Abstract
Objectives
A range of psychiatric symptoms and cognitive deficits occur in Parkinson’s disease (PD), and symptom overlap and co-morbidity complicate the classification of non-motor symptoms. The objective of this study was to use analytic-based approaches to classify psychiatric and cognitive symptoms in PD.
Design
Cross-sectional evaluation of a convenience sample of patients in specialty care.
Setting
Two outpatient movement disorders centers at the University of Pennsylvania and Philadelphia Veterans Affairs Medical Center.
Participants
177 patients with mild-moderate idiopathic PD and without significant global cognitive impairment.
Measurements
Subjects were assessed with an extensive psychiatric, neuropsychological, and neurological battery. Latent class analysis (LCA) was used to statistically delineate group-level symptom profiles across measures of psychiatric and cognitive functioning. Predictors of class membership were also examined.
Results
Results from the LCA indicated that a four-class solution best fit the data. 32.3% of the sample had good psychiatric and normal cognitive functioning, 17.5% had significant psychiatric co-morbidity but normal cognition, 26.0% had few psychiatric symptoms but had poorer cognitive functioning across a range of cognitive domains, and 24.3% had both significant psychiatric co-morbidity and poorer cognitive functioning. Age, disease severity, and medication use predicted class membership.
Conclusions
LCA delineates four classes of patients in mild-moderate PD, three of which experience significant non-motor impairments and comprise over two-thirds of patients. Neuropsychiatric symptoms and cognitive deficits follow distinct patterns in PD, and further study is needed to determine if these classes are generalizable, stable, predict function, quality of life and long-term outcomes, and are amenable to treatment at a class level.
Keywords: Parkinson’s disease, neuropsychology, psychiatry, cognition, depression
OBJECTIVES
A broad range of psychiatric disorders and cognitive deficits are common in Parkinson’s disease (PD)(5,41). Prevalence estimates for some form of depression center around 30–40%(26). Anxiety and panic attacks(28), psychotic symptoms(4), fatigue(25), and disorders of sleep and wakefulness(9) all occur at frequencies of at least 30% in PD. In addition, many PD patients, even those without dementia, experience cognitive deficits, including executive, memory, attentional, and visuospatial impairments(23).
Given the high frequencies of different non-motor complications in PD, extensive co-morbidity is expected for PD patients among different psychiatric and cognitive conditions. Frequent concomitants of depression in PD are anxiety disorders(28) and cognitive deficits(37); apathy(36) and fatigue(25) are common among PD patients experiencing other psychiatric or cognitive conditions. In one study of psychiatric co-morbidity in PD, 59% of patients experienced at least 2 non-motor symptoms, while 25% experienced at least four symptoms(33).
There are several possible explanations for psychiatric and cognitive comorbidity in PD. For example, the overlap between symptoms may simply be due to chance. Alternatively, the expected frequency of comorbid symptoms may be higher than due to chance, suggesting that psychiatric and cognitive symptoms either share common etiologies (demographic, clinical, or pathophysiological) leading to their co-occurrence or are directly, or indirectly, related to one another through biological mechanisms.
Investigating these, and other, alternative explanations of the psychiatric and cognitive comorbidity in PD requires going beyond the typical comparison of PD and non-PD groups in the majority of research on PD. Heterogeneity among PD patients in the form of unidentified subgroups with different symptom profiles may help explain the psychiatric and cognitive overlap in PD. Such subgroups may vary by the different levels at which psychiatric and cognitive symptoms may appear together. Some subgroups may exhibit either psychiatric or cognitive symptoms, while others exhibit both to different degrees. A variety of statistical approaches are available for identifying such sub-groups based on observed symptoms.
Cluster analysis is one non-model-based methodology that has been used recently to identify groups of patients with dementia who present with similar neuropsychiatric symptom profiles(3,8,20). However, cluster analysis does not provide a rule for identifying clusters in a patient sample. Alternatively, latent class analyses (LCA) are model-based clustering approaches that characterize heterogeneity in symptom profiles at the patient level with discrete unobserved subgroups of patients experiencing similar patterns of co-morbidity across measures(30). In addition, demographic and clinical predictors of class membership can be explored, enriching the clinical utility of the information to be obtained from the latent classes. Thus, LCA has the potential to inform the assessment and management of patients in routine clinical care with information from the unobserved subgroups underlying the observed psychiatric-cognitive comorbidity relationship in PD patients. Accordingly, the current study employed LCA to identify subgroups based on symptom profiles across continuous and categorical measures of psychiatric and cognitive functioning in patients with mild to moderate PD and without significant global cognitive impairment, and then to examine the association between identified subgroups and demographic and clinical characteristics commonly associated with non-motor complications in PD(40).
METHODS
Participants
Participants were a convenience sample of 177 outpatients receiving clinical care at the PD centers at the University of Pennsylvania (N=135) or the Philadelphia Veterans Affairs Medical Center (N=42). All participants had a diagnosis of possible or probable idiopathic PD(15), confirmed by a movement disorders specialist, and were participants in a study examining the frequency and correlates of psychiatric and cognitive complications in PD. Exclusion criteria included recent (within past 6 months) deep brain stimulation surgery, Mini-Mental State Examination(13) (MMSE) score <15, a current substance abuse diagnosis, and a self-reported history of bipolar disorder, schizophrenia, or schizoaffective disorder. Comparison of mean differences in patient characteristics across sites revealed that patients from the Philadelphia VAMC were significantly older (t=5.01, Mdiff =8.70, df=175, p<.001) and had a higher average daily levodopa dosage (t=2.73, Mdiff =191.59, df=175, p=.007) than patients recruited from the University of Pennsylvania. With regard to the latent class indicators (described in more detail below), while there were no site differences for any of the psychiatric measures, VA patients did score lower on attention (t=−2.02, Mdiff= −0.93, df=175, p=.05), memory (t= −2.47, Mdiff= −2.66, df=175, p=.02), and executive function (t= −2.20, Mdiff= −2.72, df=175, p=.03) than patients recruited from Penn.
