Abstract
Pseudo-rotaxane complexes of squaraine dyes and tetralactam macrocycles are converted into permanently interlocked rotaxane structures using copper-catalyzed and copper-free cycloaddition reactions with bulky stopper groups. The photophysical properties of the encapsulated squaraine depend on the structure of the macrocycle. In one case, squaraine rotaxanes are produced in near quantitative yields and with intense near-IR fluorescence. In another case, squaraine fluorescence is greatly diminished upon macrocyclic encapsulation but the signal can be restored by dye displacement with anions.
Many of the new and emerging biophotonic technologies require extremely bright and highly stable fluorescent dyes.1 One way to improve dye performance is molecular encapsulation inside a host molecule or nanoparticle.2 Our focus is on squaraine rotaxanes, which are produced by encapsulating highly fluorescent near-IR
 squaraine dyes inside surrounding macrocycles.3 This sterically protects the squaraine fluorophore such that squaraine rotaxanes exhibit increased stability and decreased intermolecular quenching.4 Furthermore, squaraine rotaxanes are well suited for structural conversion into molecular probes for bioimaging applications.5 The first generation squaraine rotaxanes were prepared in yields of 10-30% using a Leigh type clipping method;6 that is, a macrocyclization reaction that traps a squaraine template inside a tetralactam macrocycle. This synthetic strategy allows rapid production of prototype designs; however, the low yields limit the capacity to prepare more complex later-generation structures. We have explored alternative methods of preparing squaraine rotaxanes, and we report here a series of related synthetic capping strategies.7 In each case, a reversible pseudo-rotaxane complex is converted into a permanently interlocked rotaxane by covalent capping with bulky stopper groups (Scheme 1). In the best cases, squaraine rotaxanes are produced in >90% yield after column chromatography. We also find that different macrocycles alter squaraine photophysical properties in different ways; thus, the encapsulating macrocycle is a structural parameter that can be altered to control squaraine rotaxane function.
squaraine dyes inside surrounding macrocycles.3 This sterically protects the squaraine fluorophore such that squaraine rotaxanes exhibit increased stability and decreased intermolecular quenching.4 Furthermore, squaraine rotaxanes are well suited for structural conversion into molecular probes for bioimaging applications.5 The first generation squaraine rotaxanes were prepared in yields of 10-30% using a Leigh type clipping method;6 that is, a macrocyclization reaction that traps a squaraine template inside a tetralactam macrocycle. This synthetic strategy allows rapid production of prototype designs; however, the low yields limit the capacity to prepare more complex later-generation structures. We have explored alternative methods of preparing squaraine rotaxanes, and we report here a series of related synthetic capping strategies.7 In each case, a reversible pseudo-rotaxane complex is converted into a permanently interlocked rotaxane by covalent capping with bulky stopper groups (Scheme 1). In the best cases, squaraine rotaxanes are produced in >90% yield after column chromatography. We also find that different macrocycles alter squaraine photophysical properties in different ways; thus, the encapsulating macrocycle is a structural parameter that can be altered to control squaraine rotaxane function.
Scheme 1.
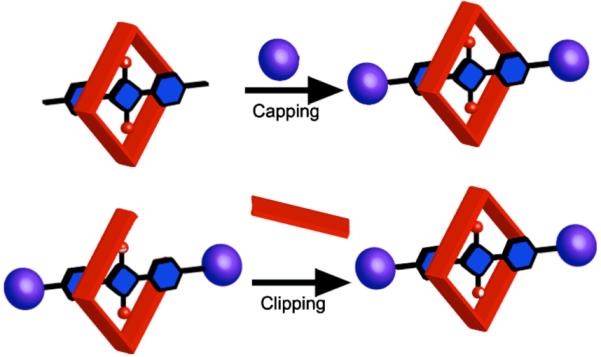
Synthesis of squaraine rotaxanes via capping (top) and clipping (bottom).
