Abstract
Age is a primary risk factor in stroke that is often overlooked in animal studies. We contend that using aged animals yields insight into aspects of stroke injury and recovery that are masked, or not elicited, in younger animals. In this study, we examined effects of co-administration of a plasminogen activator inhibitor type 1 derived peptide, EEIIMD, with tissue plasminogen activator (tPA) on infarct volume and functional outcome in aged rats following a transient middle cerebral artery occlusion. Results of our study showed aged (18–20 months) rats treated with EEIIMD along with tPA had reduced cortical infarction volume. However, aged rats showed no improvement in total infarction volume, edema formation, or functional outcome as compared to aged rats administered only tPA. Young adult rats (3–4 months) treated with EEIIMD showed significant improvement in cortical and total infarction volumes, edema formation, and functional outcome. Striatal infarction volume was unaffected by EEIIMD treatment in both young adult and aged rats. These findings emphasize that physiological differences exist between young adult and aged rats and suggest that taking aging processes into account when assessing stroke may improve our ability to discern which therapeutics can be translated from bench to bedside.
Keywords: cerebral ischemia, tissue plasminogen activator, cortex, striatum, edema, stroke
1. INTRODUCTION
Treatment of ischemic stroke remains one of the most challenging areas in medicine today. Stroke is the third leading cause of death and the leading cause of long-term disability in the United States. Since FDA approval of tissue plasminogen activator (tPA) in 1995 and despite over 100 failed clinical trials, no other therapeutic has been shown effective in treating acute ischemic stroke. As a thrombolytic agent, tPA improves cerebral reperfusion and increases functional outcome when administered within 3 h of confirmed stroke onset. Unfortunately, tPA has direct and deleterious effects on the neurovascular unit and brain parenchyma that substantially increase the risk of hemorrhage and intrinsic toxicity that limit the use of tPA [Benchenane et al., 1996; Goto et al., 2007; Kidwell et al., 2008]. Thus, only 3–4% of people who suffer an ischemic stroke are eligible for the only approved treatment [Dawson and Dawson, 2006].
While pursuit of the next clinically viable stroke drug must continue, it would seem prudent to also search for therapeutics that expand the population of patients eligible to be treated with tPA. A recent study reported that hexapeptide, EEIIMD, corresponding to amino acids 350–355 of plasminogen activator inhibitor type 1 (PAI-1), bound non-thrombolytic sites on tPA and reduced edema formation and infarction size and decreased neuronal degeneration following transient middle cerebral artery occlusion (MCAO) in young male rats [Armstead et al., 2006]. While these findings appear promising, reservations and skepticism remain due to the lack of translation of so many other agents that showed promising results in animals but failed in the clinical setting, including calcium antagonists [Funato et al., 1997; Liu et al., 2004], modulation of the glutamineric and GABAergic systems [Simon and Shirashi, 1990; Turski et al., 1998; McCracken et al., 1993; Shuaib et al., 1993; Snape et al., 1993], free radical scavengers [Schmid-Elsaesser et al., 1998; Imai et al., 2001], anti-inflammatory agents [Bertorelli et al., 1998], membrane stabilizers [Lazzaro et al., 1994], and trophic factors [Sugimori et al., 2001].
We contend that age is the primary risk factor for stroke [D’Agostino et al., 1994] and not accounting for age related processes when evaluating stroke injury in animal models is an important determinant in failure to translate promising findings from animal studies into clinically useful therapeutics. Previously, we reported that aged rats experienced larger and functionally more devastating strokes than young adult rats [Rosen et al., 2005; DiNapoli et al., 2006; DiNapoli et al., 2008]. Ischemic strokes in aged rats are characterized by earlier disruptions of the blood-brain barrier to small molecules, increased infarction volumes, increased neuronal degeneration, higher mortality rates, and decreased functional outcomes as compared to younger rats [Rosen et al., 2005; DiNapoli et al., 2006; DiNapoli et al., 2008]. In this study, we evaluated the effect of PAI-1 mimetic EEIIMD on infarct size and functional outcome in aged rats following MCAO and tPA reperfusion.
