Abstract
Chloride transport by a series of steroid-based “cholapod” receptors/carriers was studied in vesicles. The principal method involved preincorporation of the cholapods in the vesicle membranes, and the use of lucigenin fluorescence quenching to detect inward-transported Cl−. The results showed a partial correlation between anion affinity and transport activity, in that changes at the steroidal 7 and 12 positions affected both properties in concert. However, changes at the steroidal 3-position yielded irregular effects. Among the new steroids investigated the bis-p-nitrophenylthiourea 3 showed unprecedented activity, giving measurable transport through membranes with a transporter/lipid ratio of 1:250 000 (an average of <2 transporter molecules per vesicle). Increasing transporter lipophilicity had no effect, and positively charged steroids had low activity. The p-nitrophenyl monourea 25 showed modest but significant activity. Measurements using a second method, requiring the addition of transporters to preformed vesicle suspensions, implied that transporter delivery was problematic in some cases. A series of measurements employing membranes of different thicknesses provided further evidence that the cholapods act as mobile anion carriers.
Keywords: anions, biomembranes, receptors, steroids, supramolecular chemistry
Introduction
The selective transport of ions through bilayer membranes is a key process in biology.[1] Nature uses a combination of gated channels and ATP-driven transporters to establish and manage ion concentrations in cellular compartments. Disruption of this system can have major effects. For example, the malfunction of ion channels leads to a range of medical problems (channelopathies),[2] while the blocking or promotion of ion transport underlies the biological activity of many natural products.
One such family of natural products are the ionophore antibiotics (valinomycin, monensin, gramicidin A etc.).[3] These compounds provide passages for ions across cell membranes, either by self-assembling to form channels or by binding and carrying the ions. Although both cation and anion transport are important to biology, the naturally-derived ionophores are strongly biased towards cations. The above-mentioned are all cationophores, as are nearly all other ion-transporting secondary metabolites. There are very few anion-transporting natural products,[4] and none which mirror the action and availability of the major cationophores.[5] Anionophores would be useful tools for biological research and could have therapeutic applications. For example a number of channelopathies, including the well-known genetic disorder, cystic fibrosis, result from defective anion channels.[2, 6] It is realistic to hope that anionophores could compensate for the missing activity, in “channel replacement therapies”.[7]
These possibilities have stimulated recent interest in the design and study of synthetic anion transporters.[8] The work has encompassed self-assembling channels,[9] monomeric channels[10] and receptors acting as mobile carriers.[11] Among the latter, we have described the “cholapods” 1 and 2 (see below).[11a,b] These steroid-based compounds possess preorganised anion-binding sites capable of very high affinities, allied to lipophilic frameworks which promote solubility in apolar media (such as membrane interiors).[12] They can transport chloride and nitrate ions through vesicle membranes at very low loadings (down to cholapod/lipid ratios of 1:250 000), and are also active in cultured epithelial cells. The mobile carrier mechanism was supported by the dependence of rates on transporter concentration and membrane fluidity.[11a]
Practical applications of these transporters, especially in medicine, will require high activities so that useful ion fluxes can be obtained at low doses. Intuitively one might expect a correlation between anion affinities and transport activities, and early work suggested that this was the case. Affinities for Et4N+Cl− in chloroform (Ka, Cl−, CHCl3) rose from 3.4 × 106 m−1 for 1a, via 5.2 × 108 m−1 for 1e, to 1011 m−1 for 2, and transport activities increased accordingly. Compound 2 is among the strongest of the cholapod anion receptors,[13, 14] so one might conclude that further advances would be difficult. However, transporters 1 and 2 represent a small fraction of cholapod structural space, and we were interested to explore a wider range of molecules. We now describe a study of 16 cholapods which gives a clearer picture of the relationships between structure, anion affinity and chloride transport activity. Our results show that the correlation between affinity and activity is imperfect, and reveal a new champion among cholapod anion transporters. We also provide further support for the mobile carrier mechanism, and show that even simple ureas can be moderately effective as chloride anion transporters.
Results and Discussion
Cholapod structure and synthesis
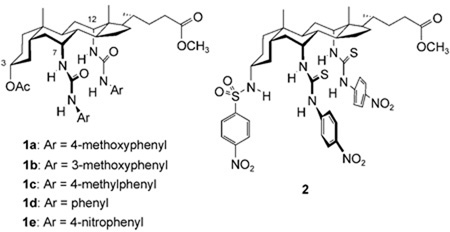
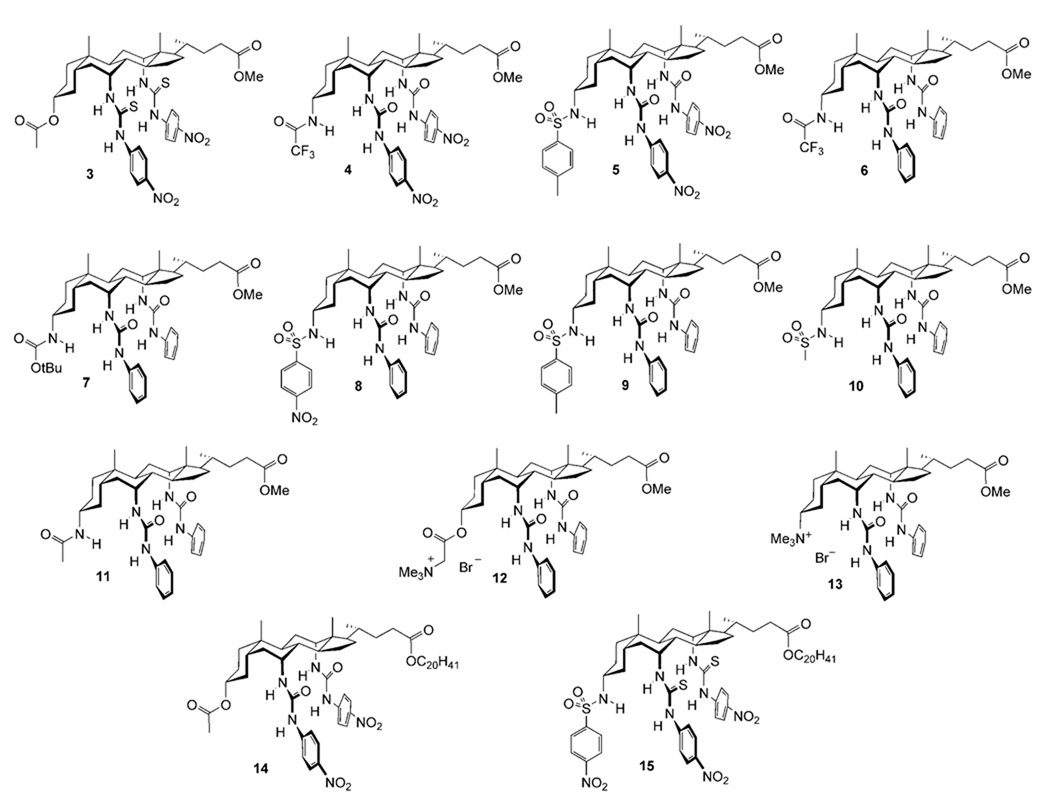
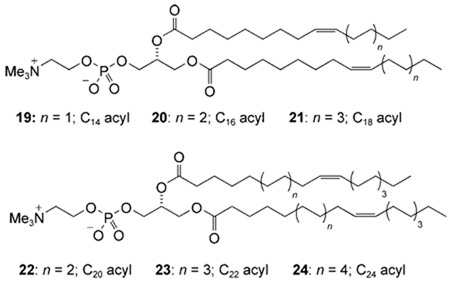
This study encompassed the previously-reported transporters 1d, 1e, and 2 along with the series of cholapods 3–15 shown in Figure 1. All possess the 7α,12α-bis(thio)ureidyl motif, which tends to afford high anion affinities.[12, 14] The choice of 7α,12α-substituent varied between phenylureidyl, p-nitrophenylureidyl and p-nitrophenylthioureidyl (for numbering, see 1). The NH acidities of these units increase in the order listed, with corresponding enhancements of anion affinities. The 3α-substituents included OAc, which makes essentially no contribution to binding,[15] and a number of H-bond donor units which again promote binding according to their acidities. Additionally, receptors 12 and 13 incorporated cationic NMe3 + groups, to test the possibility of transport via electroneutral complexes. Finally cholapods 14 and 15 were employed as eicosyl ester analogues of 1e and 2 respectively, to explore the effect of molecular size and lipophilicity.
