Abstract
The purpose of the present study was to examine the relation of total antioxidant status (TAS) to metabolic risk factors in Korean adults. Anthropometric measures, blood pressure, serum lipids and fasting glucose were determined in 406 men and women. TAS was measured by using commercially available Randox kit. Serum TAS was significantly positively correlated with body weight (p=0.004), body mass index (BMI) (p=0.033), waist circumference (p=0.017), total cholesterol (p=0.038) and triglyceride (TG) (p<0.001). The mean TAS of hypertriglyceridemic subjects (TG ≥150 mg/dl) was significantly higher than that of subjects whose TG was lower than 150 mg/dl (p=0.001). When central obesity, TG, high density lipoprotein cholesterol, fasting glucose and blood pressure were considered as metabolic risk factors, TAS was shown to be elevated with increased number of metabolic risk factors (p=0.004). The positive association between TAS and a number of metabolic risk factors suggests that increased TAS may not always indicate one's healthier condition. In order to help understand TAS as a marker of total antioxidant capacity in humans with various metabolic conditions, it is needed to clarify the factors affecting TAS in relation to changes in metabolic risk factors.
Keywords: Total antioxidant status, metabolic risk factor, obesity, triglyceride, human
Introduction
Total antioxidant capacity (TAC) assays have been designed to determine overall antioxidant power of samples contributed by antioxidant and their interactions. Evaluation of TAC in body fluid has been used as one of the biological markers for monitoring oxidative stress in humans (Kim et al., 2000; Kim et al., 2001; Mendoza-Nunez et al., 2001). In order to assess TAC, several methods have been developed and used for the last 20 years. The oxygen radical absorbance capacity (ORAC) (Cao et al., 1993), the ferric reducing ability of plasma (FRAP) (Benzie & Strain, 1996) and the trolox equivalent antioxidant capacity (TEAC)(Miller et al., 1993) are well known methods. The TEAC assay is based on the inhibition of the absorbance of ABTS (2,2'-Azino-di-[3-ethylbenzthiazoline sulphonate]) radical cation by antioxidants in the tested sample. In addition, the TEAC assay of Miller et al. (1993) has been commercialized as "Total Antioxidant Status (TAS)" kit by Randox Laboratories and introduced its automated way of assay.
While the pathological increase of free radical generation has been well recognized in various disease states, the commercially available TAS kit has been widely used to determine in vivo free radical trapping power in patients with various disease conditions. In relation with several diseases, plasma or serum TAS was consistently decreased in patients with cancer (Jeon et al., 1998), hypertension (Kim et al., 2006), osteoporosis (Altindag et al., 2007) and psoriasis (Kural et al., 2003), but was not significantly changed in patients with acute myocardial infarction (Markovic et al., 2000). Besides disease conditions, TAS assay also has been employed as a marker in determining age-related oxidative stress in healthy humans and exercise induced oxidative stress in athletes. TAS has shown to be decreased in older people (Mendoza-Nunez et al., 2007), but increased in athletes right after exercise (Kim et al., 2001; Magalhaes et al., 2007). These findings suggested that oxidative stress inducing conditions might not always results in reduction of TAS in circulation. Previous studies on antioxidant capacity in people with overweight and obesity have failed to show decreased TAS level (Bae, 2006; Kim et al., 2000; Molnar et al., 2004), although increased oxidative stress with obesity has been well presented (Mohn et al., 2007).
In previous studies, no consensus was made in the relationship between serum or plasma TAS and oxidative stress, since serum or plasma TAS has not consistently reflect the conditions which are considered to have increased oxidative stress. In addition, there are relatively large number of studies looking at changes in TAS with certain metabolic conditions such as diseases, exercise, obesity and diet alteration with antioxidant supplementation (Rasool et al., 2006; Tsakiris et al., 2007). However, there is still lack of studies to assess TAS for large numbers of people in various metabolic conditions and to examine how TAS are related with individual metabolic risk factors associated with various metabolic conditions. Therefore, the purpose of the present study was to examine the relationship between serum TAS and metabolic risk factors such as obesity, high blood pressure, high serum lipid and glucose. It is expected that the findings from the current study can be utilized as basic data to evaluate total antioxidant capacity of Korean adults using the commercially available TAS assay.