2. RESULTS
2.1 EEIIMD reduced cortical but not striatal infarction size in young adult and aged rats following MCAO and tPA reperfusion
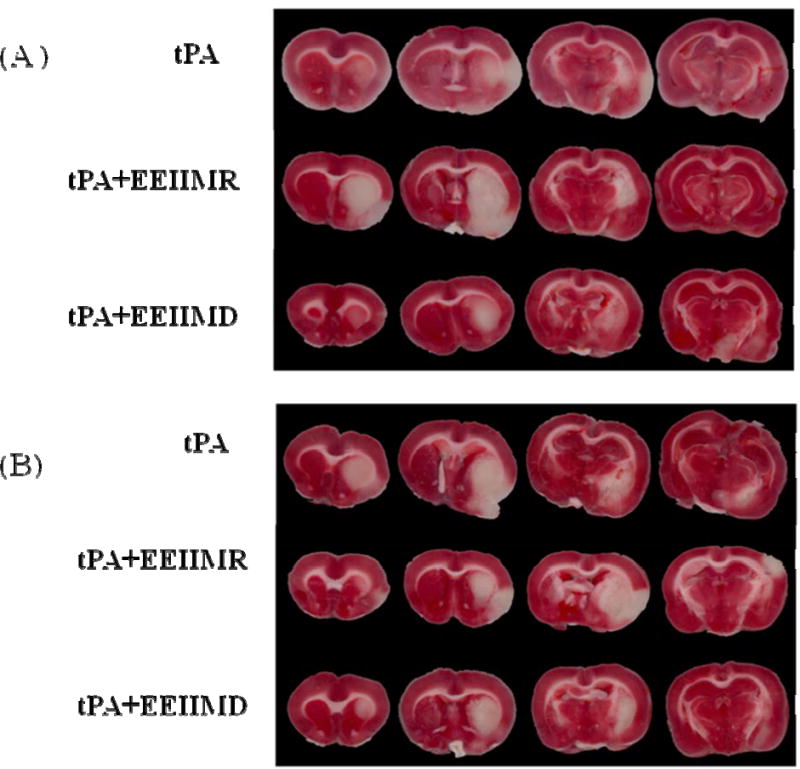
Quantification of infarction volume for cortex, striatum and total cerebral hemisphere in TTC-stained coronal sections was measured at 24 h following MCAO and tPA reperfusion (n=6 rats/group). Figure 1 is representative TTC stained sections of brain from young adult (A) and aged (B) rats. For the cortical infarction size, the results showed a significant effect of treatment group [F2, 30=19.97; P<0.001]. In Figure 2, post-hoc analyses demonstrated that the difference in treatment groups was seen in both young adult and aged rats group. In the young adult rats, results showed that the EEIIMD treated group had a significant decrease in cortical infarction size as compared to tPA alone (P=0.003) or the EEIIMR treated group (P=0.007). No difference between tPA alone and EEIIMR treated young adult rats was noted (P=0.97). In the aged rats group, results showed that EEIIMD treated rats had a significant decrease in cortical infarction size as compared tPA treated group (P=0.019) or EEIIMR treated group (P=0.001). No difference between tPA alone and EEIIMR treated aged rats was noted (P=0.40). No interaction between age and treatment was observed [F2, 30=1.14; P=0.33].
Figure 1.
Representative TTC stained coronal sections through the infarcted region of brains in (A) young adult and (B) aged rats following MCAO and tPA reperfusion with and without EEIIMR/EEIIMD co-administration. Using TTC, infarction was identified as unstained (white) and viable tissue was stained (dark).
Figure 2.
Measurement of stroke volume of the infarcted cortex, striatum, and total cerebral hemisphere following MCAO and tPA reperfusion. Results showed a significant decrease in cortical stroke volume in both young adult (1.23±1.83%) and aged (4.99±0.95%) EEIIMD treated rats as compared to age-matched controls (9.99±1.34% and 14.05±3.11%, respectively). No difference in striatal stoke volume was observed in young adult (28.12±2.91%) and aged (39.10±5.18%) EEIIMD treated rats as compared to age-matched controls (36.46±2.08% and 35.14±6.52%, respectively). Results from total stroke volume showed a significant (p<0.01) decrease in EEIIMD treated young adult rats (10.26±0.89%) as compared to young adult control rats (17.46±1.24%). No significant (p>0.05) difference in total stroke volume was demonstrated between EEIIMD treated aged rats (18.89±1.42%) and aged control rats (23.39±2.69%). No significant (p>0.05) difference in stroke volume was observed between EEIIMR treated and control rats regardless of age or brain section. Comparison of total infarct volume between young adult and aged rats showed a significant (p<0.05) increase in infarction volume in aged rats as compared to young adult rats regardless of treatment. Bars indicate mean ± S.E.M. and stroke volume is denoted as a corrected % of total hemisphere. (n=6 rats/groups). * represents p<0.05 as compared to age-matched control, ** represents p<0.01 as compared to age-matched control, *** represents p<0.001 as compared to age-matched control, and † represents p<0.05 as compared to young adult rats within treatment group.
For the striatal infarction size, the results showed no significant difference between young adult and aged rats [F1, 30=0.73; P=0.40]. No significant difference was found among treatment groups [F2, 30=0.67; P=0.52]. No interaction between age and treatment was observed [F2, 30=1.04; P=0.37] (Figure 2).
