Abstract
Vascular damage accompanying skeletal injury leads to an ischemic environment, and in clinical settings the extent of vascular damage is directly correlated with failure of skeletal repair failure. However, the exact mechanism(s) underlying ischemia-related defects in bone healing are not well understood. To better understand the mechanism and to facilitate development of novel interventions to treat ischemic fractures, a mouse model of long bone fracture healing coupled with femoral artery ligation was created. Ischemia was induced by femoral artery resection prior to tibia fracture. Fractures were left un-stabilized or were stabilized with custom-designed external fixators. Animals with intact femoral vessels served as controls. Tissues from non-stabilized fractures were analyzed at various times from 3 to 28 days after injury (n=5/time point). Femoral artery resection severely impaired blood supply to the fractured limbs and perfusion to the fracture sites did not recover until 14 days post-injury. Ischemia significantly decreased the callus size (p<0.05), and decreased bone (p<0.05) and cartilage (p<0.05) matrix production during healing of non-stabilized fracture. The decreased formation of skeletal tissues in ischemic limbs was accompanied by decreased cell proliferation and increased apoptosis at early time points, and increased fibrous and fatty tissues adjacent to the fracture site during the third and fourth week after injury. These alterations led to a delayed-union. Complete fracture healing was not achieved in the majority (day 21=4/5; day 28=5/5) of ischemic animals, while all control mice (n=5/5) had evidence of bony bridging by day 21. The ratio of cartilage to bone was similar in ischemic and control limbs at days 7 and 10 in non-stabilized fractures. In stabilized fractures, which healed through direct bone formation in the non-ischemic controls, ischemia decreased the amount of bone formation at days 10 and 14 (n=5/time point) and did not induce cartilage formation. These data reveal that an ischemic insult in the hind limb prior to fracture leads to a delayed union or a non-union, but does not favor formation of cartilage over bone. This model will be useful for testing novel therapeutic regimens to stimulate fracture healing.
Keywords: fracture, ischemia, delayed-union, non-union, vascular injury
Introduction
Inadequate blood supply is a major cause of delayed union or non-union during fracture healing. Previous work has shown that the rate of impaired healing is as high as 46% in fracture patients with concomitant vascular injuries 4, and this is significantly higher than the 10% non-union rate observed in general fractures 6. Although the relationship between ischemia and impaired fracture healing is well-established, the exact mechanisms underlying the defects are not clear. For example, how cells in the fracture callus respond to the ischemic insult is unknown. Furthermore, interventions designed specifically to treat ischemic fractures have not been well developed. A better understanding of the mechanism underlying ischemia-related defects in fracture healing will facilitate development of novel interventions.
In most clinical situations, ischemia is rarely a solitary factor that affects fracture repair. For instance, ischemia is accompanied by extensive soft tissue damage in a crush injury, and chronic ischemia can be present in patients with diabetic angiopathy. A crush injury and diabetes may have direct effects on fracture healing which makes it difficult to isolate the role of ischemia during fracture repair from other co-morbidities. To assess how ischemia impairs fracture healing without other confounding variables, an animal model is required. Rodent models are excellent for studying bone biology and skeletal repair. In this study, the mouse was chosen due to the availability of a plethora of genetically engineered strains, and the abundance and availability of reagents for cellular and molecular analyses. Previous research has shown that acute, transient ischemia after tourniquet application does not affect fracture repair 11, therefore we induced a model of more severe and persistent ischemia by resecting the femoral artery. In this model, blood flow drops precipitously and then returns through the formation of collateral blood vessels over the next 4 weeks 17. The effect of ischemia on cell proliferation, death, and differentiation, and production of cartilage and bone matrix in non-stabilized fractures were then assessed.
A secondary objective of this study was to assess the effects of ischemia on chondrocyte differentiation. Previous studies have shown that low oxygen tension increases the production of collagen type II (Col2) in cultured chondrocytes 5, and hypoxia favors cartilage but not bone formation 1. To determine whether ischemia favors chondrocyte or osteoblast differentiation, we compared the amount of cartilage and bone that formed in ischemic and control non-stabilized fractures. We also analyzed the effects of ischemia on the healing of stabilized fractures, because rigid stabilization normally induces osteoblast but not chondrocyte differentiation.
Materials and Methods
Animals
Ten to fourteen-week-old male 129J/B6 mice (25-30g) were used in this study. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of California at San Francisco.
Generation of ischemic or non-ischemic, non-stabilized tibia fractures
Animals were anesthetized by intraperitoneal injection of 2% Avertin (0.015ml/g). Femoral artery resection was performed as described previously 19. An incision was made in the right groin, the femoral artery and its branches between the inguinal ligament and saphenous/popliteal bifurcation were separated from the femoral nerve and vein by blunt dissection, ligated with 6-0 silk, and then removed. The incision was closed with 3-0 sutures. Immediately after femoral artery resection, a closed transverse mid-diaphyseal tibia fracture was created by three-point bending 14, and the animal was allowed to move freely after recovery from anesthesia. A pre-operative antibiotic (Ancef, 25mg/kg) was administered subcutaneously to prevent infection. Buprenex (0.01mg/kg) was given once pre-operatively and 2-3 times per day during the first 3 days after surgery to relieve pain. Animals that died during the post-operative period and those with foot necrosis or exposure of fracture ends were excluded from further analyses. Non-stabilized tibia fractures with intact femoral vessels were used as non-ischemic controls.