Subjects and Methods
Study participants included 406 Korean adult men and women living in the Seoul area. A single blood sample from each subject was collected in vacutainer tubes after an overnight fast. Serum samples were separated from whole blood for analyzing lipid, glucose, total antioxidant status and the sample aliquots were stored at -80℃ until analyses. Subjects' weight, height, and waist and hip circumferences were measured by trained technicians. The BMI was calculated as weight (kg) divided by height (m2). Waist circumference to hip circumference ratio (WHR) was calculated as the ratio between waist and hip circumferences. Trained technicians were assigned to measure blood pressure with a semiautomatic device, with subjects in the sitting position after at least 2 min of rest.
Serum total cholesterol (TC), low-density lipoprotein (LDL) cholesterol, high density lipoprotein (HDL) cholesterol, triglyceride (TG) and fasting glucose concentrations were measured by ADVIA® 1650 chemistry system (Bayer HealthCare LLC, Germany). Total antioxidant status (TAS) in serum was measured by total antioxidant quantification using ABTS+ (2,2'-Azino-di-[3-ethylbenzthiazoline sulphonate]) radical formation with the commercially available Randox Total Antioxidant Status test kit (Randox Lab., Ltd., UK). Assay was performed using the Victor multilabel microplate reader (PerkinElmer Life and Analytical Sciences, Inc., USA) with a slight modification (Miller et al., 1993).
Statistical analyses were done using SPSS for windows (version 12.0, SPSS Inc, Chicago). Means and standard deviation of measures were calculated for all subjects, male and female, and subjects with and without individual metabolic risk factors and metabolic syndrome. Based on metabolic risk factors which defines metabolic syndrome presented by the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III, 2001), metabolic risk factors were defined as TG (≥150 mg/dl), HDL (male <40 mg/dl, female <50 mg/dl), blood pressure (≥130/85 mmHg), impaired fasting glucose and obesity. The level of impaired fasting glucose was defined as fasting glucose ≥100 mg/dl, as presented by the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus (2003). Obesity was defined by waist circumference (male ≥90 cm, female ≥85 cm) based on the definition newly presented by the Korean Society for the Study of Obesity (Lee et al., 2007) and BMI (≥25) based on Asia-Pacific definition presented by World Health Organization (WHO) West Pacific Region (2000). Subjects were divided into two groups with and without metabolic risk factor such as obesity, dyslipidemia, increased blood pressure and fasting glucose. Of tested metabolic risk factors, TG, HDL, blood pressure, fasting glucose and central obesity, subjects who had 3 and more metabolic risk factors were considered to be the group with metabolic syndrome. Differences of mean TAS between the groups were assessed by independent two-tailed t-tests. Pearson's correlation coefficients were used to assess the relationship between two variables of TAS and metabolic risk factors. The correlation between the number of metabolic syndrome components and TAS level was assessed by Spearman's correlation coefficient by rank. In all analyses, p<0.05 was considered statistically significant.
Results
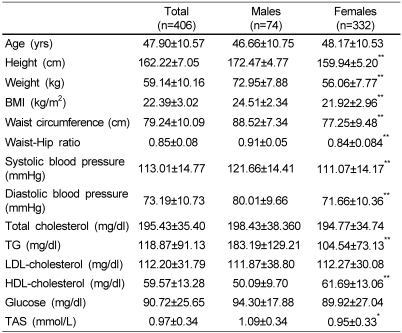
Anthropometric and biochemical measures of subjects are shown in Table 1. Four hundreds six subjects were consisted of 74 males and 332 females. Mean ages of subjects were 47.9 ± 10.6 years for all subjects, 46.7 ± 10.8 years for males, and 48.2 ± 10.5 years for females. Age ranges of male and female subjects were from 27 to 68, and from 20 to 78 years, respectively. The proportions of each age group for male subjects were 3, 26, 36, 19, and 16% for 20s, 30s, 40s, 50s and 60s, respectively. For female subjects, each proportion of 20s, 30s, 40s, 50s, 60s and 70s was 3, 20, 31, 31, 11 and 4%, respectively. Among all subjects, there were 229 subjects aged <50 years and 177 subjects aged ≥50 years. Of all measures, the mean height, weight, BMI, waist circumference, WHR, blood pressure and level of TG were significantly higher in males than females (p<0.001). In addition, serum TAS was shown to be higher in males than females (p=0.001). However, there was no significant difference in mean levels of TC, LDL and fasting glucose between gender groups, and the mean level of HDL was significantly higher in the female group (p<0.001).