Results showed significant effect of age on total infarction volume [F1, 30=19.54; P<0.001]. Aged rats had a significantly larger total infarction size than young adult rats. This finding was found regardless of treatment. A significant effect was observed among treatment groups [F2, 30=11.33; P<0.001]. In Figure 2, post-hoc analyses demonstrated that the difference in treatment groups was seen both in the young adult and aged rats group. In the young adult rats group, EEIIMD treated rats showed a significant decrease in total infarction size as compared to the tPA alone treated group (P=0.006) and EEIIMR treated group (P=0.003). No significant (P=0.95) difference between tPA alone and EEIIMR treated young adult rats was noted. In the aged rats group, no significant (P>0.05) change in total infarction volume was observed between any treatment group. No interaction between age and treatment was observed [F2, 30=0.28; P=0.76].
2.2 EEIIMD failed to affect edema formation in aged rats following MCAO and tPA reperfusion
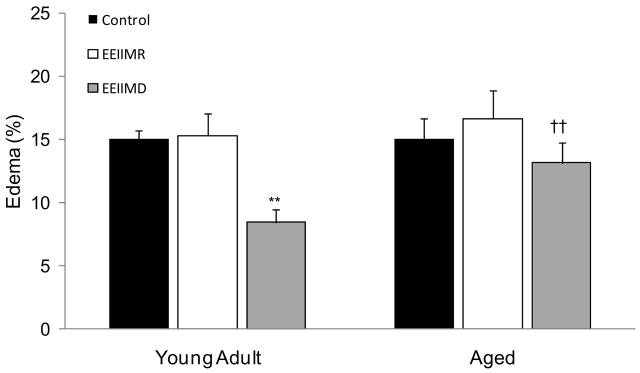
Edema formation was measured at 24 h following MCAO and tPA reperfusion (n=6 rats/group) (Figure 3). No significant effect of age on edema formation was observed [F1,30=1.1; P=0.30]. Results showed a significant effect of treatment [F2,30=3.1; P=0.046]. Post hoc analysis of treatment groups showed no significant (P>0.05) difference in edema formation between any treatment group of aged rats. A significant decrease in edema formation was found in young adult rats treated with EEIIMD as compared to age matched sham control (P=0.003) and EEIIMR treated (P=0.004) rats. No significant (P=0.99) difference in edema formation was observed between young adult sham and EEIIMR treated rats. No interaction between age and treatment was observed [F2,30=1.41 ;P=0.26].
Figure 3.
Measurement of edema formation in the infarcted hemisphere following MCAO and tPA reperfusion. Results showed a significant (p<0.01) decrease in edema formation in the EEIIMD treated young adult rats (8.42±1.02%) as compared to young adult control rats (15.03±0.68%). No significant (p>0.05) difference was observed between EEIIMD treated aged rats (13.13±1.58%) and aged control rats (15.02±1.59%). No significant (p>0.05) difference in edema formation was demonstrated between EEIIMR treated and control rats, regardless of age. No significant (p>0.05) difference in edema formation was shown between young adult and aged control rats. A significant (p<0.01) difference was demonstrated in edema formation between EEIIMD treated young adult and aged rats. Bars indicate mean ± S.E.M. (n=6 rats/group). ** represents p<0.01 as compared to age-matched control and † represents p<0.05 as compared to young adult rats within treatment group.
2.3 EEIIMD did not improve neurological function in aged rats following MCAO and tPA reperfusion
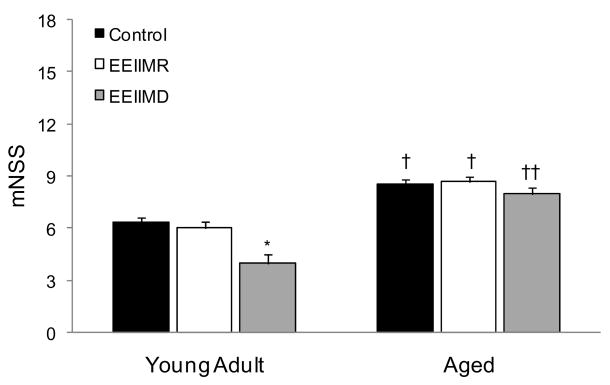
Comparison of neurological function at 24 h following MCAO and tPA reperfusion (n=6 rats/group) showed a significant effect for age [F1, 30=41.68; P<0.001] and treatment [F2,30=4.05; P=0.028]. Aged control rats had significantly increased neurological scores as compared to young adult control rats (P=0.04). In Figure 4, Tukey’s post-hoc analyses between treatment groups demonstrated that significant difference was only observed in young adult rats, which showed that EEIIMD treated young adult rats had a significant (P=0.003) decrease in functional score as compared to the tPA alone treated young adult rats and a near significant (P=0.010) decrease as compared to EEIIMR treated young adult rats. No significant (P=0.84) difference between tPA alone and EEIIMR treated young adult rats was noted. No significant difference in neurological score was observed in aged rats between treatment groups [F2,15=0.27; P=0.77]. No interaction between age and treatment was observed [F2,30=1.27; P=0.52].
Figure 4.
