Abstract
The present study evaluated the effect of various dosages of soybean isoflavone extract on body weight changes, glucose tolerance and liver function in streptozotocin-induced diabetic rats. One group of normal rats (normal control) was fed an AIN-76-based experimental diet and four groups of diabetic rats were fed the same diet supplemented with four different levels of soybean isoflavone extract for seven weeks. The daily dosages of pure isoflavone for four diabetic groups were set to be 0 mg (diabetic control), 0.5 mg (ISO-I), 3.0 mg (ISO-II) and 30.0 mg (ISO-III) per kilogram of body weight, respectively. The daily consumption of isoflavone at the level of 3.0mg per kilogram of body weight resulted in the suppression of body weight loss and increased the survival rate of diabetic animals one and half times compared to that of the diabetic control group. Blood glucose levels in a fasting state and after the oral administration of glucose were significantly lower in the ISO-II group during the oral glucose tolerance test. The ISO-II group showed a tendency to elongate the gastrointestinal transit time. The activity of serum aminotransferases, indicator of liver function, was not negatively affected by any intake level of isoflavone. The present study demonstrated that the soybean isoflavone extract may be beneficial to diabetic animals by improving their glucose tolerance and suppressing weight loss without incurring hepatotoxicity at the daily dosage of 3.0 mg per kg of body weight.
Keywords: Soybean isoflavone extract, streptozotocin, diabetic rat, glucose tolerance, survival rate
Introduction
In recent years, there has been an increased interest with regard to dietary phytoestrogens, especially isoflavones, among the public and in the medical community because of their potential role in promoting health (Ren et al., 2001; Setchell & Cassidy, 1999; Tham et al., 1998). The most abundant food sources of isoflavones are soybean and soybean products (Wang & Murphy, 1994). Soybean isoflavone has been examined for its beneficial effects on clinical and metabolic variables in animals and humans. Soybean isoflavone have been reported to act as a selective estrogen receptor modulator (Setchell, 2001), possess potential for antioxidant activity (Lajolo et al., 2005; Ruiz-Larrea et al., 1997; Wei et al., 1995), and lower blood cholesterol levels (Jenkins et al., 2002; Hermansen et al., 2001; Anderson et al., 1998). Substantial data from epidemiological surveys and clinical intervention trials involving animals and humans strongly support the beneficial effect of isoflavone-rich soy protein in preventing various chronic diseases including, cardiovascular disease (Goodman-Gruen & Kritz-Silverstein, 2001; Teede, 2001), cancer (Zhang et al., 2004; Hussain et al., 2003; Constantinou et al., 2001; Chen & Anderson, 2001), osteoporosis (Alekel et al., 2000; Arjmandi et al., 1996), and menopausal symptoms (Somekawa et al., 2001; Upmalis et al., 2000; Alekel et al., 2000; Washburn et al., 1999; Baird et al., 1995).
Evidence is also emerging that soy may have a beneficial role on obesity and diabetes in animals and humans (Lee, 2006; Bartke et al., 2004; Hsu et al., 2003; Bhathena & Velasquez, 2002; Vedavanam et al., 1999). Several studies have evaluated the effect of proteins from different sources in obese humans and found that soy protein possesses significant antiobesity activity with a reduction of serum cholesterol and triacylglycerol (Bosello et al., 1998; Yamashita et al., 1998). Studies conducted with obese animals reported that the consumption of soy products decreased body weight and plasma glucose level (Aoyama et al., 2000; Saito, 1991). It has been reported that soy protein, with its associated isoflavones and fiber, reduced lipid levels and cardiovascular risk markers in Type 2 diabetic subjects, as compared with a casein diet with cellulose but it did not have any effect on glucose metabolism (Hermansen et al, 2001). However, it was demonstrated that defatted soy flour, when added to whole-durum meal, decreased hyperglycemia as well as hyperlipidemia in Alloxan diabetic rats (Taha & Wasif, 1996). Vedavanam et al (1999) suggested that a soybean phytochemical extract displayed a range of properties which may be beneficial for diabetes, namely as an estrogenic agent, as an inhibitor of intestinal glucose-uptake and a preventive agent for glucose-induced lipid peroxidation. Hence, soybean isoflavone is expected to improve the overall metabolism in diabetes that is associated with chronic hyperglycemia and disturbances of carbohydrate and lipid metabolism.
The lipid-lowering effect of soy protein has been relatively well documented, however, studies on the effects of soy on glucose metabolism are lacking and the effect of soy protein and isoflavones on glucose metabolism is still unclear. Recently, the effect of soy isoflavone on blood glucose in streptozotocin-induced diabetic rats have been reported (Lee, 2006; Hsu et al., 2003) but the results were inconsistent. Hsu et al. (2003) supplemented a diet with isoflavone (240~1920mg/100g diet) for 24 days and found no favorable effect on plasma glucose. Lee (2006) tested genistein (600mg/kg diet) and soy protein (200g/kg diet)-supplemented diets for 3 weeks and reported a beneficial effect in correcting hyperglycemia in diabetic animals. In both studies, the dosages of isoflavones were tremendously high and the feeding periods were relatively short. Therefore, isoflavones need to be tested at more realistic dosages for a longer period of time, since the safety of isoflavones has not been established yet.
