-controlled trial of CP-316, 311 (a selective CRH1 antagonist) in the treatment of major depression. Am J Psychiat. 2008;165:617–620. doi: 10.1176/appi.ajp.2008.07071199. [DOI] [PubMed] [Google Scholar]
- Birtchnell J. Early parental death and psychiatric diagnosis. Soc Psychiat. 1972;7:202–210. [Google Scholar]
- Bluthe RM, Castanon N, Pousset F, Bristow A, Ball C, Lestage J, Michaud B, Kelley KW, Dantzer R. Central injection of IL-10 antagonizes the behavioural effects of lipopolysaccharide in rats. Psychoneuroendocrinology. 1999;24:301–311. doi: 10.1016/s0306-4530(98)00077-8. [DOI] [PubMed] [Google Scholar]
- Boisse L, Mouihate A, Ellis S, Pittman QJ. Long-term alterations in neuroimmune responses after neonatal exposure to lipopolysaccharide. J Neurosci. 2004;24:4928–4934. doi: 10.1523/JNEUROSCI.1077-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonde JP. Psychosocial factors at work and risk of depression: a systematic review of the epidemiological evidence. Occup Environ Med. 2008;65:438–435. doi: 10.1136/oem.2007.038430. [DOI] [PubMed] [Google Scholar]
- Bowlby J, Robertson J, Rosenbluth D. A two-year-old goes to the hospital. Psychoanal Study Child. 1952;7:82–94. [Google Scholar]
- Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, Gillespie CF, Berg T, Evces M, Newport DJ, Stowe ZN, Heim CM, Nemeroff CB, Schwartz A, Cubells JF, Ressler KJ. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiat. 2008;65:190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breivik T, Stephan M, Brabant GE, Straub RH, Pabst R, von Horsten S. Postnatal lipopolysacchride-induced illness predisposes to periodontal disease in adulthood. Brain, Behav Immun. 2002;16:421–438. doi: 10.1006/brbi.2001.0642. [DOI] [PubMed] [Google Scholar]
- Brown GW, Harris T, Copeland JR. Depression and loss. Brit J Psychiat. 1977;130:1–18. doi: 10.1192/bjp.130.1.1. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Carroll BJ, Martin FIR, Davies B. Resistance to suppression by dexamethasone of plasma 11-O.H.C.S. levels in severe depressive illness. Brit Med J. 1968;3:285–287. doi: 10.1136/bmj.3.5613.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadda RK, Malhotra A, Kaw N, Singh J, Sethi H. Mental health problems following the 2005 earthquake in Kashmir: findings of community-run clinics. Prehosp Disaster Med. 2007;22:541–545. doi: 10.1017/s1049023x00005409. [DOI] [PubMed] [Google Scholar]
- Coe CL. Psychosocial factors and immunity in nonhuman primates: a review. Psychosom Med. 1993;55:298–308. doi: 10.1097/00006842-199305000-00007. [DOI] [PubMed] [Google Scholar]
- Coe CL, Rosenberg LT, Levine S. Prolonged effect of psychological disturbance on macrophage chemoluminescence in the squirrel monkey. Brain Behav Immun. 1988;2:151–160. doi: 10.1016/0889-1591(88)90015-3. [DOI] [PubMed] [Google Scholar]
- Coplan JD, Andrews MW, Rosenblum LA, Owens MJ, Friedman S, Gorman JM, Nemeroff CB. Persistent elevations of cerebrospinal fluid concentrations of corticotropin-releasing factor in adult nonhuman primates exposed to early-life stressors: implications for the pathophysiology of mood and anxiety disorders. Proceed Nat Acad Sci. 1996;93:1619–1623. doi: 10.1073/pnas.93.4.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Nat Acad Sci. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: When the immune system subjugates the brain. Nature Rev Neurosci. 2008;9:46–57. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deak T, Border KA, McElderry NK, Barnum CJ, Blandino P, Deak MM, Tammariello SP. Stress-induced increases in hypothalamic IL-1: a systematic comparison of multiple stressor paradigms. Brain Res Bull. 2005;64:541–556. doi: 10.1016/j.brainresbull.2004.11.003. [DOI] [PubMed] [Google Scholar]