Assessment of neurological function using a 17 point mNSS following MCAO and tPA reperfusion. Results showed that EEIIMD treated young adult rats (4.0±0.5) had improved mNSS scores as compared to young adult control rats (6.3±0.3). No significant (p>0.05) difference in mNSS scores were demonstrated between EEIIMD treated aged rats (8.1±0.4) and aged control rats (8.5±1.1). A significant difference in mNSS scores was observed between young adult control rats and aged control rats and EEIIMD treated young adult rats and EEIIMD treated aged rats. No significant (p>0.05) difference between EEIIMR treated rats and control rats was observed regardless of treatment. Bars represent mean ± S.E.M. (n=6 rats/group). * represents p<0.05 as compared to age-matched control, † represents p<0.05 as compared to young adult rats within treatment group, and †† represents p<0.01 as compared to young adult rats within treatment group.
3. DISCUSSION
This study, for the first time, showed that administration of EEIIMD significantly improved cortical but not striatal infarct volumes in rats following embolic MCAO and tPA reperfusion. However, our findings also demonstrated that co-administration of EEIMD with tPA did not improve total infarct volume, edema formation, or neurological functional outcome in aged female rats following MCAO and tPA reperfusion. These findings are contrary to results from a previous study showing EEIIMD protected the brain and improved functional outcome following an embolic MCAO and tPA reperfusion [Armstead et al., 2006].
Consistent with previous studies [DiNapoli et al., 2006; DiNapoli et al., 2008], a mortality rate of >30% at 24 h following MCAO and tPA reperfusion was observed in aged rats (10 deaths out of 28 aged rats). No improvement in mortality was noted in EEIIMD (4 deaths) or EEIIMR (3 deaths) treated aged rats as compared to control aged (3 deaths) rats following MCAO and tPA reperfusion. Use of ketamine as an anesthetic agent during MCAO is controversial, with some studies showing no neuroprotection [Ridenour et al., 1991; Zhao et al., 2008] and others showing neuroprotection [Zhang et al., 2005], especially when administering tPA [Tsirka et al., 1995; Yanming et al., 1998]. We used ketamine in this study in order to accurately compare our results with prior studies using EEIIMD [Armstead et al., 2005; Armstead et al., 2006].
While mechanisms for the differential effects observed in brain regions and the relevance of how these findings relate to changes in neurological outcome are still unknown, results of this study reinforce the suggestion that beneficial effects seen in the cortex were due to a direct interaction between EEIIMD and non-catalytic binding sites on exogenous and endogenous tPA [Armstead et al., 2005; Armstead et al., 2006]. Administering a dose of tPA that is 50% lower than used in other studies and only administering tPA at 2 h after MCAO, we observed no signs of hemorrhage at 24 h in either young adult or aged rats, regardless of peptide treatment. No differences in infarct volume, edema formation, or neurological outcome were observed using the PAI-1 decoy peptide, EEIIMR, as compared to control; thus, supporting the suggestion of a direct interaction between tPA and EEIIMD.
The difference in age of the rats between our study and a previous study may explain the discordant results. The previous study used male Sprague-Dawley rats weighing ~250 g, which represented an age between 2–3 months. We argue age is a primary risk factor in stroke and aged rats suffer worsened brain damage and decreased functional recovery as compared to young adult rats. To test our premise, young adult rats (3–4 months of age) were administered EEIIMD along with tPA following MCAO. We showed equivalent results to the previously reported findings with decreased stroke volume and lessened edema formation. Moreover, our study showed that the improvement, which was enough to improve neurological function, was localized to the cortex in young adult rats at 24 h following MCAO. These results also argue against the disparity in findings between the two studies being related to gender, as the results obtained from young adult female rats in our study were comparable to young adult male rats used in the previous study. Thus, we are in agreement with the previous study that EEIIMD improves stroke outcome in young animals; however, relevance of these findings in relationship to the clinical population most at risk from stroke, individuals over the age of 65, suggests that experimental results in our aged model may be more clinically relevant.
To better understand the differences in brain damage following MCAO between young adult and aged rats, we evaluated regional changes in infarct volume. Results showed that the majority of infarct volume was striatal in young adult (83%) and aged rats (65%). This finding has been consistent in our studies showing aged rats not only suffered larger infarcts but a greater percentage of total infarct volume was cortical [DiNapoli et al., 2006; DiNapoli et al., 2008]. Yet in our present study, we found that EEIIMD administration improved cortical but not striatal infarct volume in both young adult and aged rats. This finding seems paradoxical to the functional outcome of the rats and underscores the importance of taking age-related changes in brain physiology into account when assessing stroke damage and recovery. The aged brain is characterized by many changes, including a 5–10% cortical atrophy, a generalized decrease in neurons, increased glia/neuron ratio [Stoll et al., 1998], decreased glucose metabolic rate [Gibson and Peterson, 1981], deficiency in handling of free radicals [Miquel et al., 1983], impaired vascular reactivity and compromised neuronal plasticity [Petcu et al., 2007]. In particular, aging has a number of adverse effects on striatal function, including decreased glutamate regulation [Nickell et al., 2007] and reduced GABAergic and cholinergic neurons [Paul et al., 1988]. Furthermore, aging also affects the functional interaction of the cellular components of the neurovascular unit. Previous studies have shown that cerebral capillaries in aged rodents become frail and lose vascular compliance. In addition, aging arterial vessels have been shown to have reduced elastin and smooth muscle cells and a thickening of the basement membrane [Popa-Wagner et al., 2006; Popa-Wagner et al., 2007; O’Rourke, 2007]. These age-related changes make the cerebral vessels in the area of an ischemic infarct more prone to neurovascular uncoupling [Zaletel et al., 2005; Schroeter et al., 2007], greater susceptibility to tPA neurotoxicity [Akkawi et al., 2006], and increased fragmentation [Knox et al., 1980], which may lead to morphological changes in the neurovascular unit including loss of pericytes, increased vascular remodeling, and separation of astrocytic endfoot processes from the capillary endothelial cells.
