Summary
Multiple subtypes of protein kinase C (PKC) isozymes are implicated in various neurological disorders including alcohol insensitivity, a trait strongly associated with alcoholism in humans, but molecular and cellular mechanisms underlying the PKC activities remain poorly understood. Here we show that functional knockdown of conventional, novel or atypical PKC in the fly nervous system each resulted in alcohol insensitivity. Neuroanatomical mapping of conventional Ca2+-sensitive PKC53E activity uncovers a previously uncharacterized role of Drosophila serotonin neurons in alcohol sensitivity. The deficiency of PKC53E but not novel Ca2+-independent PKC98E appears to reduce synaptic serotonin levels, since acute inhibition of serotonin reuptake by citalopram and Prozac reversed alcohol insensitivity in flies expressing PKC53E double-stranded RNA in serotonin neurons. Together, findings from this and our previous studies indicate that PKC53E and PKC98E differentially regulate fly alcohol sensitivity through independent modulation of conserved serotonin and neuropeptide Y-like systems.
Introduction
Insensitivity to alcohol intoxication in adolescents is a strong predictor of human alcoholism. However, our understanding of underlying genetic and neural mechanisms remains rudimentary. Drosophila, like mammals, display stereotyped behavioral responses to alcohol intoxication including hyperexcitation, uncoordination and sedation. In addition, a growing number of conserved genes and molecular pathways have been identified that mediate alcohol-related behaviors in flies and rodents. For example, it has been shown that neuropeptide Y (NPY) family peptides are critical for alcohol sensitivity. Overexpression of NPY or its fly counterpart, neuropeptide F (NPF), causes hypersensitive responses to ethanol sedation in both flies and mice (Thiele et al., 1998, Wen et al., 2005). Conversely, deficiencies in NPY/NPF signaling leads to decreased ethanol sensitivity. More recently, genetic analysis of the epidermal growth factor signaling pathway in Drosophila has lead to the discovery of its novel role in regulation of alcohol sensitivity in both insects and mammals (Corl et al., 2009). These findings provide compelling evidence that Drosophila is a powerful model for investigating mechanisms underlying alcohol use disorders.
The conserved PKC family comprises three subtypes of isozymes: conventional Ca2+/DAG-responsive PKC, novel Ca2+-independent/DAG-responsive PKC and atypical PKC. These kinases differentially mediate diverse intracellular signaling processes across evolution, and have been implicated in multiple neurological disorders including acute alcohol sensitivity (Harris et al., 1995, Hodge et al., 1999, Newton and Ron, 2007, Choi et al., 2008). Ethanol has been shown to rapidly induce PKC activity in the specific regions of the rodent brain (Deitrich et al., 1989, Mahadev and Vemuri, 1998). The demonstration of ethanol-binding sites in the regulatory domains of several conventional and novel PKCs has raised the possibility that PKC may serve as the molecular link between ethanol sensing and cellular responses (Slater et al., 1997, Das et al., 2006).
In the mouse model, genetic knockout of three conventional or novel isozymes has resulted in altered behavioral responses to alcohol intoxication (Harris et al., 1995, Hodge et al., 1999, Choi et al., 2008), but the underlying mechanisms remain unknown. The pleiotropic effects of PKC on diverse cellular processes in various tissues have also made it difficult to interpret the complex and, in some cases, opposing phenotypes of such knockout mice. Therefore, identification of physiologically relevant cellular targets of PKC responsible for observed alcohol phenotypes remains both challenging and critically important for advancing our understanding of alcohol-related behaviors.
In this study, we use the genetically tractable Drosophila model to investigate the potentially conserved roles of neural PKC isozymes and their cellular targets in acute alcohol response. We show that the neural activities of all three PKC subtypes are required for fly alcohol sensitivity. Functional mapping of conventional PKC53E activity has led to the discovery of a previously uncharacterized role of the Drosophila serotonin system in alcohol sensitivity. We also provide evidence that PKC53E deficiency-induced alcohol insensitivity is likely due to the reduction of synaptic serotonin levels, which can be reversed pharmacologically using selective serotonin reuptake inhibitors (SSRIs). Finally, findings from this and our previous work demonstrate that acute alcohol sensitivity in Drosophila requires a second evolutionarily conserved neurotransmitter system in addition to the NPY-like system, each respectively modulated by conventional PKC53E and novel PKC98E.