Generation of ischemic and non-ischemic stabilized tibia fractures
Femoral artery resection and pre- and post-operative animal care were performed as described above. Stabilized tibial fractures were created as previously described 21. Briefly, the proximal and distal metaphyses of the tibia were transfixed using four 0.25 mm pins, which were oriented perpendicular to the long axis of the tibia, 90° to each other. Two rings were positioned above the proximal and distal pins and secured to the pins using hexagonal nuts. A closed transverse fracture was then created with a three-point bending apparatus at the mid-diaphysis of tibia. Radiographs were taken immediately after fracture to confirm the extent of fracture. Stabilized tibia fractures without femoral artery surgery were used as non-ischemic controls.
Visualization of blood vessels in the hind limbs
To assess the entire vasculature of the fractured limbs at a macroscopic level, vascular casts were made at 0, 3, 5, 7, 10, 14, and 21 days post-fracture (n=2/time for both ischemic and control groups). Mice of non-stabilized fracture with or without femoral artery resection were euthanized with an overdose of Avertin and the entire vasculature was perfused by injecting Microfil (3-5ml, Flow Tech, Inc.) into the left ventricle. Legs were removed, dehydrated in ethanol, cleared in methyl salicylate, and photographed with a Leica MZFLIII dissecting microscope and digital camera.
To visualize blood vessels at a microscopic level, immunohistochemistry using an anti-PECAM (Platelet Endothelial Cell Adhesion Molecule, a marker of endothelial cells) antibody was performed 13. Ischemic and control animals with non-stabilized fractures were sacrificed at 3 and 5 days post-fracture (n=5/time for both ischemic and control groups). Fracture tissue was fixed in 4% paraformaldehyde (PFA), decalcified in 19% EDTA, and frozen sections of 20μm were prepared. Sections were incubated with anti-PECAM antibody (1:500, Pharmingen) at 4°C overnight and the antigen-antibody complex was visualized using a DAB reaction 14.
Histological and molecular analyses
Animals of non-stabilized fractures (n=5/time for both ischemic and control groups) were sacrificed at multiple time points post-injury (3, 5, 7, 10, 14, 21, and 28 days) to assess the effect of ischemia on different aspects of fracture healing. Animals of stabilized fractures (n=5 /time for both ischemic and control groups) were sacrificed at 10 and 14 days post-fracture when the cartilage should be most abundant if there is an alteration of chondrogenesis 3. Fractured tibiae were fixed in 4% paraformaldehyde (PFA) at 4°C overnight, decalcified in 19% EDTA, dehydrated, and embedded in paraffin. Sagittal sections (10μm) through the entire callus were mounted on slides (3/slide). Every tenth slide was stained with Safranin-O/Fast Green (SO/FG) and modified Milligan’s Trichrome staining (TC) was performed on adjacent slides to visualize cartilage and bone respectively 14. In situ hybridization to assess the expression of Collagen type II (Col2), Osteocalcin (Oc) and Collagen type X (Col10), markers of chondrocyte, osteoblast differentiation and chondrocyte maturation, was performed on sections adjacent to those used by histological staining 14.
Histomorphometric analysis
To assess the effect of ischemia on callus, cartilage, and bone formation during fracture healing, histomorphometry was performed as we have previously described 14. Briefly, sections (every thirtieth) stained with SO/FG or Trichrome were digitized using a Leica DMRB microscope. The number of pixels comprising each tissue component was determined by Photoshop to estimate area of callus, cartilage, or new bone. The volume of the callus, cartilage, and bone (TV, CV, or BV) was calculated using the equation for a conical frustum: TV, CV, or . Ai and Ai+1 are the area of callus, cartilage, or bone in the sequential sections; h is distance between sections (300μm), and n is total number of sections analyzed for each specimen. Two-way ANOVA analysis was used to compare the TV, CV, BV, and the ratio of cartilage to bone in the non-stabilized fracture callus of ischemic and control animals at each time point examined. Post-hoc test was performed to determine the significance of difference between ischemic and control groups at each time point.
Detection of proliferating and dying cells in ischemic and control non-stabilized fractures
To detect cell proliferation in the fracture calluses, a standard anti-BrdU staining was performed on paraffin sections of days 3 and 5 non-stabilized fractures (n=5/time for both ischemic and control animals) 15. The BrdU labeling reagent (0.3ml/mouse, Zymed) was administered intra-peritoneally two hours prior to the sacrifice of animals. TUNEL staining (Roche) was used to assess DNA strand breaks as described 10.