Figure 1.
Cholapod anion receptors assayed for chloride transport activity in this work.
The syntheses of 1d, e,[11a] 4,[14] 6,[16] 7,[16] 12,[17] 13[17] and 15[18] have been described previously. The remaining compounds were prepared from intermediates 16,[19] 17a,[19] and 17b[20] through straightforward deprotections and derivatisations.[21]
Measurement of transport rates and binding constants—methodology
Chloride transport by the cholapods was assayed using large unilamellar vesicles (LUVs, 200 nm average diameter), with back-transport of nitrate to maintain electroneutrality. The vesicles were prepared from a mixture of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) and cholesterol (7:3), chosen to mimic the outer leaflet of animal cell plasma membranes. Two methods were used to detect the passage of chloride ions across the membranes. The first involved the use of lucigenin (18), a chloride-sensitive fluorescent dye, to detect inward-flowing chloride ions.[11b] Briefly, the cholapod was mixed with lipid at the appropriate molar ratio (1:250, 1:2500, 1:25000 or 1:250 000). The mixture was solvated with an aqueous solution of NaNO3 (225 mm) containing lucigenin (1 mm) and the resulting suspension was used to prepare vesicles via freeze-thaw cycles and extrusion. The vesicle suspension was separated from external lucigenin by size exclusion chromatography, diluted with aqueous NaNO3 (225 mm), and placed in the cuvette of a fluorescence spectrometer. Aqueous NaCl was added (25 mm final concentration), and chloride influx was followed by the decrease in fluorescence intensity.
The second method employed a chloride-selective ion-exchange electrode (ISE) to detect chloride ions emerging from the vesicles.[11a] In this case the vesicles were formed from the POPC/cholesterol mixture without the addition of transporter. The lipid mixture was solvated with aqueous NaCl (500 mm), then external NaCl was replaced with NaNO3 by dialysis. The ISE was placed in the suspension and the transporter was added as a solution in a small amount of organic solvent. Chloride efflux was followed through the increase in signal from the electrode.
The methods differ in two key respects. Firstly, the lucigenin-based assay allows preincorporation of the transporter in the vesicle membranes, while the ISE method requires that transporter be added from outside to start the experiment. This results from the need to place the chloride inside the vesicles in the latter case; clearly these vesicles cannot be prepared from membranes containing transporters. The ISE-based assay is therefore affected by two factors, i) intrinsic transport ability and ii) the ability of the transporter to partition into the vesicles from an aqueous dispersion. Secondly the ISE-based assay requires quite high concentrations of chloride (≈0.5 m) in the vesicles to ensure an adequate signal. In contrast, the lucigenin method works well for the lower chloride concentrations typically found in biological systems.
Of the two methods, the lucigenin-based assay is thus simpler to interpret (measuring only intrinsic transport ability) and is also more relevant to biological conditions. It therefore served as the mainstay of the present study, while the ISE method was used to provide supporting and complementary information.
In addition to transport rates, the study also required a comparison of anion affinities. Binding constants are very sensitive to medium, and the relevant medium in this case is the apolar membrane interior. It was therefore appropriate to use an apolar, water-immiscible solvent such as chloroform. Cholapod anion affinities in water-saturated chloroform were measured using our previously-reported extraction-based method, with Et4N+ as counterion.[14] The technique involves the equilibration of cholapods dissolved in chloroform with salts Et4N+X− dissolved in water, followed by 1H NMR analysis to determine the amount of extracted salt. Binding constants Ka (X−, CHCl3) can be calculated from the extraction constant Ke, and the distribution constant Kd for the salt between chloroform and water in the absence of receptor (Ka = Ke/Kd). Depending on the anion, Kd may be determined by direct measurement, or by calibration using a receptor which can be studied by both extraction and NMR titration. The method is especially useful for powerful receptors, such as the cholapods, because of its high dynamic range; unlike most titration methods, it does not suffer from an upper limit for measurable Ka values.
Transport by cholapods pre-incorporated in vesicle membranes (lucigenin method)
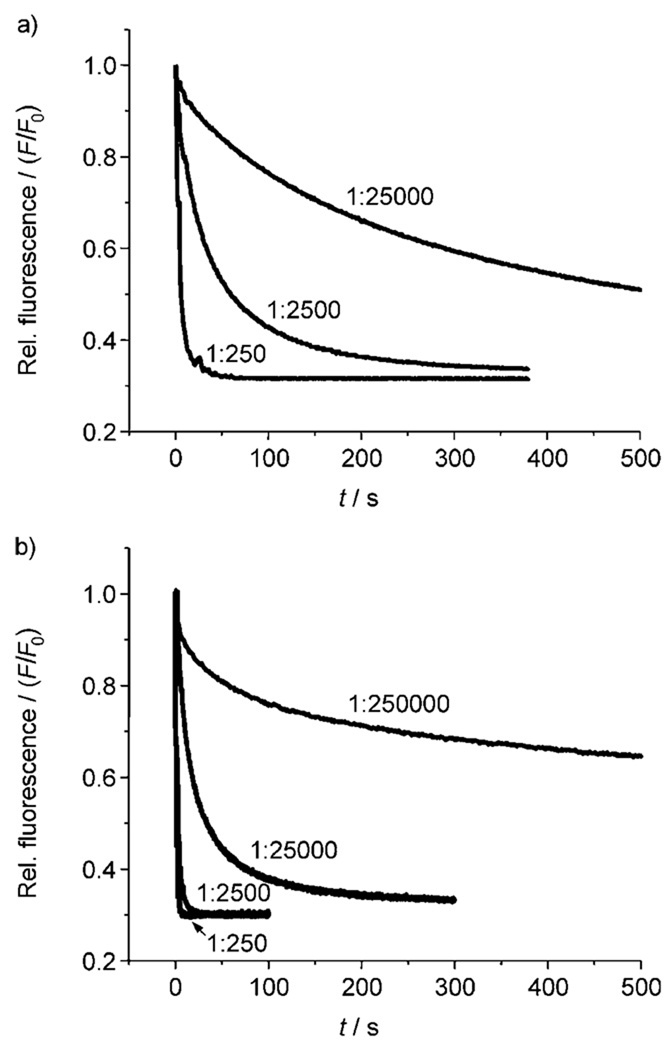
The lucigenin-based assay was applied to all the cholapods under study at a transporter/lipid mole ratio of 1:250,[22] and also to the more effective systems at lower loadings (transporter/lipid 1:2500, 1:25 000 and 1:250 000). Rates were quantified by fitting the fluorescence output to a first-order decay and thus determining an observed first-order rate constant kobs (s−1). Representative decay curves are shown in Figure 2. The rates were uncorrected for background influx of chloride, which according to control experiments occurred at ca. 0.0007 s−1 (t½ = 1430 s).
