Abstract
Patients with damage involving left ventrolateral prefrontal cortex (left VLPFC) often show syntactic deficits. They also show exaggerated interference effects during a variety of non-syntactic tasks, including picture naming and working memory. Conceivably, both deficits could arise from inadequate biasing of competitive interactions during language production. To test this hypothesis, we manipulated “positional” interference during multiword naming by priming one of the nouns in the same or different position. Experimental case studies of four left VLPFC patients revealed that two of the patients showed exaggerated positional interference, greater number of errors, including omissions during multiword production, increased production difficulty when the order of nouns did not match the predominant English pattern as well as impaired comprehension of non-canonical reversible sentences. These results suggest that these two patients had an impairment in “selection for position”. Different from the other two, their lesions included a subregion of frontal cortex (BA 44/6) that has been shown in neuroimaging studies to pay a role in sequencing.
Keywords: aphasia, language, production, syntax, sequencing, interference, prefrontal
1. Introduction
The neuropsychology of syntactic deficits has predominantly focused on agrammatism within the context of Broca’s aphasia. Agrammatism collectively refers to a number of syntactic deficits, including reduced grammatical complexity, missing grammatical elements, and impaired processing of passives and other complex sentences (Caramazza & Zurif, 1976; Goodglass, 1993; Rochon, Saffran, Berndt & Schwartz, 2000; Saffran, Schwartz, & Marin, 1980; Schwartz, Saffran & Marin, 1980). The link between agrammatism and left ventrolateral prefrontal cortex (left VLPFC), particularly Broca’s area (Brodmann 44/45), is often taken for granted; yet the precise relation is unclear. Two factors complicate the use of neuropsychological studies of agrammatism to understand the role of left VLPFC in syntax. First, the different impairments grouped under agrammatism dissociate to some degree, suggesting that agrammatism may not be a unitary psychological entity (Badecker & Caramazza, 1985). Second, the assumption that Broca’s aphasia is isomorphic with damage to Broca’s area is questionable. Some Broca’s aphasics do not have lesions in Broca’s area (Dronkers, Wilkins, van Valin, Redfern & Jaeger, 1994; Vanier & Caplan, 1990). The deficits described above are not necessarily restricted to patients with frontal damage (Caplan, Baker & Dehaut, 1985; Dick, et al., 2001). The latter is unsurprising given that sentence processing is a complex task, which involves multiple components (e.g., relational processing between lexical items, mapping between syntactic positions and semantic roles) that are likely subserved by a distributed network of areas, including temporoparietal regions.
Our goal in the current study was to investigate the role of left VLPFC in a core syntactic process that is required for the ordering of words, namely selection for position. Accordingly, we made two design decisions that were congruent with this goal. First, we chose patients based on left VLPFC damage rather than aphasia profile. This allows us to more transparently test hypotheses about left VLPFC’s role in syntax (Caplan, 2001). Second, we tested the production of a relatively simple structure (conjoined noun phrase) in order to isolate the component of interest (ordering) from other processes such as verb retrieval and syntax-semantic mappings, which are implicated in full sentence processing.
1.1. The Role of Left VLPFC in Simplified Syntax
There is prior empirical support for the utility of studying simple utterance production in aphasia. Even measures of noun production can reveal impairments that bear on the nature of patients’ sentences. For example, Schwartz A Hodgson (2002) reported a case study of a patient with frontal damage (MP.) who had difficulty naming multiple pictures presented simultaneously even when the syntactic task demands were minimal and the nouns were to be produced as a simple list. This impairment has been termed a “naming in context” or “multiword naming” deficit (Schwartz A Hodgson, 2002. See also Williams A Canter, 1982). It appears that some patients may have difficulty producing words in context that go above and beyond their difficulty in producing words in isolation and that this deficit can be detected by simper tasks than full sentence production.
Here we employed one such simple task: participants produced conjoined noun-phrases comprised of two nouns. How might left VLPFC impact the production of such phrases? When multiple nouns are co-activated, as in the above studies, interference among them may cause difficulty in the panning and execution of a response. The selection of a task-relevant representation from among multiple activated representations - in this case, the selection of the right noun at the right time - may require the deployment of left VLPFC-mediated cognitive control processes and may thus be impaired in patients with left VLPFC damage (Kimberg & Farah, 1993). Such a control process (e.g., basing activation of different representations) could both increase the activation of relevant representations and suppress the activation of irrelevant ones. A number of neuropsychological and neuroimaging studies have documented left VLPFC involvement in cognitive control -in cases where a prepotent response has to be suppressed in favor of an alternate response or when there is competition among members of an underdetermined set of options (Banich, et al., 2000; Hamilton & Martin, 2007; Jonides & Nee, 2006; Kan & Thompson-Schill, 2004; Thompson-Schill, et al., 2002).
The conclusion that emerges from these studies is that left VLPFC is critical for basing competitive interactions among multiple activated representations during the selection of the a single representation. For brevity, we will term this the “biasing hypothesis”. How might this relate to language production in left VLPFC patients? Recent experiments have explored one facet of the biasing hypothesis, namely the effect of semantic relatedness on selection. In the “semantic blocking” paradigm, participants are asked to name pictures that are presented repeatedly either in a homogenous, semantically related block or in a heterogeneous block containing items from different semantic categories. Selection of the correct noun from amongst many representations is particularly hard when the alternatives are related to one another and are activated to a similar degree. In healthy adults, the semantic blocking effect manifests as an increase in reaction times in homogenous compared to heterogeneous blocks (Belke, Meyer A Damian, 2005). Several recent studies have reported that non-fiuent and Broca’s aphasics show an exaggerated difference - in naming latencies and error rates - between homogenous and heterogeneous bocks compared to other patients and healthy controls (Biegler, Crowther A Martin, 2008; McCarthy & Kartsounis, 2000; Schnur, Schwartz, Brecher A Hodgson, 2006; Wilshire & McCarthy, 2002. See also Freedman, Martin & Biegler, 2004 for evidence from a different paradigm). Together, these results suggest that the cognitive control demands imposed by semantic interference can affect naming and phrasal panning particularly in patients with damaged left VLPFC.
Semantic interference may turn out to be one important factor in explaining the sentence production difficulties in left frontal patients. Nouns in a sentence are often taxonomically or thematically related ( I bought some spinach and lettuce; The dog is chewing his bone); semantic interference between them could lead to problems during sentence panning and execution. However, linguists have long noted that a sentence is more than a list of words. The order in which nouns appear in a sentence conform to the grammar of the language and this order must be appropriate for the meaning that is to be conveyed (John hit Bob and Bob hit John do not mean the same thing). Thus, sentence production involves not just the selection of appropriate nouns, but also the selection of appropriate nouns for appropriate positions. Sequencing whether linear or hierarchical, is a critical part of producing and understanding language. Evidence from functional neuroimaging is consistent with a role for left VLPFC - particularly the posterior portions (BA 44 and nearby BA 6) - in linguistic sequencing. These areas show increased activation for the processing of difficult word orders (Bornkessel, Zysset, Friederici, von Cramon A Schlesewsky, 2005; Roder, Stock, Neviile, Bien A Rosier, 2002).
In this study, we extend the biasing hypothesis to study sequencing. Does the requirement to order co-activated nouns in a task-appropriate way lead to a kind of interference during production? Are left VLPFC patients specifically impaired in resolving such interference? How might this relate to their sentence production difficulties? Below we present preliminary data from a novel multiword priming paradigm that taps into “position-based” interference. We present experimental case studies of four patients to investigate the relationship between this interference and other cognitive measures, and explore the anatomical correlates of these behaviors using lesion maps obtained from structural imaging.
2. Patient Information
2.1. Selection Criteria
Our strategy for participant selection was to isolate the factor of interest, namely damage to left VLPFC. With this in mind, we reviewed the records from 64 individuals with post-stroke aphasia who were enrolled in research at Moss Rehabilitation Research Institute and had given permission for their records to be stored and reviewed for future research studies. Each of the 64 had recently undergone extensive language testing and a CT or MRI scan. From this cohort we selected four who met the anatomical criterion and, in addition, demonstrated high accuracy in singe-item picture naming. There were two reasons why we required proficiency in naming. The first was theoretical: given the intent to study selection for position by means of a multi-word naming task, it was desirable to isolate - to the extent possible - sequencing-related impairments from deficits in other naming-related processes. The second reason was empirical. Because errors in multiword production are multi-determined and therefore difficult to classify, we wanted to use latency as the main dependent measure. We hoped to minimize errors and non-responses by selecting patients who were good at confrontation naming. The four patients (described in detail below) gave written permission to participate under a protocol approved by the Albert Einstein Medical Center Institutional Review Board.
