Abstract
The purpose of this study was to compare food intakes between Korean breast cancer patients and a healthy control group. We compared the intake of nutrients of 117 food items between Korean breast cancer patients (n=97) and age matched healthy controls (n=97). Nutrient intake was estimated using a quantitative food frequency questionnaire. The mean caloric intake of breast cancer patients and healthy controls was not significantly different. Breast cancer patients consumed significantly less fat and antioxidant nutrients such as vitamin A, retinol, β-carotene, vitamin C and vitamin E when compared to the control subjects. Among the food items, the intake of eggs (p<0.01), legumes (p<0.05), vegetables (p<0.05), seasonings (p<0.001), and oils and fats (p<0.01) in breast cancer patients was significantly lower than that in the controls. These results suggest that Korean breast cancer patients consumed less amount of soy and vegetables, which are rich source of antioxidant nutrients and phytosterols. Thus, dietary guidance to increase intake of these foods may be beneficial in the prevention of breast cancer.
Keywords: Breast cancer, fat, antioxidant vitamin, vegetable, soy
Introduction
Although the incidence of female breast cancer in most Asian countries is much lower than that in Western countries (Hirose et al., 2007), there has been a marked increase in recent years. Each year, it is estimated that one million women are newly diagnosed with breast cancer (Stewart & Kleihues, 2003). The incidence have been shown to be rising in Asian countries due to changes in lifestyle. In Korea, the incidence of breast cancer has doubled between 1987 and 2002 (Ministry of Health and Welfare, 2002).
Much of the international variation is due to differences in established genetic risk factors but diet might also contribute to risk and provide a potentially modifiable target for prevention. Recent efforts have focused on identifying dietary risk modulators. Both fat and fatty acids (Jakovljevi et al., 2002; Smith-Waner et al., 2001; Velie et al., 2000) have been shown to confer an increased risk, while fruits and vegetables (Gandini et al., 2001; Hanf & Gonder, 2005; Olsen et al., 2003) and phytoestrogens (Dai et al., 2002; Horn-Ross et al., 2001; McMichael-Phillips et al., 1998; Ziegler, 2004) afford a protective effect against breast cancer. Comparison studies of the food intake in breast cancer patients in Korean women are relatively rare. Therefore, we compared food intakes between Korean breast cancer patients and their age-matched controls.
Subjects and Methods
Subjects
The cases included 97 women with diagnosed breast cancer, and the age-matched controls included 97 women who were clinically healthy. Case subjects were collected women with histologically newly confirmed diagnosis of breast cancer at the inpatient or outpatient clinic of Yeouido St. Mary's Hospital, Seoul, Korea. The patients were chosen for the study after having a preliminary evaluation consisting of a brief medical history, smoking and alcohol habits and physical examinations. Patients with any history of liver diseases, diabetes mellitus, respiratory disorders and cardiovascular diseases were not included in the study. Controls were frequency matched by age and included outpatients in the department of general surgery at the same hospital during the same time period. Exclusion criteria for controls were those with known malignant, hormonal, gynaecological or endocrine diseases. All cases and controls were interviewed by a trained dietitian. The questionnaire included general information (age, sex and marital status), age at menarche, and pregnancy history.
Estimation of nutrient intake
We used an interviewer-administered quantitative food frequency questionnaire to estimate nutrient intake during the 2 year before the diagnosis for cases and before the interview for controls. The questionnaire included a list of 117 food items. Selection criteria were 1) most frequently consumed food items, 2) food items consumed in greatest amounts and 3) major food items supplying each nutrient, especially antioxidant vitamins. The selection was based on the Korean National Health and Nutrition Survey Report Ministry of Health and Welfare. Selected food items were categorized according to food groups and subdivided by food preparation methods, nutrient content and portion sizes. Categories and numbers of food items in each category were cereals and starches-15, soups-7, meats-12, egg-2 fish and other seafoods-12, legumes-4, milk and dairy products-5, vegetables-28 fruits-12 seasonings-3, oils-4, hot beverages and soft drink-10 and snacks-3. Subjects were asked to state the average frequency of consumption of each food item according to the categories of frequency, 'never or less than once per month', '1 per month', '2~3 per month', '1 per week', '2 per week', '3~4 per week', '5~6 per week', '1 per day', '2 per day', '3 per day'. The portion sizes were set as follows: a 0.5 serving size, a serving size, and a 1.5 serving size. The interviewer showed food models and photographs of the standard serving size, and asked the subjects to refer to those portions when selecting the amounts of foods consumed. The food frequency questionnaire was coded and analyzed for nutrient intake by a computer aided nutrient analysis program for professionals (CAN-Pro, APAC Intelligence, Seoul, Korea).
