Abstract
The airway epithelium is the first line of contact with the inhaled external environment and is continuously exposed to and injured by pollutants, allergens, and viruses. However, little is known about epithelial repair and in particular the identity and role of tissue resident stem/progenitor cells that may contribute to epithelial regeneration. The aims of the present study were to identify, isolate, and characterize side population (SP) cells in human tracheobronchial epithelium. Epithelial cells were obtained from seven nontransplantable healthy lungs and four asthmatic lungs by pronase digestion. SP cells were identified by verapamil-sensitive efflux of the DNA-binding dye Hoechst 33342. Using flow cytometry, CD45− SP, CD45+ SP, and non-SP cells were isolated and sorted. CD45− SP cells made up 0.12% ± 0.01% of the total epithelial cell population in normal airway but 4.1% ± 0.06% of the epithelium in asthmatic airways. All CD45− SP cells showed positive staining for epithelial-specific markers cytokeratin-5, E-cadherin, ZO-1, and p63. CD45− SP cells exhibited stable telomere length and increased colony-forming and proliferative potential, undergoing population expansion for at least 16 consecutive passages. In contrast with non-SP cells, fewer than 100 CD45− SP cells were able to generate a multilayered and differentiated epithelium in air-liquid interface culture. SP cells are present in human tracheobronchial epithelium, exhibit both short- and longterm proliferative potential, and are capable of generation of differentiated epithelium in vitro. The number of SP cells is significantly greater in asthmatic airways, providing evidence of dysregulated resident SP cells in the asthmatic epithelium.
Keywords: Epithelium, Tissue-specific stem cell, Human, Asthma
INTRODUCTION
The airway epithelium is the first line of contact with the inhaled environment and is continually exposed to infectious or noxious agents and airborne particles that can cause acute or chronic injury. It is becoming increasingly clear that in asthma, the airway epithelium responds inappropriately to challenge and displays signs of dysregulated repair, both of which may lead to airway remodeling [1, 2]. Significantly, these epithelial abnormalities can be detected prior to the onset of clinical symptoms of the disease [3]. However, the mechanisms involved in epithelial repair under normal conditions are unclear, and even less is known about these processes in asthma. Understanding these mechanisms of epithelial regeneration is crucial if we are to have an impact on the inappropriate remodeling of the airways seen in asthma.
In contrast to dermal and intestinal epithelia, which are highly proliferative and rapidly renewing [4, 5], the turnover of the airway epithelium is extremely slow unless injured [6]. Over the years, several experimental models in mice have been developed to mimic epithelial injury and repair following environmental challenges. The findings emerging from these studies demonstrate that different regions within the respiratory system (trachea and large airway, distal bronchioles, and alveoli) contain different stem cells and different repair mechanisms. To date the cells reported to be enriched for stem/progenitor cell activity include basal cells, secretory (Clara) cells, and cells residing in submucosal glands [7–10]. These cells may or may not reside within stem/progenitor niches such as the bronchioalveolar duct junction [11, 12]. However, information regarding lineage decisions, self-renewal properties, and clonality of these cells within human airways is lacking. The difficulties of identification and isolation of these cells are associated with the absence of reliable cell surface markers. Efforts to purify resident stem cells from adult tissues have focused on the Hoechst 33342 exclusion assay, which was first described by Goodell et al. [13] for hematopoietic stem cells. Side population (SP) cells are identified by flow cytometry because of a unique ability to efflux the DNA-binding dye Hoechst 33342 via a cell surface multidrug resistance/ATP-binding cassette transporter protein called breast cancer resistance protein 1/ATP-binding cassette transporter, sub family G (Bcrp-1/ABCG2), and they have been shown to exhibit properties similar to those of hematopoietic stem cells. To date, SP cells have been isolated from several human tissues, including mammary glands [14, 15], ocular epithelium [16], and skin [17]. Although SP cells have been identified in digests of whole murine lung tissue [18–20], whether SP cells exist within human airway epithelium and whether these cells play a role in epithelial repair still remain unknown. However, the structural complexity of the airway epithelium and the relative paucity of resident stem cells, their markers, and sufficient tissue for cell isolation have hindered the identification of these cells in human airways.
The aim of the present study was to identify SP cells present within human tracheal and bronchial epithelium in vivo and to isolate and characterize these cells to determine their stem/progenitor cell capacity in vitro. We demonstrate that SP cells exist in human airway epithelium, express epithelial cell markers, display increased colony-forming capacity, and are able to generate a multilayered epithelium in air-liquid interface culture. Importantly, we also demonstrate that the number of SP cells is substantially increased within asthmatic airways.
MATERIALS AND METHODS
Airway Epithelial Cell Isolation
Human transplant donor lungs deemed unsuitable for transplantation and donated for medical research were obtained through the International Institute for the Advancement of Medicine (Edison, NJ) and through the Pacific Northwest Transplant Bank (Portland, OR). The ethics committees of the institutions involved approved this study. Primary human bronchial epithelial cells were isolated by protease digestion of human airways, as previously described, with minor modifications [21]. Briefly, after surgical removal specimens were washed in Custodiol histidine-tryptophan-ketoglutarate solution (Odyssey Pharmaceuticals Inc., East Hanover, NJ, http://www.odysseypharm.com) and packed on ice for transportation. The trachea and bronchi to the fourth generations were dissected and rinsed three times in cold phosphate-buffered saline (PBS) without Ca2+ and Mg2+ to completely remove blood and mucus plugs. The epithelium on intact segments of trachea and bronchi (2–4 cm long) was then dissociated with 1.4 mg/ml pronase and 0.1 mg/ml DNase (Roche Diagnostics, Indianapolis, http://www.roche-applied-science.com) in 100 ml of minimal essential medium (MEM) (Fisher Scientific International, Philadelphia, http://www.fisherscientific.com) at 4°C for 16 hours. After digestion, the tracheal/bronchial tube was rinsed with MEM and dissociated cell clumps were strained through a 70-µm nylon mesh (Becton, Dickinson and Company, Franklin Lakes, NJ, http://www.bd.com). To neutralize pronase, cells were resuspended and incubated in MEM with 10% fetal bovine serum (FBS) for 10 minutes and washed twice with MEM by centrifugation at 4°C. Approximately 20 × 106 cells in total were recovered from both the trachea and bronchial tissues of each individual. Adherent cells were depleted by plating cell suspension on tissue culture flasks and incubation at 37°C for 30 minutes. Nonadherent cells were collected, washed with MEM, and resuspended at 1 × 106 cells per milliliter in bronchial epithelial growth medium (BEGM; Cambrex, Walkersville, MD, http://www.cambrex.com).
Hoechst 33342 Efflux Assay
Following time-course and dose-response controls based on Hoechst efflux assays, airway epithelial cells in suspension were stained with 5 µg/ml Hoechst 33342 (Sigma-Aldrich, St. Louis, http://www.sigmaaldrich.com) alone or in combination with 50 µM verapamil (Sigma-Aldrich) for 90 minutes at 37°C with 5% CO2 and intermittent mixing. Immediately after staining, cells were washed twice with ice-cold Hanks’ balanced saline solution (HBSS) (Fisher Scientific) containing 0.2 mg/ml DNase and kept on ice. Staining for specific surface markers was performed at 4°C after incubation with Hoechst 33342. Briefly, cells in suspension were incubated for 30 minutes at 4°C with 1 µg/ml mouse anti-human CD45 (fluorescein isothiocyanate-conjugated, clone 2B11; Santa Cruz Biotechnology Inc., Santa Cruz, CA, http://www.scbt.com) or isotype control (Santa Cruz Biotechnology) following washing with ice-cold HBSS containing 0.2 mg/ml DNase. Propidium iodide (2 µg/ml; Sigma-Aldrich) was added to the cell suspension 5 minutes before sorting to exclude nonviable cells. For all Hoechst efflux assays cell viability remained between 85% and 90% following the staining procedure and fluorescence-activated cell sorting (FACS) analysis.
