Abstract
Objective
Interleukin-12 (IL-12) is a pleiotropic cytokine and is elevated in affected organs of Sjögren's syndrome (SS) patients. We have previously reported in CBA mice that over-expression of IL-12 leads to mononuclear infiltration of salivary and lacrimal glands, as well as expansion of bronchial lymphoid tissue and decreased mucociliary clearance. Xerostomia is one of the most important clinical features in SS patients, therefore our main objective was to evaluate the salivary gland function in IL-12 transgenic mice. Our secondary objective was to further characterize this animal model and see if these changes are representative for SS.
Methods
Pilocarpine-stimulated salivary flow was used to address salivary gland function in a large group of mice IL-12 transgenic mice bred onto the autoimmune-prone SJL background. Furthermore, salivary glands were removed at different time points to assess the formation of infiltrates in the glands and gland morphology. Serum was also collected from these animals to investigate the formation of autoantibodies.
Results
Pilocarpine-stimulated salivary flow was significantly lower in IL-12 transgenics compared with wild type controls. Salivary glands from transgenic mice showed both a greater number and size of lymphocytic foci than those of age-matched controls. Furthermore, their acini were fewer and larger compared with controls. Anti-La antibodies showed an age-dependent increase in IL-12 transgenic mice, accompanied by a rise in anti-nuclear antibodies.
Conclusion
Our findings indicate that SJL IL-12 transgenic mice express a number of conditions associated with SS and may serve as a useful model for research on multiple aspects of the disease.
Sjögren's syndrome (SS) is an autoimmune disease of unknown etiology. Therefore, the development of animal models that mimic the disease in patients is critical not only for understanding the biology of SS but also for validating the safety and efficacy of experimental therapeutic approaches. The Non-obese Diabetic (NOD) mouse model is one of the most widely used models of SS. These mice develop age dependent histopathological changes in the salivary glands and autoantibodies in serum similar to those seen in SS patients1, 2. While the development of sialadenitis in NOD mice can be accompanied by loss of salivary gland function, this SS like phenotype is reported to be unstable3. This characteristics make it a difficult model to work with or compare results when the mice are obtained from different suppliers4, 5. Furthermore, these mice classically develop insulin dependent diabetes mellitus (IDDM), a condition not routinely observed in SS patients. Several other models have been developed over the years and each displays different aspects of SS. The histopathology of salivary gland infiltrates in SS patients shows mostly CD4-positive T lymphocytes, with some CD8-positive T lymphocytes and B lymphocytes. Some animal models have the same histopathology within the salivary glands (NOD, Id3−/− and MRL/lpr mice), others show CD4-positive T lymphocytes only (NZB/W, Aly/Aly)6, 7. A common theme in all of these models is inflammatory infiltrates that usually precede the secretory dysfunction, a condition that may not parallel the findings in patients where the extent of the inflammation does not always correlate with degree of glandular hypofunction4.
We have previously reported that transgenic expression of interleukin-12 (IL-12) in the thyroid gland also leads to elevated serum levels of IL-12 and mononuclear infiltration of lungs, salivary glands, and lacrimal glands8. The lung infiltration was characterized by expansion of the inducible Bronchus-Associated Lymphoid Tissue (iBALT), and was predominantly composed of B220-positive B lymphocytes and CD4-positive T lymphocytes. Lung infiltrates increased with age, and were associated with increased oxidative stress, TGF-β1 signaling, and impaired mucociliary clearance9. These lung findings resembled those reported in patients with SS10, 11 where lung involvement affects about 25% of these patients12.
IL-12 transgenic mice also developed primary hypothyroidism and mild spontaneous lymphocytic infiltration of the thyroid gland, which could be increased by immunization with suboptimal doses of mouse thyroglobulin8. Autoimmune thyroid diseases (ATD), represented by Hashimoto's thyroiditis and Graves' disease, occur frequently in SS, and similarly SS is common in ATD13. Overall, these findings suggested that IL-12 transgenic mice could serve as a new model to study the pathogenesis of SS. The present work was designed to better characterize the salivary gland function and histopathology in a large cohort of IL-12 transgenic mice.
MATERIALS and METHODS
Mice
The IL-12 transgenic mice that express IL-12 heterodimer (p70) in the thyroid gland have been described previously8. Transgenics were bred for more than 8 generations on the autoimmune-prone SJL background (Jackson Laboratory, Bar Harbor, ME, USA) and maintained as heterozygous. For this manuscript, a total of 31 male and 94 female SJL mice were used. Of these mice, 28 males and 20 females were used for the saliva data; 36 salivary glands and 20 lacrimal glands were used for histopathology; 14 serum samples were used for autoantibody detection and 4 salivary glands were used for staining and acinar volume detection. All experiments were conducted in accordance with the standards established by the United States Animal Welfare Acts, set forth in National Institutes of Health guidelines, and approved by the Johns Hopkins University Animal Care and Use Committee. All mice were kept in sterile conditions and received water and food ad libitum.
