Abstract
Background
The role of the wet environment in wound healing has been investigated in various studies. The current study explores the role of the wet wound environment in promoting healing of skin grafts. We hypothesized that survival of the skin grafts is not only dependent on the orientation of transplantation, but also on the environment into which the skin is transplanted.
Methods
The study included 72 full-thickness (2.5×2.5cm) wounds in 6 Yorkshire pigs. The wounds were grafted with autologous split-thickness skin grafts (meshed or sheet), placed either regularly (dermal-side-down) or inverted (dermal-side-up), and treated in wet or dry environment. Behavior of the skin grafts and healing were analyzed in histologies collected on days 4, 6, 9 and 12 postwounding. Wound contraction was quantified by photoplanimetry.
Results
In the wet environment, not only did inverted meshed skin grafts survive, but also they proliferated to accelerate reepithelialization. In this environment, wounds transplanted with inverted and regular meshed grafts showed no significant difference in reepithelialization rate and contraction. In contrast, in the dry environment, wounds transplanted with inverted meshed grafts showed a significantly lower reepithelialization and higher contraction than wounds transplanted with regular grafts. Inverted meshed grafts in dry environment and inverted sheet grafts did not survive.
Conclusions
The wound environment has an important role in the survival and proliferation of skin grafts, as demonstrated by survival of inverted meshed grafts in the wet environment and their contribution to accelerated reepithelialization, equal to the regularly placed grafts.
Introduction
History of skin grafting has its origin in ancient India about 2500 to 3000 years ago.1, 2 The modern era of skin grafting as a reconstructive option, however, was introduced by Reverdin (1869) 1, 3 and Thiersch (1886) 4 in the 19th century. Although skin grafting has become a standard procedure for resurfacing skin defects, the physiology of tissue engraftment and the mechanism of wound regeneration following grafting are not fully understood. In the ‘classic theory’ of engraftment, the transplanted skin goes through a multi-stage process of plasmatic imbibition (0 to 48 hours), inosculation (48 to 72 hours), and subsequent revascularization (> 96 hours).5, 6 It is widely accepted that for a successful revascularization and definitive survival, the transplanted piece of skin, should be placed with it dermal side down. In fact, the importance of ‘orientation’ of transplantation has become a part of the surgical tradition, that ‘the only certain way to lose a skin graft is to place it upside down’.
The impact of the environment in promoting wound re-epithelialization was first described by Winter in 1962.7 He demonstrated that when wounds were kept moist under cover of an impermeable film, re-epithelialization was about twice as rapid as the wounds exposed to the air.7 Our laboratory has studied healing in a wet environment. The wound has been enclosed in a transparent polyurethane wound chamber tightly sealed to the surrounding skin and injected with saline and tissue culture concentration of antibiotics. We demonstrated that a wet, chamber enclosed environment reduces tissue damage from desiccation and trauma and accelerates healing of partial thickness 8 and full-thickness 9 wounds. Subsequently, we showed that single cell suspensions of keratinocytes transplanted into this environment proliferate and differentiate into a multilayer epidermis.10
The current study was done to explore the role of the wound environment in promoting healing of skin grafts and to assess whether creation of a wet incubator like environment will allow survival of grafts that would otherwise fail, such as an inverted meshed graft. We hypothesized that survival of the skin graft and the rate of wound re-epithelialization are not only dependent on the orientation of transplantation but also on the environment into which the skin is transplanted.
Materials and methods
A previously established porcine wet wound model 8–12 using transparent polyurethane wound chamber injected with saline provided the environment into which autologous skin was transplanted in various ways. The similarities between human and pig skin, in terms of the structure of dermis and epidermis 13, 14 and the cell turnover time 15 make the porcine model an excellent tool for studying wound healing.16
Animals
All animal procedures were approved by the Harvard Medical Area Standing Committee on Animals. All procedures conformed to the regulations related to animal use and other federal statutes. Six female Yorkshire pigs (Parson’s Farm, MA) weighing 50–60 kg at arrival were allowed to acclimatize for 1 week prior to initiation of the experiment. Animals were kept in smooth-walled stainless steel cages to minimize wound trauma and disruption of applied wound chambers.