Figure 2.
Chloride transport into vesicles (POPC/cholesterol 7:3) induced by preincoporated cholapods, as indicated by the decay in lucigenin fluorescence. a) Cholapod 1e at transporter/lipid 1:250, 1:2500 and 1:25 000. b) Cholapod 3 at transporter/lipid 1:250, 1:2500, 1:25000 and 1:250 000.
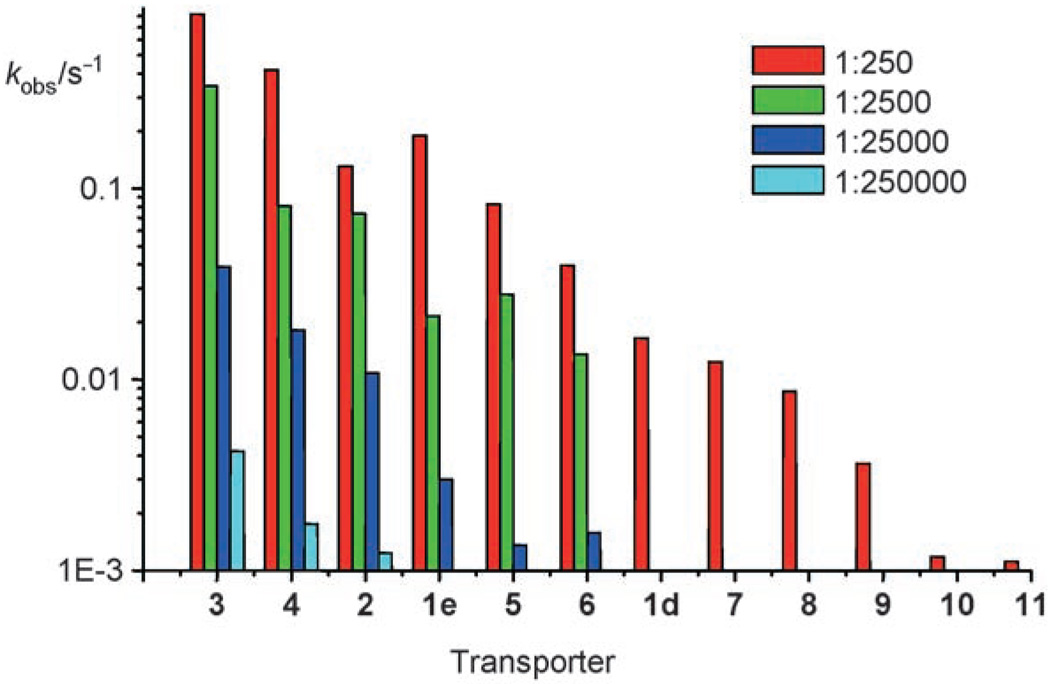
Of the 16 cholapods studied, 12 were electroneutral methyl esters (1d, 1e, 2 and 3–11). The kobs values for these compounds at transporter/lipid = 1:250 are given in Table 1, where they are related to the binding constants to Et4N+Cl− and Et4N+NO3 − in chloroform. Transport rates for the lower cholapod concentrations are listed in Table 2, and the full set of kobs is summarised as a bar chart in Figure 3. Results at the different loadings were mostly, though not fully, self-consistent. The order of presentation in Table 1 and Table 2, and Figure 3, represents a consensus activity scale, taking account of all transporter concentrations.
Table 1.
Transport activities (lucigenin method) and anion affinities for electroneutral methyl ester cholapods. Transport rates kobs are for chloride influx into vesicles with preincorporated cholapods at cholapod/lipid 1:250.[a]
| Cholapod | kobs (1:250) [s−1] | Ka to Et4N+X− in CHCl3 [m−1][b] | |
|---|---|---|---|
| X = Cl | X = NO3 | ||
| 3 | 0.82 | 2.0 × 109 | 2.5 × 108 |
| 4 | 0.43 | 1.2 × 1010 | 3.2 × 109 |
| 2 | 0.13 | 1.1 × 1011[c] | 8.5 × 109 |
| 1e | 0.19 | 5.2 × 108[d] | 1.7 × 108[d] |
| 5 | 0.083 | 1.5 × 109 | 6.6 × 108 |
| 6 | 0.040 | 2.8 × 108 | 1.1 × 108 |
| 1d | 0.017 | 1.5 × 107[d] | 1.0 × 107[d] |
| 7 | 0.012 | 1.8 × 107 | 8.5 × 106 |
| 8 | 0.0087 | 1.0 × 109 | 3.0 × 108 |
| 9 | 0.0036 | 6.3 × 107 | 2.1 × 107 |
| 10 | 0.0012 | 1.5 × 108 | 6.1 × 107 |
| 11 | 0.0011 | 5.9 × 107 | 3.7 × 107 |
Table 2.
Chloride transport rates induced by preincorporated cholapods at lower cholapod/lipid mole ratios.[a]
| Cholapod | kobs [s−1] for cholapod/lipid 1:x | ||
|---|---|---|---|
| 1:2500 | 1:25 000 | 1:250 000 | |
| 3 | 0.34 | 0.039 | 0.0042 |
| 4 | 0.081 | 0.018 | 0.0018 |
| 2 | 0.074 | 0.012 | 0.0012 |
| 1e | 0.022 | 0.0030 | |
| 5 | 0.028 | 0.0014 | |
| 6 | 0.014 | 0.0016 | |
Procedures and materials as for Table 1.
Figure 3.
Transport rates kobs from Table 1 and Table 2, shown on a logarithmic scale and colour coded according to transporter/lipid ratio.