Saliva and blood collection
Mild anesthesia was induced by ketamine (100 mg/mL, 1 mL/kg body weight; Fort Dodge Animal Health, Fort Dodge, IA, USA) and xylazine (20 mg/mL, 0.7 mL/kg body weight; Phoenix Scientific, St. Joseph, MO, USA) solution given intramuscularly (im). When anesthetized, saliva secretion was induced by subcutaneous (sc) injection of pilocarpine (Sigma-Aldrich, St. Louis, MO, USA). In these experiments two different concentrations of pilocarpine were used; 0.15 and 0.35 mg/kg bodyweight (BW). Stimulated whole saliva was gravimetrically collected at room temperature (RT) for 20 minutes from the oral cavity with a hematocrit tube (Drummond Scientific Company, Broomall, PA, USA) placed into a preweighed 0.5 ml microcentrifuge tube. The volume was determined by weight as previously described14. At the time of sacrificing, blood was collected by cardiac puncture in microcentrifuge tubes. Plasma was separated by centrifugation for 20 min at 2000 g and stored at −80°C.
Histological assessment of salivary and lacrimal glands
Salivary and lacrimal glands were removed for histological analysis from female SJL transgenic and wild-type mice at the time of sacrificing. Salivary and lacrimal glands were fixed overnight in Beckstead fixative, processed, and embedded in paraffin. At least 5 nonconsecutive 5 μm sections were cut from each gland and subsequently stained with hematoxylin and eosin (H&E). Scoring of histological sections was based on the focus score used in patients with Sjögren syndrome15. Briefly, we reviewed all sections from each gland, chose the most severely affected section, and counted the number of mononuclear cell foci. One mononuclear cell focus is an aggregate of 20 or more mononuclear cells located around vessels or ducts, with adjacent normally-appearing acini, no dilated ducts and no fibrosis. Results were expressed as the absolute number of foci.
To analyze the distribution and type of hematopoietic cells infiltrating the salivary glands, immunohistochemistry was performed on zinc-fixed, paraffin-embedded salivary glands. Fivemicrometer sections were cut, mounted onto SuperFrost plus slides (Fisher Scientific, Pittsburgh, PA), deparaffinized, and rehydrated. After blocking nonspecific binding (1 h in 5% normal goat serum and 0.02% Triton X-100), sections were incubated overnight in a humid chamber at 4°C with a biotin-conjugated monoclonal antibody recognizing B220, CD4 or CD8 (BD Biosciences, San Jose, CA). After washing, sections were incubated for 1 hour at room temperature with biotinylated goat anti-rat IgG (Jackson Immuno Research Lab, West Grove, PA). Sections were washed in PBS and incubated with peroxidase-conjugated streptavidin (Dako, Carpinteria, CA) for 30 min in the humid chamber. Reactions were visualized by the addition of a diaminobenzidine substrate (Vector Laboratories, Burlingame, CA). Finally, sections were rinsed in distilled water, counterstained with Mayer's hematoxylin (Polyscientific, Bay Shore, NY), washed in running tap water, dehydrated through increasing concentrations of ethanol, and mounted with Cytoseal (Richard-Allan Scientific, Kalamazoo, MI).
Determination of autoantibodies in plasma
Plasma samples from female SJL wild-type and transgenic mice were analyzed for autoantibodies against nuclear antigens; ANA, Ro/SSA and La/SSB. ANA (total Ig), the autoantibody against SSA/Ro (total Ig) and SSB/La (total Ig) were measured by a commercial available ELISA kit (Alpha Diagnostic International, San Antonio, TX) according the manufacturer's protocol.
Staining AQP-5, NKCC1 and tight junction complex proteins in salivary glands
Mouse salivary glands were Beckstead fixed and embedded in paraffin. Slides were cut at 6 μm. After deparafinizing the slides, heat-induced epitope retrieval (HIER) was performed by using a microwave steamer (NordicWare, Minneapolis, MN, USA) for 10 minutes in 1mM EDTA, pH 8 with 0.05% Tween 20. After cooling to room temperature, slides were blocked with 10% donkey serum in 0.5% BSA/PBS for 30 minutes and incubated with the primary antibody overnight at 4 C° in a humidified chamber. Three different primary antibodies were used: 10 μg/ml rabbit anti-claudin 3 and -Z01 (Zymed/Invitrogen, Carlsbad, CA, USA), 5 μg/ml rabbit anti-AQP-5 (Alamone Labs, Jerusalem, Israel) and 1:200 dilution of rabbit anti-NKCC1 (generous gift from Dr. R.J. Turner, NIDCR, MPTB) all in 0.5% BSA/PBS. After 5 washes in PBS, sections were incubated with a 1:100 dilution donkey anti-rabbit IgG Alexa 488 (Molecular Probes/Invitrogen, Carlsbad, CA, USA) in 0.5% BSA/PBS for 1 hour at room temperature. Slides were washed as above and mounted in Vectashield hardset mounting medium with DAPI (Vector Laboratories, Burlingame, CA, USA).
