Abstract
Objective
Rheumatoid arthritis (RA) is a destructive autoimmune disease characterized by an increased inflammation in the joint. Therapies which activate the apoptotic cascade may have potential as a future therapy for RA, however few therapeutics fit this category. Recently, therapies that mimic the action of Bcl-2 homology 3 (BH3) domain-only proteins such as Bim have shown success in preclinical studies of cancer but their potential in autoimmune disease is unknown.
Methods
Synovial tissue from RA and osteoarthritis (OA) patients were analyzed for expression of Bim and CD68 using immunohistochemistry. Macrophages from mice lacking (Bim−/−) were examined for response to lipopolysaccharide (LPS) using flow cytometry, real time PCR, ELISA, and immunoblot analysis. Bim−/− mice were stimulated with thioglycollate or LPS and examined for macrophage activation and cytokine production. Experimental arthritis was induced using the K/BxN serum-transfer model. A mimetic peptide corresponding to the BH3 domain of Bim (TAT-BH3) was administered as a prophylactic and as a therapeutic. Edema of the ankles and histopathogical analysis of ankle sections were used to determine severity of arthritis, cellular composition, and apoptosis.
Results
The expression of Bim was reduced in RA synovial tissue as compared to controls, particularly in macrophages. Bim−/− macrophages displayed elevated expression of markers of inflammation and secreted more IL-1β following stimulation with LPS or thioglycollate. TAT-BH3 ameliorated arthritis development, reduced the number of myeloid cells in the joint, and enhanced apoptosis without inducing cytotoxicity.
Conclusion
These data demonstrate that BH3 mimetic therapy may have significant potential for RA treatment.
Keywords: Bim, arthritis, macrophages, apoptosis
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic inflammatory and destructive arthropathy of unknown etiology. In RA, the synovial lining increases from 1 to 2 cell layers to as much as 10 layers thick and is composed of macrophages and fibroblasts. Since there is a paucity of apoptotic cells in RA joint, one potential mechanism responsible for the hyperplasia in synovial lining may be attributed to decreased death of fibroblasts and macrophages. However, the milieu of the RA joint contains noxious factors that are normally detrimental to the survival of the cell (1). Thus, an imbalance of apoptotic protectors and inducers may occur and lead to the pathogenesis of RA. A variety of studies have shown that induction of synoviocyte apoptosis in animal models of inflammatory arthritis ameliorates both joint inflammation and destruction (1). These data suggest that the controlled induction of apoptosis locally in the inflamed joint may be therapeutically beneficial.
There are two distinct apoptotic pathways an “extrinsic” pathway that requires binding of death ligands to their cognate receptors on the cell surface and an “intrinsic” pathway in which mitochondria play a critical role. Extrinsic apoptosis is mediated through caspase 8/10 which may activate the downstream caspases 3/7 directly or through an amplification step that requires the intrinsic apoptotic pathway. During intrinsic apoptosis, the mitochondrial inter-membrane protein cytochrome c is released into the cytosol where it binds to Apaf-1 resulting in the activation of the initiator caspase 9 and the effector caspases 3 and 7. Caspases 3 and 7 are responsible for the downstream degradative events in apoptosis (2).
The Bcl-2 protein family, which is divided into anti-apoptotic (Bcl-2, Bcl-xL, Mcl-1) and pro-apoptotic members (Bak, Bax, Bim) are central mediators of the intrinsic pathway (2). Bcl-2-related proteins contain Bcl-2-homology (BH 1-4) domains, which are critical for homodimer and heterodimer formation between the family members. While the anti-apoptotic Bcl-2-like proteins contain 3 or 4 BH domains, the pro-apoptotic Bcl-2 related proteins are subdivided into two categories: the multi-BH domain (e.g. Bak, Bax) and the BH3-only domain proteins (e.g. Bim) (2). Recent studies have shown that BH3-only domain proteins are also subdivided into two categories based on their ability to sequester anti-apoptotic Bcl-2 family members, bind to and activate Bak or Bax, and/or induce apoptosis (3-6). Apoptosis signaling through the intrinsic pathway is inhibited by overexpression of any of the Bcl-2-like pro-survival members or by loss of both multi-BH domain proteins Bak and Bax (2).
To date, almost all of the studies that have examined the potential of altering the molecular rheostat governing the Bcl-2 family through the use of BH3-mimetics have focused on xenograft tumor models (3, 7-10). Here, we show that the expression of the BH3-only protein Bim is reduced in RA tissue and that its reduction not only affects macrophage survival but also the state of activation. The expression of Bim is decreased in macrophages in RA synovial tissue. Macrophages from Bim−/− mice display increased activation as compared to control cells. Arthritic mice treated with a Bim-BH3 mimetic (TAT-BH3) peptide display reduced edema of the ankle, markedly lower histological scores of arthritis, fewer neutrophils and macrophages in joints, and enhanced numbers of TUNEL positive synoviocytes. These data document for the first time the therapeutic potential of systemic delivery of a BH3 mimetic to ameliorate inflammatory arthritis.
MATERIALS AND METHODS
Patients and tissue preparation
Synovial tissue was obtained at the time of arthroplasty from patients with the diagnosis of RA (n=8) or osteoarthritis (OA) (n=8) and fixed in 10% neutral buffered formalin. All patients met the American College of Rheumatology classification criteria for RA or OA respectively. All experiments on human tissues were approved by the internal review board at Saint Louis University, University of California San Diego, Northwestern University, and University of Michigan. Synovial sections were stained for Bim and CD68 and scored by a pathologist blinded to the study (GKH) who examined at least three fields per section with a minimum of three sections per tissue as described previously (11-15).
Mice
Bim−/− (16, 17), non-obese diabetic (NOD), homozygous KRN TCR transgenic mice, C57Bl/6, C57BL/6:129 mice were maintained at Saint Louis and Northwestern University. Mice were injected intraperitoneally with LPS (10 mg/kg of body weight) from E. Coli 0111:B4 (Sigma; St. Louis, Mo) (18). Peritonitis was induced by intraperitoneal injection of 4% aged thioglycollate. All experiments on mice were approved by the Animal Care and Use Committee at Saint Louis University and at Northwestern University.
Cell Culture
Bone marrow cells were isolated as previously described (18, 19). To induce activation, macrophages were treated with 10ng/ mL LPS. IL-1β maturation was induced by stimulating LPS-treated macrophages with 5 mM ATP (Sigma) and brefeldin A (5μg/mL) was used to inhibit release of IL-1β.
