Abstract
Elderly individuals have an increased susceptibility to microbial infections because of age-related anatomical, physiological, and environmental factors. However, the mechanism of aging-dependent susceptibility to infection is not fully understood. Here, we found that caveolae-dependent endocytosis is elevated in senescent cells. Thus, we focused on the implications of caveolae-dependent endocytosis using Salmonella typhimurium, which causes a variety of diseases in humans and animals by invading the eukaryotic host cell. Salmonella invasion increased in nonphagocytotic senescent host cells in which caveolin-1 was also increased. When caveolae structures were disrupted by methyl-β-cyclodextrin or siRNA of caveolin-1 in the senescent cells, Salmonellae invasion was reduced markedly compared to that in nonsenescent cells. In contrast, the over-expression of caveolin-1 led to increased Salmonellae invasion in nonsenescent cells. Moreover, in aged mice, caveolin-1 was found to be highly expressed in Peyer’s patch and spleen, which are targets for infection by Salmonellae. These results suggest that high levels of caveolae and caveolin-1 in senescent host cells might be related to the increased susceptibility of elderly individuals to microbial infections.
Keywords: senescence, caveolae, caveolin-1, S. typhimurium, infection, endocytosis
Introduction
Caveolae are flask-shaped invaginations in the plasma membrane on the surface of endothelial cells (Palade, 1953; Yamada, 1955). It has been suggested that caveolae mediate the extensive transcellular shuttling of serum proteins from the blood stream into tissues across the endothelial cell layer (Simionescu et al., 1975; Ghitescu & Bendayan, 1992; Schnitzer et al., 1994). Caveolae present on various types of cells consist of cholesterol and sphingolipid-rich microdomains of the plasma membrane (Brown & London, 1998; Simons & Toomre, 2000), in which many diverse signaling molecules and membrane transporters are concentrated. This organelle mediates the internalization of sphingolipids and sphingolipid-binding toxins, glycosylphisphatidylinositol (GPI)-anchored proteins, and growth hormones (Nichols & Lippincott-Schwartz, 2001).
Caveolae are composed mainly of caveolin proteins. Caveolins are a family of a 21–24 kDa integral membrane proteins consisting of caveolin-1, -2, and -3. Caveolin-1 and -2 are expressed together and form a hetero-oligomer in the plasma membrane of many cell types (Smart et al., 1999), whereas caveolin-3 is expressed only in muscle tissue (Tang et al., 1996). Caveolin-1 is a scaffolding protein within the caveolae membrane and interacts with various signaling proteins such as epidermal growth factor (EGF) receptor, G-proteins, Src-like kinases, Ha-Ras, protein kinase C, endothelial nitric-oxide synthase, and integrin (Li et al., 1996; Garcia-Cardena et al., 1997; Razani et al., 1999; Smart et al., 1999). Previously, we reported that the expressions of caveolin-1 and caveolae structures are higher in senescent cells, leading to the suppression of EGF-dependent growth activity and to the induction of senescence-associated morphological changes (Park et al., 2000; Cho et al., 2003, 2004).
Caveolae are involved in the entry of several species of viruses, parasites, and bacterial toxins. In the case of human immunodeficiency virus (HIV), the endocytosed microbial cargo is transported directly across the cell (Abrami et al., 2000). The entry of simian virus 40 (SV40) into host cells is mediated by major histocompatibility complex (MHC) class I molecules, after which the virus is transported by caveolae to the endoplasmic reticulum (ER) (Atwood & Norkin, 1989; Anderson et al., 1996). Cholera toxin (CT) binds first to the plasma membrane ganglioside (GM1), which is highly concentrated in caveolae, and subsequently this complex is transported into the ER through caveolae (Parton et al., 1994; Lencer et al., 1999). Shin et al. also provided evidence suggesting the possibility of caveolae-mediated entry of bacteria using FimH-expressing Escherichia coli. They showed that CD48, a receptor for FimH-expressing bacteria, is localized within caveolae and FimH-expressing E. coli are also co-localized with caveolae via interaction with CD48 in mast cells (Shin et al., 2000).
Because caveolae have been implicated in the entry of microbial pathogens, we examined their role in the entry of Salmonella typhimurium into host cells as this bacterium enters the eukaryotic host cell and replicates therein. The entry of Salmonella into animal host cells requires complex, syringe-like macromolecular structures termed type III secretion systems (TTSS) encoded by Salmonella Pathogenecity Island, SPI (Ohl & Miller, 2001). TTSS translocates a number of effector proteins including SopE, SopE2, and SopB, also encoded by SPI (Galan, 2001; Zhou & Galan, 2001). The SopE mimics a guanine nucleotide exchange factor that interacts with Rac1 and Cdc42, promoting actin cytoskeletal reorganization and mediating the internalization into host cells.
Therefore, in this work, we have assumed that the high level of caveolin-1 in aged cells might contribute to the higher susceptibility to microbial infection seen in older individuals. In addition, we report that the entry of Salmonella into host cells through caveolae-dependent endocytosis could be up-regulated in the senescent cells, probably resulting in the higher microbial susceptibility of older people.
