Abstract
Novel retinoids 1–3 containing perillyl alcohol were synthesized through the addition of perillyl alcohol to the activated carboxylic acids (retinoids) promoted by DCC (N, N′-Dicylohexyl cabodiimide). A set of structurally and functionally diverse perillyl alcohol derivatives of retinoids were obtained in good yields (78–82%). Biological evaluation of these novel hybrid compounds (containing retinoids and perillyl alcohol) is currently underway in our laboratory.
Apoptosis is an essential cellular process for normal development and homeostasis of multicellular organisms, to eliminate unwanted or damaged cells. Deregulated apoptosis plays a major role in many human diseases, including cancer.1–4 Defects in the apoptosis machinery can confer apoptosis resistance of cancer cells to therapeutic agents, which can render current anticancer therapies less effective, and may lead ultimately to their failure in the clinic.2–4 Accordingly, targeting critical regulators of apoptosis to overcome apoptosis resistance is a promising cancer therapeutic strategy. Perillyl alcohol and all-trans retinoic acid (ATRA) have antitumor activity toward liver, colon and hematopoietic tumors, and are used as chemotherapeutic agents.5 The chemo-preventive activity of perillyl alcohol and ATRA is associated with an increase in apoptosis of the tumor cells. Therefore, we hypothesized that hybrid molecules containing both perillyl alcohol and ATRA might further increase chemo-preventive activity through enhancing apoptosis. The hybrid concept to synthesize biological active molecules is a frontier area of research and this concept has successfully employed to synthesize new therapeutic agents for Alzheimer’s diseases.6
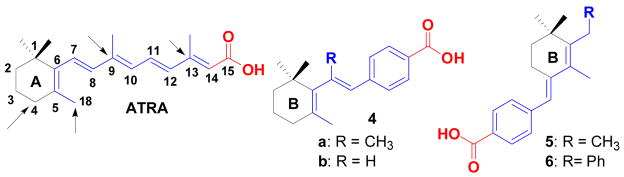
Toward this goal, we first sought to synthesize compounds 4a–b as new retinoic acid analogues by introducing a constrained phenyl ring system in the place of conjugated alkene backbone (spacers in ATRA) to inhibit the metabolism of ATRA into its isomers, 9-CRA and 13-CRA (Figure 1).
Figure 1.
Structure of ATRA and our new retinoic acid analogues. Metabolic positions of all-trans-retinoic acid (ATRA) are shown by arrows.
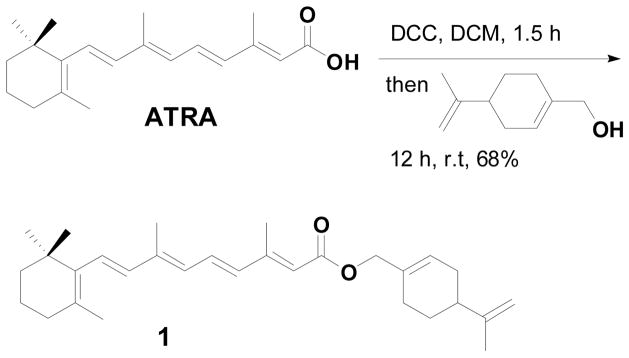
Furthermore, we considered the synthesis of novel perillyl alcohol containing retinoids (1–3) for studying tumor cell targeted apoptosis. To our surprise, a search of the literature failed to uncover any examples of perillyl alcohol containing retinoic acid derivatives. Here we report the first synthesis of perillyl alcohol containing retinoids by simple addition of perillyl alcohol to the activated carboxylic acids (retinoids), promoted by DCC (Scheme 1).
Scheme 1.