While EEIMD did show a marked improvement in protecting the cortex from ischemic damage, future studies will need to focus on the implications of striatal infarct toward neurological recovery. Furthermore, researchers may need to implement strategies to differentiate between cortical and striatal damage when assessing neurological damage and recovery following an ischemic brain injury, as modest improvement in cortical injury without concomitant changes in striatal injury may fail to improve functional status.
4. EXPERIMENTAL PROCEDURE
4.1 Chemicals and animals
All chemicals used in this study were of molecular biology grade and purchased from Sigma Chemical (St. Louis, MO), unless otherwise noted. The PAI-1 derived peptide, Am-EEIIMD-Ac, and decoy peptide, Am-EEIIMR-Ac, were synthesized by Biomatik Corporation (Hayward, CA). Two groups of female, age-matched, Sprague-Dawley rats (Harlan; Indianapolis, IN) were housed under 12-h:12-h light-dark conditions and received food and water ad libitum. Young adult (3–4 months) and aged (18–20 months) rats were randomly assigned at 2 h after MCAO to one of three treatment groups [tPA (5 mg/kg) alone; tPA + EEIIMD (1 mg/kg); tPA + EEIIMR (1 mg/kg)] and assessed at 24 h post-MCAO according to procedures detailed below. All protocols involving rats were approved by the West Virginia University Animal Care and Use Committee and abided by National Institutes of Health guidelines.
4.2 Surgical procedure for MCAO
Rats underwent a reversible embolic MCAO as previously described [DiNapoli et al., 2006; DiNapoli et al., 2008]. Briefly, rats were anesthetized with an intraperitoneal injection of ketamine (90 mg/kg; Webster Veterinary; Sterling, MA) and xylazine (5mg/kg; Webster Veterinary) and then administered at 1/5 the initial dose as needed to maintain sedation and analgesia through the duration of the surgical procedure. A servo-controlled homeothermic heating blanket, utilizing a rectal thermometer, was used to maintain body temperature at 37°C. Arterial PaO2, PaCO2, pH and hematocrit were monitored prior to embolization, during ischemia, and following recanalization using a GEM Premier 3000 blood gas analyzer (Instrumentation Laboratory; Lexington, MA). A microcatheter, outer diameter 0.3 mm, was inserted into the internal carotid artery via the external carotid artery stump and advanced until its tip occluded the ipsilateral middle cerebral artery (MCA). This mechanical occlusion was verified by laser Doppler monitoring of the MCA perfusion territory. The catheter was retracted until MCA flow was restored around the microcatheter. A 25 mm fibrin-rich, autologous blood clot was injected directly into the MCA. A drop in perfusion greater than 80% of initial baseline was determined to be ischemic. Cerebral perfusion to the MCA area was continuously monitored by laser Doppler for the duration of the procedure. After 2 h of ischemia, reperfusion was elicited by intravenous infusion (10% bolus and 90% infused over 30 min) of tPA (Genentech; South San Francisco, CA). The treatment groups consisted of tPA alone (control), and tPA together with the PAI-1 derived peptide EEIIMD or its decoy peptide EEIIMR. Reperfusion was monitored by laser Doppler and full reperfusion was determined by a return of perfusion to greater than 80% of ischemic baseline.
4.3 Composite functional score
At 24 h following MCAO and tPA reperfusion, functional assessment was examined in rats (n=6) from each treatment group using the modified neurological severity score (mNSS), which is a composite score of motor, sensory, balance and reflex measures. The mNSS scores ranged from 1 to 17, with higher scores indicating greater neurological injury [Seyfried et al., 2004].
4.4 2,3,5-Triphenyltetrazolium chloride (TTC) staining
Rats (n=6) in each treatment group were allowed to recover for 24 h following MCAO and tPA reperfusion, sacrificed by decapitation, brains removed, and sliced coronally at 2 mm intervals. Sections were incubated in 2% TTC in for 20 min at 37 °C. Following staining, the infarcted area remained unstained, appearing white, making it clearly distinguishable from viable tissue (stained red). All sections were scanned on a flatbed scanner and analyzed using Adobe Photoshop software (v7.0).