Results
Transport-related genes are affected by the level of caveolin-1
Previously, we reported that caveolin-1 increases in senescent human diploid fibroblasts (HDFs) and plays an important role in senescence-associated growth responses and morphological changes (Park et al., 2000; Cho et al., 2003, 2004). We speculated that the level of caveolin-1 alters the expression of certain genes, presumably those implicated in cellular senescence. Microarray analysis showed differences between the expression profiles of senescent HDFs and those in which the expression of specific siRNAs was reduced by caveolin-1. After confirming the effect of siRNA targeting to caveolin-1 (Fig. 1A; Park et al., 2000; Cho et al., 2003, 2004), we isolated mRNA from senescent and control siRNA-treated senescent or caveolin-1 siRNA-treated senescent cells and performed microarray analysis. Using a microarray analysis chip composed of 8000 human EST clones, we found 65 genes whose expressions were altered more than 2-fold by treatment with siRNA targeting caveolin-1, compared to control siRNA (Table 1). Of these, 23 genes were grouped into three classes of known genes: those involved in the cell cycle, cell growth and maintenance, and signal transduction. The remainder consisted of genes of unknown function. Among the genes involved in cell growth and maintenance, several transport-related genes were notably reduced, including those involved in iron transport (ATP2B1, SLC26A11), lipid transport (APOE, HDLBP), protein transport (CHM, NPC1), and endocytosis (SLC6A1, VAMP3), suggesting that the level of caveolin-1 expression primarily affected transport-related genes. We speculated that in the senescent cell, these transport-related genes are elevated in accordance with increased expression of caveolin-1, although it is unlikely that caveolin-1 would activate these genes directly.
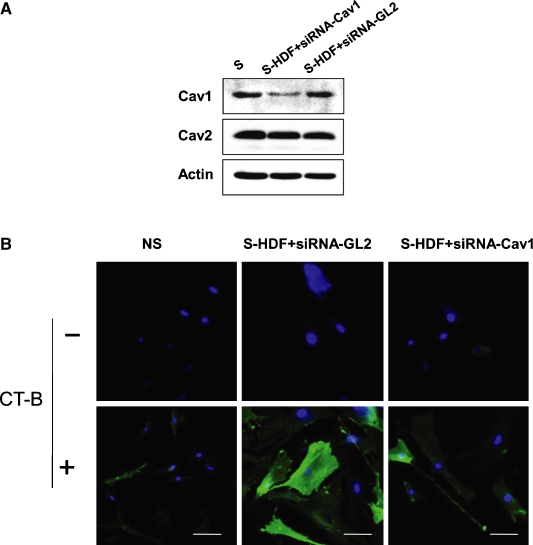
Fig. 1.
Caveolae-dependent uptake of fluorescent cholera toxin (CT) B subunits. Senescent human diploid fibroblasts were transfected with siRNA targeting of caveolin-1 (Cav1) or firefly luciferase (GL2), as a control. (A) Down-regulation of caveolin-1 expression was confirmed by Western blotting with anti-caveolin-1, -caveolin-2, -caveolin-3, and -actin antibody. (B) Cells were treated with fluorescent CT-B subunits (green) for 30 min and then fixed with 4% paraformaldehyde solution. Fixed cells were stained with DAPI (blue) and analyzed by confocal microscopy. Upper panel shows CT-B untreated cells, and lower panel shows CT-B-treated cells. Data are representative of > 100 cells observed in at least five independent experiments. Scale bar, 10 μm.
Table 1.
Caveolin-1 modulates transport-related genes
| Group | Fold induction | Gene | Functions |
|---|---|---|---|
| Apoptosis | 2.4 | STK17B | Induction of apoptosis, protein amino acid phosphorylation |
| Cell cycle | 2.0 | TOP1 | Cell growth and/or maintenance |
| 2.0 | SMC5L1 | Chromosome segregation | |
| −3.8 | CENPE | Mitotic metaphase plate congression, DNA replication and chromosome cycle | |
| −2.0 | PFDN1 | Cell cycle | |
| −2.8 | CENPB | Centromere/kinetochore complex maturation | |
| Cell growth and maintenance | −2.1 | ATP2B1 | Cation transport, calcium ion transport |
| 2.6 | MPHOSPH10 | rRNA processing | |
| −2.2 | CHM | C-terminal protein geranylgeranylation, intracellular protein transport | |
| −3.4 | SLC6A1 | Synaptic transmission, neurotransmitter transport | |
| −2.4 | SLC26A11 | Sulfate transport | |
| −2.8 | APOE | Lipid transport, cholesterol metabolism | |
| −4.0 | NPC1 | Intracellular protein transport, cholesterol transport | |
| −2.0 | VAMP3 | Nonselective vesicle docking, membrane fusion | |
| −2.8 | HDLBP | Lipid transport | |
| −2.0 | IGFBP5 | Signal transduction, regulation of cell growth | |
| −2.2 | ACTN4 | Invasive growth, cell motility | |
| −2.4 | PFN2 | Regulation of actin polymerization and/or depolymerization | |
| −2.6 | TGFBR2 | TGFβ ligand binding to type II receptor, positive regulation of cell proliferation | |
| Signal transduction | −2.0 | XPR1 | Pathogenesis |
| −2.0 | ACCN3 | Sensory perception, small molecule transport, signal transduction | |
| −2.4 | RASAL2 | Signal transduction | |
| −2.4 | ELK2 | Signal transduction, regulation of transcription from PolII promoter | |
| Transcription | −2.4 | LOC90233 | Regulation of transcription, DNA-dependent |
Subsequently, we examined the extent of endocytosis by senescent HDF by determining the uptake of fluorescein isothiocyanate (FITC)-conjugated CT B subunits (CT-B; Fig. 1B). CT-B is taken up by animal cells through caveolae-dependent endocytosis (Parton et al., 1994). The uptake of FITC-conjugated CT-B was detected in senescent HDFs compared to nonsenescent HDFs. We also examined the uptake of CT-B in senescent HDFs in which caveoline-1 was down-regulated by specific siRNA and found that the uptake of CT-B was reduced to the level of nonsenescent HDFs. Taken together, these findings suggest that elevated caveolin-1 in senescent cells would be responsible for the increased transport-related gene expression, including that involved in endocytosis, which results in increased caveolae-dependent endocytosis.