The present study examined the effect of the supplementation of soybean isoflavone extract at various dose levels for seven weeks on glucose tolerance, body weight changes and diabetic symptoms in streptozotocin-induced diabetic rats. The second purpose of this study was to determine the dosage of dietary isoflavone, which may exhibit beneficial effect on diabetic animal without hepatic toxicity.
Materials and Methods
Animals and diet
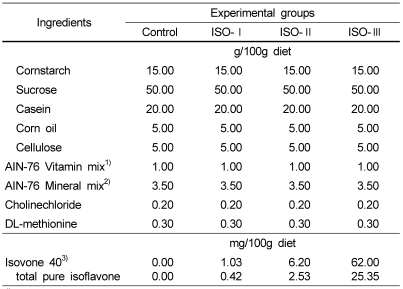
Eighty weanling male Sprague-Dawley rats (Biogenomics Co., Korea) were raised on a chow diet (Jeil Animal Feed Co., Korea) until they weighed over 200 g and then they were fed an AIN 76-based diet (American Institute of Nutrition, 1977) for 1 week prior to the experiment. Ten rats were assigned to the normal control group and seventy were experimentally induced to a diabetic condition. Diabetic rats were divided into four groups according to body weight and blood glucose levels in order to have similar weights and levels in each of the groups. The normal control group (n=10) was fed an AIN-76-based experimental diet and four groups of diabetic rats were fed the same diet supplemented with four different levels of soybean isoflavone extract, 0 mg (diabetic control, n=16), 0.42 mg (ISO-I, n=18), 2.53mg (ISO-II, n=18), and 25.35mg (ISO-III, n=18) isoflavone per 100g diet, respectively. When assuming that the average daily feed intake of a mature rat is approximately 30 g, the daily dosages of isoflavone are estimated to be 0 mg (normal and diabetic control), 0.5 mg (ISO-I), 3.0 mg (ISO-II), and 30.0 mg (ISO-III) per kilogram of body weight, respectively. The daily dose of isoflavone for the ISO-I group (0.5 mg/kg body weight) was set to be approximately equivalent to the average daily isoflavone intake of Korean adult women, (Lee et al., 2000) and the dose for the ISO-II group (3.0 mg/kg body weight) was 6 times that of ISO-I group which was approximately equivalent to the maximum daily isoflavone intake of Korean adult women (Lee et al., 2000). The dose for the ISO-III group (30 mg/kg body weight) was 60 times that of the ISO-I group and this level was set in order to evaluate any possible additive or adverse effects of the high dose of isoflavone. The source of isoflavone was ISOVONE 40 (Bioland, Seoul, Korea) which was a soybean isoflavone extract that contained 40.9% of total pure isoflavone. All diets contained an identical concentration of energy and nutrients (Table 1). The animals were fed experimental diets and filtered tap water ad libitum for seven weeks.
Table 1.
The composition of the experimental diets
1)AIN-76 vitamin mix (g/kg mix): thiamin·HCl 0.6, riboflavin 0.6, pyridoxine HCl 0.7, nicotinic acid 3, D-calcium pantothenate 1.6, folic acid 0.2, cyanocobalamin 0.001, retinyl palmitate 0.8 (500,000 IU/g), DL-α-tocopherol acetate 20 (2501 IU/g), cholecalciferol 0.0025, menaquinone 0.005, and sucrose to make 1kg
2)AIN-76 mineral mix (g/kg mix) : calcium phosphate dibasic 500, sodium chloride 74, potassium citrate monohydrate 220, potassium sulfate 52, magnesiumoxide 24, manganous carbohydrate 3.5, ferric citrate 6, zinc carbonate 1.6, cupric carbonate 0.3, potassium iodate 0.01, sodium selenite 0.01, chromium potassium sulfate 0.55, and sucrose to make 1kg.
3)Isovone 40 (Product No. HF-2002-038, Bioland Inc. Seoul, Korea) contains 40.9% of the total pure isoflavone which consists of 36.9% total aglycone type (22% daidzein, 11.7% glycitein, 6.39% genistein) and 4% of a total glycoside type.
The induction of diabetes and the evaluation of changes in body weight
Diabetes was induced by a single femur intramuscular injection of streptozotocin (STZ, Sigma Chemical, St Louis, MO, USA) at a dose of 50 mg/kg BW. STZ was dissolved immediately before use in a 0.1 M citrate buffer (pH 4.5). The same amount of citrate buffer (0.1 M, pH 4.5) was injected into the normal control animals. Twenty-four hours later, blood was drawn from the tail vein of the rats in the fasting state. The rats were considered to be diabetic only if their fasting blood glucose levels exceeded 180 mg/dL. The maintenance of a diabetic state was confirmed by measuring the fasting blood glucose level at week 5 of the experimental period. The weights of the animals were measured using an animal balance once a week during the seven week period.
The Survival rates
The Survival rate was calculated as the ratio of the number of animals survived at the end of experimental period to the number of animals at the beginning of the experiment.