Experimental Procedures
Flies
Synchronized fly eggs were collected onto apple juice agar plates with yeast paste. Larvae and adults were reared on the same food at room temperature with exposure to natural lighting. Adult females, synchronized by collecting flies enclosed within a 12-h period, were aged for 7 days. All fly lines are in the w1118 genetic background. The Ddc-GAL4 line drives expression in both serotonergic and dopaminergic neurons (Li et al., 2000), while the TH-GAL4 line drives expression only in dopaminergic neurons (Friggi-Grelin et al., 2003). The UAS-nsyb-GFP encodes GFP fused with neuronal synaptobrevin (Vosshall et al., 2000). The UAS-PKCi encodes a pseudo-substrate fragment inhibitory to PKC isozymes (Broughton et al., 1996), 1996). The UAS-PKC53EdsRNA, UAS-PKC98EdsRNA and UAS-DaPKCdsRNA encode double strand RNA sequences of respective PKC isozymes (Dietzl et al., 2007). The UAS-shits1 encodes a semi-dominant-negative form of dynamin (Kitamoto, 2002).
Behavioral assay
The procedures for behavioral assay were described previously (Chen et al., 2008). Unless indicated otherwise, 1ml of 45% ethanol solution was applied to each behavioral test. After exposing 20 female flies to ethanol vapor, the number of uncoordinated or anesthetized flies was recorded at specified time intervals. At least 3 independent trials were performed per experiment. Data were analyzed by using PROC PHREG in statistical software SAS 9.1 (SAS Institute, Cary, NC).
Pharmacological treatment of flies
Prozac (fluoxetine hydrochloride), citalopram (citalopram hydrobromide), 5-HTP (5-hydroxy-L-tryptophan), and α-MTP (α-methyl-DL-tryptophan) were purchased from Sigma (St. Louis, MO). Drugs were mixed with autoclave-killed yeast paste food. The concentration of Prozac or citalopram is 0.1% (w/w) in food. The concentration of 5-HTP or α-MTP was ca. 50 mM (1%, w/w) in food. Flies were fed with drugs for 72 hours prior to behavioral assays.
Quantitative RT-PCR (qRT-PCR)
The procedures of RNA extraction, first-strand cDNA synthesis, and qRT-PCR were described previously (Chen et al., 2008). The quantification of PKC53E, PKC98E, DaPKC and gapdh2 transcripts in fly heads was done by using TaqMan primer Dm01816273_m1, Dm02151736_g1, Dm01843305_g1 and Dm0184377_s1, respectively (Applied Biosystems Inc, Foster City, CA). The relative RNA quantification was normalized against gapdh2. Significance was analyzed by using PROC GLM with post hoc Tukey's test.
Ethanol content assay
The assays were performed as previously described (Moore et al., 1998, Wen et al., 2005). The ethanol assay kit was from Genzyme Diagnostics P.E.I. Inc (Charlottetown, Canada). The protein assay kit was from Thermo Scientific (Rockford, IL).
Results
Roles of conventional, novel and atypical PKCs in alcohol sensitivity
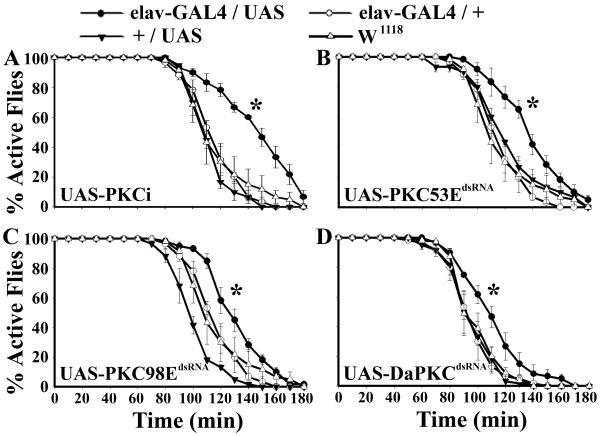
To investigate the regulatory roles of PKC isozymes in fly ethanol sensitivity, we attempted to knock down the activity of Ca2+-sensitive PKC53E, Ca2+-independent PKC98E and atypical DaPKC in the nervous system. A pan neural elav-GAL4 driver was used to direct expression of UAS-PKCi, which encodes a pseudo-substrate peptide that competitively inhibits different subtypes of PKC (Broughton et al., 1996), and the double-stranded RNA (dsRNA) of PKC53E, PKC98E and DaPKC (Figure 1). The assay involves exposing 20 adult flies to an increasing concentration of ethanol vapor in a sealed bottle and scoring for ethanol-induced uncoordination and sedation. We found that flies deficient in multiple (elav-GAL4/UAS-PKCi) or a single PKC activity (e.g., elav-GAL4/UAS-PKC53EdsRNA) were significantly more resistant to acute ethanol intoxication than the paired controls (e.g. UAS-PKCi alone). We also found that each dsRNA construct reduced the transcripts level of the corresponding PKC isozyme ((Chen et al., 2008); Figure S1). These results suggest that the neural activity of each of the three isozymes is required for ethanol sensitivity.