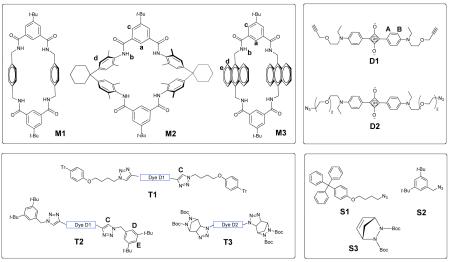
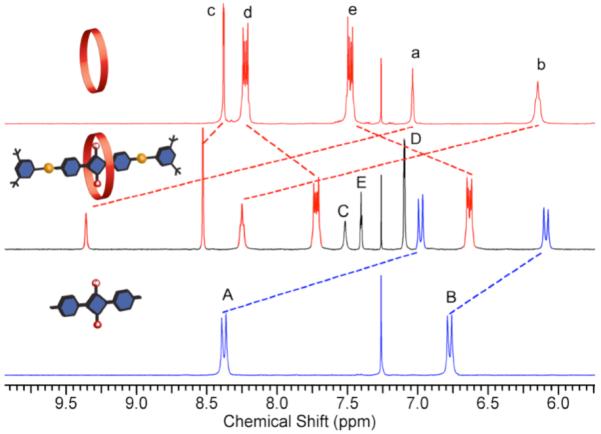
Capping strategies with phenylene-containing tetralactam macrocycles such as M1 are not effective because of very poor macrocycle solubility.8 To circumvent this problem we investigated other macrocycles that have a similar isophthalamide tetralactam motif but exhibit more favorable solubility in organic solvents. We initially examined the well-known M2, which is soluble in methylene chloride and has been extensively studied as a macrocyclic component in various interlocked molecules.9,10 NMR titration experiments showed that M2 can encapsulate squaraine dye D1 with an association constant (Ka) of 5×103 M−1 in methylene chloride at 25 °C.11 We then covalently capped the protruding alkyne ends of the pseudo-rotaxane complex by conducting a copper mediated alkyne azide cycloaddition reaction.12 After some experimentation, we found that mixing macrocycle M2 with excess dye D1 and stopper S1, followed by treatment with Cu(Ph3P)3Br catalyst generated the “clicked rotaxane” M2⊃T1 with an isolated yield of 40%. The rotaxane structure was verified by standard spectroscopic methods including 1H NMR (Figure 1). The downfield changes in chemical shift for the macrocycle amide and aryl protons are indicative of deshielding by the encapsulated squaraine thread T1, and are consistent with previously observed changes in 1H NMR spectra for squaraine rotaxanes.
Figure 1.
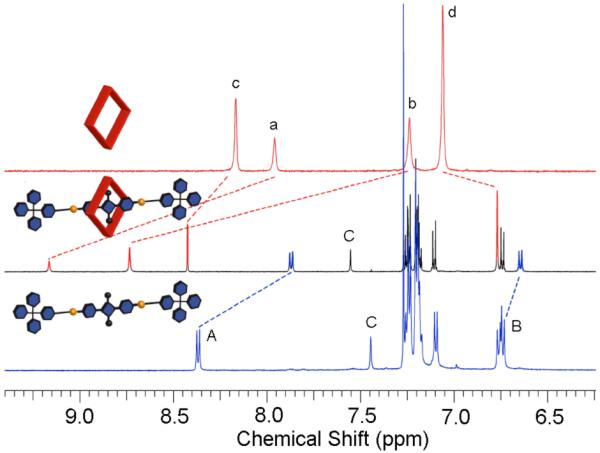
Partial 1H NMR spectra of M2 (top), T1 (bottom), and the resulting clicked rotaxane M2⊃T1 (middle).