4.5 Determination of infarction volume
Following TTC staining, infarction volumes were quantified according to methods previously described [Yang et al., 1998]. On each brain slice, the ischemic area (nonstained) was marked and infarct volume calculated. To avoid overestimation of infarct volume, the corrected infarction volume (CIV) was calculated using the following equation: CIV = (LA−[RA−RI]) × d, where LA was area of the left hemisphere (mm2), RA was area of the right hemisphere (mm2), RI was the infarcted area (mm2), and d was slice thickness (2 mm). Since total brain volume in the treatment groups varied because of different body weight of animals at baseline, we calculated relative infarction volumes expressed as percentage of total brain volume.
4.6 Edema index (%)
Rats (n=6) in each treatment group were allowed to recover for 24 h following MCAO and tPA reperfusion, sacrificed by decapitation, and brains removed. Brain swelling was calculated as edema index (%) according to the following formula by a previously described method [Maier et al., 1998]. Briefly, edema index = (RV – LV)/LV x 100%, where RV was volume of the right hemisphere (mm3) and LV was volume of the left hemisphere (mm3).
4.7 Statistical analysis
Data are presented as mean ± S.E.M. Physiologic parameters and functional data were compared using two-way analysis of variance (ANOVA) and comparisons between groups determined using Tukey’s post hoc analysis. Level of significance was set at p<0.05.
Acknowledgments
The authors would like to thank the National Institutes of Health for their support of this project through the National Institute of Neurological Diseases and Stroke (RO1-NS061954).
References
- Akkawi S, Nassar T, Tarshis M, Cines DB, Higazi AA. LRP and alphavbeta3 mediate tPA activation of smooth muscle cells. Am J Physiol Heart Circ Physiol. 2006;291:H1351–H1359. doi: 10.1152/ajpheart.01042.2005. [DOI] [PubMed] [Google Scholar]
- Armstead WM, Cines DB, Higazie AA. Plasminogen activators contribute to age-dependent impairment of NMDA cerebrovasodilation after brain injury. Brain Res Dev Brain Res. 2005;156:139–46. doi: 10.1016/j.devbrainres.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Armstead WM, Nassar T, Akkawi S, Smith DH, Chen XH, Cines DB, Higazi AA. Neutralizing the neurotoxic effects of exogenous and endogenous tPA. Nat Neurosci. 2006;9:1150–1155. doi: 10.1038/nn1757. [DOI] [PubMed] [Google Scholar]
- Benchenane K, Berezowski V, Ali C, Fernandez-Monreal M, Lopez-Atalaya JP, Brillault J, Chuquet J, Nouvelot A, MacKenzie ET, Bu G, Cecchelli R, Touzani O, Vivien D. Tissue-type plasminogen activator crosses the intact blood-brain barrier by low-density lipoprotein receptor-related protein-mediated transcytosis. Circulation. 2005;111:2241–2249. doi: 10.1161/01.CIR.0000163542.48611.A2. [DOI] [PubMed] [Google Scholar]
- Bertorelli R, Adami M, Di Santo E, Ghezzi P. MK 801 and dexamethasone reduce both tumor necrosis factor levels and infarct volume after focal cerebral ischemia in the rat brain. Neurosci Letter. 1998;246:41–4. doi: 10.1016/s0304-3940(98)00221-3. [DOI] [PubMed] [Google Scholar]
- D’Agostino RB, Wolf PA, Belanger AJ, Kannel WB. Stroke risk profile: adjustment for antihypertensive medication. The Framington Study. Stroke. 1994;25:40–3. doi: 10.1161/01.str.25.1.40. [DOI] [PubMed] [Google Scholar]
- Dawson TM, Dawson VL. Taming the clot-buster tPA. Nat Med. 2006;12:993–994. doi: 10.1038/nm0906-993. [DOI] [PubMed] [Google Scholar]
- DiNapoli VA, Huber JD, Houser K, Li X, Rosen CL. Early disruptions of the blood-brain barrier may contribute to exacerbated neuronal damage and prolonged functional recovery following stroke in aged rats. Neurobiol Aging. 2008;29:753–764. doi: 10.1016/j.neurobiolaging.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNapoli VA, Rosen CL, Nagamine T, Crocco T. Selective MCA occlusion: a precise embolic stroke model. J Neurosci Methods. 2006;154:233–238. doi: 10.1016/j.jneumeth.2005.12.026. [DOI] [PubMed] [Google Scholar]