Salmonella entry increase in senescent host cells over-expressing caveolin-1
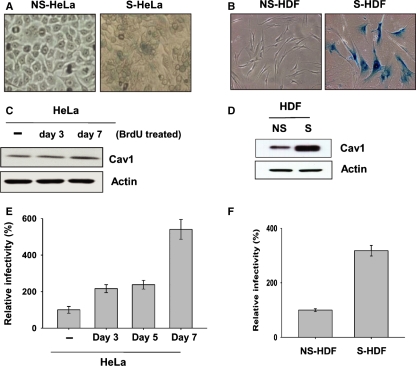
Because caveolae have been implicated in the endocytosis of microbial pathogens into host cells (Shin & Abraham, 2001; Pelkmans & Helenius, 2002; Laude & Prior, 2004), we investigated whether microbial invasion into senescent host cells would be elevated because of increased caveolin-1 expression using S. typhimurium. It is well known that Salmonella entry into nonphagocytotic intestinal epithelial cells is an essential early event in pathogenesis (Finlay & Falkow, 1997), a process that can be approximated in vitro using cultured cells. HeLa cells originating from epithelial cells have been used as representative models for the study of Salmonella invasion and virulence (Steele-Mortimer et al., 2002). Thus, we first attempted to induce the senescent phenotype in HeLa cells using 5-bromo-2-deoxyuridine (BrDU) treatment. BrDU induces the senescent phenotype through its incorporation into DNA, which produces damage in cancer cells and results in phenotypic changes that resemble senescence, including reduced proliferation (Masterson & O’Dea, 2007). After 7 days, the HeLa cells treated with BrDU started to exhibit the senescent phenotype, as revealed by β-Gal staining, although the phenotype was slightly less pronounced than in naturally senescent HDFs (Fig. 2A,B). Subsequently, we examined the level of caveolin-1 expression in BrDU-treated HeLa cells with young and naturally senescent HDFs using a specific antibody. We found that caveolin-1 increased, in a time-dependent manner, to about 3-fold on Day 7 (Fig. 2C). A direct comparison revealed a notable increase in caveolin-1 expression in senescent HDFs (Fig. 2D). Next, we determined the extent of Salmonella invasion using nonsenescent and senescent HeLa cells (Fig. 2E). Salmonella invasion increased in BrDU-treated HeLa cells in a time-dependent manner; a gradual increase was observed up to 7 days (∼5.5-fold). Similarly, Salmonella invasion was elevated in senescent HDFs by approximately 3.5-fold compared to that in nonsenescent HDFs (Fig. 2F).
Fig. 2.
Increased Salmonella infection to senescent nonphagocytotic host cells Senescent cells were confirmed by senescence-associated β-galactosidase staining. (A) Senescent HeLa cells (S-HeLa) were induced with 5-bromo-2-deoxyuridine (BrDU) treatment over 7 days. Nonsenescent HeLa (NS-HeLa) and S-HeLa cells were stained with senescence-associated β-galactosidase staining. (B) Senescent human diploid fibroblasts (HDFs) were also confirmed by senescence-associated β-galactosidase staining. The expression levels of caveolin-1 (cav-1) in BrDU-treated S-HeLa (C) and nonsenescent (NS) and senescent (S) HDFs (D) were analyzed by Western blotting with anti-caveolin-1 (top) or anti-actin (bottom) antibodies. Data are representative of at least five independent experiments. Salmonella invasion of BrDU-treated senescent HeLa cells (E) or senescent HDFs (F) was quantified as described in Methods. Invasion is expressed as a percentage of wild-type bacteria internalized by control cells after 60 min. Data are the means (±SD) of three independent filters, and experiments were performed at least five times.
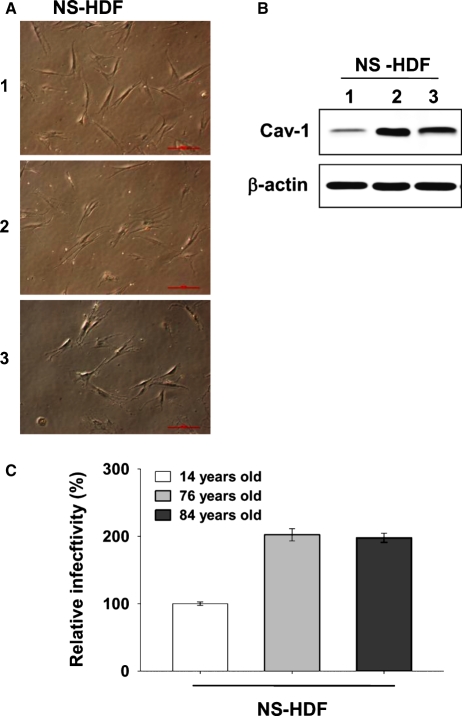
We examined the extent of Salmonella invasion in isolated primary human fibroblasts from young (14 years) and elderly donors (75, 84 years). Although primary fibroblasts from elderly donors did not show senescent phenotypes (Fig. 3A), Salmonella invasion was increased 2-fold in these fibroblasts compared to the fibroblasts from young donors (Fig. 3C). Interestingly, caveolin-1 was also highly expressed in primary fibroblasts from elderly donors (Fig. 3B).
Fig. 3.
Increase of Salmonella infection of nonsenescent fibroblasts with age. Primary fibroblasts were isolated from donors aged 14, 76, or 84 years old. (A) Cells were stained with senescence-associated β-galactosidase staining solution. (B) Expression levels of caveolin-1 (cav-1) in nonsenescent (NS) cells were analyzed by Western blotting with anti-caveolin-1 (top) or anti-actin (bottom) antibodies. (C) Salmonella invasion to primary fibroblasts was quantified and expressed as a percentage of wild-type bacteria internalized by control cells after 60 min. 1: 14 years old; 2: 76 years old; 3: 84 years old. Data are the means (±SD) of three independent filters, and experiments were performed at least five times.
