Abstract
An increase in cytosolic Ca2+ via a capacitative calcium entry mediated pathway, attributed to members of the transient receptor potential (TRP) superfamily, TRPC1 and TRPC3, have been reported to play an important role in regulating cardiomyocyte hypertrophy. Increased cytosolic Ca2+ also plays a critical role in mediating cell death in response to ischemia/reperfusion (I/R). Therefore, we tested the hypothesis that overexpression of TRPC3 in cardiomyocytes will increase sensitivity to I/R injury. Adult cardiomyocytes isolated from wild-type (WT) mice and from mice over expressing TRPC3 in the heart were subject to 90 min ischemia and 3hr reperfusion. After I/R, viability was 51 ± 1% in WT and 42 ± 5% in TG (p < 0.05). Apoptosis assessed by Annexin-V was significantly increased in TRPC3 group compared to WT (32 ± 1% vs. 21 ± 3%; p < 0.05); however, there was no significant difference in necrosis between groups. Treatment of TRPC3 cells with the CCE inhibitor SKF96365 (0.5 μM), significantly improved cellular viability (54 ± 4%) and decreased apoptosis (15 ± 4%); in contrast, the L-type Ca2+ channel inhibitor verapamil (10 μM) had no effect. Calpain-mediated cleavage of α-fodrin was increased ~3-fold in the TG group following I/R compared to WT (p < 0.05); this was significantly attenuated by SKF96365. The calpain inhibitor, PD150606 (25 μM) attenuated the increase in both α-fodrin cleavage and apoptosis in the TPRC3 group. Increased TRPC3 expression also increased sensitivity to Ca2+ overload stress, but did not affect the response to TNF-α induced apoptosis. These results suggest that CCE mediated via TRPC may play a role in cardiomyocyte apoptosis following I/R due, at least in part, by increased calpain activation.
Keywords: Capacitative calcium entry (CCE), calpain, Tumor necrosis factor (TNF)-alpha
INTRODUCTION
In cardiomyocytes calcium plays a central role not only in regulating excitation contraction coupling (3), but also in modulating the response to range of stress stimuli (6). An increase in cytosolic Ca2+ plays a critical role in initiating cardiomyocyte apoptosis and necrosis that occurs in response to ischemia/reperfusion (4, 6). Although a number of Ca2+ entry pathways, including the Na+/Ca2+ exchanger and the L-type Ca2+ channel have been implicated in mediating cardiomyocyte Ca2+ overload (4, 6, 32) there is little consensus as to which pathways are critical in mediating this process. We have identified a capacitative calcium entry (CCE) or store-operated calcium (SOC) entry pathway in neonatal and adult rat cardiomyocytes (12, 13) that not only influences the response to hypertrophic stimuli but may also play a role in mediating Ca2+ overload in the heart (20). CCE or SOC, which was first described in nonexcitable cells (34), refers to the influx of Ca2+ through plasma membrane calcium channels, activated in response to depletion of calcium in the endo/sarcoplasmic reticulum (ER/SR). We have demonstrated that in cardiomyocytes Ca2+ entry via CCE-mediated pathway is clearly distinct from both L-type voltage-gated channels and the reverse mode of Na+/Ca2+ exchanger (12, 13).
The specific proteins responsible for facilitating CCE have yet to be fully characterized; however, there is growing evidence that members of the transient receptor potential (TRP) protein superfamily of proteins may be involved in regulating CCE (14). Mammalian TRP channels are divided in six subfamilies and the TRPC (canonical) family in particular has been implicated in contributing to cellular Ca2+ homeostasis (9, 14, 23, 24, 43). There are seven members of the TRPC family (TRPC1-7), 6 of which (TRPC1-6) have been shown, at least by RT-PCR, to be present in the heart (9). Structurally TRP channels consist of six transmembrane domains containing a putative Ca2+ pore region between the fifth and sixth domains; TRPC channels have ankyrin repeat domains in their N-terminus, which may mediate protein-protein interactions (23). Prototypical activation of TRPC channels occurs in response to agonist stimulation resulting in the generation of diacylglycerol (DAG) and inositol 1,4,5-triphosphate (IP3). Subsequent activation of the ER/SR IP3 receptor (IP3R) can mediate depletion of Ca2+ from internal stores thereby triggering Ca2+ entry across the sarcolemma via TRPCs. Independent of store depletion TRPC activity can be regulated by an increase in DAG; alternatively, IP3R bound to IP3 may activate TRPC via direct interaction (23).
As noted above, until relatively recently CCE was considered to be primarily a feature of non-excitable cells and consequently, the majority of data supporting a role for TRPCs in mediating CCE is also from non-excitable cells. However, a number of recent studies have provided evidence that TRPCs may also be functional in the heart (18, 28, 30). Ohba et al., demonstrated that hypertrophic stimuli increased TRPC1 expression both in the intact heart and in isolated cardiomyocytes (30). They also showed that in isolated cardiomyocytes the increase in TRPC1 expression was associated with increased CCE. The functional significance of TPRC1 was confirmed by using siRNA techniques to prevent agonist induced increase in TRPC1 expression, which attenuated both the hypertrophic response and CCE (30). Conversely, Nakayama et al. demonstrated that overexpression of TRPC3 in cardiomyocytes increased CCE and this was associated with increased cardiac hypertrophy in response to neuroendocrine agonists or pressure overload stimulation (28). Kuwahara et al., (18) also reported that cardiac specific overexpression of TRPC6 increased the propensity for cardiac hypertrophy and heart failure; however, they did not determine whether this was associated with an alteration in CCE.
Therefore, in light of the growing evidence linking TRPC proteins to CCE in cardiomyocytes, combined with our finding that CCE inhibition attenuated cardiac injury in response to calcium overload (20), we tested the hypothesis that increased expression of TRPC3 in cardiomyocytes would result in increased injury due to ischemia/reperfusion. We found that TRPC3 overexpression increased apoptosis and calpain mediated proteolysis resulting from ischemia/reperfusion injury and also increased sensitivity to Ca2+ overload; however, TRPC3 had no effect on the response to TNF-α induced apoptosis. These data strongly suggest that CCE mediated via TRPC may contribute to Ca2+-induced cardiomyocyte apoptosis resulting from ischemia/reperfusion.
MATERIALS AND METHODS
Materials
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise stated.