Results
A model of hindlimb ischemia
Femoral artery resection followed by tibial fracture appeared to be a major insult to the animals. To alleviate pain, Buprenex was provided as an analgesic during the first 3 days post-surgery. A total of 84 mice underwent femoral artery resection and tibia fracture. Complications included death during the post-operative period (n=9), necrosis of the foot and/or limb (n=2), necrosis of the skin at the fracture site leading to exposure of the fracture ends (n=3), necrosis of the toes (n=6), and open incision (n=4). The open incisions were re-sutured. Animals that exhibited signs of foot and/or limb necrosis, that had open fractures, or had died were excluded from analysis. Non-ischemic fractures were generated in 75 mice, and 6 animals were excluded due to post-operative death. No limb or skin necrosis was observed in non-ischemic mice. Immediately after femoral artery resection the ischemic hind limb became dark red, and this color usually attenuated within 2 to 3 days of injury. Swelling of the fractured ischemic limbs occurred during the first 5-7 days, which was longer than the 3 to 4 days that was typically observed in control fractures.
PECAM immunohistochemistry and blood vessel casting demonstrated that femoral artery resection significantly decreased blood supply to the fractured limbs and created a persistent ischemic environment at the fracture site. In control animals, PECAM immunostaining revealed an extensive vascular network at days 3 and 5 post injury (Fig. 1A, C). In contrast, the ischemic mice exhibited little evidence of vasculature in the lower leg at day 3 (Fig. 1B), and by day 5 limited PECAM staining was observed near the fracture site (Fig. 1D). Within 10 days of injury, microfil injection failed to fill blood vessels at fracture sites in ischemic animals (Fig 1E). However, by days 14 (Fig. 1F) and 21 (Fig. 1G) blood vessel casting demonstrated an increasing number of blood vessels around the fracture site, reflecting the recovery of blood supply to the injured limb.
Figure 1. Perfusion of the lower limb gradually returns in ischemic animals.
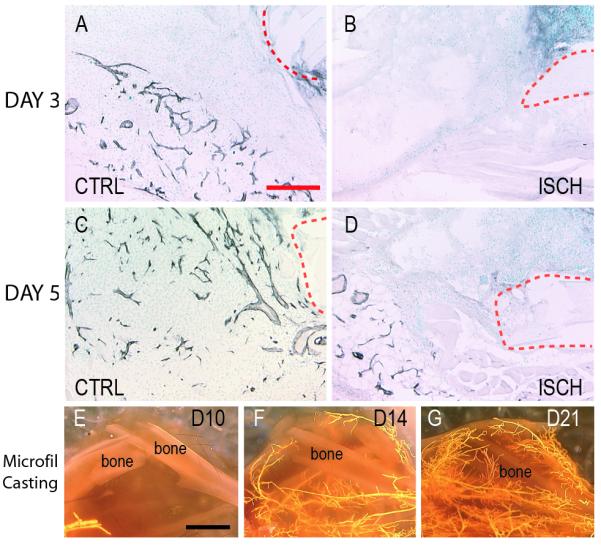
(A) Immunohistochemical detection of PECAM (black) at day 3 post-injury in the fracture callus illustrates the presence of a vascular network near the fracture site in control animals. (B) Little PECAM staining was evident adjacent to the fracture site in ischemic mice at this time. (C) By 5 days after injury a large number of PECAM-positive profiles are present in the fracture callus of control animals. (D) In ischemic animals, blood vessels are visualized adjacent to but not in the fracture callus. (E) Microfil injection at 10 days after fracture illustrates little perfusion to the limb of ischemic fractures. (F) By day 14, and (G) day 21, more perfusion of the lower leg is evident. Dotted red line=fracture ends. CTRL=control. ISCH=ischemic. Scale bar A-D=200μm, E-G =2mm.
Effects of ischemia on non-stabilized fracture repair
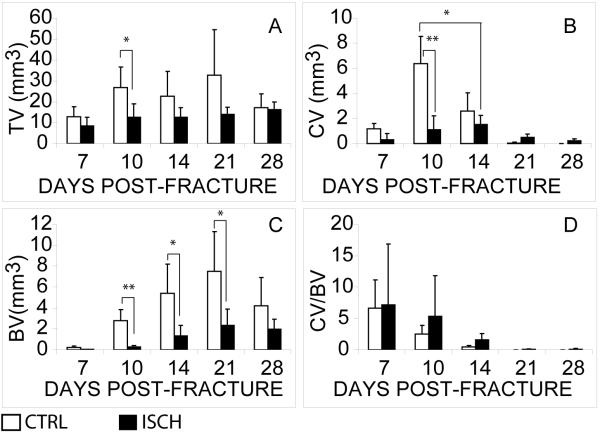
Histomorphometric and statistical analyses illustrated that ischemia had significant effects on the volume of callus (Fig. 2A, TV, p<0.01), cartilage (Fig. 2B, CV, p<0.01), and bone formation (Fig. 2C, BV, p<0.01), which demonstrated that ischemia significantly reduced the capacity of fracture repair. Further post-hoc statistical analyses demonstrated that ischemic mice formed significantly less callus than control animals at 10 days post-fracture (Fig. 2A, p<0.05). Ischemic animals had significantly less cartilage in fracture calluses at days 10 (Fig. 2B, p<0.001) and 14 (Fig. 2B, p<0.05). A similar decrease in formation of bone matrix was also observed in ischemic limbs at days 10 (Fig. 2C, p<0.01), 14 (Fig. 2C, p<0.05), and 21 (Fig. 2C, p<0.05). By day 28 remodeling of the callus in control animals had reduced the amount of bone to the levels of bone that formed in the ischemic limbs. Ischemia did not exhibit a significant impact on the ratio of cartilage to bone formed in fracture calluses (Fig. 2D), suggesting that ischemia may not favor the formation of cartilage over bone during fracture repair.