Salmonella infected senescent and primary cells from elderly donors more readily than nonsenescent and primary cells from young donors, presumably because of the increased expression of caveolin-1. This suggests that the entry of Salmonella is not completely random but is mediated through caveolae.
Caveolae mediate Salmonella invasion into host cells
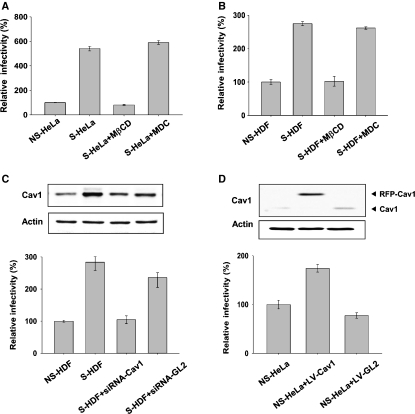
We further examined the role of caveolae in Salmonella invasion by treating host cells with methyl-β-cyclodextrin (MβCD) to inhibit caveolae-dependent endocytosis (Yancey et al., 1996) or with monodansylcadaverin (MDC) to inhibit clathrin-dependent endocytosis (Ray & Samanta, 1996). Senescent HeLa cells exhibited ∼5.5-fold higher Salmonella invasion compared to nonsenescent HeLa cells (Fig. 4A). This increase was abolished when the senescent HeLa cells were treated with MβCD, but not when they were treated with MDC. Senescent HDFs were invaded by Salmonella approximately three times more readily than were nonsenescent HDFs (Fig. 4B). Again, the higher infection rate was abolished when senescent HDFs were treated with MβCD, but not with MDC. These results suggested that caveolae-dependent endocytosis is indeed implicated in the Salmonella infection of senescent host cells.
Fig. 4.
Implication of caveolae in Salmonella invasion of senescent host cells. Nonsenescent (NS) and senescent (S) HeLa cells (A) or human diploid fibroblasts (HDFs) (B) were treated with methyl-β-cyclodextrin (MβCD) or monodansylcadaverine (MDC). Salmonella infection of these cells was quantified as described. (C) Senescent HDFs were infected with lentivirus carrying the siRNA of caveolin-1 or firefly luciferase (GL2), as a control. The protein level was confirmed by Western blotting. Salmonella invasion was quantified after disruption of caveolae by siRNA in senescent HDFs. (D) To induce caveolin-1 in HeLa cells, we constructed a lentivirus carrying the caveolin-1 gene. Salmonella invasion was quantified in the HeLa cells that over-expressed caveolin-1 from the lentivirus. Invasion is expressed as a percentage of wild-type bacteria internalized by control cells after 60 min. Data are the means (±SD) of at least five independent filters, and experiments were performed at least three times. siRNA-CAV1, lentivirus carrying the siRNA of caveolin-1; siRNA-GL2, lentivirus carrying siRNA of GL2; LV-CAV-1, lentivirus carrying cavelin-1; LV-GL2, lentivirus carrying GL2.
Subsequently, we treated senescent HDFs with siRNA targeting caveolin-1 and determined the level of Salmonellae invasion. The extent of Salmonella invasion in siRNA-treated senescent cells was reduced to the level seen in nonsenescent HDFs (Fig. 4C). Conversely, increased expression of caveolin-1 in nonsenescent HeLa cells by infection with lentivirus carrying caveolin-1 led to an approximately 1.8-fold increase in entry by Salmonella (Fig. 4D). We concluded that Salmonella enter senescent host cells through caveolae-dependent endocytosis.
Elevated expression of caveolin-1 in old mice
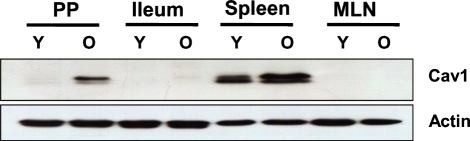
It is known that Salmonellae infect animals through specialized M cells in the follicle-associated epithelium of intestinal Peyer’s patches (PP), which serve as portals for diverse particulates (Kerneis et al., 1997; Sansonetti & Phalipon, 1999). Thus, we examined the expression of caveolin-1 in PP, spleen, mesenteric lymph node (MLN), and ileum in young (2 months old) and old (24 months old) mice, using a specific antibody. The level of caveolin-1 in PP and spleen was elevated significantly in the old mice, whereas caveolin-1 was virtually undetected in ileum and MLN in both young and old mice (Fig. 5A).
Fig. 5.
Differential expression of caveolin-1 in young and old organs. Organs were isolated from ‘young’ (2 months old, Y) and ‘old’ (25 months old, O) mice and analyzed for the expression of caveolin-1 by Western blot using anti-caveolin-1 antibody and anti-actin antibody. Data are representative of at least five individually analyzed mice. PP, Peyer’s patch; MLN, mesenteric lymph node.
This suggests that the increased susceptibility of aged hosts to microbial infection could be attributable to the elevated expression of caveolin-1 and the subsequent increase in caveolae in PP and spleen.
Discussion
In this study, we demonstrated that the level of caveolin-1 expression affects the expression of transport-related genes (Table 1). The elevated expression of transport-related genes in senescent HDFs was reduced by the down-regulation of caveolin-1 to the level observed in young HDFs. Consistently, significantly more FITC-conjugated CT-B was taken up by caveolae in the senescent cells than in the young cells, and this was reduced by the down-regulation of caveolin-1 to the level observed in the young cells (Fig. 1B). Although the underlying mechanism of the regulation of transport-related genes by caveolin-1 has yet to be elucidated, caveolin-1 expression appears directly related to molecule transport.