Pigs were fasted for 12 hours prior to the surgical procedure. On the day of the procedure, pigs were transferred to a Panepinto sling (Universal Metals, Milford, MA). The animals received induction anaesthesia with Telazol (10 mg/kg BW, Wyeth Madison, NJ)/Xylazine (2.5 mg/kg BW, Xyla-Ject, Phoenix, St. Josephs, MO) via intramuscular injection. General anaesthesia was performed with inhaled Isoflurane (2–3 Vol%; Novaplus, Hospira, IL). During the operations, the animals received Sodium Chloride 0.9% (Hospira, Lake Forest, IL) infusion intravenously at the rate of 150 ml per hour through a 21-gauge intravenous catheter (Becton Dickinson, NJ) inserted into an ear vein. The paraspinal area of the porcine dorsum was waxed (Nair, Church & Dwight, Princeton, NJ) and shaved. The outlines of the wounds were tattooed using a tattoo gun and black ink (Special Electric Tattoo Marker, Huck Spaulding Enterprises, Inc., Voorheesville, NY). The paraspinal area was washed with alcohol and thoroughly disinfected using 10% povidone iodine and then washed with 70% isopropanol.
Split-Thickness Skin Graft harvest
Prior to skin harvest, the designated area was wetted with normal saline. A split thickness skin graft of 50 cm2 was harvested from the dorsal neck region, using a Zimmer pneumatic dermatome (Zimmer Inc, Warsaw IN) set at the thickness of 0.014 inch.
Meshing procedure
For the meshed graft group, each skin piece measured 1×3 cm. For the sheet graft group, each piece measured 2.5 × 2.5 cm. The skin was meshed on the ratio of 2:1 using Brennen Skin Graft Mesher (Brennen Med, St Paul MN).
Wounding procedure
Twelve full-thickness excisional wounds (2.5 × 2.5 × 0.8 cm) were created on dorsum of each pig using a No. 11 blade. Complete hemostasis was achieved before skin grafting.
Skin grafting and Wound chamber treatment
The graft was placed at the base of the wound (Fig. 1). Then the graft margins were sutured with eight 5-0 Vicryl (Ethicon Inc., Stonerville, NJ) to the deep margins of the wound. In the case of the sheet graft, an extra suture was placed in the center.
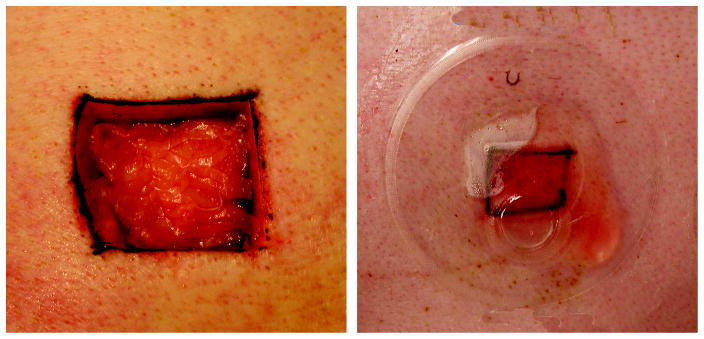
Fig. 1.
A full-thickness wound measuring 2.5cm × 2.5cm, transplanted with meshed split thickness skin graft with the dermal side up in a 1:2 expansion ratio (left). The wet environment (right) is provided by a transparent polyurethane wound chamber sealed to the surrounding skin. The chamber has an injection port for daily fluid exchange that allows for biochemical analysis of wound environment.
Application of wound chambers
Subsequent to the skin grafting process, a thin layer of medical adhesive (Hollister, Libertyville, IL) was applied to the skin surrounding the wound and an adhesive polyurethane chamber (Corium International, Grand Rapids, MI) was placed over each wound (Fig. 1). Wound chambers were injected with 2 ml of keratinocyte growth medium on the first day. Subsequently, wound fluid was collected on a daily basis and replaced with 2 ml of 0.9% sterile saline containing 100 μg/ml Penicillin and 100 μg/ml Streptomycin (Invitrogen, Carlsbad, CA). Dry wounds were covered with dry sterile gauze (Kendall Healthcare, Mansfield MA) under an adhesive Leukotape bandage (BSN medical Ltd, Brierfield, BB9 5NJ, England).
Experimental groups
This study included a total of 72 wounds in 6 pigs divided into 6 experimental groups: 1) Inverted (stratum corneum facing the wound bed) meshed split thickness skin graft in wet environment; 2) Regular (dermis facing the wound) meshed split thickness skin graft in wet environment; 3) Inverted sheet split thickness skin graft in wet environment; 4) Regular sheet split thickness skin graft in wet environment; 5) Inverted meshed split thickness skin graft in dry environment; 6) Regular meshed split thickness skin graft in dry environment.
Light and electron microscopy histology
8 mm cross-sectional wound biopsies were obtained on days 4, 6, 9 and 12. All biopsied wounds were excluded from further study. On the final day of the experiment, pigs were euthanized (Euthasol; Virbac AH, Fort Worth, TX). Samples were fixed in 4% buffered paraformaldehyde. Specimens were processed for hematoxylin-eosin to measure reepithelialization rate. Cytokeratin staining was done to evaluate the behaviour and migration of keratinocytes. Part of the biopsies on day 9 was fixed with 2.5% glutaraldehyde in a buffered solution of 0.1 M sodium cacodylate buffer, pH 7.3, for 2 h at room temperature for electron microscopy.