Figure 1. Downregulation of Neural PKC Isozymes Attenuates Ethanol Sensitivity.
A) Inhibition of multiple PKC isozymes by expressing a pseudo-substrate peptide PKCi in the nervous system. B-D) RNA interference mediated knockdown of PKC53E, PKC98E and DaPKC, respectively. The asterisks indicate the data set that is statistically different from controls (PKCi: χ21,4 = 36.53, P < 0.0001; PKC53EdsRNA: χ21,4 = 21.04, P < 0.0001; PKC98EdsRNA: χ21,4 = 11.32, P < 0.001; DaPKCdsRNA: χ21,4 = 12.00, P < 0.001). Cox's regression model-based Wald test was used for the analysis of anesthetization data in this and other figures. The selectivity of each RNAi line against the corresponding PKC isozyme was confirmed using qRT-PCR ((Chen et al., 2008), Figure S1 of supplemental data). All PKC knockdown flies also exhibited delayed display of ethanol-induced uncoordination (data not shown). PKC deficient flies also showed normal pharmacokinetics of ethanol catabolism (see Figure S2 of supplemental data).
Mapping of PKC53E activity to serotonin neurons
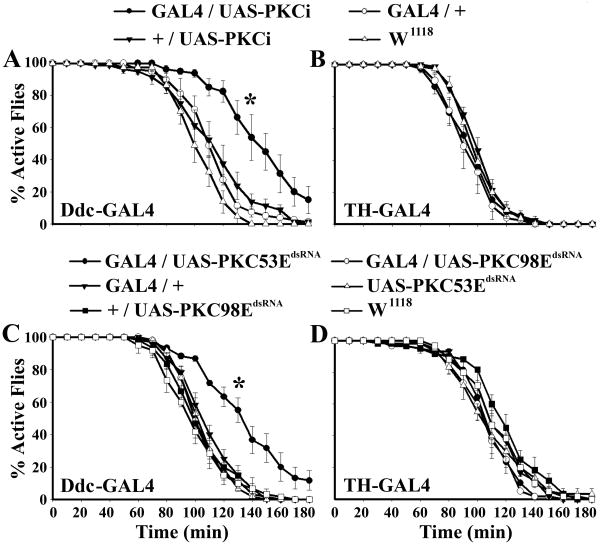
To determine how PKC53E regulates fly ethanol response, we expressed PKCi or PKC53EdsRNA in different subsets of neurons using various GAL4 drivers. We found that the fly lines expressing PKCi or PKC53EdsRNA, driven by Ddc-GAL4, displayed significantly reduced alcohol sensitivity (Figure. 2A, C). However, expression of PKC98EdsRNA directed by the same driver showed no alteration in ethanol sensitivity. Importantly, Ca2+-independent PKC98E, but not PKC53E, has been shown to modulate the activity of NPF neurons, the only previously known neurotransmitter system critical for fly ethanol sensitivity (Wen et al., 2005, Chen et al., 2008). Thus, our result suggests that PKC98E and PKC53E differentially regulate alcohol sensitivity through mutually exclusive modulation of NPF and Ddc-GAL4 neurons.
Figure 2. PKC53E but not PKC98E Deficiency in Serotonin Neurons Results in Ethanol Insensitivity.
A, B) Inhibition of PKC activity by a pseudo-substrate peptide in Ddc-GAL4 but not TH-GAL4 neurons leads to ethanol sensitivity. C) RNA interference-mediated knockdown of PKC53E but not PKC98E activity in Ddc-GAL4 neurons caused ethanol insensitivity. D) Knockdown of PKC53E or PKC98E activity in TH-GAL4 neurons showed no altered ethanol sensitivity. Ddc-GAL4/UAS-PKCi: χ21,4 = 33.11, P< 0.0001; Ddc-GAL4/UAS-PKC53EdsRNA: χ21,4 = 31.36, P < 0.0001.