Imaging and image analysis
Sections were imaged with a Leica SP2 AOBS confocal system using an upright DM-RE7 microscope with 40x 1.25 NA objective (Exton, PA, USA). Images were collected as XYZ stacks and viewed and analyzed using Volocity (Improvision, Waltham, MA, USA). For each measurement 3–5 stacks (Image thickness 0.1628 μm, voxel width and height 0.366 μm) were analyzed from at least 3 different slides from each tissue sample. After setting the threshold limits for the sample group, samples were analyzed using the following measurement options: object volume measurements, sum of pixel × intensity, and skeletal length.
Statistical analysis
Differences in salivary flow, histopathology scores, and confocal measurements among experimental groups were assessed using the non-parametric Wilcoxon's ranksum test. Other experiments were assessed using unpaired student's t-test to compare differences between groups. The histopathology score analysis was performed with Stata statistical software (release 9; Stata, College Station, TX, USA) and all the other analyses were performed with GraphPad Prism statistical software (GraphPad Software Inc. version 4.02, La Jolla, CA, USA). Correlations were assessed using the non-parametric Spearman's rank test in SPSS statistical software (release 15.0.1; SPSS Inc., Chicago, IL, USA). A p value ≤0.05 is scored as statistically significant.
RESULTS
SJL IL-12 transgenic mice display salivary gland dysfunction and sex specific growth retardation
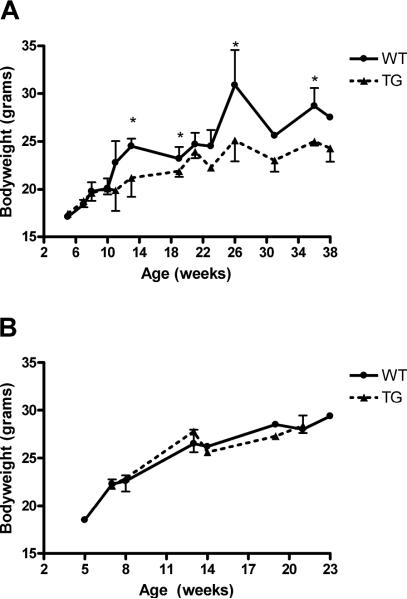
As a marker for the overall health of the animals, weight was measured at several time points for both sexes. Starting at 13 weeks of age female IL-12 TG mice showed a significant decrease in weight compared with WT littermates. This was not seen in males, suggesting a gender specific effect of IL12 over-expression (p=0.0260, fig. 1A–B). The female TG mice did not display any signs of ill health and no change in feeding or behavior was observed in these animals.
Figure 1. Gender dependent growth retardation in SJL IL-12 TG mice.
The weight of the mice was measured at the indicate times for WT and TG female or male mice. A) Growth retardation in female SJL IL-12 TG mice (N=58) compared with SJL WT mice (N=36). B) Normal growth curve in male SJL IL-12 TG mice (N=12) compared with WT littermates (N=19). Data shown are mean values +/− SD. Significant differences are indicated (*) and were determined by unpaired student's t-test. WT = wild type, TG = IL-12 transgenic.
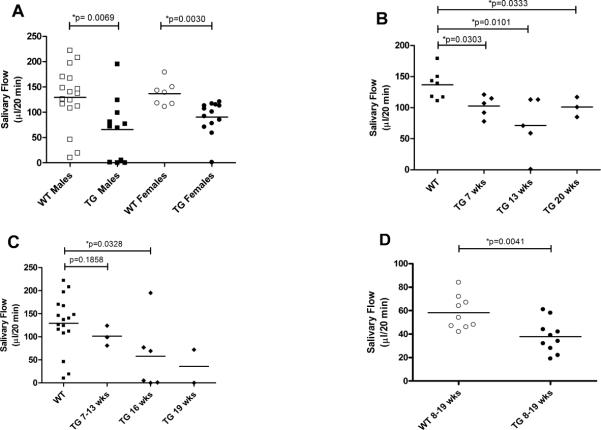
To investigate the effect of IL-12 over-expression on salivary gland function in both sexes, stimulated saliva flow was measured in four groups; male and female SJL WT and IL-12 TG mice. In this experiment, 0.35 mg/kg BW pilocarpine was used. Both female and male SJL mice showed a significant decrease in stimulated salivary flow in the IL-12 TG group compared to the WT group (p=0.0030 and p=0.0069 respectively, fig. 2A). This change in salivary gland activity was also statistically significant when adjusted for the weight of the animals (p=0.0113 and p=0.0165 for females and males, respectively (data not shown). These data suggest that thyroid targeted IL-12 over-expression results in a loss of salivary gland function in both males and females and growth retardation in females.