2.2. Imaging Methods
All scans were obtained at least 6 months post-onset and within 5-years of testing. Patient CBD underwent a magnetic resonance imaging (MRI) scan on a 1.5 Tesla Siemens Sonata scanner. Whole brain T1-weighted images were acquired (TR = 3000 ms, TE = 3.54, FOV = 24 cm) with a slice thickness of 1mm, along with a FLAIR (TR = 10000 ms, TE = 154 ms, FOV = 1.75 cm) with 3 mm thick slices using a standard radio-frequency head coil. The lesion was segmented manually on a 1x1x1 mm T1-weighted structural image, after which the scan and lesion were sequentially registered to a standard template (“Colin27”; Holmes, Hoge, Collins, Woods, Toga & Evans, 1998) using a symmetric diffeomorphic registration algorithm (Avants, Schoenemann & Gee, 2006; see also http://www.picsl.upenn.edu/ANTS/). The final lesion map was quantized to produce a 0/1 map, using 0.5 as the cutoff value.
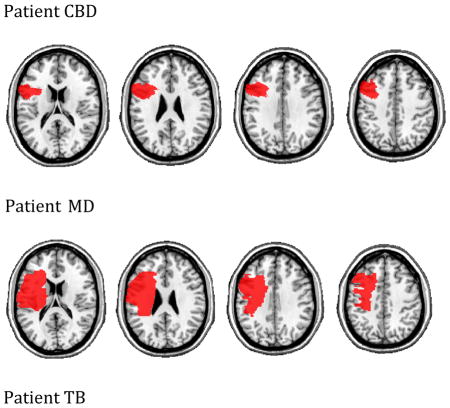
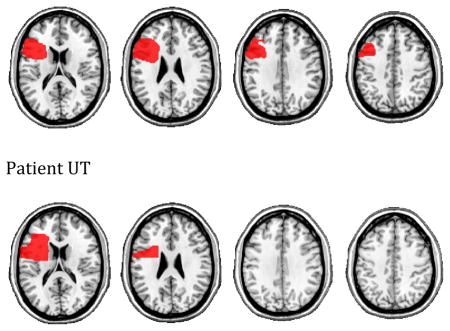
For the remaining three patients, MRI was contra-indicated. These participants (UT, TB, MD) underwent whole brain CT scans without contrast (60 axial slices, 3 mm thick) on a 64-Slice SOMATOM Sensation Scanner. Their lesions were drawn directly onto the common Colin27 volume, after rotating (pitch only) the template to approximate the slice plane of the patient’s scan. We have previously demonstrated excellent intra-and inter-rater reliability with this method (Schnur, et al., 2009). The lesions maps were all drawn or approved by an experienced neurologist who had no knowledge of the behavioral data. Representative images are shown in the Appendix.
We determined each patient’s total lesion volume and percentage of damage to several a priori ROIs using VoxBo (http://www.voxbo.org) and MRICron (http://www.sph.sc.edu/comd/rorden/mricron). These data are shown in Table 1. We selected the four patients based on their having substantial damage to left VLPFC (BA 44, 45, and/or 47) and minimal damage to posterior areas that are typically associated with language (BA 21, 22, 39, 40). We did not consider damage to thalamus or basal ganglia in selecting patients, nor did we see any obvious relation between the extent of this damage and patients’ performance on our tasks (details in Appendix).
Table 1.
Patient overall lesion volume and % damage for selected Brodmann areas
| CBD | MD | TB | UT | |
|---|---|---|---|---|
| Lesion vol. (cc) | 46.28 | 111.95 | 51.86 | 51.6 |
| Left VLPFC | ||||
| BA 44 % | 91.8 | 81.5 | 87.3 | 14.7 |
| BA 45 % | 5 | 27.9 | 42.9 | 47.3 |
| BA 47 % | 0.2 | 44.1 | 19 | 38.4 |
| Other Frontal | ||||
| BA 46 % | 6.9 | 9.3 | 6.2 | 3 |
| BA 9 % | 21.2 | 7.7 | 13.4 | 0 |
| BA 6 % | 21 | 22.5 | 11.8 | 5.1 |
| Temporal/Parietal | ||||
| BA 21 % | 0 | 0 | 0 | 0 |
| BA 22 % | 0 | 2 | 0 | 0 |
| BA 39 % | 1.1 | 0 | 0 | 0 |
| BA 40 % | 0 | 1.1 | 0 | 0 |
2.3. Standardized Language Tasks
In addition to the experimental results, we report patients’ scores on the following language tests and measures (Table 2):
Table 2.
Patient test scores
| CBD | MD | TB | UT | 64 Patient Cohort Mean (SD) | Control Norms Mean (SD) | |
|---|---|---|---|---|---|---|
| WAB Fluency | 8 | 9 | 9 | 9 | 7 (2.36) | |
| WAB Comp. | 9.75 | 9.6 | 8.85 | 9.8 | 8.73 (1.1) | |
| PNT | 95 | 88 | 91 | 90 | 69.77 (25.37) | |
| STM Span | 3.2 | 3.4 | 4.6 | 5 | 2.92 (1.02) | |
| Lex-Comp | 100 | 100 | 100 | 100 | 93.67 (9.26) | |
| Map-Comp | 73 | 97 | 63 | 100 | 74.59 (16.31) | |
| QPA Measures | ||||||
| Words/min | 42.55 | 45.9 | 96.09 | 160.82 (37) | ||
| Prop. closed class | 0.48 | 0.56 | 0.5 | 0.54 (0.04) | ||
| Determiner index | 0.96 | 0.96 | 0.95 | 0.99 (0.02) | ||
| Prop. Verbs | 0.42 | 0.43 | 0.48 | 0.48 (0.06) | ||
| Prop. well-formed | 0.73 | 0.95 | 0.62 | 0.95 (0.08) | ||
The Western Aphasia Battery (WAB. Kersetz, 1982) fluency score is a loose measure of sentence length and complexity obtained from a spontaneous speech sample. The maximum score is 10.
The WAB comprehension score is calculated from patients’ responses yes/no questions and sequential commands, and their ability to match words to real objects. The maximum score is 10.
The Philadelphia Naming Test (PNT. http://www.ncrrn.org) is a test of confrontation naming using 175 pictures. The maximum score is 100% correct.
The short-term memory (STM) score measures the string length that patients are able to correctly encode and repeat. Participants listened to 10 strings each for lengths 1 to 5 words. The maximum score is 5. A score of <x>.<y> indicates at least 50% correct at length <x> with more limited success at length <x+1> (y/2 items correct).
Sentence comprehension scores were obtained from a 2 alternative forced choice sentence-to-picture-matching task that is part of the Philadelphia Comprehension Battery (Saffran, Schwartz, Linebarger, Martin & Bochetto, 1998). Patients were tested on 60 sentences, 10 each of actives, actives with prepositional phrases, passives, locatives, subject relative clauses and object relative clauses. For eachsentence type, half of the trials could be answered by paying attention to lexical content alone (Lex-Comp). For example, for the sentence “The dog chases the boy”, the two accompanying pictures were of a dog chasing a boy (target) and of a dog chasing a rabbit (distractor). In the other half of the trials, the lexical content in the target and distractor pictures was the same; participants could succeed only by correctly mapping syntactic positions to semantic roles (Map-Comp). For example, for the sentence “The man serves the woman”, one picture showed a man serving a woman and the other showed a woman serving a man. We report % correct for each of the test types (Lex-Comp and Map-Comp).
Quantitative Production Analysis (QPA) scores were obtained from free-form narrations of well-known stories (Cinderella, Red Riding Hood; Saffran, Berndt & Schwartz, 1989). This is a standardized analysis that produces several measures of fluency and grammatical competence. We report the following - words per minute, proportion of closed class words, determiner index, proportion of verbs, proportion of well-formed sentences. In all cases, smaller numbers indicate greater difficulty with sentence production.