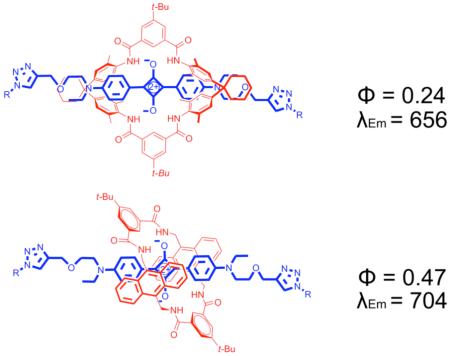
The yield of squaraine rotaxane was sufficient to allow photophysical measurements. A direct comparison of squaraine thread T1 and rotaxane M2⊃T1 revealed a modest red shift in both absorption (+10 nm) and fluorescence emission (+7 nm) and approximately a three fold decrease in fluorescence quantum yield. It is also noteworthy that addition of two trizaole rings did not significantly alter the quantum yield in thread T1 as compared to dye D1 (Table 1). The macrocycle-induced quenching effect was verified by fluorescence titration experiments that added aliquots of M2 to a solution of squaraine D1 in methylene chloride. Addition of excess M2 lowers the emission intensity of D1 by about a factor of four (Figure 2 (a) vs. (b)). Additional evidence that the quenching is due to inclusion of D1 inside M2 was gained by conducting anion displacement experiments.13 As shown in Figure 2, treatment of the M2⊃D1 psuedorotaxane system with the tetrabutylammonium salts of chloride, acetate or benzoate leads to displacement of squaraine D1 from the macrocyclic cavity and nearly complete restoration of its fluorescence intensity. These anions are known to bind strongly to the NH residues in M2 and form hydrogen bonded complexes (Ka>105 M−1).14 Interestingly, tetrabutylammonium dihydrogenphosphate was less effective at squaraine displacement suggesting that the M2 cavity does not readily accommodate this larger anion. The fluorescence quenching induced by M2 means that this system is unlikely to have utility as a bioimaging probe; however, the pseudo-rotaxane system M2⊃D1 is an effective and selective anion sensor with near-IR fluorescence.15
Table 1.
Absorption, emission, extinction coefficients and quantum yields of select squaraine derivatives in CHCl3
| compound | λAbs (nm) | log ε | λEm (nm) | Φfa |
|---|---|---|---|---|
| D1 | 631 | 5.65 | 651 | 0.68 |
| T1 | 635 | 4.91 | 649 | 0.65 |
| M2⊃[T1] | 645 | 5.23 | 656 | 0.24 |
| M3⊃[T2] | 661 | 5.24 | 704 | 0.47 |
error ± 5%.
Figure 2.
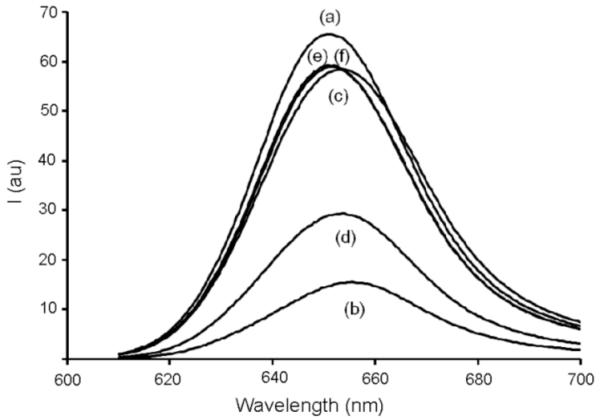
Fluorescence emissions of separate CH2Cl2 solutions of (a) D1, (b) M2⊃D1, and M2⊃D1 in the presence of (c) TBA+·Cl−, (d) TBA+·H2PO4−, (e) TBA+·CH3COO−, and (f) TBA+·C6H5COO− at 5 mM for each anion. The concentrations of D1 and M2 were 1 μM and 3 mM, respectively.