Statistical analysis
All data were analyzed through the SAS (Version 8.1) statistical package. The results are given as mean ± SD values. The significance of the mean difference between the two groups was assessed by the Student's t-test.
Results
Demographic characteristics
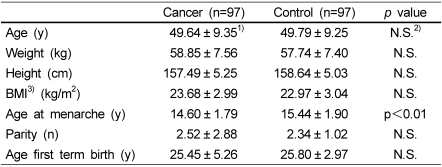
The demographic characteristics of breast cancer patients and their age-matched controls were similar (Table 1). Statistical analysis showed no significant differences between breast cancer patients and the control group in age, anthropometric variables, parity, or age at first full-term birth. However, the mean age at menarche was significantly younger in the patient group (p<0.01).
Table 1.
Descriptive characteristics of patients with breast cancer and their age matched controls
1)values are mean ± SD.
2)not significant
3)body mass index
Nutrient intake of study subjects
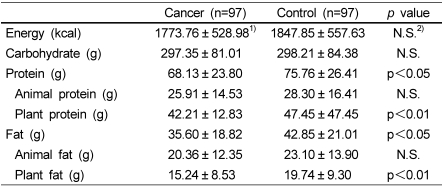
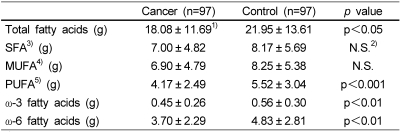
Daily nutrient intake is shown in Table 2. The mean energy intake of breast cancer patients was not significantly different than that of the control subjects. However, the intake of total protein and fat was significantly lower among breast cancer patients (p<0.05). Breast cancer patients consumed significantly less polyunsaturated fatty acids (p<0.001), ω-3 fatty acids (p<0.001) and ω-6 fatty acids (p<0.01) compared to the controls (Table 3).
Table 2.
Daily intake of energy and nutrients assessed by the quantitative food frequency questionnaire
1)values are mean ± SD.
2)not significant
Table 3.
Mean daily fatty acids intake of patients with breast cancer and their age-matched controls
1)values are mean ± SD.
2)not significant
3)saturated fatty acids
4)monounsaturated fatty acids
5)polyunsaturated fatty acids
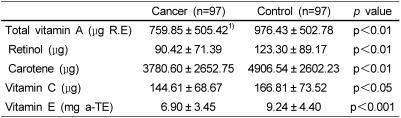
The association between major antioxidant vitamin intake and the risk of breast cancer is presented in Table 4. The patient group consumed significantly less vitamin A (p<0.01), retinol (p<0.01), carotene (p<0.01), vitamin C (p<0.05) and vitamin E (p<0.001) compared to the control group.
Table 4.
Daily vitamin intake assessed by the quantitative food frequency questionnaire
1)values are mean ± SD.