2.4. Patient CBD
Patient CBD is a Caucasian male who was 71 years old and 14 months post left CVA at the time of testing. His structural MRI revealed damage to the frontal lobe, including left VLPFC, dorsolateral prefrontal cortex (DLPFC) and premotor cortex (PMC) (Table 1). Patient CBD was diagnosed as anomic, and had high WAB comprehension and Lex-Comp scores (Table 2). His WAB fluency score was 8, which indicates that his sentences were mostly compete, but with marked word finding difficulty. His singe picture naming was excellent however, as indicated by his accuracy on the PNT.
2.5. Patient MD
Patient MD is a Caucasian male who was 60 years old and 164 months post left CVA at testing. His CT scan revealed extensive damage to left VLPFC and also damage to DLPFC and PMC (Table 1). Patient MD was diagnosed as anomic, and had high WAB comprehension, Lex-Comp and Map-Comp scores (Table 2). His WAB fluency score was 9, which indicates that he produced mostly complete sentences, with occasional word finding difficulty. His PNT accuracy was a little lower than that of the other patients, but was still very much in the functional range.
2.6. Patient TB
Patient TB is an African American female who was 40 years old and 75 months post left CVA at testing. Her CT scan revealed damage to left VLPFC, DLPFC and PMC (Table 1). Pattent TB was classified as anomic, and had high WAB fluency, Lex-Comp and PNT scorrs (Table 2). Her WAB comprehension score was high, but not as high as the other three patients. She made errors on some items that required relational processing (e.g., “Do you eat a banana before you peel it?”, “Point to the comb with the pen.”).
2.7. Patient UT
Patient UT is a Caucasian male who was 55 years old and 27 months post left CVA at testing. His CT scan revealed frontal lobe damage restricted mostiy to left VLPFC, particularly to the anterior and ventral portion. Compared to the other three patients, UT had less damage to BA 44 (Table 1). His diagnosis was anomia. He was the least impaired amongst the patients reported here, with uniformly high scores demonstrating nearly unimpaired language production and comprehension (Table 2). We could not obtain QPA samples from patient UT; but see Appendix for his speech sample from a free-form picture description task, which shows that UT’s speech is coherent, well formed and grammatical.
3. Multiword Priming Experiment
As outlined in the Introduction, our primary aim was to investigate the role of left VLPFC in resolving interference during “selection for position”. Accordingly, in this experiment we asked whether switching the phrasal position of a primed noun in a multiword naming task would lead to interference and whether in the left frontal patients such interference would be exaggerated.
3.1. Control Participants
In addition to the four patients, we tested eight healthy older adults from the Philadelphia area (4 female. Ages 52–70). Al participants gave informed consent in accordance with a protocol approved by the local institutional review board.
3.2. Stimuli
We used 80 color pictures adapted from Snodgrass A Vanderwart’s line drawings (Rossion A Pourtois, 2004). The 80 items were categorized into 8 categories of 10 items (see Appendix). All had name agreement > 90%.
3.3. Procedure
Participants were instructed to name two pictures presented on a computer screen from left to right, using simple “the x and the y” phrases (Fig. 1). Not all patients used this phrasal form, substituting “a x and a y” or “x and y” instead. However, each patient was largely consistent in using a particular form in multiple testing bocks. Prior to the start of the experiment, participants were familiarized with the pictures one at a time. The experimenter provided feedback if a participant was unable to name a picture or used a different name. Participants then practiced multiword naming before proceeding to the main experiment.
Figure 1.
Example trial (“the eye and the pencil”)
Each testing session contained two naming blocks. Each bock contained 200 naming trials. Stimuli were presented using E-Prime (Schneider, Eschman, & Zuccolotto, 2002). Each trial began with a 1-second fixation cross that was accompanied by a beep. Subsequently, two pictures appeared side by side. The participants’ task was to name the two pictures using a conjoined noun-phrase. The pictures disappeared upon speech onset in order to encourage concurrent panning of the two nouns (cf. Freedman et al., 2004; Martin, et al., 2004). The pictures stayed on screen for 5 seconds or until response onset, whichever came sooner. Participants were then allowed 3 seconds to finish their response before the next trial began with a fixation cross.
The 200 trials in each block included 80 filers, and 40 experimental three-trial sets (“triads”) in which one of the two nouns repeated. We were primarily interested in effects during the third trial of the triad (“target”). During these target trials, the position of the repeated noun was either consistent or inconsistent with the previous two trials in the triad (“primes”) (see Table 3 for examples). Our prediction was that participants would be quicker, and possibly more accurate, in producing the target utterance if the repeated noun had been produced in the same position before than if it had been produced in the other position. Each bock contained 20 “consistent” and 20 “inconsistent” targets. Within each type, half of the trials contained the repeated noun in the first position.
Table 3.
Example experimental triads
| Consistent Target | Inconsistent Target |
|---|---|
| Example 1 | Example 1 |
| Prime: eye, pencil | Prime: glove, duck |
| Prime: eye, toaster | Prime: glove, carrot |
| Target: eye, camel | Target: whistle, glove |
| Example 2 | Example 2 |
| Prime: chair, snowman | Prime: bowl, flag |
| Prime: dress, snowman | Prime: skirt, flag |
| Target: balloon, snowman | Target: flag, nail |
Pictures presented together in a trial were never from the same semantic category. In experimental triads, the 3 non-repeating nouns were never from the same category as one another nor were they from the category of the singe repeating noun. This was done in order to minimize the possibility of semantic interference. Items were counterbalanced across 4 lists such that the target noun appeared in the first or second position, and was consistent or inconsistent with the primes. Participants were randomly assigned to one of the 4 lists. We used two randomly determined orders of filler and experimental trials in the two blocks.
3.4. Trial Types
In the rest of the paper, we allude to three types of trials when discussing the results. Target trials, as described above, refer to the third trial of triads. Unprimed trials refer to filler trials and the first trial of triads. Primed trials refer to the second and third trial of triads (so they include target trials).
3.5. Dependent Measure
We measured latency to onset of the first word of target trials using Praat (http://www.praat.org).1 Our primary prediction was that patients and controls would experience interference on trials where a primed noun appeared in the previously unprimed position compared to trials where it appeared in the consistent, primed position. Therefore, we calculated a positional interference score for each participant as follows:
Patients competed two different sessions - of 2 blocks each2 - separated by 4 days or more. In addition to measures averaged across the 4 bocks, we report interference scores from the two sessions separately in order to evaluate the within-patient replicability of latency patterns. Controls completed one session (2 blocks) only. We excluded error trials (see below) and trials where the latency was more than 2 standard deviations away from the baseline for each participant (see below).
3.6. Error Coding
We counted an utterance as an error if any of the following were true: one or both nouns were missing or wrong; the order of nouns was wrong; there were extraneous phonemes or syllables before the onset of a noun (e.g.,/f/button); there were major disfluencies involving entire words (e.g., the the cat). Using fillers such as “um” and “er” and minor disfluencies involving parts of the target word (e.g.,/ri/ring) were not considered errors.3 Using a different morphological form from the expected one (e.g., clouds for cloud) was also not counted as an error.
3.7. Results
3.7.1. Response Latencies
Controls showed a marginally significant interference effect [t(7)=2.072; p=.077]. On average, their response latencies were 34.2 ms longer on inconsistent target trials than on consistent target trials. This is consistent with our prediction that switching the position of a repeated noun would lead to positional interference and delayed responses. Next, we compared the interference effect in the four patients to the controls. Table 4 shows the mean interference score per session for the patients as well as the mean and standard deviation for the controls. To determine whether patients showed exaggerated positional interference, we used a modified t-test for comparing individual scores to small sample norms (Crawford A Howell, 1998). Two of the four patients (CBD and TB) had significant larger interference scores compared to the controls in both sessions. One patient (UT) was not significantly different from the controls in either session. The fourth patient (MD) showed the opposite pattern, i.e., a negative interference score, in the first session. In the second session, his interference score was negative but not reliably different from controls. Table 4 shows that this patient (MD) had faster latencies on inconsistent than on consistent trials. A more fine-grained analysis of the inconsistent trials revealed the following: when a noun was switched from the first to the second position, MD’s latencies were not particularly faster than for consistent trials (inconsistent 1 → 2: 1384 ms, consistent: 1393 ms). In contrast, when a noun was switched from the second to the first position, his latencies were the fastest among all trials (inconsistent 2 → 1: 1124 ms). Thus, this patient seemed to derive the greatest benefit from priming for the shortest lag between prime and target uses of a noun. We will turn to this in more detail in Discussion.
