Abstract
Rho-associated protein kinases (ROCKs) play key roles in mediating the control of the actin cytoskeleton by Rho family GTPases in response to extracellular signals. Such signaling pathways contribute to diverse neuronal functions from cell migration to axonal guidance to dendritic spine morphology to axonal regeneration to cell survival. In this review, the authors summarize biochemical knowledge of ROCK function and categorize neuronal ROCK-dependent signaling pathways. Further study of ROCK signal transduction mechanisms and specificities will enhance our understanding of brain development, plasticity, and repair. The ROCK pathway also provides a potential site for therapeutic intervention to promote neuronal regeneration and to limit degeneration.
Keywords: Rho GTPase, Cytoskeleton, Rho-associated kinase, Signal transduction, Axonal outgrowth
The cytoplasmic Rho family of GTPases plays a critical role in regulating actin microfilament dynamics. The control of cell shape and cell migration by Rho GTPases has attracted intense interest from the fields of cancer biology and cardiovascular medicine. More recently, it has become clear that Rho family proteins and their intracellular effectors participate in a range of neuronal functions from axonal growth to neuronal differentiation to neuronal survival to regeneration.
The Rho-associated kinases (ROCKs) are principal mediators of RhoA activity, especially in the nervous system, and are the focus of this review. We explore current knowledge regarding the downstream targets and functions of ROCKs. In addition, we summarize known transduction mechanisms from extracellular neuronal signals to ROCK. By analyzing the upstream receptors, effectors, and mediators of Rho GTPase-related neuronal morphogenesis, we group these pathways functionally and biochemically.
Rho-GTPases
By sequence homology, the Ras-superfamily of GTPases can be subdivided into 9 families: Ras, Rab, Arf, Ran, Rad, Rheb, Rag, Rit and Rho (Blumenstein 2004). Clustal W algorithm alignment of the RhoGTPase domains allowed the 22 mammalian RhoGTPases to be grouped into eight subclasses (Aspenstrom and others 2004). A revised classification employing Clustal X neighborjoining combined with ProML maximum likelihood methods indicated that two out of these 22 Rho GTPases—Miro-1 and Miro-2, or Wrch-1 and Wrch-2—are best considered an autonomous Ras-like family (Boureux and others 2007). Hence, 20 mammalian RhoGTPases constitute eight subclasses, fulfilling tasks that include cell growth and transformation control (Zohn and others 1998), actin filament (re)organization in fibroblasts (Voncken and others 1995; Zohn and others 1998; Johnson 1999; Cerione 2004; Jaffe and Hall 2005; Vardouli and others 2005), mitogen-activated protein kinase cascade activation, G1-S cell cycle regulation, gene transcription (Zohn and others 1998; Olofsson 1999; Narumiya and others 2004), cytokinesis regulation, microtubule dynamics, cell adhesion, endo- and exocytosis, migration, and survival (Van Aelst and D’Souza-Schorey 1997; Etienne-Manneville and Hall 2002; Cerione 2004).
An overview of the eight subclasses of RhoGTPases are shown in Table 1, together with their common expression patterns (Wennerberg and Der 2004; Ridley 2006) and effects on the actin filament system after transfection into porcine aortic endothelial cells (Aspenstrom and others 2004). For a recent SAGE analysis of the mRNA expression of 17 RhoGTPases in mouse tissue, refer to Boureux and others (2007).
Table 1.