Flow Cytometry
Positively stained cells were sorted on a FACS DiVA (BD Biosciences, San Diego, http://www.bdbiosciences.com), using FACS DiVA software. A UV laser and an argon laser (excitation 350 and 488 nm) were used to excite the Hoechst 33342, and emissions of the dye were detected using 670/30-nm band-pass and 450/65-nm band-pass filters. SP cells were identified as described by Goodell et al. [22]. Detection of CD45+ SP cells was performed using a 530/40-nm band-pass filter. Compensation was set manually using single-color control. CD45− SP cells and non-SP cells were then re-sorted to ensure purity and collected directly into tissue culture plates (Corning Costar, Acton, MA, http://www.corning.com/lifesciences) or fixed in 4% paraformaldehyde (Fisher Scientific) for 20 minutes for further FACS analysis. For this, cells were stained with antibodies for either 1 µg/ml Bcrp-1/ABCG2 (clone 5D3; StemCell Technologies, Vancouver, BC, Canada, http://www.stemcell.com), 1 µg/ml pan-cytokeratin phycoerythin conjugated (clone C-11; Abcam, Cambridge, U.K., http://www.abcam.com), 1 µg/ml pan-p63 (clone 4A4; Santa Cruz Biotechnology), or isotype controls in PBS with 0.1% saponin for 30 minutes at room temperature. Following washing with 0.1% saponin and 0.1% Tween20 in PBS, secondary antibody goat anti-mouse IgG Alexa Fluor 488 (Invitrogen, Carlsbad, CA, http://www.invitrogen.com) was used to detect Bcrp-1/ABCG2 and pan-p63 before final washing. Detection of Bcrp-1 and pan-cytokeratin CD45+ SP cells was performed using a 530/40-nm band-pass filter. Compensation was set manually using single-color control.
Clonogenic Assay
Colony formation capacity of CD45− SP, non-SP, and unsorted cells was determined using the method previously published by Jones and Watt [23]. Briefly 1, 10, 100, and 1,000 cells were plated in 300 µl of bronchial epithelial growth media (BEGM) onto a 24-well culture plate. Clonogenic assays were carried out three times for each cell population isolated, to obtain an average. Cells were allowed to adhere and propagate for 12 days and were then fixed in 4% paraformaldehyde (Fisher Scientific) for 20 minutes, stained with Giemsa stain (Sigma-Aldrich) for 30 minutes, washed with water, and air-dried. Colonies were counted using a Zeiss Stemi 2000-C microscope (Carl Zeiss, Toronto, http://www.zeiss.com). Colony formation was calculated as a ratio of numbers of colonies with > 32 cells per colony to total numbers of cells plated. Colonies were also characterized by morphology: cobblestone, stellate, or cells with high cytoplasmic-to-nuclear ratio. The colony-forming capacity was followed over 16 passages for each cell population isolated from an individual lung donor.
Immunofluorescent Staining
At each passage of the clonogenic assay, protocol cells were also seeded into eight-well chamber slides for characterization by immunofluorescent staining. Following 12 days in culture, cells were fixed in 4% paraformaldehyde (Fisher Scientific) for 20 minutes and stained with antibodies for cytokeratin-5 (clone D5/16 B4; Dako, Mississauga, ON, Canada, http://www.dako.com), cytokeratin-18 (rabbit polyclonal; Calbiochem, Temecula, CA, http://www.emdbiosciences.com), ΔNp63 isoform (sc-8609), TAp63 isoform (D-20), E-cadherin (clone G-10), and vimentin (clone C-20; all from Santa Cruz Biotechnology) all at a concentration of 1 µg/ml in PBS with 0.1% saponin for 2 hours at room temperature. Following washing with 0.1% saponin and 0.1% Tween-20 in PBS, secondary antibodies conjugated with either goat anti-mouse IgG Alexa Fluor 488 or goat anti-rabbit IgG Alexa Fluor 594 (Invitrogen) were incubated for 2 hours at room temperature. Cells were visualized with a Nikon fluorescent microscope and imaged with a C-spot camera (Nikon Instruments, Melville, NY, http://www.nikon.com).
Quantitative Polymerase Chain Reaction to Determine Telomere Length
Genomic DNA was extracted from CD45− SP and non-SP cells after passages 1, 2, and 3 using QIAamp DNA mini kit (Qiagen, Valencia, CA, http://www1.qiagen.com). Telomere length was determined using the method previously described [24]. Polymerase chain reaction (PCR) assays were performed on a Prism 7700 sequence detection system (Applied Biosystems, Foster City, CA, http://www.appliedbiosystems.com).
TaqMan Gene Expression Assays
RNA was isolated from CD45− and non-SP cells following the initial Hoechst sort and at passage using RNeasy Plus Mini-Kits (Qiagen) as per the manufacturer’s instructions. Purified RNA was reverse-transcribed into cDNA using TaqMan reverse transcription reagents (Applied Biosystems). E-cadherin and vimentin gene expression was determined by real-time PCR using the TaqMan Universal PCR Master Mix (Applied Biosystems) and predeveloped TaqMan Gene Expression Assays (Applied Biosystems) as per the manufacturer’s instructions. Gene expression was normalized to glyceraldehyde-3-phosphate dehydrogenase. Transcript expression of genes of interest was calculated, with 16-human bronchial epithelial 14o-cell line (16HBE14o-) cells serving as the calibrator.
Air-Liquid Interface Culture
For air-liquid interface (ALI) cultures, CD45− and non-SP cells were seeded on 12-well polyester membrane inserts (0.4-µm pore size; Corning Costar) at a density of 1 × 102 cells per cm2. Cells were submerged in 0.5 ml of BEGM at the apical surface, with 1 ml added to the basal compartment and cultured at 37°C with 5% CO2. Media was replaced every 2nd day until a confluent monolayer was formed. The media was then removed from the apical compartment to bring the culture to air-liquid interface. Cells were then differentiated for a further 25–35 days in a 1:1 mix of Dulbecco’s modified Eagle’s media and bronchial epithelial basal media (BEBM) with insulin (5.0 µg/ml), transferrin (10 µg/ml), hydrocortisone (0.5 µg/ml), bovine pituitary extract (52 µg/ml), epithelial growth factor (0.5 ng/ml), epinephrine (0.5 ng/ml) (all from Cambrex), 0.1µM all-trans retinoic acid, 100 units/ml penicillin, and 100 µg/ml streptomycin (all from Sigma-Aldrich). The media was replaced every 2 days, and cultures were incubated at 37°C in humidified air with 5% CO2. Membranes were processed for histological and immunohistochemical analysis following 25 days in culture. All independent experiments were performed in triplicate.
Immunohistochemical Staining of ALI Sections
Sections were deparaffinized, rehydrated, and subjected to antigen retrieval by autoclaving (15 minutes, 120°C, 30 psi) for 20 minutes in citrate target retrieval solution (Dako). Subsequently, endogenous peroxidase was quenched with 3% H2O2 and blocked for 20 minutes with 50% goat serum. Primary mouse anti-human cytokeratin-5 (1 µg/ml clone D5/16 B4; Dako), cytokeratin-18 (1 µg/ml rabbit polyclonal; Calbiochem), or p63 (1 µg/ml clone 4A4; Santa Cruz Biotechnology) was added overnight at 4°C in 25% goat serum. Sections were then incubated with a biotinylated goat anti-mouse secondary antibody (1:100; Vector Laboratories, Burlingame, CA, http://www.vectorlabs.com) for 60 minutes followed by a 10-minute treatment with streptavidin-horseradish peroxidase (Dako). The antigen of interest was visualized by using the brown chromogen 3,3-diaminobenzidine (Dako) and counterstained with Harris Hematoxylin Solution (Sigma-Aldrich). Sections were then dehydrated and mounted with Cytoseal 60 (Richard-Allan Scientific, Kalamazoo, MI, http://www.rallansci.com). Antibody dilutions and all washes were in Tris-buffered saline solution.
Statistical Analysis
The mean percentages of CD45− SP cells, clonogenic capacity, and mean telomere length were compared using an unpaired Student’s t test. A p value < .05 was considered to be statistically significant.