Table 4.
Latencies and interference scores for controls and patients
| Measure | Controls (N=8) | CBD | MD | TB | UT | |
|---|---|---|---|---|---|---|
| Session 1 | Unprimed | 849.8 | 2081.6 | 1684.7 | 1522.4 | 2210.1 |
| Consistent | 755.5 | 1397.8 | 1441.6 | 1218.4 | 1870.2 | |
| Inconsistent | 789.7 | 1846.7 | 1232.0 | 1437.1 | 1980.4 | |
| Interference (incons. - cons.) | 34.2 (SD=46.7) | 448.9 | −209.6 | 218.7 | 110.2 | |
| Inferential statistic | N/A | t=8.37* | t=−4.92* | t=3.72* | t=1.53 | |
| Session 2 | Unprimed | N/A | 1937.4 | 1527.0 | 1682.2 | 1783.7 |
| Consistent | N/A | 1387.8 | 1339.5 | 1361.8 | 1529.7 | |
| Inconsistent | N/A | 1925.9 | 1272.9 | 1520.2 | 1597.9 | |
| Interference (incons. - cons.) | N/A | 538.1 | −66.6 | 158.4 | 68.2 | |
| Inferential statistic | N/A | t=10.17* | t=−2.04 | t=2.51* | t=0.69 |
Latencies are shown in milliseconds. Inferential statistic computed using the Crawford test for comparing individual scores to small sample norms.
denotes a significant effect at p<.05.
Individuals with longer baseline latencies often show larger differences between conditions than individuals with shorter latencies, so we evaluated whether baseline differences might account for the difference between patients. For each control participant and patient, we calculated the baseline latency from all unprimed trials (see Table 4). All patients had significantly longer baselines compared to controls. Critically however, there was no suggestion that the two patients who showed exaggerated positional interference (CBD and TB) had consistently longer baselines than the two patients who did not (MD and UT). To further ensure that baseline differences were not the primary reason for the difference between patients, we calculated percent interference scores as raw interference score divided by baseline latency for each participant (collapsed across both sessions for patients). These percent scores showed roughly the same pattern as the raw scores (Controls mean=3.4%; CBD=24.7%; MD=−8.4%; TB=11.9%; UT=4.4%). Having normalized using baseline latency, patients CBD and TB still showed interference effects that were 7 and 3 times the effect in controls. The other two patients did not show this pattern.
3.7.2. Errors
Controls made few errors (M=2.8%) while the error rate for the four patients was higher (CBD: 17.43%; MD: 12.75%; TB: 14.38%; UT: 8.75%). We tabulated patients’ errors separately for unprimed and primed trials. Because there were more unprimed than primed trials in each block (120 unprimed, 80 primed), we calculated errors as a percent of the total number of trials of each type. Three out of the four patients made proportionally fewer errors during primed than during unprimed trials (Table 5), suggesting that priming one noun helped patients in the production of a multi-noun utterance. Next, we compared the number of errors during consistent and inconsistent target trials. This showed no consistent pattern; specifically, there was no suggestion that the patients as a group committed more errors during inconsistent than during consistent target trials (Table 5). Patients seldom made order reversal errors (e.g., pencil and eye instead of eye and pencil). Overall, this pattern of errors suggests that priming, whether consistent or inconsistent, helped patients make the correct response.
Table 5.
Patients’ errors
| Patient | # errors unprimed trials | # errors primed trials | % unprimed trials with errors | % primed trials with errors | # errors consistent targets | # errors inconsistent targets | % unprimed errors that were omissions |
|---|---|---|---|---|---|---|---|
| CBD | 90 | 32 | 21.43a | 11.43a | 10 | 5 | 33.33 |
| MD | 61 | 41 | 12.71 | 12.81 | 11 | 10 | 9.84 |
| TB | 78 | 37 | 16.25 | 11.56 | 4 | 12 | 42.31 |
| UT | 53 | 17 | 11.04 | 5.31 | 5 | 5 | 1.89 |
These percentages are adjusted for the fact that we analyzed 3.5 instead of 4 blocks for patient CBD.
Because priming had a facilitative effect independent of position and errors were a lot fewer in this condition, we restricted the more fine-grained analysis of error types to unprimed trials. These trials are potentially informative about patients’ “basic” multiword production outside of the priming manipulation. During unprimed trials, patients CBD and TB had a higher error rate overall compared to patients MD and UT. The difference in error types was even more telling (last column of Table 5). Patients MD and UT were seldom unable to compete their utterances. Omission of one noun in the attempted conjoined noun phrase comprised <10% of their errors. Instead, their errors largely consisted of disfluencies (e.g., windmill and /s/ /er/ kite) and semantic substitutions (e.g., clouds and fork /er/ knife). This is similar to the pattern of naming errors in the control participants (83% of their errors were semantic substitutions or disfluencies or a combination of the two. There were only 2 omissions). In contrast, more than quarter of CBD’s and TB’s errors were omissions (e.g., a book and a —;— and a bus). Thus, the exaggerated positional interference reported in the latency results strikingly correlated with overall difficulty with basic multiword naming (as indexed by omissions during unprimed trials) in patients CBD and TB.
3.8. Speeded Single Word and Cued Dyad Naming
As described above, patients CBD and TB had considerable difficulty with multiword naming despite good proficiency on the PNT. The most relevant difference between the PNT and the current task is the nature of the production (singe word vs. multiword). But the two tasks also differ in the stimuli used and the presence of a naming deadline in the multiword task. In order to assess the effect of task parameters, we tested patients CBD and TB in two other speeded naming tasks using the same picture stimuli as those used in the multiword naming experiment.
In speeded singe word naming, participants saw pictures one at a time, just as in the PNT, but with a deadline to respond within 3.5 seconds (see Table 6 for details). CBD and TB each competed 2 bocks of 400 trials. As can seen from Table 6, both patients had little difficulty with this task. This suggests that time pressure is unlikely to account for their poor performance in multiword naming, where the maximum time allotted for making a response was more than double the time allotted here (8000 ms vs. 3500 ms).
Table 6.
Comparison of CBD’s and TB’s performance in three different naming tasks
| Task | Parameters | Number pictures visible | Number pictures named | CBD error % | TB error % |
|---|---|---|---|---|---|
| Single word naming | Fixation cross with beep: 500 ms. Picture: 2000 ms max Blank screen for finishing utterance: 1500 ms. |
1 | 1 | 2.5 | 2.125 |
| Cued dyad naming | Fixation cross with beep: 500 ms Pictures: 1250 ms Time after cue appears: 750 ms max Blank screen for finishing utterance: 1000 ms |
2 | 1 | 7.188 | 5.313 |
| Multiword naming (unprimed trials only) | Fixation cross with beep: 1000 ms Pictures: 5000 ms max Blank screen for finishing utterance: 3000 ms |
2 | 2 | 21.43 | 16.25 |
In the speeded cued dyad naming task, patients looked at two pictures, waited until a border appeared around one of the pictures, and then named the highlighted picture (see Table 6 for details). Each patient completed 2 bocks of 160 trials. Patients were told to prepare the responses for both pictures, but only output one of them as required. In some ways, this task could have been more difficult than both the single word naming task (where no attentional switch was required because there was only one picture) and the multiword naming task (where the two pictures could be attended and named in a predictable, consistent order). Nevertheless, patients did much better here than at multiword naming (Table 6). Compared to single word namely made 2–3 times more errors in cued dyad naming versus 7–9 times more errors in multiword naming.
Together, data from the three naming tasks suggest that CBD and TB had specific difficulty in the planning and execution of multi-noun utterances that go beyond any impairment in their single word naming and attentional abilities.
3.9. Effects of Animacy
Transitive sentences in English (e.g., John kicked the ball) typically place an animate agent (someone who performs an action) before an inanimate patient (something affected by the action). We hypothesized that if CBD and TB are impaired in selection for position, they may have particular difficulty when the order of nouns goes counter to long-term experience e.g., in pacing an inanimate noun first during multiword naming. Accordingly, we evaluated participants’ performance separately in animate-first and inanimate-first trials.4 We only looked at unprimed trials, to avoid complications arising from the interaction between priming and any long-term ordering biases.