Table 1.
Anthropometric and biochemical indicators of subjects Mean ± SD
*p<0.01, **p<0.001, significantly different from males by independent samples t-test
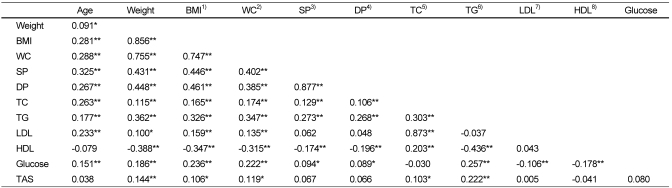
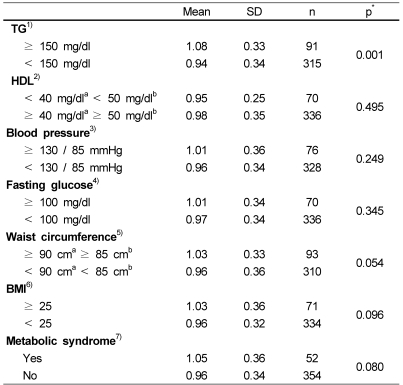
Table 2 shows the Pearson's correlation coefficient between TAS and tested metabolic risk factors. TAS was significantly positively correlated with weight (p=0.004), BMI (p=0.033), waist circumference (p=0.017), TC (p=0.038) and TG (p<0.001). Table 3 shows the comparison of TAS between groups with and without metabolic risk factors, such as hypertriglyceridemia (≥150 mg/dl), low HDL concentration (male <40 mg/dl, female <50 mg/dl), high blood pressure (≥130/85 mmHg), hyperglycemia (fasting glucose ≥100 mg/dl), central obesity (waist circumference, male ≥90 cm, female ≥85 cm) and BMI (≥25). Among the 406 subjects, 91 (male 39, female 52) subjects had serum TG level ≥150 mg/dl, and their mean TAS was significantly higher (p=0.001) than mean TAS of subjects whose serum TG levels were <150 mg/dl. In addition, the mean TAS of 93 (male 30, female 63) central obese subjects and of 71 (male 28, female 43) obese subjects (BMI ≥25) were shown to be 7-8% higher than relative mean TAS of non-obese subjects, but this difference did not reach the significance (p=0.054, 0.096). Unlike to these results, mean TAS of a group with low HDL concentrations (15 males and 55 females) showed no notable difference as compared to the group with normal HDL concentrations. A total of 76 (male 26, female 50) and 70 (male 20, female 50) subjects were found to have high blood pressure (≥130/85 mmHg) and fasting hyperglycemia (fasting glucose ≥100 mg/dl), respectively. When groups with and without these metabolic risk factors, the groups with high blood pressure or hyperglycemia showed a small tendency to have higher (4-5%) mean TAS than groups without these metabolic risk factors.
Table 2.
Pearson's correlation coefficients between measures
*Correlation is significant at the 0.05 level
**Correlation is significant at the 0.01 level
1)BMI: Body mass index, 2)WC: Waist circumference, 3)SP: Systolic blood pressure, 4)DP: Diastolic blood pressure, 5)TC: Total cholesterol, 6)TG: Triglyceride, 7)LDL: Low density lipoprotein, 8)HDL: High density lipoprotein
Table 3.