RESULTS
Cells with an SP Phenotype Exist Within Human Tracheal and Bronchial Epithelium
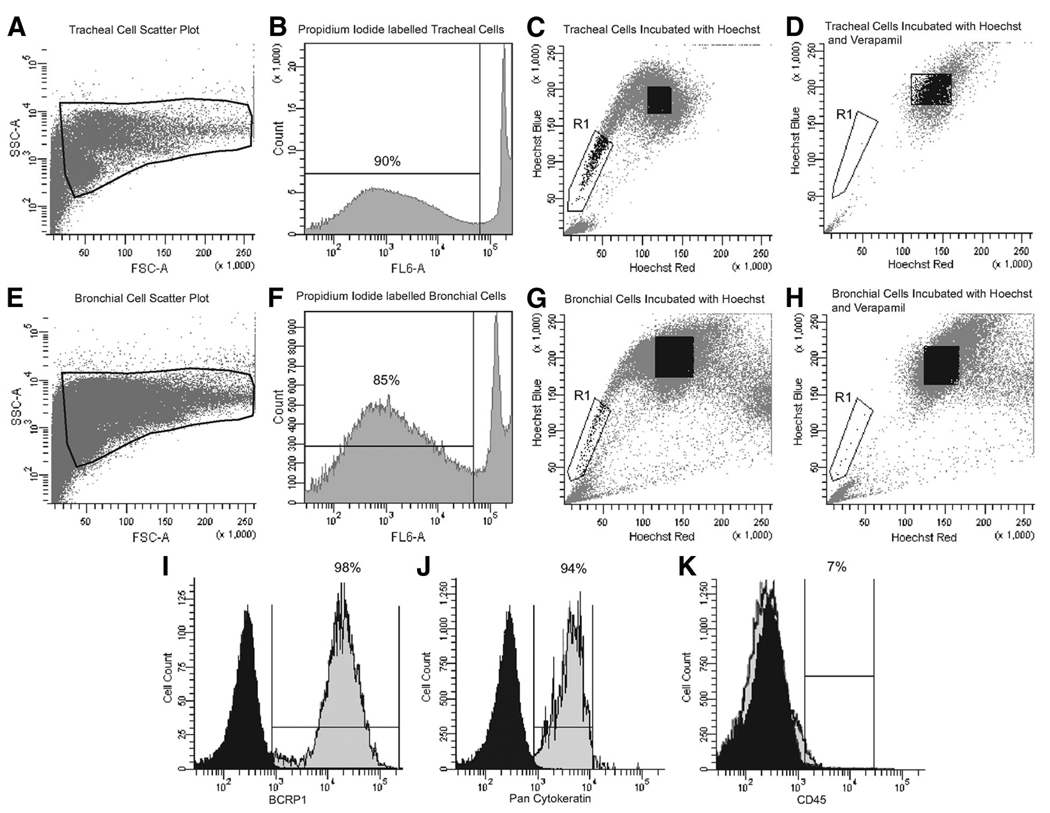
Human tracheal and bronchial epithelial cells were obtained via pronase digestion of airway tissue from five normal adults; patient demographics are presented in Table 1. On average, 5 × 106 epithelial cells were obtained from the trachea and 15 × 106 cells from the bronchial tissue, as shown by the forward and side scatter plots (Fig. 1A, 1E). SP cells were then immediately isolated from both tracheal and bronchial preparations by flow cytometry and cell sorting on the basis of Hoechst 33342 exclusion. In each experiment, cell viability remained 85%–90% following Hoechst 33342 and verapamil treatment, as shown by the histogram plots for propidium iodide staining (Fig. 1B, 1F). Density dot plot analysis confirmed the presence of a defined SP profile in both tracheal and bronchial cell preparations (Fig. 1C, 1G, region R1). The average percentage of SP cells isolated from normal donors was 0.12% ± 0.01%, irrespective of anatomical localization (n = 7). The specificity of Hoechst efflux by Bcrp-1 was confirmed by incubation of cells with the calcium channel inhibitor verapamil (Fig. 1D, 1H, region R1). As we observed no difference in the percentage of SP cells obtained from either the trachea or bronchus, cells were pooled for additional experiments.
Table 1.
Patient demographics and mean airway CD45− SP cell frequency
| Patient identification |
Sex | Age (years) | Disease | Medication | % of CD45− SP cells in total cell population |
|---|---|---|---|---|---|
| N1 | Male | 20 | None | None | 0.10 |
| N2 | Male | 21 | None | None | 0.15 |
| N3 | Female | 22 | None | None | 0.12 |
| N4 | Male | 18 | None | None | 0.11 |
| N5 | Male | 24 | None | None | 0.13 |
| N6 | Female | 4 | None | None | 0.11 |
| N7 | Male | 14 | None | None | 0.13 |
| A1 | Female | 8 | Fatal asthma | Albuterol, Singulair | 4.12 |
| A2 | Male | 11 | Mild asthma | Albuterol | 4.01 |
| A3 | Female | 21 | Mild asthma | Albuterol, Advair | 4.23 |
| A4 | Female | 15 | Fatal asthma | Albuterol, Advair | 3.84 |
Patients are identified by disease status, denoted as either N or A.
Abbreviations: A, asthmatic; N, none; SP, side population.
Figure 1.
Identification of side population (SP) cells in human airways. Isolated airway epithelial cells from the trachea and bronchus, as shown by FSC and SSC plots (A, E), were 90% viable by propidium iodide staining (B, F). Cells from both the trachea and bronchus were stained with 5 µg/ml Hoechst 33342 alone ([C, G], respectively) or in combination with 50 µg/ml verapamil ([D, H], respectively). Results indicate that a SP cells exist in both the trachea ([C], region R1) and bronchus ([G], region R1) and represent 0.12% ± 0.01% of the epithelial population (n = 7). Detection of SP cells was inhibited by the calcium channel blocker verapamil ([D, H], gate R1). The data shown are representative of all the normal airways analyzed in this study. Following incubation with Hoechst 33342, cells were stained and analyzed by fluorescence-activated cell sorting (FACS) for Bcrp-1 and pan-cytokeratin to confirm epithelial SP phenotype (I, J). Airway SP cells were further segregated using FACS by positive staining for CD45; 93% of airway SP cells were CD45− compared with 7% that were CD45+ (K). Abbreviations: Bcrp-1, breast cancer resistance protein; FSC, forward scatter; SSC, side scatter.
All SP cells stained positive for surface expression of Bcrp-1 and pan-cytokeratin, which is found only in the intracytoplasmic cytoskeleton of epithelial tissue, as shown in Figure 1I and 1J, compared with the isotype controls. Airway SP cells were also sorted for the presence of the hematopoietic stem cell marker CD45. The SP was heterogeneous for CD45 expression, containing on average 7% CD45-positive (CD45+) cells (Fig. 1K). Only CD45-negative (CD45−) SP cells were collected for further analysis, as our study was focused on epithelial resident SP cells, and CD45+ SP cells represented a minute fraction of the total SP.