Since Ddc-GAL4 is predominantly expressed in both dopamine and serotonin neurons (see Figure S3 of supplemental data), we also tested whether dopamine neuron-specific TH-GAL4-driven expression of PKCi or PKC53EdsRNA has a similar effect on ethanol behavior. We found that the ethanol sensitivity of TH-GAL4/UAS-PKC53EdsRNA flies was similar to those of paired controls (Figure 2B, D). This finding suggests that serotonin neurons have a previously uncharacterized role in fly ethanol insensitivity, and PKC53E regulates serotonin signaling.
Acute regulation of ethanol sensitivity by serotonin neurons
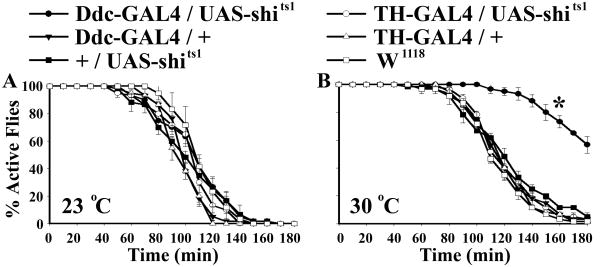
To provide evidence that PKC53E positively regulates serotonin signaling, we performed two additional experiments. First, we expressed, in Ddc-GAL4 neurons, UAS-shits1, which encodes a temperature-sensitive, semidominant-negative form of dynamin that blocks neuronal activity at a restrictive temperature (Kitamoto, 2002). When assayed at the restrictive temperature (30°C), Ddc-GAL4/UAS-shits1 but not TH-GAL4/UAS-shits1 flies showed significantly reduced ethanol sensitivity (Figure 3), suggesting that functional knockdown of PKC53E attenuates activity of serotonin neurons which are acutely required for fly response to ethanol intoxication.
Figure 3. Conditional Inhibition of Ddc-GAL4 and TH-GAL4 Neurons Reveals an Acute Requirement of Serotonin Signaling in Ethanol Sensitivity.
A) Expression of a temperature-sensitive, semidominant negative form of dynamin (UAS-shits1) in Ddc-GAL4 and TH-GAL4 neurons did not reduce ethanol sensitivity at a permissive temperature (23 °C). B) Ddc-GAL4/UAS-shits1 but not TH-GAL4/UAS-shits1 flies displayed decreased ethanol sensitivity. Ddc-GAL4/UAS-shits1: χ21,4 = 49.88, P < 0.0001. 1 ml of 30% ethanol solution was applied in each test.
Reversal of ethanol insensitivity by acute inhibition of serotonin reuptake
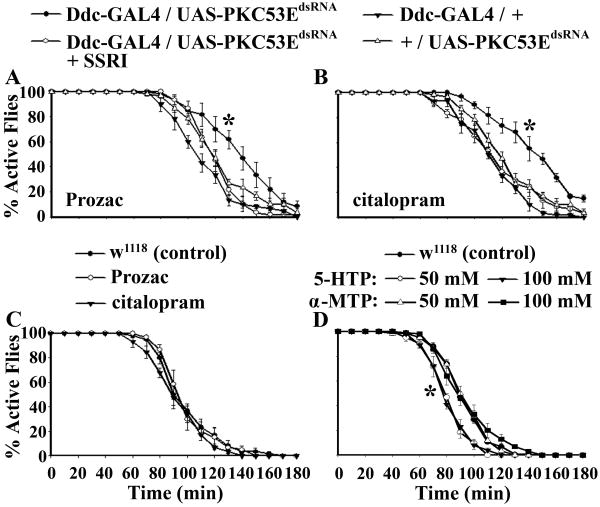
In the second experiment, we attempted to treat PKC53E deficiency-induced ethanol insensitivity by feeding Ddc-GAL4/UAS-PKC53EdsRNA flies with citalopram or Prozac, two SSRIs used clinically as antidepressants. Strikingly, each pharmacological treatment restored ethanol sensitivity of Ddc-GAL4/UAS-PKC53EdsRNA flies to the normal level (Figure 4A, B). These results suggest that PKC53E deficiency leads to a significant reduction in the synaptic serotonin level. On the other hand, the drug treatment of wild type flies did not significantly alter ethanol sensitivity (Figure 4C), suggesting that the level of synaptic serotonin is normally at or near saturation for fly ethanol sensitivity.