Procedures
The institutional review boards of both institutions approved all study procedures, and all subjects provided their own informed consent. Potential participants were screened with the 15-item Geriatric Depression Screen (GDS-15)(32) and the Mini-Mental State Examination(13) (MMSE) at a regularly scheduled clinic appointment, and patients who met basic study eligibility criteria were scheduled for a follow-up detailed neuropsychiatric, neuropsychological, and neurological assessment with trained research staff and one of the authors (DW).
Measures
Demographic and clinical characteristics
Patients provided the following information during the screening interview: age, sex, race, marital status, years of education, disease duration of PD, history of deep brain stimulation (DBS) surgery (yes/no), current levodopa (mg/day) and dopamine agonist (DA) use (yes/no).
Psychiatric assessments
The following psychiatric measures were used: Inventory for Depressive Symptomatology(31) (IDS; scores 0–84, higher scores indicating greater depression severity); Spielberger State Anxiety Inventory(34) (STAI; scores 20–80, higher scores indicating greater anxiety severity); Apathy Scale(36) (AS; scores 0 to 42, higher scores indicating greater apathy severity); and the Epworth Sleepiness Scale (ESS; scores 0 to 24, higher scores indicating greater severity of daytime sleepiness). Psychosis was assessed with a modified version of the Parkinson’s Psychosis Rating Scale(14) (PPRS). In light of the fact that the distribution of PPRS scores was highly skewed (i.e., there were few scores in the high range) and nonspecificity of certain PPRS items for psychosis, a positive response to either the hallucination (modified to include auditory hallucinations) or paranoia item was considered a positive response for psychosis (entered as a dichotomous variable).
Cognitive assessments
The sum score for trials 1–3 on the free recall component of the Hopkins Verbal Learning Task(7) (HVLT; scores 0–36, higher scores indicating better performance) was used as the memory measure. Executive functioning was assessed with the Semantic Verbal Fluency Test (number of animals generated in one minute)(16). Attention was assessed with the backward score on the Digit Span test(1), which is thought to be more specific to attentional abilities and working memory compared with the forward Digit Span(24).
PD assessments
Severity of PD was assessed with the Unified Parkinson’s Disease Rating Scale(12) (UPDRS) motor score and the Hoehn and Yahr Scale(17).
Other assessments
Olfaction was assessed with the University of Pennsylvania Smell Identification Test(11) (UPSIT; scores 0–40, lower scores indicating greater impairment).
Analyses
Descriptive statistics included means and standard deviations (SDs) for continuous variables, and frequencies and percentages for categorical variables. In addition, we conducted latent class analysis (LCA;(29,30)), a special case of finite mixture modeling whereby observed multiple dependent variables are used to obtain categorical latent (i.e., unobserved) variables, or classes. Specifically, we used LCA to estimate, 1) the number of classes that best captured subgroups of psychiatric and cognitive symptom profiles across patients’ observed values, 2) the proportion of the sample that belonged to each class, and 3) each individual’s class membership. We also used LCA to determine the extent to which various PD patient and disease characteristics predicted differential group membership across the identified latent symptom classes.
The first step in the LCA procedure involved determining the optimal number of categorical latent classes for the observed patterns of data across the 8 measures of psychiatric and cognitive functioning (i.e., latent class indicators). The continuous indicators included in this step of the procedure were daytime sleepiness, apathy, depression, state anxiety, memory, executive function, and attention. Psychosis was specified as a dichotomous indicator (yes/no) in the model. We compared a series of LCA models with increasing number of latent classes from two to six, using the Bayesian Information Criteria (BIC) as the model fit index (smaller values denote better model fit). Although the five (BIC=8050.23) and six (BIC=8050.23) class solutions yielded smaller BIC values than the four class solution (BIC=8068.12), the additional classes were parallel profiles of those identified in the four class solution and did not impart new clinically-relevant information. Thus, we selected the four class model as the best fitting model. The final LCA model produced separately for each of the four latent symptom classes means and standard deviations for the seven observed measures of psychiatric and cognitive functioning and thresholds for the categorical indicator psychosis. To test whether the means of the psychiatric and cognitive outcomes differed across the four identified classes, we ran a series of post-hoc ANOVA’s. Pairwise comparisons between all class means were evaluated using a Bonferroni adjustment for the error rate. For indicators with unequal variances across classes (e.g., depression and anxiety), the Games-Howell multiple comparisons test was evaluated. The test of differences among the four classes with respect to the proportion of patients with psychosis was tested with the chi-square test.
Following identification of the optimal number of classes, we examined predictors of class membership by adding covariates to the LCA model. We estimated the probability of membership in each of the latent classes conditional on the following patient-level variables: age, PD duration, Hoehn and Yahr stage, UPDRS motor score, levodopa daily dosage, and DA use (yes/no). Specifically, this structural model was specified as a multinomial logistic regression equation whereby the set of four categorical latent classes identified in the first step was regressed on the set of predictors.