Animals
All animal experiments were approved by the University of Alabama at Birmingham (UAB) Institutional Animal Care and Use Committee and conformed to the Guide for the Care and Use of Laboratory Animals published by National Institute of Health (NIH publication no. 85-23, 1996). Mice overexpressing TRPC3 in the heart, were a kind gift from Dr. Jeffrey D. Molkentin (University of Cincinnati), and have been characterized in detail elsewhere (28); the mice used in this study were derived from line 23 described in the original study (28). All animals for this study were bred at UAB and were genotyped prior to use. Normal, non-transgenic (WT) littermates were used as controls.
Cardiomyocytes Isolation
Male mice, 2-4months of age (25g - 40g) were heparinized (5000 U/kg, i.p.) 20 min prior to being anesthetized with ketamine (100 mg/kg i.p.). Hearts were rapidly excised and arrested in ice-cold, Ca2+-free perfusion buffer, consisting of (in mM) NaCl (113), KCl (4.7), KH2PO4 (0.6), Na2HPO4 (0.6), MgSO4.7H2O (1.2), Phenol red (0.032), NaHCO3 (12), KHCO3 (10), HEPES (10), Taurine (30), 2,3-Butanedione monoxime (10), Glucose (5.5), pH 7.46. The aorta was cannulated, and the heart was perfused retrogradely with Ca2+-free perfusion buffer at a constant flow of 3 ml/min at 37°C, for 4 min, followed by the same perfusion buffer containing 12.5 μM CaCl2 and 0.4 mg/ml collagenase type 2 (Worthington). After 15-25 min perfusion with collagenase containing buffer, the heart appeared swollen, pale, and flaccid at which time the ventricles were removed and finely minced. Dispersed myocytes were filtered through a 100 μm mesh and allowed to sediment by gravity for 10 min. The supernatant was removed and centrifuged for 1 min at 180 X g. The pellet was resuspended and combined with the original sedimented myocytes in perfusion buffer containing 5% bovine calf serum and 12.5 μM CaCl2. The calcium concentration was increased gradually from 12.5 μM to 1 mM in 5 steps over approximately 20 min. Freshly isolated cardiomyocytes were kept in 2% CO2 incubator at 37C. All experiments were performed at least 1 hour after myocyte isolation.
Experimental protocols
Modified cell pelleting model of ischemia
We used a cell-pelleting model to assess hypoxia- and reoxygenation-induced cell death as described in detail by Yamawaki et al. (39). Briefly, an aliquot of cardiomyocytes suspended in MEM (0.5 ml) was placed into a microcentrifuge tube and centrifuged at 80 × g for 60 s. After centrifugation 0.2 ml of the supernatant was removed and replaced by 0.2 mls of mineral oil, which was layered on top of the cell pellet to prevent diffusion of oxygen into the sample. The cell pellet was maintained at 37°C for 90 min at which time cells were either assess for apoptosis and necrosis as described below or reoxygenated for 3 hours by re-suspending in fresh MEM. In all experiments additional aliquots of cardiomyocytes were incubated under time-controlled normoxic conditions.
Calcium-induced cell death
To determine the effects of TRPC3 expression on the response to calcium-induced cell death, cardiomyocytes were placed on coverslips, and preincubated with 5 μM Thapsigargin for 5 min followed by addition of 2.5 mM extracellular calcium. The cells were imaged on an Olympus I × 70 inverted microscope through a X40 Uplan APO objective. Rounded cells (defined as cells when the ratio between the length and width was < 2) were consider irreversible injured and dead(5, 26).
TNF-α induced apoptosis
Cardiomyocytes were incubated with 10 ng/ml TNF-α in serum-free MEM media (2). Apoptosis was assessed by Annexin V-propidium iodide staining 2h or 18h after treatment with TNF-α.
Assessment of cell viability, apoptosis and necrosis
Several different methods were used to assess cell viability, apoptosis and necrosis.
Cell Morphology
Healthy viable adult cardiomyocytes typically exhibit rod-shaped morphology whereas non-viable cells are usually rounded in nature; therefore the percentage of rod shaped cells was used as an indicator of cell viability.
Annexin V and propidium iodide staining
Myocytes were stained with fluorescein isothiocyanate (FITC), annexin V, and propidium iodide, as per the manufacturer's directions (Vybrant Apoptosis Assay Kit 2, Molecular Probes). The cells were visualized under a fluorescence microscope and were counted regardless of morphology.
Cells not binding FITC-annexin V and excluding propidium iodide were classified as annexin V-negative (29, 33) and were deemed viable. Myocytes that bound FITC-annexin V [excitation wavelength (λex) = 488 nm and emission wavelength (λem) = 520 nm] but excluded propidium iodide (λex = 540 nm and λem = 630 nm) were deemed apoptotic. Myocytes permeant to propidium iodide (regardless of whether or not they bound FITC-annexin V) were deemed necrotic (29, 33). For quantification, 300 randomly distributed cells were counted in each experiment (29) and the percentage of total number of apoptotic and necrotic cells determined.
Lactate dehydrogenase (LDH) release
As previously described (7), necrosis was assessed by determining the release of lactate dehydrogenase (LDH) in the medium using an LDH assay kit (Sigma). Briefly, the percentage of LDH release was calculated by the ratio of the released LDH into the media to the total LDH (release plus cellular content).
DNA Fragmentation
The Cell Death Detection ELISA plus kit (Roche Molecular Biochemicals, Mannheim, Germany) was used as another indicator of apoptosis. In this assay internucleosomal DNA fragmentation was quantitatively assayed by antibody-mediated capture and detection of cytoplasmic mononucleosome- and oligonucleosome-associated histone-DNA complexes. Briefly, after centrifugation (200 g), cardiomyocytes (1 × 104 cells in each well) resuspended in 200 μL of the lysis buffer supplied by the manufacturer, and incubated for 30 minutes at room temperature. After pelleting nuclei (200 g, 10 minutes), 20 μL of the supernatant (cytoplasmic fraction) was used in the enzyme-linked immunosorbent assay (ELISA) following the manufacturer's standard protocol. Following, incubation with peroxidase substrate for 5 minutes, absorbance at 405 nm and 490 nm (reference wavelength) was determined with a microplate reader (Bio-Tec Instruments, Winooski, VT). Signals in wells containing the substrate only were subtracted as background (19).