Clonogenic Capacity of Airway SP Cells to Form Epithelial Colonies In Vitro
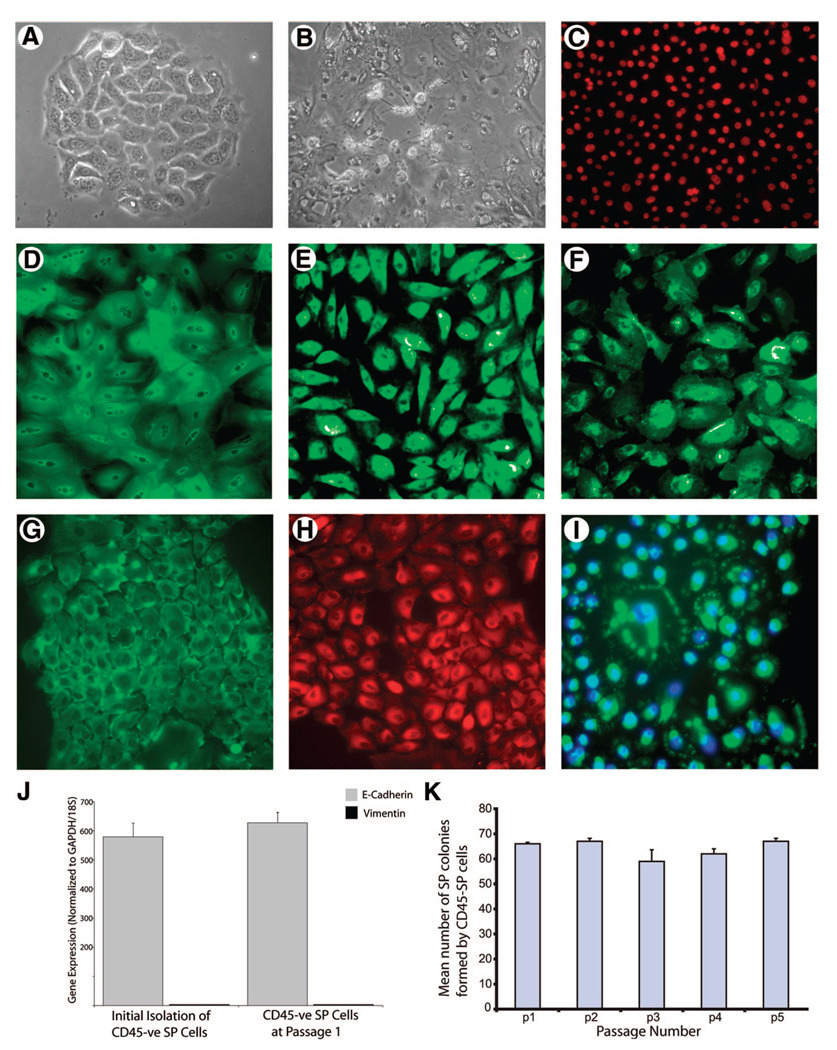
To determine the functional properties of airway SP cells, their clonogenic capacity was determined in vitro. To ensure cell purity, both CD45− SP and non-SP cells were re-sorted for Hoechst 33342 staining before plating. As shown in Table 2, 69.4% ± 2.7% of CD45− SP cells, seeded at any cell density, were able to form colonies, which were all cobblestone in appearance (Fig. 2A). In contrast, only 20% ± 0.2% of non-SP cells and 22% ± 1.2% of unsorted cells, seeded at 1,000 cells per well, were able to form colonies > 32 cells, but these were stellate in appearance and failed to divide further (Fig. 2B).
Table 2.
Number of colonies (mean ± SEM) formed by CD45− SP and non-SP cells in culture
| Cell seeding density |
CD45− SP cells |
Non-SP cells |
Unsorted cells |
|---|---|---|---|
| 1 | |||
| Cobblestone | 66.7 ± 1.2 | 0 | 0 |
| Stellate | 0 | 0 | 0 |
| 10 | |||
| Cobblestone | 69.1 ± 2.7 | 0 | 0 |
| Stellate | 0 | 0 | 0 |
| 100 | |||
| Cobblestone | 72.3 ± 4.2 | 0 | 0 |
| Stellate | 0 | 0 | 0 |
| 1,000 | |||
| Cobblestone | 69 ± 4.2 | 0 | 0 ± 0.2 |
| Stellate | 0 | 20 ± 0.2 | 22 ± 1.2 |
The average number of colonies formed by CD45− SP, non-SP, and unsorted cells was counted and characterized by appearance as either cobblestone or stellate. Values given are mean ± SEM.
Abbreviation: SP, side population.
Figure 2.
Clonogenic capacity and phenotypic characterization of airway SP cells. CD45− SP and non-SP colonies were characterized as either cobblestone (A) or stellate (B) in appearance. CD45− SP colonies were stained with antibodies for basal epithelial markers ΔNp63 (C), cytokeratin-5 (D), CD151 (E), tissue factor (F), E-cadherin (G), ZO-1 (H), and breast cancer resistance protein 1 (I). There was no expression of mesenchymal marker vimentin, as determined by quantitative polymerase chain reaction (J). (K): Colony-forming capacity of 1 × 102 CD45− SP cells over five passages. This staining pattern was consistent over 16 passages. Abbreviations: GAPDH, glyceraldehyde-3-phosphate dehydrogenase; p, passage; SP, side population; -ve, negative.
Immunofluorescent staining of CD45− SP cells at initial passage demonstrated expression of epithelial basal cell markers transcription factor ΔNp63, cytokeratin-5, CD151, and tissue factor (Fig. 2C–2F, respectively); epithelial adherens junction protein E-cadherin (Fig. 2G); and tight junctional protein ZO-1 (Fig. 2H), but were negative for the mesenchymal marker vimentin (data not shown), indicating that all cells were of epithelial phenotype. However, we also found no evidence for cytokeratin-18 or Muc5AC expression (data not shown). Following passage, 5% of cells retain Bcrp-1 positivity on the cell membrane in a pattern that may represent channels. In the remaining cells that stained positive for Bcrp-1, the immunofluorescence exhibited a distinct perinuclear staining pattern (Fig. 2I). The expression of E-cadherin (gray bars) and the lack of mesenchymal marker vimentin (black bars) were also confirmed by quantitative reverse transcription PCR (Fig. 2J). Furthermore, CD45− SP cells were able to maintain their colony-forming capacity over five passages (Fig. 2K), in contrast to non-SP cells, which were capable of limited propagation over 3–4 passages when seeded at 20,000 cells per well (data not shown).
Telomere Length Is Maintained in SP Cells over Subsequent Passages
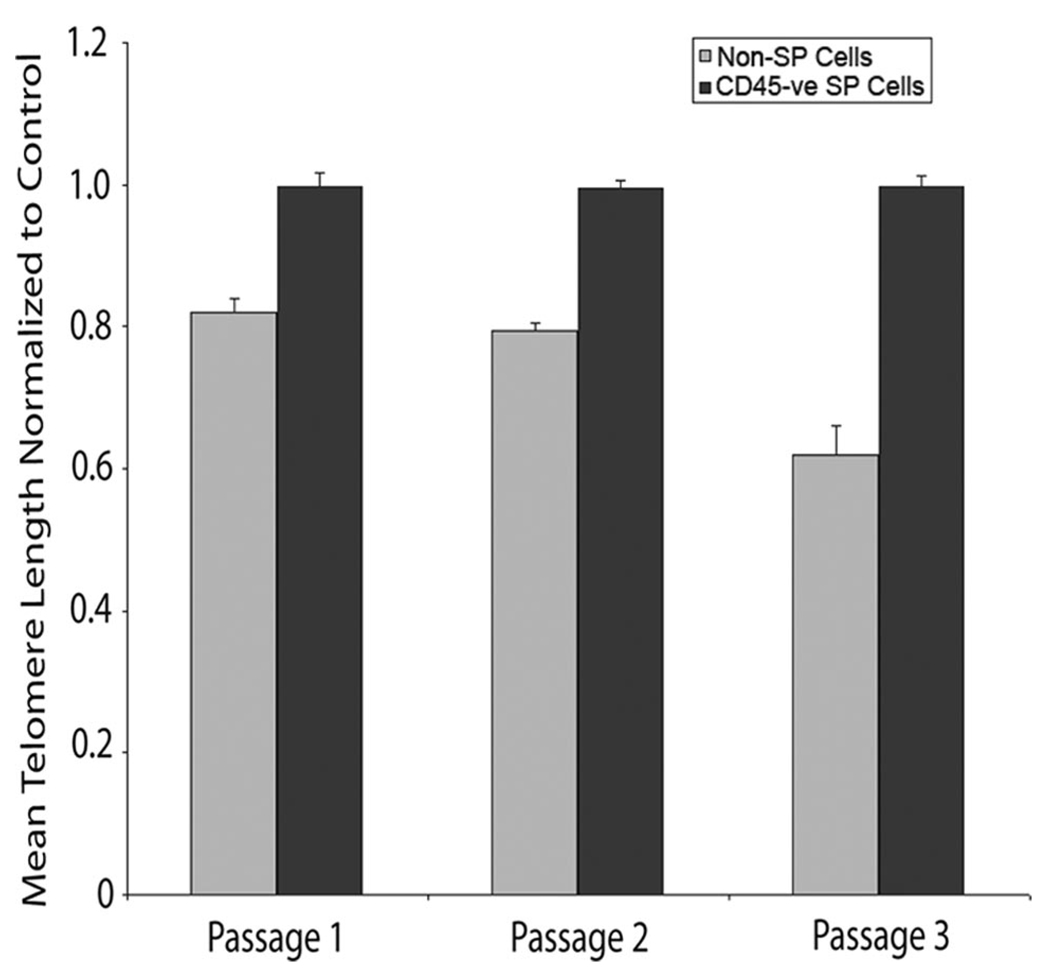
Multiple cell divisions in differentiated cells result in telomere shortening and dysfunction, which lead to DNA damage responses and ultimately irreversible cellular senescence [25, 26]. We analyzed telomere length in CD45− SP and non-SP cells over sequential passages. As shown in Figure 3, mean telomere length normalized to control in CD45− SP cells remained constant (0.997 ± 0.02) over three passages compared with non-SP cells, in which the mean telomere length shortened to 0.62 ± 0.04 by passage 3 (p < .05).