- del Rey A, Furukawa H, Monge-Arditi G, Kabiersch A, Voigt KH, Besedovsky HO. Alterations in the pituitary-adrenal axis of adult mice following neonatal exposure to interleukin-1. Brain Behav Immun. 1996;10:235–248. doi: 10.1006/brbi.1996.0021. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH, de Beaurepaire R. Cytokines as mediators of depression: what can we learn from animal studies? Neurosci Biobehav Rev. 2005;29:891–909. doi: 10.1016/j.neubiorev.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Ellis S, Mouihate A, Pittman QJ. Neonatal programming of the rat neuroimmune response: stimulus specific changes elicited by bacterial and viral mimetics. J Physiol. 2006;571:695–701. doi: 10.1113/jphysiol.2005.102939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis S, Mouihate A, Pittman QJ. Early life immune challenge alters innate immune responses to lipopolysaccharide: implications for host defense as adults. FASEB J. 2005;19:1519–1521. doi: 10.1096/fj.04-3569fje. [DOI] [PubMed] [Google Scholar]
- Felger JC, Alagbe O, Hu F, Mook D, Freeman AA, Sanchez MM, Kalin NH, Ratti E, Nemeroff CB, Miller AH. Effects of interferon-alpha on rhesus monkeys: a nonhuman primate model of cytokine-induced depression. Biol Psychiat. 2007;62:1324–1333. doi: 10.1016/j.biopsych.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis DD, Diorio J, Plotsky PM, Meaney MJ. Environmental enrichment reverses the effects of maternal separation on stress reactivity. J Neurosci. 2002;22:7840–7843. doi: 10.1523/JNEUROSCI.22-18-07840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie CF, Nemeroff CB. Corticotropin-releasing factor and the psychobiology of early-life stress. Curr Dir Psychol Sci. 2007;16:85–89. [Google Scholar]
- Gilman SE, Kawachi I, Fitzmaurice GM, Buka SL. Family disruption in childhood and risk of adult depression. Am J Psychiat. 2003;160:939–946. doi: 10.1176/appi.ajp.160.5.939. [DOI] [PubMed] [Google Scholar]
- Gold PW, Goodwin FK, Chrousos GP. Clinical and biochemical manifestations of depression: relation to the neurobiology of stress (Part 2) New Eng J Med. 1988;319:413–420. doi: 10.1056/NEJM198808183190706. [DOI] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T, Yirmiya R. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiat. 2008;13:717–728. doi: 10.1038/sj.mp.4002055. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J, Minor KL, Hallmayer J. HPA axis reactivity: a mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biol Psychiat. 2008;63:847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DA, Hood KE, Dreschel NA, Sergeant E, Likos A. Developmental effects of early immune stress on aggressive, socially reactive, and inhibited behaviors. Dev Psychopath. 2001;13:599–610. doi: 10.1017/s0954579401003108. [DOI] [PubMed] [Google Scholar]
- Granger DA, Hood KE, Ikeda SC, Reed CL, Block ML. Neonatal endotoxin exposure alters the development of social behavior and the hypothalamic-pituitary-adrenal axis in selectively bred mice. Brain Behav Immun. 1996;10:249–259. doi: 10.1006/brbi.1996.0022. [DOI] [PubMed] [Google Scholar]
- Harlow HF, Harlow MK. Effects of various mother-infant relationships on rhesus monkey behaviors. In: Foss BM, editor. Determinants of Infant Behavior IV. Methuen; London: 1969. pp. 15–36. [Google Scholar]
- Harper LV. Behavior. In: Wagner JV, Manning PJ, editors. The Biology of the Guinea Pig. Academic Press; New York: 1976. pp. 31–51. [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Hayley S, Lacosta S, Merali Z, van Rooijen N, Anisman H. Central monoamine and plasma corticosterone changes induced by a bacterial endotoxin: sensitization and cross-sensitization effects. Eur J Neurosci. 2001;13:1155–1165. doi: 10.1046/j.0953-816x.2001.01496.x. [DOI] [PubMed] [Google Scholar]