Most intracellular microbes enter host cells through clathrin-dependent endocytosis, an intracellular pathway in which lysosome fusion with internalized vesicles leads to the degradation of their contents (Veiga & Cossart, 2006). The microbes that enter host cells through this classical clathrin-dependent endocytosis must avoid degradation by the endosome–lysosome pathway, either by escaping from their endocytic vacuoles (phagosomes) into the cytoplasm before lysosomes fuse with phagosomes, or by actively neutralizing the microbicidal agents inside the phagosomes after fusion (Alpuche Aranda et al., 1992). Unlike clathrin-dependent endocytosis, the caveolae-dependent endocytic vesicles do not fuse with lysosomes (Shin & Abraham, 2001); thus, microbes can readily avoid intracellular degradation. Various microbes use caveolae as a pathway for escaping degradation. The caveolae levels change dramatically with cellular conditions or functions. Accordingly, the extent of caveolae-dependent endocytosis of pathogenic microbes can also vary depending on the physiological status of the target organ. Although Salmonella entry into animal cells has been considered random, we have found that it occurs through caveolae-dependent endocytosis, which increases with animal age.
It is well known that elderly individuals have an increased susceptibility to infections. Here, we implemented a model to study age-related microbial infection using Salmonella that invaded host cells. We showed that the extent of Salmonella invasion was higher in two types of cellular aging models: HDFs and HeLa cells (Fig. 2E,F), and treatment with MβCD, an inhibitor of caveolae-dependent endocytosis reduced this to the level seen in young cells (Fig. 4A,B). Because MβCD depletes cholesterol in the cell membrane, whole cholesterol-rich membrane domains, including both lipid rafts and caveolae, are affected by treatment with MβCD (Yancey et al., 1996; Xia et al., 2004). Lipid rafts and caveolae are putative membrane microdomains with compositions different from the surrounding regions of the membrane. Both are enriched in cholesterol, glycosphingolipids, shpingomyelin, and GPI-linked proteins. Caveolae are a subset of lipid rafts characterized by flask-/omega-shaped membrane invaginations with caveolin proteins. To distinguish the role of caveolae and lipid rafts, we treated the cells with specific siRNA for caveolin-1, which specifically disrupted caveolae structures in senescent HDFs (Cho et al., 2003). The degree of Salmonella entry in siRNA-treated senescent HDFs was reduced to the level observed in the young HDFs (Fig. 4C). In contrast, the over-expression of caveolin-1 in HeLa cells led to increased Salmonella entry (Fig. 4D). Taken together, these results suggest that caveolae are directly implicated in Salmonella infection.
Once Salmonella are ingested by the host animal, they travel through the gastrointestinal tract until they encounter M cells of the PP, through which they traverse the underlying tissue and then infect macrophages (Jensen et al., 1998; Sansonetti & Phalipon, 1999). Salmonella not only survive but proliferate in macrophages and spread to other organs including liver and spleen. We observed that caveolin-1 expression was higher in the PP and spleen of old mice (Fig. 5). Taken together, these results provide the first evidence that elevated caveolin-1 and caveolae may be related to the age-dependent increase in susceptibility to microbial infection, although further study is needed to elucidate the underlying mechanism of caveolae-dependent Salmonella entry.
Experimental procedures
Reagents
Monoclonal anti-caveolin-1 antibody (C43420) was purchased from Transduction Laboratories (San Jose, CA, USA). Secondary horseradish peroxidase-conjugated anti-rabbit and anti-mouse antibodies were purchased from Jackson Immunochemicals (West Grove, PA, USA). Other biochemical reagents were purchased from Sigma Chemical (St Louis, MO, USA) or Life Technologies (Carlsbad, CA, USA).
Cellular senescence model
HDFs were isolated from the foreskin of a 6-year-old boy. HDFs were maintained in 10-cm plates in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% antibiotics. HDFs were subcultured at a ratio of 1:4. We defined young cells as those resulting from fewer than 25 population doublings and senescent cells from more than 60 population doublings. HeLa cells were induced senescent phenotypes, resulting from BrDU treatment over 7 days, as described by Michishita et al. (1999). Senescent cells were confirmed by their delayed population-doubling times and through a senescence-associated β-galactosidase activity assay, as described by Dimri et al. (1995). After reaching a semi-confluent state, senescence-associated β-galactosidase activity (pH 6.0) was examined. Briefly, cells were washed with phosphate-buffered saline (PBS) and then fixed with 2% paraformaldehyde containing 0.2% glutaraldehyde in PBS for 5 min at room temperature. After washing with PBS, cells were incubated with β-galactosidase reagent [1 mg mL−1 X-gal, 40 mm citric acid/sodium phosphate buffer (pH 6.0), 5 mm potassium ferrocyanide/potassium ferricyanide, 150 mm NaCl, 2 mm MgCl2] for 4 h at 37°C.
To study human aging, primary fibroblasts were obtained from buttock skin samples of young (14 years old) and elderly (76 and 84 years old) male donors. These cells were provided by Dr J. H. Chung (The Seoul National University College of Medicine).
Animals
C57BL/6J mice were obtained from the Jackson Laboratory and maintained on a standard diet with food and water ad libitum in an animal facility and were used in accordance with the guidelines of the Institutional Animal Care and Use Committee of Chonnam National University Medical School. Old mice were maintained for more than 24 months; tumor-free female mice were used for this experiment. Two 1-month-old female mice were used as young controls.