Measuring gastrointestinal (GI) transit time
At the week 4 of the experiment, the animals were fed experimental diets, mixed with 0.5% Carmine red (Sigma Chemical, St Louis, MO. USA), which acts as a marker of GI transit. The excretion of the marker in the feces was checked every hour after feeding. The time interval between the initiation of feeding and the first detection of the marker in the feces was considered to be the GI transit time.
Assessing diabetic symptoms
During week 6 of the experimental period, each animal was held in a metabolic cage for 48 hours. Food intake, water intake and excreted urine volume were measured in order to assess the diabetic symptoms of the animals. Urine was collected in a 50 mL centrifuge tube treated with a few drops of toluene as preservative.
Oral glucose tolerance test (OGTT)
During week 7 of the experiment, the animals were deprived of their diets for 12 hours and the fasting blood sugar levels were measured from the tail vein. Subsequently, the oral administration of a 50% glucose solution (0.1 g glucose/100 g BW), using an intubation tube, was followed, and the postprandial blood sugar levels were measured from the tail vein at 30, 60, 120 and 180 minutes after ingestion. The blood glucose levels were measured using a blood glucose measuring instrument (Accutrend GC, Boehringer Mannheim, Germany).
Preparing blood and tissue samples
After fasting for 12 hours, animals were anesthetized by an intraperitoneal injection of 0.2 mL of 1% Ketamin hydrochloride per 100 g body weight, and the abdomen was opened. Blood was drawn from the inferior vena cava by heparinized syringes, and plasma was obtained by centrifugation at 3,000 rpm for 20 minutes, and stored frozen at -70℃ until analyses were performed. Liver, kidney, heart, and lung tissue samples were excised, and fat was removed. The samples were rinsed 3 times with a cold saline and blotted dry, weighed, and then frozen in liquid nitrogen.
Measuring plasma activities of glutamic oxaloacetic transaminase (GOT) and glutamic pyruvic transaminase (GPT).
The activity of GOT and GPT in the plasma samples was measured using an enzymatic analysis kit (Asan Pharmaceuticals, Hwasung, Korea), according to the Reitman-Frankel method (Reitman & Frankel, 1957).
Statistical analysis
Data were presented as means and standard errors. Group means were compared by an Analysis of Variance using Duncan's multiple range test and differences were considered to be statistically significant at a p value of less than 0.05. All statistical tests were performed using the SPSS program for Windows (SPSS, Chicago, IL, USA: Version 10.0)
Results
Body weight change and survival rates
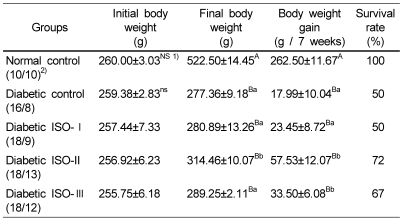
There were no significant differences in the initial body weight among all of the groups, however, after seven weeks of the testing, the final body weights and the mean body weight gains were significantly lower (p<0.05) in all diabetic groups, as compared with the normal control group. Among the diabetic groups, the mean weight gains of the ISO-II and ISO-III groups were significantly higher (p<0.05) than those of the diabetic control and ISO-I groups. The survival rates of the diabetic groups were lower than that of the normal control group. Among the diabetic groups, the ISO-II and ISO-III groups showed higher survival rate than that of the diabetic control or ISO-I groups (Table 2).
Table 2.
The effect of soybean isoflavone extract supplementation on the body weight changes and survival rate of STZ-induced diabetic rats
Abbreviations: ISO : isoflavone, STZ : streptozotocin
1)Mean±S.E., 2)Number of animals (initial/final)
Different capital superscripts in the same column indicate significant differences (p<0.05) among 5 groups by Duncan's multiple comparison test.
Different small superscripts in the same column indicate significant differences (p<0.05) among 4 diabetic groups by Duncan's multiple comparison test.
NS Not significantly different among the 5 groups (p<0.05)
ns Not significantly different among the 4 diabetic groups (p<0.05)
Organ weights
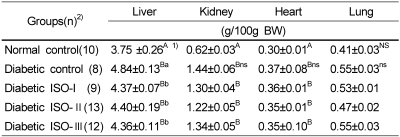
The mean relative weights of the liver, kidney and heart of all diabetic groups were significantly higher (p<0.05) than those of the normal control group. All of the isoflavone-supplemented groups tended to have lower mean organ weights than those of the diabetic control group even though the differences were not statistically significant (Table 3).
Table 3.
The effect of soybean isoflavone extract supplementation on organ weights in STZ-induced diabetic rats
Abbreviations: ISO: soybean isoflavone extract, STZ : streptozotocin
1)Mean±S.E., 2)Number of animals
Different capital superscripts in the same column indicate significant differences (p<0.05) among 5 groups by Duncan's multiple comparison test.
Different small superscripts in the same column indicate significant differences (p<0.05) among 4 diabetic groups by Duncan's multiple comparison test.
NS Not significantly different among the 5 groups (p<0.05)
ns Not significantly different among the 4 diabetic groups (p<0.05)
Diabetic symptoms
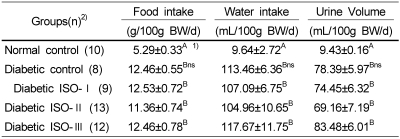
Feed and water consumption, and the volume of urine excretion for all diabetic groups were significantly (p<0.05) higher than those of the normal control group. Among the diabetic groups, the ISO-II group showed the lowest consumption of feed and water, and the least urine volume compared to other diabetic groups (Table 4).