IL-1β synthesis
RNA isolation, and real time PCR for IL-1β and GAPDH were previously described (20). Data were normalized to the housekeeping gene GAPDH and analyzed using the ΔΔCT method to obtain fold increase over the untreated control for each genotype. For detection of IL-1β in cell supernatants, sandwich ELISAs were performed as previously described (18). All ELISA data (pg/mL) were normalized by number of cells per well.
Flow cytometry
Phenotyping of macrophages, splenocytes, peripheral blood leukocytes, bone marrow cells, or peritoneal cells was performed as previously described (17, 21),(17, 19, 22) Apoptosis was measured by staining with annexin V-APC. Cells were acquired on a BD LSRII (BD Biosciences) at the Saint Louis University Core Flow Cytometry Facility or the Translational Medicine Flow Cytometry Core Facility at Northwestern University. All analysis was performed using FlowJo software (Tree Star Inc.). Total leukocyte numbers were determined using an automated hematology analyzer ABX Pentra 60 (Diamond Diagnostics, Inc, Holliston, MA). .
K/BxN serum transfer-induced arthritis
K/BxN serum was collected at 7-8 weeks of age and pooled and at the time of injection serum was again pooled and then divided appropriately for injections. One hundred and fifty microliters of K/BxN serum were injected intraperitoneally into each flank of 6-8 week old mice as previously described (19, 22-24). In all studies, mice were matched prior to addition of the serum or peptide and were coded. For the prophylactic study, one hour before injection of serum and at days 2 and 4 post-serum injection, 2 mg/kg of TAT-BH3 peptide were injected intraperitoneally. For the therapeutic study, 10 mg/kg of TAT-BH3 peptide were injected intraperitoneally at days 2, 3, 4, 5, and 6 post-serum injection. The mice at day 2 were The variant TAT sequence is composed of D-amino acids and has a glutamine to ornithine substitution, which has been shown to markedly enhance (10-fold) the uptake of the peptides by cells (25). The peptide from the BH3 domain of Bim was constructed as follows: TAT-BH3: Ac- RKKRR-Orn-RRR-EIWIAQELRRIGDEFNAYYAR-OH, TAT-BIM inactive (TAT-inactive BH3): Ac- RKKRR-Orn-RRR-EIWIAQEARRIGAEFNAYYAR-OH or Ac- RKKRR-Orn-RRR-DMPEIWIEQEARRIEAEFNAYYARR-OH) and purchased from the Peptide Synthesis group at Tufts University. In addition, a fluorescein conjugated TAT-BH3 peptide was also generated. At each time point and prior to euthanasia, the degree of arthritis as indicated by the increase in ankle circumference was measured (19, 22-24). The change in ankle circumference at each time point is defined as the difference between the ankle circumference and the measurement at day 0. Following euthanasia, serum were isolated from peripheral blood by cardiac puncture and ankle joints were removed, fixed in 10% neutral buffered formalin, decalcified in EDTA, embedded in paraffin, and sectioned. To examine toxicity due to systemic delivery of TAT-conjugated peptide, alkaline phosphatase (ALP), alanine transaminase (AST), alanine aminotransferase (ALT), and blood urea nitrogen (BUN) levels were measured by the Department of Comparative Medicine, Saint Louis University.
Immunohistochemistry
Ankles section staining and scoring by a pathologist blinded to the study was previously described (19, 22-24, 26). Photographs were taken on a Nikon microscope equipped with the Nikon digital camera DMX1200.
Statistical Analysis
Results were expressed as the mean ± standard error. Differences between groups were analyzed using Student's t test or ANOVA analysis.
RESULTS
Decreased expression of Bim in RA synovial tissue
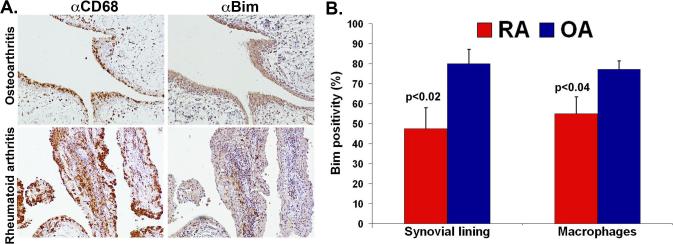
Previous studies have focused on the expression pattern of the pro-survival Bcl-2 family members (1) in order to define a potential mechanism responsible for resistance to apoptosis in RA synovial tissue. Here, we focused on determining the expression level of Bim, a known Bcl-2 pro-apoptotic family member that is vital for leukocyte survival and inflammation (16). The staining pattern for Bim was granular cytoplasmic staining consistent with localization in the cytoplasm and mitochondria. There was no positive staining in control RA and osteoarthritis (OA) synovial tissue (data not shown) stained with IgG. Fewer numbers of RA synovial lining cells were positive for Bim (48 ± 10.4 % vs. 80 ± 7.2%; p<0.02) as compared to OA synovial tissue (Figure 1A, B). Further examination of adjacent sections stained for CD68 (macrophages) and Bim revealed a decrease in Bim expression in the macrophage population in synovial lining and sublining of RA compared to OA synovial tissue (Figure 1A, B). There was no difference in expression of Bim in lymphocytes, fibroblasts, endothelial cells, or blood vessels in RA and OA synovial tissue (data not shown). Since there was an increase in average synovial lining thickness (2.4 ± 0.2 vs. 1.7± 0.4; p<0.06) and inflammation score (3.4 ± 0.5 vs. 1.6 ± 0.3; p<0.01) in RA compared to OA synovial tissue, the decreased expression of Bim is associated with the increase in inflammatory score and synovial lining thickness.
Figure 1. Bim expression in RA and OA synovial tissue.
(A) Bim expression is decreased in RA compared to OA synovial tissue. Representative photomicrographs of adjacent RA (n=8) and OA (n=8) synovial tissue sections stained for Bim (brown) or CD68 (brown) and counterstained with hematoxylin (blue). (B) Quantitative analysis of expression of Bim in RA and OA synovial tissue. RA and OA synovial tissue adjacent sections stained for Bim and CD68 were scored by a pathologist blinded to the study.