Western Blot Analysis
Proteins were separated by electrophoresis on 6% or 8% SDS gel, transferred onto a polyvinylidenedifluoride (PVDF) membrane, and immunoblotted with antibodies against β-actin (1:20000, Abcam), α-fodrin (1:2000, Millipore), TRPC1 and TRPC3 (1:200, Alomone Labs). The immunoblots were developed with chemiluminescence (Pierce) and the signal was recorded on X-ray film. Densitometry analysis was performed on the entire lane of each sample using Labworks Analysis Software (UVP).
Data Analysis
All data are presented as means ± SEM from 3-6 separate experiments, where each experiment represents cells isolated from a single heart. Statistical analysis was performed by either an unpaired t-tests or one-way analysis of variance (ANOVA) followed by Dunnett's Multiple Comparisons Test as appropriate. Statistically significant differences between groups were defined as p < 0.05 and are indicated in the legends to the figures.
RESULTS
Expression of TRPC1 and TRPC3
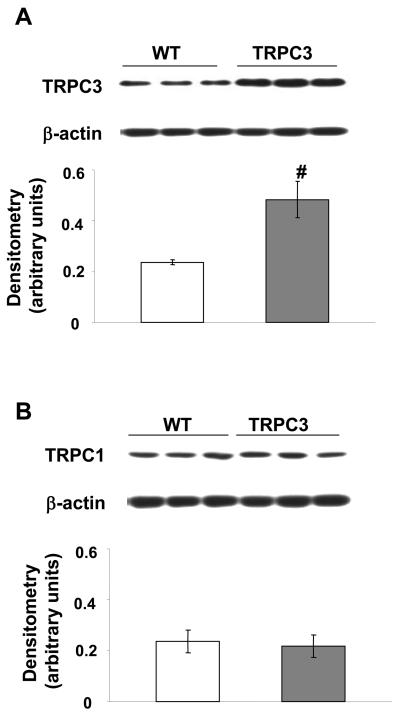
Immunoblot analysis demonstrated that TRPC3 protein levels were significantly increased in hearts from TRPC3 mice compared to wild-type mice (Fig.1A); however, there was no change in the expression of levels of TRPC1, the other predominant TRPC isoform present in the mouse heart (Fig.1B). This is consistent with the original study (28), which showed that increased TRPC3 expression was not associated with any changes in other TRPC proteins.
Figure 1.
A) TRPC3 and B) TRPC1 protein expression in the hearts from wild-type (WT) and TRPC3 transgenic mice. Upper panels are representative immunoblots and the lower panels are mean densitometric data from 6 individual experiments normalized to β-actin. # = p < 0.05.
TRPC3 overexpression leads to increased apoptosis in response to ischemia/reperfusion
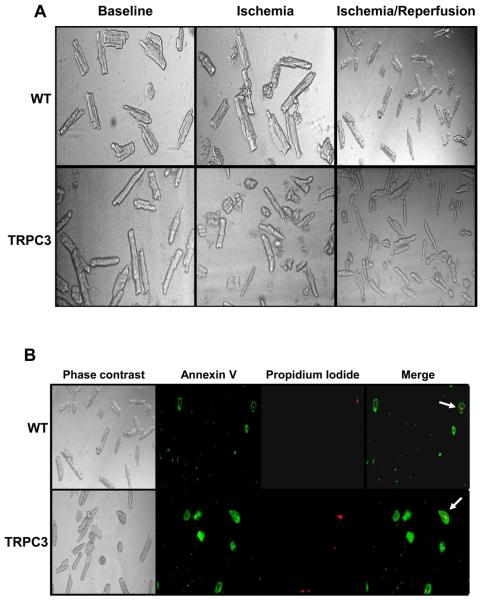
Adult cardiomyocytes isolated from WT and TRPC3 mice prior to ischemia exhibited predominantly rod-shaped morphology (> 80%; Fig. 2A, 3A). At the end of ischemia and ischemia/reperfusion the number of rod-shaped cells in both groups significantly decreased compared to both baseline conditions (Fig 2A, Fig 3A) and time-controlled normoxic controls. At the end of ischemia/reperfusion, the % rod shaped cells were significantly reduced in the TRPC3 group compared to WT (42 ± 5% vs. 51 ± 1%, p < 0.06; Fig 3A). Prior to and at the end of ischemia there were no significant differences in apoptosis, as assessed by annexin V positive, propidium iodide negative stained cells, between WT and TRPC3 groups; however, at the end of ischemia/reperfusion apoptosis was approximately 50% higher in the TRPC3 group (32 ± 1% vs. 21 ± 3%, p < 0.05; Fig 2B, 3B) compared to WT. Necrosis as indicated by propidium iodide positive cells was not different between groups at any time point (Fig 2B, 3C). In time-controlled normoxic incubations for the I/R protocol, both WT and TRPC3 groups compared to baseline there were small, but non-significant increases in apoptosis (from ~3.5 to 4.5%) and necrosis (from ~15-18%); however, there were no differences between WT and TRPC3 groups at any time-point in during time-controlled normoxic incubations. The apparent decrease in necrosis from the end of ischemia to the end of I/R (Fig 2C) is presumably a consequence of a loss of cells early during reperfusion; however, since total cell number was not assessed at each time point this cannot be confirmed. It is also possible, although we believe unlikely, that the higher necrosis in the ischemia only cells was an artifact associated of the preparation of these cells for annexin V and propidium iodide staining.
Figure 2.
A) Bright field phase contrast images of cardiomyocytes from wild-type (WT) and TRPC3 transgenic mice at baseline, at the end of 90 min ischemia and after 90 min ischemia and 3 hours reperfusion; B) cardiomyocytes from wild-type (WT) and TRPC3 transgenic mice after 90 min ischemia and 3 hours reperfusion showing Annexin V and propidium iodide staining. Arrows in merged image indicate necrotic cells, staining positive for both Annexin V and propidium iodide.
Figure 3.
A) Cell viability assessed by % rod shaped cells; B) % apoptotic cells indicated by Annexin V positive and propidium iodide negative staining; C) % necrotic cells indicated by Annexin V and propidium iodide positive staining in cardiomyocytes from wild-type (WT) and TRPC3 transgenic mice at baseline, at the end of 90 min ischemia and after 90 min ischemia and 3 hours reperfusion (I/R). Data presented as mean ± SEM of six individual experiments (i.e., six separate mouse cardiomyocytes isolations; with at least 300 cells counted per experiment under each condition). # = p < 0.05 vs. WT I/R.