Figure 2. Decreased salivary flow in SJL IL-12 TG mice.
Saliva was collected as described in the Materials and Methods section over a 20-min period after stimulation with either 0.35 mg/kg BW (A, B, C) or 0.15mg/kg BW (D) pilocarpine. Animals ranged in age from 7–20 weeks. For this experiment, 28 male and 20 female SJL mice were used. Female and male SJL IL-12 TG mice show decreased salivary flow (A). Change in salivary flow in female mice (B) is not age or dose (D) dependent. On the contrary, male mice (C) showed an age dependent change in salivary flow. Horizontal bars are mean values for each group. Significant differences are indicated (*) and were determined by non-parametric Wilcoxon's ranksum test. WT = wild type, TG = IL-12 transgenic.
SJL IL-12 TG mice have decrease salivary gland activity
To determine whether the decreased stimulated saliva flow in both female and male IL-12 TG mice is age dependent, we tested the salivary gland function between 7 and 20 weeks. At all ages the stimulated salivary flow in female IL-12 TG mice was significantly lower than in the WT group (fig. 2B). We were not able to detect age-dependent differences within the female transgenic mice. On the contrary, young male IL-12 TG mice showed comparable salivary flow data with WT littermates (p=0.1858), but starting from 16 weeks of age decreased saliva production was detected in transgenic mice (p=0.0328), suggesting an age dependent decreased salivary flow (fig. 2C).
The decreased salivary flow in SJL IL-12 TG mice is independent of the dose of pilocarpine
To determine if the change in salivary gland activity was dependent on the dose of pilocarpine, saliva flow was tested using 0.15 mg/kg BW pilocarpine (~43% of previous dose) in female SJL mice only. Similar to what we have seen with 0.35 mg/ml pilocarpine, stimulation with 0.15 mg/kg BW pilocarpine produced significantly lower amounts of saliva (p=0.0041, fig. 2D) in IL-12 transgenic mice compared to controls. These data suggest the lower stimulated saliva production in female IL-12 TG mice is not a function of pilocarpine concentration.
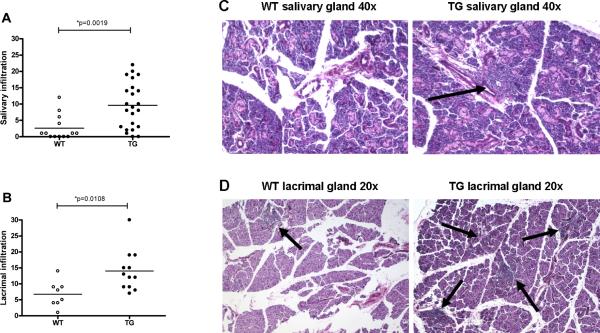
SJL IL-12 TG mice display increased mononuclear infiltration in the salivary and lacrimal glands
To determine the effect of elevated levels of IL-12 on salivary gland morphology, we analyzed glands of female transgenic mice and controls sacrificed at various ages. The mononuclear infiltration was significantly greater in IL-12 transgenics than controls (p=0.0019, fig. 3A), and appeared as focal collection of hematopoietic cells scattered throughout the acinar background (fig. 3C). Similar findings were observed for the lacrimal glands (p=0.0108, fig. 3B and 3D). Moreover, mononuclear cell type characterization in the salivary glands showed predominantly B220-positive B lymphocytes and CD4-positive T lymphocytes (B220>CD4), without differences between TG and WT mice (data not shown). These data are similar to what we have reported in the lungs of these mice9. Further analysis showed a correlation between mononuclear infiltration and glandular hypofunction in female IL-12 TG mice (correlation coefficient = −0.600, p=0.030), not seen in WT mice (correlation coefficient = 0.638, p=0.173, data not shown).
Figure 3. Salivary and lacrimal gland histopathology and scores.
A total number of 13 WT SGs, 23 IL-12 TG SGs, 8 WT LGs and 12 TG LGs were analyzed. Infiltrates in TG mice appear periductal and dispersed in the acinar tissue. Salivary (A) and lacrimal (B) histopathology scores; salivary (C) and lacrimal (D) histopathology in female SJL mice. TG mice show increased mononuclear infiltrates in both salivary and lacrimal glands. Horizontal bars are the mean value of each group. Significant differences are indicated (*) and were determined by non-parametric Wilcoxon's ranksum test. Arrows indicate lymphocytic infiltrates.