Bim functions to limit the activation of macrophages
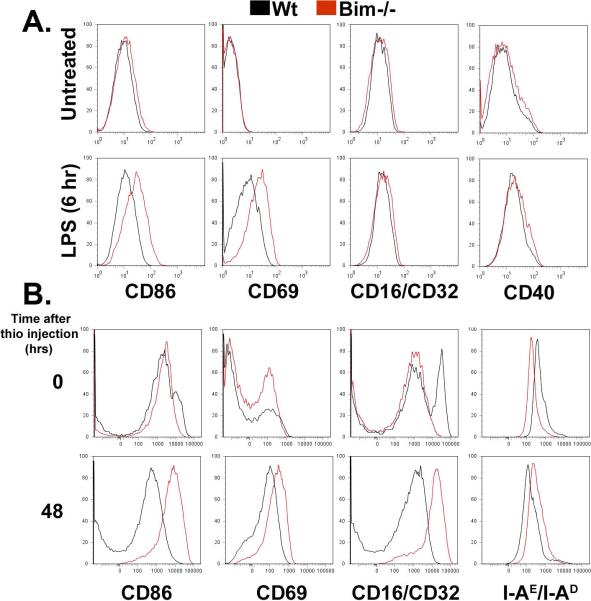
Since macrophages in the RA joint express lower levels of Bim, are highly activated, secrete high levels of TNFα and IL-1β, and their numbers correlate with disease outcome (27), we examined whether Bim may play a role in the activation of macrophages. Bone marrow-derived macrophages isolated from wild-type (Wt) and Bim−/− mice were stimulated with the toll-like receptor 4 agonist lipopolysaccharide (LPS) and expression of CD86, CD69, CD16/CD32, CD40, and MHC class II were examined by flow cytometry. There was no change in viability in response to stimulation with LPS in any of the treated macrophages as compared to untreated cells (Supplemental Figure 1). While LPS-induced the expression of CD86, CD69, CD16/CD32, and CD40 on Wt and Bim−/− macrophages compared to untreated cells, there was an increase in mean fluorescence intensity (MFI) in CD86 (44%, p<0.003), CD69 (232% p<0.0003), and CD40 (23%, p<0.02) in Bim−/− compared to Wt macrophages (Figure 2A). To determine whether in vivo Bim−/− macrophages also display elevated expression in markers of inflammation, Bim−/−and Wt mice were injected intraperitoneally with aged 4% thioglycollate. There was an increase in the expression (Figure 2B) and in the number of cells positive for CD86 (3.0-fold) , CD69 (2.-fold), MHC class II (1.3-fold), and CD16/CD32 (4.8-fold) in Bim−/− macrophages as compared to Wt macrophages at 48 hours following post-thioglycollate stimulation. These data suggest that Bim may function not only to induce apoptosis but also to limit the extent of the inflammatory response in macrophages.
Figure 2. Deficiency for Bim leads to increased activation of macrophages.
(A) Increased LPS-induced expression of activation markers on Bim−/− BMDMs. Wt and Bim−/− macrophages stimulated with LPS were examined by flow cytometry. Data are representative of four independent experiments. (B) Thioglycollate elicited Bim−/− peritoneal macrophages exhibit enhanced expression of markers of activation. Wt (n=4/time point) and Bim−/− (n=4/time point) peritoneal macrophages were isolated prior and following thioglycollate injection and analyzed by flow cytometry.
Bim deficiency leads to increased production of IL-1β in LPS-stimulated macrophages
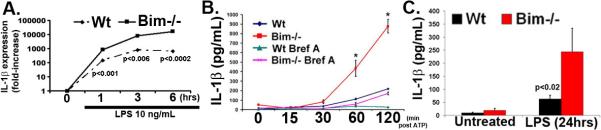
We have shown that Bim−/− mice display increased levels of IL-1β in arthritic joints (23). Further, the increased secretion of IL-1β in the joint was associated with more macrophages in the pannus in Bim−/− mice (data not shown). Expression of IL-1β transcripts was undetectable under resting conditions in isolated Wt and Bim−/− macrophages. However, there was a 6-fold (p<0.001), 11-fold (p<0.006), and 25-fold (p<0.0002) increase at 1, 3, and 6 hours post-LPS stimulation respectively, in the level of IL-1β mRNA in Bim−/− as compared to Wt macrophages (Figure 3A). Since IL-1β is synthesized as a precursor and requires active caspase 1 for processing, we performed immunoblot analysis on Wt and Bim−/− macrophages stimulated with LPS and activated with ATP. ATP is required to activate the inflammasome, a multiprotein complex including caspase 1. Analysis of the secreted isoform of IL-1β showed that there was a 6.0-fold (p<0.01) increase in IL-1β (Figure 3B) in supernatants isolated from Bim−/− as compared to Wt macrophages following stimulation with LPS/ATP. In addition, there was a 4.0-fold (p<0.02) increase in serum IL-1β (Figure 3C) in Bim−/− as compared to Wt mice injected intraperitoneally with LPS (10 mg/kg of body weight). Collectively, these data suggest that the reduced expression of Bim may contribute to increased macrophage activation.
Figure 3. Increased synthesis of IL-1β in Bim−/− macrophages.
(A) The mRNA levels of IL-1β are increased in Bim−/− BMDMs following LPS stimulation. Macrophages stimulated with LPS were examined over time for IL-1β and GAPDH by real time PCR. Data are representative of two independent experiments. (B) Bim−/− macrophages secrete increased IL-1β production following stimulation with LPS. LPS-treated macrophages were examined for IL-1β secretion using ELISA. * indicates p<0.05. (C) Bim−/− mice have increased in vivo production of IL-1β following LPS injection. Serum from untreated (Wt, n=21: Bim−/−, n=15) or LPS injected (Wt, n=12: Bim−/−, n=7) mice were examined for IL-1β levels using a Luminex based assay.