Figure 2. Ischemia reduces formation of skeletal tissues during the healing of non-stabilized fractures.
(A) Total volume of the callus (TV). (B) Total volume of the cartilage (CV). (C) Total volume of bone (BV). (D) Ratio of cartilage to bone (CV/BV). CTRL=control. ISCH=ischemic. Data shown are mean ± SD. * p<0.05, ** p<0.01.
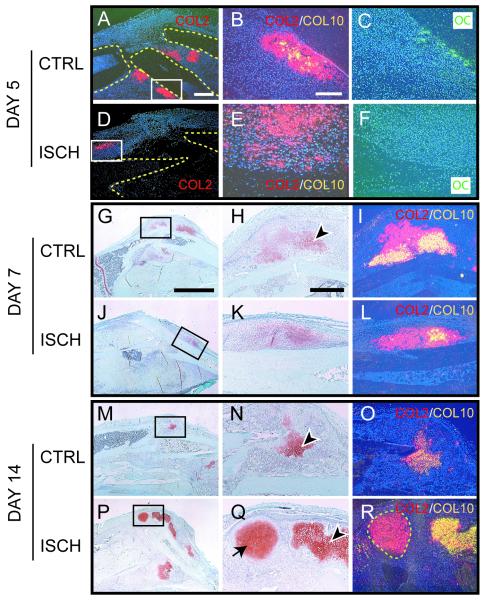
To determine whether the differences observed in production of skeletal matrices were accompanied by alterations to the cellular program of skeletal repair, the time course of chondrocyte and osteoblast differentiation, and chondrocyte maturation was compared between control and ischemic limbs. In control mice, a robust periosteal reaction close to the fracture site was observed at day 5 post-fracture. Col2 and oc transcripts were broadly expressed at this time (Fig. 3A-C). Additionally, Col10 expression (Fig. 3B) was observed in a small number of the Col2-positive cells in control mice indicating that chondrocyte maturation had begun. In contrast, in ischemic mice the periosteal reaction was minimal at day 5 and no cartilage or new bone was observed in the callus close to the fracture site (Fig. 3D-F). Rather, small loci of Col2 expression were observed in the periosteum at a distance from the fracture site (Fig. 3D). Col10 transcripts were not present in any of these chondrocytes (Fig. 3E).
Figure 3. Differentiation of chondrocytes and osteoblasts and maturation of chondrocytes are delayed in Ischemic Limbs.
(A) Col2 transcripts (red) are present in cells adjacent to the fracture ends (outlined) in control limbs at day 5 post-injury. Boxed area is shown in B and C. (B) A superimposition of adjacent in situ hybridization images reveals that some Col2 positive cells (red) are expressing Col10 transcripts (yellow). (C) Osteoblasts are evident and express oc transcripts (green) in the periosteum. (D) A small domain of Col2 expression (red) is observed at a distance from the fracture site (outlined) in ischemic limbs at 5 days post-injury. The boxed area is shown in E and F. (E) Superimposition of Col2 (red) and Col10 (yellow) in situ hybridization images reveals that no mature chondrocytes are present at this time. (F) Lack of oc expression indicates that no new bone is formed in the periosteum at day 5. (G) Low magnification of control fracture callus 7 days after injury stained with Safranin-O/Fast Green (SO/FG). Cartilage matrix is red. (H) High magnification of box in G illustrates that some chondrocytes are hypertrophic (arrowhead). (I) In situ hybridization reveals that a lot of chondrocytes are expressing Col2 (red) and Col10 (yellow). (J) Low magnification of ischemic fracture callus stained with SO/FG shows the location of cartilage (red). (K) High magnification of boxed area in J reveals that few chondrocytes are hypertrophic, and (L) superimposition of Col2 (red) and Col10 (yellow) in situ hybridization images indicate fewer mature chondrocytes at day 7 post-injury. (M) By day 14, a small amount of cartilage remains in control fractures. (N) High magnification of boxed area in M shows nearly all chondrocytes (arrowhead) exhibit a hypertrophic appearance. (O) In situ hybridization reveals that the chondrocytes are co-expressing Col2 (red) and Col10 (yellow). (P) A large amount of cartilage is present in ischemic fractures at day 14. (Q) High magnification of boxed area in P shows both hypertrophic (arrowhead) and immature (arrow) chondrocytes are present in the fracture site of ischemic limbs. (R) Chondrocytes expressing only Col2 (red, outlined) and chondrocytes expressing Col2 (red) and Col10 (yellow) are present at this time. CTRL=control. ISCH=ischemic. Scale bars: A, D=0.5mm; B, C, E, F=200μm; G, J, M, P=2.5mm; H, I, K, L, N, O, Q, R =500μm.