Overview Concerning RhoGTPases, Their Classification into Subclasses, Expression Patterns, and Effects on Actin Filaments
| Rho- GTPase- Subclass |
RhoGTPases (Official Symbol) |
Loci of Expression (Wennerberg and Der 2004) and Cellular Localization (Ridley 2006) |
Effects on Actin Filament System after Transfection into Porcine Aortic Endothelial Cells (Aspenstrom and Others 2004) |
|---|---|---|---|
| Rho | RhoA (RHOA) | Ubiquitous/PM,CS | Assembly of stress fibers |
| RhoB (RHOB) | Ubiquitous/PM,ES | Assembly of stress fibers | |
| RhoC (RHOC) | Ubiquitous/PM,CS | Assembly of stress fibers | |
| Rac | Rac1 (RAC1) | Ubiquitous/PM | Formation of lammelipodia and thick bundles of actin |
| Rac2 (RAC2) | Hematopoietic/PM,CS | Formation of lammelipodia and thick bundles of actin |
|
| Rac3 (RAC3) | Brain, Heart, Placenta, Pancreas/PM, EM |
Formation of lammelipodia | |
| RhoG (RHOG) | Ubiquitous/PM, ES | Formation of lammelipodia | |
| Cdc42 | TCL (RHOJ) | Heart, Lung, Liver/PM, ES | Formation of lammelipodia |
| TC10 (RHOQ) | Heart, Skeletal muscle/ PM, PN |
Formation of focal adhesion-like structures |
|
| Cdc42 (CDC42) |
Ubiquitous/PM, GO | Formation of lammelipodia and thick bundles of actin |
|
| Wrch1 (RHOV) | n.a./PM, EM | Formation of long and thin filopodia | |
| Chp/Wrch2 (RHOU) |
Brain, Spleen, Lung, Testis/PM, EM |
Formation of focal adhesion-like structures |
|
| Rnd | Rnd1/Rho6 (RND1 |
Brain, Liver/PM | Loss of stress fibers, formation of actin-/ezrin-containing dorsal microvilli |
| Rnd2/Rho7 (RND2 |
Brain, Testis/ES, CS? | Loss of stress fibers, formation of actin-/ezrin-containing dorsal microvilli |
|
| Rnd3/RhoE/ MemB (RND3) |
Ubiquitous/ PM, GO, CS |
Loss of stress fibers, formation of actin-/ezrin-containing dorsal microvilli |
|
| RhoD | RhoD (RHOD) | n.a./PM, ES | Formation of very long and flexible filopodia |
| Rif (RHOF) | Ubiquitous/PM | Formation of very long and flexible filopodia |
|
| RhoH | RhoH/TTF (RHOH) |
Hematopoietic/n.a. | n.a. |
| RhoBTB | RhoBTB1 (RHOBTB1) |
Ubiquitous/VS | No effects (but present in vesicular structures) |
| RhoBTB2 (RHOBTB2) |
Brain/VS | No effects (but present in vesicular structures) |
The official symbols, shown in brackets, are provided by HUGO gene nomenclature committee. PM = plasma membrane; CS = cytosol; ES = endosomes; PN = perinuclear; EM = endomembranes; GO = golgi; VS = vesicular.
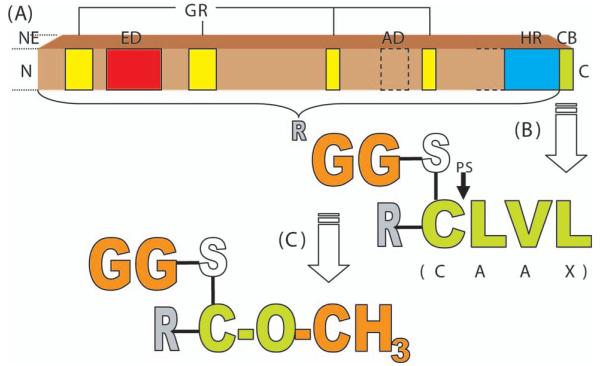
The general structure of RhoGTPases, as shown in Figure 1A, consists of several core components. An effector domain is made accessible by guanosine triphosphate (GTP)-induced conformational changes in two “switch” domains between residues 28-44 and 62-69 of RhoA (Hakoshima and others 2003). The hypervariable region differs not only between the RhoGTPase subclasses but also between the isoforms within one subclass in terms of the presence of a polybasic region or a palmitoylation site (Ridley 2006). The carboxy-terminal CAAX-box (C, cysteine; A, aliphatic amino acid; X, any amino acid) is crucial for posttranslational modifications, including proteolysis, carboxyl group methylation, and prenylation (Adamson and others 1992).
Fig. 1.