Food stuff intake of study subjects
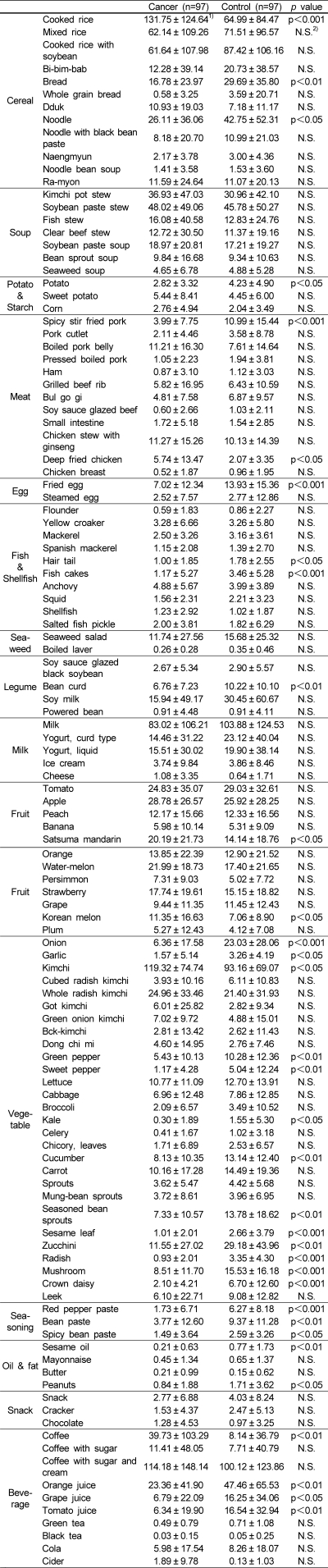
The mean daily selective food stuff intake is shown in Table 5. The breast cancer patients consumed significantly greater quantities of cooked rice, noodles, deep fried chicken, satsuma mandarin, Korean melon, kimchi and coffee. While they consumed a significantly lower quantity of bean curd in the legume category, onion, garlic, green pepper, sweet pepper, kale, cucumber, seasoned bean sprouts, sesame leaf, zucchini, radish, mushroom, crown daisy in the vegetable category, red pepper paste, bean paste, spicy bean paste in the seasonings category, and orange juice, grape juice, tomato juice in the beverages category compared to controls.
Table 5.
Food intakes in controls and patients with breast cancer (unit: g)
1)values are mean ± SD.
2)not significant
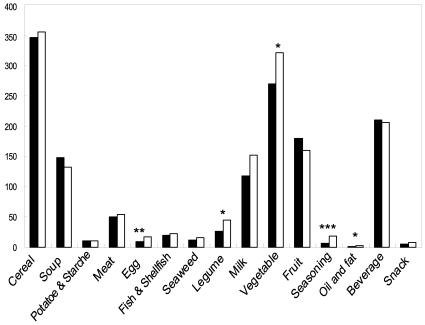
Among the food items from each group, the intake of eggs (p<0.01), legumes (p<0.05), vegetables (p<0.05), seasonings (p<0.001), and oils and fats (p<0.01) in the breast cancer patients was significantly lower than those of the controls (Figure 1).
Fig. 1.
Food intakes from each food group in patients with breast cancer (■) and their age-matched controls (□).
*p<0.05, **p<0.01, ***p<0.001
Discussion
This study compared with in food intakes between Korean breast cancer patients and their age-matched controls. The demographic characteristics of breast cancer patients and their age-matched controls were similar. However the age at menarche was younger in breast cancer patients, implying that earlier exposure to hormones may be a key determinant in these subjects. A younger age at menarche is associated with a higher risk of breast cancer and increases lifetime exposure to estrogens. There has been a decrease in the average age of menarche in Western countries (Yoo et al., 2006). This, coupled with the increase in body size and fat percentage, has been attributed, in part, to nutritional factors. It has been argued that over-nutrition, in early life, causes rapid growth resulting in early menarche and in turn an increased risk of breast cancer (Law, 2000). There is evidence from cohort studies, after control for body sizes and energy intakes, that higher consumptions of grains, nuts, and legumes are associated with later menarche whereas higher consumptions of meat are associated with earlier menarche (Law, 2000).