By 7 days post-fracture, a large amount of cartilage had formed in calluses of control mice, and chondrocytes were expressing Col2 and Col10 (Fig. 3G-I). Newly formed bone was evident adjacent to these cartilage islands (data not shown). In ischemic mice, only a small amount of cartilage (Fig. 3J-L) and new bone (data not shown) was observed at this time. Further, there was no evidence of invasion of the cartilage by endothelial cells yet (data not shown). Ten days after fracture, abundant cartilage expressing Col2 and Col10 was present and endochondral ossification was well underway in both ischemic and control animals (data not shown), but in ischemic limbs less cartilage was observed (Fig. 2B).
Fourteen days after fracture, replacement of the cartilaginous callus in the control animals was well underway (Fig. 3M-O). The chondrocytes that remained in these animals were hypertrophic (Fig. 3N) and expressed both Col2 and Col10 (Fig. 3O). In ischemic limbs cartilage matrix increased in abundance through this period. Chondrocytes exclusively expressing Col2 were abundant (Fig. 3P-R) and hypertrophic chondrocytes co-expressing Col2 and Col10 were also apparent (Fig. 3R).
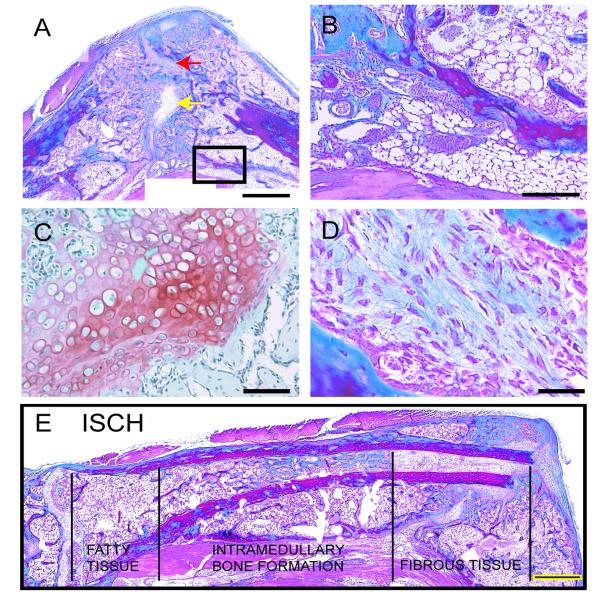
By 21 and 28 days after fracture, bridging callus composed of new bone was observed in all control animals analyzed (n=5 each for days 21 and 28). Only a small amount of cartilage remained in the calluses (data not shown). However, in ischemic animals, fractures failed to heal in 4/5 mice at day 21 and 5/5 mice at day 28. In the animals that had not healed, a bridging callus was not present and a large amount of fibrous and adipose tissue was located adjacent to the ends of the fractured bone (Fig. 4A, B, D). Additionally, a large amount of cartilage was still present in the calluses of ischemic limbs (Fig. 4C).
Figure 4. Adipose and Fibrous Tissue Infiltrate the Fracture Site of Ischemic Limbs at Day 28.
(A) Trichrome staining of ischemic fracture callus reveals the presence of cartilage (yellow arrow, shown in C) and fibrous tissues (red arrow, shown in D) in the fracture gap at day 28 post-injury. Boxed area is shown in B. (B) Fatty tissues (arrowheads) are present in areas of the injured leg. (C) Safranin-O/Fast Green staining demonstrates the presence of hypertrophic chondrocytes (red) in the fracture gap of ischemic animals. (D) Trichrome staining of fibrous tissue in the fracture gap. (E) A composite image of the injured tibia 28 days after injury illustrates heterogeneity in the composition of the bone marrow cavity. Near the knee joint the marrow cavity is filled with fatty tissues. Bone is evident in the marrow cavity more distally, and fibrous tissue comprises the marrow cavity adjacent to the fracture site. CTRL=control. ISCH=ischemic. Scale bar A, G=1mm; B=500μm; C=100μm; D=50μm.
Ischemia also affected the soft tissues adjacent to the fracture site. The skeletal muscle appeared necrotic at early time points after injury (data not shown). At later time points an extensive amount of lipid-containing cells that appeared to be adipocytes were present in the degenerated muscle beds and in areas near the fracture site (Fig.4B).
The bone marrow cavity also exhibited marked alterations in ischemic animals (Fig. 4E). At the site of bone injury and in more distal locations the bone marrow cavity was filled with fibrous tissue. In more proximal locations bone matrix and fatty tissue filled the marrow space. In contrast, control animals had no fibrous tissue or bone in the marrow cavity and adipocytes were restricted to the marrow cavity distal to the fracture site (data not shown).