A peptide corresponding to the BH3 domain of Bim suppresses the clinical severity of arthritis
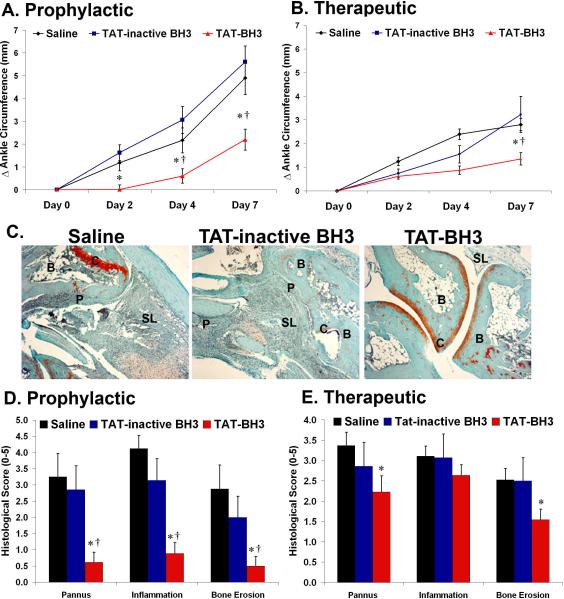
Our data suggest that Bim may play a central role in limiting the development of inflammatory arthritis, therefore a mimetic to Bim may be a potential therapy for the disease. To mimic the expression of Bim, a peptide corresponding to the BH3 domain of Bim, which has been shown to duplicate the functions of Bim (3, 4, 6), was generated. A polycationic peptide derived from HIV-1 TAT (9) was fused to the BH3 peptide of Bim (TAT-BH3) or to an altered-inactive BH3 peptide (TAT-inactive BH3). Mice were injected intraperitoneally with K/BxN serum which induces the effector phase of inflammatory arthritis. For the prophylactic study mice were injected intraperitoneally with saline, TAT-inactive BH3, or TAT-BH3 peptide at one hour before and at days 2 and 4 post-injection of serum. Saline and TAT-inactive BH3 peptide treated mice had 1.2 mm (p<0.007) and 1.6 mm (p<0.001) change in ankle circumference at day 2, respectively, while TAT-BH3 peptide-treated mice had only a 0.1 mm change in ankle circumference (Figure 4A). TAT-BH3 peptide dramatically reduced the development of arthritis by 80% (p<0.02) and 72% (p<0.001) compared to saline or TAT-inactive BH3 peptide treatment, respectively, at day 4. Further, there was a 55% (p<0.003) and 61% (p<0.001) inhibition of arthritis in TAT-BH3 peptide as compared to saline or TAT-inactive BH3 peptide treated groups, respectively, at day 7 post-K/BxN serum injection (Figure 4A). There was no statistical difference in saline and TAT-inactive BH3 peptide treatment. In addition, a therapeutic study was performed to test the efficacy of TAT-BH3 on mice with established arthritis (Figure 4B). Mice were injected intraperitoneally with K/BxN serum and then at day 2 post-serum injection, a time point where all mice displayed overt characteristics of inflammatory arthritis, were injected daily with saline, TAT-inactive peptide, or TAT-BH3 peptide. A higher dose of the TAT-BH3 peptide as compared to prophylactic study was used for the therapeutic regimen, since lower doses or fewer injections had minimal effects on development of arthritis (data not shown). TAT-BH3 peptide treated mice exhibited a 63% (p<0.001) and 46% (p<0.07) decrease at day 4 and a 52% (p<0.0001) and 63% (p<0.008) decrease at day 7, in ankle circumference compared to saline-treated mice and TAT-inactive BH3 peptide treated mice, respectively (Figure 4B). The TAT-BH3 peptide was non-toxic and had no consistent effect on renal or hepatocyte function (Supplemental Figure 2).
Figure 4. TAT-BH3 peptide is effective as a (A) prophylactic or as a (B) therapeutic.
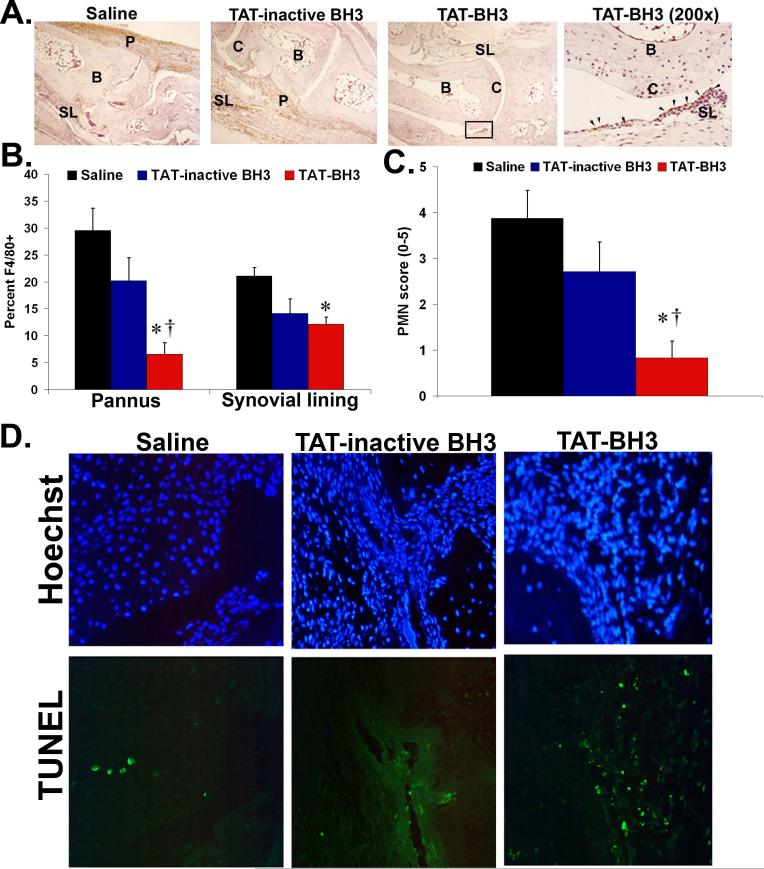
For prophylactic (saline, n=24; TAT-inactive BH3, n=16; or TAT-BH3 peptide, n=32) and therapeutic study (saline, n=36; TAT-inactive BH3, n=18; or TAT-BH3 peptide, n=22). (C) Representative photomicrographs of ankle sections from the prophylactic study. C= cartilage, B= bone, P=pannus, SL= synovial lining. (D, E) Histological scores of ankle sections from the prophylactic (saline, n=8);TAT-inactive BH3, n=16; TAT-BH3 peptide, n=18 and therapeutic study (saline, n=20; TAT-inactive BH3, n=18;TAT-BH3, n=22). * =p<0.05 as compared to saline; †=p<0.05 as compared to TAT-inactive BH3.