Earlier findings from international comparisons and case-control studies suggested a positive association between a Western-style diet and breast cancer risk (Zaridze et al., 1991). However, a large scale prospective cohort of 8 years has failed to prove that breast cancer risk is reduced by a low-fat diet (Prentice et al., 2006). Results from the present study showed a lower total fat intake in the control subjects and no difference was found in total caloric intake. In our study, means of fat intake of the breast cancer patients and the controls were 8.6% and 10.1% of total energy intakes, respectively. And which is much a smaller quantity than the study population of other nations, especially European and Western countries. In a recent study carried out in the United States (Velie et al., 2000), means of daily intake of total fat was 35.0% of total energy, which is almost three times higher than those of our study population. The level of energy from fat is relatively low in Korean patients compared to their Western counterparts, and fat consumption may not be an independent risk factor at this level. Venkatraman et al. (2000) suggested that lipids are mediators of the immune system and that they may modulate its immune-regulatory effects. That study has shown that a low-fat/high-carbohydrate diet may increase inflammatory and decrease antiinflammatory immune factors, and depresses antioxidants. An association between the specific type of dietary fat consumed or the ω3/ω6 fatty acids ratio and breast cancer risk should also be considered.
In the current study, the breast cancer patient group consumed significantly less major antioxidant vitamins including vitamin A, retinol, carotene, vitamin C and vitamin E compared to the control group. And they consumed significantly lower quantities of onion, garlic, green pepper, sweet pepper, kale, cucumber, seasoned bean sprouts, sesame leaf, zucchini, radish, mushroom and crown daisy. These foods contain vitamins, minerals, fiber and non-nutritional components that may reduce the risk of cancer that can be used together or alone. Several studies have found that the consumption of vegetables and fruit is associated with a lower risk of cancer (Ching et al., 2002; Nissen et al., 2003; Rock et al., 2005). The study by Ronco et al. (1999) showed that high intake of raw and cooked vegetables seemed to be inversely associated with breast cancer. A follow-up study by Dorgan et al. (1998) reported that 105 cases of histologically confirmed breast cancer patients were diagnosed during 9.5 years, and intake and serum carotenoids were associated inversely with risk. About the study of the relationship between dietary intakes and death after breast cancer diagnosis, McEligot et al. (2006) reported nutrient including vitamin C and carotenoids intakes were significantly associated with reduced mortality. Vitamin C, vitamin E, and the carotenoids act as antioxidants, which protect DNA from oxidative damage (Rock et al., 2000). In addition, each has other chemopreventive properties (Rock et al., 2000). Carotenoids found in orange vegetables and fruits, may inhibit cell proliferation and inhibit cellular growth via conversion into retinols (Borek, 2004). A pooled analysis of 12 early case-control studies found that women in the highest fifth percentile of β-carotene consumption had a 19% reduction in breast cancer incidence compared to other postmenopausal women (Howe et al., 1990). A meta-analysis of literature from 1982 to 1997 found a similar result (Gandini et al., 2001).
In addition to its antioxidant potential, vitamin C is also crucial for immune function (Rock et al., 2000). A meta-analysis of case-control studies found a 37% reduction in breast cancer risk among postmenopausal women in the highest fifth percentile of consumption of vitamin C (Willett, 2001). In animal models, vitamin E has been found to inhibit tumors and reduce cell proliferation (Rock et al., 2000). However, available epidemiologic data do not support this effect (Willet, 2001).
In our study, breast cancer patients consumed lower quantities of red pepper paste, bean paste and spicy bean paste. Pepper is also a major source of flavonols, which may have a protective effect on breast cancer risk in laboratory and animal studies (Acklane et al., 2005; Zhong et al., 2003).