RNA interference and transfection
A synthetic siRNA duplex corresponding to the caveolin-1 mRNA sequence (5′-AACCAGAAGGGACACACAGUU-3′) was used to inhibit caveolin-1 protein expression (Cho et al., 2003). A synthetic siRNA duplex corresponding to firefly luciferase (GL2) mRNA (5′-AACGUACGCGGAAUACUUCGA-3′) was used as a negative control. The siRNA duplexes were purchased from Dharmacon Research (Lafayette, CO, USA). The cells were grown to 50% confluence in 100-mm dishes and transfected with annealed siRNA using OligofectAMINE (Invitrogen, Carlsbad, CA, USA) for 6 h, as described by the manufacturer. The cells were then transfected with 0.5 nmole (7 μg) siRNA and Oligofectamine™ reagent in serum-free DMEM and incubated for 4 h at 37°C in a CO2 incubator. Following incubation, the cells were supplied with DMEM containing 10% FBS and harvested 48 or 72 h later for further analysis.
cDNA microarray analysis
DNA microarray chips composed of 8000 human EST clones were provided by Digital Genomics Inc. (Seoul, Republic of Korea). RNA samples from senescent HDFs were used to make cDNAs labeled with Cy3, which served as reference probes. RNA samples from siRNA-GL transfected senescent and siRNA-CAV transfected senescent HDFs were used to make cDNA labeled with Cy5. Microarray experiments were performed according to the manufacturer’s standard protocol. Normalized data were obtained by the method of intensity/location-dependent normalization (Yang et al., 2002). We compiled the genes from the normalized data that displayed 2-fold changes and ruled out the genes that changed after treatment with siRNA-GL in senescent HDFs.
CT uptake assay
Cells were cultured on coverslips in 24-well plates and treated with CT-B conjugated with FITC (CT-B–FITC) for 30 min at 4°C. The cells were washed with cold DMEM and incubated at 37°C for 30 min. The cells were processed for immunocytochemistry as described previously (Lim et al., 2000). Cells were fixed with 4% paraformaldehyde in PBS for 60 min and then mounted for detection by confocal microscopy.
Salmonella invasion assay
Bacterial invasion assays were performed as described previously (Lee et al., 1992). Overnight cultures of S. typhimurium were grown at 37°C in LB medium. Salmonella typhimurium were inoculated into fresh cultures and grown for 4 h at 37°C and then resuspended at the appropriate dilution in cell culture medium for infection of cell monolayers (HeLa multiplicity of infection (MOI) 1:10, HDF MOI 1:100) for 30 min. Cells were seeded (HDF, 1 × 104 cells; HeLa, 1 × 105 cells) in a 24-well dish and grown in DMEM with 10% FBS at 37°C in a 5% CO2 incubator. Infected cells were washed three times with PBS (pH 7.4). Then, DMEM containing gentamicin (10 μg mL−1; Sigma Chemical) was added, and the mixtures were incubated for 30 min. Intracellular bacteria were harvested by extraction with lysis buffer (0.05% Triton X-100 in PBS, pH 7.4) in triplicate for colony counting on brain–heart infusion agar plates.
Inhibition of endocytosis
Senescent HDFs were seeded (1 × 104) in 24-well plates and treated with 1% MβCD (Sigma Chemical) to disrupt cholesterol-rich membrane domains for 45 min in serum-free medium at 37°C in a CO2 incubator. Then, the cells were washed with PBS (pH 7.4) and used immediately for S. typhimurium invasion assays. Senescent HDFs were seeded (1 × 104) in a 24-well plate and treated with 1% MDC (Sigma Chemical), to inhibit clathrin-coated pit formation for 30 min in serum-free medium at 37°C in a CO2 incubator. Then, the cells were washed with PBS (pH 7.4) and immediately used for S. typhimurium invasion assays.
Infection of lentivirus
Lentivirus expressing full-length caveolin-1 gene was manufactured by Macrogen Co. (Seoul, Korea). Senescent HDFs were seeded (4 × 104) in a 6-well plate and cultured until 60–70% confluent. The cells were infected with 1 mL of lentivirus for 8 h. After incubation, the cells were supplied with growth medium containing 10% FBS and harvested 48 or 72 h later for further assay.
Western blot analysis
Peyer’s patches, ileum, MLN, and spleen were isolated from young (6–7 weeks old) and old (23–24 months old) mice. Tissues were homogenized in lysis buffer [50 mm Tris–HCl, pH7.5, 150 mm NaCl, 1 mm EDTA, 60 mm octyl β-d-glucopyranoside, 1 mm phenylmethysulfonyl fluoride, protease inhibitor cocktail (1:500; Sigma Chemical), 50 mm NaF, and 1 mm Na3VO4] with polytron homogenizers and then sonicated briefly. Protein samples were separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride transfer membrane (Pall Co., Pensacola, FL, USA). The membranes were incubated with a primary antibody overnight in a cold room, incubated with a peroxidase-conjugated anti-mouse or anti-rabbit secondary antibody for 1 h at room temperature and then visualized using an enhanced chemiluminescence detection kit (Amersham ECL kit; GE Healthcare, Buckinghamshire, UK).
Acknowledgments
This project was funded by grant No. RTI05-01-01 from the Regional Technology Innovation Program of the Ministry of Knowledge Economy (MKE). J. S. L. was supported by a National Research Foundation of Korea Grant funded by the Korean Government (KRF-2007-521-C00224), and S. C. P. was supported by grants from the Korea Research Foundation for Health Science and the Korea Science and Engineering Foundation (KOSEF) through the Center for Ageing and Apoptosis Research. This work was supported, in part, by the Korea Science and Engineering Foundation grant funded by MOST (No. 2007-04213) to H. E. C.
Author contributions
J. S. L.: collection and assembly of data; H. E. C.: collection and design of data; S. C. P. and I. S. J.: provision of old animal; J. M. H.: provision of experimental materials and data analysis; K. A. C.: conception and design, financial support, data analysis and interpretation, manuscript writing, final approval of manuscript.