Apoptosis was also assessed at the end of ischemia/reperfusion by quantifying internucleosomal DNA fragmentation. Consistent with the annexin V assays apoptosis was significantly increased TRPC3 group compared to WT controls (4.3 ± 0.2% vs. 2.7 ± 0.3%, p < 0.05; n = 6). As an alternative index of necrosis, LDH released during ischemia and reperfusion was measured in both groups and quantified as a percentage of total LDH. Consistent with the propidium iodide results (Fig 3C) there was no significant difference between TRPC3 and WT groups (27 ± 1 % vs. 26 ± 3%; n = 6).
Effect of L-type channel inhibitor and CCE inhibitor on TRPC3-induced apoptosis
Treatment of TRPC3 cells with 0.5 μM SKF96365, an inhibitor of CCE, significantly improved cellular viability following ischemia/reperfusion (54 ± 4% vs. 42 ± 5%, p < 0.05; n = 6), to a level similar to that seen in WT cells following ischemia/reperfusion (Fig. 3A). SKF96365 also significantly decreased apoptosis in TRPC3 cells (15 ± 4% vs. 32 ± 1.4%, p < 0.05; n = 6) to levels similar to that seen in WT cells (Fig. 4). Similar to SKF96365, PD150606, an inhibitor of calpain, also attenuated apoptosis in the TRPC3 group (Fig 4). However, the L-type Ca2+ channel inhibitor verapamil (10 μM) had no significantly effect on either viability (data not shown) or apoptosis in TRPC3 cells (Fig. 4). The untreated WT and TRPC3 data in Fig. 4 are from the same experiments as those shown in Fig 3B; however, it should be noted that the experiments in Fig. 4 were performed simultaneously with, and on cells from the same isolations as those used in Fig 3, thereby permitting direct comparisons.
Figure 4.
Apoptosis after 90 min ischemia and 3 hours reperfusion in cardiomyocytes from wild-type (WT) and TRPC3 transgenic mice and in TRPC3 cardiomyocytes treated with the L-type calcium channel inhibitor verapamil (VER, 10 μM), the CCE inhibitor SKF96365 (SKF, 0.5 μM) and the calpain inhibitor PD150606 (PD, 25 μM). The untreated WT and TRPC3 data are from the same experiments as those shown in Fig 3B; however the experiments with VER, SKF and PD were performed simultaneously with, and on cells from the same isolations as those in Fig 3. Data presented as mean ± SEM of six individual experiments, i.e., six separate mouse cardiomyocytes isolations; with at least 300 cells counted per experiment under each condition. # = p < 0.05 vs. TRPC3.
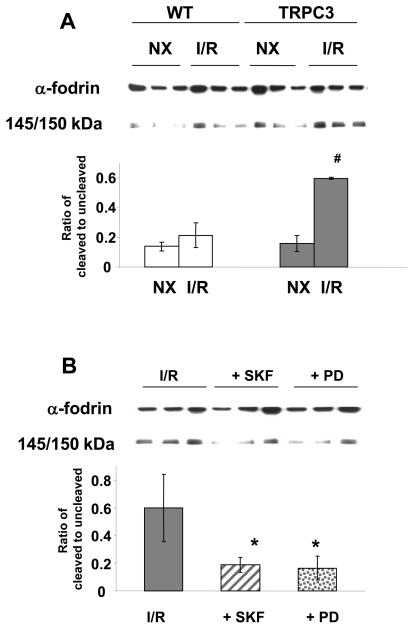
TRPC3-induced apoptosis associated with increased calpain activation
One pathway by which increased cytosolic Ca2+ contributes to apoptosis is by activation of the calcium sensitive protease calpain, leading to proteolysis of structural proteins (10, 11). The cytoskeletal protein α-fodrin is a well-characterized substrate for calpain, and increased calpain activity has been shown to lead to the formation of 140/150kD cleavage fragment of α-fodrin (37, 40, 41). In Fig 5A it can be seen that in the TRPC3 group there is significant increase in α-fodrin cleavage following ischemia/reperfusion compared to WT group (p < 0.05). The increase in α-fodrin cleavage in the TRPC3 cells was attenuated by the CCE inhibitor SKF96365 and the calpain inhibitor PD150606 (Fig 5B).
Figure 5.
A) Total and cleaved α-fodrin (145/150kD) in cardiomyocytes from wild-type (WT) and TRPC3 transgenic mice under normoxic conditions (NX) and following 90 min ischemia and 3 hours reperfusion (I/R); B) total and cleaved α-fodrin (145/150kD) following 90 min ischemia and 3 hours reperfusion (I/R) in untreated cardiomyocytes from TRPC3 transgenic mice and in TRPC3 cardiomyocytes treated with the CCE inhibitor SKF96365 (SKF, 0.5 μM) and the calpain inhibitor PD150606 (PD, 25 μM). Upper panels are representative immunoblots and the lower panels are mean densitometric data from 3 individual experiments. # = p < 0.05 vs. TRPC NX and WT I/R; * = p < 0.05 vs. I/R.
TRPC3 overexpression has an increased sensitivity to high extracellular calcium induced cell death
Cell death induced by ischemia/reperfusion is a multi-factorial process involving metabolic inhibition, intracellular acidosis as well as increased cytosolic calcium. Therefore, we determine whether increased TRPC3 expression would increase the sensitivity to cell death induced solely by Ca2+-overload. Cells were treated with 5 μM thapsigargin in the absence of extracellular calcium for 5 mins followed by addition of 2.5 mM extracellular Ca2+ and the ratio of cell length/width followed as a function of time. In Fig 6 it can be seen that in the WT group, cells maintained a rod shaped viable form for at least 6 minutes and complete rounding of the cells took occurred between 7 and 9 minutes following addition of extracellular calcium. In contrast TRPC3 cells were completely rounded after only 6 minutes (p < 0.05).
Figure 6.
Cardiomyocytes from wild-type (WT) and TRPC3 transgenic mice were treated with 5 μM thapsigargin for 5 mins in the absence of extracellular Ca2+ and were then exposed to 2.5 mM extracellular calcium. Upper panels show a typical time course of myocyte rounding following addition of 2.5 mM Ca2+ in WT and TRPC3 cardiomyocytes. Data are mean ± SEM from 15 cells of at least 3 individual experiments. # = p < 0.05 vs. TRPC3 6min.