- Hayley S, Poulter MO, Merali Z, Anisman H. The pathogenesis of clinical depression: stressor- and cytokine-induced alterations of neuroplasticity. Neurosci. 2005;135:659–678. doi: 10.1016/j.neuroscience.2005.03.051. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiat. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinol. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Hennessy MB. Hypothalamic-pituitary-adrenal responses to brief social separation. Neurosci Biobehav Rev. 1997;21:11–29. doi: 10.1016/s0149-7634(96)00013-9. [DOI] [PubMed] [Google Scholar]
- Hennessy MB. Enduring maternal influences in a precocial rodent. Dev Psychobiol. 2003;42:225–336. doi: 10.1002/dev.10095. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Deak T, Schiml-Webb PA. Stress-induced sickness behaviors: An alternative hypothesis for responses during maternal separation. Dev Psychobiol. 2001;39:76–83. doi: 10.1002/dev.1031. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Deak T, Schiml-Webb PA, Barnum CJ. Immune influences on behavior and endocrine activity in early-experience and maternal separation paradigms. In: Czerbska MT, editor. Psychoneuroendocrinology Research Trends. Nova Science Publishers; Hauppauge, NY: 2007a. pp. 293–319. [Google Scholar]
- Hennessy MB, Deak T, Schiml-Webb PA, Carlisle C, O’Brien E. A second, daily maternal separation evokes a separation-specific rise in core temperature and sensitized behavioral response in guinea pig pups. Paper presented at the meeting of the International Society for Developmental Psychobiology; Washington, DC. 2008. [Google Scholar]
- Hennessy MB, Deak T, Schiml-Webb PA, Wilson SE, Greenlee TM, McCall E. Responses of guinea pig pups during isolation in a novel environment may represent stress-induced sickness behaviors. Physiol Behav. 2004;81:5–13. doi: 10.1016/j.physbeh.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Long SJ, Nigh CK, Williams WT, Nolan DJ. Effects of peripherally administered corticotropin-releasing factor (CRF) and a CRF antagonist: Does peripheral CRF activity mediate behavior of guinea pig pups during isolation? Behav Neurosci. 1995;109:1137–1145. doi: 10.1037//0735-7044.109.6.1137. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Ritchey RL. Hormonal and behavioral attachment responses in infant guinea pigs. Dev Psychobiol. 1987;20:613–625. doi: 10.1002/dev.420200607. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Schiml-Webb PA, Miller EE, Maken DS, Bullinger KL, Deak T. Anti-inflammatory agents attenuate the passive responses of guinea pig pups: Evidence for stress-induced sickness behavior during maternal separation. Psychoneuroendocrinol. 2007b;32:508–515. doi: 10.1016/j.psyneuen.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy MB, Vogt J, Levine S. Strain of mother determines long-term effects of early handling: Evidence for maternal mediation. Physiol Psychol. 1982;10:153–157. [Google Scholar]
- Hodgson DM, Knott B, Walker FR. Neonatal endotoxin exposure influences HPA responsivity and impairs tumor immunity in Fischer 344 rats in adulthood. Pediatr Res. 2001;50:750–755. doi: 10.1203/00006450-200112000-00020. [DOI] [PubMed] [Google Scholar]
- Hofer MA. Shaping forces within early social relationships. In: Krasnegor NA, Blass EM, Hofer MA, Smotherman WP, editors. Perinatal Development: a psychobiological perspective. Academic Press; Orlando: 1987. pp. 251–274. [Google Scholar]