Control participants were faster when an animate noun was placed (animate-first trials=796.51 ms, inanimate-first trials=857.89 ms, F(1,7)=10.177, p<.02). Analysis of RTs as a percent of baseline latency revealed the same pattern (animate-first percent RT=0.94, Non-animate first percent RT=1.01, F(1,7)=15.085, p<.01). Thus, results from healthy controls were consistent with our prediction.
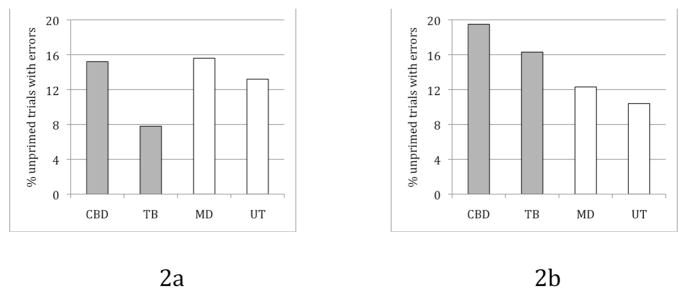
Patients’ RTs did not show any effects, but their errors showed an interesting pattern. Because inanimate-first were more numerous than animate-first trials (~7:1), we report error data as a percent of all trials with a particular configuration. During animate-first trials, which tested the statistically prevalent order in English, the impaired patients (CBD and TB) did not make any more errors than the other two patients (Fig 2a. Fisher’s exact p>.5). This is in contrast to inanimate-first trials, which were incongruent with the dominant English order. Here, the impaired patients made more errors (Fig. 2b. Fisher’s exact p<.001).
Figure 2. Patient error percentages for two different types of unprimed trials.
a. Animate-first trials; b. Inanimate-first trials.
These results suggest that patients CBD and TB have difficulty in adjusting their linguistic behavior: the priming analysis suggests that they have difficulty in overriding patterns seen in recent experience, specifically in switching a noun from a primed to an unprimed position; the animacy analysis suggests that they have difficulty in overriding patterns seen in long-term experience, specifically in beginning utterances with inanimate nouns.
3.10. Correlations with Standardized Tests
CBD and TB’s impairment in the experimental task strikingly correlates with their poor performance on two standardized language tests (Table 2).5 First, CBD and TB had poorer Map-Comp scores than the other two patients. This is notable because these two patients had perfect scores on the lexical condition of the sentence comprehension test (Lex-Comp). More detailed examination of CBD’s and TB’s Map-Comp scores showed that their poor performance was limited to non-canonical sentence structures that do not have the predominant Subject<->Agent mapping found in English. Patient CBD got 8/15 correct on passives, locatives and object relative clauses and 14/15 correct on actives, actives containing prepositional phrases and subject relative clauses. Patient TB got 5/15 correct in the former and 14/15 correct in the latter group. In conjunction with the position-priming interference effect and the animacy results, this suggests a general inability to override prepotent syntactic or ordering biases.
Second, CBD and TB show unexpectedly low proportions of well-formed sentences in the QPA compared to the other two patients (Table 2). According to other QPA measures, their sentences did not patently lack grammatical elements such as verbs and determiners. Nevertheless, many of these patients’ sentences were judged as ill formed by coders who were trained in QPA procedures and were blind to the experimental results (e.g., Cinderella had to what was going to the ball). Future studies should undertake more detailed linguistic analysis of such deviant sentences in order to understand the possible connection between impairment in selection for position and difficulty in sentence formulation. For now, we simply note the discrepancy with typical grammaticality measures (e.g., verb or closed class production), which suggests that the various impairments that are collapsed under agrammatism may be caused by dissociable mechanisms.
3.11. Anatomical Correlates
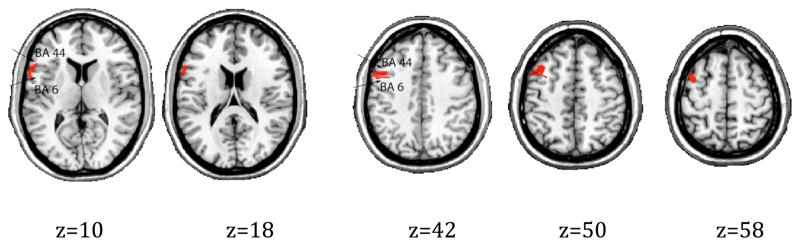
So far, we have presented data that suggest that a subset of the left VLPFC patients show a coherent set of behavioral impairments that may all be related to impaired selection for position. Next we explore the patients’ lesions in greater detail to determine the possible anatomical basis of differences among patients. As a first pass, unimpaired patient UT’s damage was mostly confined to the anterior and inferior parts of left VLPFC (see Appendix for lesion maps of individual patients). He has much less damage in the posterior portion (BA 44) compared to the other patients (Table 1). Many neuroimaging studies have implicated the BA 44 region of left VLPFC in syntactic processing (Dapretto & Bookheimer, 1999; Bookheimer, 2002; Caplan, Alpert & Waters, 1998). Following from those findings, it is perhaps not surprising that patient UT appears unimpaired when considering syntactic/sequential measures. The more surprising contrast is between patients CBD/TB and patient MD. The latter has the greatest overall lesion volume as well as substantial damage to BA 44 (Table 1). We constructed lesion subtraction maps using MRIcron to determine how the impaired patients differed from MD. Figure 3 shows areas where CBD and TB have damage and MD does not. All coordinates are in the Montreal Neurological Institute (MNI) space. The first two axial slices show that CBD and TB have additional damage to the junction between BA 44 and BA 6. The next three slices show similar damage more dorsally, extending into BA 9. There were no other differences (in the intermediate slices z=19 to 41, or other slices more ventral or dorsal to what is shown here). Overall, the subtraction maps in Figure 3 indicate that the impaired patients had some damage to the junction between pars opercularis and ventral premotor cortex that was not present in patient MD. This pattern also applies to the comparison between UT and the impaired patients. As noted earlier, UT had little overall damage to the posterior portion of left VLPFC.
Figure 3.
Lesion subtraction maps. Areas where CBD and TB have damage and MD does not. MNI coordinates.
This locus (BA 44/6) is intriguing because it has been implicated in a number of functional imaging studies that have looked at syntactic processing. For example, Fiebach and colleagues found increased activation at this junction for the processing of non-canonical sentences in German (object wh- sentences e.g., He asks himself, who the doctor has called?) when contrasting long vs. short displacement of the object noun-phrase (Fiebach, Schlesewsky, Lohmann, von Cramon & Friederici, 2005). Using an artificial grammar, Bahlmann and colleagues found increased activation in BA 44/6 for the processing of hierarchical versus adjacent dependencies (Bahlmann, Schubotz & Friederici, 2008). Some authors have focused on cytoarchitectonic and connectivity differences between BA 6 and BA 44, while admitting some continuity. It has been suggested that ventral premotor cortex (BA 6) is agranular, phylogenetically older, and may underlie local processing (as in simple noun-phrases in language, and perhaps sequence processing in non-linguistic domains) while newer portions of left VLPFC (BA 44/45) are granular and may underlie the processing of hierarchical structure (Friederici, 2006; Friederici, Bahlmann, Heim, Schubotz & Anwander, 2006). Other researchers have stressed the similarities between premotor cortex and classic language areas (e.g., Rizzolatti & Arbib, 1998). When integrating gesture and speech, activity in Broca’s area can be modulated by action information and activity in premotor cortex can be influenced by the linguistic context (Willems, Ozyurek & Hagoort, 2007). Damage to Broca’s area results in not just linguistic deficits, but also deficits in action comprehension (Fazio, et al., 2009; Saygin, Wilson, Dronkers & Bates, 2004).6 Overall, these results hint at a tight interconnection between the motor and language functions of left VLPFC.
What role might BA 44/6 pay in language production? Two recent fMRI studies suggest a role for this locus in sequencing/linearization. Grewe and colleagues (2006) compared German sentences where an inanimate subject was placed either before or after an animate object (both orders are unmarked for the German construction that was used). They found increased activation at the junction of pars opercularis and ventral PMC in the former condition, when an inanimate subject appeared in sentence-initial position. They found no such increase when comparing subject-before-object and object-before-subject sentences where both nouns were animate. This suggests that the pars opercularis and ventral PMC are sensitive to violations of linear order based on animacy. These results bear a striking resemblance to our finding that patients with additional damage to BA 44/6 (CBD and TB) have specific difficulty with the production of inanimate-first multiword utterances as well as the comprehension of non-canonical reversible sentences (where both noun-phrases are animate and animacy is therefore not helpful for the assignment of semantic roles).