Effects of ischemia on cell proliferation and cell death
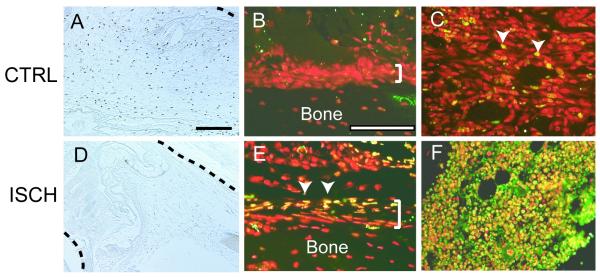
Cell proliferation and apoptosis were investigated to assess the extent to which the delays in cell differentiation could result from decreased proliferation or increased death of skeletal progenitor cells in ischemic limbs. A dramatic decrease in the amount of proliferating cells was observed at 3 days post-fracture in ischemic animals compared to control animals (Fig.5A, D). In control animals, proliferating cells were observed throughout the area near the fracture site (Fig. 5A), but in ischemic animals few BrdU positive cells were present near the injured bone (Fig. 5D).
Figure 5. Ischemia Reduces Cell Proliferation and Increases Cell Death.
(A) Immunohistochemical detection of BrdU incorporation in control animals at 3 days after fracture. (B) The periosteum (bracket) exhibits no signs of cell death, but (C) a few nuclei in the bone marrow cavity are TUNEL positive (arrowheads, yellow) in control animals. Nuclei (red) are counterstained with propidium iodide. (D) Immunohistochemistry identifies few cells that have incorporated BrDU in ischemic limbs. (E) Dying cells (arrowheads, yellow) are present in the periosteum (bracket) of the fractured bone and the adjacent mesenchyme of ischemic limbs. (F) In the bone marrow a large amount of cell death is evident (yellow). Black dotted lines: fractured bone. CTRL=control. ISCH=ischemic. Scale bar=200μm.
Increased cell death was also evident around the fracture site in ischemic animals. In control animals, rare periosteal cells and few bone marrow cells were TUNEL-positive (Fig. 5B, C). In contrast a large number of cells in the periosteum and bone marrow exhibited signs of apoptosis in ischemic animals (Fig. 5E, F).
Effects of ischemia on stabilized tibial fractures
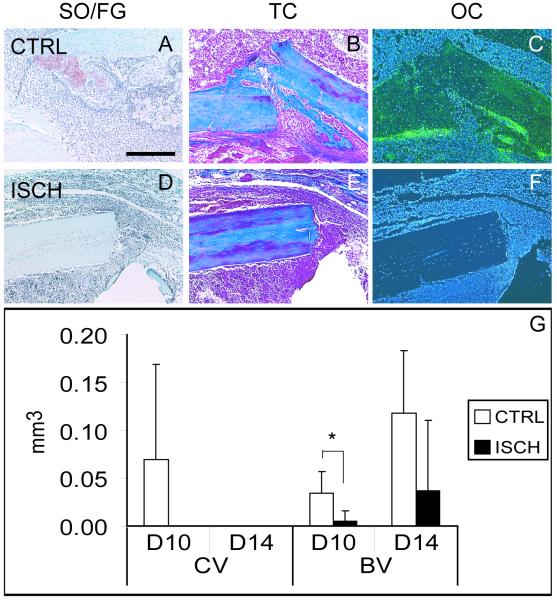
In control stabilized fractures, a periosteal reaction, a small amount of cartilage in some animals (n=3/5), and new bone was observed at 10 days post-injury (Fig. 6A-C, G), and at day 14 a substantial amount of bone was observed in the callus with no cartilage present (Fig. 6G). However, in ischemic fractures a less robust periosteal reaction and less bone were present at both of these time points (Fig. 6D-G). Statistical analyses demonstrated that ischemia had significant effects on the amount of bone formation in the callus (Fig. 6G, p<0.05); ischemic mice formed significantly less bone at 14 days post-fracture compared to the control ones (Fig. 6G, p<0.05). Further, cartilage was not present in the callus of stabilized ischemic fractures (Fig. 6D,G) indicating that cartilage formation was not stimulated by ischemia in the mechanically stable environment.
Figure 6. Ischemia does not Alter the Mode of Healing in Stabilized Fractures.
(A) Safranin-O/Fast Green (SO/FG) staining of control, stabilized fractures reveals a small cartilage island (red) 10 days after fracture, and (B) Trichrome staining (TC) illustrates new bone formation. (C) New bone is confirmed by the presence of osteocalcin (oc, green). (D) In ischemic limbs, there is no evidence of cartilage after SO/FG staining. (E) Likewise, no new bone is observed by TC staining. (F) In situ hybridization reveals no oc transcripts. (G) Histomorphometric analysis illustrates the volume of cartilage (CV) and bone (BV) present at days 10 and 14. At day 10 control animals have significantly more bone than ischemic animals. CTRL=control. ISCH=ischemic. Scale bar=500μm. * p<0.05.