A, General structure of RhoGTPases (adapted from Ridley 2006), and B, Posttranslational modification of RhoA CAAX-Box by geranylgeranyl-prenylation, proteolysis, and C, Methylation. NE = N-terminal extension in some RhoGTPases; ED = effector domain; GR = GTP/GDP-binding regions; AD = additional domains in RhoBTB; HR = hypervariable region (varies in length for different RhoGTPases); CB = CAAX-Box; N = N-terminal end; C = C-terminal end; GG = geranylgeranyl residue; PS = proteolysis site; R = rest of protein N-terminal of CAAX-Box; CLVL = amino acids Cys, Leu, Val, Leu (note: even though S and O are atoms belonging to Cys, they are shown separately in order to visualize the posttranslational bonds).
RhoA (Structure and Posttranslational Modification)
Here, we focus on RhoA, a small GTPase of about 24 kDa (Kamai and others 2004) that has been extensively studied. This protein’s CAAX-box is composed of the amino acids cysteine (C), leucine (L), valine (V), and leucine (L). Mutation of the X-amino acid of a ras peptide to leucine (Seabra and others 1991), as well as the examination of a variety of small guanine nucleotide binding proteins in which X = leucine or phenylalanine (Kawata and others 1990; Maltese and Sheridan 1990; Maltese and others 1990; Buss and others 1991; Yamane and others 1991; Leung and others 2007), revealed that these amino acids specify prenylation with geranylgeranyl by protein geranylgeranyltransferase type 1. In Rho family proteins where X = serine, methionine, glutamine, or alanine, prenylation is catalyzed by protein farnesyl transferase to add a farnesyl group. As predicted by its sequence, RhoA is modified by a thioether linkage of C20 geranylgeranyl to the COOH-terminal cysteine residue (Fig. 1B) (Katayama and others 1991). Protein prenylation is known to be required for membrane targeting and function (Lane and Beese 2006; Leung and others 2006). Prenylated RhoA initially associates with the membrane of the endoplasmic reticulum (ER), where proteolysis, catalyzed by the CAAX protease Ras and α-factor-converting enzyme (Rce1), occurs immediately C-terminal to the geranylgeranylated cysteine (Fig. 1B) (Lane and Beese 2006). The carboxyl-terminal geranylgeranylcysteine of RhoA can be methylated at its alpha-carboxy-residue by the ER-membrane bound protein-S-isoprenylcysteine O-methyltransferase (systematic name, S-adenosyl-L-methionine: protein-C-terminal-S-farnesyl-L-cysteine O-methyltransferase)—an enzyme with a high activity for C-terminal S-farnesyl-L-cysteine and lower affinities for C-terminal S-geranylgeranyl-L-cysteine and S-geranyl-L-cysteine (Stephenson and Clarke 1990; Volker and others 1991; Perez-Sala and others 1992). This reversible process of methylation (see Fig. 1C) is thought to facilitate membrane attachment and interactions with effector proteins, as described for other proteins (Hancock and others 1991; Silvius and l’Heureux 1994; Parish and others 1995) and for RhoGTPases (Lane and Beese 2006). Amino terminal to the CAAX box there is another region thought to contribute to proper subcellular targeting (not shown in Fig. 1). This region contains either a series of basic amino acids or cysteine residues acting as palmitoylation sites (Hancock and others 1990; Michaelson and others 2001).
ROCKs (Structure, Regulators, and Localization)
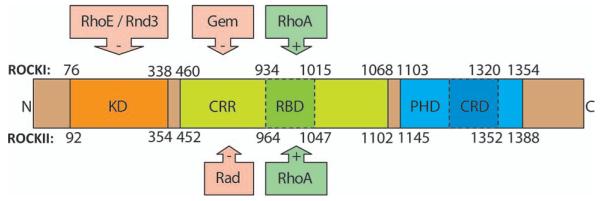
The first identified and most exhaustively studied downstream targets of RhoA are the Rho-associated coiled-coil-containing protein kinases (also known as ROCKs) (Leung and others 1995; Fujisawa and others 1996; Leung and others 1996; Matsui and others 1996; Nakagawa and others 1996). There are two genes encoding two protein isoforms: ROKα (Leung and others 1996) (also termed ROCKII [Matsui and others 1996; Nakagawa and others 1996] or Rho kinase [Matsui and others 1996]) and ROKβ (Leung and others 1995) (also referred to as ROCKI or p160ROCK [Palmer and others 1994]). The ROCKs are two of the more than 50 members of the ACG kinase family (Proud 2007). The ROCK serine/threonine kinases consist of three major domains: a RhoA binding domain (RBD) situated in a coiled-coil region (CCR), a kinase domain that is responsible for its catalytic activity and a cysteine-rich domain encompassing pleckstrin-homology domain that is thought to participate in protein localization (Riento and Ridley 2003). Both ROCK isoforms are activated by RhoA binding to the RBD (Leung and others 1995; Fujisawa and others 1996). Intracellular second messengers such as arachidonic acid and sphingosylphosphorylcholine also have activating effects on ROCKs (Fu and others 1998; Shirao and others 2002). Furthermore, the autoinhibitory C-terminus of ROCKI can be cleaved proteolytically by caspase 3, a process occurring during apoptosis and leading to increased ROCKI activity (Coleman and others 2001; Sebbagh and others 2001). Inhibitors for ROCKI include Gem, which is thought to change the substrate specificity by binding to the CCR (Ward and others 2002), and RhoE/Rnd3, which acts as an inhibitor by binding to the kinase domain (Riento and others 2003). In the case of ROCKII, Rad serves as an inhibitor by binding to the CCR of the protein (Ward and others 2002). The structure and regulation of ROCKI and ROCKII are summarized in Figure 2.