Among the food items from each group, the intake of legumes in breast cancer patients was significantly lower than those of the controls. Adlercreutz (2002) reported breast cancer rates in Asian women consuming soy-containing diets have been noted to be lower than in Western nations. In one study, women who reported having consumed soy at least once a week during adolescence showed a statistically significant reduced risk of breast cancer (Wu et al., 2002). Yamamoto et al. reported that increased miso soup and/or isoflavone consumption was associated with a reduced risk of breast cancer in Japanese women. In Ingram et al., US women were the breast cancer risk reduction associated with greater urinary excretion of phytoestrogens than in Australian women aged 30-84 years, among whom the level of consumption of traditional soy-based foods is low. Studies have investigated the effect of soy consumption on cell proliferation or biomarkers of cell proliferation in breast tissue (Hargreaves et al., 1999; Jenkins et al., 2002; Nishio et al., 2007). These data suggest that short-term dietary soy supplementation can induce proliferation of breast tissue in premenopausal women with breast disease. In contrast, soy consumption has been associated with a reduction inmammographic density (Atkinson & Bingham, 2002) whereas Maskarinec & Meng (2001) reported a positive correlation between self-reported soy food intake and percentage of breast density of women living in Hawaii. Increased mammographic density has been associated with a four to six fold increased risk of breast cancer (Atkinson & Bingham, 2002). In addition, the epidemiology of breast cancer in relation to dietary intake of phytoestrogens has been adequately reviewed and suggests that early exposure to relatively high concentrations of soy phytoestrogens may have a protective effect against breast cancer in later life (Adlercreutz, 2002). Despite the evidence that some phytoestrogens at low doses can promote the growth of breast cancer cell lines and increase biomarkers of cellular proliferation in human breast cells, current evidence suggests that a high dietary intake of soy decreases the risk of breast cancer (Maskarinec et al., 2004).
In summary, we compared the intake of nutrients and foods between Korean breast cancer patients and age matched controls. Fat intake was lower in the breast cancer patients and no difference was found in total caloric intake compared those of controls. However, that breast cancer patients consumed lesser amounts of legumes and vegetables, which are rich sources of antioxidant nutrients and phytosterols than those of the controls. Thus, dietary guidance to increase legume and vegetable intake may be beneficial in the prevention of breast cancer.
Footnotes
This work was supported by the SRC/ERC program of MOST/KOSEF (RESEARCH CENTER FOR WOMEN'S DISEASES-R11-2005-017).
References
- 1.Ackland ML, van de Waarsenburg S, Jones R. Synergistic antiproliferative action of the flavonols quercetin and kaempferol in cultured human cancer cell lines. In Vivo. 2005;19:69–76. [PubMed] [Google Scholar]
- 2.Adlercreutz H. Phytoestrogens and breast cancer. J Steroid Biochem Mol Biol. 2002;83:113–118. doi: 10.1016/s0960-0760(02)00273-x. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson C, Bingham SA. Mammographic breast density as a biomarker of effects of isoflavones on the female breast. Breast Cancer Res. 2002;4:1–4. doi: 10.1186/bcr410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borek C. Dietary antioxidants and human cancer. Integr Cancer Ther. 2004;3:333–341. doi: 10.1177/1534735404270578. [DOI] [PubMed] [Google Scholar]