- Holsboer F, Ising M. Central CRH system in depression and anxiety—evidence from clinical studies with CRH1 receptor antagonists. Eur J Pharmacol. 2008;583:350–357. doi: 10.1016/j.ejphar.2007.12.032. [DOI] [PubMed] [Google Scholar]
- Huot RL, Thrivikraman KV, Meaney MJ, Plotsky PM. Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long Evans rats and reversal with antidepressant treatment. Psychopharmacol. 2001;158:366–373. doi: 10.1007/s002130100701. [DOI] [PubMed] [Google Scholar]
- Ilias I, Mastorakos G. The emerging role of peripheral corticotropin-releasing hormone (CRH) J Endocrinol Invest. 2003;26:364–371. doi: 10.1007/BF03345186. [DOI] [PubMed] [Google Scholar]
- Jackel M, Trillmich F. Olfactory individual recognition of mothers by young guinea pigs (Cavia porcellus) Ethol. 2003;109:197–208. [Google Scholar]
- Johnson RW, Curtis SE, Dantzer R, Kelley KW. Central and peripheral prostaglandins are involved in sickness behavior in birds. Physiol Behav. 1993;53:127–131. doi: 10.1016/0031-9384(93)90020-g. [DOI] [PubMed] [Google Scholar]
- Kaufman IC, Rosenblum LA. The reaction to separation in infant monkeys: Anaclitic depression and conservation withdrawal. Psychosom Med. 1967;29:648–675. doi: 10.1097/00006842-196711000-00010. [DOI] [PubMed] [Google Scholar]
- Keen-Rhinehart E, Michopoulos V, Toufexis DJ, Martin EI, Nair H, Ressler KJ, Davis M, Owens MJ, Nemeroff CB, Wilson ME. Continuous expression of corticotropin-releasing factor in the central nucleus of the amygdala emulates the dysregulation of the stress and reproductive axes. Mol Psychiat. 2008 doi: 10.1038/mp.2008.91. E-pub, ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Pecora PJ, Williams J, Hiripi E, O’Brien K, English D, White J, Zerbe R, Downs AC, Plotnick R, Hwang I, Sampson NA. Effects of enhanced foster care on the long-term physical and mental health of foster care alumni. Arch Gen Psychiat. 2008;65:625–633. doi: 10.1001/archpsyc.65.6.625. [DOI] [PubMed] [Google Scholar]
- Konig B. Maternal activity budget during lactation in two species of Caviidae (Cavia porcellus and Galea musteloides) Zeit Tierpsychol. 1985;68:215–230. [Google Scholar]
- Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci USA. 2008;105:751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronfol Z. Immune dysregulation in major depression: A critical review of existing evidence. Int J Neuropsychopharm. 2002;5:333–343. doi: 10.1017/S1461145702003024. [DOI] [PubMed] [Google Scholar]
- Kubera M, Holan V, Mathison R, Maes M. The effect of repeated amitriptyline and desipramine administration on cytokine release in C57BL/6 mice. Psychoneuroendocrinol. 2000;25:785–797. doi: 10.1016/s0306-4530(00)00026-3. [DOI] [PubMed] [Google Scholar]
- Laudenslager ML, Reite M, Harbeck RJ. Suppressed immune response in infant monkeys associated with maternal separation. Behav Neur Biol. 1982;36:40–48. doi: 10.1016/s0163-1047(82)90223-0. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Feldon J. Long-term biobehavioral effects of maternal separation in the rat: consistent or confusing. Rev Neurosci. 2000;11:383–408. doi: 10.1515/revneuro.2000.11.4.383. [DOI] [PubMed] [Google Scholar]
- LeMay LG, Vander AJ, Kluger MJ. The effects of psychological stress on plasma interleukin-6 activity in rats. Physiol Behav. 1990;47:957–961. doi: 10.1016/0031-9384(90)90024-x. [DOI] [PubMed] [Google Scholar]
- Levine S. Maternal and environmental influences on the adrenocortical response to stress in weanling rats. Science. 1967;156:258–260. doi: 10.1126/science.156.3772.258. [DOI] [PubMed] [Google Scholar]