TRPC3 overexpression does not contribute TNF-α induced apoptosis
To determine whether TRPC3 overexpression increased sensitivity to other apoptotic stimuli, cardiomyocytes isolated from WT and TRPC3 mice were incubated with 10 ng/ml TNF-α in serum-free media for 2h and 18h. Apoptosis, assessed by Annexin V-propidium iodide staining, increased in both groups following TNF-α treatment; however, in contrast to ischemia/reperfusion, there was no difference in apoptosis between the two groups (Fig. 7). In time-controlled normoxic incubations there were no differences in apoptosis or % rod shaped cells between WT or TRPC3 groups at any time point (data not shown). There was also no increase in either parameter at 2 or 18 hours compared to baseline in either WT or TRPC3 groups (data not shown).
Figure 7.
After 1h of isolation, cardiomyocytes from WT and TRPC3 transgenic mice were treated with 10 ng/ml TNF-α for 2h or 18h, viability and apoptosis were measured by Annexin V-propidium iodide staining. Data presented as mean ± SEM of six individual experiments, i.e., six separate mouse cardiomyocytes isolations; with at least 300 cells counted per experiment under each condition. * = p < 0.05 vs. baseline.
DISCUSSION
First described in non-excitable cells, CCE has now been shown to co-exist with L-type channels in smooth and skeletal muscle cells (17, 35) and in both neonatal and adult cardiomyocytes (12, 13, 31). The TRPC protein family have been prime candidates for CCE channel proteins (14) and TRPC1, 3, 4, 5 and 6 have all been identified in rat and mouse hearts (14, 28, 30). Here we demonstrate for the first time that TRPC3 overexpression increased the sensitivity of cardiomyocytes to apoptosis following ischemia/reperfusion, which was due, at least in part, by an increase in calpain-mediated proteolysis. We also demonstrated that increased TRPC3 expression sensitizes cardiomyocytes to Ca2+ overload induced cell death, but did not alter the response to TNF-α mediates apoptosis. This is consistent with our report that CCE inhibition markedly attenuated Ca2+-overload injury in the intact heart (20) and suggests that in addition to playing a role in regulating cardiomyocyte hypertrophy, TRPC-mediated CCE also contributes to cardiomyocyte apoptosis. These data provide further support for both TRPC and CCE in regulating the response of cardiomyocytes to a range of pathophysiological stimuli.
In vitro studies showed that CCE played a critical role in mediating the hypertrophic response to IP3-generating agonists such as angiotensin and phenylephrine in neonatal cardiomyocytes (11). Hunton et al. (12) also demonstrated that the increase in cytosolic Ca2+ induced by these agonists was clearly independent of Ca2+-entry mediated via L-type Ca2+ channels and the reverse mode of the Na+/Ca2+ exchanger. Further evidence, demonstrating a physiological role for CCE in the heart was provided by Ohba et al. who showed that TRPC1 expression increased in the intact heart following pressure overload and in isolated cardiomyocytes in response to hypertrophic stimuli; the latter was associated with increased CCE (30). Consistent with the study by Ohba et al., overexpression of TRPC3 in the mouse heart enhanced CCE at the cardiomyocyte level and was associated with increased hypertrophy in response to either pressure overload or neuroendocrine agonists in vivo (28).
Although a number of Ca2+ entry pathways have been implicated in mediating cardiomyocyte Ca2+ overload following ischemia reperfusion, including the Na+/Ca2+ exchanger and the L-type Ca2+ channel (4, 8, 32), there is still considerable controversy as to which pathways are critical in mediating this process. In cardiomyocytes, angiotensin- and phenylephrine-induced CCE is inhibited by glucosamine and SKF96365 (12, 27); glucosamine also protected the isolated perfused heart from calcium-overload induced by the calcium paradox (20). We have also shown that glucosamine improves recovery following ischemia/reperfusion including lowering end-diastolic pressure (20) and decreasing Ca2+-mediated proteolysis, consistent with attenuation of ischemia/reperfusion induced increase in cytosolic Ca2+. Given that glucosamine inhibited CCE in isolated cardiomyocytes (12, 27), these studies raised the possibility that Ca2+ entry mediated via CCE pathways may contribute to injury resulting from ischemia/reperfusion. Consistent with this notion we found that following simulated ischemia and reperfusion cardiomyocytes overexpressing TRPC3 had increased levels of apoptosis assessed by both annexin V staining (Fig 3) and DNA fragmentation, as well as decreased viability as indicated by percentage of rod-shaped cells. Interestingly, however, TRPC3 overexpression did not contribute to increased necrosis as indicated by either LDH release or propidium iodide/annexin V staining (Fig 3).
Additional evidence for a role of TRPCs in regulating cell death has been described in other cell types. For example, Marasa et al., reported that increased TRPC1 expression sensitized intestinal epithelial cells to apoptosis as a result of increased Ca2+ influx (21). It is also worth noting that oxidative stress has been shown to activate TRPC3 and TRPC4 in endothelial cells (23) and another submember of TRP channels, TRPM, has been reported to contribute to oxidative stress induced cell death (23). However, in contrast to these reports suggesting that TRPCs contribute to increased cell death, Jia et al., showed that TPRC3 and TRPC6 played a role in promoting neuronal survival in response to serum deprivation (16). Taken together with our results, these studies, support the notion that TRPCs play a role in regulating cell survival; however, whether they contribute to cell death or survival may be both cell and stress specific.
Further supporting a role of Ca2+ via CCE in contributing to the increase in apoptosis in the TRPC3 group, we also found that the CCE inhibitor SKF96365 attenuated the increase in apoptosis associated with TRPC3 overexpression, whereas the L-type Ca2+ channel inhibitor verapamil had no such effect (Fig. 4). Elevated intracellular Ca2+ levels activate numerous Ca2+-regulated enzymes including protein kinases, protein phosphatases, phospholipases, NO synthases, Ca2+/calmodulin-dependent protein kinase II (CaMKII) and the cysteine protease calpain (42). There is increasing evidence that calpain plays a major role in post-ischemic injury (15, 36), in part because of proteolysis of structural proteins (10, 11) including the cytoskeletal protein α-fodrin (37, 40, 41). We found that in WT cardiomyocytes ischemia/reperfusion did not significantly increase cleavage of α-fodrin; in contrast, TRPC3 overexpression was associated with ~3-fold increase in α-fodrin cleavage consistent with increased calpain activity (Fig 5A). The calpain inhibitor PD150606, attenuated both α-fodrin cleavage and apoptosis in cardiomyocytes overexpressing TRPC3 (Fig. 4, 5B); furthermore, consistent with its effect on apoptosis, SKF96365 markedly attenuated ischemia/reperfusion-induced increase in α-fodrin cleavage In the TRPC3 group (Fig 5B). Taken together these data support the concept that in cardiomyocytes overexpressing TRPC3 Ca2+ entry via CCE contributes to increased apoptosis, at least in part by calpain activation; however, while there is considerable data demonstrating that α-fodrin cleavage is calpain-specific (37, 40, 41), a direct measure of calpain activity would have further substantiated this conclusion. Nakayama et al., (28), have shown that TRPC3 overexpression was also associated with increased calcineurin activity; consequently, it is possible that calcineurin inhibition may also attenuate the increased apoptosis seen in the TRPC3 group. It should be noted that because we did not evaluate the effects of SKF96365 or PD150606 in WT cells we cannot comment about the potential role of CCE in contributing to cardiomyocyte apoptosis where TRPC3 levels are not increased. However, in preliminary experiments in the normal intact perfused heart, SKF96365 markedly attenuated tissue injury resulting from the calcium paradox (Marchase et al., unpublished data), suggesting that CCE may contribute to calcium mediated cell death in the normal intact heart.