Figure 3.
Telomere length is maintained in SP cells. Telomere length normalized to control was analyzed in CD45− SP (black bars) and non-SP cells (gray bars) over three consecutive passages. Values are expressed as mean ± SEM, as results were done in triplicate (n = 3). Abbreviations: SP, side population; -ve, negative.
SP Cells Seeded at Low Densities Are Capable of Generating a Differentiated Epithelium in Air-Liquid Interface Cultures
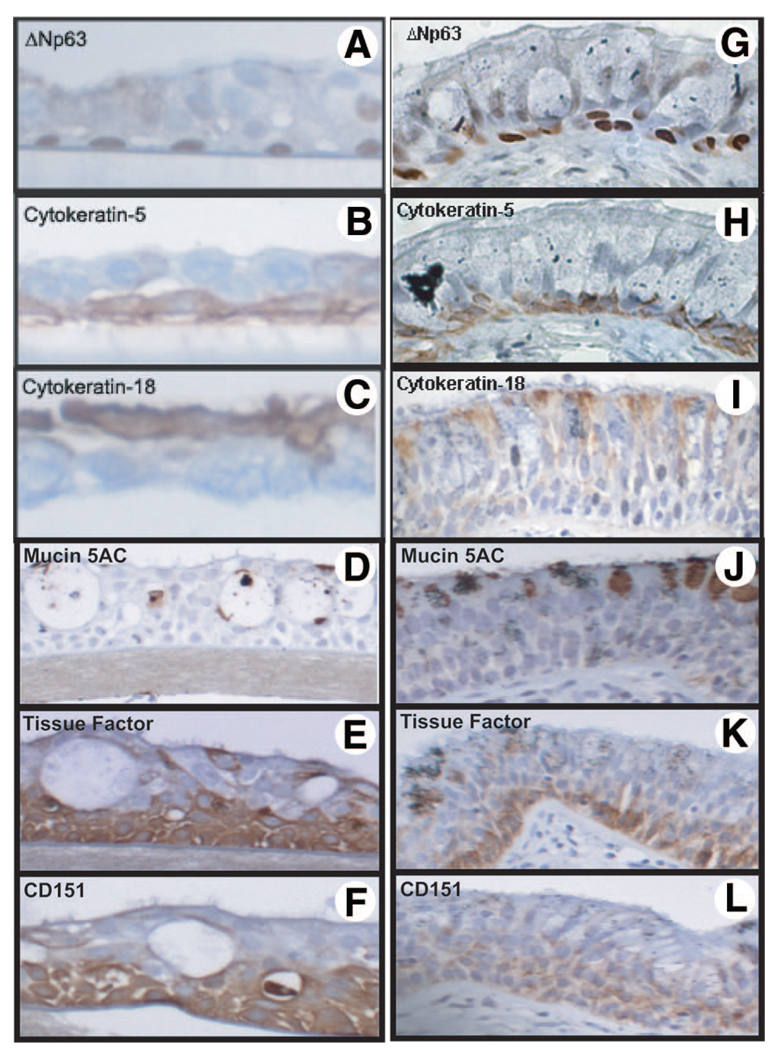
As CD45− SP cells show substantial colony formation capacity in monolayer culture, it was important to determine whether these cells could produce a multilayered differentiated epithelium. CD45− SP cells were capable of differentiating into a multilayered epithelium consisting of basal cells that stained positive for the epithelium-restricted transcription factor ΔNp63, which is important in epithelial proliferation and differentiation, and cytokeratin (CK)-5, as well as ciliated cells characterized by positive staining for CK-18 (Fig. 4A–4C, respectively). We also observed positive staining for muc5AC (Fig. 4D), as well as the basal cell markers tissue factor (Fig. 4E) and CD151 (Fig. 4F). The localization of ΔNp63, CK-5, and CK-18 observed within the ALI cultures was comparable to that seen in histological sections of the same donor airways that were embedded prior to epithelial cell isolation (Fig. 4G–4L).
Figure 4.
Capacity of CD45− side population (SP) cells to generate differentiated epithelium. CD45− SP cells seeded at 1 × 102 cells in transwell insert (0.45 µm pore, 12 mm diameter) were able to form a stratified epithelium in air-liquid-interface culture. Differentiation of CD45− SP cells was confirmed by immunostaining with antibodies for basal cell markers ΔNp63 (A), cytokeratin-5 (B), cytokeratin-18 (C), Muc5AC (D), tissue factor (E), and CD151 (F), and the staining was compared with patient matched airway sections (G–L).
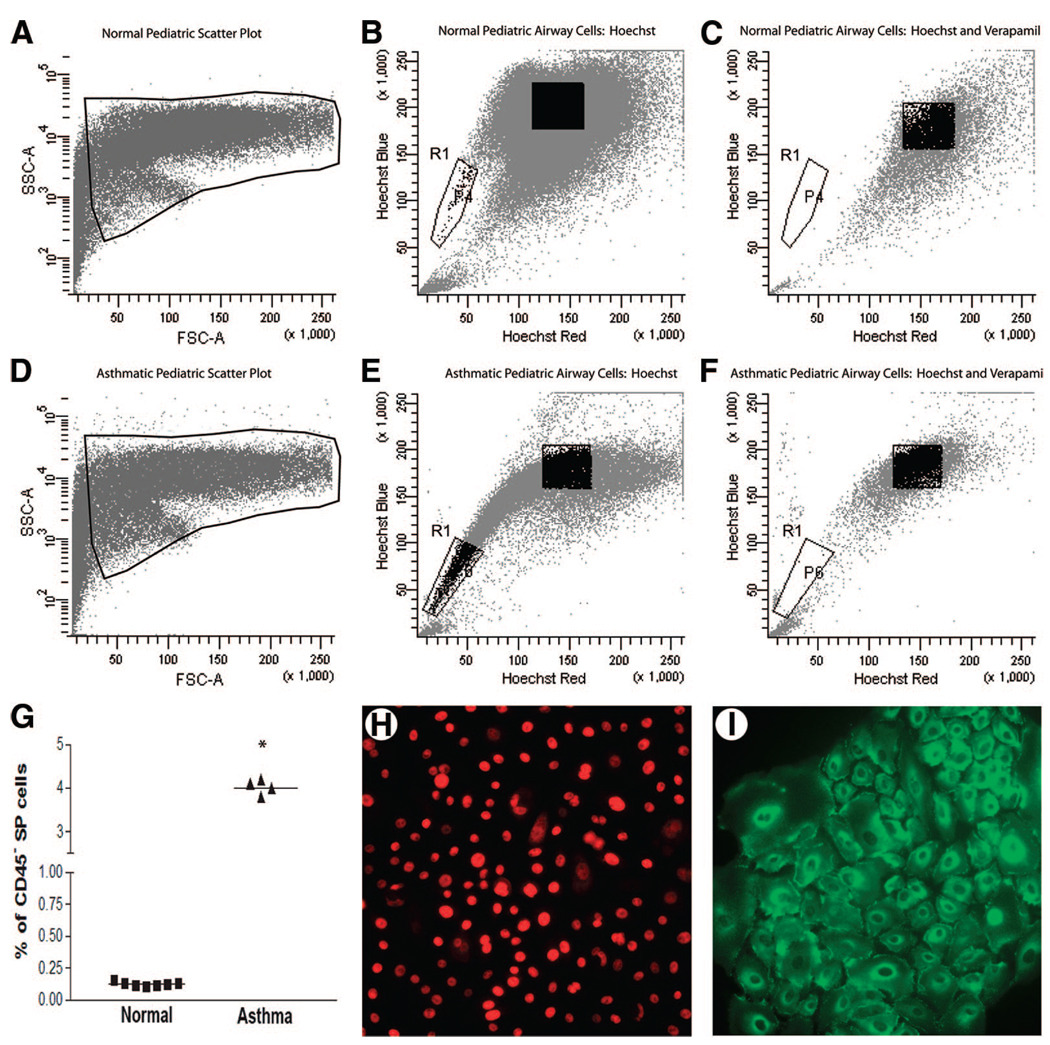
Asthmatic Epithelium Has Elevated Numbers of Airway SP Cells
In both pediatric and adult airways from normal donors, SP cells represented 0.12% ± 0.01% of the cell population (Fig. 5B, region R1). In contrast, we observed a 40-fold increase in the number of SP cells within the asthmatic airways (Fig. 5E, region R1). Although the asthmatic airways had a greater number of SP cells, Hoechst efflux was still inhibited by incubation with verapamil (Fig. 5C, 5F), and cell viability remained ≥90% during analysis. When the percentage of SP cells was plotted by disease for each patient, the mean number of SP cells within the asthmatic airway epithelium was 4.0% ± 0.08% (n = 4), compared with the normal airway mean of 0.12% ± 0.01% (n = 7) (Fig. 5G). Despite the substantial increase in overall SP number observed in asthmatic airways, the proportion of CD45+ SP cells remained at approximately 7% (data not shown). The epithelial markers demonstrated in Figure 2 were also found to be expressed in asthmatic CD45− SP cell colonies, and representative images for ΔNp63 and E-cadherin are shown in Figure 5H and 5I. In addition, the mean colony-forming capability of CD45− SP cells was 63.7% ± 1.2%, which was not significantly different from that of CD45− SP cells from healthy normal subjects.