Anti-SSB and nuclear antibodies are increased in SJL IL-12 TG mice
Sjögren's syndrome patients typically develop antibodies against Ro (Sjögren's syndrome antigen A, SSA), La (Sjögren's syndrome antigen B, SSB), and nuclear antigens (ANA). We compared the serum levels of these three antibodies in IL-12 transgenics and wild type controls at 13, 32, and 36 weeks of age. At 13 weeks of age, increased ANA levels were detected in IL-12 TG mice (35.61 ± 1.60 μg/ml) compared with WT mice (26.48 ± 1.86 μg/ml; p=0.0098, fig. 4A). Increased ANA levels were stable over time, showing at 32 weeks 22.48 ± 5.78 μg/ml and 36.65 ± 1.76 μg/ml in WT and TG mice respectively (p=0.0082, fig. 4A). The same plasma samples were assayed for anti-Ro (60-kDa) antibodies. Although there was a trend towards increased anti-Ro (60-kDa) antibodies in IL-12 TG mice, the change was not statistically significant for either age (fig. 4B; p=0.1211 and p=0.0743 for 13 weeks and 32 weeks of age respectively). However, age dependent increases in anti-La antibody levels were observed (fig. 4C). At 32 weeks of age, anti-La antibody levels were 4.71 ± 0.04 ng/ml and 5.03 ± 0.05 ng/ml in WT and IL-12 TG mice respectively (p=0.0007; fig. 4C). These data suggest a consistent upregulation of ANA and an age dependent increase in autoantibodies against La in mice over-expressing IL-12.
Figure 4. Increased autoantibodies in female SJL IL-12 TG mice.
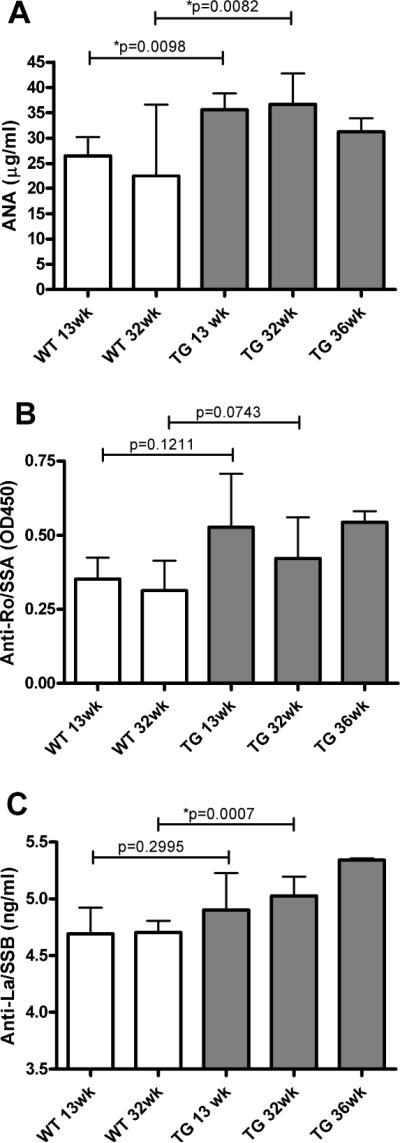
Plasma samples were collected and autoantibody levels were measured as described in the Material and Methods section. Plasma analysis (N=5 for WT and N=9 for TG) showed increased ANA levels (A) and age-dependent increased levels of anti-La/SSB (C). The levels of anti-Ro(60-kDa)/SSA (B) showed a trend to increased levels in TG compared to WT mice, but was not significant. Data shown are mean values +/− SD. Significant differences are indicated (*) and were determined by unpaired student's t-test. WT = wild type, TG = IL-12 transgenic.
Salivary acinar cells show increased volume and decreased number in SJL IL-12 TG mice
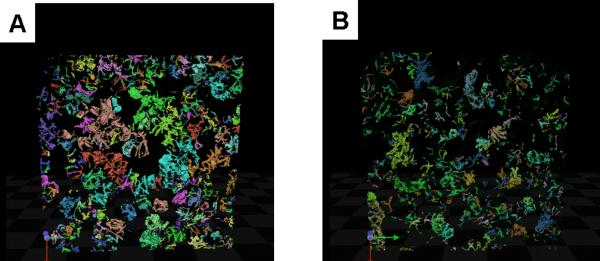
To better understand the functional changes seen in the salivary glands after IL-12 over-expression, the expression of tight junction complex (TJC) proteins (zona occludens 1 (ZO1) and claudin 3 (C3)), a water channel aquaporin-5, (AQP-5), an acinar luminal membrane marker, and Na+/K+/2Cl− cotransporter, (NKCC1), an acinar basolateral membrane marker were examined in 8, 13 and 19 week old female SJL mice by confocal immunofluorescence. Although no significant change in expression of ZO1 or C3 expression levels was detected, changes in AQP5, and NKCC1 expression were observed. AQP-5 expression, was increased 3.33 fold in IL-12 TG mice compared with controls (p=0.0233, table 1). Moreover, immunofluorescence detection of NKCC1 also showed a 4.00 fold increase in levels in IL-12 TG mice compared with controls (p=0.0018, table 1), suggesting either an increase in the number of acinar cells or an increase in acinar cell volume. To test for a change in acinar cell size, the skeletal length of AQP-5 immunofluorescence was measured as an indication of cell surface area and was found to be 1.14 fold greater in TG mice compared with WT mice (p=0.0304, table 1). To confirm this finding, 3D image stacks of confocal microscopy labeling for NKCC1 were reconstructed (with each acinus individually colored for ease of visualization) and the average acinus volume was measured for both WT and TG mice (fig. 5 A–B). In agreement with the increase in protein expression and skeletal length measurements in IL-12 TG mice, the volume of each acinus increased 1.60 fold in TG compared with WT (p=0.0371, table 1). Moreover, nuclei counts indicated a decrease (0.89 fold) in the numbers of cells per acinus, suggesting a loss of acinar cells (p=0.0004, table 1).