References
- Abrami L, Fivaz M, van der Goot FG. Adventures of a pore-forming toxin at the target cell surface. Trends Microbiol. 2000;8:168–172. doi: 10.1016/s0966-842x(00)01722-4. [DOI] [PubMed] [Google Scholar]
- Alpuche Aranda CM, Swanson JA, Loomis WP, Miller SI. Salmonella typhimurium activates virulence gene transcription within acidified macrophage phagosomes. Proc. Natl. Acad. Sci. U S A. 1992;89:10079–10083. doi: 10.1073/pnas.89.21.10079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson HA, Chen Y, Norkin LC. Bound simian virus 40 translocates to caveolin-enriched membrane domains, and its entry is inhibited by drugs that selectively disrupt caveolae. Mol. Biol. Cell. 1996;7:1825–1834. doi: 10.1091/mbc.7.11.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood WJ, Norkin LC. Class I major histocompatibility proteins as cell surface receptors for simian virus 40. J. Virol. 1989;63:4474–4477. doi: 10.1128/jvi.63.10.4474-4477.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, London E. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- Cho KA, Ryu SJ, Park JS, Jang IS, Ahn JS, Kim KT, Park SC. Senescent phenotype can be reversed by reduction of caveolin status. J. Biol. Chem. 2003;278:27789–27795. doi: 10.1074/jbc.M208105200. [DOI] [PubMed] [Google Scholar]
- Cho KA, Ryu SJ, Oh YS, Park JH, Lee JW, Kim HP, Kim KT, Jang IS, Park SC. Morphological adjustment of senescent cells by modulating caveolin-1 status. J. Biol. Chem. 2004;279:42270–42278. doi: 10.1074/jbc.M402352200. [DOI] [PubMed] [Google Scholar]
- Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, Peacocke M, Campisi J. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. U S A. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay BB, Falkow S. Common themes in microbial pathogenicity revisited. Microbiol. Mol. Biol. Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan JE. Salmonella interactions with host cells: type III secretion at work. Annu. Rev. Cell Dev. Biol. 2001;17:53–86. doi: 10.1146/annurev.cellbio.17.1.53. [DOI] [PubMed] [Google Scholar]
- Garcia-Cardena G, Martasek P, Masters BS, Skidd PM, Couet J, Li S, Lisanti MP, Sessa WC. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the nos caveolin binding domain in vivo. J. Biol. Chem. 1997;272:25437–25440. doi: 10.1074/jbc.272.41.25437. [DOI] [PubMed] [Google Scholar]
- Ghitescu L, Bendayan M. Transendothelial transport of serum albumin: a quantitative immunocytochemical study. J. Cell Biol. 1992;117:745–755. doi: 10.1083/jcb.117.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen VB, Harty JT, Jones BD. Interactions of the invasive pathogens Salmonella typhimurium, Listeria monocytogenes, and Shigella flexneri with M cells and murine Peyer’s patches. Infect. Immun. 1998;66:3758–3766. doi: 10.1128/iai.66.8.3758-3766.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerneis S, Bogdanova A, Kraehenbuhl JP, Pringault E. Conversion by Peyer’s patch lymphocytes of human enterocytes into M cells that transport bacteria. Science. 1997;277:949–952. doi: 10.1126/science.277.5328.949. [DOI] [PubMed] [Google Scholar]
- Laude AJ, Prior IA. Plasma membrane microdomains: organization, function and trafficking. Mol. Membr. Biol. 2004;21:193–205. doi: 10.1080/09687680410001700517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CA, Jones BD, Falkow S. Identification of a Salmonella typhimurium invasion locus by selection for hyperinvasive mutants. Proc. Natl. Acad. Sci. U S A. 1992;89:1847–1851. doi: 10.1073/pnas.89.5.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencer WI, Hirst TR, Holmes RK. Membrane traffic and the cellular uptake of cholera toxin. Biochim. Biophys. Acta. 1999;1450:177–190. doi: 10.1016/s0167-4889(99)00070-1. [DOI] [PubMed] [Google Scholar]
- Li S, Couet J, Lisanti MP. Src tyrosine kinases, Galpha subunits, and H-Ras share a common membrane-anchored scaf protein, caveolin. Caveolin binding negatively regulates the auto-activation of Src tyrosine kinases. J. Biol. Chem. 1996;271:29182–29190. doi: 10.1074/jbc.271.46.29182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim IK, Won Hong K, Kwak IH, Yoon G, Park SC. Cytoplasmic retention of p-Erk1/2 and nuclear accumulation of actin proteins during cellular senescence in human diploid fibroblasts. Mech. Ageing Dev. 2000;119:113–130. doi: 10.1016/s0047-6374(00)00167-6. [DOI] [PubMed] [Google Scholar]
- Masterson JC, O’Dea S. 5-Bromo-2-deoxyuridine activates DNA damage signalling responses and induces a senescence-like phenotype in p16-null lung cancer cells. Anticancer Drugs. 2007;18:1053–1068. doi: 10.1097/CAD.0b013e32825209f6. [DOI] [PubMed] [Google Scholar]
- Michishita E, Nakabayashi K, Suzuki T, Kaul SC, Ogino H, Fujii M, Mitsui Y, Ayusawa D. 5-Bromodeoxyuridine induces senescence-like phenomena in mammalian cells regardless of cell type or species. J. Biochem. 1999;126:1052–1059. doi: 10.1093/oxfordjournals.jbchem.a022549. [DOI] [PubMed] [Google Scholar]