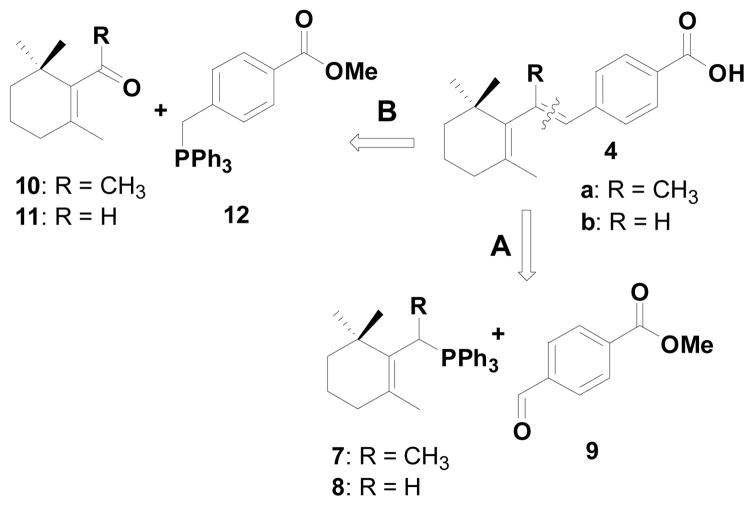
We next explored the synthesis of the constrained retinoids 4a–b and the possible retro-synthetic analysis as shown in Scheme 2.
Scheme 2.
Retrosynthetic analysis
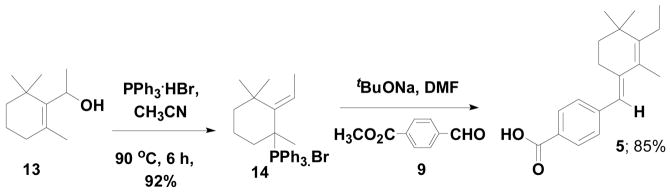
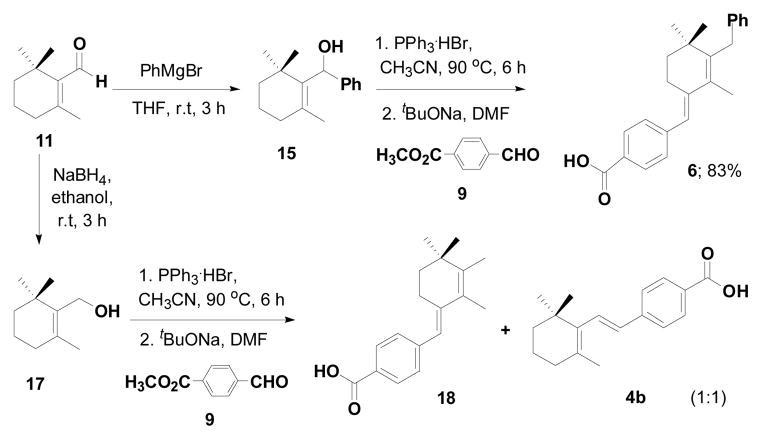
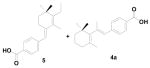
In the process of synthesizing 4a, based on disconnection route A (Scheme 2) starting from the allylic alcohol 13, we encountered the unusual Wittig salt 14 which led to the formation of the unusual compound 5 when the ylide 14 was treated with aldehyde 9 (Scheme 3).7
Scheme 3.
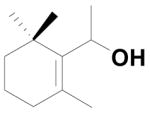
To explore the mechanism for forming the unusual Wittig salt 14, we first tried to understand the substitution effect at the α-position of the allylic alcohol 13. For that purpose we synthesized the allylic alcohol 15 from Grignard reaction of β-cyclocitral 11 with phenyl magnesium bromide in THF. The allylic alcohol 15 was converted into the corresponding ylide using PPh3HBr followed by the Wittig reaction with aldehyde 9 furnishing the unusual product 6 with 83% yield (Scheme 4).
Scheme 4.
Results obtained from route A
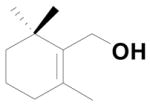
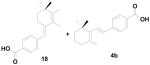
When the α-position of the allylic alcohol was substituted with hydrogen 17 (1° alcohol) we found both unusual and usual Wittig products 18 and 4b with the ratio of 1:0.6 respectively (Scheme 4). From these experiments we found that when the α-position is substituted with methyl, phenyl gives exclusively the unusual Wittig salt, whereas when the α-position is substituted with hydrogen, both usual and unusual products are obtained.
To further explore the formation of the unusual Wittig product, we examined various solvents. The allylic alcohol 13 gave the unusual Wittig product (5) in methanol, DMF and acetonitrile (Table 1; entry 1). Interestingly, allylic alcohol 17 gave the normal Wittig product (4b) in DMF (Table 1; entry 2) but produced mixture of usual (4b) and unusual (18) Wittig products in both methanol and acetonitrile (Table 1; entry 2). This observation indicates that the α-methyl group plays an important role in the reaction. We next examined the mechanistic insight to study the unusual Wittig salt formation using the cyclic allylic alcoholic systems (the alcohol group is inside the ring system).