To accurately assess the degree of inflammation and destruction of cartilage and bone, ankle sections were examined using an established histopathological scoring system (19, 22-24, 26). Prophylactically, TAT-BH3 peptide suppressed pannus formation by 79% compared to saline or TAT-inactive BH3 peptide treatment (Figure 4C, D). The lack of pannus formation in TAT-BH3 peptide-treated mice was associated with a 71% decrease in bone erosion score compared to saline or TAT-inactive BH3 peptide treatment (Figure 4C, D). Further, there was a 72% reduction in inflammation in TAT-BH3 peptide compared to saline or TAT-inactive BH3 peptide treatment (Figure 4C, D). There was no statistical difference in histological scores in saline or TAT-inactive BH3 peptide treatment (Figure 4D). In the therapeutic study, TAT-BH3 peptide reduced pannus formation by 34% (p<0.03) and 22% (NS) and bone erosion scores by 39% (p<0.01) and 38% (p<0.06) as compared to saline- or TAT-inactive BH3 peptide treated mice, respectively (Figure 4E). These data demonstrate that systemic delivery of TAT-BH3 peptide leads to decreased arthritis and reduced bone destruction.
TAT-BH3 peptide induces apoptosis of myeloid cells in vivo
To determine the mechanism of TAT-BH3 peptide-mediated suppression of arthritis, we treated single cell suspensions of splenocytes with medium, DMSO, TAT-BH3, or TAT-inactive BH3 and analyzed leukocyte viability 24 hours later. Only the TAT-BH3 peptide induced apoptosis in the myeloid cell populations but had no effect on the lymphocytes (Supplemental Figure 3). There was no difference between the level of apoptosis in TAT-inactive BH3 peptide and the DMSO-control treated cells (Supplemental Figure 3). These data suggest that the myeloid cells may be more susceptible to TAT-BH3 peptide and that the inactive peptide is relatively inert.
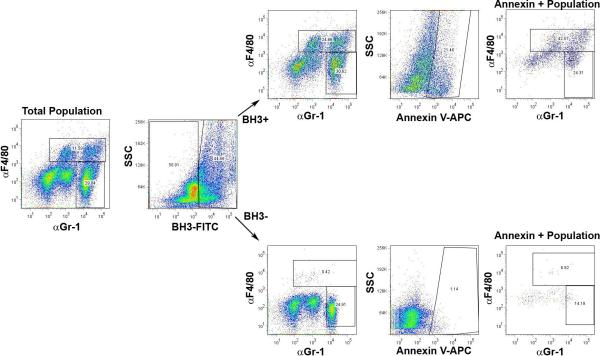
In addition to the cell culture studies, apoptosis was examined in K/BxN treated mice. One hour prior to intraperitoneal injection of K/BxN serum, a FITC-conjugated TAT-BH3 peptide was injected intraperitoneally into mice. The cells in the peritoneal cavity were examined at 6 and 24 hours post K/BxN serum injection for FITC-positivity, annexin V staining, and cell surface expression of F4/80 and Gr-1. At 6 hours post-serum injection, 44% of the peritoneal cells were positive for the FITC-TAT-BH3 peptide. Surprisingly, 88% of the macrophages incorporated the peptide, while only 46% of the neutrophils and 53% of the lymphocytes were positive for the TAT-BH3 peptide. Further, 50% of the macrophages that incorporated the peptide were also positive for Annexin V (Figure 5 and Supplemental Figure 4). Similar results were obtained at 24 hours post-K/BxN serum transfer (data not shown). These data demonstrate that the macrophages preferentially take-up the TAT-BH3 peptide and are sensitive to the death inducing effects of the BH3-peptide.
Figure 5. TAT-BH3 peptide induces apoptosis in myeloid cells.
Peritoneal cells from mice treated with the prophylactic regimen were harvested at 6 hours post K/BxN serum injection, stained with annexin V-APC, anti-F4/80 antibody, and anti-Gr-1 antibody, and analyzed by flow cytometry. Shown are the cells gated for total population, FITC-TAT-BH3, or annexin V-APC.
TAT-BH3 peptide treatment reduces the number of myeloid cells in arthritic joints
Since macrophages and neutrophils are required for the development of K/BxN serum transfer-induced arthritis (23, 28, 29) and since TAT-BH3 peptide has a substantial effect on the fate of myeloid cells in the peritoneum, we quantified the numbers of macrophages (F4/80+) and granulocytes (Gr-1+F4/80neg, PMN) in peripheral blood (Supplemental Table 1) and in joints of arthritic mice. While there was no decrease in the total number of circulating monocytes in peripheral blood in TAT-BH3 peptide-treated mice, there was a 77% (p<0.001) and 68% (p<0.02) decrease in numbers of macrophages within the synovial lining and pannus region of TAT-BH3 peptide compared to saline-treated arthritic mice, respectively (Figure 6A, B). Analysis of bone marrow (Supplemental Figure 5) and peripheral blood (Supplemental Table 1) from saline- and TAT-BH3 peptide-treated arthritic mice revealed a 40% (p<0.05) decrease in the numbers of granulocytes in TAT-BH3 peptide-treated mice compared to saline-treated mice. Further, TAT-BH3 peptide-treated mice showed a 69% reduction in joint PMN score compared to saline-treated mice (Figure 6C). In addition, there were increased numbers of TUNEL+ cells in the joints of TAT-BH3 peptide-treated mice as compared to saline-treated mice from the therapeutic regimen (Figure 6D). Taken together, these data suggest that TAT-BH3 peptide may modulate the immune response presumably through decreasing the activation potential of and/or the induction of apoptosis in inflammatory cells leading to the suppression of arthritis.
Figure 6. Fewer myeloid cells are recruited to joints of TAT-BH3 peptide-treated arthritic mice.
(A) Representative photomicrographs of macrophages in ankle sections from the prophylactic study. C=cartilage, B=bone, P=pannus, SL=synovial lining. Arrows represent F4/80-positive cells in the untreated mouse. (B, C) TAT-BH3 peptide-treated mice have decreased numbers of macrophages and PMNs. * =p<0.05 as compared to saline; †=p<0.05 as compared to TAT-inactive BH3. (D) Representative photomicrographs of TUNEL and Hoechst stained ankle sections from therapeutic study.
DISCUSSION
In the rheumatoid joint there is a shift in the balance of pro- and anti-Bcl-2 apoptotic factors towards increased expression of the anti-apoptotic Bcl-2 members. There are elevated numbers of cells positive for Bcl-2, Mcl-1, and Bcl-xL in RA compared to control synovial tissue (11, 12, 30, 31). Although the expression of Bax is shown to be elevated in RA synovial tissue compared to healthy controls, the activated form of Bax was not examined in these tissues (32). Recently, the expression of Puma was shown to be localized to the sublining and not to the synovial lining region (33). Since there are few apoptotic cells in the joint, these data suggest that the increased expression of Bax and Puma is insufficient to induce apoptosis in the RA joint. We show for the first time that expression of Bim is reduced in RA compared to control OA synovial tissue. Thus, these data are consistent with the concept that the molecular rheostat that governs the survival of cells in the RA joint is shifted toward the Bcl-2 pro-survival proteins.