- Nichols BJ, Lippincott-Schwartz J. Endocytosis without clathrin coats. Trends Cell Biol. 2001;11:406–412. doi: 10.1016/s0962-8924(01)02107-9. [DOI] [PubMed] [Google Scholar]
- Ohl ME, Miller SI. Salmonella: a model for bacterial pathogenesis. Annu. Rev. Med. 2001;52:259–274. doi: 10.1146/annurev.med.52.1.259. [DOI] [PubMed] [Google Scholar]
- Palade GE. An electron microscope study of the mitochondrial structure. J. Histochem. Cytochem. 1953;1:188–211. doi: 10.1177/1.4.188. [DOI] [PubMed] [Google Scholar]
- Park WY, Park JS, Cho KA, Kim DI, Ko YG, Seo JS, Park SC. Up-regulation of caveolin attenuates epidermal growth factor signaling in senescent cells. J. Biol. Chem. 2000;275:20847–20852. doi: 10.1074/jbc.M908162199. [DOI] [PubMed] [Google Scholar]
- Parton RG, Joggerst B, Simons K. Regulated internalization of caveolae. J. Cell Biol. 1994;127:1199–1215. doi: 10.1083/jcb.127.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkmans L, Helenius A. Endocytosis via caveolae. Traffic. 2002;3:311–320. doi: 10.1034/j.1600-0854.2002.30501.x. [DOI] [PubMed] [Google Scholar]
- Ray E, Samanta AK. Dansyl cadaverine regulates ligand induced endocytosis of interleukin-8 receptor in human polymorphonuclear neutrophils. FEBS Lett. 1996;378:235–239. doi: 10.1016/0014-5793(95)01462-4. [DOI] [PubMed] [Google Scholar]
- Razani B, Rubin CS, Lisanti MP. Regulation of cAMP-mediated signal transduction via interaction of caveolins with the catalytic subunit of protein kinase A. J. Biol. Chem. 1999;274:26353–26360. doi: 10.1074/jbc.274.37.26353. [DOI] [PubMed] [Google Scholar]
- Sansonetti PJ, Phalipon A. M cells as ports of entry for enteroinvasive pathogens: mechanisms of interaction, consequences for the disease process. Semin. Immunol. 1999;11:193–203. doi: 10.1006/smim.1999.0175. [DOI] [PubMed] [Google Scholar]
- Schnitzer JE, Oh P, Pinney E, Allard J. Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J. Cell Biol. 1994;127:1217–1232. doi: 10.1083/jcb.127.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JS, Abraham SN. Caveolae as portals of entry for microbes. Microbes Infect. 2001;3:755–761. doi: 10.1016/s1286-4579(01)01423-x. [DOI] [PubMed] [Google Scholar]
- Shin JS, Gao Z, Abraham SN. Involvement of cellular caveolae in bacterial entry into mast cells. Science. 2000;289:785–788. doi: 10.1126/science.289.5480.785. [DOI] [PubMed] [Google Scholar]
- Simionescu N, Siminoescu M, Palade GE. Permeability of muscle capillaries to small heme-peptides. Evidence for the existence of patent transendothelial channels. J. Cell Biol. 1975;64:586–607. doi: 10.1083/jcb.64.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Toomre D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Smart EJ, Graf GA, McNiven MA, Sessa WC, Engelman JA, Scherer PE, Okamoto T, Lisanti MP. Caveolins, liquid-ordered domains, and signal transduction. Mol. Cell. Biol. 1999;19:7289–7304. doi: 10.1128/mcb.19.11.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele-Mortimer O, Brumell JH, Knodler LA, Meresse S, Lopez A, Finlay BB. The invasion-associated type III secretion system of Salmonella enterica serovar Typhimurium is necessary for intracellular proliferation and vacuole biogenesis in epithelial cells. Cell Microbiol. 2002;4:43–54. doi: 10.1046/j.1462-5822.2002.00170.x. [DOI] [PubMed] [Google Scholar]
- Tang Z, Scherer PE, Okamoto T, Song K, Chu C, Kohtz DS, Nishimoto I, Lodish HF, Lisanti MP. Molecular cloning of caveolin-3, a novel member of the caveolin gene family expressed predominantly in muscle. J. Biol. Chem. 1996;271:2255–2261. doi: 10.1074/jbc.271.4.2255. [DOI] [PubMed] [Google Scholar]
- Veiga E, Cossart P. The role of clathrin-dependent endocytosis in bacterial internalization. Trends Cell Biol. 2006;16:499–504. doi: 10.1016/j.tcb.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia F, Gao X, Kwan E, Lam PP, Chan L, Sy K, Sheu L, Wheeler MB, Gaisano HY, Tsushima RG. Disruption of pancreatic beta-cell lipid rafts modifies Kv2.1 channel gating and insulin exocytosis. J. Biol. Chem. 2004;279:24685–24691. doi: 10.1074/jbc.M314314200. [DOI] [PubMed] [Google Scholar]
- Yamada E. The fine structure of the gall bladder epithelium of the mouse. J. Biophys. Biochem. Cytol. 1955;1:445–458. doi: 10.1083/jcb.1.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey PG, Rodrigueza WV, Kilsdonk EP, Stoudt GW, Johnson WJ, Phillips MC, Rothblat GH. Cellular cholesterol efflux mediated by cyclodextrins. Demonstration Of kinetic pools and mechanism of efflux. J. Biol. Chem. 1996;271:16026–16034. doi: 10.1074/jbc.271.27.16026. [DOI] [PubMed] [Google Scholar]
- Yang YH, Dudoit S, Luu P, Lin DM, Peng V, Ngai J, Speed TP. Normalization for cDNA microarray data: a robust composite method addressing single and multiple slide systematic variation. Nucleic Acids Res. 2002;30:e15. doi: 10.1093/nar/30.4.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Galan J. Salmonella entry into host cells: the work in concert of type III secreted effector proteins. Microbes Infect. 2001;3:1293–1298. doi: 10.1016/s1286-4579(01)01489-7. [DOI] [PubMed] [Google Scholar]