The first point to note is that activities rise to very high levels. The most powerful transporter is the 3-acetoxy-7,12-bis-p-nitrophenylthiourea 3. As shown in Figure 2b, this compound promotes chloride equilibration in a few seconds, even at 3/lipid 1:2500. At 3/lipid 1:25000, the half-life is 26 seconds, while at 1:250000 the effect is still measurable. In this last case, the transporter is operating at the level of a few molecules per vesicle. A rough calculation[21] suggests that a 200 nm diameter vesicle should be composed of ≈400 000 lipid molecules, so that the average loading at 1:250 000 is < 2 cholapods per vesicle.
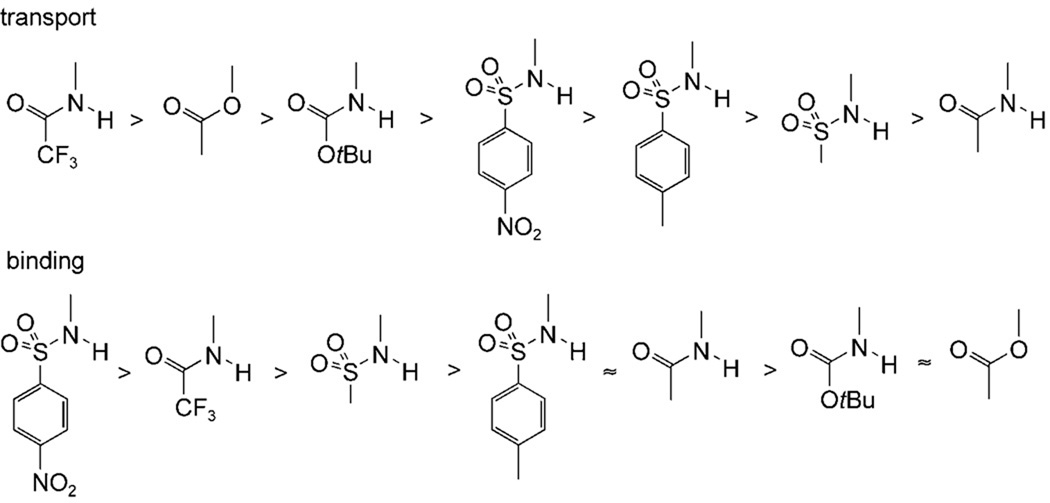
Turning to the relationship between structures and activities, it seems that variation of the urea groups in positions 7 and 12 has predictable, concordant effects on both anion affinities and transport effectiveness. For the sequence (1d;1e;3), mutation from phenylurea to p-nitrophenylurea to p-nitrophenylthiourea yielded the expected increases in binding constants and corresponding increases in transport rates. The same correlation was observed for the pairings (6;4), (8;2) and (9;5). In contrast, the effect of the substituent in position 3 is more difficult to understand. From the series of bis-phenylureas 6, 1d, and 7–11, one concludes that the 3-substituents promote transport in the order shown in Figure 4 (top row). However, their effects on anion affinity are quite different (Figure 4, bottom row). Thus 3α-acetoxy (as present in 3) is a good substituent for transport but does not promote binding, 3α-trifluoroacetamido is good for both functions, and 3α-p-nitrophenylsulfonamido is the most effective for binding but only moderate for transport. The results from 7α,12α-bis-p-nitrophenylureas 4, 1e and 5, and thioureas 3 and 2, are also consistent with Figure 4.
Figure 4.
Relative effects of cholapod 3α substituents on chloride transport (top row) and anion binding in chloroform (bottom row).
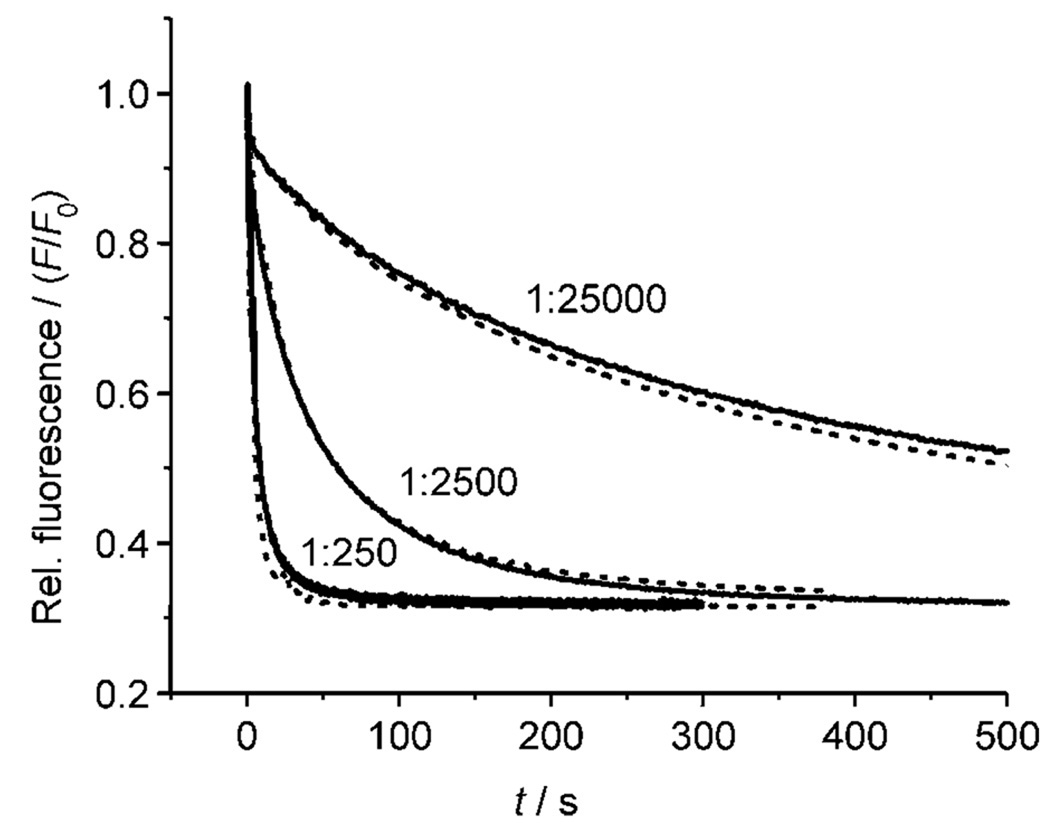
This irregular correlation between affinities and transport rates cannot yet be fully explained. Two of the simpler hypotheses seem unlikely. Firstly, an increase in receptor strength beyond a certain point can be counterproductive for transport, because high affinities inhibit anion release. However, in this case the transport rates should pass through a maximum as affinities are raised, and no such relationship is observed for the cholapods. Secondly, the results could be affected by differential partitioning of the cholapods between membrane and aqueous phase. However, experiments employing eicosyl esters 14 and 15 demonstrated that increasing hydrophobicity made very little difference to transport activity. Results for 14 are shown in Figure 5, superimposed upon the almost identical data obtained for methyl ester analogue 1e. Such similarities are only likely if both methyl and eicosyl esters are fully concentrated in the membrane. If this is true for both 14/1e and 15/2, it is probably also true for the remaining cholapods.