Deficiency in Bim results in enhanced activation of dendritic cells (34) and macrophages (Figures 2, 3), which are known to be major contributors to RA. The transcription of IL-1β in Bim−/− macrophages is elevated in response to LPS as compared to WT cells under parallel conditions and loss of Bim results in a marked increase in the secretion of IL-1β by LPS-treated macrophages and mice. Since IL-1β production is a multistep process, Bim may affect the synthesis of IL-1β at various levels. One potential pathway that Bim may affect is the inflammasome, a multiprotein complex including active caspase 1 (35) that is responsible for the processing of pro-IL-1β. Recently, Bcl-2 and Bcl-xL were shown to interact with the NALP1 inflammasome (36), which responds to the bacterial ligand, muramyl-dipeptide to activate IL-1β synthesis and release (37). Interestingly, preliminary studies revealed that the TAT-BH3 peptide led to a decrease in the release of IL-1β in Bim−/− macrophages, indicating that the BH3 mimetic may partially compensate for the loss of full-length Bim (data not shown). Thus, future studies are required to determine whether Bim may interact with the NALP3-inflammasome which is responsible for the activation of IL-1β in response to LPS (37).
The role that multi-BH domain and BH3-only domain proteins play in the development inflammatory arthritis has been recently investigated. Mice deficient in Bak or Bax develop inflammatory arthritis similar to control mice (23). In contrast, mice lacking Bim develop a more aggressive and severe form of inflammatory arthritis (23) even compared to Bid−/− mice (24). These data are consistent with the notion that a decrease in expression of Bim may enhance the progression of inflammatory arthritis. Here, we show that systemic delivery of a peptide corresponding to the BH3 domain of Bim is dramatically effective at preventing and suppressing the progression of K/BxN serum transfer-induced arthritis, which closely resembles the effector phase of RA. However, in the prophylactic study it appears that at later time points the TAT-BH3 treated mice show mild development of inflammatory arthritis as indicated by joint edema. A potential explanation for the observation may be attributed to the half-life of the peptide since in the prophylactic study the TAT-BH3 peptide was administered at a lower concentration and with fewer injections as compared to the therapeutic study. Interestingly, in the therapeutic study the TAT-BH3 peptide maintained and even partially decreased the edema of the joint but had minimal effect on the inflammatory infiltrate. However, TAT-BH3 peptide is effective at reducing pannus and bone destruction scores. Thus, the TAT-BH3 peptide may not be sufficient to inhibit the inflammation in the synovium but may render the cells that are destroying the joint ineffective by inducing apoptosis. Future studies will be required to examine potential modifications of the BH3 peptide i.e. using hydrocarbon stapled BH3 peptide (8) and the utility of the TAT-BH3 peptide as a dual agent i.e. co-administration with a DMARD.
The level of IL-1β but not TNFα (data not shown) is reduced in the joints of TAT-BH3 peptide treated mice. A decrease in the numbers of macrophages and neutrophils appears to account for this decline in IL-1β. It is not surprising that neutrophils are susceptible to the BH3 peptide since it was shown to have a high affinity for Mcl-1, unlike other BH3 mimetics (3, 4, 6), and Mcl-1 is required for development of neutrophils but not monocytes or macrophages (38). Previous investigations have suggested a staging model for the development of K/BxN serum transfer-induced arthritis (39) in which neutrophils are required for one of the early steps in the K/BxN serum transfer-induced arthritis model (29). A decrease in the numbers of neutrophils in circulation and joint may also contribute to a reduction in the numbers of macrophages recruited to the joint by the lowering the levels of secreted chemotactic factors. Our data also indicate that macrophages are more susceptible to TAT-BH3 peptide as compared to lymphocytes. The decrease in macrophages in the joint is consistent with studies that showed the importance of macrophages in the K/BxN serum transfer-induced arthritis model (19, 28). While the K/BXN model of arthritis closely resembles the effector phase of RA future studies will be required to examine the broad impact of BH3 peptide on different models of inflammatory arthritis.
BH3 mimetic therapy has gained considerable momentum over the past several years (4). Almost all of the studies using BH3 mimetic therapy have focused on the feasibility in treating oncogenesis (4). The most well characterized BH3 mimetic is ABT-737 (4, 40-46). Recently, ABT-737 was shown to induce lymphocytopenia and thereby suppress the development of collagen-induced arthritis (47), a model which requires lymphocytes (48). While ABT-737 mechanistically behaves similarly to the sensitizer class of BH3-only domain proteins (Bad, Noxa), TAT-BH3 functions like Bim, a promiscuous BH3-only initiator protein. These data suggest that TAT-BH3 has a broader range of anti-apoptotic targets, and thus may prove to be more effective as a therapy than other BH3 mimetics. Although the TAT-BH3 peptide has a minimal but statistically significant reduction in platelet counts (Supplemental Table 1), ABT-737 was shown to have a dramatic effect on platelet numbers (49). These data also indicate that TAT-BH3 may have fewer adverse side effects than ABT-737. Thus, these data suggest that a Bim-BH3 mimetic has therapeutic value in autoimmune diseases such as RA, in which a failure to delete the autoreactive cells leads to its pathogenesis.
Supplementary Material
Acknowledgments
We thank Joy Eslick and Sherri Koehm for their assistance with flow cytometry (Saint Louis University Flow Cytometry Core Facility).