Ischemia/reperfusion is a multifactorial process that includes intracellular acidosis and energy depletion, which may contribute to both necrosis and apoptosis either directly or via altered Ca2+ homeostasis, mediated via Ca2+ entry pathways such as the Na+/Ca2+ exchanger. Therefore, we asked whether TRPC3 overexpression also increased sensitivity to a specific Ca2+ overload stress. Cells were first exposed to thapsigargin (5 μM) in the absence of extracellular Ca2+ followed by the addition of 2.5 mM extracellular Ca2+, this is a protocol that has used previously to increase CCE (12, 27). We found that in the absence of extracellular Ca2+ cardiomyocytes maintained a normal rod-shaped morphology; however, the addition of extracellular Ca2+ resulted in a loss of viability as indicated by rounding of the cells, which was markedly accelerated in cells with increased TRPC3 expression. In contrast, TRPC3 overexpression had no effect on the response of cardiomyocytes to TNF-α, which induces apoptosis via a Ca2+ independent pathway by means of the TNF type-1 receptor and Fas activation (22) (Fig 7). Thus, the increased sensitivity of TRPC3 to ischemia/reperfusion and Ca2+ overload was not a consequence of a general decreased tolerance to stress. However, TNF-α has been shown to increase TRPC3 expression in smooth muscle cells (38). Since, we did not determine whether TNF-α affected TRPC3 levels here, we cannot rule out the possibility that a preferential increase in TRPC3 expression in TNF-α treated WT cells could mask potential differences in the response of WT and TRPC3 cardiomyocytes to TNF-α.
The role of CCE and TRPC in mediating cardiomyocyte function remains somewhat controversial, in part due to the fact that the predominant models describing Ca2+ handling in the heart are focused on understanding the regulation of excitation-contraction coupling (3). The importance of Ca2+ as a second messenger regulating a diverse array of cellular functions, including activation of gene transcription and cell growth, initiation of apoptosis and necrosis (6), raises the fundamental question as to how the cardiomyocyte distinguishes between the fluctuations in Ca2+ that occur in response contraction and relaxation from calcium signals (25). One characteristic of CCE is that it leads to a low-amplitude, sustained elevation in cytosolic Ca2+, which could be distinguished from the high frequency and high amplitude oscillations in Ca2+ associated with contraction and relaxation. A number of studies have demonstrated a role for CCE mediated via TRP channel in mediating the responses to hypertrophic stimuli (12, 28, 30); here, we provide evidence to that calcium entry via TRPC may also contribute to Ca2+-mediated apoptosis. Clearly, there are limitations in extrapolating studies on isolated cardiomyocytes to the intact heart; furthermore, the fact that TRPC3 overexpression is associated with increased apoptosis does not necessarily imply that Ca2+ entry via TRP channels contributes to apoptosis in the normal heart. However, hypertrophic agonists such as angiotensin and phenylephrine, which have been shown to stimulate CCE, are also known to induce apoptosis. Furthermore, cardiac hypertrophy, which increases TRPC1 expression levels in the normal intact adult heart (30) is also associated with decreased tolerance to ischemic injury (1).
In this study we have focused entirely on isolated cardiomyocytes and thus any extrapolation with regard to the role of TRPCs in mediating cell death in the intact heart must be made with caution. In the intact heart, tissue injury following I/R is a result not only of metabolic and ionic events, but also a consequence of mechanical stress that occurs due to muscle contraction mediated in part by cell-to-cell connections. Such events are clearly absent in studies of cultured adult cardiomyocytes, which do not contract spontaneously and where there are no cellular connections. Conversely, cardiomyocyte isolation is itself an appreciable stress and this could potentially have a greater effect on cells overexpressing TRPC3 than on WT cells. It is conceivable that this could make TRPC3 cells more susceptible to subsequent I/R injury; however, it is likely that this would be manifest by increased basal cell death or a significantly greater increase in necrosis or apoptosis in time-controlled normoxic incubations, neither of which were seen here. Clearly, studies in the isolated perfused heart or in vivo would have demonstrated a more definitive role for TRPC3 in mediating I/R reperfusion in the intact heart. Another limitation of this study is the potential for chronic changes in cardiomyocytes overexpressing TPRC3 such as hypertrophy or increased calcineurin activity (28) that could also increase sensitivity to I/R injury independent of acute CCE-mediated increase in cytosolic Ca2+. Future studies using acute adenoviral transfection approaches or conditional overexpression models would help resolve this issue. The use of siRNA to decrease TRPC expression in normal cells would also provide valuable insight into the contribution of TRPCs to cardiomyocyte injury.
In conclusion, we have shown that increased TPRC3 expression contributes to increased apoptosis but not necrosis in cardiomyocytes subjected to ischemia/reperfusion. This increase in apoptosis was associated with increased calpain-mediated proteolysis that was attenuated by the calpain inhibitor PD150606 and the CCE inhibitor SKF96365. However, while increased TRPC3 expression increased sensitivity to Ca2+ stress, it did not increase the sensitivity to TNF–α induced apoptosis, demonstrating that TRPC3 overexpression did not result in general decreased tolerance to stress. These results provide further evidence for a role of TRP channels in mediating cardiomyocyte function and suggest that TRPC mediated CCE may contribute to increased tolerance to injury seen in cardiac hypertrophy.
