Abstract
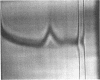
Formyltetrahydrofolate synthetase from Clostridium thermoaceticum had optimal activity at 55 to 60 C. Km values were: adenosine triphosphate (ATP), 6.25 × 10−4m; formate, 1.67 × 10−3m; and tetrahydrofolate, 1.00 × 10−3m; at pH 8.0. There was a requirement for divalent metals and specifically for ATP.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- HIMES R. H., RABINOWITZ J. C. Formyltetrahydrofolate synthetase. II. Characteristics of the enzyme and the enzymic reaction. J Biol Chem. 1962 Sep;237:2903–2914. [PubMed] [Google Scholar]
- Kuratomi K., Poston J. M., Stadtman E. R. Synthesis of Co-methyl cobalamin by cell-free extracts of Clostridium thermoaceticum. Biochem Biophys Res Commun. 1966 Jun 13;23(5):691–695. doi: 10.1016/0006-291x(66)90455-4. [DOI] [PubMed] [Google Scholar]
- Li L. F., Ljungdahl L., Wood H. G. Properties of Nicotinamide Adenine Dinucleotide Phosphate-Dependent Formate Dehydrogenase from Clostridium thermoaceticum. J Bacteriol. 1966 Aug;92(2):405–412. doi: 10.1128/jb.92.2.405-412.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungdahl L., Irion E., Wood H. G. Role of corrinoids in the total synthesis of acetate from CO-2 by Clostridium thermoaceticum. Fed Proc. 1966 Nov-Dec;25(6):1642–1648. [PubMed] [Google Scholar]
- Ljungdahl L., Irion E., Wood H. G. Total synthesis of acetate from CO2. I. Co-methylcobyric acid and CO-(methyl)-5-methoxybenzimidazolylcobamide as intermediates with Clostridium thermoaceticum. Biochemistry. 1965 Dec;4(12):2771–2780. doi: 10.1021/bi00888a030. [DOI] [PubMed] [Google Scholar]
- Poston J. M., Kuratomi K., Stadtman E. R. The conversion of carbon dioxide to acetate. I. The use of cobalt-methylcobalamin as a source of methyl groups for the synthesis of acetate by cell-free extracts of Clostridium thermoaceticum. J Biol Chem. 1966 Sep 25;241(18):4209–4216. [PubMed] [Google Scholar]
- Poston J. M., Stadtman E. R. The conversion of carbon dioxide to acetate. 3. Demonstration of ferredoxin in the system converting Co-14Ch3-cobalamin to acetate. Biochem Biophys Res Commun. 1967 Mar 9;26(5):550–555. doi: 10.1016/0006-291x(67)90100-3. [DOI] [PubMed] [Google Scholar]
- RABINOWITZ J. C., PRICER W. E., Jr Formyltetrahydrofolate synthetase. I. Isolation and crystallization of the enzyme. J Biol Chem. 1962 Sep;237:2898–2902. [PubMed] [Google Scholar]