REFERENCES
- 1.Liu H, Pope RM. Apoptosis in rheumatoid arthritis: friend or foe. Rheum Dis Clin North Am. 2004;30(3):603–25. x. doi: 10.1016/j.rdc.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Strasser A. The role of BH3-only proteins in the immune system. Nat Rev Immunol. 2005;5(3):189–200. doi: 10.1038/nri1568. [DOI] [PubMed] [Google Scholar]
- 3.Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2(3):183–92. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- 4.Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9(5):351–65. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 5.Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17(3):393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 6.Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, et al. BH3 Domains of BH3-Only Proteins Differentially Regulate Bax-Mediated Mitochondrial Membrane Permeabilization Both Directly and Indirectly. Mol Cell. 2005;17(4):525–35. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Goldsmith KC, Liu X, Dam V, Morgan BT, Shabbout M, Cnaan A, et al. BH3 peptidomimetics potently activate apoptosis and demonstrate single agent efficacy in neuroblastoma. Oncogene. 2006;25(33):4525–33. doi: 10.1038/sj.onc.1209489. [DOI] [PubMed] [Google Scholar]
- 8.Walensky LD, Kung AL, Escher I, Malia TJ, Barbuto S, Wright RD, et al. Activation of apoptosis in vivo by a hydrocarbon-stapled BH3 helix. Science. 2004;305(5689):1466–70. doi: 10.1126/science.1099191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kashiwagi H, McDunn JE, Goedegebuure PS, Gaffney MC, Chang K, Trinkaus K, et al. TAT-Bim Induces Extensive Apoptosis in Cancer Cells. Ann Surg Oncol. 2007;14(5):1763–71. doi: 10.1245/s10434-006-9298-z. [DOI] [PubMed] [Google Scholar]
- 10.Gavathiotis E, Suzuki M, Davis ML, Pitter K, Bird GH, Katz SG, et al. BAX activation is initiated at a novel interaction site. Nature. 2008;455(7216):1076–81. doi: 10.1038/nature07396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu H, Huang Q, Shi B, Eksarko P, Temkin V, Pope RM. Regulation of Mcl-1 expression in rheumatoid arthritis synovial macrophages. Arthritis Rheum. 2006;54(10):3174–81. doi: 10.1002/art.22132. [DOI] [PubMed] [Google Scholar]
- 12.Perlman H, Georganas C, Pagliari LJ, Koch AE, Haines K, Pope RM. Bcl-2 expression in synovial fibroblasts is essential for maintaining mitochondrial homeostasis and cell viability. Journal of Immunology. 2000;164:5227–5235. doi: 10.4049/jimmunol.164.10.5227. [DOI] [PubMed] [Google Scholar]
- 13.Perlman H, Nguyen N, Liu H, Eslick J, Esser S, Walsh K, et al. Rheumatoid arthritis synovial fluid macrophages express decreased tumor necrosis factor-related apoptosis-inducing ligand R2 and increased decoy receptor tumor necrosis factor-related apoptosis-inducing ligand R3. Arthritis Rheum. 2003;48(11):3096–101. doi: 10.1002/art.11302. [DOI] [PubMed] [Google Scholar]
- 14.Koch AE, Kunkel SL, Harlow LA, Mazarakis DD, Haines GK, Burdick MD, et al. Macrophage inflammatory protein-1α: a novel chemotactic cytokine for macrophages in rheumatoid arthritis. J Clin Invest. 1994;93:921. doi: 10.1172/JCI117097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koch AE, Kunkel SL, Harlow LA, Mazarakis DD, Haines GK, Burdick MD, et al. Epithelial neutrophil activating peptide-78: a novel chemotactic cytokine for neutrophils in arthritis. J. Clin. Invest. 1994;94:1012–1018. doi: 10.1172/JCI117414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Kontgen F, et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286(5445):1735–8. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 17.Hutcheson J, Scatizzi JC, Bickel E, Brown NJ, Bouillet P, Strasser A, et al. Combined loss of proapoptotic genes Bak or Bax with Bim synergizes to cause defects in hematopoiesis and in thymocyte apoptosis. J Exp Med. 2005;201(12):1949–60. doi: 10.1084/jem.20041484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scatizzi JC, Mavers M, Hutcheson J, Young B, Shi B, Pope RM, et al. The CDK domain of p21 is a suppressor of IL-1beta-mediated inflammation in activated macrophages. Eur J Immunol. 2009 doi: 10.1002/eji.200838683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scatizzi JC, Hutcheson J, Bickel E, Woods JM, Klosowska K, Moore TL, et al. p21Cip1 is required for the development of monocytes and their response to serum transfer-induced arthritis. Am J Pathol. 2006;168(5):1531–41. doi: 10.2353/ajpath.2006.050555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Savard CE, Blinman TA, Choi HS, Lee SK, Pandol SJ, Lee SP. Expression of cytokine and chemokine mRNA and secretion of tumor necrosis factor-alpha by gallbladder epithelial cells: response to bacterial lipopolysaccharides. BMC Gastroenterol. 2002;2:23. doi: 10.1186/1471-230X-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hutcheson J, Scatizzi JC, Siddiqui AM, Haines GK, 3rd, Wu T, Li QZ, et al. Combined deficiency of proapoptotic regulators Bim and Fas results in the early onset of systemic autoimmunity. Immunity. 2008;28(2):206–17. doi: 10.1016/j.immuni.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 22.Brown NJ, Hutcheson J, Bickel E, Scatizzi JC, Albee LD, Haines GK, 3rd, et al. Fas death receptor signaling represses monocyte numbers and macrophage activation in vivo. J Immunol. 2004;173(12):7584–93. doi: 10.4049/jimmunol.173.12.7584. [DOI] [PubMed] [Google Scholar]
- 23.Scatizzi JC, Bickel E, Hutcheson J, Haines GK, 3rd, Perlman H. Bim deficiency leads to exacerbation and prolongation of joint inflammation in experimental arthritis. Arthritis Rheum. 2006;54(10):3182–93. doi: 10.1002/art.22133. [DOI] [PubMed] [Google Scholar]