Figure 5.
Identification of lung SPs in asthmatic airways. Representative fluorescence-activated cell sorting FSC and SSC plots of airway epithelial SP cells in normal and asthmatic pediatric patients ([A, D], region R1) demonstrate a distinct SP that represented 4.01% of the epithelial population in asthmatic pediatric airways ([B], region R1) and 0.11% in normal pediatric airways ([E], region R1). Detection of SP cells was inhibited in the presence of 50 µg/ml verapamil ([C, F], region R1). (G): Mean percentages of SP cells for all normal (■) and asthmatic (▲) individuals in the study; * indicates p < .05. (H, I): Representative images demonstrating ΔNp63 and E-cadherin staining in asthmatic CD45− SP colonies. Abbreviations: FSC, forward scatter; SP, side population; SSC, side scatter.
DISCUSSION
The results of this study demonstrate for the first time the existence of resident SP cells within human airway epithelium. We report that SP cells in normal airways represent approximately 0.1% of the total epithelial cell population. We found that 93% of the SP cells were CD45−, had sustained colony-forming capacity, maintained telomere length over serial passage, and, importantly, were able to form a differentiated, multilayered epithelium, confirming their stem cell capacity. In addition, we report that asthmatic airway epithelium contains 40-fold more SP cells compared with normal airways. However, despite the increase in SP numbers, functional differences between SP cells isolated from asthmatic and nonasthmatic subjects were not observed.
The epithelium is constantly exposed to a vast array of environmental stimuli, including airborne allergens, pathogens, and potential toxic agents and, as such, must respond effectively to cellular damage and the resultant inflammatory cytokine response to restore adequate barrier function. One approach to further our understanding of the repair process is to characterize the resident stem/progenitor cells that contribute to epithelial repair. We isolated epithelial cells from human trachea and fourth-generation bronchi and found that the number of SP cells, characterized by verapamil-sensitive efflux of Hoechst 33342 and Bcrp-1 expression, represents less than 0.12% of the total population, irrespective of anatomical localization. That SP cells represent a small fraction of epithelial cells is consistent with data from other organs, including breast, lung, and skeletal tissue [14, 20, 27]. In addition to Bcrp-1, several other ABC transporter proteins have been identified on SP cells. For example, Loebinger et al. recently showed, by quantitative PCR, that carcinomas express ABCG2 (Bcrp-1) and ABCC1 (MRP1) transporters [28]. However, Summer et al. showed that Bcrp-1-null mice do not have lung SP cells [18], and Scharenberg et al. compared mRNA levels for MDR1, MRP1, and Bcrp-1 in bone marrow SP cells and showed that Bcrp-1 is the predominant form in these cells [29]. For these reasons, we focused on Bcrp-1 in this study.
SP cells were further sorted into hematopoietic and nonhematopoietic lineage according to CD45 expression, which is uniformly found on bone marrow [30] SP cells. We found that the overwhelming majority (93%) of SP cells were CD45−. These data are in keeping with studies in murine tissues, such as muscle [27], kidney [30], and lung [19], where 84%–96% of the SP is CD45−, whereas in tissues such as heart [31, 32], testis [33], and brain [34] CD45+ SP cells have not been identified. In contrast, Summer et al. [18] reported that up to 60%–70% of SP cells within mouse lung were CD45+. The reasons underlying this discrepancy are unclear but likely relate to cell extraction and sampling methodology and the use of whole minced and enzyme-digested lung versus isolation from a specific tissue compartment. It has been proposed that, in skeletal muscle at least, CD45+ SP cells represent a circulating, transiently resident hematopoietic stem cell population distinct from tissue-specific SP, since only CD45+ SP cells were capable of hematopoietic differentiation [27]. As epithelial resident stem/progenitor cells are likely to be CD45− and this population represents more than 90% of the airway epithelial SP, we focused our attention on these cells.
CD45− SP cells were found to be highly clonogenic in colony formation assays over both short- and long-term passage. Importantly, a single CD45− SP cell was able to form colonies of > 32 cells following 12 days in culture without the addition of extracellular matrix or a feeder layer of cells. However, non-SP cells were able to form viable epithelial colonies only when seeded at densities > 5,000 cells per cm2. These findings are consistent with previous studies in murine lungs and skin, which reported lung SP cells to have high clonogenic potential in xenografts and in vitro cell culture [18, 20]. Both tracheal and bronchial CD45− SP cells formed cobblestone colonies characteristic of cultured epithelial cells. An epithelial phenotype of CD45− SP cells was further confirmed by positive immunohistochemical staining for the epithelial markers pan-cytokeratin, ZO-1, and E-cadherin. However, these cells did not stain for the mesenchymal marker vimentin or express vimentin mRNA. In contrast, studies using murine SP cells isolated from both trachea and lung have reported vimentin expression and a stellate appearance of tracheal SP cells [20]. These contrasting findings could be due to differences in culturing conditions. Reynolds et al. [20] used FBS in their media for both tracheal (10%) and lung (2%) SP cell cultures, whereas in our study, serum-free media was used to propagate SP cultures.
On further investigation, CD45− SP colonies expressed the epithelial-restricted protein CK-5 and the transcription factor p63. Previous studies using human epithelium have demonstrated that CK-5-expressing epithelial cells have progenitor properties [9, 35]. p63, which has been previously demonstrated to play a critical role in the normal morphogenesis and differentiation of the tracheobronchial epithelia, is thought to be important for the differentiation of early stem cells into the basal cell population. Indeed, studies in murine mammary gland [36], rat limbal [37], and rabbit limbocorneal [38] cells have shown p63 to be expressed in SP cells. p63 belongs to the p53 family of transcription factors, and the gene encodes two major isoforms, TAp63, which is transcribed from the 5′-promoter, and ΔNp63, which lacks the N-terminal transcription-activating domain and is transcribed from the intronic internal promoter. Each isoform has been shown to have separate functions and form complicated networks in different systems [39, 40]. We observed that the most abundant isoform expressed in CD45− SP cells was ΔNp63, which is in keeping with other epithelial tissues [41], and we did not observe expression of the transiently amplifying (TA) isoform. It is temping to conclude that p63 is important in maintaining the resident airway CD45− SP epithelial population. Attempts at staining airway sections to investigate SP cell localization did not allow specific discrimination of SP cells since CK-5+/p63+ cells are expressed throughout the entire basal airway epithelial compartment and this represents approximately 25% of the total airway epithelium. Similarly, Bcrp-1 expression is not confined to SP cells. Further work is required to determine to the relationship between p63 and stem cell function in SP cells and to characterize specific markers that could be used to determine the exact anatomical localization of resident SP cells within the airways.