Compared to M2, the anthrylene macrocycle M3 is a more promising building block for squaraine rotaxane fabrication and probe development. In a prior study, we showed that an admixture of M3 and D1 self-assembles quantitatively at millimolar concentration in chloroform solution (Ka of 1.8×105 M−1) to produce an inclusion complex whose absorption and emission maxima are red-shifted by +40 nm.16 We now report that “clicking” both ends of this pseudo-rotaxane with two molar equivalents of stopper S2 produces the squaraine rotaxane M3⊃T2 in near quantitative yield. The 1H NMR spectra in Figure 3 exhibit the expected changes in chemical shift for a squaraine rotaxane and the fluorescence spectra in Figure 4 demonstrate that it is a permanently interlocked structure. Pseudo-rotaxane M3⊃D1 partially dissociates at micromolar concentrations in chloroform and produces two emission peaks, one at 638 nm which corresponds to free squaraine D1 and one at 694 nm, corresponding to pseudo-rotaxane. In contrast, the squaraine rotaxane M3⊃T2 does not dissociate under these conditions or in more polar solvents such as pure methanol (see supporting information).
Figure 3.
Partial 1H NMR spectra of M3 (top), D1 (bottom), and the resulting clicked rotaxane M3⊃T2 (middle).
Figure 4.
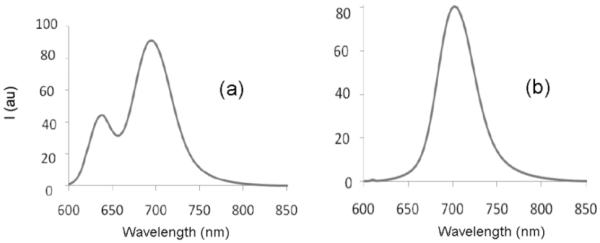
Fluorescence spectra in chloroform (ex: 590 nm); (a) pseudo-rotaxane M3⊃D1 (6 μM) yields free D1 (638 nm) and M3⊃D1 (694 nm); (b) M3⊃T2 (6 μM).
One of our long term research goals is to assemble highly stable squaraine rotaxane probes in complex biological environments such as the interior of cells. This requires the development of covalent capping methods that are biocompatible; that is, they do not use cytotoxic copper(I). One possible approach is to employ strain promoted cycloadditions with cyclic alkynes that undergo uncatalyzed reactions at practically useful rates.17 We were attracted to the related idea of a strained bicyclic alkene azide cycloaddition which produces five membered triazoline ring structures.18 As a biocompatible method for capping pseudo-rotaxanes, the reaction is appealing because it is perfectly atom economical and does not require copper catalysis. As a proof of concept, we heated a mixture of bis-azide dye D2, macrocycle M3 and stopper S3 (1:1:2 molar ratio) in chloroform for 3 days and produced the squaraine rotaxane M3⊃T3 in near quantitative yield (Scheme 2). The compound is stable enough for immediate characterization but it slowly decomposes if left standing under laboratory lights.19 Thus, the capping reaction is quite efficient but the product instability may possibly limit applications.
Scheme 2.
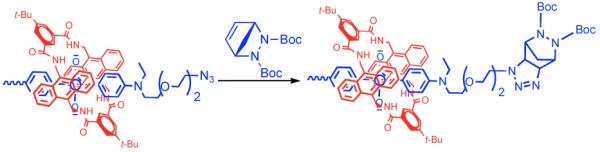
Capping of pseudorotaxane M3⊃D2 to produce M3⊃T3.
In summary, we report that squaraine rotaxanes with the anthrylene-containing macrocycle M3 can be prepared in high yield by capping the alkyne groups that protrude from pseudo-rotaxane precursors using either copper catalyzed azide-alkyne cycloaddition or uncatalyzed azide-alkene cycloaddition reactions. The resulting squaraine rotaxanes exhibit intense near-IR absorption/emission maxima and it should be possible to develop them into molecular probes for many types of photonic and bioimaging applications. In contrast, squaraine fluorescence intensity is greatly diminished when the dye is encapsulated by macrocycle M2. The fluorescence is restored when a suitable anionic guest is used to displace the squaraine dye from a pseudo-rotaxane complex; thus, the multicomponent system can be employed as a fluorescent anion sensor.
Supplementary Material
Acknowledgment
We are grateful for useful discussions with Professor Marvin J. Miller and his coworkers. This work was funded by the NSF.