- 24.Scatizzi JC, Hutcheson J, Bickel E, Haines GK, 3rd, Perlman H. Pro-apoptotic Bid is required for the resolution of the effector phase of inflammatory arthritis. Arthritis Res Ther. 2007;9(3):R49. doi: 10.1186/ar2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gammon ST, Villalobos VM, Prior JL, Sharma V, Piwnica-Worms D. Quantitative analysis of permeation peptide complexes labeled with Technetium-99m: chiral and sequence-specific effects on net cell uptake. Bioconjug Chem. 2003;14(2):368–76. doi: 10.1021/bc0256291. [DOI] [PubMed] [Google Scholar]
- 26.Pettit AR, Ji H, von Stechow D, Muller R, Goldring SR, Choi Y, et al. TRANCE/RANKL knockout mice are protected from bone erosion in a serum transfer model of arthritis. Am J Pathol. 2001;159(5):1689–99. doi: 10.1016/S0002-9440(10)63016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wijbrandts CA, Vergunst CE, Haringman JJ, Gerlag DM, Smeets TJ, Tak PP. Absence of changes in the number of synovial sublining macrophages after ineffective treatment for rheumatoid arthritis: Implications for use of synovial sublining macrophages as a biomarker. Arthritis Rheum. 2007;56(11):3869–71. doi: 10.1002/art.22964. [DOI] [PubMed] [Google Scholar]
- 28.Solomon S, Rajasekaran N, Jeisy-Walder E, Snapper SB, Illges H. A crucial role for macrophages in the pathology of K/B x N serum-induced arthritis. Eur J Immunol. 2005;35(10):3064–73. doi: 10.1002/eji.200526167. [DOI] [PubMed] [Google Scholar]
- 29.Wipke BT, Allen PM. Essential role of neutrophils in the initiation and progression of a murine model of rheumatoid arthritis. J Immunol. 2001;167(3):1601–8. doi: 10.4049/jimmunol.167.3.1601. [DOI] [PubMed] [Google Scholar]
- 30.Liu H, Eksarko P, Temkin V, Haines GK, 3rd, Perlman H, Koch AE, et al. Mcl-1 is essential for the survival of synovial fibroblasts in rheumatoid arthritis. J Immunol. 2005;175(12):8337–45. doi: 10.4049/jimmunol.175.12.8337. [DOI] [PubMed] [Google Scholar]
- 31.Busteed S, Bennett MW, Molloy C, Houston A, Stone MA, Shanahan F, et al. Bcl-x(L) expression in vivo in rheumatoid synovium. Clin Rheumatol. 2006 doi: 10.1007/s10067-005-0191-0. [DOI] [PubMed] [Google Scholar]
- 32.Hilbers I, Hansen T, Petrow PK, Gaumann A, Brauer R, Salzmann G, et al. Expression of the apoptosis accelerator Bax in rheumatoid arthritis synovium. Rheumatol Int. 2003;23(2):75–81. doi: 10.1007/s00296-002-0255-2. [DOI] [PubMed] [Google Scholar]
- 33.Cha HS, Rosengren S, Boyle DL, Firestein GS. PUMA regulation and proapoptotic effects in fibroblast-like synoviocytes. Arthritis Rheum. 2006;54(2):587–92. doi: 10.1002/art.21631. [DOI] [PubMed] [Google Scholar]
- 34.Chen M, Huang L, Wang J. Deficiency of Bim in dendritic cells contributes to over-activation of lymphocytes and autoimmunity. Blood. 2007 doi: 10.1182/blood-2006-11-056424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petrilli V, Papin S, Tschopp J. The inflammasome. Curr Biol. 2005;15(15):R581. doi: 10.1016/j.cub.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 36.Bruey JM, Bruey-Sedano N, Luciano F, Zhai D, Balpai R, Xu C, et al. Bcl-2 and Bcl-XL regulate proinflammatory caspase-1 activation by interaction with NALP1. Cell. 2007;129(1):45–56. doi: 10.1016/j.cell.2007.01.045. [DOI] [PubMed] [Google Scholar]
- 37.Sutterwala FS, Ogura Y, Flavell RA. The inflammasome in pathogen recognition and inflammation. J Leukoc Biol. 2007 doi: 10.1189/jlb.1206755. [DOI] [PubMed] [Google Scholar]
- 38.Dzhagalov I, St John A, He YW. The antiapoptotic protein Mcl-1 is essential for the survival of neutrophils but not macrophages. Blood. 2007;109(4):1620–6. doi: 10.1182/blood-2006-03-013771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wipke BT, Wang Z, Nagengast W, Reichert DE, Allen PM. Staging the initiation of autoantibody-induced arthritis: a critical role for immune complexes. J Immunol. 2004;172(12):7694–702. doi: 10.4049/jimmunol.172.12.7694. [DOI] [PubMed] [Google Scholar]
- 40.Chauhan D, Velankar M, Brahmandam M, Hideshima T, Podar K, Richardson P, et al. A novel Bcl-2/Bcl-X(L)/Bcl-w inhibitor ABT-737 as therapy in multiple myeloma. Oncogene. 2006 doi: 10.1038/sj.onc.1210028. [DOI] [PubMed] [Google Scholar]
- 41.Chen S, Dai Y, Harada H, Dent P, Grant S. Mcl-1 down-regulation potentiates ABT-737 lethality by cooperatively inducing Bak activation and Bax translocation. Cancer Res. 2007;67(2):782–91. doi: 10.1158/0008-5472.CAN-06-3964. [DOI] [PubMed] [Google Scholar]
- 42.Del Gaizo Moore V, Brown JR, Certo M, Love TM, Novina CD, Letai A. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J Clin Invest. 2007;117(1):112–21. doi: 10.1172/JCI28281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10(5):375–88. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 44.Tahir SK, Yang X, Anderson MG, Morgan-Lappe SE, Sarthy AV, Chen J, et al. Influence of Bcl-2 family members on the cellular response of small-cell lung cancer cell lines to ABT-737. Cancer Res. 2007;67(3):1176–83. doi: 10.1158/0008-5472.CAN-06-2203. [DOI] [PubMed] [Google Scholar]
- 45.Trudel S, Stewart AK, Li Z, Shu Y, Liang SB, Trieu Y, et al. The Bcl-2 family protein inhibitor, ABT-737, has substantial antimyeloma activity and shows synergistic effect with dexamethasone and melphalan. Clin Cancer Res. 2007;13(2 Pt 1):621–9. doi: 10.1158/1078-0432.CCR-06-1526. [DOI] [PubMed] [Google Scholar]
- 46.van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10(5):389–99. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bardwell PD, Gu J, McCarthy D, Wallace C, Bryant S, Goess C, et al. The Bcl-2 family antagonist ABT-737 significantly inhibits multiple animal models of autoimmunity. J Immunol. 2009;182(12):7482–9. doi: 10.4049/jimmunol.0802813. [DOI] [PubMed] [Google Scholar]
- 48.Luross JA, Williams NA. The genetic and immunopathological processes underlying collagen-induced arthritis. Immunology. 2001;103(4):407–16. doi: 10.1046/j.1365-2567.2001.01267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang H, Nimmer PM, Tahir SK, Chen J, Fryer RM, Hahn KR, et al. Bcl-2 family proteins are essential for platelet survival. Cell Death Differ. 2007 doi: 10.1038/sj.cdd.4402081. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.