Table 4.
The effect of soybean isoflavone extract supplementation on food and water intake and urine excretion in STZ-induced diabetic rats at week 5 of the experimental diet feeding
Abbreviations : ISO: isoflavone, STZ: streptozotocin
1)Mean±S.E., 2)Number of animals
Different capital superscripts in the same column indicate significant difference (p<0.05) among 5 groups by Duncan's multiple comparison test.
Different small superscripts in the same column indicate significant difference (p<0.05) among 4 diabetic groups by Duncan's multiple comparison test.
ns Not significantly different among the 4 diabetic groups (p<0.05)
The gastrointestinal (GI) transit time
The mean GI transit time of the diabetic groups was significantly (p<0.05) shorter than that of the normal control group. Among the diabetic groups, the mean GI transit time of the ISO-II group was longer than that of the other diabetic groups, but similar to that of the normal control group (Table 5).
Table 5.
The effect of soybean isoflavone extract supplementation on gastrointestinal (GI) transit time in STZ-induced diabetic rats
Abbreviations ; ISO : isoflavone, STZ : streptozotocin
1)Mean±S.E., 2)Number of animals
Different superscripts in the same column indicate significant differences (p<0.05) between the 5 groups by Duncan's multiple comparison test.
Glucose tolerance
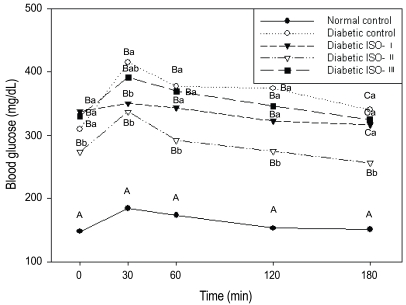
In the oral glucose tolerance test, the diabetic groups had significantly higher levels of blood glucose (p<0.05) than the normal control group. Among the diabetic groups, the ISO-II group showed significantly lower (p<0.05) fasting and postprandial blood glucose levels at all time points compared with the other diabetic groups. With regard to the time-course change, blood glucose reached its highest level at 30 minutes after the administration of glucose regardless of the group type. The levels were decreased gradually and returned to their fasting levels at 120 minutes for the ISO-I and ISO-II groups, and at 180 minutes for the ISO-III group (Figure 1).
Fig. 1.
Blood glucose curves during OGTT in normal and diabetic rats.
At week 7 of the experimental period, the rats were administered with a glucose solution (0.1 g/100g B.W.) and the plasma glucose was determined at 0, 30, 60, 120, 180min after glucose load. Abbreviations: ISO: soybean isoflavone extract. Different capital letters at the same time point indicate significant differences (p<0.05) among the five groups and different small letters at the same time point indicate significant differences (p<0.05) among the four diabetic groups by Duncan's multiple comparison test.
The activity of GOT and GPT in plasma
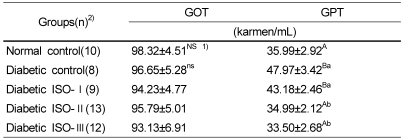
There were no significant differences regarding the plasma GOT activity in any of the groups, however, GPT activity was significantly lower (p<0.05) in the normal, ISO-I, ISO-II groups than in the diabetic control and ISO-I groups (Table 6).
Table 6.
The effect of soybean isoflavone extract supplementation on the activity of plasma transaminases in STZ-induced diabetic rats
Abbreviations: ISO: isoflavone, STZ: streptozotocin, GOT: glutamic oxaloacetic transaminase, GPT: glutamic pyruvate transaminase
1)Mean±S.E., 2)Number of animals
Different capital superscripts in the same column indicate significant differences (p<0.05) among the 5 groups by Duncan's multiple comparison test.
Different small superscripts in the same column indicate significant differences (p<0.05) among the 4 diabetic groups by Duncan's multiple comparison test.
NS Not significantly different among the 5 groups (p<0.05)
ns Not significantly different among the 4 diabetic groups (p<0.05)
Discussion
The present study investigated the effect of diet that contained different levels of soy isoflavone extract with regard to changes in body weight and the survival rate of diabetic animals, as an indicator of overall health conditions. It has been observed that the supplementation of soybean isoflavone, at the intake levels of 3.0 and 30 mg per kilogram of body weight per day, resulted in the suppression of body weight loss and an increase in the survival rate of the diabetic animals compared to the diabetic control group. Even though animal model was different, Bartke et al. (2004) recently reported that a dietary isoflavone intake can prolong lifespan and enhance the capacity for glucose tolerance in normal mice. Hermansen et al. (2001) demonstrated that soybean and its isoflavones had a beneficial effect on carbohydrate and lipid metabolism in diabetic animals. Other studies (Ali et al., 2004; Bhatena & Velasquez, 2002) also provided evidence regarding a wide range of health benefits of soybean isoflavones in diabetes including favorable altering of insulin resistance, glycemic control, and serum lipid control. Based on these results, it appears that soybean isoflavones could improve the overall metabolism of diabetic animals, thus resulting in suppressing weight loss and increasing the survival rates. However, the exact mechanisms by which isoflavones exhibited their beneficial effect on reducing death rate of diabetic animals remain unclear.