Assessment of reepithelialization
Reepithelialization was calculated by scanning the slides (Epson Perfection 3600, Epson, CA) and measuring the epithelial tongues from the computerized image with Paintshop Pro 7.0 software (Jasc Software, MN) using the formula: (Sum of epithelial tongues) / (Distance between tattoo marks) x 100.
Assessment of rete formations in the newly formed epithelium
The number of rete formations per each millimetre of neo-epithelium was counted under the microscope from 5 standardized locations in each wound.
Wound Contraction
The wound contraction was determined by digitized planimetry of the tattooed margins as described earlier.9 The area of the wounds on specific days was measured using Scion image software (Scion, MD), and the percentage of contraction was calculated by the formula (Area at biopsy day)/ (Area at wounding) x 100.
Statistic analysis
Values are calculated as means ±SE. Statistical analysis between groups of interest were compared using t-test and statistical calculations were performed with SigmaPlot 10.0. Statistical significance was defined as p-value of <0.05.
Results
Macroscopic appearance of the healing wounds
By day 4, a fibrinous clot had developed in the wet wounds. At 6 days after wounding, this was replaced by reddish-pink granulation tissue that was more prominent in the wet wounds. By day 9, wet wounds were completely filled with granulation tissue. No crust formed in wet wounds; instead they were covered with a thin layer of fibrin that gradually detached during the process of reepithelialization. On day 12, we did not observe any macroscopic difference between the regularly-placed and inverted meshed skin graft groups in the wet environment, both of which appeared fully re-epithelialized. At this point, the dry treated wounds with regularly placed meshed skin graft also looked completely re-epithelialized, but revealed a thick desiccated clot on the surface and healed with significant depression. The wounds that healed in a liquid environment showed a surface that was even or close to even to the rest of the skin surface. Wounds treated with inverted meshed graft in the dry environment showed no difference with their non-transplanted counterparts and remained to a large degree unepithelialized.
Histological assessment and evaluation of reepithelialization
As the meshed skin grafts were expanded to twice the wound surface area (1:2 expansion ratio), the regularly placed skin grafts with the dermis down started out covering 50% of the wound. In this group, epithelialization of the non-grafted interstices resulted from migration and proliferation of keratinocytes from the edges of the meshed skin. On day 4 these same skin grafts had expanded and on day 6 covered approximately 93%, and on day 9, 96% of the surface area of the wounds in the wet environment (Fig. 2). In comparison, the wounds treated with regularly placed meshed grafts in the dry environment revealed a slightly retarded reepithelialization rate (79%) on day 9 (Fig. 3).
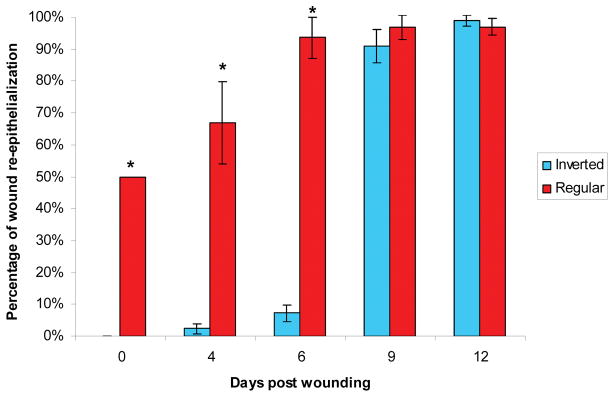
Fig. 2.
Comparison of re-epithelialization between regular and inverted meshed skin graft treated wounds in the wet environment. The bars represent the mean percentage of re-epithelialization of each wound on different biopsy days. Re-epithelialization was significantly higher in wounds treated with regular meshed graft than those with inverted meshed graft on days 0, 4 and 6 (* p<0.01). Re-epithelialization of wounds treated with inverted meshed graft on days 9 and 12 equalized with wounds treated with regular meshed grafts.
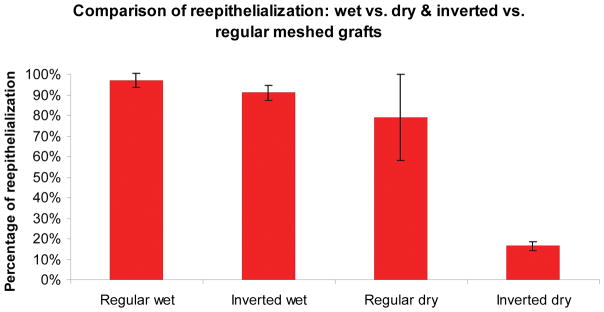
Fig. 3.