Comparison of TAS between groups with and without metabolic risk factors
1),2),3)National Cholesterol Education Program Adult Treatment Panel III (2001)
4)Expert Committee on the Diagnosis and Classification of Diabetes Mellitus (2003)
5)Korean Society for the Study of Obesity (2007)
6)Asia-Pacific definition presented by World Health Organization West Pacific Region (2000)
7)Subjects who had 3 and more metabolic risk factors (Waist circumference, TG, HDL, blood pressure and fasting glucose)
aMale, bFemale
*Significance by independent samples t-test
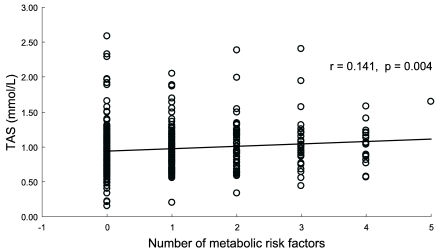
Table 3 also shows a comparison of mean TAS between subjects with and without metabolic syndrome. Twenty two males and 30 females had 3 and more metabolic risk factors among increased TG, fasting glucose, blood pressure, low HDL and central obesity. Mean TAS of 52 subjects with metabolic syndrome was 9% higher than that of subjects without metabolic syndrome, but the difference did not reach its significance (p=0.08). When subjects were divided into two groups (older ≥50 y, younger <50 y), 34 out of 177 older subjects and 18 out of 229 younger subjects had metabolic syndrome. In each older and younger age group, the mean TAS of subjects with metabolic syndrome showed 8 and 10% higher than that of subjects without metabolic syndrome, respectively. However, these differences did not reach their significance (p>0.1). Of all subjects, 183 did not have any of the 5 metabolic risk factors, and their mean TAS (0.94 ± 0.36) tended to be lower than the 52 subjects with ≥3 metabolic risk factors (p=0.052). Fig. 1 illustrates the distribution of TAS according to the number of metabolic syndrome components, and the median TAS was elevated with increasing metabolic risk factors (p=0.004).
Fig. 1.
Distribution of the serum TAS according to the number of metabolic risk factors
The correlation between the number of metabolic syndrome components and TAS level by Spearman's rank correlation coefficient.
Discussion
TAC measured by commercially available TAS tended to be increased with various metabolic risk factors, especially obesity and increased serum lipids. TAS was significantly positively related with increased weight, BMI and waist circumference, and tended to be higher in obese subjects. Obesity has shown to increase oxidative stress by increased LDL oxidation and malondialdehyde (MDA), and these markers of oxidative stress have found to be related with BMI and WHR (Mohn et al., 2007). However, TAC measured with various methods has not seemed to decrease in overweight and obese people (Bae, 2006; Kim et al., 2000; Molnar et al., 2004; Sharifian et al., 2005). Similar to the findings from the present study, TAS was significantly higher in overweight adult males (Kim et al., 2000) and tended to be, but not significantly, higher in obese children (Molnar et al., 2004), as compared to controls. In addition, TAS was found to be significantly positively correlated with weight and BMI in 158 adults with and without hypertension (Kim et al., 2006). These findings including the results from the present study consistently suggest that weight gain may increase serum or plasma TAS level.
In previous studies, obesity has often been accompanied with increased serum TG and TC (Ahn et al., 2005; Kim & Oh, 2007). The present study also showed a significantly positive association of serum TG and TC with weight, BMI and waist circumference. In conjunction with obesity, serum TG and TC significantly positively correlated with TAS, and subjects with high serum TG (≥150 mg/dl) showed that their mean TAS was significantly higher than subjects whose serum TG were lower than 150 mg/dl. However, the findings in the association between TAS and increased TG and/or TC in various metabolic conditions have shown to vary. When sedentary ex-athletes were compared with physically active ex-athletes, there was no difference in TAS, while TG and TC were significantly higher in sedentary ex-athletes (Pihl et al., 2003). In a study with psoriasis patients, there was a lower TAS observed along with higher TG and TC than controls (Kural et al., 2003). When dyslipidemia patients were treated with lipid lowering drug, TAS was increased as TG and TC were decreased (Kural et al., 2004). However, in a study by Belo et al. (2004), TG and TC were significantly increased with increased gestation period, as well as TAS and uric acid. Uric acid was also significantly correlated with TAS in each gestation periods (1st, 2nd and 3rd trimester). This significantly positive relationship between TAS and uric acid has been well recognized in previous studies (Kim et al., 2000; Kim et al., 2006).