Figure 4. Identification of the Cellular Targets of PKC53E Activity Underlying the Ethanol Insensitive Phenotype.
A, B) Treatment of Ddc-GAL4/UAS-PKC53EdsRNA flies with Prozac or citalopram restores ethanol sensitivity. Ddc-GAL4/UAS-PKC53EdsRNA: χ21,4 = 10.11, P = 0.0015. C) Treatment of wild type flies with Prozac or citalopram. D) Treatment of wild type flies with the serotonin precursor (5-HTP) and serotonin synthesis inhibitor (α-MTP). 5-HTP treated w1118 flies: χ21,4 = 10.84, P = 0.001 (50 mM); χ21,6 = 16.97, P < 0.0001 (100mM). 1 ml of 40% ethanol solution was applied in each test.
It has been shown that ingestion of food containing 50 mM of serotonin precursor 5-HTP caused 15- to 20-fold increases in serotonin level in the fly head (Dierick and Greenspan, 2007). We found that wild type files fed with ca. 50 or 100 mM 5-HTP food consistently displayed a small but significant increase in ethanol sensitivity (Figure 4D). In addition, flies treated with ca. 50 or 100 mM serotonin synthesis inhibitor α-MTP, which reduces the serotonin level by about two fold (Dierick and Greenspan, 2007), showed no significant changes in ethanol sensitivity. Taken together, these findings suggest that ethanol sensitivity of normal flies is relatively independent of the fluctuations in tissue serotonin levels.
Discussion
We have shown that the acute alcohol sensitivity of Drosophila depends on all three subtypes of PKC isozymes in the nervous system. Functional analysis of a conventional PKC (PKC53E) has led to the discovery of a previously uncharacterized role of Drosophila serotonin neurons in acute alcohol response. Our findings suggest that further characterization of the cellular targets of different PKC isozymes related to ethanol sensitivity in the Drosophila model may hold great promise for gaining novel insights into the genetic and neural mechanisms underlying alcohol dependence.
In addition to the conserved NPY-like system (Wen et al., 2005), we have identified the serotonin system as another major signaling component in the fly neural circuit for alcohol sensitivity. In this and our previous studies (Chen et al., 2008), we have also shown that serotonin and NPY-like signaling activities are differentially modulated by conventional PKC53E and novel PKC98E in a mutually exclusive manner (Figure 5). It has been reported that conventional PKCγ and novel PKCδ knockout mice display reduced sensitivity to ethanol intoxication (Harris et al., 1995, Choi et al., 2008). However, how the deficiencies of these two PKC isozymes impact mouse alcohol sensitivity are not yet clear. Since both serotonin and NPY signaling systems have been implicated in alcohol sensitivity of rodents (Myers and Veale, 1968, McBride et al., 1990, Thiele et al., 1998), it will be interesting to examine whether PKCγ and PKCδ isozymes also differentially modulate the serotonin and NPY pathways.
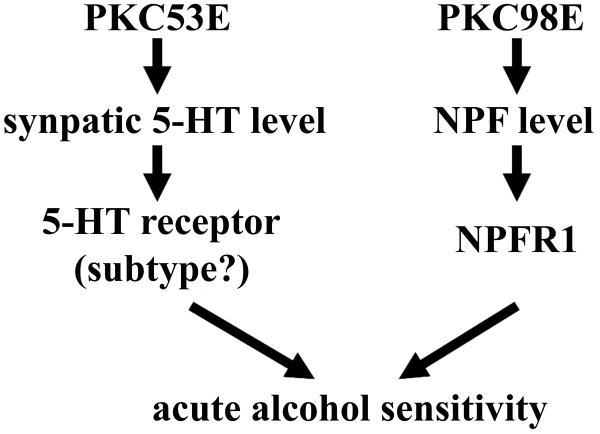
Figure 5. A Schematic Presentation of the Differential Role of PKC53E and PKC98E in Ethanol Sensitivity.
In this model, it is proposed that conventional PKC53E and novel PKC98E modulate independently conserved serotonin and NPF signaling activities, which may function, in parallel, through their receptor neurons to positively regulate ethanol sensitivity.