- 5.Ching S, Ingram D, Hahnel R, Beilby J, Rossi E. Serum levels of micronutrients, antioxidants and total antioxidant status predict risk of breast cancer in a case control study. J Nutr. 2002;132:303–306. doi: 10.1093/jn/132.2.303. [DOI] [PubMed] [Google Scholar]
- 6.Dai Q, Franke AA, Jin F, Shu XO, Hebert JR, Custer LJ, Cheng J, Gao YT, Zheng W. Urinary excretion of phytoestrogens and risk of breast cancer among Chinese women in Shanghai. Cancer Epidemiol Biomarkers Prev. 2002;11:815–821. [PubMed] [Google Scholar]
- 7.Dorgan JF, Sowell A, Swanson CA, Potischman N, Miller R, Schussler N, Stephenson HE., Jr Relationships of serum carotenoids, retinol, alpha-tocopherol, and selenium with breast cancer risk: results from a prospective study in Columbia, Missouri (United States) Cancer Causes Control. 1998;9:89–97. doi: 10.1023/a:1008857521992. [DOI] [PubMed] [Google Scholar]
- 8.Gandini S, Merzenich H, Robertson C, Boyle P. Metaanalysis of studies on breast cancer risk and diet: the role of fruit and vegetable consumption and the intake of associated micronutrients. Eur J Cancer. 2001;36:636–646. doi: 10.1016/s0959-8049(00)00022-8. [DOI] [PubMed] [Google Scholar]
- 9.Hanf V, Gonder U. Nutrition and primary prevention of breast cancer: foods, nutrients and breast cancer risk. Eur J Obstet Gynecol Reprod Biol. 2005;123:139–149. doi: 10.1016/j.ejogrb.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Hargreaves DF, Potten CS, Harding C, Shaw LE, Morton MS, Roberts SA, Howell A, Bundred NJ. Two-week dietary soy supplementation has an oestrogenic effect on normal premenopausal breast. J Clin Endocrinol Metab. 1999;84:4017–4024. doi: 10.1210/jcem.84.11.6152. [DOI] [PubMed] [Google Scholar]
- 11.Hirose K, Matsuo K, Iwata H, Tajima K. Dietary patterns and the risk of breast cancer in Japanese women. Cancer Sci. 2007:1–8. doi: 10.1111/j.1349-7006.2007.00540.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horn-Ross PL, John EM, Lee M, Stewart SL, Koo J, Sakoda LC, Shiau AC, Goldstein J, Davis P, Perez-Stable EJ. Phytoestrogen consumption and breast cancer risk in a multiethnic population: the Bay Area Breast Cancer Study. Am J Epidemiol. 2001;154:434–441. doi: 10.1093/aje/154.5.434. [DOI] [PubMed] [Google Scholar]
- 13.Howe GR, Hirohata T, Hislop TG. Dietary factors and risk of breast cancer: combined analysis of 12 case-control studies. J Natl Cancer Inst. 1990;82:561–569. doi: 10.1093/jnci/82.7.561. [DOI] [PubMed] [Google Scholar]
- 14.Ingram D, Sanders K, Kolybaba M. Case-control study of phyto-oestrogens and breast cancer. Lancet. 1997;350:990–994. doi: 10.1016/S0140-6736(97)01339-1. [DOI] [PubMed] [Google Scholar]
- 15.Jakovljevic J, Touillaud MS, Bondy ML, Singletary SE, Pillow PC, Chang S. Dietary intake of selected fatty acids, cholesterol and carotenoids and estrogen receptor status in premenopausal breast cancer patients. Breast Cancer Res Treat. 2002;75:5–14. doi: 10.1023/a:1016588629495. [DOI] [PubMed] [Google Scholar]
- 16.Jenkins DJ, Kendall CW, Connelly PW, Jackson CJ, Parker T, Faulkner D, Vidgen E. Effects of high- and low-isoflavone (phytoestrogen) soy foods on inflammatory biomarkers and proinflammatory cytokines in middle-aged men and women. Metabolism. 2002;51:919–924. doi: 10.1053/meta.2002.33352. [DOI] [PubMed] [Google Scholar]