Fig. 2.
Structure of ROCKI/ROCKII and regulatory binding sites. The upper and lower numbers represent the amino acids of ROCKI and ROCKII, respectively. The following major domains are shown: the catalytic kinase domain (KD), the pleckstrin-homology domain (PHD) that encompasses a cysteine-rich domain (CRD), and the Rho binding domain (RBD) situated within the coiled-coil region. RhoA activates ROCKs by binding to the RBD, whereas Gem and RhoE/Rnd3 inactivate ROCKI preferably by binding to the CCR and KD, respectively. Rad was found to inactivate ROCKII preferably by binding to its CCR. N = N-terminal end; C = C-terminal end.
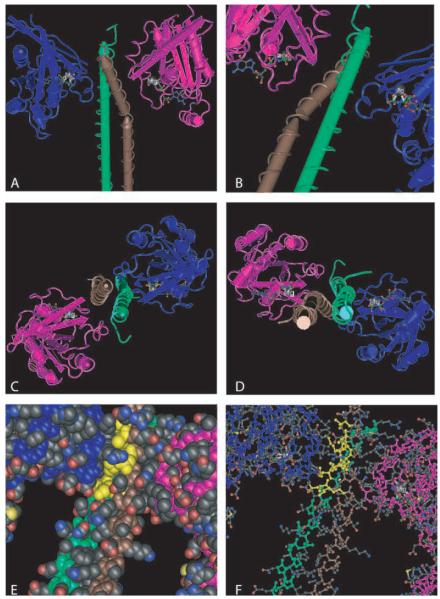
Although the two ROCK isoforms share 65% identity in their amino-acid sequences, the highest similarities are found in their kinase domains, which are 92% identical (Nakagawa and others 1996). ROCK autophosphorylation implies that autoregulation contributes a physiological function for these kinases. The ROCKs also phosphorylate a range of effector proteins (Ishizaki and others 1996; Leung and others 1995). Downstream targets that can be phosphorylated by ROCK include the consensus amino acid sequences R/KXS/T or R/KXXS/T (R, arginine; K, lysine; S, serine; T, threonine; X, any amino acid) (Kawano and others 1999; Sumi and others 2001). As shown in Figure 3, the α-helical RBD assembles into a parallel coiled-coil in ROCKI (Dvorsky and others 2004) and ROCKII (Chen and others 2002; Shimizu and others 2003). Even though differences in the spatial arrangement of the coiled-coil do occur between the two isoforms, the C-terminal structures of the RBD are highly similar. The minimal RhoA-interacting motif (residues 998-1010 of ROCKI), located in the C-terminal part of the ROCK-RBD, is identical in ROCKs of different organisms (Dvorsky and others 2004). The structure of the ROCKI/RBD interaction is illustrated in Figure 3.
Fig. 3.