All LCA analyses were conducted using Mplus version 5.0(30). The ANOVA and chi-square analyses were performed in SPSS(2).
RESULTS
Participant Characteristics
Participants on average were 64.5 (SD = 10.5) years old, and primarily male (73.4%) and Caucasian (93.8%) (Table 1). Overall, patients were representative of PD patients in specialty care settings, with a mean disease duration of 6.8 (SD = 5.4) years, PD of mild-moderate severity (mean Hoehn & Yahr Stage = 2.3 (SD = 0.7)), and a mean UPDRS motor score of 21.9 (SD = 10.6). Approximately half (53.1%) of the sample was taking a DA, and the mean levodopa dosage was 519.9 (SD = 404.3) mg/day.
Table 1.
Demographic and clinical characteristics of study population
| Variables | Mean (SD) or N (%) | Range |
|---|---|---|
|
Sociodemographics | ||
| Age (# years) | 64.5 (10.5) | 37–87 |
| Education (# years formal education) | 15.9 (3.1) | 8–24 |
| Sex (% male) | 130 (73.4%) | - |
| Race (% white) | 166 (93.8%) | - |
|
Parkinson’s disease and other clinical indices | ||
| PD Duration (# years) | 6.8 (5.4) | 0–31 |
| Hoehn & Yahr stage | 2.3 (0.7) | 1–5 |
| UPDRS motor score | 21.9 (10.6) | 0–60 |
| Levodopa dosage (mg/day) | 519.9 (404.3) | 0–2480 |
| Dopamine agonist use (% yes) | 94 (53.1%) | - |
| MMSE score | 28.3 (2.0) | 18–30a |
| UPSIT scoreb | 19.3 (6.9) | 7–38 |
96.6% of subjects had an MMSE score ≥24
N=171
Results from univariate analyses of the sample means and frequencies, standard deviations, and ranges for the 8 latent class indicators included in the LCA models are presented in Table 2. On average, patients reported mild-moderate symptom severity on measures of apathy, depression, anxiety, and daytime sleepiness, and 28% of the sample experienced psychotic symptoms. Mean performance on cognitive assessments suggested average global cognitive abilities overall, consistent with the mean (SD) MMSE score of 28.3 (2.0), with 97% of subjects having an MMSE score ≥24.
Table 2.
Descriptive statistics for latent class analysis indicators
| Variables | Mean (SD) or N (%) | Observed Range |
|---|---|---|
| Excessive daytime sleepiness severity scorea | 10.2 (5.0) | 0–22 |
| Apathy severity score | 13.0 (6.9) | 0–39 |
| Depression severity score | 20.1 (13.7) | 0–60 |
| Anxiety severity scoreb | 41.7 (15.0) | 20–76 |
| Psychosis (percentage yes) | 50 (28.4%) | - |
| Memory (# items free recall) | 21.9 (6.2) | 5–35 |
| Executive function (# animals generated) | 19.2 (7.1) | 1–36 |
| Attention (# items correct backward digit span) | 6.6 (2.6) | 2–13 |
N=176
N=174
Determination of the Optimal Number of Psychiatric and Cognitive Symptom Classes
Comparing LCA models ranging from 2 to 6 classes, a 4-class solution best fit the data (BIC = 8258.13). The resulting proportion of the sample belonging to each of the four classes was: 32.0% in Class 4, 26.0% in Class 3, 24.3% in Class 2, and 17.5% in Class 1.
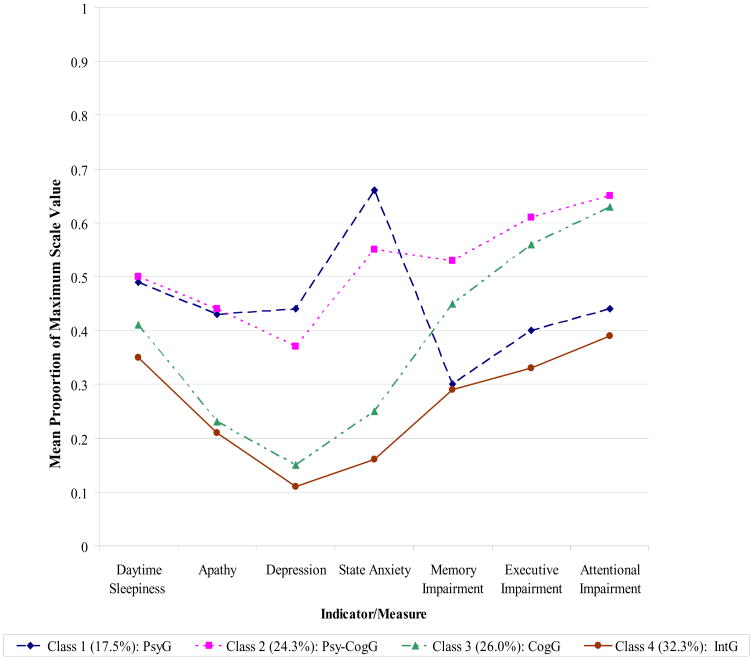
Figure 1 displays symptom profiles across the 7 continuous latent class indicators in each of the 4 classes, and Table 3 displays the corresponding estimated means and standard deviations for each of these classes. In order to facilitate comparison across measures, mean observed values for the latent class indicators presented in Figure 1 were transformed and scaled as the proportion of the maximum possible score for each measure. The distinctions among the four classes displayed in Figure 1 and Table 3 indicate that the four classes have the following interpretations: Class 1 is the Psychiatric Group (PsyG); Class 2 is the Psychiatric-Cognitive Group (Psy-CogG); Class 3 is the Cognitive Group (CogG); and finally Class 4 is the Intact Group (IntG). More specifically, the symptom profiles suggest that patients in both Classes 1 and 2 experienced moderate-severe symptoms of apathy (F (3,173)=46.48, P <.001), depression (F (3,173)=143.95, P <.001), and anxiety (F (3,170)=117.03, P <.001) relative to Classes 3 and 4 (please refer to Table 3 for results of post hoc, pairwise comparisons). However, compared to patients in Class 1 (PsyG), patients belonging to Class 2 (Psy-CogG) evidenced significantly worse performance across the 3 cognitive indicators (memory: F (3,173)=33.31, P <.001; executive function: F (3,173)=35.26, P <.001; attention: F (3,173)=38.98, P <.001; see Table 3 for post hoc pairwise comparisons) and were more likely to experience symptoms of psychosis (57.3% vs. 27.8%, x2 (1, N=73) = 5.69, P =.02). Similarly, while patients in Classes 3 and 4 both reported absent-mild psychiatric symptoms, patients in Class 4 (IntG) performed significantly better on the three cognitive tasks than those in Class 3 (CogG). There were no significant between-class differences among the four classes in terms of severity of daytime sleepiness.