Table 1.
Studies of the solvent effect of the unusual Wittig reaction
| entry | allylic alcohol | product | Yield (%)a (product ratio)b |
||
|---|---|---|---|---|---|
| MeOH | DMF | CH3CN | |||
| 1 |
 13 |
 |
85 (5: 4a = 1:0) | 83 (5: 4a =1:0) | 90 (5: 4a =1:0) |
| 2 |
 17 |
 |
80 (18: 4b =1:0.6) | 78 (18: 4b = 0:1) | 76 (18: 4b = 1:0.6) |
Isolated yield refers to aldehyde.
The ratios of the usual and unusual Wittig products were calculated from the 1H NMR.
To our surprise reactions utilizing allylic alcohols inside the ring system (entries 1–4; Table 2) generated the more normal Wittig products (20a–d). We examined cyclic 5-, 6- and 7- member ring systems and in all cases we isolated the regular Wittig products.
Table 2.
Studies of the Wittig reactions alcohol attached to the ring systema
 | |||
|---|---|---|---|
| entry | allylic alcohol (19a–d) | product | Yield (%)b (E:Z)c |
| 1 | 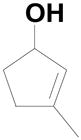 |
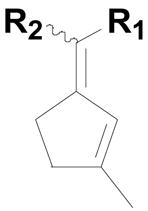 20a: (R1 = H, R2 = 4-HO2CC6H4) |
76 (99:1)d |
| 2 | 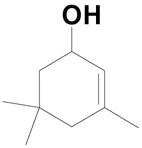 |
 20b: (R1 = H, R2 = 4-HO2CC6H4) |
83 (85:15) |
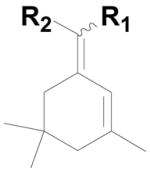
| 3 | 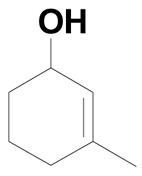 |
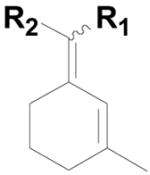 20c: (R1 = H, R2 = 4-HO2CC6H4) |
85 (72:28) |
| 4 | 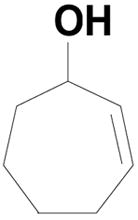 |
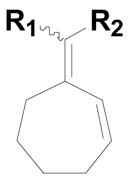 20d: (R1 = H, R2 = 4-HO2CC6H4) |
69 (99:1)d |
All reactions were performed using 1 equivalent of aldehyde and 3 equivalents of tBuONa in DMF for 8–12 h.
Isolated yield (mixture of E and Z) refers to aldehyde.
The E:Z ratios were determined by 1H NMR.
Trace amount of Z isomer was observed in 1H NMR.
These new derivatives 20a–d also have the hydrophobic cycloalkene ring and constrained phenyl ring system in the place of conjugated alkene backbone in ATRA, and the carboxylic acid group remains intact. We compared the structural relation of our original target constrained retinoids 4a–b with the new unusual products 5–6 and these new compounds 20a–d. Having an extension of the double bond conjugation via the 4th position of the cycloalkene ring unit may block or limit retinoic acid metabolism caused by cytochrome P450 enzymes (CYPs). This could be very advantageous, since it is believed that the most prominent physiological degradation pathway starts with the rate-limiting hydroxylation at the C-4 position of the cyclohexenyl ring, leading to formation of 4-hydroxy-ATRA, followed by a reductase enzyme into 4-oxo-ATRA and further transformation by CYP(s) into more polar metabolites.8
Then we tried to synthesize our original target constrained retinoids 4a–b through the disconnection route B (Scheme 1). First we tried with the ylide 4-[(Triphenyl-phosphanyl)-methyl]-benzoic acid methyl ester 12 with β-cyclocitral 11 with different reaction conditions and the reactions failed to obtain the constrained retinoid 4a. We then prepared the reactive ylide 4-(diethoxy-phosphorylmethyl)-benzoic acid methyl ester 21, and treating with β-cyclocitral 11 using tBuOK as a base yielded the final product 4b with 79% yield (Table 3; entry 3).
Table 3.