Table 1.
Membrane protein expression and acinar cell number and volume in the salivary gland of female SJL mice
| WT | TG | P-value | Fold | |
|---|---|---|---|---|
| AQP-5 | 4.8 (4.9) | 16 (9.6) | 0.0233* | 3.33 |
| NKCC1 | 4.8 (2.5) | 19 (8.4) | 0.0018* | 4.00 |
| Z01 | 0.9 (.36) | 1.2 (.75) | 0.3912 | 1.33 |
| C3 | 3.1 (3.6) | 1.9 (1.2) | 0.3838 | 0.61 |
| Skeletal Length | 17.6 (2.1) | 20.0 (3.6) | 0.0304* | 1.14 |
| NKCC1 Volume | 34.4 (18.5) | 55.2 (24.3) | 0.0371* | 1.60 |
| Nuclei Count | 8.9 (2.8) | 7.9 (2.3) | 0.0004* | 0.89 |
The membrane protein expression data shown are the mean of the number of pixels x their intensity (107) +/− (SD). Ten different fields were imaged on at least 3 different non-consecutive slides (N=4). The volume, length and nuclei count data shown are the mean of the number of cubic microns (NKCC1 volume), microns (skeletal length) and the number of nuclei per acinus (nuclei count) +/− (SD). Between 3–5 stacks were imaged on at least 3 different non-consecutive slides (N=3). Significant differences are indicated (*) and were determined by unpaired student's t-test. AQP-5=Aquaporin-5, NKCC1= Na+/K+/2Cl- cotransporter, ZO1= zona occludens 1, C3= claudin 3, WT = wild type, TG = IL-12 transgenic.
Figure 5. Acinar cell staining by NKCC1.
A representative 3D stack of confocal microscopy labeling was reconstructed with each acinus individually colored for ease of visualization. IL-12 TG female mice (A) showed on average larger acinus volume compared to WT mice (B).
DISCUSSION
Interleukin-12 (IL-12) is a heterodimeric cytokine produced predominantly by activated monocytes and dendritic cells. It enhances proliferation and cytolytic activity of natural killer (NK) and T cells, and stimulates their IFN-γ production16. IL-12 production plays a key role in promoting type 1 T helper cell (Th1) responses, however, IL-12 can also trigger Th1-mediated immunopathology and induction of Th1-mediated organ-specific autoimmune diseases, such as rheumatoid arthritis, autoimmune thyroiditis and insulin-dependent diabetes mellitus17. The role of IL-12 in Th-1 mediated autoimmune diseases has been suggested in other animal models. Administration of IL-12 induces rapid onset of IDDM in female NOD mice and severe collagen induced arthritis in DBA/1 mice18, 19.
In order to better understand the role of IL-12 in SS, we have studied the affect of IL12 on salivary gland function, histopathology, and autoantibody formation, in SJL IL-12 TG mice and observed a number of changes also observed in SS patients. In addition we have noted morphological changes in the salivary glands of TG mice (increased acinar cell volume, decrease in the number of cells per acinus) that have previously been associated with a loss of gland activity20.
Previously it was shown that CBA IL-12 TG mice develop mononuclear infiltrates in lungs, salivary, and lacrimal glands9. Similarly, SJL IL-12 TG mice had increased lymphocytic infiltration in salivary and lacrimal glands predominantly composed of B220-positive B lymphocytes and CD4-positive T lymphocytes. Interestingly, SJL WT mice also had a low degree of mononuclear infiltration in salivary and lacrimal glands, suggesting an activated immune system in this strain. Our study suggests a stimulating role of IL-12 on the infiltration rates in exocrine glands from SJL mice. Although statistical analysis showed a correlation between the degree of mononuclear infiltration and salivary gland dysfunction in SJL IL-12 TG mice, the saliva measurement preceded the tissue collection by several weeks to months. Therefore, the level of infiltration in the glands at the time of the saliva collection may not be the same as observed at the time of gland removal and histological analysis which may limit the utility of this observation on understanding the pathophysiology of salivary gland dysfunction.