After seven weeks of being fed a diet, all diabetic groups showed significantly higher organ weights (liver, kidney and heart), as compared to those of the normal control group. In particular, the average size of the kidney of the diabetic groups was more than twice that of the normal group, indicating that renal enlargement commonly occurs in diabetes. Among the diabetic groups, however, the isoflavone-supplemented groups showed significantly lower liver weight and relatively lower overall organ weight compared to the diabetic control group. Similar results were reported by Ali et al. (2004), in which isoflavone significantly decreased liver and kidney weights in both lean and obese SHR/N-cp rats which are a genetic animal model of obesity and type 2 diabetes. Even though the fat content in organs was not measured, we could guess that soybean isoflavones may reduce the accumulation of fat in the liver and kidney since the antilipogenic effect of isoflavones had been reported in previous studies (Naaz et al., 2003; Duncan et al., 1999; Kawano-Takahashi, 1986). In diabetes, long-lasting hyperglycemia has been known to lead to chronic renal complications and finally, proteinuria and uremia (Humphrey & Ballard, 1990). There are several studies which have reported on the beneficiary effect of dietary soybean and soy protein with regard to improving kidney function in Type II diabetes with the nephropathy (Teixeira et al., 2004; Azadbakht et al, 2003) or retardation of the development and progression of chronic renal disease in human and animals (Anderson et al., 1999). The results of this study support the possibility that isoflavones in soybeans might be effective in preventing renal enlargement in diabetic animals.
Although our data demonstrated that supplementing with isoflavone is favorable to body weight maintenance and surviving of diabetic animals, there was no significantly positive effect on the alleviating classical diabetic symptoms, the so called "3 polys (polydipsia, polyuria, polyphagia)". However, the ISO-II group showed the least consumption of water and feed and the least excretion of urine among the diabetic groups. Therefore, it is expected that, when the period of supplementation is longer, soybean isoflavones may reveal a more evident effect on lessening diabetic symptoms through improving the overall metabolism in the diabetic animals.
The present study demonstrated that a certain level of soybean isoflavones supplement may improve glycemic control in diabetic rats. The intake level of isoflavones, which exerted a favorable effect on glucose tolerance, was 3.0 mg per kilogram of body weight (ISO-II group). With regard to glycemic control by soy isoflavones, there have been inconsistent reports. Hsu et al. (2003) examined the effect of diets that contained different levels of isoflavones on diabetic animals and reported that there were no differences in plasma glucose or insulin levels. Animals were fed with tremendously high levels of isoflavones (240, 480, 1920 mg/100g diet) in that experiment, compared with this study (0.4, 2.5, 25 mg/100g diet). When considering that the daily feed intake of mature rats is around 30 gram, the range of daily intake of isoflavones in the previous study was approximately equivalent to 360~2880 mg/kg of body weight. It is much higher levels compared with this study (0.5~30 mg per kilogram of body weight), however, no favorable effect on plasma glucose was found. At this point, it is not clear whether different dosages significantly alter glucose tolerance results. On the other hand, there have been several studies which have shown that isoflavone is favorable toward carbohydrate metabolism. Lee and Lee (2001) demonstrated a beneficial effect of soy isoflavone on glycemic control in diabetes, by acting as α-glucosidase inhibitors in the intestinal brush border uptake of glucose. Also, the benefit of soy protein and genistein has been proven in diabetic rats (Lee, 2006), in which the plasma insulin level was increased and the blood glucose level was significantly decreased during the oral glucose tolerance test. Dyrskog et al. (2005) demonstrated that a soy diet lowered levels of fasting blood glucose and improved insulin sensitivity in Type 2 diabetic obese Zucker fatty rats. The beneficial effect of soy protein on glucose tolerance and insulin sensitivity has been reported also in normal rats (Lavigne et al., 2000). From these studies, it appears that soy protein including isoflavone may have favorable effect on glucose metabolism in either normal or diabetic conditions. The effective intake level of soy isoflavones which could exhibit a favorable action on glucose metabolism is not suggested from the previous reports. In this study, it has been observed that soy isoflavones improved glucose tolerance in diabetic rats only at a specific dose level (3.0 mg per kilogram of body weight per day). Moreover, the diabetic group which showed a hypoglycemic response demonstrated a significantly longer GI transit time compared with the other diabetic groups. This result suggests the possibility of a correlation between GI transit time and glucose tolerance in diabetic animals. In other words, the addition of soybean isoflavones to the diet may have an effect on the control of postprandial blood glucose level by prolonging the GI transit time, resulting in slowing the rate of digestion as well as absorption of meal components.
To examine the effect of an isoflavone supplement on liver function, the activity of plasma transaminases, GOT and GPT, was measured. In the present study, the mean GPT activity levels of the ISO-II and ISO-III groups were similar to that of the normal group and they were significantly (p<0.05) lower than that of the diabetic control and ISO-I groups. The activity of GOT was not affected by isoflavone supplement. Ali et al. (2005) also observed a significant decrease in AST (=GOT) and ALT (=GPT) in rats that were fed isoflavones (0.1% isoflavone mixture). Therefore, we can conclude that isoflavones are not harmful to the liver at the dosage level up to 30 mg per kilogram of body weight because the activity of these enzymes increases when the liver is damaged.