References
- 1.(a) Shen X, Van Wijk R, editors. Biophotonics: Optical Science and Engineering for the 21st Century. Springer; New York: 2005. [Google Scholar]; (b) Cox GC, editor. Optical Imaging Techniques in Cell Biology. CRC Press; Boca Raton: 2007. [Google Scholar]; (c) Wolfbeis OS. Fluorescence Methods and Applications: Spectroscopy, Imaging, and Probes. Wiley-Blackwell; New York: 2008. [Google Scholar]
- 2.(a) Arunkumar E, Forbes CC, Smith BD. Eur. J. Org. Chem. 2005:4051–4059. [Google Scholar]; (b) Buston JEH, Young JR, Anderson HL. Chem. Commun. 2000:905–906. [Google Scholar]; (c) Park JS, Wilson JN, Hardcastle KI, Bunz UHF, Srinivasarao M. J. Am. Chem. Soc. 2006;128:7714–7715. doi: 10.1021/ja0584121. [DOI] [PubMed] [Google Scholar]; (d) Mohanty J, Pal H, Ray AK, Kumar S, Nau WM. ChemPhysChem. 2007;8:54–56. doi: 10.1002/cphc.200600625. [DOI] [PubMed] [Google Scholar]; (e) Comes M, Marcos MD, Martínez-Máñez R, Millán MC, Ros-Lis JV, Sancenón F, Soto J, Villaescusa LA. Chem. Eur. J. 2006;12:2162–2170. doi: 10.1002/chem.200500932. [DOI] [PubMed] [Google Scholar]; (f) Constantin TP, Silva GL, Robertson KL, Hamilton TP, Fague K, Waggoner AS, Armitage BA. Org. Lett. 2008;10:1561–1564. doi: 10.1021/ol702920e. [DOI] [PubMed] [Google Scholar]
- 3.Arunkumar E, Forbes CC, Noll BC, Smith BD. J. Am. Chem. Soc. 2005;127:3288–3289. doi: 10.1021/ja042404n. [DOI] [PubMed] [Google Scholar]
- 4.(a) Arunkumar E, Fu N, Smith BD. Chem. Eur. J. 2006;12:4684–4690. doi: 10.1002/chem.200501541. [DOI] [PubMed] [Google Scholar]; (b) Arunkumar E, Sudeep PK, Kamat KV, Bruce Noll B, Smith BD. New J. Chem. 2007;31:677–683. doi: 10.1039/b616224j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson JR, Fu N, Arunkumar E, Leevy WM, Gammon ST, Piwnica-Worms D, Smith BD. Angew. Chem. Int. Ed. 2007;46:5528–5531. doi: 10.1002/anie.200701491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Leigh DA, Murphy A, Smart JP, Slawain AMZ. Angew. Chem., Int. Ed. 1997;36:728. [Google Scholar]; (b) Gatti FG, Leigh DA, Nepogodiev SA, Slawin AMZ, Teat SJ, Wong JKY. J. Am. Chem. Soc. 2001;123:5983–5989. doi: 10.1021/ja001697r. [DOI] [PubMed] [Google Scholar]; (c) Leigh DA, Wong JKY, Dehez F, Zerbetto F. Nature. 2003;424:174–179. doi: 10.1038/nature01758. [DOI] [PubMed] [Google Scholar]
- 7.(a) Klotz EJF, Claridge TDW, Anderson HL. J. Am. Chem. Soc. 2006;128:15374–15375. doi: 10.1021/ja0665139. [DOI] [PubMed] [Google Scholar]; (b) Hübner GM, Reuter C, Seel C, Vögtle F. Synthesis. 2000;1:103–108. [Google Scholar]; (c) Dunnwald T, Jäger R, Vögtle F. Chem. Eur. J. 1997;3:2043–2051. [Google Scholar]
- 8.Inoue Y, Kanbara T, Yamamoto T. Tetrahedron Lett. 2003;44:5167–5169. [Google Scholar]
- 9.(a) Schalley CA, Weilandt T, Bruggemann J, Vögtle F. Top. Curr. Chem. 2004;248:141–200. [Google Scholar]; (b) Affeld A, Hübner GM, Seel C, Schalley CA. Eur. J. Org. Chem. 2001:2877–2890. [Google Scholar]; (c) Fischer C, Nieger M, Mogck O, Böehmer V, Ungaro R, Vögtle FJ. Eur. J. Org. Chem. 1998:155–161. [Google Scholar]; (d) Handel M, Plevoets M, Gestermann S, Vögtle FJ. Angew. Chem Int. Ed. 1997;109:1248–1250. [Google Scholar]
- 10.(a) Hunter CA. Chem. Commun. 1991:749–751. [Google Scholar]; (b) Hunter CA. J. Am. Chem. Soc. 1992;114:5303–5311. [Google Scholar]
- 11.Macrocycle M2 binds uncharged carbonyl-containing guests with association constants < 103 M−1 in CH2Cl2. For further information see: Seel C, Parham AH, Safarowsky O, Hübner GM, Vögtle F. J. Org. Chem. 1999;64:7236–7242.