- Levine S, Cohen C. Differential survival to leukemia as a function of infantile stimulation in DBA/s mice. Proceed Soc Exp Med. 1959;102:53–54. doi: 10.3181/00379727-102-25140. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Cytokines for psychologists: implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol Rev. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- Mason WA, Berkson G. Effects of maternal mobility on the development of rocking and other behaviors in rhesus monkeys: a study with artificial mothers. Dev Psychobiol. 1975;8:197–211. doi: 10.1002/dev.420080305. [DOI] [PubMed] [Google Scholar]
- McArthur R, Borsini F. Animal models of depression in drug recovery: A historical perspective. Pharmacol Biochem Behav. 2006;84:436–452. doi: 10.1016/j.pbb.2006.06.005. [DOI] [PubMed] [Google Scholar]
- McInturf SM, Hennessy MB. Peripheral administration of corticotropin-releasing factors antagonists increases the vocalizing and locomotor activity of isolated guinea pig pups. Physiol Behav. 1996;60:707–710. doi: 10.1016/0031-9384(96)00091-1. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Diorio J, Francis D, Widdowson J, LaPlante P, Caldji C, Sharma S, Seckl JR, Plotsky PM. Early environmental regulation of forebrain glucocorticoid receptor gene expression: implications for adrenocortical responses to stress. Dev Neurosci. 1996;18:49–72. doi: 10.1159/000111395. [DOI] [PubMed] [Google Scholar]
- Mineka S, Suomi SJ. Social separation in monkeys. Psychol Bull. 1978;85:1376–1400. [PubMed] [Google Scholar]
- Miura H, Ozaki N, Sawada M, Isobe K, Ohta T, Nagatsu T. A link between stress and depression: shift in the balance between the kynurenine and serontonin pathways of tryptophan metabolism and the etiology and pathophysiology of depression. Stress. 2008;11:198–209. doi: 10.1080/10253890701754068. [DOI] [PubMed] [Google Scholar]
- Miyaoka H, Otsubo T, Kamijima K, Ishii M, Onuki M, Mitamura K. Depression from interferon therapy in patients with Hepatitis C. Am J Psychiat. 1999;156:1120. doi: 10.1176/ajp.156.7.1120. [DOI] [PubMed] [Google Scholar]
- Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, Goodkin RS, Greiner K, Nemeroff CB, Miller AH. Paroxetine for the prevention of depression induced by high-dose interferon Alfa. N Engl J Med. 2001;344:961–966. doi: 10.1056/NEJM200103293441303. [DOI] [PubMed] [Google Scholar]
- Nava F, Calapai G, Facciolá G, Cuzzocrea S, Marciano MC, De Sarro A, Caputi AP. Effects of interleukin-10 on water intake, locomotory activity, and rectal temperature in rat treated with endotoxin. Int J Immunopharmacol. 1997;19:31–38. doi: 10.1016/s0192-0561(97)00006-4. [DOI] [PubMed] [Google Scholar]
- Pace TWW, Mletzko TC, Alagabe O, Musselman DL, Nemeroff CB, Miller AH, Heim CM. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiat. 2006;163:1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- Patchev VK, Montkowski A, Rouskova D, Koranyi L, Holsboer F, Almeida OFX. Neonatal treatment of rats with the neuroactive steroid tetrahydrodeoxycorticosterone (THDOC) abolishes the behavioral and neuroendocrine consequences of adverse early life events. J Clin Invest. 1997;99:962–966. doi: 10.1172/JCI119261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkeybile AM, Schiml-Webb PA, O’Brien E, Deak T, Hennessy MB. Anti-inflammatory influences on behavioral, but not cortisol, responses during maternal separation. Psychoneuroendocrinol. doi: 10.1016/j.psyneuen.2009.02.014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacol. 2005;30:2192–2204. doi: 10.1038/sj.npp.1300769. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Feldon J. Long-term neurobehavioural impact of the postnatal environment in rats: manipulations, effects and mediating mechanisms. Neurosci Biobehav Rev. 2003;27:57–71. doi: 10.1016/s0149-7634(03)00009-5. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Nguyen KT, Gonyea JL, Fleshner M, Watkins LR, Maier SF, Rudy JW. Role if interleukin-1 beta in impairment of contextual fear conditioning caused by social isolation. Behav Brain Res. 1999;106:109–118. doi: 10.1016/s0166-4328(99)00098-4. [DOI] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz HZ, Giaconia RM, Carmola Hauf AM, Wasserman MS, Silverman AB. Major depression in the transition to adulthood: risks and impairments. J Abnor Psychol. 1999;108:500–510. doi: 10.1037//0021-843x.108.3.500. [DOI] [PubMed] [Google Scholar]