Figure 1. Latent class profiles of continuous psychiatric and cognitive measures.
Note: Value (Y) axis represents mean observed values for LCA indicators scaled as the proportion of the maximum possible score for each measure. For free memory, executive function, and attention, proportions were transformed such that higher proportions indicate greater cognitive impairment.
Table 3.
Latent class indicator mean values and significant differences across identified latent classes
| Variablesa | Class 1 (PsyG; n=31) | Class 2 (Psy-CogG; n=43) | Class 3 (CogG; n=46) | Class 4 (IntG; n=57) | Test (df), p-value |
|---|---|---|---|---|---|
| Excessive daytime sleepiness | 11.66 (1.06) | 11.89 (0.77) | 9.74 (1.03) | 8.51 (0.81) | F (3, 172)=4.93, p=.003 |
| Apathyb | 18.15 (1.19) | 18.45 (1.09) | 9.72 (0.88) | 8.78 (0.76) | F (3, 173)=46.48, p<.001 |
| Depressionc | 37.08 (2.69) | 30.90 (1.62) | 12.40 (1.86) | 8.98 (0.88) | F (3, 173)=143.95, p<.001 |
| Anxietyd | 59.87 (2.60) | 52.88 (1.53) | 34.99 (2.25) | 29.33 (1.43) | F (3, 170)=117.03, p<.001 |
| Psychosis (n (%) yes)e | 9 (27.8%) | 25 (57.3%) | 10 (21.6%) | 7 (12.6%) | x2 (3, N=176)=25.35, p<.001 |
| Memoryf | 25.15 (0.85) | 16.84 (1.10) | 19.97 (1.03) | 25.65 (0.99) | F (3, 173)=33.31, p<.001 |
| Executive Functiong | 22.07 (1.52) | 13.99 (0.81) | 16.34 (1.24) | 24.05 (1.07) | F (3, 173)=35.26, p<.001 |
| Attentionh | 7.82 (0.46) | 4.97 (0.45) | 5.23 (0.32) | 8.52 (0.42) | F (3, 173)=38.98, p<.001 |
Cell values represent means (standard deviations) and frequencies (percentages) estimated by the LCA procedure for continuous and categorical indicators, respectively.
Post hoc analyses revealed significant differences between Classes 1 & 3 (Mdiff = 8.22, SE=1.20, P<.001), Classes 1 & 4 (Mdiff =9.22, SE=1.15, P<.001), Classes 2 & 3 (Mdiff = 9.00, SE=1.09, P<.001), and Classes 2 & 4 (Mdiff = 10.00, SE=1.04, P<.001).
Post hoc analyses revealed significant differences between Classes 1 & 2 (Mdiff = 5.79, SE=2.11, P=.04), Classes 1 & 3 (Mdiff =24.27, SE=2.01, P<.001), Classes 1 & 4 (Mdiff = 27.94, SE=1.82, P<.001), Classes 2 & 3 (Mdiff = 18.48, SE=1.67, P<.001), Classes 2 & 4 (Mdiff = 22.15, SE=1.44, P<.001), and Classes 3 & 4 (Mdiff = 3.67, SE=1.28, P=.03).
Post hoc analyses revealed significant differences between Classes 1 & 2 (Mdiff = 6.73, SE=2.26, P=.02), Classes 1 & 3 (Mdiff =24.63, SE=2.32, P<.001), Classes 1 & 4 (Mdiff = 30.70, SE=2.17, P<.001), Classes 2 & 3 (Mdiff = 17.90, SE=1.79, P<.001), Classes 2 & 4 (Mdiff = 23.97, SE=1.60, P<.001), and Classes 3 & 4 (Mdiff = 6.07, SE=1.68, P=.003).
Chi square analyses revealed significant differences between Classes 1 & 2 (x2 (1, N=73)=5.69, p=.02), Classes 2 & 3 (x2 (1, N=88)=11.61, p=.001), and Classes 2 & 4 (x2 (1, N=99)=22.63, p<.001).