The lineage commitment decisions of SP cells have obvious implications for the composition of the airway epithelium, and it is presently unclear whether human SP cells function as progenitors for glandular and/or airway epithelium. To begin to address this, we have determined the capacity of SP cells to form a multilayered differentiated epithelium in ALI culture. We show that < 100 CD45− SP cells were able to proliferate and differentiate into a multilayer differentiated ALI culture containing both basal, ciliated cells and mucus-secreting cells. In contrast, as many as 5 × 105 non-SP cells were required to form a differentiated epithelium in culture. Several previous studies have shown, on the basis of expression of CD151 and tissue factor, that TA cells or putative progenitor cells have the capacity to generate a differentiated epithelium in vitro and xenograft models in vivo [8, 42, 43]. The contribution of SP cells to this process was not reported. Because of limited material, we did not examine the expression of CD151 or tissue factor on SP cells. However, given that SP, CD151+, TFF+, and p63+ cells are localized to the basal cell compartment, it is likely that SP cells represent the true progenitor cell within this mixed population. Interestingly, dermal SP cells have been shown to form a pluristratified epidermis when added to an epithelium-denuded rat tracheal xenograft model, suggesting that resident SP cells assume tissue specificity and require the appropriate environment for correct tissue regenerative function.
In the stem cell compartment of several tissues, heightened telomerase activity has been demonstrated [44]. In particular, telomerase activity has been shown to be concentrated in epithelial cells near the basal lamina during human fetal lung gestation [45], as well as in human adult bronchial basal cells [46]. We found that airway epithelial CD45 −SP cells were able to maintain telomere length over passage compared with non-SP cells. This finding is consistent with studies investigating mesenchymal stem cells aging in vitro [47]. Given that telomerase activity is detected in the basal cells of adult nasal epithelium, which retain transit-amplifying cell properties [42], our data provide further evidence that SP cells are enriched for progenitor activity.
Studies focused on the airway epithelium in asthma have previously reported markers of damage and dysregulated repair even in the early stages of the disease in childhood. As such, it was of importance to determine whether SP cell number or function was altered in asthmatic airways. To begin to address these questions, we obtained the lungs of four asthmatic subjects. Our findings show that asthmatic airways contained 40-fold more CD45− SP cells than normal airways. The increase in SP cells within the asthmatic airways did not appear to be an age-related factor since nondiseased pediatric airways had the same frequency of SP cells compared with those of normal adults. However, despite the substantial increase in numbers, we were unable to detect any differences in the function of SP cells isolated from asthmatic airways. Until recently airway remodeling in the development of asthma was considered a secondary phenomenon due to persistent inflammation. However, tissue remodeling, especially within the epithelium, has now been convincingly shown to be an early and consistent component of childhood asthma [3]. As this was a prospective study we were unable to address the underlying cause of the increased numbers of CD45− SP cells in asthmatic epithelium. However, it is an important clinical finding that supports the hypothesis that early pathological changes related to disturbances in the local epithelial environment due to stress and or injury occur within the airways of asthmatics. As such, further understanding of epithelial- resident progenitor cells involved in normal regeneration, as well as disease, is essential and is the focus of long-term future studies.
CONCLUSION
In conclusion, we demonstrate for the first time the presence of resident CD45− SP cells within the human tracheobronchial epithelium. CD45− SP cells exhibited epithelial cell markers cytokeratin-5, E-cadherin, ZO-1, and p63. These cells show robust colony-forming and clonogenic capacity and well-maintained telomere length, all of which are indicative of stem/progenitor cell function. Importantly, these cells give rise to a multilayered differentiated epithelium in ALI culture. Our preliminary data suggest that SP cells are overexpressed in asthmatic epithelium, which may indicate an abnormality in the wound repair process that could contribute to the pathogenesis of the disease. Further work is required to determine the role of resident SP cells in epithelial repair in vivo and how this may be altered by disease.
ACKNOWLEDGMENTS
This work was supported by Allergen-National Centres of Excellence/Canadian Institute of Health Research (CIHR) 79632; National Health Medical Research Council Grant 303145; NIH Grants HL-071795, HL-54659, and RR-023424; and an unrestricted educational grant from Johnson & Johnson Corporate Office of Science and Technology. T.-L.H. is a recipient of a Canadian lung association/CIHR Trainee Fellowship. The authors would like to thank Yuexin Li for her guidance with the telomere assay.
Footnotes
Author contributions: T.-L.H.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; F.S., A.J., and S.W.: collection and/or assembly of data, data analysis and interpretation; D.V.P.: conception and design, data analysis and interpretation; D.B.J.: provision of study material or patients; A.K. and S.M.S.: provision of study material or patients, data analysis and interpretation; D.A.K.: conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
The authors indicate no potential conflicts of interest.
REFERENCES
- 1.Knight DA, Holgate ST. The airway epithelium: Structural and functional properties in health and disease. Respirology. 2003;8:432–446. doi: 10.1046/j.1440-1843.2003.00493.x. [DOI] [PubMed] [Google Scholar]
- 2.Hackett TL, Knight DA. The role of epithelial injury and repair in the origins of asthma. Curr Opin Allergy Clin Immunol. 2007;7:63–68. doi: 10.1097/ACI.0b013e328013d61b. [DOI] [PubMed] [Google Scholar]
- 3.Barbato A, Turato G, Baraldo S, et al. Epithelial damage and angiogenesis in the airways of children with asthma. Am J Respir Crit Care Med. 2006;174:975–981. doi: 10.1164/rccm.200602-189OC. [DOI] [PubMed] [Google Scholar]
- 4.Alonso L, Fuchs E. Stem cells of the skin epithelium. Proc Natl Acad Sci U S A. 2003;100 suppl 1:11830–11835. doi: 10.1073/pnas.1734203100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lipkin M. Growth and development of gastrointestinal cells. Annu Rev Physiol. 1985;47:175–197. doi: 10.1146/annurev.ph.47.030185.001135. [DOI] [PubMed] [Google Scholar]
- 6.Blenkinsopp WK. Proliferation of respiratory tract epithelium in the rat. Exp Cell Res. 1967;46:144–154. doi: 10.1016/0014-4827(67)90416-8. [DOI] [PubMed] [Google Scholar]
- 7.Zepeda ML, Chinoy MR, Wilson JM. Characterization of stem cells in human airway capable of reconstituting a fully differentiated bronchial epithelium. Somat Cell Mol Genet. 1995;21:61–73. doi: 10.1007/BF02255823. [DOI] [PubMed] [Google Scholar]
- 8.Engelhardt JF, Schlossberg H, Yankaskas JR, et al. Progenitor cells of the adult human airway involved in submucosal gland development. Development. 1995;121:2031–2046. doi: 10.1242/dev.121.7.2031. [DOI] [PubMed] [Google Scholar]
- 9.Boers JE, Ambergen AW, Thunnissen FB. Number and proliferation of basal and parabasal cells in normal human airway epithelium. Am J Respir Crit Care Med. 1998;157:2000–2006. doi: 10.1164/ajrccm.157.6.9707011. [DOI] [PubMed] [Google Scholar]
- 10.Borthwick DW, Shahbazian M, Krantz QT, et al. Evidence for stem-cell niches in the tracheal epithelium. Am J Respir Cell Mol Biol. 2001;24:662–670. doi: 10.1165/ajrcmb.24.6.4217. [DOI] [PubMed] [Google Scholar]