- Robertson J. Some responses of young children to the loss of maternal care. Nursing Times. 1953 April;:382–386. [Google Scholar]
- Rosenfeld P, Suchecki D, Levine S. Multifactorial regulation of the hypothalamic-pituitary-adrenal axis during development. Neurosci Biobehav Rev. 1992;16:553–568. doi: 10.1016/s0149-7634(05)80196-4. [DOI] [PubMed] [Google Scholar]
- Sachar EJ, Hellman L, Fukushima DK, Gallagher TF. Cortisol production in depressive illness. Arch Gen Psychiat. 1970;23:289–298. doi: 10.1001/archpsyc.1970.01750040001001. [DOI] [PubMed] [Google Scholar]
- Schanberg SM, Ingledue VF, Lee JY, Hannun YA, Bartolome JV. PKC-α mediates maternal touch regulation of growth-related gene expression in infant rats. Neuropsychopharmacol. 28:1026–1030. doi: 10.1038/sj.npp.1300125. [DOI] [PubMed] [Google Scholar]
- Schiepers OJG, Wichers MC, Maes M. Cytokines and major depression. Prog Neuro-Psychopharmacol Biol Psychiat. 2005;29:201–217. doi: 10.1016/j.pnpbp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Schiml PA, Hennessy MB. Light-dark variation and changes across the lactational period in the behavior of undisturbed mother and infant guinea pigs (Cavia porcellus) 1990;104:283–288. doi: 10.1037/0735-7036.104.3.283. [DOI] [PubMed] [Google Scholar]
- Schiml-Webb PA, Deak T, Greenlee TM, Maken DS, Hennessy MB. Alpha-melanocyte stimulating hormone reduces putative stress-induced sickness behaviors in isolated guinea pig pups. Behav Brain Res. 2006;168:326–330. doi: 10.1016/j.bbr.2005.08.022. [DOI] [PubMed] [Google Scholar]
- Schiml-Webb PA, Miller EE, Deak T, Hennessy MB. Alpha-melanocyte stimulating hormone attenuates behavioral effects of corticotropin-releasing factor in isolated guinea pig pups. doi: 10.1002/dev.20379. (manuscript submitted for publication) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt ED, Aguilera G, Binnekade R, Tilders FJH. Single administration of interleukin-1 increased corticotropin releasing hormone and corticotropin releasing hormone-receptor mRNA in the hypothalamic paraventricular nucleus which paralleled long-lasting (weeks) sensitization to emotional stressors. Neurosci. 2003;116:275–283. doi: 10.1016/s0306-4522(02)00555-9. [DOI] [PubMed] [Google Scholar]
- Shanks N, Larocque S, Meaney MJ. Neonatal endotoxin exposure alters the development of the hypothalamic-pituitary-adrenal axis: early illness and later responsivity to stress. J Neurosci. 1995;15:376–384. doi: 10.1523/JNEUROSCI.15-01-00376.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks N, Windle RJ, Perks PA, Harbuz MS, Jessop DS, Ingram CD, Lightman SL. Early-life exposure to endotoxin alters hypothalamic-pituitary-adrenal function and predisposition to inflammation. Proc Nat Acad Sci. 2000;97:5645–5650. doi: 10.1073/pnas.090571897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton RC. The molecular neurobiology of depression. Psychiat Clin North Am. 2007;30:1–11. doi: 10.1016/j.psc.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EM, Cadet P, Stefano GB, Opp MR, Hughes TK., Jr IL-10 as a mediator in the HPA axis and brain. J Neuroimmunol. 1999;100:140–148. doi: 10.1016/s0165-5728(99)00206-4. [DOI] [PubMed] [Google Scholar]