Results obtained from route B
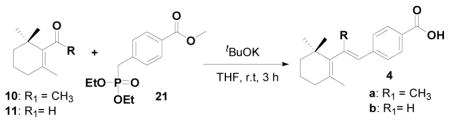 | |||
|---|---|---|---|
| entry | R | Reaction condition | Yield (%)a |
| 1 | H | tBuONa in DMF | - |
| 2 | H | tBuONa in THF | 16% |
| 3 | H | tBuOK in THF | 79% |
| 4 | CH3 | tBuONa in DMF | - |
| 5 | CH3 | tBuONa in THF | - |
| 6 | CH3 | tBuOK in THF | - |
| 7 | CH3 | TMG, THF | - |
Isolated yield
However, very surprisingly the Wittig reaction of ylide 4-(diethoxy-phosphorylmethyl)-benzoic acid methyl ester 21 with ketone 10 clearly failed to give the olefination product 4a.
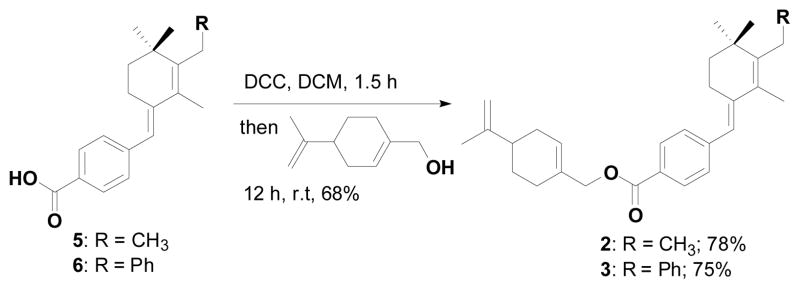
Encouraged by these results and having established a new strategy for synthesizing the new retinoid derivatives we synthesized the perillyl alcohol containing constrained retinoids 2–3. These were synthesized from the acids 5 and 6 using DCC as a carboxylic acid activating agent in DCM. The activated carboxylic acid was treated with perillyl alcohol in DCM at room temperature and generated the products 2 and 3 with 78% and 75% yields respectively (Scheme 5).
Scheme 5.
In conclusion, we have synthesized novel constrained retinoids (4b, 5, 6 and 20a–d) and found when alcohols are outside the ring produce unusual Wittig salts, whereas when alcohols are inside the ring produce usual Wittig salts. These acids were further esterified using perillyl alcohol to synthesize new perillyl alcohol containing retinoids through DCC coupling. This is based on the addition of perillyl alcohol to the activated carboxylic acids, promoted by DCC. A novel set of structurally and functionally diverse perillyl alcohol containing retinoids were obtained in moderate to good yields. Biological evaluation of these novel hybrid retinoids is currently underway in our laboratory.
Supplementary Material
Acknowledgments
The author B.C.D. is thankful to AECOM for start up funding. The instrumentation in the AECOM Structural NMR Resource is supported by the Albert Einstein College of Medicine and in part by Grants from the NSF (DBI9601607 and DBI0331934), the NIH (RR017998) and the HHMI Research Resources for Biomedical Sciences. T.E. is supported by the NIH (HL056182, HL064282).
Footnotes
The supplementary data (copies of 1H, 13C NMR and Mass spectra) are available online with this paper in Science Direct.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kerr JF, Wyllie AH, Currie AR. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson CB. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 3.Nicholson DW. Nature. 2000;407:810–816. doi: 10.1038/35037747. [DOI] [PubMed] [Google Scholar]
- 4.Reed JC. Nat Rev Drug Discovery. 2002;1:111–121. doi: 10.1038/nrd726. [DOI] [PubMed] [Google Scholar]
- 5.Keith RS, Jamie HM, Yvette DB, Yvonne AB, Pamela LC. Carcinogenesis. 1997;18:1655–1658. [Google Scholar]
- 6.Pelayo C, Xavier F, Carles G, Tània G, Diego M, Michele S, Elisabet V, Albert B, Victòria Clos M, Antoni C, Mercè P, Manuela B, Francesca M, Vincenza A, Joan E, Mònica L, Axel B, Javier Luque F. J Med Chem. 2008;51:3588–3598. [Google Scholar]