A second fMRI study, by Gelfand and Bookheimer (2003), found activation at the BA 44/6 junction during a sequence manipulation task. Participants performed either an easy sequencing task (judge whether two consecutive 3-item sequences match) or a hard sequencing task (judge whether one sequence is the reverse of another, or involves the deletion of the middle item). The contrast between the hard tasks and the easy task revealed activation in BA 44/6 and BA 44/6/9. Similar patterns of activation were found for sequence manipulation of linguistic and non-linguistic items (syllables/hummed tones). The authors concluded that the posterior part of left VLPFC might support domain-general sequencing operations. Again, this is consistent with our hypothesis that parts of left VLPFC may play a critical role in selection for position.
3.12. Discussion
Going in, we made two predictions: a) that switching the position of a noun from a previously primed position would result in “positional” interference, and b) that left VLPFC patients would be specially impaired in resolving this interference. The first prediction was largely borne out. Control participants showed a marginal interference effect, with longer latencies during inconsistent target trials than consistent target trials. Three of the four patients also showed this pattern. The one exception was patient MD, who showed unexpectedly fast latencies on some inconsistent trials (see 3.7.1). Interestingly, MD had a much lower score than the other patients on two standardized screening tasks that are presumed to measure phonological STM (non-word repetition: CBD (68), MD (52), TB (90), UT (88); rhyme span probe: CBD (6.3), MD (3.7), TB (5), UT (6.8)). Patients with poor phonological STM sometimes show patterns consistent with overly rapid decay of representations (see patient EA in Hamilton & Martin, 2007). We speculate that MD may have relied upon such fast decaying phonological representations to name the pictures, thereby deriving the most facilitation for the shortest lag between repeated uses of the same noun even though the position was inconsistent.
With respect to our second prediction, based on prior evidence for impaired interference resolution with left VLPFC damage (e.g., Hamilton & Martin, 2007), we had originally hypothesized that all of our patients would show exaggerated positional interference. As it happens, we found this pattern in only two of the four patients. Reassuringly however, three different measures within the multiword experiment indicated the same grouping of patients. Patients CBD and TB showed exaggerated position-based interference compared to controls; they made more errors (including omissions) during the unprimed trials of the multiword task compared to the other two patients; and the pattern of increased errors applied specifically to trials where the order of nouns went counter to the prevalent order in English. The results from the multiword experiment also correlated with two standardized language measures. As detailed in section 3.10, patients CBD and TB showed poor comprehension of non-canonical sentences as well as difficulty in producing well-formed utterances in a free-form story-telling task. Finally, our lesion analysis suggests that there were relevant anatomical differences between patients that might correlate with their behavioral profiles. The two impaired patients – CBD and TB – had damage to the junction of BA 44 and BA 6 that the unimpaired patients did not. Together, the behavioral and anatomical data are consistent with a role for parts of left VLPFC in selection for position. In the rest of this section, we discuss in more detail the link between the hypothesized mechanism, and the behavioral effects on the one hand and the anatomical locus on the other.
3.12.1. The Link between Selection for Position and the Behavioral Effects
How might impaired selection for position relate to CBD’s and TB’s behavioral effects? In the multiword task, the activation level of a noun at any given point during the utterance could be influenced by: baseline activation that reflects, among other things, the long-term frequency of noun use in that particular position; short-term activation due to priming of the noun in a particular position; and task-induced “bottom-up” activation that say, maps the entity on the left to the first uttered noun (see Kimberg & Farah, 1993 for an example implementation of this model). As an example, consider when participants produce a prime (cup and pencil) followed by an incongruent target (eye and cup). During the target, both “eye” and “cup” are presumably activated because participants are planning a conjoined noun phrase. In selecting the first noun to be uttered, “cup” would have an activation advantage due to position-priming while “eye” would be better activated due to bottom-up activation from the visual scene (because EYE is on the left). Under these conditions, left VLPFC involvement may be necessary for biasing the activations in accordance with task goals such that “eye” is selected. If so, damage to this area may result in longer times for resolving the competition, particularly during inconsistent target trials. This could explain the RT effects seen in CBD and TB. While this competition can be exaggerated or minimized by the priming manipulation, some amount of competition exists whenever concurrently activated words have to be ordered into a grammatical utterance. This could explain why CBD and TB had trouble during unprimed trials – an inability to quickly resolve competition amongst nouns may have led to errors and omissions by these patients. Along the same lines, long-term mappings between animacy features and sentential positions may have affected the relative activation and competition between the two nouns, leading to the patients’ difficulty with inanimate-first phrases (see Bock, Loebell & Morey, 1992 for related evidence). Lastly, the statistical patterns of English may lead to greater default activation of the subject ↔ agent relative to the subject ↔ patient mapping. Under less than effective biasing of competition by left VLPFC, this may have caused CBD and TB difficulty in comprehending non-canonical sentences.
3.12.2. The Link between Selection for Position and the Anatomical Locus
The anatomical locus suggested by our lesion analysis (BA 44/6) is consistent with the selection for position mechanism. As reviewed above, this area is implicated in syntactic processing as well as sequencing of linguistic and non-linguistic stimuli. It is possible however that a general-purpose competition-biasing mechanism i.e., one that is not specific to sequencing might explain our results. Many areas within left prefrontal cortex, including but not limited to VLPFC, have been implicated in resolving competition among alternate characterizations of an input. Chief among such areas are the dorsolateral PFC (DLPFC) and the inferior frontal junction (IFJ) (Brass, Derrfuss, Forstmann & von Cramon, 2005; Derrfuss, Brass & Yves von Cramon, 2004). Many of the tasks that recruit these areas (e.g., Stroop) have no obvious sequencing component. The anatomical differences between our impaired and unimpaired patients are seen in BA 44/6 but also extend dorsally to the IFJ (BA 44/6/9). Thus, our anatomical data cannot help disambiguate between a broadly applicable selection mechanism that is subserved by ventral and dorsal PFC, and a more specific “selection for position” mechanism hypothesized to be supported by BA 44/6. Future studies could address this question through concurrent examination of different kinds of interference (e.g., positional, semantic) in the same set of patients. For the present, we have information on semantic interference for one of the patients discussed here (TB). This patient, who showed exaggerated interference in the current sequencing-related task, showed no indication of an interference effect in a semantic Mocking paradigm that required no sequencing (Schnur, et al., 2006: Appendix C). Therefore, we tentatively predict possible specialization within PFC for the biasing of different kinds of representations.
4. Conclusion
Our results suggest that parts of left VLPFC (BA 44/6) may underlie a selection for position mechanism, which could play a role in syntactic production and comprehension. Future research can shed light on the relation between this mechanism and the resolution of semantic interference, the relation between left VLPFC-supported control processes and full sentence production, and the extent to which the purported mechanisms are language-specific. While available fMRI evidence provides tentative answers to some of these questions, the causal link between posterior VLPFC and the proposed selection mechanisms may be better established by studying brain-damaged individuals.
Acknowledgments
The authors thank Adelyn Brecher, Laurel Brehm and Rachel Jacobson for help in recruiting participants and coding data. This research was supported in part by NIH/NICHD grant 5-T32-HD-007425 to the University of Pennsylvania and by RO1 DC000191 to MFS.