Crystal structure of ROCKI interaction with RhoA (adapted from Dvorsky and others 2004). Shown in the figures are complexes between human ROCKI-RBDs (residues 947-1015; labeled green and brown) and the truncated forms of human RhoA (residues 1-181; labeled magenta and cyan) bound to the nonhydrolyzable GTP analogue Gpp (NH)p (stabilized by Mg2 ). A-D, Display of the complex of two RhoA molecules and two ROCKI molecules from different angles. The close up in B shows the area of ROCKI-RBD/RhoA interaction. The same perspective, only turned 180 degrees around the helix-axis, is employed in E and F, where the residues of ROCKI-RBD, interacting with RhoA, are labeled yellow. These residues include K999, V1003, N1004, L1006, A1007, and M1010 in the brown-labeled α-helix and L998, Q1001, A1002, and K1005 in the green-labeled α-helix. Whereas A-D are rendered in “worms-style,” E uses the “space-fill-style” and F the “ball-and-stick-style.”
Even though the ROCK isoforms share the structural similarities mentioned above, their localization in the body differs. The ROCKII mRNA is preferentially found in brain and heart tissue, whereas ROCKI mRNA is predominant in other tissues (Leung and others 1996; Nakagawa and others 1996; Di Cunto and others 2000; Wei and others 2001; Riento and Ridley 2003). Pyramidal neurons of the hippocampus and cerebral cortex and Purkinje cells of the cerebellum are the cells with the highest ROCKII expression (Hashimoto and others 1999). By immuno-fluorescence and cell fractionation, ROCKII was found to be primarily cytoplasmic and a minor fraction is associated with cellular membranes (Leung and others 1995; Matsui and others 1996; Kimura and others 1998). A subset of ROCKII colocalizes with actin stress fibers (Katoh and others 2001; Chen and others 2002), and with the vimentin intermediate-filament network in serum-starved cells (Sin and others 1998). A portion of ROCKI is reported to colocalize with centrosomes (Chevrier and others 2002).
ROCK-Effectors and Cytoskeleton
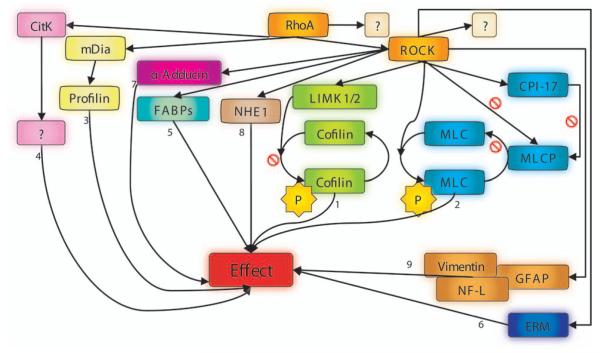
A variety of effectors for ROCKs have been described (Fig. 4). Downstream targets include phosphatidylinositol-3-kinase/protein kinase akt (leading to decreased activation of the endothelial nitric oxide synthase) (Ming and others 2002; Wolfrum and others 2004), the insulin receptor substrate-1 (resulting in uncoupling of insulin receptor from phosphatidylinositol-3-kinase and potentially GLUT4 activation) (Farah and others 1998), and various cytoskeletonorganization regulating proteins (discussed in detail below).
Fig. 4.
Main targets of RhoA and ROCK, involved in organization of the cytoskeleton. Arrows carrying a stop sign symbolize inactivation; arrows without a stop sign indicate activation. Molecules attached to numbered arrows lead to the following effects: 1 = decreased depolymerization of actin filaments; 2 = stress fiber formation and increased contractility; 3 = actin polymerization regulation → contractile ring → cytokinesis; 4 = also involved in formation of contractile ring → cytokinesis; 5 = disrupted actin binding activity/signaling mainly unknown so far; 6 = actin-cytoskeletal reorganization; 7 = assembly of actin filaments (spectrin-actin meshwork beneath plasma membranes); 8 = actin organization and focal adhesions; 9 = disruption of intermediate filaments. ? = pathway that is not yet known; P = phosphorylated molecule; RhoA = Ras homology gene family member A; ROCK = Rho-associated coiled-coil-containing protein kinase; CitK = citron kinase; mDia = mammalian diaphanous; CPI-17 = protein kinase C-potentiated inhibitor of 17 kDa; LIMK1/2 = LIM-domaincontaining protein kinases 1 and 2; MLC = myosin-II regulatory light chain; MLCP = myosin-II regulatory light chain phosphatase; NHE1 = Na+/H+-exchanger-1; FABPs = F-actin binding proteins; ERM = ezrin-radixin-moesin; GFAP = glial fibrillary acidic protein; NF-L = neurofilament protein.