We have shown that acute inhibition of serotonin signaling with SSRIs reversed the alcohol insensitivity of Ddc-GAL4/UAS-PKC53ERNAi flies, suggesting that the PKC53E deficiency reduces serotonin levels at presynaptic sites. In mammalian cells, PKC activity has also been shown to downregulate serotonin reuptake by its transporters (Qian et al., 1997, Jayanthi et al., 2005). On the other hand, Ca2+-independent PKC98E may be specialized to regulate neuropeptide release. Consistent with this notion, PKC-1, the C. elegans homolog of PKC98E, has been shown to promote neuropeptide secretion (Sieburth et al., 2007). Ethanol has been shown to bind and activate mammalian PKC isozymes (Slater et al., 1997, Das et al., 2006, Das et al., 2009). It would be interesting to determine whether PKC53E and PKC98E may function as intracellular ethanol sensors to mediate cellular responses.
Although acutely blocking neurotransmission of serotonin neurons by a dominant-negative form of dynamin (encoded by shits1) caused significant reduction of fly alcohol sensitivity, feeding wild type flies with up to 100 mM α-MTP failed to show any significant effect. One possible explanation for these observations is that the serotonin receptor(s) responsible for fly ethanol sensitivity may bind to serotonin with a high affinity. Despite the fact that feeding of α-MTP reduces the serotonin level in head tissues by about two fold (Dierick and Greenspan, 2007), it may not reduce sufficiently the serotonin levels at presynaptic sites to trigger ethanol insensitivity. Consistent with this notion, we also found that feeding flies with 50 mM or 100 mM 5-HTP, which increases the serotonin level in head tissues by 15-20 fold, had similarly smaller effects (Figure 4D).
The serotonin and NPF neurons appear to define two parallel neuronal pathways critical for fly ethanol sensitivity. However, it remains unclear how these two pathways mediate the behavioral effects of ethanol. One possibility is that serotonin and NPF signaling may act upon a common set neurons expressing both serotonin and NPF receptors in defined brain regions that control motor coordination/consciousness (Yuan et al., 2005, Yuan et al., 2006). We are currently investigating this hypothesis.
Supplementary Material
The transcripts levels were expressed relative to that of the control (elav-GAL4 / +). The abundance of RNA has been normalized against that of the gapdh2 gene, and the asterisk indicates a significant reduction relative to elav-GAL4/+ and respective +/UAS controls (P < 0.05; post hoc Tukey's test). The transcript level of PKC53E was selectively reduced by 70% in elav-GAL4/UAS-PKC53EdsRNA, and the transcript level of PKC98E was selectively reduced by 47% in elav-GAL4/UAS-PKC98EdsRNA (Chen et al., 2008). The transcript level of DaPKC was selectively reduced by 31% in elav-GAL4/UAS-DaPKCdsRNA (F2,17 = 35.73, P = 0.0005).
To measure the body content of alcohol, flies were exposed to ethanol vapor for 12 min and recovered for 0,100 or 150 min ((Wen et al., 2005); also see Experimental Procedures). Body ethanol concentration was normalized against fly protein content in the body extracts. The volume of each fly was assumed to be 2 μl (Moore et al., 1998). Bars at each time point are not significantly different from one another at α = 0.05 nominal criterion level in post hoc Tukey's test.
The intact brains were dissected and fixed in Formalin Solution 10% Neutral Buffered pH 7.0 (SLMP, Inc., Lewisville, TX, USA) for 2 hours on ice. The immunostaining procedure and antibodies have been described previously (Wen et al., 2005, Chen et al., 2008). The Ddc-GAL4 but not TH-GAL4 neurons displayed extensive innervation at the subesophageal ganglia. Scale bar: 50 μm.