Discussion
Ischemia Creates a Delayed-union in Mice
Results from this study demonstrate that a murine model of ischemic fracture can be created by femoral artery resection prior to the generation of tibia fractures. Femoral artery ligation induces a severe ischemic environment in the hind limbs 17, 20 and our data show that blood supply to the fracture site did not recover until 14 days post-injury. This acute, persistent ischemia led to multiple defects in fracture repair. Immediately after fracture, cell proliferation and survival were adversely affected by ischemia, and at later time points cell differentiation at the fracture site was aberrant. These alterations led to a smaller fracture callus that did not bridge the fracture gap within 28 days after injury, and also led to an increase in fibrous and adipose tissue adjacent to the fracture site. The large amount of non-skeletal tissue that regenerated adjacent to the fracture site suggests that either the number of osteo- and chondro-progenitor cells are reduced by ischemia, that cells have a reduced capacity to form bone and cartilage after an ischemic insult, or that the ischemic environment directs cells to differentiate along non-skeletogenic pathways. Each of these ideas require further exploration, because understanding factors that control cell differentiation during fracture repair is essential for developing new therapies.
Clinically, ischemia caused by a lack of blood supply to the fracture site is a major contributor to the formation of delayed or non-union of skeletal fractures 4. In this study, we analyzed fracture healing in an ischemic environment induced by femoral artery resection. The tibia fractures were created by three-point bending in our model. Compared to a crush injury, three-point bending disrupts the integrity of skeletal structure without causing much damage to the surrounding soft tissues. Femoral artery resection immediately before the creating of tibia fracture created an acute ischemic environment and the tissue in the hind limb was normal at the time of injury. Therefore, this model allows us distinguish the effect of ischemia itself on fracture healing without the confounding effect of extensive soft tissue damage that occurs in a crush injury.
Cell Differentiation at the Fracture Site is Influenced by Ischemia
In this study, reduced blood flow significantly hindered fracture healing but did not alter the mode of repair. Previous investigators have suggested that chondrocyte differentiation can be induced by hypoxia 1, 8, therefore ischemia was expected to favor differentiation of chondrocytes instead of osteoblasts during fracture healing. However, this is not confirmed by our data and ischemia did not influence differentiation of skeletal cell types. Infiltration of the muscle bed and marrow cavity with fatty tissues, and the appearance of fibrous tissue at the fracture site were apparent at later stages of healing. Thus, ischemia did not simply create an injury to the limb that compromised tissue regeneration. Rather, impaired healing likely resulted from a suppression of skeletogenic and a stimulation of adipogenic and fibrogenic proliferation and/or differentiation.
“Adipogenic healing,” where adipose tissue rather than scar tissue forms in injured muscle beds, has recently been described in rats 22. Very rapid rates of distraction of murine tibiae using external Ilizarov fixators created non-unions, and adipocytes were observed in the marrow cavity 2. In addition to adipose tissue, fibrous tissue is commonly observed in fracture non-union2, 9, 16. Collagen type III, a marker of fibrous tissue, is found expressed by mature osteoblasts in human fracture non-unions 12, suggesting that the factors causing non-union may alter the behavior of cells in fracture calluses. Similarly, in the experiments described in the current paper, adipose and fibrous tissues appear to form in place of skeletal tissues. The mechanism controlling adipocyte and fibroblast differentiation in these scenarios is not known. Adipocytes, fibroblast, osteoblasts, and chondrocytes in the limb are derived from mesoderm during embryonic development. Hence, increased differentiation of adipocytes and fibroblasts at the expense of bone and cartilage is conceivable. Additional experimentation will determine the lineage relationship among the adipocytes and fibroblasts and the osteo-and chondro-progenitor cells.
Avenues of Future Study and Utility of this Model
The model of delayed-union described in this work will allow a detailed investigation into many processes that are critical for bone repair. For instance, the necessity of the blood supply for bone repair is well recognized, but the exact mechanism through which the blood supply influences skeletal repair is unknown. In addition to providing nutrients and oxygen that are obviously required for cell survival, the blood supply is also a route for migratory inflammatory cells that are being recruited to the fracture site. An inflammatory reaction is required for fracture repair 7, 18, and the reduction in blood flow should alter the recruitment of inflammatory cells to the injury site. Additionally, the circulatory system could supply stem cells that would normally participate in or regulate fracture healing. The next objectives are to explore these possibilities and begin to assess mechanisms that underlie recalcitrant fracture repair.
Acknowledgements
We would like to thank A. Sapozhnikova, Z. Xing, J. Cone, E Hansen for technical help and R. Yee for statistical analysis. This work was aided by the Zimmer Corporation through a grant provided by the Orthopaedic Trauma Association (Basic Research Grant to R.M.) and NIH-NIAMS (KO8-AR002164 and RO1-AR053645-01 to T.M.).
Footnotes
Presented as an Abstract at the ORS Meeting, March 2006, Chicago IL.