One intensively studied downstream target of ROCKs is the myosin-II regulatory light chain phosphatase (MLCP), an enzyme consisting of a 20kDa noncatalytic subunit (M21), a 37kDa catalytic subunit (PP1δ), and a 110-130kDa targeting domain, termed the myosin binding site (MBS) or myosin phosphatase targeting peptide. After phosphorylation of the MBS at threonine 697 and threonine 855 by ROCKII (Kawano and others 1999), the enzyme activity of MLCP is decreased via inhibition of MBS’s ability to activate the PP1δ, resulting in less dephosphorylation and thus lower inactivation of myosin-II regulatory light chain (MLC) (Noda and others 1995; Kimura and others 1996; Hartshorne and others 1998; Somlyo and Somlyo 2000). Increased cellular contractility and stress fiber formation are the main effects of this signaling due to induced interaction of MLC with actin. The net effect is an activation of the myosin ATPase (Zhao and Manser 2005). ROCKII is also able to phosphorylate MLC at serine 19, a phosphorylation site shared with MLC kinase, to shift MLC into the active form. ROCK-mediated phosphorylation of threonine 38 in the 17 kDa protein kinase C-potentiated inhibitor (CPI-17) potentiates this protein’s inhibitory action on MLCP (Somlyo and Somlyo 2000). Additionally, RhoA can bind to the C-terminal site of the MBS (Kimura and others 1996; Hartshorne and others 1998), providing a parallel ROCK-independent mechanism to regulate cellular contractility (for clarity, not shown in Fig. 4).
LIM-domain (Lin11, Isl1, Mec3)-containing protein kinases 1 and 2 (LIMK1/2) are phosphorylated by ROCKI at threonine 508 and threonine 505, respectively, enabling these enzymes to phosphorylate and thus inactivate cofilin at serine 3. The reduced binding to F-actin by phosphorylated cofilin/actin depolymerizing factor attenuates its ability to catalyze F-actin depolymerization and severing (Maekawa and others 1999; Stanyon and Bernard 1999; Ohashi and others 2000; Sumi and others 2001).
Another ROCKII effector is α-Adducin, a membrane skeletal protein that binds to spectrin and promotes the association of that molecule with F-actin (Kimura and others 1998; Fukata and others 1999). Thought to play its role in spectrin-actin network assembly by capping of actin and by spectrin recruitment to the growing ends of actin filaments, α-Adducin shows enhanced binding activity to F-actin after phosphorylation by ROCKI. This has been shown to occur preferentially beneath to the plasma membrane at ruffles (Fukata and others 1999).
Cross-linking of membrane proteins and actin filaments near the cell surface is promoted by head-to-tail association of ezrin, radixin, and moesin (ERM) proteins. The ERM proteins are additional substrates of the ROCKs. Following the phosphorylation of the ERM proteins at threonine 567, threonine 564, and threonine 558, respectively, cross-linking of actin filaments is disrupted, permitting cytoskeletal reorganization (Matsui and others 1998).
Activated by ROCKI, the Na+/H+-exchanger-1 functions in RhoA-induced reorganization of actin filaments (Denker and others 2000; Tominaga and Barber 1998) and also as an ERM-binding protein and ion exchanger, contributing to fibroblast migration (Tominaga and Barber 1998; Denker and Barber 2002).
Intermediate filament proteins such as vimentin, glial fibrillary acidic protein (GFAP), and neurofilament protein (NF-L) are phosphorylated by ROCKII at multiple residues. In vitro, vimentin filament disassembly and impaired GFAP- or NF-L filament formation have been reported after phosphorylation by ROCKII (Kosako and others 1997; Goto and others 1998; Hashimoto and others 1998).
Other downstream targets of ROCKII include F-actin binding proteins such as eukaryotic elongation factor 1α (EF-1α), myristylated alanine-rich C kinase substrate (MARCKS), and calponin. For these proteins, phosphorylation leads to decreased actin binding activity (Winder and Walsh 1990; Izawa and others 2000; Nagumo and others 2001).