Figure 5.
Chloride transport into vesicles (POPC/cholesterol 7:3) by preincorporated eicosyl ester cholapod 14 (––) compared to methyl ester 1e (-----). The lucigenin method was used. Results are shown for transporter/lipid = 1:250, 1:2500 and 1:25 000. Similar results were obtained in a comparison between eicosyl ester 15 and methyl ester 2.
Other factors affecting transport activities could be; a) variations in the kinetics of anion binding at the membrane/aqueous interfaces; b) variations in cholapod–anion complex mobility within the membrane; c) different levels and/or effects of cholapod aggregation within the membrane;[23] or d) variations in binding selectivity for the anions involved in the transport process (chloride and nitrate), and also for the anionic centres in the membrane phospholipids.[24] Although further work will be required to clarify the picture, the trends in Figure 4 should serve as empirical rules for the design of high performance cholapods.
Finally, the two cationic cholapods 12 and 13 were found to have little or no transport activity. Again, this could be due in principle to the cholapods partitioning into the aqueous phase. However, previous work has shown that 12 can mediate the translocation of phosphatidylserine head groups in vesicles, and that the corresponding eicosyl ester is equally active.[17] By the argument used previously, this strongly suggests that 12 and (presumably) 13 locate within the membrane. The most likely explanation for their low chloride transport activity is the formation of extremely stable electroneutral complexes[25] which only slowly release anions into the aqueous phase.
Transport by externally added cholapods (ISE method)
A majority of the cholapods were also studied using the ISE-based method, in which the transporter was added externally to preformed vesicles. The results are gathered in Table 3. In this case, initial rates were determined from the slope of the ISE output, expressed as % of total signal variance per second. The relative activities are significantly different from those obtained by the lucigenin method. For example, while 4 and 1e retain their positions near the top of the table, 2 and 3 are among the least effective. The major source of difference is presumed to be the mode of transporter addition. While some cholapods disperse readily in the aqueous medium and rapidly partition into the membranes, others appear to form aggregates or precipitates which are not available to the vesicles. Biological applications will require delivery to cell membranes, so the data highlight an issue which should be addressed in future work. In the mean time cholapods 1e, 6 and especially 4, provide good levels of activity with both modes of addition.
Table 3.
Transport activities for externally added cholapods (ISE method).[a]
| Cholapod | Initial transport rate [% s−1] |
Cholapod | Initial transport rate [% s−1] |
|---|---|---|---|
| 4 | 0.61 | 10 | 0.033 |
| 6 | 0.54 | 11 | 0.026 |
| 1e | 0.52 | 9 | 0.017 |
| 1d | 0.085 | 13 | 0.010 |
| 8 | 0.084 | 2 | 0.010 |
| 5 | 0.059 | 3 | 0.008 |
Cholapod/lipid mole ratio 1:250. Lipids were composed of POPC/cholesterol 7:3. Rates are the average of three individual experiments with S.D. ± 5.0%.
Effect of bilayer thickness on transport rate—Further evidence for the carrier mechanism
Mechanistic understanding is critical for the optimisation of cholapod transport activities. A key question is that of mobile carrier vs. stationary channel. Thus far, two lines of evidence have suggested that the cholapods act as carriers.[11a] Firstly, concentration–activity studies have shown no sign of cooperative behaviour. More than one cholapod would be required to form a stationary channel and, unless self-association is exceptionally strong, this should lead to a second- or higher-order concentration dependence. In fact, the concentration dependence for 1d was found to be less than first-order.[11a] Secondly, transport rates were sensitive to the fluidity of the membrane. In dipalmitoylphosphatidylcholine (DPPC) vesicles, which show a gel/liquid crystalline phase transition at 41 °C, the cholapods were inactive in the less fluid gel phase. However they transported chloride effectively above the transition temperature, implying that mobility within the membrane was required for activity.[11a]
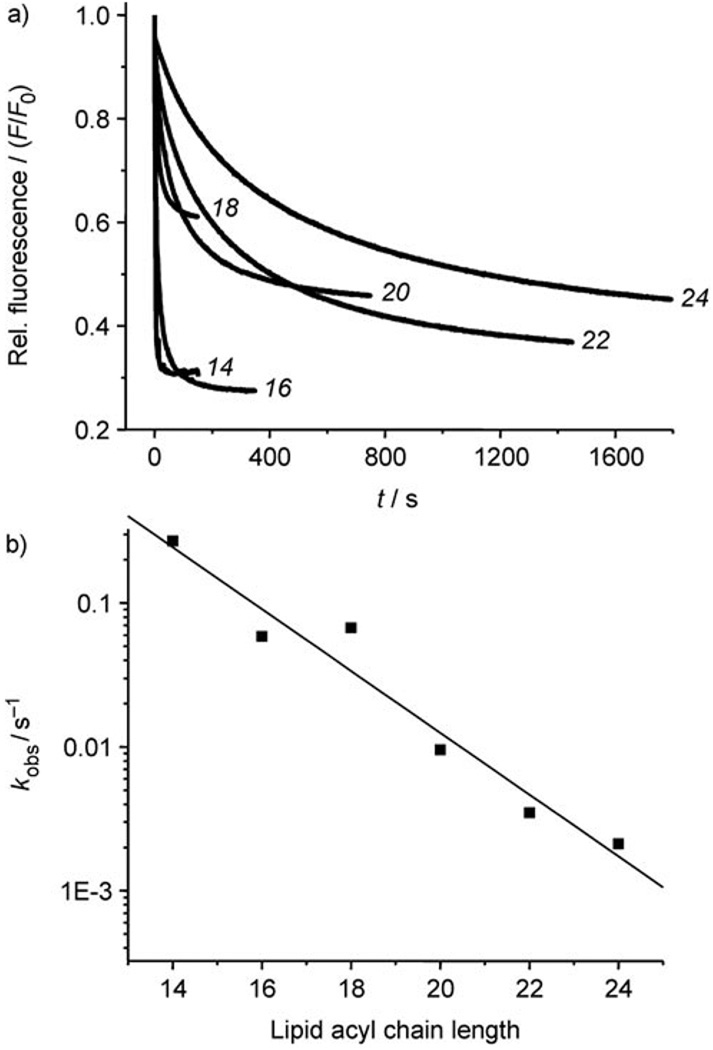
Given the importance of this issue, we have sought further confirmation that the carrier mechanism is indeed correct. A useful probe is the dependence of transport rates on membrane thickness. Research by Läuger and co-workers on the cation carrier valinomycin showed a simple correlation between transport rates and membrane hydrocarbon chain lengths.[26] For a stationary channel, this type of relationship is not expected. Rates should be similar for a range of membrane thicknesses, but should fall off dramatically once the channel can no longer span the bilayer.[27] We therefore tested cholapods for chloride transport in vesicles formed from the six phosphatidylcholines (PCs) 19–24 (acyl chain lengths C14–C24). Unsaturated lipids were employed to maintain the fluid phase. One set of experiments employed the ISE method with cholapod 6, and membranes formed from pure PC and also from PC/cholesterol 7:3. A second series employed the lucigenin method with cholapod 1e and the PC/cholesterol vesicles.