- Doerfler A, Schwab S, Hoffmann TT, Engelhorn T, Forsting M. Combination of decompressive craniectomy and mild hypothermia ameliorates infarction volume after permanent focal ischemia in rats. Stroke. 2001;32:2675–2681. doi: 10.1161/hs1101.098369. [DOI] [PubMed] [Google Scholar]
- Funato H, Kawano H, Akada Y, Katsuki Y, Sato M, Uemura A. Effects of a calcium antagonist, lacidipine, on experimental focal cerebral ischemia in rats. Jpn J Pharmacol. 1997;75:415–23. doi: 10.1254/jjp.75.415. [DOI] [PubMed] [Google Scholar]
- Gibson GE, Peterson C. Aging decreases oxidative metabolism and the release and synthesis of acetylcholine. J Neurochem. 1981;37:978–984. doi: 10.1111/j.1471-4159.1981.tb04484.x. [DOI] [PubMed] [Google Scholar]
- Gladstone DJ, Black SE, Hakim AM. Toward wisdom from failure: lessons from neuroprotective stroke trials and new therapeutic directions. Stroke. 2002;33:2123–2136. doi: 10.1161/01.str.0000025518.34157.51. [DOI] [PubMed] [Google Scholar]
- Imai H, Masayasu H, Dewar D, Graham DI, Macrae IM. Ebselen protects both gray and white matter in a rodent model of focal cerebral ischemia. Stroke. 2001;32:2149–54. doi: 10.1161/hs0901.095725. [DOI] [PubMed] [Google Scholar]
- Knox CA, Yates RD, Chen I, Klara PM. Effects of aging on the structural and permeability characteristics of cerebrovasculature in normotensive and hypertensive strains of rats. Acta Neuropathol. 1980;51:1–13. doi: 10.1007/BF00688844. [DOI] [PubMed] [Google Scholar]
- Lazzaro A, Seren MS, Koga T, Zanoni R, Schiavo N, Manev H. GM1 reduces infarct volume after focal cerebral ischemia. Exp Neurol. 1994;125:278–85. doi: 10.1006/exnr.1994.1030. [DOI] [PubMed] [Google Scholar]
- Lin TN, He YY, Wu G, Khan M, Hsu CY. Effect of brain edema on infarct volume in a focal cerebral ischemia model in rats. Stroke. 1993;24:117–121. doi: 10.1161/01.str.24.1.117. [DOI] [PubMed] [Google Scholar]
- Liu LS, Zhao Y, Lei YP, Wang W, Zhang XE, Jin L. Calcium antagonists in prevention of hypertension and stroke in stroke-prone spontaneously hypertensive rats. Chin Med J. 1989;102:106–13. [PubMed] [Google Scholar]
- Miquel J, Binnard R, Fleming JE. Role of metabolic rate and DNA-repair in Drosophila aging: implications for the mitochondrial mutation theory of aging. Exp Gerontol. 1983;18:167–171. doi: 10.1016/0531-5565(83)90009-8. [DOI] [PubMed] [Google Scholar]
- National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. Stroke. 1997;28:2109–2118. doi: 10.1161/01.str.28.11.2109. [DOI] [PubMed] [Google Scholar]
- Nickell J, Salvatore MF, Pomerleau F, Apparsundaram S, Gerhardt GA. Reduced plasma membrane surface expression of GLAST mediates decreased glutamate regulation in the aged striatum. Neurobiol Aging. 2007;28:1737–1748. doi: 10.1016/j.neurobiolaging.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Nyberg P, Waller S. Age-dependent vulnerability of brain choline acetyltransferase activity to transient cerebral ischemia in rats. Stroke. 1989;20:495–500. doi: 10.1161/01.str.20.4.495. [DOI] [PubMed] [Google Scholar]
- O’Rourke MF. Arterial aging: pathophysiological principles. Vasc Med. 2007;12:329–41. doi: 10.1177/1358863X07083392. [DOI] [PubMed] [Google Scholar]
- Petcu EB, Sfredel V, Platt D, Herndon JG, Kessler C, Popa-Wagner A. Cellular and molecular events underlying the dysregulated response of the aged brain to stroke: a mini-review. Gerontology. 2008;54:6–17. doi: 10.1159/000112845. [DOI] [PubMed] [Google Scholar]
- Popa-Wagner A, Dinca I, Yalikun S, Walker L, Kroemer H, Kessler C. Accelerated delimitation of the infarct zone by capillary-derived nestin-positive cells in aged rats. Curr Neurovasc Res. 2006;3:3–13. doi: 10.2174/156720206775541732. [DOI] [PubMed] [Google Scholar]
- Popa-Wagner A, Badan I, Walker L, Groppa S, Patrana N, Kessler C. Accelerated infarct development cytogenesis and apoptosis following transient cerebral ischemia in aged rats. Acta Neuropathol. 2007;113:277–93. doi: 10.1007/s00401-006-0164-7. [DOI] [PubMed] [Google Scholar]
- Ridenour TR, Warner DS, Todd MM, Baker MT. Efects of ketamine on outcome from temporary middle cerebral artery occlusion in the spontaneously hypertensive rat. Brain Res. 1991;565:116–22. doi: 10.1016/0006-8993(91)91742-j. [DOI] [PubMed] [Google Scholar]