The effect of wound environment on inverted and regular meshed skin graft and the rate of wound re-epithelialization on day 9. There is no statistical difference between regular and inverted meshed grafts in the wet environment (96% & 91% respectively). In the dry environment, regular meshed skin grafts showed a slightly lower reepithelialization rate (79%), though this difference did not achieve statistical significance. Re-epithelialization rate of the inverted graft wounds in the dry environment (14%) was significantly lower than the other 3 groups (*p<0.05).
Inverted meshed skin grafts in wet environment survived and showed a different pattern of proliferation. By day 4, several clusters of migrating and proliferating keratinocytes that seemed to have originated from the basal layer, dermal appendages and the edges of the grafted skin were seen moving through the provisional matrix of the newly formed fibrovascular tissue toward the surface of the wound (Fig. 4). This resulted in the formation of multiple advancing tongues of proliferating and differentiating keratinocytes that eventually coalesced with each other and the advancing epithelium from the wound edges to form the new epidermis, the majority of which originated from the transplanted skin (Fig. 5). Following a period of delay of approximately 6 days, the rate of re-epithelialization increased remarkably, catching up with the regularly-placed meshed graft by day 9 and reaching almost 100% on day 12 (Fig. 2 &6). The slight reepithelialization observed before day 6 was from the wound edges. The majority of the surface epithelium seen on day 9, however, came from cells that had migrated from the transplanted skin located in deeper portions of the wound rather than the wound edges. The origin of this epithelium could further be confirmed in many of the transplanted wounds based upon the observations of isolated islands of epidermis not being physically connected to the migrating epithelium originating from the surrounding unwounded skin. After giving rise to newly formed epithelium, the nonviable upper epidermal layers of the skin graft, including its stratum corneum were ultimately eliminated (Fig. 6).
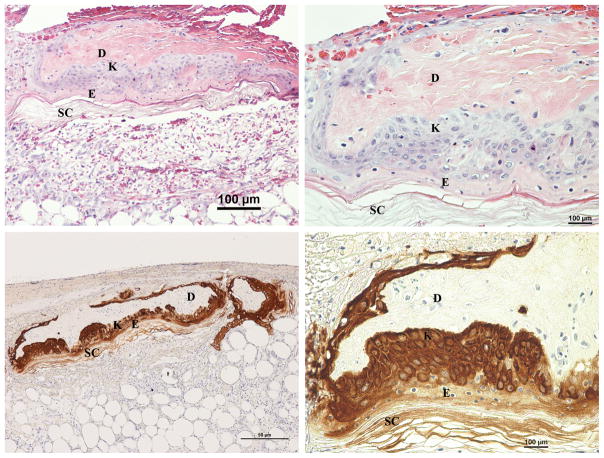
Fig. 4.
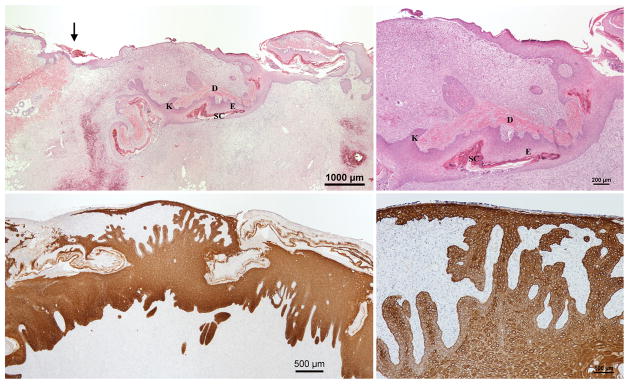
Day 4 H&E (Above left & right) and Cytokeratin (Below left & right) stained sections of wounds with inverted meshed skin graft in the wet environment. H&E sections show an inverted section of the graft with proliferation of the basal keratinocytes throughout its entire length. The upper layers of the original epidermis are not viable at this point. Migrating keratinocytes can be seen moving upward toward the wound surface. D, original dermis, E, original epidermis and SC, original stratum corneum of the skin graft; K, proliferating keratinocytes.
Fig. 5.
Day 9 H&E (Above left & right) and Cytokeratin (Below left & right) stained sections of wounds with inverted meshed skin graft in the wet environment. (Above left & right). Proliferation of keratinocytes arising from the skin graft and moving upward to form the new epidermis can be seen. There is not yet connection between the migrating epithelium originating from the surrounding unwounded skin and the migrating keratinocytes from the skin graft. The arrow indicates the wound margin. The dermis and epidermis of the skin graft appear completely separated. (Below left) The newly formed epithelium at this stage is thick. Some segments of the skin graft are being eliminated from the surface. (Below right) An advancing layer of migrating keratinocytes on the surface with several connecting bridges of keratinocytes from the deeper portions of the wound.