In conclusion, the present study has demonstrated that the soybean isoflavone extract may be beneficial to diabetic animals by improving their glucose tolerance and suppressing weight loss without incurring hepatotoxicity at the daily dosage of 3.0 mg per kilogram of body weight.
Footnotes
This work was supported by grant No. R01-2000-00084 from the Basic Research Program of the Korea Science and Engineering Foundation. We extend our appreciation to Bioland Inc. (Seoul, Korea) for their generous donation of soybean isoflavone extracts.
References
- 1.Alekel DL, Germain AS, Peterson CT, Hanson KB, Stewart JW, Toda T. Isoflavone-rich soy protein isolate attenuates bone loss in the lumbar spine of perimenopausal women. Am J Clin Nutr. 2000;72:844–852. doi: 10.1093/ajcn/72.3.844. [DOI] [PubMed] [Google Scholar]
- 2.Ali AA, Velasquez MT, Hansen CT, Mohamed AI, Bhathena SJ. Modulation of carbohydrate metabolism and peptide hormones by soybean isoflavones and probiotics in obesity and diabetes. J Nutr Biochem. 2005;16:693–699. doi: 10.1016/j.jnutbio.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Ali AA, Velasquez MT, Hansen CT. Effects of soy bean isoflavones, probiotics, and their interactions on lipid metabolism and endocrine system in an animal model of obesity and diabetes. J Nutr Biochem. 2004;15:583–590. doi: 10.1016/j.jnutbio.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Anderson JW, Smith BM, Washnock CS. Cardiovascular and renal benefits of dry bean and soybean intake. Am J Clin Nutr. 1999;70:464S–474S. doi: 10.1093/ajcn/70.3.464s. [DOI] [PubMed] [Google Scholar]
- 5.Anderson JW, Blake JE, Turner J, Smith BM. Effects of soy protein on renal function and proteinuria in patients with type 2 diabetes. Am J Clin Nutr. 1998;68:1347S–1353S. doi: 10.1093/ajcn/68.6.1347S. [DOI] [PubMed] [Google Scholar]
- 6.Aoyama T, Fukui K, Takamatsu K, Hashimoto Y, Yamamoto T. Soy protein isolate and its hydrolysate reduce body fat of dietary obese rats and genetically obese mice (yellow KK) Nutrition. 2000;16:349–354. doi: 10.1016/s0899-9007(00)00230-6. [DOI] [PubMed] [Google Scholar]
- 7.Arjmandi BH, Alekel L, Hollis BW, Amin D, Stacewicz-Sapuntzakis M, Guo P, Kukreja SC. Dietary soybean protein prevents bone loss in an ovariectomized rat model of osteoporosis. J Nutr. 1996;126:161–167. doi: 10.1093/jn/126.1.161. [DOI] [PubMed] [Google Scholar]
- 8.Azadbakht L, Shakerhosseini R, Atabak S, Jamshidian M, Mehrabi Y, Esmaill-Zadeh A. Beneficiary effect of dietary soy protein on lowering plasma level lipids and improving kidney function in type II diabetes with nephropathy. Eur J Clin Nutr. 2003;57:1292–1294. doi: 10.1038/sj.ejcn.1601688. [DOI] [PubMed] [Google Scholar]
- 9.Baird DD, Umbach DM, Lansdell L, Hughes CL, Setchell KD, Weinberg CR, Haney AF, Wilcox AJ, Mclachlan JA. Dietary intervention study to assess estrogenicity of dietary soy among postmenopausal women. J Clin Endocrinol Metab. 1995;80:1685–1690. doi: 10.1210/jcem.80.5.7745019. [DOI] [PubMed] [Google Scholar]
- 10.Bartke A, Peluso MR, Moretz N, Wright C, Bonkowski M, Winters TA, Shanahan MF, Kopchick JJ, Banz WJ. Effects of soy-derived diets on plasma and liver lipids, glucose tolerance, and longevity in normal, long-lived and short-lived mice. Horm Metab Res. 2004;36:550–558. doi: 10.1055/s-2004-825796. [DOI] [PubMed] [Google Scholar]
- 11.Bhatena SJ, Velasquez MT. Beneficial role of dietary phytoestrogens in obesity and diabetes. Am J Clin Nutr. 2002;76:1191–1201. doi: 10.1093/ajcn/76.6.1191. [DOI] [PubMed] [Google Scholar]
- 12.Bosello O, Cominacini L, Zocca I, Garbin U, Compri R, Davol A, Brunetti L. Short- and long-term effects of hypocaloric diets containing proteins of different sources on plasma lipids and apoproteins of obese subjects. Ann Nutr Metab. 1998;32:206–214. doi: 10.1159/000177443. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Anderson JJB. Isoflavones inhibit proliferation of ovarian cancer cells in vitro via an estrogen receptor-dependent pathway. Nutr Cancer. 2001;41:156–170. doi: 10.1080/01635581.2001.9680628. [DOI] [PubMed] [Google Scholar]