Acknowledgments
We thank the Bloomington Drosophila Stock Center (Bloomington, IN), and Vienna Drosophila RNAi Center (Vienna, Austria) for providing fly lines. This work is supported by National Institute of Health grant AA-014348 (to P.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Broughton SJ, Kane NS, Arthur B, Yoder M, Greenspan RJ, Robichon A. Endogenously inhibited protein kinase C in transgenic Drosophila embryonic neuroblasts down regulates the outgrowth of type I and II processes of cultured mature neurons. J Cell Biochem. 1996;60:584–599. doi: 10.1002/(sici)1097-4644(19960315)60:4<584::aid-jcb14>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang Y, Shen P. A protein kinase C activity localized to neuropeptide Y-like neurons mediates ethanol intoxication in Drosophila melanogaster. Neuroscience. 2008;156:42–47. doi: 10.1016/j.neuroscience.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Choi DS, Wei W, Deitchman JK, Kharazia VN, Lesscher HM, McMahon T, Wang D, Qi ZH, Sieghart W, Zhang C, Shokat KM, Mody I, Messing RO. Protein kinase Cdelta regulates ethanol intoxication and enhancement of GABA-stimulated tonic current. J Neurosci. 2008;28:11890–11899. doi: 10.1523/JNEUROSCI.3156-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corl AB, Berger KH, Ophir-Shohat G, Gesch J, Simms JA, Bartlett SE, Heberlein U. Happyhour, a Ste20 family kinase, implicates EGFR signaling in ethanol-induced behaviors. Cell. 2009;137:949–960. doi: 10.1016/j.cell.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Das J, Pany S, Rahman GM, Slater SJ. PKC epsilon has an alcohol-binding site in its second cysteine-rich regulatory domain. Biochem J. 2009;421:405–413. doi: 10.1042/BJ20082271. [DOI] [PubMed] [Google Scholar]
- Das J, Zhou X, Miller KW. Identification of an alcohol binding site in the first cysteine-rich domain of protein kinase Cdelta. Protein Sci. 2006;15:2107–2119. doi: 10.1110/ps.062237606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitrich RA, Bludeau PA, Baker RC. Investigations of the role of protein kinase C in the acute sedative effects of ethanol. Alcohol Clin Exp Res. 1989;13:737–745. doi: 10.1111/j.1530-0277.1989.tb00413.x. [DOI] [PubMed] [Google Scholar]
- Dierick HA, Greenspan RJ. Serotonin and neuropeptide F have opposite modulatory effects on fly aggression. Nat Genet. 2007;39:678–682. doi: 10.1038/ng2029. [DOI] [PubMed] [Google Scholar]
- Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, Couto A, Marra V, Keleman K, Dickson BJ. A genome-wide transgenic RNAi library for conditional gene inactivationi n Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- Friggi-Grelin F, Coulom H, Meller M, Gomez D, Hirsh J, Birman S. Targeted gene expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. J Neurobiol. 2003;54:618–627. doi: 10.1002/neu.10185. [DOI] [PubMed] [Google Scholar]
- Harris RA, McQuilkin SJ, Paylor R, Abeliovich A, Tonegawa S, Wehner JM. Mutant mice lacking the gamma isoform of protein kinase C show decreased behavioral actions of ethanol and altered function of gamma-aminobutyrate type A receptors. Proc Natl Acad Sci U S A. 1995;92:3658–3662. doi: 10.1073/pnas.92.9.3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge CW, Mehmert KK, Kelley SP, McMahon T, Haywood A, Olive MF, Wang D, Sanchez-Perez AM, Messing RO. Supersensitivity to allosteric GABA(A) receptor modulators and alcohol in mice lacking PKCepsilon. Nat Neurosci. 1999;2:997–1002. doi: 10.1038/14795. [DOI] [PubMed] [Google Scholar]
- Jayanthi LD, Samuvel DJ, Blakely RD, Ramamoorthy S. Evidence for biphasic effects of protein kinase C on serotonin transporter function, endocytosis, and phosphorylation. Mol Pharmacol. 2005;67:2077–2087. doi: 10.1124/mol.104.009555. [DOI] [PubMed] [Google Scholar]
- Kitamoto T. Targeted expression of temperature-sensitive dynamin to study neural mechanisms of complex behavior in Drosophila. J Neurogenet. 2002;16:205–228. doi: 10.1080/01677060216295. [DOI] [PubMed] [Google Scholar]
- Li H, Chaney S, Roberts IJ, Forte M, Hirsh J. Ectopic G-protein expression in dopamine and serotonin neurons blocks cocaine sensitization in Drosophila melanogaster. Curr Biol. 2000;10:211–214. doi: 10.1016/s0960-9822(00)00340-7. [DOI] [PubMed] [Google Scholar]