In the present study, since we did not make an attempt to measure serum antioxidant parameters including uric acid, it limited us to evaluating magnitude of antioxidant contributions to TAS. However, we might be able to assume that uric acid did contribute serum TAS level, when we consider the report that 19.3% of Randox-TEAC called as TAS was contributed by uric acid (Cao & Prior, 1998). Uric acid was shown to be the second highest contributor among the known serum antioxidants, albumin (28.0%), ascorbic acid (3.08%), α-tocopherol (1.74%) and bilirubin (1.0%). In addition, uric acid was found to be elevated with overweight, obesity and visceral fat area (Hikita et al., 2007; Kim et al., 2000; Molnar et al., 2004). These previous findings may suggest the partial effect of increased uric acid on TAS in overweight and obese conditions, and explain in part the positive association among obesity indices and TAS shown in the current study. In addition, we also observed elevated TAS with increased TG and metabolic risk factors. Hikita et al., (2007) demonstrated that uric acid was elevated with increased number of metabolic risk factors, as well as TG, one of the most influential parameters on serum uric acid elevation. With respect to the relation between serum uric acid and metabolic risk factors, there was proportional elevation of uric acid levels when number of metabolic risk factors increased as 0, 1, 2 and ≥3 (Desai et al., 2005). In the present study, we observed similar tendency in elevated TAS with increased number of metabolic risk factors.
Based on these observations, we might take it for granted that changes in serum uric acid affect TAS levels. Besides uric acid, serum albumin was also found to contribute TAS level by 28% (Cao & Prior, 1998), and significantly correlated with TAS of 17 normal weight young males (Kim et al., 2000). Further, there are still unknown serum antioxidants which hold the greatest proportion of contribution to TAS by 47% (Cao & Prior, 1998). Other than TAS, various biomarkers of oxidative stress have been employed in studies on antioxidant status in humans. Of those markers, plasma MDA and LDL oxidation are well-recognized biomarkers of oxidative stress which have shown to reflect changes in oxidative stress caused by increased body weight (Mohn et al., 2005), aging (Mendoza-Nunez et al., 2007), exercise (Kim et al., 2001) and antioxidant supplementation (Giannini et al., 2007). Unlike these biomarkers of oxidative stress, TAS did not consistently reflect the conditions with increased oxidative stress (Bae, 2006; Belo et al., 2004; Kim et al., 2000; Kim et al., 2001; Magalhaes et al., 2007; Mendoza-Nunez et al., 2007). Further, with increased gestation period, changes in TAS were shown to be negatively correlated with changes in oxidized LDL, while increased oxidized LDL was accompanied with increased TG and LDL (Beloe et al., 2004). In the current study, TAC measure by TAS was significantly positively associated with obesity, as well as number of metabolic risk factors, although these conditions have known to be high in oxidative stress. Therefore, it seems that TAS appears to be affected by changes in individual antioxidant parameters which might be altered by various metabolic conditions. These antioxidant parameters affecting TAS values could be increased not only by body's increased antioxidant status, but by body's compensatory mechanism to counteract increased oxidative stress (Ames et al., 1985). Since there are no individual antioxidant data available in the present study, we may not be able to strongly assume the factors directly affecting TAS in subjects with and without metabolic risk factors. However, it is worthy to note that positive associations between TAS and increased metabolic risk factors, especially obesity related parameters, were consistently found in the present study and a few previous studies. This implies that increased TAS level may not always represent one's healthier condition or condition with a low oxidative stress, suggesting that TAS may not be used as a sole indicator of oxidative stress marker. Further, it is necessary to clarify the unknown factors affecting TAS with relation to various metabolic conditions in order to have better understanding what the TAS level implies in certain metabolic conditions.
Acknowledgments
We thank Soyoung Lim for excellent technical assistance in the laboratory.
Footnotes
This was supported by Korea National Open University Research Fund.