References
- 1.Bassett CA, Herrmann I. Influence of oxygen concentration and mechanical factors on differentiation of connective tissues in vitro. Nature. 1961;190:460–1. doi: 10.1038/190460a0. [DOI] [PubMed] [Google Scholar]
- 2.Choi P, Ogilvie C, Thompson Z, et al. Cellular and molecular characterization of a murine non-union model. J Orthop Res. 2004;22:1100–7. doi: 10.1016/j.orthres.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Colnot C, Thompson Z, Miclau T, et al. Altered fracture repair in the absence of MMP9. Development. 2003;130:4123–4133. doi: 10.1242/dev.00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dickson KF, Katzman S, Paiement G. The importance of the blood supply in the healing of tibial fractures. Contemp Orthop. 1995;30:489–93. [PubMed] [Google Scholar]
- 5.Domm C, Schunke M, Christesen K, et al. Redifferentiation of dedifferentiated bovine articular chondrocytes in alginate culture under low oxygen tension. Osteo Cart. 2002;10:13–22. doi: 10.1053/joca.2001.0477. [DOI] [PubMed] [Google Scholar]
- 6.Einhorn TA. Enhancement of fracture-healing. J Bone Joint Surg Am. 1995;77:940–56. doi: 10.2106/00004623-199506000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Gerstenfeld LC, Thiede M, Seibert K, et al. Differential inhibition of fracture healing by non-selective and cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs. J Orthop Res. 2003;21:670–675. doi: 10.1016/S0736-0266(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 8.Grimshaw MJ, Mason RM. Bovine articular chondrocyte function in vitro depends upon oxygen tension. Osteo Cart. 2000;8:386–92. doi: 10.1053/joca.1999.0314. [DOI] [PubMed] [Google Scholar]
- 9.Hausman MR, Schaffler MB, Majeska RJ. Prevention of fracture healing in rats by an inhibitor of angiogenesis. Bone. 2001;29:560–4. doi: 10.1016/s8756-3282(01)00608-1. [DOI] [PubMed] [Google Scholar]
- 10.Hirai K, Hayashi T, Chan PH, et al. PI3K inhibition in neonatal rat brain slices during and after hypoxia reduces phospho-Akt and increases cytosolic cytochrome c and apoptosis. Brain Res Mol Brain Res. 2004;124:51–61. doi: 10.1016/j.molbrainres.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Kase T, Skjeldal S, Nordsletten L, et al. Healing of tibial fractures is not impaired after acute hindlimb ischemia in rats. Arch Orthop Trauma Surg. 1998;117:273–276. doi: 10.1007/s004020050245. [DOI] [PubMed] [Google Scholar]
- 12.Lawton DM, Andrew JG, Marsh DR, et al. Mature osteoblasts in human non-union fractures express collagen type III. Mol Pathol. 1997;50:194–7. doi: 10.1136/mp.50.4.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu C, Miclau T, Hu D, et al. Cellular basis for age-related changes in fracture repair. J Orthop Res. 2005;23:1300–7. doi: 10.1016/j.orthres.2005.04.003.1100230610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu C, Huang S, Miclau T, et al. Mepe is expressed during skeletal development and regeneration. Histochem Cell Biol. 2004;121:493–9. doi: 10.1007/s00418-004-0653-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marcucio RS, Cordero DR, Hu D, et al. Molecular interactions coordinating the development of the forebrain and face. Dev Biol. 2005;284:48–61. doi: 10.1016/j.ydbio.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 16.Oni OO. A non-union model of the rabbit tibial diaphysis. Injury. 1995;26:619–22. doi: 10.1016/0020-1383(95)00132-s. [DOI] [PubMed] [Google Scholar]
- 17.Stabile E, Kinnaird T, la Sala A, et al. CD8+ T Lymphocytes Regulate the Arteriogenic Response to Ischemia by Infiltrating the Site of Collateral Vessel Development and Recruiting CD4+ Mononuclear Cells Through the Expression of Interleukin-16. Circulation. 2006;113:118–124. doi: 10.1161/CIRCULATIONAHA.105.576702. [DOI] [PubMed] [Google Scholar]
- 18.Sudmann E, Dregelid E, Bessesen A, et al. Inhibition of fracture healing by indomethacin in rats. Eur J Clin Invest. 1979;9:333–9. doi: 10.1111/j.1365-2362.1979.tb00893.x. [DOI] [PubMed] [Google Scholar]
- 19.Tang G, Charo DN, Wang R, et al. CCR2-/- knockout mice revascularize normally in response to severe hindlimb ischemia. J Vasc Surg. 2004;40:786–795. doi: 10.1016/j.jvs.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 20.Tang G, Charo DN, Wang R, et al. CCR2-/- knockout mice revascularize normally in response to severe hindlimb ischemia. J Vasc Surg. 2004;40:786–95. doi: 10.1016/j.jvs.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 21.Thompson Z, Miclau T, Hu D, et al. A model for intramembranous ossification during fracture healing. J Orthop Res. 2002;20:1091–8. doi: 10.1016/S0736-0266(02)00017-7. [DOI] [PubMed] [Google Scholar]
- 22.Xaymardan M, Gibbins JR, Zoellner H. Adipogenic healing in adult mice by implantation of hollow devices in muscle. Anat Rec. 2002;267:28–36. doi: 10.1002/ar.10072. [DOI] [PubMed] [Google Scholar]