- 14.Constantinou AI, Lantvit D, Hawthorne M, Xu X, van Breemen RB, Pezzuto JM. Chemopreventive effects of soy protein and purified isoflavones on DMBA-induced mammary tumors in female Sprague-Dawley rats. Nutr Cancer. 2001;41:75–81. doi: 10.1080/01635581.2001.9680615. [DOI] [PubMed] [Google Scholar]
- 15.Duncan AM, Underhill KE, Xu X, Lavalleur J, Phipps WR, Kurzer MS. Modest hormonal effects of soy isoflavones in postmenopausal women. J Clin Endocrinol Metab. 1999;84:3479–3484. doi: 10.1210/jcem.84.10.6067. [DOI] [PubMed] [Google Scholar]
- 16.Dyrskog SEU, Jeppesen PB, Colombo M, Abudula R, Hermansen K. Preventive effects of a soy-based diet supplemented with stevioside on the development of the metabolic syndrome and type 2 diabetes in Zucker diabetic fatty rats. Metabolism. 2005;54:1181–1188. doi: 10.1016/j.metabol.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 17.Goodman-Gruen D, Kritz-Silverstein D. Usual dietary isoflavone intake is associated with cardiovascular disease risk factors in postmenopausal women. J Nutr. 2001;131:1202–1206. doi: 10.1093/jn/131.4.1202. [DOI] [PubMed] [Google Scholar]
- 18.Hermansen K, Sondergaard M, Hoie L, Carstensen M, Brock B. Beneficial effects of a soy-based dietary supplement on lipid levels and cardiovascular risk markers in type 2 diabetic subjects. Diabetes Care. 2001;24:228–233. doi: 10.2337/diacare.24.2.228. [DOI] [PubMed] [Google Scholar]
- 19.Hsu CS, Chiu WC, Yeh SH. Effects of soy isoflavone supplementation on plasma glucose, lipids and antioxidant enzyme activities in streptozotocin-induced diabetic rats. Nutr Res. 2003;23:67–75. [Google Scholar]
- 20.Humphrey LL, Ballard DJ. Renal complications in non-insulin-dependent diabetes mellitus. Clin Geriatr Med. 1990;6:807–825. [PubMed] [Google Scholar]
- 21.Hussain M, Banerjee M, Sarkar FH, Djuric Z, Pollak MN, Doerge D, Fontana J, Chinni S, Davis J, Forman J, Wood DP, Kucuk O. Soy isoflavones in the treatment of prostate cancer. Nutr Cancer. 2003;47:111–117. doi: 10.1207/s15327914nc4702_1. [DOI] [PubMed] [Google Scholar]
- 22.Jenkins DJ, Kendall CW, Jackson CJ, Connelly PW, Parker T, Faulkner D, Vidgen E, Cunnane SC, Leiter LA, Josse RG. Effects of high- and low-isoflavone soyfoods on blood lipids, oxidized LDL, homocysteine, and blood pressure in hyperlipidemic men and women. Am J Clin Nutr. 2002;76:365–372. doi: 10.1093/ajcn/76.2.365. [DOI] [PubMed] [Google Scholar]
- 23.Kawano-Takahashi Y, Ohminami H, Okuda I. Effect of soy saponins on thioglucose (GTG) induced obesity in mice. Int J Obes. 1986;10:293–302. [PubMed] [Google Scholar]
- 24.Lajolo FM, Hassimotto NMA, Genovese MI. Isoflavone profile and antioxidant activity of Brazilian soybean varieties. Food Sci Technol Int. 2005;11:205–211. [Google Scholar]
- 25.Lavigne C, Marette A, Jacques H. Cod and soy proteins compared with casein improve glucose tolerance and insulin sensitivity in rats. Am J Physiol Endocrinol Metab. 2000;278:E491–E500. doi: 10.1152/ajpendo.2000.278.3.E491. [DOI] [PubMed] [Google Scholar]
- 26.Lee DS, Lee SH. Genistein, a soy isoflavone, is a potent alpha-glucosidase inhibitor. FEBS Lett. 2001;501:84–86. doi: 10.1016/s0014-5793(01)02631-x. [DOI] [PubMed] [Google Scholar]
- 27.Lee JS. Effects of soy protein and genistein on blood glucose, antioxidant enzyme activities, and lipid profile in streptozotoc-ininduced diabetic rats. Life Sci. 2006;79:1578–1584. doi: 10.1016/j.lfs.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 28.Lee SK, Yoon S, Kwon DJ. Estimated isoflavone intake from soy products in Korean middle-aged women. J Korean Soc Food Sci Nutr. 2000;29:948–956. [Google Scholar]
- 29.Naaz A, Yellayi S, Zakroczymski MA. The soy isoflavone genistein decreases adipose deposition in mice. Endocrinology. 2003;144:3315–3320. doi: 10.1210/en.2003-0076. [DOI] [PubMed] [Google Scholar]
- 30.Reitman S, Frankel S. A colorimetic method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminase. Am J Clin Pathol. 1957;2:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 31.Ren MQ, Kuhn G, Wegner J, Chen J. Isoflavone, substances with multi-biological and clinical properties. Eur J Nutr. 2001;40:135–146. doi: 10.1007/pl00007388. [DOI] [PubMed] [Google Scholar]