Autoantibody formation is one of the characteristics in SS patients. Interestingly, over-expression of IL-12 in SJL mice resulted in elevated levels of ANA and an age-dependent increase in anti-SSB/La autoantibodies. Previous publications concerning the role of IL-12 in autoantibody formation in autoimmune disease prone mice showed conflicting results. Recently, beneficial effects of intermittent IL-12 plasmid administration was reported on autoantibody levels and autoimmunity in MRL/lpr mice21. In contrast, daily injection of recombinant IL-12 led to accelerated glomerulonephritis in MRL/lpr mice, illustrating the importance of the mode of IL-12 administration22. Also, an association is described between IL-12 (p40) levels and anti-SSA and – SSB in serum23. Our data suggest that over-expression of IL-12 in autoimmune prone mice can induce autoantibody formation, as also seen in SS patients.
Although the functional changes seen in IL-12 TG salivary glands might be explained by the autoantibodies or increased infiltrates, we have also detected morphological changes in the structure of the glands of TG mice. Increased AQP-5 and NKCC1 expression correlated with an increase in acinus volume and apical surface length. Interestingly a similar increase in acinus volume and loss of gland function was also observed in AQP-5 null mice20. In this study the authors proposed a connection between the change in permeability of the tight junction complexes associated with a decrease in claudin 3 expression. While we also see a slight decrease in claudin 3 expression, it was not statistically significant. In addition to the increase in acinus volume, the IL12 TG mice also have a decrease in the number of cells/acinus. This could be the result of either a developmental abnormality or apoptosis of gland tissue. Although we have not specifically studied the transcellular and paracellular transport routes in the IL12 TG mice, it remains a possibility that the IL-12 TG mice could also have a similar change in permeability. Many phenomena can affect salivary flow (e.g. change in acinus, cell number, transport properties, signaling cascades etc.) and our results do not address the ultimate cause of the decrease in flow observed in these TG mice. In addition, further study will be required in order to understand if similar changes in salivary gland morphology are observed in the major salivary glands of SS patients.
In addition to the SS-like characteristics in this animal model, the SJL IL-12 TG mice also develop mild autoimmune thyroid disease (ATD)8. Prevalence studies and clinical observations strongly suggest a common etiology and similar pathogenetic mechanisms between SS and ATD. Thyroid and salivary glands show a number of functional similarities, including the uptake and concentration of iodine. Moreover, lymphocytic infiltrates in the salivary and lacrimal glands and clonal B cell expansion observed in SS are similar to those seen in ATD24. Our data support an association between thyroid disease and SS, also seen in patients.
Acknowledgments
This work is supported by a Dutch Arthritis Association grant [NR 07-1-406] to JLV, a Sjögren's Foundation grant to PC, and an NIH NIDCR intramural research grant to JAC.
REFERENCES
- 1.Hu Y, Nakagawa Y, Purushotham KR, Humphreys-Beher MG. Functional changes in salivary glands of autoimmune disease-prone NOD mice. Am J Physiol. 1992;263(4 Pt 1):E607–14. doi: 10.1152/ajpendo.1992.263.4.E607. [DOI] [PubMed] [Google Scholar]
- 2.Humphreys-Beher MG, Brinkley L, Purushotham KR, et al. Characterization of antinuclear autoantibodies present in the serum from nonobese diabetic (NOD) mice. Clin Immunol Immunopathol. 1993;68(3):350–6. doi: 10.1006/clin.1993.1137. [DOI] [PubMed] [Google Scholar]
- 3.Lodde BM, Mineshiba F, Kok MR, et al. NOD mouse model for Sjogren's syndrome: lack of longitudinal stability. Oral Dis. 2006;12(6):566–72. doi: 10.1111/j.1601-0825.2006.01241.x. [DOI] [PubMed] [Google Scholar]
- 4.Yamano S, Atkinson JC, Baum BJ, Fox PC. Salivary gland cytokine expression in NOD and normal BALB/c mice. Clin Immunol. 1999;92(3):265–75. doi: 10.1006/clim.1999.4759. [DOI] [PubMed] [Google Scholar]
- 5.Lodde BM, Mineshiba F, Wang J, et al. Effect of human vasoactive intestinal peptide gene transfer in a murine model of Sjogren's syndrome. Ann Rheum Dis. 2006;65(2):195–200. doi: 10.1136/ard.2005.038232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vosters JL, Baum BJ, Tak PP, Illei GG, Chiorini JA. Developing a gene therapy for Sjogren's syndrome. Future Reumatol. 2006;1(4):433–40. [Google Scholar]