- Rosen CL, DiNapoli VA, Nagamine T, Crocco T. Influence of age on stroke outcome following transient focal ischemia. J Neurosurg. 2005;103:687–694. doi: 10.3171/jns.2005.103.4.0687. [DOI] [PubMed] [Google Scholar]
- Schmid-Elsaesser R, Zausinger S, Hungerhuber E, Baethmann A, Reulen HJ. Monotherapy with dexamethasone or trilizad- but not a combination of both improves outcome after transient focal cerebral ischemia in rats. Exp Brain Res. 1998;122:121–7. doi: 10.1007/s002210050498. [DOI] [PubMed] [Google Scholar]
- Schroeter ML, Cutini S, Wahl MM, Scheid R, Yves von Cramon D. Neurovascular coupling is impaired in cerebral microangiopathy- An event-related Stroop study. Neuroimage. 2007;34:26–34. doi: 10.1016/j.neuroimage.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Seyfried D, Han Y, Lu D, Chen J, Bydon A, Chopp M. Improvement in neurological outcome after administration of atorvastatin following experimental intracerebral hemorrhage in rats. J Neurosurg. 2004;101:104–107. doi: 10.3171/jns.2004.101.1.0104. [DOI] [PubMed] [Google Scholar]
- Shuaib A, Ijaz S, Mazagri R, Senthilsevlvan A. CGS-19755 is neuroprotective during repetitive ischemia: this effect is significantly enhanced when combined with hypothermia. Neuroscience. 1993;56:915–20. doi: 10.1016/0306-4522(93)90137-5. [DOI] [PubMed] [Google Scholar]
- Simon R, Shiraishi K. N-methyl-D-aspartate antagonist reduces stroke size and regional glucose metabolism. Ann Neurol. 1990;27:606–11. doi: 10.1002/ana.410270604. [DOI] [PubMed] [Google Scholar]
- Snape MF, Baldwin HA, Cross AJ, Green AR. The effects of chlormethiazole and nimodipine on cortical infarct area after focal cerebral ischaemia in the rat. Neuroscience. 1993;53:837–44. doi: 10.1016/0306-4522(93)90628-s. [DOI] [PubMed] [Google Scholar]
- Stoll G, Jander S, Schroeter M. Inflammation and glial responses in ischemic brain lesions. Prog Neurobiol. 1998;56:149–171. doi: 10.1016/s0301-0082(98)00034-3. [DOI] [PubMed] [Google Scholar]
- Sugimori H, Speller H, Finkelstein SP. Intravenous basic fibroblast factor produces a persistent reduction in infarct volume in a permanent focal ischemia in rats. Neuroscience Lett. 2001;300:13–6. doi: 10.1016/s0304-3940(01)01549-x. [DOI] [PubMed] [Google Scholar]
- Tsirka SE, Gualandris A, Amaral DG, Strickland S. Excitotoxin-induced neuronal degeneration and seizure are mediated by tissue plasminogen activator. Nature. 1995;377:340–44. doi: 10.1038/377340a0. [DOI] [PubMed] [Google Scholar]
- Turski L, Huth A, Sheardown M, McDonald F, Neuhaus R, Schneider HH, Dirnagl U, Wiegand F, Jacobsen P, Ottow E. ZK200775: a phosphonate quinoxalinedione AMPA anatagonist for neuroprotection in stroke and trauma. Proc Natl Acad Sci USA. 1998;95:10960–5. doi: 10.1073/pnas.95.18.10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Shuaib A, Li Q. Quantification of infarct size on focal cerebral ischemia model of rats using a simple and economical method. J Neurosci Methods. 1998;84:9–16. doi: 10.1016/s0165-0270(98)00067-3. [DOI] [PubMed] [Google Scholar]
- Yanming WF, Tsirka SE, Strickland S, Steig PE, Soriano SG, Lipton SA. Tissue plasminogen activator (tPA) increase neuronal damage after focal cerebral ischemia in wild-type and tPA-deficient mice. Nature Med. 1998;4:228–31. doi: 10.1038/nm0298-228. [DOI] [PubMed] [Google Scholar]
- Zaletel M, Struci M, Pretnar-Oblak J, Zvan B. Age-related changes in the relationship between visual evoked potentials and visually evoked cerebral blood flow velocity responses. Funct Neurol. 2005;20:115–20. [PubMed] [Google Scholar]
- Zhang P, Liu Y, Li J, Chen X, Tian Y, Sun J, Liu J. Effects of ketamine and midazolam combination anesthesia on focal cerebral ischemia injury in rats. Sichuan da xue bao. 2005;36:351–4. [PubMed] [Google Scholar]
- Zhao H, Mayhan WG, Sun H. A modified suture technique produces consistent cerebral infarction in rats. Brain Res. 2008;1246:158–66. doi: 10.1016/j.brainres.2008.08.096. [DOI] [PMC free article] [PubMed] [Google Scholar]