- 7.(a) Das BC, Kabalka GW. Tetrahedron Lett. 2008;49:4695. [Google Scholar]; (b) Das BC, Mahalingam SM, Evans T, Kabalka GW, Anguiano J, Hema K. Chem Comm. 2009:2133. doi: 10.1039/b823063c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Napoli JL. FASEB J. 1996;10:993–1001. doi: 10.1096/fasebj.10.9.8801182. [DOI] [PubMed] [Google Scholar]
- 9.General procedure for synthesis of perillyl alcohol containing new retinoids (1–3): A mixture of all trans-retinoic acid (100 mg, 0.35 mmol) in dry CH2Cl2 (5 mL) was stirred under nitrogen atmosphere was added DCC (79 mg, 0.38 mmol) and cat. DMAP at room temperature. The reaction mixture was stirred for 2 h at room temperature under nitrogen atmosphere. To this stirred solution perillyl alcohol (58.6 mg, 0.38 mmol) was added drop wise. The reaction mixture was stirred at room temperature until TLC analysis indicated none remaining (about 10–12 h). The reaction was quenched with saturated NH4Cl and extracted with ethyl acetate. The extracts were washed with H2O and brine, then dried overage Na2SO4, and evaporated. The residue was purified by flash column chromatography using hexane/ethyl acetate (8/1) as the eluent to give 1 as colourless oil.compound 1: 1H NMR (300 MHz, 1.05 (s, 6H), CDCl3): δ 1.45–1.52 (m, 2H), 1.58 –1.67 (m, 2H), 1.73 (s, 3H), 1.76 (s, 3H), 1.97–2.10 (m, 6H), 2.10–2.20 (m, 4H), 2.38 (s, 3H), 4.53 (s, 2H), 4.76 (m, 2H), 5.78–5.82 (m, 2H), 6.12–6.15 (m, 1H), 6.20 (s, 1H), 6.25–6.31 (2H, d, J = 18 Hz), 6.92–7.05 (m, 1H). 13C NMR (75 MHz, CDCl3): δ 13.3, 14.3, 19.6, 21.2, 22.1, 26.8, 27.7, 29.4, 30.9, 33.5, 34.6, 39.9, 41.3, 68.2, 109.1, 118.7, 125.8, 129.1, 129.8, 130.4, 131.4, 133.4, 135.5, 137.6, 138.1, 140.0, 150.1, 153.4, 167.5.compound 2: 1H NMR (300 MHz, 1.07–1.15 (m, CDCl3): δ 9H), 1.23–1.38 (m, 2H), 1.50–1.54 (m, 3H), 1.77 (s, 3H), 1.94 (s, 4H), 2.18–2.30 (m, 6H), 2.60–2.68 (m, 2H), 4.70–4.79 (m, 4H), 5.59 (s, 1H), 6.48 (s, 1H), 7.33–7.36 (d, J = 9 Hz, 2H), 8.00–8.03 (d, J = 9 Hz, 2H). 13C NMR (75 MHz, CDCl3): δ 15.3, 15.7, 21.0, 23.5, 24.2, 25.1, 26.0, 27.1, 27.8, 28.0, 31.1, 35.8, 36.4, 39.2, 41.7, 69.4, 109.2, 120.8, 125.9, 127.8, 128.0, 129.8, 130.0, 133.8, 142.6, 144.7, 149.2, 150.8, 166.0. ESI MS:[M+H]+ 419.2946, calcd 419.2950 calcd for C29H39O2.compound 3: 1H NMR (300 MHz, 1.05 (s, 6H), CDCl3): δ 1.58–1.65 (m, 5H), 1.77 (s, 4H), 1.87 (s, 4H), 2.15–2.30 (m, 5H), 2.70–2.76 (m, 2H), 3.71 (s, 2H), 4.73–4.77 (m, 4H), 5.88 (br s, 1H), 6.57 (s, 1H), 7.17–7.25 (m, 3H), 7.29–7.33 (m, 2H), 7.38–7.41 (d, J = 9 Hz, 2H), 8.02–8.05 (d, J = 9 Hz, 2H). 13C NMR (75 MHz, CDCl3): δ 16.8, 21.2, 24.4, 25.0, 25.8, 27.0, 27.7, 28.2, 30.9, 35.3, 35.8, 36.1, 39.0, 41.6, 56.0, 69.7, 110.0, 121.8, 125.4, 128.7, 129.6, 129.7, 130.8, 133.0, 140.3, 142.5, 144.2, 145.0, 150.1, 166.8. ESI MS:[M+H]+ 481.3160, calcd 481.3107 for C34H40O2.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.