- 17.Law M. Dietary fat and adult diseases and the implications for childhood nutrition: an epidemiologic approach. Am J Clin Nutr. 2000;72:1291S–1296S. doi: 10.1093/ajcn/72.5.1291s. [DOI] [PubMed] [Google Scholar]
- 18.Maskarinec G, Meng L. An investigation of soy intake and mammographic characteristics in Hawaii. Breast Cancer Res. 2001;3:134–141. doi: 10.1186/bcr285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maskarinec G, Franke AA, Williams AE, Hebshi S, Oshiro C, Murphy S, Stanczyk FZ. Effects of a 2-year randomized soy intervention on sex hormone levels in premenopausal women. Cancer Epidemiol Biomarkers Prev. 2004;13:1736–1744. [PubMed] [Google Scholar]
- 20.McEligot AJ, Largent J, Ziogas A, Peel D, Anton-Culver H. Dietary fat, fiber, vegetable, and micronutrients are associated with overall survival in postmenopausal women diagnosed with breast cancer. Nutr Cancer. 2006;55:132–140. doi: 10.1207/s15327914nc5502_3. [DOI] [PubMed] [Google Scholar]
- 21.McMichael-Phillips DF, Harding C, Morton M, Roberts SA, Howell A, Potten CS, Bundred NH. Effects of soy-protein supplementation on epithelial proliferation in the histologically normal human breast. Am J Clin Nutr. 1998;68:1431S–1435S. doi: 10.1093/ajcn/68.6.1431S. [DOI] [PubMed] [Google Scholar]
- 22.Ministry of Health and Welfare. Report on 2001 Korea National Health and Nutrition Survey. Seoul. Republic of Korea: 2002. [Google Scholar]
- 23.Nissen SB, Tjonneland A, Stripp C, Olsen A, Christensen J, Overvad K, Dragsted LO, Thomsen B. Intake of vitamins A, C, and E from diet and supplements and breast cancer in postmenopausal women. Cancer Causes Control. 2003;14:695–704. doi: 10.1023/a:1026377521890. [DOI] [PubMed] [Google Scholar]
- 24.Nishio K, Niwa Y, Toyoshima H, Tamakoshi K, Kondo T, Yatsuya H, Yamamoto A, Suzuki S, Tokudome S, Lin Y, Wakai K, Hamajima N, Tamakoshi A. Consumption of soy foods and the risk of breast cancer: findings from the Japan Collaborative Cohort (JACC) Study. Cancer Causes Control. 2007;18:801–808. doi: 10.1007/s10552-007-9023-7. [DOI] [PubMed] [Google Scholar]
- 25.Olsen A, Tjonneland A, Thomsen BL. Fruits and vegetables intake differentially affects estrogen receptor negative and positive breast cancer incidence rates. J Nutr. 2003;133:2342–2347. doi: 10.1093/jn/133.7.2342. [DOI] [PubMed] [Google Scholar]
- 26.Prentice RL, Caan B, Chlebowski RT, Patterson R, Kuller LH, Ockene JK, Margolis KL, Limacher MC, Manson JE, Parker LM, Paskett E, Phillips L, Robbins J, Rossouw JE, Sarto GE, Shikany JM, Stefanick ML, Thomson CA, Van Horn L, Vitolins MZ, Wactawski-Wende J, Wallace RB, Wassertheil-Smoller S, Whitlock E, Yano K, Adams-Campbell L, Anderson GL, Assaf AR, Beresford SA, Black HR, Brunner RL, Brzyski RG, Ford L, Gass M, Hays J, Heber D, Heiss G, Hendrix SL, Hsia J, Hubbell FA, Jackson RD, Johnson KC, Kotchen JM, LaCroix AZ, Lane DS, Langer RD, Lasser NL, Henderson MM. Low-fat dietary pattern and risk of invasive breast cancer: the Women's Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295:629–642. doi: 10.1001/jama.295.6.629. [DOI] [PubMed] [Google Scholar]
- 27.Rock CL, Lampe JW, Patterson RE. Nutrition, genetics, and risks of cancer. Annu Rev Public Health. 2000;21:47–64. doi: 10.1146/annurev.publhealth.21.1.47. [DOI] [PubMed] [Google Scholar]