D, original dermis, E, original epidermis and SC, original stratum corneum of the skin graft; K, proliferating keratinocytes.
Fig. 6.
Day 12 H&E (Above) and Cytokeratin (Center & Below) stained sections of the wounds with inverted meshed skin graft in the wet environment. Parts of the skin graft are still seen underneath and connected to the newly formed epidermis. (Center) The newly formed epidermis. (Below) A piece of the skin graft is being eliminated. D, original dermis, E, original epidermis and SC, original stratum corneum of the skin graft; K, proliferating keratinocytes.
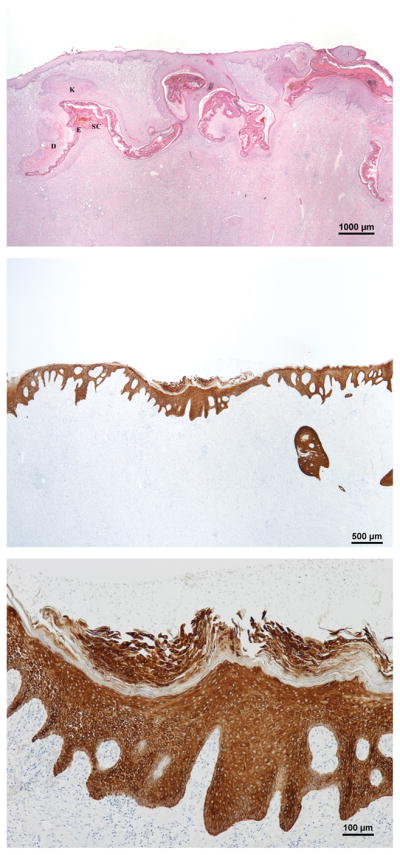
Inverted meshed grafts in the dry environment failed completely. The rate of reepithelialization of the inverted skin graft group in the dry environment was comparable to untransplanted wounds, approximately 14% on day 9 (Fig. 3&7). In the wet environment, regular sheet grafts survived and the corresponding wounds remained fully reepithelialized on day 9. Inverted sheet grafts in wet environments, however, did not survive. Overall, there appeared to be proliferation from all skin grafts except for the inverted meshed grafts in the dry environment and inverted sheet grafts.
Fig. 7.
Day 9 H&E section of a wound treated with inverted meshed graft in the dry environment. There is a central granulation tissue with a lack of significant re-epithelialization aside from short epithelial tongues migrating from the wound margins.
Thickness of the epithelium, rete ridges formation and development of basement membrane
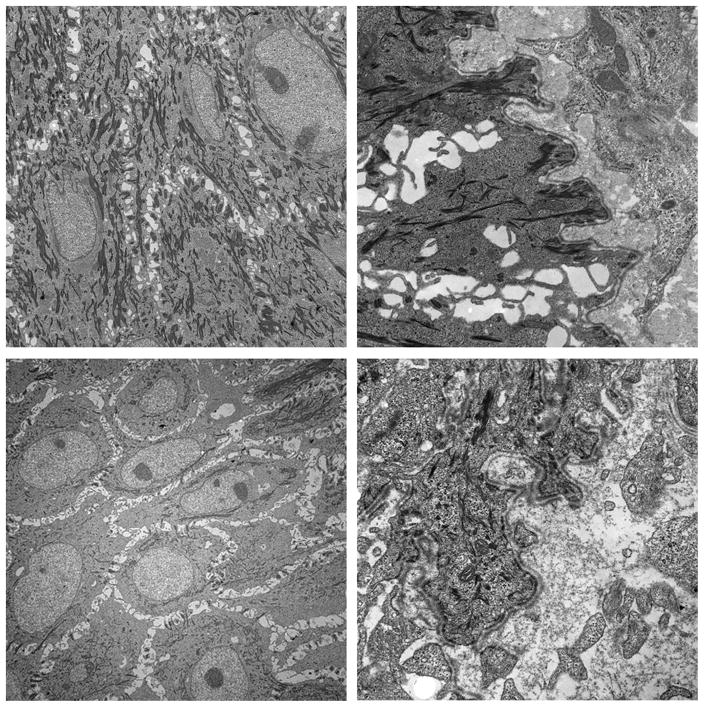
When assessing epithelial thickness in the wet environment, it was found that the initially formed epithelium in the wounds transplanted with inverted meshed skin graft had an irregular shape with a long distance between the deepest part of the epithelium and the surface on day 9. This irregular and thick newly formed epithelium transformed to a thin layer of epithelium with more mature dermo-epidermal junction and orderly formation of rete ridges by day 12. Epithelial thickness in the wounds treated with inverted mesh grafts was 432 μ, in comparison to 312 μ in the wounds treated with regular meshed grafts (p<0.05). The number of rete ridges per linear millimeter on day 12 was 5.8 for the regularly placed skin grafts and 5.4 for the inverted skin graft. This is to be compared with 2.3 per millimeter in normal pig skin (Fig. 8). Additionally, using electron microscopy, we evaluated the development of basement membrane and hemidesmosomes on day 9, which revealed a well formed basement membrane but much fewer hemidesmosomes in the inverted compared with regularly placed skin graft group (Fig. 9).