- 32.Ruiz-Larrea MB, Mohan AR, Paganga G., Miller NJ, Bolwell GP, Rice-Evans CA. Antioxidant activity of phytoestrogenic isoflavones. Free Radic Res. 1997;26:63–70. doi: 10.3109/10715769709097785. [DOI] [PubMed] [Google Scholar]
- 33.Satio M. Effect of soy peptides on energy metabolism in obese animals. Nutr Sci Soy Protein. 1991;12:91–94. [Google Scholar]
- 34.Setchell KD. Soy isoflavones-benefits and risks from nature's selective estrogen receptor modulators (SERMs) J Am Coll Nutr. 2001;20:354S–362S. doi: 10.1080/07315724.2001.10719168. [DOI] [PubMed] [Google Scholar]
- 35.Setchell KD, Cassidy A. Dietary isoflavones: Biological effects and relevance to human health. J Nutr. 1999;129:758S–767S. doi: 10.1093/jn/129.3.758S. [DOI] [PubMed] [Google Scholar]
- 36.Somekawa Y, Chiguchi M, Ishibashi T, Aso T. Soy intake related to menopausal symptoms, serum lipids, and bone mineral density in postmenopausal Japanese women. Obstet Gynecol. 2001;97:109–115. doi: 10.1016/s0029-7844(00)01080-2. [DOI] [PubMed] [Google Scholar]
- 37.Teede HJ, Dalais FS, Kotsopoulos D, Liang YL, Davis S, McGrath BP. Dietary soy has both beneficial and potentially adverse cardiovascular effects: a placebo-controlled study in men and postmenopausal women. J Clin Endocrinol Metab. 2001;86:3053–3060. doi: 10.1210/jcem.86.7.7645. [DOI] [PubMed] [Google Scholar]
- 38.Teixeira SR, Tappenden KA, Carson L, Jones R, Parabhudesai M, Marsl WP, Erdman JW., Jr Isolated soy protein consumption reduces urinary albumin excretion and improves the serum lipid profile in men with type 2 diabetes mellitus and nephropathy. J Nutr. 2004;134:1874–1880. doi: 10.1093/jn/134.8.1874. [DOI] [PubMed] [Google Scholar]
- 39.Taha SA, Wasif MM. Hypoglycemic effect and protein nutritive quality of soy and methionine supplemented whole durum pasta products. Nahrung. 1996;40:281–287. doi: 10.1002/food.19960400512. [DOI] [PubMed] [Google Scholar]
- 40.Tham DM, Gardner CD, Haskell WL. Potential health benefits of dietary phytoestrogens: A review of the clinical, epidemiological, and mechanistic evidence. J Clin Endocrinol Metab. 1998;83:2223–2235. doi: 10.1210/jcem.83.7.4752. [DOI] [PubMed] [Google Scholar]
- 41.Upmalis DH, Lobo R, Bradley L, Warren M, Cone FL, Lamia CA. Vasomotor symptom relief by soy isoflavone extract tablets in postmenopausal women: A multicenter, double-blind, randomized, placebo-controlled study. Menopause. 2000;7:236–242. doi: 10.1097/00042192-200007040-00005. [DOI] [PubMed] [Google Scholar]
- 42.Vedavanam K, Srijayanta S, O'Reilly J, Raman A, Wiseman H. Antioxidant action and potential antidiabetic properties of an isoflavonoid-containing soyabean phytochemical extract (SPE) Phytother Res. 1999;13:601–608. doi: 10.1002/(sici)1099-1573(199911)13:7<601::aid-ptr550>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 43.Wang HJ, Murphy PA. Isoflavone content in commercial soybean foods. J Agric Food Chem. 1994;42:1666–1673. [Google Scholar]
- 44.Washburn S, Burke GL, Morgan T, Anthony M. Wrem BG, editor. Effect of soy protein supplementation on serum lipoproteins, blood pressure, and menopausal symptoms in premenopausal women. Menopause. 1999;6:7–13. [PubMed] [Google Scholar]
- 45.Wei H, Bowen R, Cai Q, Barnes S, Wang Y. Antioxidant and antipromotional effects of the soybean isoflavone genistein. Proc Soc Exp Biol Med. 1995;208:124–130. doi: 10.3181/00379727-208-43844. [DOI] [PubMed] [Google Scholar]
- 46.Yamashita T, Sasahara T, Pomeroy SE, Collier G, Nestel PG. Arterial compliance, blood pressure, plasma leptin, and plasma lipids in women are improved with weight reduction equally with a meat-based diet and a plant-based diet. Metabolism. 1998;47:1308–1314. doi: 10.1016/s0026-0495(98)90297-9. [DOI] [PubMed] [Google Scholar]
- 47.Zhang M, Xie X, Lee AH, Binns CW. Soy isoflavone intake are associated with reduced risk of ovarian cancer in southeast China. Nutr Cancer. 2004;49:125–130. doi: 10.1207/s15327914nc4902_2. [DOI] [PubMed] [Google Scholar]