Appendix
Patient % damage for selected subcortical areas
| CBD | MD | TB | UT | |
|---|---|---|---|---|
| Caudate % | 0.05 | 15.4 | 0 | 3.88 |
| Putamen % | 0 | 18.77 | 22.78 | 26.03 |
| Thalamus % | 0.47 | 1.98 | 0 | 0.02 |
| Pallidum % | 0 | 0 | 0.09 | 0.39 |
List of pictures used in Experiment I
| Edible things | Clothing | Kitchen things | Animals | Toy/Games | Body parts | Hardware/Tools | Misc. |
|---|---|---|---|---|---|---|---|
| apple | belt | bottle | camel | football | ear | nail | cloud |
| banana | button | bowl | cat | balloon | heart | ladder | sun |
| carrot | dress | fork | dog | kite | nose | chain | star |
| lemon | glove | glass | duck | swing | thumb | scissors | candle |
| mushroom | hat | spoon | fish | whistle | arm | ruler | ashtray |
| onion | ring | toaster | horse | bell | foot | pen | bus |
| pear | shirt | cup | monkey | book | eye | pencil | cigar |
| grapes | skirt | knife | owl | drum | hair | hammer | pipe |
| pumpkin | sock | table | snake | clown | hand | saw | windmill |
| sandwich | shoe | chair | zebra | fag | lips | screw | snowman |
Connected speech sample from UT
Well the husband and wife are arguing in the first one. She’s yelling at him with her arms pointed at him. He’s got his arm on the chair as he’s reading the newspaper, yelling back. Then she leaves the house. She’s got her coat and bag and he’s just reading the newspaper, and she’s leaving. Now, she’s left the house and he’s sitting there, all alone, hand on his head, head on his hand, the paper on his one arm and he’s sitting there, just/dIs-/disgusted. Next, she comes back. She looks different now, her hair’s all messed up, (laughs) but she comes back in, and he’s still sitting there in the chair, and the door’s behind him. He gets up and goes over and hugs her. He’s hugging her and she’s crying. Her bag’s there, she, he’s hugging her and she’s alright. Now the last one, he looks out the door and he sees the car wrapped around a tree. That’s why she came back. She wasn’t angry, she wasn’t unhappy, she came back because she smashed the car into a tree. And they’re hugging.
Lesion maps for individual patients. Four axial slices (z=18, 26, 34, 42)
Footnotes
For example, for “the cup and the zebra” the measured latency was to the onset of the utterance i.e., to the first “the”. This measure seemed to us to be the most appropriate given that participants were instructed to plan the entire utterance and the pictures disappeared upon utterance onset. We did not measure latencies to the first and second nouns.
For patient CBD’s first session, we analyzed data from 1.5 blocks. This patient began the first block in this session using “the x and the y” phrases but switched half through the block to “x and y” because he had difficulty with producing the former.
On these trials, latencies were measured to the first attempted word in the utterance (e.g., “ri ring…”: to “ri”, “er a cup and …”: to “a”).
Our stimuli were not designed to test the animacy hypothesis specifically. However, post-hoc analyses suggested no systematic differences between animate and inanimate nouns (MRC database. Coltheart, 1981). They did not differ in concreteness, written frequency or number of syllables. Familiarity (inanimate>animate, p<.01) and imageability (animate>inanimate, p=.09) went in opposite directions.
Some studies have suggested a link between language production and STM span, particularly semantic STM (sSTM) (see e.g., Hamilton & Martin, 2007; Martin, Miller & Vu, 2004). However, we found no correlation of multiword performance with either the overall span (Table 2) or patients’ scores on a category probe test of sSTM (CBD=3; TB=3.5; UT=4.1).
Willems, et al. tested semantic integration between language and gesture. The strongest results were obtained in BA 45. Fazio, et al. tested 6 patients with agrammatic speech, but normal praxic abilities. The maximum overlap of patient’s lesions was in BA 44. Saygin, et al. conducted a voxel-based lesion symptom mapping analysis of 29 patients. Lesion to BA 44/6/4 was significantly correlated with poor non-linguistic action comprehension.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Avants BB, Schoenemann PT, Gee JC. Lagrangian frame diffeomorphic image registration: Morphometric comparison of human and chimpanzee cortex. Medical Image Analysis. 2006;10:397–412. doi: 10.1016/j.media.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Badecker W, Caramazza A. On considerations of method and theory governing the use of clinical categories in neurolinguistics and cognitive neuropsychology: the case against agrammatism. Cognition. 1985;20:97–125. doi: 10.1016/0010-0277(85)90049-6. [DOI] [PubMed] [Google Scholar]
- Bahlmann J, Schubotz RI, Friederici AD. Hierarchical artificial grammar processing engages Broca’s area. Neuroimage. 2008;42:525–534. doi: 10.1016/j.neuroimage.2008.04.249. [DOI] [PubMed] [Google Scholar]
- Banich MT, Milham MP, Atchley RA, Cohen NJ, Webb A, Wszalek T, et al. Prefrontal regions play a predominant role in imposing an attentional ‘set’: Evidence from fMRI. Cognitive Brain Research. 2000;10:1–9. doi: 10.1016/s0926-6410(00)00015-x. [DOI] [PubMed] [Google Scholar]
- Belke E, Meyer AS, Damian MF. Refractory effects in picture naming as assessed in a semantic blocking paradigm. The Quarterly Journal of Experimental Psychology. 2005;58A:667–692. doi: 10.1080/02724980443000142. [DOI] [PubMed] [Google Scholar]
- Biegler KA, Crowther JE, Martin RC. Consequences of an inhibition deficit for word production and comprehension: Evidence from the semantic blocking paradigm. Cognitive Neuropsychology. 2008;25:493–527. doi: 10.1080/02643290701862316. [DOI] [PubMed] [Google Scholar]
- Bock K, Loebell H, Morey R. From conceptual roles to structural relations: Bridging the syntactic cleft. Psychological Review. 1992;99:150–171. doi: 10.1037/0033-295x.99.1.150. [DOI] [PubMed] [Google Scholar]
- Bookheimer S. Functional MRI of language: New approaches to understanding the cortical organization of semantic processing. Annu Rev Neurosci. 2002;25:151–188. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- Bornkessel I, Zysset S, Friederici AD, von Cramon DY, Schlesewsky M. Who did what to whom? The neural basis of argument hierarchies during language comprehension. Neuroimage. 2005;26(1):221–233. doi: 10.1016/j.neuroimage.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Brass M, Derrfuss J, Forstmann B, von Cramon DY. The role of the inferior frontal junction area in cognitive control. Trends in Cognitive Sciences. 2005;9(7):314–316. doi: 10.1016/j.tics.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Caplan D. The measurement of chance performance in aphasia, with specific reference to the comprehension of semantically reversible passive sentences: A note on issues raised by Caramazza, Capitani, Rey, and Berndt (2001) and Drai, Grodzinsky, and Zurif (2001) Brain and Language. 2001;76:193–201. doi: 10.1006/brln.2000.2354. [DOI] [PubMed] [Google Scholar]
- Caplan D, Baker C, Dehaut F. Syntactic determinants of sentence comprehension in aphasia. Cognition. 1985;21:117–175. doi: 10.1016/0010-0277(85)90048-4. [DOI] [PubMed] [Google Scholar]
- Caplan D, Alpert N, Waters G. Effects of syntactic structure and propositional number on patterns of regional cerebral blood flow. Journal of Cognitive Neuroscience. 1998;10:541–52. doi: 10.1162/089892998562843. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Zurif EB. Dissociation of algorithmic and heuristic processes in language comprehension: Evidence from aphasia. Brain and Language. 1976;3:572–582. doi: 10.1016/0093-934x(76)90048-1. [DOI] [PubMed] [Google Scholar]
- Coltheart M. The MRC Psycholinguistic Database. Quarterly Journal of Experimental Psychology. 1981;33A:497–505. [Google Scholar]
- Crawford JR, Howell DC. Comparing an individual’s test score against norms derived from small samples. The Clinical Neuropsychologist. 1998;12:482–486. [Google Scholar]
- Dapretto M, Bookheimer SY. Form and content: dissociating syntax and semantics in sentence comprehension. Neuron. 1999;24:427–32. doi: 10.1016/s0896-6273(00)80855-7. [DOI] [PubMed] [Google Scholar]