Fig. 8.

Comparison of epithelial thickness (Left) and number of rete ridges formation (Right) between inverted and regularly placed meshed skin graft treated wounds in the wet environment on day 12. The epithelium in the inverted graft wounds (432 μ) is thicker than regular wounds (312 μ) on day 12 (*p<0.05). There is no significant difference in the number of rete ridges between the two groups (regular, 5.4/mm; inverted, 5.8/mm).
Fig. 9.
Electron microscopy of the wounds treated in the wet environment on day 9. Wounds treated with regular meshed graft show well developed basement membrane (Above right) and hemidesmosomes (Above left). Wounds with inverted meshed graft also revealed well-developed basement membrane (Below right) but much fewer hemidesmosomes (Below left).
Contraction analysis
The area of each wound, as defined by the tattooed outer margins, was expressed as a percent of the area on day 0. On day 12, there was no significant difference in the rate of contraction between the regular (31%) and inverted (29%) meshed graft wounds in the wet environment. On the other hands, the rate of contraction in dry wounds with inverted meshed skin graft (41%) was significantly higher than the regular skin grafts (31%). Overall, the least amount of contraction was observed in the wounds treated with regular sheet graft (17%).
Discussion
The role of the wet wound environment as an important promoter of wound healing has been described previously. Winter in 1962 reported accelerated epithelialization of superficial wounds under the moist conditions.7 In several reports, we showed reduced inflammation and tissue loss from desiccation, and accelerated healing of partial thickness and full-thickness wounds in the wet chamber enclosed environment. 8, 9, 17 Consequently, we showed that transplanted cultured 10, 11 or non-cultured 11 keratinocytes in suspension migrate, proliferate and differentiate to form a multilayer epithelium in the wet wound environment.
The current study was undertaken in order to provide a more definitive proof that the environment is crucial in wound regeneration following skin grafting. It is a common belief that in order to survive, skin graft must be transplanted with its dermal side down. When Meek described his microdermagrafting technique for expansion of skin grafts in 1958, 18 he found that minced pieces of skin would fail unless they were placed with the raw side down. At that time, moist and wet dressings were not available, as Winter reported his findings about healing in a moist environment in 1962. 7 As it was very cumbersome to maintain the orientation of the small skin particles throughout the process of mincing and transplantation, Meek’s method of skin expansion never gained widespread clinical application. Subsequently, in 1964 Tanner described the meshing procedure as it is being used today.19
In this study, we demonstrated that survival of the meshed skin graft is not only dependent on the orientation, but also on the environment into which the skin is transplanted. We achieved this by transplanting skin in an inverted fashion into our established wet wound healing model and evaluated the behavior of the skin graft and wound re-epithelialization at various time points. Clinically, it has been proven repeatedly that meshed skin grafts placed upside down will invariably fail completely. We showed that inverted meshed skin grafts in the wet environment survive and accelerate wound re-epithelialization. We observed a unique pattern of proliferation that has not been described previously. Keratinocytes migrated and proliferated not only from the edges but also from the basal layer and dermal appendages. Basal keratinocytes were seen infiltrating the dermis of the skin graft and forming tongues of migrating and proliferating cells through the provisional matrix of granulation tissue. Following an initial delay, the re-epithelialization of the wounds transplanted in an inverted fashion increased dramatically to catch up with the wounds transplanted with regularly placed grafts. It should be emphasized that regularly placed skin grafts were transplanted in 1:2 expansion ratio and were already 50% re-epithelialized on day 0.
Inverted sheet grafts did not survive. The pattern of proliferation described above was only observed in the inverted meshed grafts in the wet environment. The liquid environment not only allowed the inverted meshed skin grafts to survive but also proliferate and cover the wounds almost as fast as regularly placed skin grafts. It appears that this environment promotes proliferation and migration of the basal keratinocytes not only toward the edges and the skin appendages but toward the dermis of the graft. It is unclear at this point if this is due to liquid itself or due to factors being allowed to diffuse into the graft in the liquid environment.