Post hoc analyses revealed significant differences between Classes 1 & 2 (Mdiff = 8.69, SE=1.17, P<.001), Classes 1 & 3 (Mdiff =5.20, SE=1.16, P<.001), Classes 2 & 3 (Mdiff = −3.48, SE=1.06, P=.007), Classes 2 & 4 (Mdiff = −8.94, SE=1.00, P<.001), and Classes 3 & 4 (Mdiff = −5.46, SE=0.99, P<.001).
Post hoc analyses revealed significant differences between Classes 1 & 2 (Mdiff = 8.38, SE=1.32, P<.001), Classes 1 & 3 (Mdiff =6.02, SE=1.30, P<.001), Classes 2 & 4 (Mdiff = −10.31, SE=1.13, P<.001), and Classes 3 & 4 (Mdiff = −7.95, SE=1.11, P<.001).
Post hoc analyses revealed significant differences between Classes 1 & 2 (Mdiff = 2.80, SE=0.52, P<.001), Classes 1 & 3 (Mdiff =2.69, SE=0.46, P<.001), Classes 2 & 4 (Mdiff = −3.62, SE=0.44, P<.001), and Classes 3 & 4 (Mdiff = −3.51, SE=0.37, P<.001).
Patient-level Predictors of Class Membership
The extent to which patient-level characteristics predicted class membership is reflected in the parameter estimates and corresponding significance levels generated by the Mplus LCA procedure and reported in Table 4. Adjusting for all other variables in the model, increasing age was associated with a greater likelihood of belonging to Psy-CogG (Class 2) relative to the PsyG (Class 1) (OR=1.26, 95% CI=1.08–1.47, Wald χ2=8.87, df=1, P=.003) and IntG (Class 4) (OR=1.21, 95% CI=1.04–1.41, Wald χ2=5.83, df=1, P=.02). Increasing Hoehn & Yahr stage was associated with an increased likelihood of belonging to PsyG (OR=5.15, 95% CI=1.67–15.83, Wald χ2=8.12, df=1, P=.004) and Psy-CogG (OR=21.55, 95% CI=1.67–277.59, Wald χ2=5.55, df=1, P=.02) than IntG, and increasing UPDRS scores were associated with a greater likelihood of membership in Psy-CogG (OR=1.14, 95% CI=1.04–1.25, Wald χ2=7.88, df=1, P=.005), CogG (OR=1.09, 95% CI=1.01–1.20, Wald χ2=4.12, df=1, P=.04) and IntG (OR=1.09, 95% CI=1.01–1.19, Wald χ2=4.35, df=1, P=.04) relative to PsyG.
Table 4.
Membership in latent classes as a function of patient-level characteristics
| Classa | Variable | OR | 95% Confidence Interval | Wald χ2 (df=1) | p-value |
|---|---|---|---|---|---|
| Class 1 (PsyG; n=31) | Age (# years) | 0.96 | (0.90, 1.02) | 1.83 | 0.18 |
| PD duration (# years) | 0.96 | (0.78, 1.17) | 0.20 | 0.65 | |
| Hoehn & Yahr Stage | 5.15 | (1.67, 15.83) | 8.12 | 0.004 | |
| UPDRS motor score | 0.92 | (0.84, 0.99) | 4.35 | 0.04 | |
| Levadopa dosage (per 100 mg increase) | 1.00 | (0.82, 1.22) | 0.04 | 0.85 | |
| Dopamine agonist use (% yes) | 0.83 | (0.25, 2.80) | 0.001 | 0.76 | |
| Class 2 (Psy-CogG; n=43) | Age (# years) | 1.21 | (1.04, 1.41) | 5.83 | 0.02 |
| PD duration (# years) | 1.18 | (0.94, 1.49) | 1.95 | 0.16 | |
| Hoehn & Yahr Stage | 21.55 | (1.67, 277.59) | 5.55 | 0.02 | |
| UPDRS motor score | 1.05 | (0.98, 1.12) | 1.56 | 0.21 | |
| Levadopa dosage (per 100 mg increase) | 1.35 | (0.91, 2.00) | 4.33 | 0.04 | |
| Dopamine agonist use (% yes) | 0.08 | (0.01, 0.86) | 4.35 | 0.04 | |
| Class 3 (CogG; n=46) | Age (# years) | 1.17 | (0.96, 1.43) | 2.33 | 0.13 |
| PD duration (# years) | 1.08 | (0.85, 1.37) | 0.41 | 0.52 | |
| Hoehn & Yahr Stage | 7.43 | (0.69, 79.65) | 2.75 | 0.10 | |
| UPDRS motor score | 1.00 | (0.94, 1.08) | 0.01 | 0.91 | |
| Levadopa dosage (per 100 mg increase) | 1.36 | (1.34, 2.01) | 11.03 | 0.001 | |
| Dopamine agonist use (% yes) | 0.45 | (0.03, 5.83) | 0.38 | 0.54 |
Class 4 (IntG) is the reference category.
In addition to predicting membership in Class 2 relative to Class 4, increasing age was associated with a greater likelihood of belonging to Class 2 relative to Class 1 (OR=1.26, 95% CI= 1.08–1.47, Wald χ2=8.87, df=1, P=.003). Increasing UPDRS scores were associated with a greater likelihood of membership in Class 2 (OR=1.14, 95% CI=1.04–1.25, Wald χ2=7.88, df=1, P=.005) and Class 3 (OR=1.09, 95% CI= 1.01–1.20, Wald χ2=4.12, df=1, P=.04) relative to Class 1. Higher levodopa dosages were associated with a greater likelihood of belonging to Class 3 relative to Class 1 (OR=1.65, 95% CI=1.11–2.44, Wald χ2=8.20, df=1, P=.004). Patients taking a DA were more likely to belong to Class 3 than Class 2 (OR=5.85, 95% CI=1.16–29.08, Wald χ2=4.62, df=1, p=.03).