- Derrfuss J, Brass M, von Cramon DY. Cognitive control in the posterior frontolateral cortex: evidence from common activations in task coordination, interference control, and working memory. Neuroimage. 2004;23:604–612. doi: 10.1016/j.neuroimage.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Dick F, Bates E, Wulfeck B, Utman J, Dronkers N, Gernsbacher M. Language deficits, localization, and grammar: Evidence for a distributive model of language breakdown in aphasic patients and neurologically intact individuals. Psychological Review. 2001;108:759–788. doi: 10.1037/0033-295x.108.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dronkers NF, Wilkins DP, van Valin RD, Redfern BB, Jaeger JJ. A reconsideration of the brain areas involved in the disruption of morphosyntactic comprehension. Brain and Language. 1994;47:461–463. [Google Scholar]
- Fazio P, Cantagallo A, Craighero L, D’Ausilio A, Roy AC, Pozzo T, et al. Encoding of human action in Broca’s area. Brain. 2009;132:1980–1988. doi: 10.1093/brain/awp118. [DOI] [PubMed] [Google Scholar]
- Freedman ML, Martin RC, Biegler K. Semantic relatedness effects in conjoined noun phrase production: Implications for the role of short-term memory. Cognitive Neuropsychology. 2004;21:245–265. doi: 10.1080/02643290342000528. [DOI] [PubMed] [Google Scholar]
- Fiebach CJ, Schlesewsky M, Lohmann G, von Cramon DY, Friederici AD. Revisiting the role of Broca’s area in sentence processing: Syntactic integration versus syntactic working memory. Human Brain Mapping. 2005;24:79–91. doi: 10.1002/hbm.20070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD. Broca’s area and the ventral premotor cortex in language: Functional differentiation and specificity. Cortex. 2006;42:472–475. doi: 10.1016/s0010-9452(08)70380-0. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Bahlmann J, Heim S, Schubotz RI, Anwander A. The brain differentiates human and non-human grammars: Functional localization and structural connectivity. Proceedings of the National Academy of Sciences. 2006;103:2458–2463. doi: 10.1073/pnas.0509389103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand J, Bookheimer S. Dissociating Neural Mechanisms of Temporal Sequencing and Processing Phonemes. Neuron. 2003;38:831–842. doi: 10.1016/s0896-6273(03)00285-x. [DOI] [PubMed] [Google Scholar]
- Goodglass H. Understanding aphasia. San Diego, CA: Academic Press; 1993. [Google Scholar]
- Grewe T, Bornkessel I, Zysset S, Wiese R, von Cramon DY, Schlesewsky M. Linguistic prominence and Broca’s area: the influence of animacy as a linearization principle. NeuroImage. 2006;32:1395–1402. doi: 10.1016/j.neuroimage.2006.04.213. [DOI] [PubMed] [Google Scholar]
- Hamilton AC, Martin RC. Proactive interference in a semantic short-term memory deficit: Role of semantic and phonological relatedness. Cortex. 2007;43:112–123. doi: 10.1016/s0010-9452(08)70449-0. [DOI] [PubMed] [Google Scholar]
- Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. Enhancement of MR images using registration for signal averaging. Journal of Computer Assisted Tomography. 1998;22:324–33. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- Jonides J, Nee DE. Brain mechanisms of proactive interference in working memory. Neuroscience. 2006;139:181–193. doi: 10.1016/j.neuroscience.2005.06.042. [DOI] [PubMed] [Google Scholar]
- Kan IP, Thompson-Schill SL. Effect of name agreement on prefrontal activity during overt and covert picture naming. Cognitive, Affective and Behavioral Neuroscience. 2004;4:43–57. doi: 10.3758/cabn.4.1.43. [DOI] [PubMed] [Google Scholar]
- Kertesz A. Western aphasia battery. New York: Grune & Stratton; 1982. [Google Scholar]
- Kimberg DY, Farah MJ. A unified account of cognitive impairments following frontal lobe damage: The role of working memory in complex, organized behavior. Journal of Experimental Psychology: General. 1993;112:411–428. doi: 10.1037//0096-3445.122.4.411. [DOI] [PubMed] [Google Scholar]
- Martin RC, Miller M, Vu H. Lexical semantic retention and speech production: Further evidence from normal and brain-damaged participants for a phrasal scope of planning. Cognitive Neuropsychology. 2004;21:625–644. doi: 10.1080/02643290342000302. [DOI] [PubMed] [Google Scholar]
- McCarthy RA, Kartsounis LD. Wobbly words: Refractory anomia with preserved semantics. Neurocase. 2000;6:487–497. [Google Scholar]
- Rizzolatti G, Arbib M. Language within our grasp. Trends in Neurosciences. 1998;21:188–194. doi: 10.1016/s0166-2236(98)01260-0. [DOI] [PubMed] [Google Scholar]
- Rochon E, Saffran EM, Berndt RS, Schwartz MF. Quantitative analysis of aphasic sentence production: Further development and new data. Brain and Language. 2000;72(3):193–218. doi: 10.1006/brln.1999.2285. [DOI] [PubMed] [Google Scholar]
- Roder B, Stock O, Neville H, Bien S, Rosier F. Brain activation modulated by the comprehension of normal and pseudo-word sentences of different processing demands: a functional magnetic resonance imaging study. NeuroImage. 2002;15:1003–1014. doi: 10.1006/nimg.2001.1026. [DOI] [PubMed] [Google Scholar]
- Rossion B, Pourtois G. Revisiting Snograss and Vanderwort’s object pictorial set: The role of surface detail in basic-level object recognition. Perception. 2004;33:217–236. doi: 10.1068/p5117. [DOI] [PubMed] [Google Scholar]
- Saffran EM, Schwartz MF, Marin OS. The word order problem in agrammatism: II. Production. Brain and Language. 1980;10(2):263–280. doi: 10.1016/0093-934x(80)90056-5. [DOI] [PubMed] [Google Scholar]
- Saffran EM, Berndt RS, Schwartz MF. The quantitative analysis of agrammatic production: Procedure and data. Brain and Language. 1989;37(3):440–479. doi: 10.1016/0093-934x(89)90030-8. [DOI] [PubMed] [Google Scholar]
- Saffran EM, Schwartz MF, Linebarger MC, Martin N, Bochetto P. Philadelphia comprehension battery. 1998. Unpublished. [Google Scholar]
- Saygin AP, Wilson SM, Dronkers NF, Bates E. Action comprehension in aphasia: Linguistic and non-linguistic deficits and their lesion correlates. Neuropsychologia. 2004;42:1788–1804. doi: 10.1016/j.neuropsychologia.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A. E-Prime reference guide. Pittsburgh: Psychology Software Tools, Inc; 2002. [Google Scholar]
- Schnur TT, Schwartz MF, Brecher A, Hodgson C. Semantic interference during blocked-cyclic naming: Evidence from aphasia. Journal of Memory and Language. 2006;54:199–227. [Google Scholar]
- Schnur TT, Schwartz MF, Kimberg DY, Hirshorn E, Coslett HB, Thompson-Schill SL. Localizing interference during naming: Convergent neuroimaging and neuropsychological evidence for the function of Broca’s area. Proceedings of the National Academy of Sciences. 2009;106:322–327. doi: 10.1073/pnas.0805874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MF, Saffran EM, Marin OS. The word order problem in agrammatism: I. Comprehension. Brain and Language. 1980;10(2):249–262. doi: 10.1016/0093-934x(80)90055-3. [DOI] [PubMed] [Google Scholar]
- Schwartz MF, Hodgson C. A new multiword naming deficit: Evidence and interpretation. Cognitive Neuropsychology. 2002;19(3):263–288. doi: 10.1080/02643290143000187. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Jonides J, Marshuetz C, Smith EE, D’Esposito M, Kan IP, et al. Effects of frontal lobe damage on interference effects in working memory. Cognitive, Affective, & Behavioral Neuroscience. 2002;2:109–120. doi: 10.3758/cabn.2.2.109. [DOI] [PubMed] [Google Scholar]
- Vanier M, Caplan D. CT-scan correlates of agrammatism. In: Menn L, Obler LK, editors. Agrammatic aphasia. Amsterdam: Benjamins; 1990. pp. 97–114. [Google Scholar]
- Williams SE, Canter GJ. The influence of situational context on naming performance in aphasic syndromes. Brain and Language. 1982;17:92–106. doi: 10.1016/0093-934x(82)90007-4. [DOI] [PubMed] [Google Scholar]
- Willems RM, Ozyurek A, Hagoort P. When language meets action: The neural integration of gesture and speech. Cerebral Cortex. 2007;17:2322–2333. doi: 10.1093/cercor/bhl141. [DOI] [PubMed] [Google Scholar]
- Wilshire CE, McCarthy RA. Evidence for a context-sensitive word retrieval disorder in a case of nonfluent aphasia. Cognitive Neuropsychology. 2002;19:165–186. doi: 10.1080/02643290143000169. [DOI] [PubMed] [Google Scholar]