Several possibilities can explain failure of the inverted sheet grafts. With the stratum corneum adjacent to the raw surface to the wound, the cells would have to receive their nutrition through the liquid medium of the wound. In the early hours after transplantation when the survival of the skin graft is solely dependent on diffusion, it appears that diffusion distance could explain the failure of the inverted sheet grafts, whose basal keratinocytes have the greatest functional distance from the wound bed, given that keratin can act as a barrier against diffusion. It is possible that this type of proliferation can only be initiated if the diffusion distance does not exceed a certain number. An additional important factor, in particular after the first 48 hours, is the time factor as blood vessels will start sprouting from the inner surfaces of the wound soon after wounding 20 and will grow into the interstices of the meshed graft while they can not do so in the inverted sheet graft, as keratin acts as a barrier to vessel in-growth.
Several factors have been proposed to contribute to the improved healing rate in the wet environment. Epidermal cells migrate much easier over moist wound surface instead of under the scab. 7, 21 It is believed that epidermal regeneration may be enhanced in the clot-inducing environment of occlusive dressings,17, 22 perhaps due to increased precipitation of fibrinogen and fibronectin.23 In the wet environment created by a completely sealed wound chamber evaporation from the wound surface does not take place.24 This minimizes unnecessary heat loss and helps the wound maintain a higher metabolism. In addition, presence of growth factors and other signaling substances in the wound exudate retained under the occlusive dressings and in particular distributed in the liquid environment play an important role in stimulating wound repair.25 The chamber wound fluid reaches equilibrium with the interior of the wounds and contains concentrations of electrolytes, glucose and total protein very similar to those found in serum.8 This liquid in turn has strong similarity to common keratinocyte growth medium. Conceptually, the chamber becomes an in vivo incubator in which the wound can be treated as an in vivo tissue culture. Taken together, we believe that this environment offers favorable conditions for cell proliferation and wound healing.
It should be emphasized that the blood clot that forms in the wound upon wounding will form a scaffold in the wet environment and appears to promote not only the formation of granulation tissue but also the migration of skin particles and keratinocytes and fibroblasts.7, 21 In the dry environment this clot would rapidly desiccate and form an almost impermeable crust.7 It has been shown that in the fetus, wounds that heal before the third trimester leave very little scar. 26, 27 It has been assumed that some of this is due to the characteristics of the fetal tissue to regenerate and to the fetal environment.27 This study clearly shows that the liquid environment is essential to healing when for instance the meshed graft is placed upside down. It also appears essential to allowing the wound to be programmed to heal without an uneven surface.
Acknowledgments
This study was supported by Public Health Service Grant RO1GM5144904 from the National Institutes of Health to Dr. Elof Eriksson.
Footnotes
Conflict of interest statement:
E. Eriksson has partial ownership in patents related to wound chamber treatment. There are no financial and personal relationships with other people or organizations that could inappropriately influence this manuscript.
References
- 1.Davis JS. Address Of The President: The Story Of Plastic Surgery. Ann Surg. 1941 May;113(5):641–656. doi: 10.1097/00000658-194105000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pagella P. Sucruta Samhita: an original chapter of surgery or an interdependence of values in the history of medicine? Ann Osp Maria Vittoria Torino. 1977 Jul-Dec;20(7–12):354–363. [PubMed] [Google Scholar]
- 3.Reverdin J. Greffe epidermique. Bull Soc Imperiale Chir Paris. 1869:493. [Google Scholar]
- 4.Smahel J. The healing of skin grafts. Clin Plast Surg. 1977 Jul;4(3):409–424. [PubMed] [Google Scholar]
- 5.Converse JM, Uhlschmid GK, Ballantyne DL., Jr “Plasmatic circulation” in skin grafts. The phase of serum imbibition. Plast Reconstr Surg. 1969 May;43(5):495–499. [PubMed] [Google Scholar]