- 11.Giangreco A, Reynolds SD, Stripp BR. Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction. Am J Pathol. 2002;161:173–182. doi: 10.1016/S0002-9440(10)64169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim CF, Jackson EL, Woolfenden AE, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 13.Goodell MA, Brose K, Paradis G, et al. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alvi AJ, Clayton H, Joshi C, et al. Functional and molecular characterisation of mammary side population cells. Breast Cancer Res. 2003;5:R1–R8. doi: 10.1186/bcr547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smalley MJ, Clarke RB. The mammary gland “side population”: A putative stem/progenitor cell marker? J Mammary Gland Biol Neoplasia. 2005;10:37–47. doi: 10.1007/s10911-005-2539-0. [DOI] [PubMed] [Google Scholar]
- 16.Budak MT, Alpdogan OS, Zhou M, et al. Ocular surface epithelia contain ABCG2-dependent side population cells exhibiting features associated with stem cells. J Cell Sci. 2005;118:1715–1724. doi: 10.1242/jcs.02279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larderet G, Fortunel NO, Vaigot P, et al. Human side population keratinocytes exhibit long-term proliferative potential and a specific gene expression profile and can form a pluristratified epidermis. STEM CELLS. 2006;24:965–974. doi: 10.1634/stemcells.2005-0196. [DOI] [PubMed] [Google Scholar]
- 18.Summer R, Kotton DN, Sun X, et al. Side population cells and Bcrp1 expression in lung. Am J Physiol Lung Cell Mol Physiol. 2003;285:L97–L104. doi: 10.1152/ajplung.00009.2003. [DOI] [PubMed] [Google Scholar]
- 19.Giangreco A, Shen H, Reynolds SD, et al. Molecular phenotype of airway side population cells. Am J Physiol Lung Cell Mol Physiol. 2004;286:L624–L630. doi: 10.1152/ajplung.00149.2003. [DOI] [PubMed] [Google Scholar]
- 20.Reynolds SD, Shen H, Reynolds PR, et al. Molecular and functional properties of lung SP cells. Am J Physiol Lung Cell Mol Physiol. 2007;292:L972–L983. doi: 10.1152/ajplung.00090.2006. [DOI] [PubMed] [Google Scholar]
- 21.Karp PH, Moninger TO, Weber SP, et al. An in vitro model of differentiated human airway epithelia. Methods for establishing primary cultures. Methods Mol Biol. 2002;188:115–137. doi: 10.1385/1-59259-185-X:115. [DOI] [PubMed] [Google Scholar]
- 22.Goodell MA, McKinney-Freeman S, Camargo FD. Isolation and characterization of side population cells. Methods Mol Biol. 2005;290:343–352. doi: 10.1385/1-59259-838-2:343. [DOI] [PubMed] [Google Scholar]
- 23.Jones PH, Watt FM. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell. 1993;73:713–724. doi: 10.1016/0092-8674(93)90251-k. [DOI] [PubMed] [Google Scholar]
- 24.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:1–6. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bodnar AG, Ouellette M, Frolkis M, et al. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 26.d’Adda di Fagagna F, Reaper PM, Clay-Farrace L, et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- 27.Asakura A, Seale P, Girgis-Gabardo A, et al. Myogenic specification of side population cells in skeletal muscle. J Cell Biol. 2002;159:123–134. doi: 10.1083/jcb.200202092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loebinger MR, Giangreco A, Groot KR, et al. Squamous cell cancers contain a side population of stem-like cells that are made chemosensitive by ABC transporter blockade. Br J Cancer. 2008;98:380–387. doi: 10.1038/sj.bjc.6604185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scharenberg CW, Harkey MA, Torok-Storb B. The ABCG2 transporter is an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood. 2002;99:507–512. doi: 10.1182/blood.v99.2.507. [DOI] [PubMed] [Google Scholar]
- 30.Challen GA, Bertoncello I, Deane JA, et al. Kidney side population reveals multilineage potential and renal functional capacity but also cellular heterogeneity. J Am Soc Nephrol. 2006;17:1896–1912. doi: 10.1681/ASN.2005111228. [DOI] [PubMed] [Google Scholar]
- 31.Hierlihy AM, Seale P, Lobe CG, et al. The post-natal heart contains a myocardial stem cell population. FEBS Lett. 2002;530:239–243. doi: 10.1016/s0014-5793(02)03477-4. [DOI] [PubMed] [Google Scholar]
- 32.Oh H, Chi X, Bradfute SB, et al. Cardiac muscle plasticity in adult and embryo by heart-derived progenitor cells. Ann N Y Acad Sci. 2004;1015:182–189. doi: 10.1196/annals.1302.015. [DOI] [PubMed] [Google Scholar]
- 33.Falciatori I, Borsellino G, Haliassos N, et al. Identification and enrichment of spermatogonial stem cells displaying side-population phenotype in immature mouse testis. Faseb J. 2004;18:376–378. doi: 10.1096/fj.03-0744fje. [DOI] [PubMed] [Google Scholar]
- 34.Murayama A, Matsuzaki Y, Kawaguchi A, et al. Flow cytometric analysis of neural stem cells in the developing and adult mouse brain. J Neurosci Res. 2002;69:837–847. doi: 10.1002/jnr.10339. [DOI] [PubMed] [Google Scholar]
- 35.Boecker W, Buerger H. Evidence of progenitor cells of glandular and myoepithelial cell lineages in the human adult female breast epithelium: A new progenitor (adult stem) cell concept. Cell Prolif. 2003;36 suppl 1:73–84. doi: 10.1046/j.1365-2184.36.s.1.7.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Behbod F, Xian W, Shaw CA, et al. Transcriptional profiling of mammary gland side population cells. STEM CELLS. 2006;24:1065–1074. doi: 10.1634/stemcells.2005-0375. [DOI] [PubMed] [Google Scholar]
- 37.Umemoto T, Yamato M, Nishida K, et al. Rat limbal epithelial side population cells exhibit a distinct expression of stem cell markers that are lacking in side population cells from the central cornea. FEBS Lett. 2005;579:6569–6574. doi: 10.1016/j.febslet.2005.10.047. [DOI] [PubMed] [Google Scholar]
- 38.Epstein SP, Wolosin JM, Asbell PA. P63 expression levels in side population and low light scattering ocular surface epithelial cells. Trans Am Ophthalmol Soc. 2005;103:187–199. discussion 199. [PMC free article] [PubMed] [Google Scholar]
- 39.Perez CA, Pietenpol JA. Transcriptional programs regulated by p63 in normal epithelium and tumors. Cell Cycle. 2007;6:246–254. doi: 10.4161/cc.6.3.3801. [DOI] [PubMed] [Google Scholar]
- 40.Senoo M, Pinto F, Crum CP, et al. p63 Is essential for the proliferative potential of stem cells in stratified epithelia. Cell. 2007;129:523–536. doi: 10.1016/j.cell.2007.02.045. [DOI] [PubMed] [Google Scholar]
- 41.Lee HO, Lee JH, Kim TY, et al. Regulation of DeltaNp63alpha by tumor necrosis factor-alpha in epithelial homeostasis. FEBS J. 2007;274:6511–6522. doi: 10.1111/j.1742-4658.2007.06168.x. [DOI] [PubMed] [Google Scholar]
- 42.Hajj R, Lesimple P, Nawrocki B-Raby, et al. Human airway surface epithelial regeneration is delayed and abnormal in cystic fibrosis. J Pathol. 2007;211:340–350. doi: 10.1002/path.2118. [DOI] [PubMed] [Google Scholar]
- 43.Ford JR, Terzaghi-Howe M. Basal cells are the progenitors of primary tracheal epithelial cell cultures. Exp Cell Res. 1992;198:69–77. doi: 10.1016/0014-4827(92)90150-7. [DOI] [PubMed] [Google Scholar]
- 44.Chan SW, Blackburn EH. New ways not to make ends meet: Telomerase, DNA damage proteins and heterochromatin. Oncogene. 2002;21:553–563. doi: 10.1038/sj.onc.1205082. [DOI] [PubMed] [Google Scholar]
- 45.Ulaner GA, Giudice LC. Developmental regulation of telomerase activity in human fetal tissues during gestation. Mol Hum Reprod. 1997;3:769–773. doi: 10.1093/molehr/3.9.769. [DOI] [PubMed] [Google Scholar]
- 46.Yashima K, Litzky LA, Kaiser L, et al. Telomerase expression in respiratory epithelium during the multistage pathogenesis of lung carcinomas. Cancer Res. 1997;57:2373–2377. [PubMed] [Google Scholar]
- 47.Bonab MM, Alimoghaddam K, Talebian F, et al. Aging of mesenchymal stem cell in vitro. BMC Cell Biol. 2006;7:14. doi: 10.1186/1471-2121-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]