- 12.(a) Aucagne V, Hanni KD, Leigh DA, Lusby PJ, Walker DB. J. Am. Chem. Soc. 2006;128:2186–2187. doi: 10.1021/ja056903f. [DOI] [PubMed] [Google Scholar]; (b) Dichtel WR, Miljanić OS, Spruell JM, Heath JR, Stoddart JF. J. Am. Chem. Soc. 2006;128:10388–10390. doi: 10.1021/ja063127i. [DOI] [PubMed] [Google Scholar]; (c) Miljanić OS, Dichtel WR, Khan SI, Mortezaei S, Heath JR, Stoddart JF. J. Am. Chem. Soc. 2007;129:8236–8246. doi: 10.1021/ja071319n. [DOI] [PubMed] [Google Scholar]
- 13.(a) Montalti M, Prodi L. Chem. Commun. 1998:1461–1462. [Google Scholar]; (b) Wiskur SL, Ait-Haddou H, Lavigne JJ, Anslyn EV. Acc. Chem. Res. 2001;34:963–972. doi: 10.1021/ar9600796. [DOI] [PubMed] [Google Scholar]
- 14.Hübner GM, Gläser J, Seel C, Vögtle F. Angew. Chem. Int. Ed. 1999;38:383–386. doi: 10.1002/(SICI)1521-3773(19990201)38:3<383::AID-ANIE383>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 15.Preliminary anion titration experiments with the rotaxane M2⊃T2 show that it acts as a fluorescent anion sensor. Full details will be reported when the studies are complete.
- 16.Gassensmith JJ, Arunkumar E, Barr L, Baumes JM, DiVittorio KM, Johnson JR, Noll BC, Smith BD. J. Am. Chem. Soc. 2007;129:15054–15059. doi: 10.1021/ja075567v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.(a) Agard NJ, Prescher JA, Bertozzi CR. J. Am. Chem. Soc. 2004;126:15046–15047. doi: 10.1021/ja044996f. [DOI] [PubMed] [Google Scholar]; (b) Baskin JM, Prescher JA, Laughlin ST, Agard NJ, Chang PV, Miller IA, Lo A, Codelli JA, Bertozzit CR. Proc. Natl. Acad. Sci. USA. 2007;104:16793–16797. doi: 10.1073/pnas.0707090104. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Ning XH, Guo J, Wolfert MA, Boons GJ. Angew. Chem. Int. Ed. 2008;47:2253–2255. doi: 10.1002/anie.200705456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.(a) Bodnar BS, Miller MJ. J. Org. Chem. 2007;72:3929–3932. doi: 10.1021/jo0701987. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Shea KJ, Kim JS. J. Am. Chem. Soc. 1992;114:4846–4855. [Google Scholar]; (c) Stout DM, Takaya T, Meyers AI. J. Org. Chem. 1974;40:563–569. [Google Scholar]
- 19.Triazolines can liberate N2 producing acid sensitive aziridines. See ref 19(c)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.