- 6.Smahel J. Revascularization of a free skin autograft. Acta Chir Plast. 1962;4:102–112. [PubMed] [Google Scholar]
- 7.Winter GD. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature. 1962 Jan 20;193:293–294. doi: 10.1038/193293a0. [DOI] [PubMed] [Google Scholar]
- 8.Breuing K, Eriksson E, Liu P, Miller DR. Healing of partial thickness porcine skin wounds in a liquid environment. J Surg Res. 1992 Jan;52(1):50–58. doi: 10.1016/0022-4804(92)90278-8. [DOI] [PubMed] [Google Scholar]
- 9.Svensjo T, Pomahac B, Yao F, Slama J, Eriksson E. Accelerated healing of full-thickness skin wounds in a wet environment. Plast Reconstr Surg. 2000 Sep;106(3):602–612. discussion 613–604. [PubMed] [Google Scholar]
- 10.Vogt PM, Thompson S, Andree C, et al. Genetically modified keratinocytes transplanted to wounds reconstitute the epidermis. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9307–9311. doi: 10.1073/pnas.91.20.9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Svensjo T, Yao F, Pomahac B, Eriksson E. Autologous keratinocyte suspensions accelerate epidermal wound healing in pigs. J Surg Res. 2001 Aug;99(2):211–221. doi: 10.1006/jsre.2001.6197. [DOI] [PubMed] [Google Scholar]
- 12.Svensjo T, Pomahac B, Yao F, Slama J, Wasif N, Eriksson E. Autologous skin transplantation: comparison of minced skin to other techniques. J Surg Res. 2002 Mar;103(1):19–29. doi: 10.1006/jsre.2001.6331. [DOI] [PubMed] [Google Scholar]
- 13.Hengge UR, Chan EF, Hampshire V, Foster RA, Vogel JC. The derivation and characterization of pig keratinocyte cell lines that retain the ability to differentiate. J Invest Dermatol. 1996 Feb;106(2):287–293. doi: 10.1111/1523-1747.ep12340722. [DOI] [PubMed] [Google Scholar]
- 14.Sullivan TP, Eaglstein WH, Davis SC, Mertz P. The pig as a model for human wound healing. Wound Repair Regen. 2001 Mar-Apr;9(2):66–76. doi: 10.1046/j.1524-475x.2001.00066.x. [DOI] [PubMed] [Google Scholar]
- 15.Weinstein GD. Autoradiographic Studies on Turnover Time and Protein Synthesis in Pig Epidermis. J Invest Dermatol. 1965 Jun;44:413–419. [PubMed] [Google Scholar]
- 16.Donovan W. experimental models in skin flap research. Boston: Little Brown; 1975. [Google Scholar]
- 17.Vogt PM, Andree C, Breuing K, et al. Dry, moist, and wet skin wound repair. Ann Plast Surg. 1995 May;34(5):493–499. doi: 10.1097/00000637-199505000-00007. discussion 499–500. [DOI] [PubMed] [Google Scholar]
- 18.Meek CP. Successful microdermagrafting using the Meek-Wall microdermatome. Am J Surg. 1958 Oct;96(4):557–558. doi: 10.1016/0002-9610(58)90975-9. [DOI] [PubMed] [Google Scholar]
- 19.Tanner JC, Jr, Vandeput J, Olley JF. The Mesh Skin Graft. Plast Reconstr Surg. 1964 Sep;34:287–292. [PubMed] [Google Scholar]
- 20.Converse JM, Filler M, Ballantyne DL., Jr Vascularization Of Split-Thickness Skin Autografts In The Rat. Transplantation. 1965 Jan;3:22–27. doi: 10.1097/00007890-196501000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Winter GD, Scales JT. Effect of air drying and dressings on the surface of a wound. Nature. 1963 Jan 5;197:91–92. doi: 10.1038/197091b0. [DOI] [PubMed] [Google Scholar]
- 22.Jonkman MF, Bruin P, Hoeksma EA, et al. A clot-inducing wound covering with high vapor permeability: enhancing effects on epidermal wound healing in partial-thickness wounds in guinea pigs. Surgery. 1988 Sep;104(3):537–545. [PubMed] [Google Scholar]
- 23.Jonkman MF, Hoeksma EA, Nieuwenhuis P. Accelerated epithelization under a highly vapor-permeable wound dressing is associated with increased precipitation of fibrin(ogen) and fibronectin. J Invest Dermatol. 1990 Apr;94(4):477–484. doi: 10.1111/1523-1747.ep12874624. [DOI] [PubMed] [Google Scholar]
- 24.Vranckx JJ, Slama J, Preuss S, et al. Wet wound healing. Plast Reconstr Surg. 2002 Dec;110(7):1680–1687. doi: 10.1097/01.PRS.0000033181.56887.61. [DOI] [PubMed] [Google Scholar]
- 25.Chen WY, Rogers AA, Lydon MJ. Characterization of biologic properties of wound fluid collected during early stages of wound healing. J Invest Dermatol. 1992 Nov;99(5):559–564. doi: 10.1111/1523-1747.ep12667378. [DOI] [PubMed] [Google Scholar]
- 26.Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2006;22:287–309. doi: 10.1146/annurev.cellbio.22.010305.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin P, Parkhurst SM. Parallels between tissue repair and embryo morphogenesis. Development. 2004 Jul;131(13):3021–3034. doi: 10.1242/dev.01253. [DOI] [PubMed] [Google Scholar]