Results from the lucigenin experiments are shown in Figure 6. As expected for the carrier mechanism, the transport rates decreased steadily as the lipid chain lengths increased. The scale of the change was roughly similar to that for valinomycin. Moving from PC 20 (C16 acyl) to PC 23 (C22 acyl), kobs decreased by a factor of 16, while the corresponding figure for valinomycin was 10.[26, 28] The results from the ISE experiments were essentially similar to those obtained by the lucigenin method. The presence of cholesterol in the membranes slowed transport by a small amount in most cases, but did not affect the general trend. These experiments provide a third argument for favouring the carrier, as opposed to channel, mechanism for anion transport by cholapods.
Figure 6.
Chloride transport by cholapod 1e into vesicles formed from lipids 19–24, assayed by the lucigenin method. a) Fluorescence decay curves. Traces are labelled according to the number of carbons in the lipid acyl chains. b) Dependence of kobs (logarithmic scale) on lipid acyl chain length.
Chloride transport by a monourea—A remarkably simple anionophore
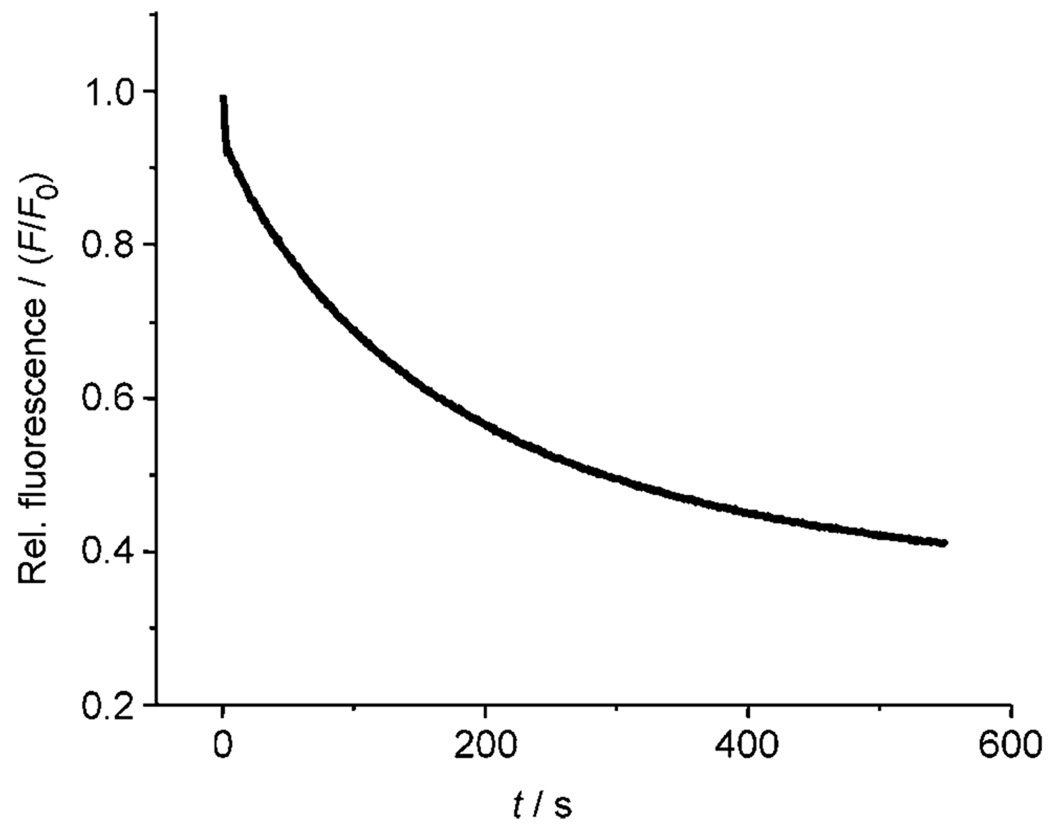
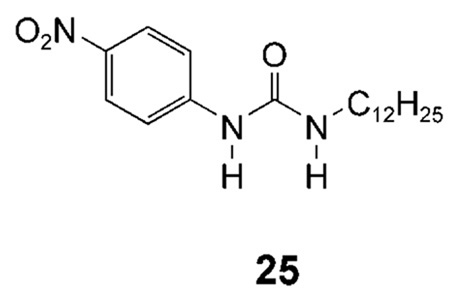
All the cholapod transporters discussed in this paper feature two axially directed urea groups, positioned such that they can cooperate in anion binding. As a final step in our structure–activity studies, we were interested to determine how a single urea group might perform. p-Nitrophenylurea 25 was chosen as a lipophilic monourea with relatively powerful H-bond donors. The extraction method was used to determine an apparent Ka of ≈8000 m−1 for 25 + Et4N+Cl− in water-saturated chloroform.[29] At relatively high concentrations, 25 was found to be a fairly effective chloride transporter. At a transporter/lipid ratio of 1:150, it promoted chloride efflux from vesicles with kobs = 0.0056 s−1 (see Figure 7). Comparison with Table 1, and considering the difference in loadings, suggests that it is roughly equivalent to cholapod 9 (which is a far stronger receptor). Although it is interesting that such a simple structure can show significant activity, the results confirm the value of the cholapod design. The monourea 25 is about fifty times less efficient than cholapod 1e, in which two p-nitrophenylurea units are preorganised on the steroidal scaffold.
Figure 7.
Chloride transport into vesicles (POPC/cholesterol, 7:3) by p-nitrophenylurea 25 at transporter/lipid 1:150, assayed by the lucigenin method.
Conclusion
In previous work we have shown that cholapods can be extremely effective as chloride transporters. Herein we present a study encompassing an extended and varied range of these anionophores. Structure–activity relationships have been investigated using a method in which transporters are preincorporated in vesicle membranes. The results are puzzling in some respects; some imply that binding strength and transport rates are correlated, while others show that additional factors are also significant (see Figure 4). However, importantly, we have found that very high transport activities can be achieved. Our new champion, 3, achieves a transport half life of 26 seconds at the very low transporter/lipid ratio of 1:25 000. It is measurably effective even at transporter/lipid 1:250 000 (less than two transporter molecules per vesicle). We have also shown that increased lipophilicity confers no advantage, and that a positive charge is strongly deleterious. The comparison with simple monourea 25 confirms the benefit of the preorganized cholapod architecture. Results with a second measurement method, involving the addition of cholapod to preformed vesicles, demonstrate that transporter delivery to the vesicle membranes occurs with varying effectiveness. Finally, experiments with membranes of different thicknesses have provided further support for the mobile carrier mechanism.
In future work, we will improve our understanding of cholapod anion transport by applying further techniques, especially conductivity measurements in membrane patches. This new knowledge will aid the optimisation of transport properties. We will also seek to improve transporter delivery to preformed synthetic and biological membranes. Optimised cholapod anionophores should be valuable as tools for membrane transport research, and may uncover new modes of biological activity.