With respect to medication use, higher daily levodopa dosages were associated with a greater likelihood of belonging to CogG than either of the groups with intact cognitive functioning (PsyG: OR (per 100-mg increase in dosage) =1.65, 95% CI=1.11–2.44, Wald χ2=8.20, df=1, P=.004; IntG: OR =1.36, 95% CI=1.34–2.01, Wald χ2=11.03, df=1, P=.001), as well as predicting membership in Psy-CogG as opposed to IntG (OR =1.35, 95% CI=0.91–2.00, Wald χ2=4.33, df=1, P=.04). On the other hand, patients taking a DA were more likely to belong to the two classes with fewer psychiatric symptoms (i.e., CogG (OR=5.85, 95% CI=1.16–29.08, Wald χ2=4.62, df=1, P=.03) and IntG (OR=13.07, 95% CI=1.40–146.45, Wald χ2=4.35, df=1, P=.04)) than Psy-CogG.
CONCLUSION
Psychiatric and cognitive disorders are common and frequently co-occur in PD. In order to empirically delineate distinct classes of PD patients presenting with similar symptom profiles across both psychiatric and cognitive assessments, we utilized latent variable modeling to characterize profiles of non-motor symptoms among PD patients without significant global cognitive impairment. Overall, the findings lend support to the notion of multiple, distinct classes of PD patients, with each class characterized by a distinct psychiatric and cognitive profile. Furthermore, patient-level characteristics appeared to serve as significant predictors of PD patients’ likelihood of membership in the various identified classes.
Results from the LCA models indicated that a 4-class model best captured group-level variability in PD patients’ psychiatric and cognitive symptom profiles. Only one-third of the study sample could be considered intact from this standpoint (Class 4; IntG). Close to one-fifth of the sample (Class 1; PsyG) demonstrated a profile of numerous, significant psychiatric symptoms with intact cognition, and approximately one-quarter (Class 3; CogG) had worse cognitive abilities across a range of domains, without significant co-morbid psychiatric symptoms. The final quarter of the sample (Class 2; Psy-CogG) had both significant psychiatric symptoms and worse cognitive abilities across a range of domains. Our results are consistent with two recent cluster analyses of Neuropsychiatric Inventory(10) (NPI) scores in PD patients(3,8) that identified several clusters of patients, including one with minimal neuropsychiatric symptoms, one or more clusters with primarily affective symptoms, and another with primarily psychotic symptoms.
A number of notable associations emerged when examining the relationship between patient-level characteristics and latent class membership. Patients in Class 2 (Psy-CogG), the most impaired group overall, were older, had more advanced disease, and higher UPDRS motor scores on average than all the other groups, with many of these differences meeting statistical significance. This is consistent with previous research demonstrating that such factors are associated with both cognitive impairment and some psychiatric symptoms in PD(22,41). Patients in Class 1, who had significant psychiatric symptoms but were intact cognitively, were the youngest group with the shortest disease duration and fewest motor symptoms on average, consistent with some research findings that younger PD patients may have an elevated risk of psychiatric disorders early in the disease course(35,38).
Class membership was also predicted by medication use. Specifically, higher levodopa dosages were associated with a greater likelihood of belonging to both classes with impaired cognitive functioning, and in the case of Class 2 (Psy-CogG), also a higher frequency of psychosis, as opposed to classes with relatively intact cognitive functioning. This is consistent with previous research demonstrating that higher levodopa dosages can be associated with cognitive impairment and psychosis, particularly in older patients and in those with more advanced disease(19,39,42). Conversely, patients taking a dopamine agonist were significantly more likely to belong to the groups characterized by low psychiatric symptom severity, consistent with preliminary evidence that this medication class may improve certain psychiatric symptoms (e.g., depression)(6), although the association between infrequent dopamine agonist use and high psychosis prevalence in Class 2 (Psy-CogG) suggests that some medication treatment decisions in PD are made in response to the presence of certain psychiatric symptoms.
There are several limitations to this study. First, our convenience sample, which was drawn from tertiary care clinics, was predominately male, white, college educated, and had mild to moderate severity of PD. Second, while there were significant site differences for age, levodopa dose, attention, and executive function, we chose not to include site as a predictor in the multinomial logistic regression portion of the LCA model due to the fact that the majority of patients were recruited from one of the two sites (i.e., the University of Pennsylvania). Had we included site as a predictor, we would have run the risk of small, or empty, cells when estimating the joint distribution of the four categorical latent variables and independent variables. Thus, additional studies in larger numbers of PD patients with greater demographic and clinical diversity are needed to verify and generalize our findings. Larger samples would also allow for more flexibility when modeling predictors and covariates. Third, although our study looked at a range of neuropsychiatric and cognitive variables, the ones included were those available from our assessment battery. Future studies should consider other non-motor symptoms or impairments for inclusion in symptom profiling. Finally, we were not able to assess the clinical impact of the psychiatric symptoms or worse cognitive performance in the affected groups, but the scores on the depression, anxiety, apathy and daytime sleepiness scales in the two psychiatric groups were above the cut-offs recommended to indicate clinically-significant symptoms(18,21,31,36), and in general neuropsychiatric symptoms in non-demented PD patients are associated with poorer quality of life(27).