References
- 1.Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci USA. 1985;78:6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn NY, Shin YJ, Kim KJ. Blood lipids concentration and body composition as the different age in obese women. Journal of Korean Physical Education Association for Girls and Women. 2005;19:55–65. [Google Scholar]
- 3.Altindag O, Erel O, Soran N, Celik H, Selek S. Total oxidative/anti-oxidative status and relation to bone mineral density in osteoporosis. Rheumatol Int. 2007 doi: 10.1007/s00296-007-0452-0. DOI: 10.1007/s00296-007-0452-0. [DOI] [PubMed] [Google Scholar]
- 4.Bae HS. Body mass index, dietary intake, serum lipids and antioxidant status if young females. Korean Journal of Community Nutrition. 2006;11:479–487. [Google Scholar]
- 5.Belo L, Caslake M, Santos-Silva A, Castro EMB, Pereira-Leite L, Quintanilha A, Rebelo I. LDL size, total antioxidant status and oxidised LDL in normal human pregnancy: a longitudinal study. Atherosclerosis. 2004;177:391–399. doi: 10.1016/j.atherosclerosis.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 6.Benzie IFF, Strain JJ. The Ferric Reducing Ability of Olasma. (FRAP) as a Measure of "Antioxidant Power": The FRAP Assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 7.Cao G, Alessio HM, Cutler RG. Oxygen-radical absorbance capacity assay for antioxidants. Free Radic Biol Med. 1993;14:303–311. doi: 10.1016/0891-5849(93)90027-r. [DOI] [PubMed] [Google Scholar]
- 8.Cao G, Prior RL. Comparison of differns analytical methods for assessing total antioxidant capacity of human serum. Clin Chem. 1998;44:1309–1315. [PubMed] [Google Scholar]
- 9.Desai MY, Santos RD, Dalal D, Carvalho JA, Martin DR, Flynn JA, Nasir K, Blumenthal RS. Relation of serum uric acid with metabolic risk factors in asymptomatic middle-aged Brazilian men. Am J Cardiol. 2005;95:865–868. doi: 10.1016/j.amjcard.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 10.Giannini C, Lombardo F, Currò F, Pomilio M, Bucciarelli T, Chiarelli F, Mohn A. Effects of high-dose vitamin E supplementation on oxidative stress and microalbuminuria in young adult patients with childhood onset type 1 diabetes mellitus. Diabetes Metab Res Rev. 2007;23:539–546. doi: 10.1002/dmrr.717. [DOI] [PubMed] [Google Scholar]
- 11.Hikita Miho, Ohno Iwao, Mori Yutaka, Ichida K, Yokose Takuo, Hosoya T. Relationship between hyperuricemia and body fat distribution. Intern Med. 2007;46:1353–1358. doi: 10.2169/internalmedicine.46.0045. [DOI] [PubMed] [Google Scholar]
- 12.Jeon CH, Lee EH, Lee HI. Blood total antioxidant capacity in patients with stomach and colorectal cancer. Korean Journal of Clinical Pathology. 1998;18:151–155. [Google Scholar]
- 13.Kim HS, Oh JA. Comparison of the Metabolic Syndrome risk factor prevalence forty and fifty something women. Korean Academy of Nursing Journal. 2007;37:453–458. doi: 10.4040/jkan.2007.37.4.453. [DOI] [PubMed] [Google Scholar]
- 14.Kim JH, Kim MJ, Kwak HK. Obesity indices and plasma total antioxidant status in hypertensive elderly living in Ulsan area. Korean Journal of Community Nutrition. 2006;11:279–288. [Google Scholar]
- 15.Kim SH, Yi SM, Chang MJ. The Study of antioxidant enzyme malondialdehyde and total antioxidant status of the blood in athletics. The Korean Journal of Exercise Nutrition. 2001;5:43–56. [Google Scholar]
- 16.Kim SK, Park YS, Byoun KE. Comparison of the total antioxidant status and usual dietary intake in normal and overweight males. Korean Journal of Community Nutrition. 2000;5:633–641. [Google Scholar]
- 17.Kural BV, Orem A, Cimsit G, Yandi YE, Calapoglu M. Evaluation of the atherogenic tendency of lipids and lipoprotein content and their relationship with oxidant-antioxidant system in patients with psoriasis. Clinica Chimica Acta. 2003;328:71–82. doi: 10.1016/s0009-8981(02)00373-x. [DOI] [PubMed] [Google Scholar]