- Solomon GF, Levine S, Kraft JK. Early experience and immunity. Nature. 1968;220:821–822. doi: 10.1038/220821a0. [DOI] [PubMed] [Google Scholar]
- Soszynski D. Inhibition of nitric oxide synthase delays the development of tolerance to LPS in rats. Physiol Behav. 2002;76:159–169. doi: 10.1016/s0031-9384(02)00693-5. [DOI] [PubMed] [Google Scholar]
- Spencer SJ, Heida JG, Pittman QJ. Early life immune challenge—effects on behavioural indices of adult rat fear and anxiety. Behav Brain Res. 2005;164:231–238. doi: 10.1016/j.bbr.2005.06.032. [DOI] [PubMed] [Google Scholar]
- Spinelli S, Schwandt ML, Lindell SG, Newman TK, Heilig M, Suomi SJ, Higley JD, Goldman D, Barr CS. Association between the recombinant human serotonin transporter linked promoter region polymorphism and behavior in rhesus macaques during a separation paradigm. Dev Psychopathol. 2007;19:977–987. doi: 10.1017/S095457940700048X. [DOI] [PubMed] [Google Scholar]
- Spitz RA. Anaclitic depression: an inquiry into the genesis of psychiatric conditions in early childhood: II. Psychoanal Study Child. 1946;2:313–342. [PubMed] [Google Scholar]
- Takeuchi H, Hiroe T, Kanai T, Morinobu S, Kitamura T, Takahashi K, Furukawa TA. Childhood parental separation experiences and depressive symptomatology in acute major depression. Psychiat Clin Neurosci. 2002;53:215–219. doi: 10.1046/j.1440-1819.2003.01103.x. [DOI] [PubMed] [Google Scholar]
- Tilders FH, Schmidt ED. Cross-sensitization between immune and non-immune stressors: a role in the etiology of depression? Adv Exp Med Biol. 1999;461:179–197. doi: 10.1007/978-0-585-37970-8_11. [DOI] [PubMed] [Google Scholar]
- Valdez GR. Development of CRF1 receptor antagonists as antidepressants and anxiolytics: progress to date. CNS Drugs. 2006;20:887–896. doi: 10.2165/00023210-200620110-00002. [DOI] [PubMed] [Google Scholar]
- Vanbesien-Mailliot CCA, Wolowczuk I, Mairesse J, Viltart O, Delacre M, Khalife J, Chartier-Harlin M-C, Maccari S. Prenatal stress has pro-inflammatory consequences on the immune system in adult rats. Psychoneuroendocrinol. 2007;32:114–124. doi: 10.1016/j.psyneuen.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Walker FR, Hodyl NA, Krivanek KM, Hodgson DM. Early life host-bacteria relations and development: long-term individual differences in neuroimmune function following neonatal endotoxin challenge. Physiol Behav. 2006;87:126–134. doi: 10.1016/j.physbeh.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Walker FR, March J, Hodgson DM. Endotoxin exposure in early life alters the development of anxiety-like behaviour in the Fischer 344 rat. Behav Brain Res. 2004;154:63–69. doi: 10.1016/j.bbr.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Widom CS, DuMont K, Czaja SJ. A prospective investigation of major depressive disorder and comorbidity in abused and neglected children grown up. Arch Gen Psychiat. 2007;64:49–56. doi: 10.1001/archpsyc.64.1.49. [DOI] [PubMed] [Google Scholar]
- Wigger A, Neumann ID. Periodic maternal deprivation induces gender-dependent alterations in behavioral and neuroendocrine responses to emotional stress in adult rats. Physiol Behav. 1999;66:293–302. doi: 10.1016/s0031-9384(98)00300-x. [DOI] [PubMed] [Google Scholar]
- Yirmiya R. Endotoxin produces a depressive-like episode in rats. Brain Res. 1996;711:163–174. doi: 10.1016/0006-8993(95)01415-2. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Barak O, Avitsur R, Gallily R, Weidenfeld J. Intracerebral administration of Mycoplasma fermentans produces sickness behavior: role of prostaglandins. Brain Res. 1997;749:7–81. doi: 10.1016/s0006-8993(96)01295-4. [DOI] [PubMed] [Google Scholar]