Experimental Section
General
Where not previously reported, cholapods were prepared and characterised as described in the Supporting Information. Other materials and equipment were sourced as follows: lipids, Avanti Polar Lipids, Inc.; POPC, Genzyme; cholesterol, Sigma; lucigenin, Molecular Probes; Lipofast hand held extruder, Avestin; polycarbonate membranes (200 nm), Avestin; quartz cuvettes, Fischer-Scientific; chloride selective electrode, Fisher-Scientific. Fluorescence spectra were obtained using a Jobin-Yvon Fluoromax-3 fluorimeter with FT WinLab software and external water bath cooling unit.
Preparation of unilamellar vesicles
All lipids and cholesterol were stored in a −20°C freezer as solids or as chloroform solutions (10 or 20 mg mL−1). The chloroform solutions were combined with a solution of cholapod as appropriate to give the desired lipid or lipid–cholapod mixture in a ten mL round bottom flask. The chloroform was removed using a rotary evaporator followed by evacuation on a high vacuum pump line for 1–3 h. The aqueous solution to be placed inside the vesicles (1 mL) was added and the lipid film was resolvated by vortexing in the presence of a glass ring. The lipid solution subsequently underwent nine freeze-thaw cycles and was extruded twenty-nine times through a 200 nm polycarbonate membrane to give the vesicle suspension.
Lucigenin assay (typical procedure)
A suspension of unilamellar vesicles (200 nm mean diameter, 20 mm lipid concentration) composed of POPC/Cholesterol (7:3 molar ratio) and cholapod, and containing aqueous NaNO3 (225 mm) and lucigenin (1 mm), was prepared. A portion of this suspension (500 µL) was loaded onto a Sephadex G-50 column and eluted with aqueous NaNO3 (225 mm) to remove the un-encapsulated lucigenin. The elution process was monitored with a hand-held UV/Vis lamp. The vesicles were then diluted with aqueous NaNO3 (225 mm) to a final 25 mL total volume (0.4 mm lipid). An aliquot of this suspension (3 mL) was placed in a cuvette, and aqueous NaCl (25 mm) was added. The lucigenin fluorescence was monitored at 450/505 ex/em with a 3 nm slit width, with the temperature maintained at 25 °C. The results shown in the Figures are representative traces of three individual experiments. The lucigenin fluorescence quenching curves were fitted by non-linear computer methods to the first order decay equation (Io − It)/(Io − If) = 1 − e−kobst where Io is initial fluorescence intensity, It is intensity at time t, If is intensity at time final. The results for kobs in Table 1 and Table 2 are the average of three individual experiments with S.D. ± 5.0%.
Ion-selective electrode (ISE) assay (typical procedure)
A suspension of unilamellar vesicles (1 mL, 30 mm in lipid) composed of POPC/cholesterol (7:3 molar ratio) prepared using aqueous NaCl (500 mm) was dialysed against aqueous NaNO3 (500 mm) using spectra/pore 12–14 000 MWCO dialysis tubing (Fisher Scientific) for ten to twelve hours. The resulting suspension was diluted to 1 mm lipid concentration with aqueous NaNO3 (500 mm). A solution of the appropriate cholapod (5 mm) was prepared in THF. Typically, 4 µL of this solution was added to 5 mL of the vesicle suspension for a final transporter concentration of 4 µm. Chloride release was monitored for six minutes using a chloride selective electrode with the temperature maintained at 25°C. At the end of the experiment the vesicles were lysed with polyoxyethylene 8 lauryl ether detergent to obtain 100 percent chloride release. The results in Table 3 are the average of three individual experiments with S.D. ± 5.0%. Additional experimental details and typical transport data are described in ref. [11a].
Acknowledgements
Financial support from the BBSRC (BBS/B/11044 and BBS/S/E/2006/13241), the EPSRC (GR/R04584/01 and EP/F03623X/1), NIH (USA) and the University of Bristol is gratefully acknowledged.
Footnotes
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/chem.200801163.
Contributor Information
Bradley D. Smith, Email: smith.115@nd.edu.
Anthony P. Davis, Email: Anthony.Davis@bristol.ac.uk.
References
- 1.Hille B. Ion Channels of Excitable Membranes. Sunderland, MA: Sinauer; 2001. [Google Scholar]; Hucho F, Weise C. Angew. Chem. 2001;113:3194. doi: 10.1002/1521-3773(20010903)40:17<3100::AID-ANIE3100>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2001;40:3100. [Google Scholar]
- 2.Ashcroft FM. Ion Channels and Disease. London: Academic Press; 2000. [Google Scholar]
- 3.Riddell FG. Chem. Br. 1992:533. [Google Scholar]; Dobler M. In: Comprehensive Supramolecular Chemistry, Vol. 1 (Molecular recognition: receptors for cationic guests) Gokel GW, editor. Oxford: Pergamon; 1996. p. 267. [Google Scholar]
- 4.Examples and leading references: a) Prodigiosins: Ohkuma S, Sato T, Okamoto M, Matsuya H, Arai K, Kataoka T, Nagai K, Wasserman HH. Biochem. J. 1998;334:731. doi: 10.1042/bj3340731. b) Duramycin: Sheth TR, Henderson RM, Hladky SB, Cuthbert AW. Biochim. Biophys. Acta. 1992;1107:179. doi: 10.1016/0005-2736(92)90345-m. c) Pamamycin: Jeong EJ, Kang EJ, Sung LT, Hong SK, Lee E. J. Am. Chem. Soc. 2002;124:14655. doi: 10.1021/ja0279646.
- 5.Prodigiosins (ref. [4a]) are relatively well-studied, but transport H+ along with Cl−. They do not therefore serve as counterparts to ionophores like valinomycin, with purely cation-transporting properties.