Identifying discrete classes of patients with similar psychiatric and cognitive profiles and also certain demographic and clinical characteristics may shed light on the underlying etiology of different patterns of non-motor impairment. For instance, the group with psychiatric symptoms only (Class 1), younger on average with mild PD of short duration, may have psychosocially-mediated psychiatric symptoms, whereas the group with both psychiatric symptoms and worse cognitive functioning (Class 2), older and with longer disease duration, may have psychiatric and cognitive symptoms secondary to progressive disease-related neuropathological changes. As a result, more appropriate assessment and treatment plans may be developed that target discrete patient subgroups, addressing the shortcomings of traditional approaches that target individual symptoms (e.g., “target symptom” approach) or empirically derived clusters of highly correlated symptoms (e.g., factor analysis). Further research is needed to determine if identified classes are stable across time, and if the classes help predict function, quality of life, and long-term patient outcomes. Additionally, studies should also assess whether symptom profiles are amenable to treatment at a class level.
Acknowledgments
Funding: This research was supported by National Institute of Mental Health (NIMH) grant #067894.
Footnotes
Disclosure: No Disclosures to Report.
References
- 1.Comprehensive norms for an expanded Halstead-Reitan Battery: Demographic corrections, research findings, and clinical applications. Lutz, Fl: Psychological Assessment Resources, Inc; 1991. [Google Scholar]
- 2.SPSS for Windows. Version 15.0. Chicago: SPSS Inc; 2007. [Google Scholar]
- 3.Aarsland D, Bronnick K, Ehrt U, et al. Neuropsychiatric symptoms in patients with Parkinson’s disease and dementia: frequency, profile and associated caregiver stress. J Neurol Neurosurg Psychiatry. 2007;78:36–42. doi: 10.1136/jnnp.2005.083113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aarsland D, Larsen JP, Cummings JL, et al. Prevalence and clinical correlates of psychotic symptoms in Parkinson disease: a community-based study. Arch Neurol. 1999;56:595–601. doi: 10.1001/archneur.56.5.595. [DOI] [PubMed] [Google Scholar]
- 5.Aarsland D, Larsen JP, Lim NG, et al. Range of neuropsychiatric disturbances in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1999;67:492–496. doi: 10.1136/jnnp.67.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barone P, Scarzella L, Marconi R, et al. Pramipexole versus sertraline in the treatment of depression in Parkinson’s disease. J Neurol. 2006;253:601–607. doi: 10.1007/s00415-006-0067-5. [DOI] [PubMed] [Google Scholar]
- 7.Brandt J, Benedict RHB. The Hopkins Verbal Learning Test-Revised. Odessa, FL: Psychological Assessment Resources; 2001. [Google Scholar]
- 8.Bronnick K, Aarsland D, Larsen JP. Neuropsychiatric disturbances in Parkinson’s disease clusters in five groups with different prevalence of dementia. Acta Psychiatr Scand. 2005;112:201–207. doi: 10.1111/j.1600-0447.2005.00562.x. [DOI] [PubMed] [Google Scholar]
- 9.Caap-Ahlgren M, Dehlin O. Insomnia and depressive symptoms in patients with Parkinson’s disease. Relationship to health-related quality of life. An interview of patients living at home. Arch Gerontol Geriatr. 2001;32:23–33. doi: 10.1016/s0167-4943(00)00087-x. [DOI] [PubMed] [Google Scholar]
- 10.Cummings JL, Mega M, Gray K, et al. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 11.Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav. 1984;32:489–502. doi: 10.1016/0031-9384(84)90269-5. [DOI] [PubMed] [Google Scholar]
- 12.Fahn S, Elton RL the UPDRS Development Committee. In: Unified Parkinson’s disease rating scale, in Recent developments in Parkinson’s disease. Fahn S, Marsden CD, Calne D, et al., editors. Florham Park, NJ: Macmillan Health Care Information; 1987. pp. 153–163. [Google Scholar]
- 13.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:196–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 14.Friedberg G, Zoldan J, Weizman A, et al. Parkinson Psychosis Rating Scale: A practical instrument for grading psychosis in Parkinson’s disease. Clin Neuropharmacol. 1998;21:280–284. [PubMed] [Google Scholar]
- 15.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson’s disease. Arch Neurol. 1999;56:33–39. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- 16.Gladsjo JA, Shuman CC, Evans JD, et al. Norms for letter and category fluency: demographic corrections for age, education, and ethnicity. Assessment. 1999;6:147–178. doi: 10.1177/107319119900600204. [DOI] [PubMed] [Google Scholar]
- 17.Hoehn MH, Yahr MD. Parkinsonism: onset, progression, and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 18.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 19.Kulisevsky J. Role of dopamine in learning and memory: implications for the treatment of cognitive dysfunction in patients with Parkinson’s disease. Drugs and Aging. 2000;16:365–379. doi: 10.2165/00002512-200016050-00006. [DOI] [PubMed] [Google Scholar]