ACKNOWLEDGMENTS
The TRPC3 transgenic mice were a kind gift from Dr. Jeffrey D. Molkentin, University of Cincinnati. We thank Voraratt Champattanachai and Charlye Brocks for technical assistance. This work was supported by grants from the NHLBI HL-076175 (RBM); HL-67464 and HL079364 (JCC) and HL-077100.
REFERENCES
- 1.Anderson PG, Allard MF, Thomas GD, Bishop SP, Digerness SB. Increased ischemic injury but decreased hypoxic injury in hypertrophied rat hearts. Circ Res. 1990;67:948–959. doi: 10.1161/01.res.67.4.948. [DOI] [PubMed] [Google Scholar]
- 2.Bajaj G, Sharma RK. TNF-alpha-mediated cardiomyocyte apoptosis involves caspase-12 and calpain. Biochem Biophys Res Commun. 2006;345:1558–1564. doi: 10.1016/j.bbrc.2006.05.059. [DOI] [PubMed] [Google Scholar]
- 3.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 4.Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 5.Budas GR, Jovanovic S, Crawford RM, Jovanovic A. Hypoxia-induced preconditioning in adult stimulated cardiomyocytes is mediated by the opening and trafficking of sarcolemmal KATP channels. Faseb J. 2004;18:1046–1048. doi: 10.1096/fj.04-1602fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carafoli E. Calcium signaling: a tale for all seasons. Proc Natl Acad Sci U S A. 2002;99:1115–1122. doi: 10.1073/pnas.032427999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Champattanachai V, Marchase RB, Chatham JC. Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein-associated O-GlcNAc. Am J Physiol Cell Physiol. 2007;292:C178–187. doi: 10.1152/ajpcell.00162.2006. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Zhang X, Kubo H, Harris DM, Mills GD, Moyer J, Berretta R, Potts ST, Marsh JD, Houser SR. Ca2+ influx-induced sarcoplasmic reticulum Ca2+ overload causes mitochondrial-dependent apoptosis in ventricular myocytes. Circ Res. 2005;97:1009–1017. doi: 10.1161/01.RES.0000189270.72915.D1. [DOI] [PubMed] [Google Scholar]
- 9.Freichel M, Schweig U, Stauffenberger S, Freise D, Schorb W, Flockerzi V. Store-operated cation channels in the heart and cells of the cardiovascular system. Cell Physiol Biochem. 1999;9:270–283. doi: 10.1159/000016321. [DOI] [PubMed] [Google Scholar]
- 10.Gao WD, Atar D, Liu Y, Perez NG, Murphy AM, Marban E. Role of troponin I proteolysis in the pathogenesis of stunned myocardium. Circ Res. 1997;80:393–399. [PubMed] [Google Scholar]
- 11.Gao WD, Liu Y, Mellgren R, Marban E. Intrinsic myofilament alterations underlying the decreased contractility of stunned myocardium. A consequence of Ca2+-dependent proteolysis? Circ Res. 1996;78:455–465. doi: 10.1161/01.res.78.3.455. [DOI] [PubMed] [Google Scholar]
- 12.Hunton DL, Lucchesi PA, Pang Y, Cheng X, Dell'Italia LJ, Marchase RB. Capacitative calcium entry contributes to nuclear factor of activated T-cells nuclear translocation and hypertrophy in cardiomyocytes. J Biol Chem. 2002;277:14266–14273. doi: 10.1074/jbc.M107167200. [DOI] [PubMed] [Google Scholar]
- 13.Hunton DL, Zou LY, Pang Y, Marchase RB. Adult Rat Cardiomyocytes Exhibit Capacitative Calcium Entry. Am J Physiol Heart Circ Physiol. 2004;286:H1124–H1132. doi: 10.1152/ajpheart.00162.2003. [DOI] [PubMed] [Google Scholar]
- 14.Inoue R, Jensen LJ, Shi J, Morita H, Nishida M, Honda A, Ito Y. Transient receptor potential channels in cardiovascular function and disease. Circ Res. 2006;99:119–131. doi: 10.1161/01.RES.0000233356.10630.8a. [DOI] [PubMed] [Google Scholar]
- 15.Iwamoto H, Miura T, Okamura T, Shirakawa K, Iwatate M, Kawamura S, Tatsuno H, Ikeda Y, Matsuzaki M. Calpain inhibitor-1 reduces infarct size and DNA fragmentation of myocardium in ischemic/reperfused rat heart. J Cardiovasc Pharmacol. 1999;33:580–586. doi: 10.1097/00005344-199904000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Jia Y, Zhou J, Tai Y, Wang Y. TRPC channels promote cerebellar granule neuron survival. Nat Neurosci. 2007;10:559–567. doi: 10.1038/nn1870. [DOI] [PubMed] [Google Scholar]
- 17.Kurebayashi N, Ogawa Y. Depletion of Ca2+ in the sarcoplasmic reticulum stimulates Ca2+ entry into mouse skeletal muscle fibres. J Physiol (London) 2001;533:185–199. doi: 10.1111/j.1469-7793.2001.0185b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuwahara K, Wang Y, McAnally J, Richardson JA, Bassel-Duby R, Hill JA, Olson EN. TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J Clin Invest. 2006;116:3114–3126. doi: 10.1172/JCI27702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu CY, Takemasa A, Liles WC, Goodman RB, Jonas M, Rosen H, Chi E, Winn RK, Harlan JM, Chuang PI. Broad-spectrum caspase inhibition paradoxically augments cell death in TNF-alpha -stimulated neutrophils. Blood. 2003;101:295–304. doi: 10.1182/blood-2001-12-0266. [DOI] [PubMed] [Google Scholar]
- 20.Liu J, Pang Y, Chang T, Bounelis P, Chatham JC, Marchase RB. Increased hexosamine biosynthesis and protein O-GlcNAc levels associated with myocardial protection against calcium paradox and ischemia. J Mol Cell Cardiol. 2006;40:303–312. doi: 10.1016/j.yjmcc.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Marasa BS, Rao JN, Zou T, Liu L, Keledjian KM, Zhang AH, Xiao L, Chen J, Turner DJ, Wang JY. Induced TRPC1 expression sensitizes intestinal epithelial cells to apoptosis by inhibiting NF-kappaB activation through Ca2+ influx. Biochem J. 2006;397:77–87. doi: 10.1042/BJ20060124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meldrum DR. Tumor necrosis factor in the heart. Am J Physiol Regulatory Integrative Comp Physiol. 1998;274:R577–R595. doi: 10.1152/ajpregu.1998.274.3.R577. [DOI] [PubMed] [Google Scholar]