- 6.Rowe SM, Miller S, Sorscher EJ. New Engl. J. Med. 2005;352:1992. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]; Welsh MJ, Ramsey BW, Accurso F, Cutting GR. In: The Metabolic and Molecular Basis of Inherited Disease. Scriver CR, Beaudet AL, Sly WS, Valle D, editors. New York: McGraw-Hill; 2001. p. 5121. [Google Scholar]
- 7.Broughman JR, Shank LP, Takeguchi W, Schultz BD, Iwamoto T, Mitchell KE, Tomich JM. Biochemistry. 2002;41:7350. doi: 10.1021/bi016053q. [DOI] [PubMed] [Google Scholar]
- 8.Davis AP, Sheppard DN, Smith BD. Chem. Soc. Rev. 2007;36:348. doi: 10.1039/b512651g. [DOI] [PMC free article] [PubMed] [Google Scholar]; Sisson AL, Shah MR, Bhosale S, Matile S. Chem. Soc. Rev. 2006;35:1269. doi: 10.1039/b512423a. [DOI] [PubMed] [Google Scholar]; Boon JM, Smith BD. Curr. Opin. Chem. Biol. 2002;6:749. doi: 10.1016/s1367-5931(02)00399-x. [DOI] [PubMed] [Google Scholar]; Sessler JL, Allen WE. Chemtech. 1999;29:16. [Google Scholar]
- 9.Merritt M, Lanier M, Deng G, Regen SL. J. Am. Chem. Soc. 1998;120:8494. [Google Scholar]; Schlesinger PH, Ferdani R, Liu J, Pajewska J, Pajewski R, Saito M, Shabany H, Gokel GW. J. Am. Chem. Soc. 2002;124:1848. doi: 10.1021/ja016784d. [DOI] [PubMed] [Google Scholar]; Sidorov V, Kotch FW, Abdrakhmanova G, Mizani R, Fettinger JC, Davis JT. J. Am. Chem. Soc. 2002;124:2267. doi: 10.1021/ja012338e. [DOI] [PubMed] [Google Scholar]; Sakai N, Houdebert D, Matile S. Chem. Eur. J. 2003;9:223. doi: 10.1002/chem.200390016. [DOI] [PubMed] [Google Scholar]; Gorteau V, Bollot G, Mareda J, Perez-Velasco A, Matile S. J. Am. Chem. Soc. 2006;128:14788. doi: 10.1021/ja0665747. [DOI] [PubMed] [Google Scholar]; Li X, Shen B, Yao XQ, Yang D. J. Am. Chem. Soc. 2007;129:7264. doi: 10.1021/ja071961h. [DOI] [PubMed] [Google Scholar]
- 10.Madhavan N, Robert EC, Gin MS. Angew. Chem. 2005;117:7756. doi: 10.1002/anie.200501625. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2005;44:7584. [Google Scholar]
- 11.a) Koulov AV, Lambert TN, Shukla R, Jain M, Boon JM, Smith BD, Li HY, Sheppard DN, Joos JB, Clare JP, Davis AP. Angew. Chem. 2003;115:5081. doi: 10.1002/anie.200351957. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2003;42:4931. [Google Scholar]; b) McNally BA, Koulov AV, Smith BD, Joos JB, Davis AP. Chem. Commun. 2005:1087. doi: 10.1039/b414589e. [DOI] [PubMed] [Google Scholar]; c) Sessler JL, Eller LR, Cho WS, Nicolaou S, Aguilar A, Lee JT, Lynch VM, Magda DJ. Angew. Chem. 2005;117:6143. doi: 10.1002/anie.200501740. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2005;44:5989. [Google Scholar]; d) Gale PA, Light ME, McNally B, Navakhun K, Sliwinski KE, Smith BD. Chem. Commun. 2005:3773. doi: 10.1039/b503906a. [DOI] [PubMed] [Google Scholar]; e) Santacroce PV, Davis JT, Light ME, Gale PA, Iglesias-Sanchez JC, Prados P, Quesada R. J. Am. Chem. Soc. 2007;129:1886. doi: 10.1021/ja068067v. [DOI] [PubMed] [Google Scholar]
- 12.Davis AP, Joos J-B. Coord. Chem. Rev. 2003;240:143. [Google Scholar]; Davis AP. Coord. Chem. Rev. 2006;250:2939. [Google Scholar]
- 13.Just one relative, a tris-p-nitrophenylthiourea, is slightly more powerful. See ref. [14].
- 14.Clare JP, Ayling AJ, Joos JB, Sisson AL, Magro G, Pérez-Payán MN, Lambert TN, Shukla R, Smith BD, Davis AP. J. Am. Chem. Soc. 2005;127:10739. doi: 10.1021/ja0524144. [DOI] [PubMed] [Google Scholar]
- 15.In a separate work we have studied cholapods with 3α-H, and have found that their chloride affinities are similar to analogues with 3α-OAc.
- 16.Sisson AL, del Amo Sanchez V, Magro G, Griffin AME, Shah S, Charmant JPH, Davis AP. Angew. Chem. 2005;117:7038. doi: 10.1002/anie.200502330. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2005;44:6878. [Google Scholar]
- 17.Boon JM, Lambert TN, Sisson AL, Davis AP, Smith BD. J. Am. Chem. Soc. 2003;125:8195. doi: 10.1021/ja029670q. [DOI] [PubMed] [Google Scholar]
- 18.Ayling AJ, Pérez-Payán MN, Davis AP. J. Am. Chem. Soc. 2001;123:12716. doi: 10.1021/ja016796z. [DOI] [PubMed] [Google Scholar]
- 19.del Amo V, Siracusa L, Markidis T, Baragaña B, Bhattarai KM, Galobardes M, Naredo G, Pérez-Payán MN, Davis AP. Org. Biomol. Chem. 2004;2:3320. doi: 10.1039/B412298D. [DOI] [PubMed] [Google Scholar]
- 20.Lambert TN, Boon JM, Smith BD, Pérez-Payán MN, Davis AP. J. Am. Chem. Soc. 2002;124:5276. doi: 10.1021/ja026022y. [DOI] [PubMed] [Google Scholar]
- 21.For more details see Supporting Information.
- 22.In calculating transporter/lipid mole ratios, both phospholipid and cholesterol are included in the lipid molecule count.
- 23.Aggregation effects might explain some of the variations in transporter performance at different loadings. For example, cholapod 2 performs relatively poorly at the high loading of receptor/cholapod 1:250, and this might reflect self-association to form inactive assemblies.
- 24.A possible role for chloride/nitrate selectivity was suggested by a referee. With relatively simple liquid organic membranes it is known that, if the rate determining step is membrane diffusion, then transport rate is a maximum when the ion and counter-ion have the same carrier affinities (T. M. Fyles, in Inclusion Aspects of Membrane Chemistry, Vol. 2 (Eds.: T. Osa, J. Atwood), Kluwer, Dordrecht, 1991, p. 59). This correlation is not observed for the data in Table 1. However, this is not surprising because diffusion through thin vesicle membranes may not be fully rate-determining, and the association kinetics may be affected by additional factors such as carrier – phospholipid and ion – phospholipid interactions.
- 25.For a crystal structure of a complex of 13, see: Sisson AL, Clare JP, Taylor LH, Charmant JPH, Davis AP. Chem. Commun. 2003:2246. doi: 10.1039/b305261c.
- 26.Benz R, Frohlich O, Läuger P. Biochim. Biophys. Acta. 1977:464–465. doi: 10.1016/0005-2736(77)90023-2. [DOI] [PubMed] [Google Scholar]
- 27.Weber ME, Schlesinger PH, Gokel GW. J. Am. Chem. Soc. 2005;127:636. doi: 10.1021/ja044936+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.The measurements on valinomycin employed monoglyceride lipids, as opposed to the PCs used in the present work.
- 29.Very recently, Pescatori et al. have measured the association constant for another lipophilic p-nitrophenylurea to Bu4NCl in (dry) CHCl3, by UV titration. Their value of 14000 m−1 is consistent with our result for 25. See: Pescatori L, Arduini A, Pochini A, Ugozzoli F, Secchi A. Eur. J. Org. Chem. 2008:109.