- 7.van Blokland SC, Versnel MA. Pathogenesis of Sjogren's syndrome: characteristics of different mouse models for autoimmune exocrinopathy. Clin Immunol. 2002;103(2):111–24. doi: 10.1006/clim.2002.5189. [DOI] [PubMed] [Google Scholar]
- 8.Kimura H, Tzou SC, Rocchi R, et al. Interleukin (IL)-12-driven primary hypothyroidism: the contrasting roles of two Th1 cytokines (IL-12 and interferon-gamma) Endocrinology. 2005;146(8):3642–51. doi: 10.1210/en.2005-0275. [DOI] [PubMed] [Google Scholar]
- 9.McGrath-Morrow S, Laube B, Tzou SC, et al. IL-12 overexpression in mice as a model for Sjogren lung disease. Am J Physiol Lung Cell Mol Physiol. 2006;291(4):L837–46. doi: 10.1152/ajplung.00134.2006. [DOI] [PubMed] [Google Scholar]
- 10.Mathieu A, Cauli A, Pala R, et al. Tracheo-bronchial mucociliary clearance in patients with primary and secondary Sjogren's syndrome. Scand J Rheumatol. 1995;24(5):300–4. doi: 10.3109/03009749509095167. [DOI] [PubMed] [Google Scholar]
- 11.Rangel-Moreno J, Hartson L, Navarro C, Gaxiola M, Selman M, Randall TD. Inducible bronchus-associated lymphoid tissue (iBALT) in patients with pulmonary complications of rheumatoid arthritis. J Clin Invest. 2006;116(12):3183–94. doi: 10.1172/JCI28756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davidson BK, Kelly CA, Griffiths ID. Ten year follow up of pulmonary function in patients with primary Sjogren's syndrome. Ann Rheum Dis. 2000;59(9):709–12. doi: 10.1136/ard.59.9.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jara LJ, Navarro C, Brito-Zeron Mdel P, Garcia-Carrasco M, Escarcega RO, Ramos-Casals M. Thyroid disease in Sjogren's syndrome. Clin Rheumatol. 2007;26(10):1601–6. doi: 10.1007/s10067-007-0638-6. [DOI] [PubMed] [Google Scholar]
- 14.Kok MR, Yamano S, Lodde BM, et al. Local adeno-associated virus-mediated interleukin 10 gene transfer has disease-modifying effects in a murine model of Sjogren's syndrome. Hum Gene Ther. 2003;14(17):1605–18. doi: 10.1089/104303403322542257. [DOI] [PubMed] [Google Scholar]
- 15.Vivino FB, Gala I, Hermann GA. Change in final diagnosis on second evaluation of labial minor salivary gland biopsies. J Rheumatol. 2002;29(5):938–44. [PubMed] [Google Scholar]
- 16.Trinchieri G. Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood. 1994;84(12):4008–27. [PubMed] [Google Scholar]
- 17.Adorini L. Interleukin-12, a key cytokine in Th1-mediated autoimmune diseases. Cell Mol Life Sci. 1999;55(12):1610–25. doi: 10.1007/s000180050400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trembleau S, Germann T, Gately MK, Adorini L. The role of IL-12 in the induction of organ-specific autoimmune diseases. Immunol Today. 1995;16(8):383–6. doi: 10.1016/0167-5699(95)80006-9. [DOI] [PubMed] [Google Scholar]
- 19.Germann T, Szeliga J, Hess H, et al. Administration of interleukin 12 in combination with type II collagen induces severe arthritis in DBA/1 mice. Proc Natl Acad Sci U S A. 1995;92(11):4823–7. doi: 10.1073/pnas.92.11.4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawedia JD, Nieman ML, Boivin GP, et al. Interaction between transcellular and paracellular water transport pathways through Aquaporin 5 and the tight junction complex. Proc Natl Acad Sci U S A. 2007;104(9):3621–6. doi: 10.1073/pnas.0608384104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hagiwara E, Okubo T, Aoki I, et al. IL-12-encoding plasmid has a beneficial effect on spontaneous autoimmune disease in MRL/MP-lpr/lpr mice. Cytokine. 2000;12(7):1035–41. doi: 10.1006/cyto.1999.0662. [DOI] [PubMed] [Google Scholar]
- 22.Huang FP, Feng GJ, Lindop G, Stott DI, Liew FY. The role of interleukin 12 and nitric oxide in the development of spontaneous autoimmune disease in MRL/MP-lpr/lpr mice. J Exp Med. 1996;183(4):1447–59. doi: 10.1084/jem.183.4.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathsson L, Ahlin E, Sjowall C, Skogh T, Ronnelid J. Cytokine induction by circulating immune complexes and signs of in-vivo complement activation in systemic lupus erythematosus are associated with the occurrence of anti-Sjogren's syndrome A antibodies. Clin Exp Immunol. 2007;147(3):513–20. doi: 10.1111/j.1365-2249.2006.03313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adamson TC, 3rd, Fox RI, Frisman DM, Howell FV. Immunohistologic analysis of lymphoid infiltrates in primary Sjogren's syndrome using monoclonal antibodies. J Immunol. 1983;130(1):203–8. [PubMed] [Google Scholar]