- 23.Miller BA. The role of TRP channels in oxidative stress-induced cell death. J Membr Biol. 2006;209:31–41. doi: 10.1007/s00232-005-0839-3. [DOI] [PubMed] [Google Scholar]
- 24.Minke B, Cook B. TRP channel proteins and signal transduction. Physiol Rev. 2002;82:429–472. doi: 10.1152/physrev.00001.2002. [DOI] [PubMed] [Google Scholar]
- 25.Molkentin JD. Dichotomy of Ca2+ in the heart: contraction versus intracellular signaling. J Clin Invest. 2006;116:623–626. doi: 10.1172/JCI27824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mora A, Davies AM, Bertrand L, Sharif I, Budas GR, Jovanovic S, Mouton V, Kahn CR, Lucocq JM, Gray GA, Jovanovic A, Alessi DR. Deficiency of PDK1 in cardiac muscle results in heart failure and increased sensitivity to hypoxia. Embo J. 2003;22:4666–4676. doi: 10.1093/emboj/cdg469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagy T, Champattanachai V, Marchase RB, Chatham JC. Glucosamine inhibits angiotensis II induced cytoplasmic Ca2+ elevation in neonatal cardiomyocytes via protein-associated O-GlcNAc. Am J Physiol Cell Physiol. 2006;290:C57–65. doi: 10.1152/ajpcell.00263.2005. [DOI] [PubMed] [Google Scholar]
- 28.Nakayama H, Wilkin BJ, Bodi I, Molkentin JD. Calcineurin-dependent cardiomyopathy is activated by TRPC in the adult mouse heart. Faseb J. 2006;20:1660–1670. doi: 10.1096/fj.05-5560com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narayan P, Mentzer RM, Jr., Lasley RD. Annexin V staining during reperfusion detects cardiomyocytes with unique properties. Am J Physiol Heart Circ Physiol. 2001;281:H1931–1937. doi: 10.1152/ajpheart.2001.281.5.H1931. [DOI] [PubMed] [Google Scholar]
- 30.Ohba T, Watanabe H, Murakami M, Takahashi Y, Iino K, Kuromitsu S, Mori Y, Ono K, Iijima T, Ito H. Upregulation of TRPC1 in the development of cardiac hypertrophy. J Mol Cell Cardiol. 2007;42:498–507. doi: 10.1016/j.yjmcc.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 31.Pang Y, Bounelis P, Chatham JC, Marchase RB. The hexosamine pathway is responsible for the inhibition by diabetes of phenylephrine-induced inotropy. Diabetes. 2004;53:1074–1081. doi: 10.2337/diabetes.53.4.1074. [DOI] [PubMed] [Google Scholar]
- 32.Piper HM, Abdallah Y, Schafer C. The first minutes of reperfusion: a window of opportunity for cardioprotection. Cardiovasc Res. 2004;61:365–371. doi: 10.1016/j.cardiores.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 33.Porter AG, Janicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 34.Putney JW, Jr., Broad LM, Braun FJ, Lievremont JP, Bird GS. Mechanisms of capacitative calcium entry. J Cell Sci. 2001;114:2223–2229. doi: 10.1242/jcs.114.12.2223. [DOI] [PubMed] [Google Scholar]
- 35.Trepakova ES, Csutora P, Hunton DL, Marchase RB, Cohen RA, Bolotina VM. Calcium influx factor directly activates store-operated cation channels in vascular smooth muscle cells. J Biol Chem. 2000;275:26158–26163. doi: 10.1074/jbc.M004666200. [DOI] [PubMed] [Google Scholar]
- 36.Trumbeckaite S, Neuhof C, Zierz S, Gellerich FN. Calpain inhibitor (BSF 409425) diminishes ischemia/reperfusion-induced damage of rabbit heart mitochondria. Biochem Pharmacol. 2003;65:911–916. doi: 10.1016/s0006-2952(02)01610-6. [DOI] [PubMed] [Google Scholar]
- 37.Tsuji T, Ohga Y, Yoshikawa Y, Sakata S, Abe T, Tabayashi N, Kobayashi S, Kohzuki H, Yoshida KI, Suga H, Kitamura S, Taniguchi S, Takaki M. Rat cardiac contractile dysfunction induced by Ca2+ overload: possible link to the proteolysis of alpha-fodrin. Am J Physiol Heart Circ Physiol. 2001;281:H1286–1294. doi: 10.1152/ajpheart.2001.281.3.H1286. [DOI] [PubMed] [Google Scholar]
- 38.White TA, Xue A, Chini EN, Thompson M, Sieck GC, Wylam ME. Role of transient receptor potential C3 in TNF-alpha-enhanced calcium influx in human airway myocytes. Am J Respir Cell Mol Biol. 2006;35:243–251. doi: 10.1165/rcmb.2006-0003OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamawaki M, Sasaki N, Shimoyama M, Miake J, Ogino K, Igawa O, Tajima F, Shigemasa C, Hisatome I. Protective effect of edaravone against hypoxia-reoxygenation injury in rabbit cardiomyocytes. Br J Pharmacol. 2004;142:618–626. doi: 10.1038/sj.bjp.0705775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshida K. Myocardial ischemia-reperfusion injury and proteolysis of fodrin, ankyrin, and calpastatin. Methods Mol Biol. 2000;144:267–275. doi: 10.1385/1-59259-050-0:267. [DOI] [PubMed] [Google Scholar]
- 41.Yoshida K, Inui M, Harada K, Saido TC, Sorimachi Y, Ishihara T, Kawashima S, Sobue K. Reperfusion of rat heart after brief ischemia induces proteolysis of calspectin (nonerythroid spectrin or fodrin) by calpain. Circ Res. 1995;77:603–610. doi: 10.1161/01.res.77.3.603. [DOI] [PubMed] [Google Scholar]
- 42.Zhang T, Miyamoto S, Brown JH. Cardiomyocyte calcium and calcium/calmodulin-dependent protein kinase II: friends or foes? Recent Prog Horm Res. 2004;59:141–168. doi: 10.1210/rp.59.1.141. [DOI] [PubMed] [Google Scholar]
- 43.Zhu X, Jiang M, Peyton M, Boulay G, Hurst R, Stefani E, Birnbaumer L. trp, a novel mammalian gene family essential for agonist-activated capacitative Ca2+ entry. Cell. 1996;85:661–671. doi: 10.1016/s0092-8674(00)81233-7. [DOI] [PubMed] [Google Scholar]