- 18.Kural BV, Orem C, Uydu HA, Alver A, Orem A. The effects of lipid-lowering therapy on paraoxonase activities and their relationships with the oxidant-antioxidant system in patients with dyslipidemia. Coron Artery Dis. 2004;15:277–283. doi: 10.1097/01.mca.0000135221.32523.a1. [DOI] [PubMed] [Google Scholar]
- 19.Lee SY, Park HS, Kim DJ, Han JH, Kim SM, Cho GJ, Kim DY, Kwon HS, Kim SR, Lee CB, Oh SJ, Park CY, Yoo HJ. Appropriate waist circumference cutoff points for central obesity in Korean adults. Diabetes Res Clin Pract. 2007;75:72–80. doi: 10.1016/j.diabres.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 20.Magalhaes J, Ferreira R, Marques F, Olivera E, Soares J, Ascensao A. Indoor climbing elicits plasma oxidative stress. Med Sci Sports Exerc. 2007;39:955–963. doi: 10.1249/mss.0b013e318038f728. [DOI] [PubMed] [Google Scholar]
- 21.Markovic S, Dordevic J, Majkic-Singh N, Vasiljevic Z, Petrovic M, Glavinic L, Letic S, Milosevic A. The importance of antioxidant enzyme and total antioxidant status of patients with acute myocardial infarction on thrombolytic therapy. Clin Lab. 2000;46:495–499. [PubMed] [Google Scholar]
- 22.Mendoza-Nunez VM, Ruiz-Ramos M, Sanchez-Rodriguez MA, Retana-Ugalde R, Munoz-Sanchez JL. Aging-related oxidative stress in healthy humans. Tohoku J Exp Med. 2007;213:261–268. doi: 10.1620/tjem.213.261. [DOI] [PubMed] [Google Scholar]
- 23.Mendoza-Nunez VM, Sanchez-Rodriguez MA, Retana-Ugalde R, Vargas-Guadarrama LA, Altamirano-Lozano MA. Total antioxidant levels, gender, and age as risk factors for DNA damage in lymphocytes of the elderly. Mech Ageing Dev. 2001;122:835–847. doi: 10.1016/s0047-6374(01)00240-8. [DOI] [PubMed] [Google Scholar]
- 24.Miller NJ, Rice-Evans C, Davies MJ, Gopinathan V, Milner A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin Sci (Lond) 1993;84:407–412. doi: 10.1042/cs0840407. [DOI] [PubMed] [Google Scholar]
- 25.Mohn A, Catino M, Capanna R, Giannini C, Marcovecchio M, Chiarelli F. Increased oxidative stress in prepubertal severely obese children: Effect of a dietary restriction-weight loss program. J Clin Endocrinol Metab. 2007;90:2653–2658. doi: 10.1210/jc.2004-2178. [DOI] [PubMed] [Google Scholar]
- 26.Molnar D, Decsi T, Koletzko B. Reduced antioxidant status in obese children with multimetabolic syndrome. Int J Obes Relat Metab Disord. 2004;28:1197–1202. doi: 10.1038/sj.ijo.0802719. [DOI] [PubMed] [Google Scholar]
- 27.Pihl E, Zilmer K, Kullisaar T, Kairane C, Pulges A, Zilmer M. High-sensitive C-reactive protein level and oxidative stress-related status in former athletes in relation to traditional cardiovascular risk factors. Atherosclerosis. 2003;171:321–326. doi: 10.1016/j.atherosclerosis.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 28.Rasool AHG, Yuen KH, Yusoff K, Wong AR, Rahman ARA. Dose dependent elevation of plasma tocotrienol levels and its effect on arterial compliance, plasma total antioxidant status, and lipid profile in healthy humans supplemented with Tocotrienol rich vitamin E. J Nutr Sci Vitaminol. 2006;52:473–478. doi: 10.3177/jnsv.52.473. [DOI] [PubMed] [Google Scholar]
- 29.Sharifian A, Farahani S, Pasalar P, Gharavi M, Aminian O. Shift work as an oxidative stressor. J Circadian Rhythms. 2005;3:15. doi: 10.1186/1740-3391-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsakiris S, Karikas GA, Parthimos T, Tsakiris T, Bakogiannis C, Schulpis KH. Alpha-tocopherol supplementation prevents the exercise-induced reduction of serum paraoxonase1/arylesterase activities in healthy individuals. Eur J Clin Nutr. 2007 doi: 10.1038/sj.ejcn.1602918. DOI: 10.1038/sj.ejcn.1602918. [DOI] [PubMed] [Google Scholar]
- 31.World Health Organization, International Association for the Study of Obesity & International Obesity Task Force. The Asia-Pacific Perspective: Redefining obesity and its treatment. Geneva. Switzerland: Health Communications Australia Pty Limited; 2000. [Google Scholar]