- 20.Kulisevsky J, Pagonabarraga J, Pascual-Sedano B, et al. Prevalence and correlates of neuropsychiatric symptoms in Parkinson’s disease without dementia. Mov Disord. 2008;23:1889–1896. doi: 10.1002/mds.22246. [DOI] [PubMed] [Google Scholar]
- 21.Kvaal K, Ulstein I, Nordhus IH, et al. The Spielberger State-Trait Anxiety Inventory (STAI): the state scale in detecting mental disorders in geriatric patients. Int J Geriatr Psychiatry. 2005;20:629–634. doi: 10.1002/gps.1330. [DOI] [PubMed] [Google Scholar]
- 22.Lauterbach EC. The neuropsychiatry of Parkinson’s disease and related disorders. Psychiatr Clin North Am. 2004;27:801–825. doi: 10.1016/j.psc.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Levin BE, Katzen HL. Early cognitive changes and nondementing behavioral abnormalities in Parkinson’s disease. In: Weiner WJ, Lang AE, editors. Behavioral Neurology of Movement Disorders. New York: Raven Press, Ltd; 1995. pp. 85–95. [PubMed] [Google Scholar]
- 24.Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. New York: Oxford University Press; 2004. [Google Scholar]
- 25.Lou J-S, Kearns G, Oken B, et al. Exacerbated physical fatigue and mental fatigue in Parkinson’s disease. Mov Disord. 2001;16:190–196. doi: 10.1002/mds.1042. [DOI] [PubMed] [Google Scholar]
- 26.McDonald WM, Richard IH, Delong MR. Prevalence, etiology, and treatment of depression in Parkinson’s disease. Biol Psychiatry. 2003;54:363–375. doi: 10.1016/s0006-3223(03)00530-4. [DOI] [PubMed] [Google Scholar]
- 27.McKinlay A, Grace RC, Dalrymple-Alford JC, et al. A profile of neuropsychiatric problems and their relationship to quality of life for Parkinson’s disease patients without dementia. Parkinsonism and Related Disorders. 2008;14:37–42. doi: 10.1016/j.parkreldis.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 28.Menza MA, Robertson-Hoffman DE, Bonapace AS. Parkinson’s disease and anxiety: comorbidity with depression. Biol Psychiatry. 1993;34:465–470. doi: 10.1016/0006-3223(93)90237-8. [DOI] [PubMed] [Google Scholar]
- 29.Muthen BO. Advances in latent variable mixture models. Charlotte, NC: Information Age Publishing, Inc; 2008. Latent variable hybrids: Overview of old and new models. [Google Scholar]
- 30.Muthen LK, Muthen BO. Mplus User’s Guide. Los Angeles, CA: Muthen & Muthen; 2007. [Google Scholar]
- 31.Rush AJ, Giles DE, Schlesser MA, et al. The Inventory for Depressive Symptomatology (IDS): preliminary findings. Psychiatry Res. 1985;18:65–87. doi: 10.1016/0165-1781(86)90060-0. [DOI] [PubMed] [Google Scholar]
- 32.Sheikh JI, Yesavage JA. In: Geriatric Depression Scale (GDS): recent evidence and development of a shorter version, in Clinical Gerontology: A Guide to Assessment and Intervention. Brink TL, editor. New York: The Haworth Press; 1986. pp. 165–173. [Google Scholar]
- 33.Shulman LM, Taback RL, Bean J, et al. Comorbidity of the nonmotor symptoms of Parkinson’s disease. Mov Disord. 2001;16:507–510. doi: 10.1002/mds.1099. [DOI] [PubMed] [Google Scholar]
- 34.Spielberger CD, Gorsuch RL, Lushene R, et al. Manual for the State-Trait Anxiety Inventory (Form Y) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 35.Starkstein SE, Berthier ML, Bolduc PL, et al. Depression in patients with early versus late onset of Parkinson’s disease. Neurology. 1989;39:1441–1445. doi: 10.1212/wnl.39.11.1441. [DOI] [PubMed] [Google Scholar]
- 36.Starkstein SE, Mayberg HS, Preziosi TJ, et al. Reliability, validity, and clinical correlates of apathy in Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 1992;4:134–139. doi: 10.1176/jnp.4.2.134. [DOI] [PubMed] [Google Scholar]
- 37.Starkstein SE, Preziosi TJ, Berthier ML, et al. Depression and cognitive impairment in Parkinson’s Disease. Brain. 1989;112:1141–1153. doi: 10.1093/brain/112.5.1141. [DOI] [PubMed] [Google Scholar]
- 38.Starkstein SE, Preziosi TJ, Bolduc PL, et al. Depression in Parkinson’s disease. J Nerv Ment Dis. 1990;178:27–31. doi: 10.1097/00005053-199001000-00005. [DOI] [PubMed] [Google Scholar]
- 39.Weintraub D, Morales KH, Duda JE, et al. Frequency and correlates of co-morbid psychosis and depression in Parkinson’s disease. Parkinsonism and Related Disorders. 2006;12:427–431. doi: 10.1016/j.parkreldis.2006.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weintraub D, Stern MB. Psychiatric complications in Parkinson’s disease. Am J Geriatr Psychiatry. 2005;13:844–851. doi: 10.1176/appi.ajgp.13.10.844. [DOI] [PubMed] [Google Scholar]
- 41.Weintraub D, Stern MB. Intervening in the neuropsychiatric features of Parkinson’s disease. Expert Review of Neurotherapeutics. 2007;7:699–710. doi: 10.1586/14737175.7.6.699. [DOI] [PubMed] [Google Scholar]
- 42.Young BK, Camicioli R, Ganzini L. Neuropsychiatric adverse effects of antiparkinsonian drugs: Characteristics, evaluation and treatment. Drugs and Aging. 1997;10:367–383. doi: 10.2165/00002512-199710050-00005. [DOI] [PubMed] [Google Scholar]