- 28.Rock CL, Flatt SW, Natarajan L, Thomson CA, Bardwell WA, Newman VA, Hollenbach KA, Jones L, Caan BJ, Pierce JP. Plasma carotenoids and recurrence-free survival in women with a history of breast cancer. J Clin Oncol. 2005;23:6631–6638. doi: 10.1200/JCO.2005.19.505. [DOI] [PubMed] [Google Scholar]
- 29.Ronco A, De Stefani E, Boffetta P, Deneo-Pellegrini H, Mendilaharsu M, Leborgne F. Vegetables, fruits, and related nutrients and risk of breast cancer: a case-control study in Uruguay. Nutr Cancer. 1999;35:111–119. doi: 10.1207/S15327914NC352_3. [DOI] [PubMed] [Google Scholar]
- 30.Smith-Warner SA, Spiegelman D, Adami HO, Beeson WL, van den Brandt PA, Folsom AR, Fraser GE, Freudenheim JL, Goldbohm RA, Graham S, Kushi LH, Miller AB, Rohan TE, Speizer FE, Toniolo P, Willett WC, Wolk A, Zeleniuch-Jacquotte A, Hunter DJ. Types of dietary fat and breast cancer: a pooled analysis of cohort studies. Int J Cancer. 2001;92:767–774. doi: 10.1002/1097-0215(20010601)92:5<767::aid-ijc1247>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 31.Stewart BW, Kleihues P. World Cancer Report. Lyon. France: IRAC Press; 2003. [Google Scholar]
- 32.UK Department of Health. Nutritional aspects of the development of cancer. London. UK: Her Majesty's Stationery Office; 1998. Working Group on Diet and Cancer of the Committee on Medical Aspects of Food and Nutrition Policy. [Google Scholar]
- 33.Velie E, Kulldorff M, Schairer C, Block G, Albanes D, Schatzkin A. Dietary fat, fat subtypes, and breast cancer in postmenopausal women: a prospective cohort study. J Natl Cancer Inst. 2000;92:833–839. doi: 10.1093/jnci/92.10.833. [DOI] [PubMed] [Google Scholar]
- 34.Venkatraman JT, Leddy J, Pendergast D. Dietary fats and immune status in athletes: clinical implications. Med Sci Sports Exerc. 2000;32:S389–S395. doi: 10.1097/00005768-200007001-00003. [DOI] [PubMed] [Google Scholar]
- 35.Verhoeven DT, Assen N, Goldbohm RA, Dorant E, van 't Veer P, Sturmans F, Hermus RJ, van den Brandt PA. Vitamins C and E, retinol, beta-carotene and dietary fibre in relation to breast cancer risk: a prospective cohort study. Br J Cancer. 1997;75:149–155. doi: 10.1038/bjc.1997.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Willett WC. Diet and breast cancer. J Intern Med. 2001;249:395–411. doi: 10.1046/j.1365-2796.2001.00822.x. [DOI] [PubMed] [Google Scholar]
- 37.Wu AH, Wan P, Hankin J, Tseng CC, Yu MC, Pike MC. Adolescent and adult soy intake and risk of breast cancer in Asian-Americans. Carcinogenesis. 2002;23:1491–1496. doi: 10.1093/carcin/23.9.1491. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto S, Sobue T, Kobayashi M, Sasaki S, Tsugane S. Soy, isoflavones and breast cancer risk in Japan. J Natl Cancer Inst. 2003;95:906–913. doi: 10.1093/jnci/95.12.906. [DOI] [PubMed] [Google Scholar]
- 39.Yoo KY, Kim Y, Park SK, Kang D. Lifestyle, genetic susceptibility and future trends of breast cancer in Korea. Asian Pac J Cancer Prev. 2006;7:679–682. [PubMed] [Google Scholar]
- 40.Zaridze D, Lifanova Y, Maximovitch D, Day NE, Duffy SW. Diet, alcohol consumption and reproductive factors in a case-control study of breast cancer in Moscow. Int J Cancer. 1991;48(4):493–501. doi: 10.1002/ijc.2910480404. [DOI] [PubMed] [Google Scholar]
- 41.Zhong X, Wu K, He S, Ma S, Kong L. Effects of quercetin on the proliferation and apoptosis in transplantation tumor of breast cancer in nude mice. Sichuan Da Xue Xue Bao Yi Xue Ban. 2003;34:439–442. [PubMed] [Google Scholar]
- 42.Ziegler RG. Phytoestrogens and breast cancer. Am J Clin Nutr. 2004;79:183–184. doi: 10.1093/ajcn/79.2.183. [DOI] [PubMed] [Google Scholar]