- Mahadev K, Vemuri MC. Selective changes in protein kinase C isoforms and phosphorylation of endogenous substrate proteins in rat cerebral cortex during pre- and postnatal ethanol exposure. Arch Biochem Biophys. 1998;356:249–257. doi: 10.1006/abbi.1998.0773. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Murphy JM, Lumeng L, Li TK. Serotonin, dopamine and GABA involvement in alcohol drinking of selectively bred rats. Alcohol. 1990;7:199–205. doi: 10.1016/0741-8329(90)90005-w. [DOI] [PubMed] [Google Scholar]
- Moore MS, DeZazzo J, Luk AY, Tully T, Singh CM, Heberlein U. Ethanol intoxication in Drosophila: Genetic and pharmacological evidence for regulation by the cAMP signaling pathway. Cell. 1998;93:997–1007. doi: 10.1016/s0092-8674(00)81205-2. [DOI] [PubMed] [Google Scholar]
- Myers RD, Veale WL. Alcohol preference in the rat: reduction following depletion of brain serotonin. Science. 1968;160:1469–1471. doi: 10.1126/science.160.3835.1469. [DOI] [PubMed] [Google Scholar]
- Newton PM, Ron D. Protein kinase C and alcohol addiction. Pharmacol Res. 2007;55:570–577. doi: 10.1016/j.phrs.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Qian Y, Galli A, Ramamoorthy S, Risso S, DeFelice LJ, Blakely RD. Protein kinase C activation regulates human serotonin transporters in HEK-293 cells via altered cell surface expression. J Neurosci. 1997;17:45–57. doi: 10.1523/JNEUROSCI.17-01-00045.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieburth D, Madison JM, Kaplan JM. PKC-1 regulates secretion of neuropeptides. Nat Neurosci. 2007;10:49–57. doi: 10.1038/nn1810. [DOI] [PubMed] [Google Scholar]
- Slater SJ, Kelly MB, Larkin JD, Ho C, Mazurek A, Taddeo FJ, Yeager MD, Stubbs CD. Interaction of alcohols and anesthetics with protein kinase Calpha. J Biol Chem. 1997;272:6167–6173. doi: 10.1074/jbc.272.10.6167. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Marsh DJ, Ste Mane L, Bernstein IL, Palmiter RD. Ethanol consumption and resistance are inversely related to neuropeptide Y levels. Nature. 1998;396:366–369. doi: 10.1038/24614. [DOI] [PubMed] [Google Scholar]
- Vosshall LB, Wong AM, Axel R. An olfactory sensory map in the fly brain. Cell. 2000;102:147–159. doi: 10.1016/s0092-8674(00)00021-0. [DOI] [PubMed] [Google Scholar]
- Wen T, Parrish CA, Xu D, Wu Q, Shen P. Drosophila neuropeptide F and its receptor, NPFR1, define a signaling pathway that acutely modulates alcohol sensitivity. Proc Natl Acad Sci U S A. 2005;102:2141–2146. doi: 10.1073/pnas.0406814102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q, Joiner WJ, Sehgal A. A sleep-promoting role for the Drosophila serotonin receptor 1A. Curr Biol. 2006;16:1051–1062. doi: 10.1016/j.cub.2006.04.032. [DOI] [PubMed] [Google Scholar]
- Yuan Q, Lin F, Zheng X, Sehgal A. Serotonin modulates circadian entrainment in Drosophila. Neuron. 2005;47:115–127. doi: 10.1016/j.neuron.2005.05.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The transcripts levels were expressed relative to that of the control (elav-GAL4 / +). The abundance of RNA has been normalized against that of the gapdh2 gene, and the asterisk indicates a significant reduction relative to elav-GAL4/+ and respective +/UAS controls (P < 0.05; post hoc Tukey's test). The transcript level of PKC53E was selectively reduced by 70% in elav-GAL4/UAS-PKC53EdsRNA, and the transcript level of PKC98E was selectively reduced by 47% in elav-GAL4/UAS-PKC98EdsRNA (Chen et al., 2008). The transcript level of DaPKC was selectively reduced by 31% in elav-GAL4/UAS-DaPKCdsRNA (F2,17 = 35.73, P = 0.0005).
To measure the body content of alcohol, flies were exposed to ethanol vapor for 12 min and recovered for 0,100 or 150 min ((Wen et al., 2005); also see Experimental Procedures). Body ethanol concentration was normalized against fly protein content in the body extracts. The volume of each fly was assumed to be 2 μl (Moore et al., 1998). Bars at each time point are not significantly different from one another at α = 0.05 nominal criterion level in post hoc Tukey's test.
The intact brains were dissected and fixed in Formalin Solution 10% Neutral Buffered pH 7.0 (SLMP, Inc., Lewisville, TX, USA) for 2 hours on ice. The immunostaining procedure and antibodies have been described previously (Wen et al., 2005, Chen et al., 2008). The Ddc-GAL4 but not TH-GAL4 neurons displayed extensive innervation at the subesophageal ganglia. Scale bar: 50 μm.