RhoA-Effectors and Cytoskeleton
Activated RhoA does not signal through ROCK alone, although in many systems, ROCK is essential for signal transduction. Other effectors include mammalian diaphanous (mDia) and citron kinase (CitK). mDia possesses an actin-nucleating region (FH2 domain) and is able to nucleate parallel, unbranched actin filaments after binding of RhoA to the protein’s N-terminus, a process that results in unfolding and thus activation of the nucleating activity (Ridley 2006). CitK is suggested to be involved in the formation of the contractile ring, consequently playing a role in cytokinesis, possibly by participation in regulating di-phosphorylation of MLC (Goto and others 2000; Yamashiro and others 2003).
ROCKs in the CNS
As stated above, certain intermediate filament proteins are regulated by ROCKII. Belonging to the type IV family of intermediate filaments, NF-L is found in high concentrations along axons of vertebrate neurons. NF-L undergoes depolymerization after phosphorylation by ROCKII (Kosako and others 1997; Goto and others 1998; Hashimoto and others 1998). An increase in NF-L is reported to result in a higher number of filaments and decreased interfilament distance. The intriguing hypothesis that ROCKII/neurofilament interactions have an effect on axonal diameter remains to be tested.
Microtubule-associated proteins (MAPs), such as Tau or MAP2, play major roles in shaping neuronal morphology by altering microtubule dynamics (Hirokawa 1994; Mandelkow and Mandelkow 1995). Tau and MAP2 can be phosphorylated by ROCKII to decrease microtubule polymerization (Amano and others 2003). Suppression of microtubule and neurofilament assembly is seen after RhoA/ROCKII activation in neuroblastoma cells (Amano and others 1998; Hirose and others 1998; Katoh and others 1998), consistent with Tau and MAP2 participating in neurite retraction.
Collapsin response mediator protein 2 (CRMP2), a neuronal protein that is associated with semaphorin-3A and lysophosphatidic acid-induced growth cone collapse and axon outgrowth (Goshima and others 1995; Arimura and others 2000; Inagaki and others 2001), is another ROCKII effector. The axon guidance functions that are associated with ROCKII/CRMP2 signaling illustrate a role for ROCKs during the development of the central nervous system. As much as a lipid-kinase-mutant form of PtdIns(4)P5K inhibits ROCK-induced growth cone collapse, the native form might also function downstream of ROCKII-induced neurite retraction (Yamazaki and others 2002). Actin-myosin-interaction regulating proteins like LIMK1/2 and MLC (Fig. 4) are known to participate in growth-cone collapse (Amano and others 1998; Aizawa and others 2001; Birkenfeld and others 2001; Fujita and others 2001; Mueller and others 2005), further emphasizing the potential regulatory role of ROCKII in axon guidance.
To evaluate the in vivo functions of CNS ROCKs, inhibitors have been administered in several model systems. Beneficial neurological effects were observed in animal models of Alzheimer’s disease, neuropathic pain, demyelinating/inflammatory diseases (eg, multiple sclerosis), stroke, and in spinal-cord injuries (reviewed in Mueller and others 2005). As a caveat, it must be appreciated that the ROCK small molecule inhibitors do not distinguish between ROCKI and ROCKII. Moreover, the close relationship of ROCKs to other ACG kinases leaves open the possibility that some of these effects are mediated by other kinases. Thus, many of these pharmacological experiments should be confirmed by genetically specific RNAi or knock-out animal models.
Rho-GTPase Regulators
Over the past 30 years, a growing interest for small GTP-binding proteins (GTPases) and their influence on cell morphogenesis has developed. Regarded as important molecular switches for the developing and reorganizing cytoskeleton, Rho family GTPases were soon found to play a key role in neuronal morphogenesis. As mentioned above, the Rho family GTPases comprise a set of related proteins—more than 20 have been identified in humans, and at least 7 of them play a role in dendrite development (Negishi and Katoh 2005). For clarity, we focus here primarily on RhoA—one of the most important GTPases for modulation of cell morphology and motility in neurons. After examining the downstream effects of RhoA, the question of how this molecular switch is regulated shall be addressed.
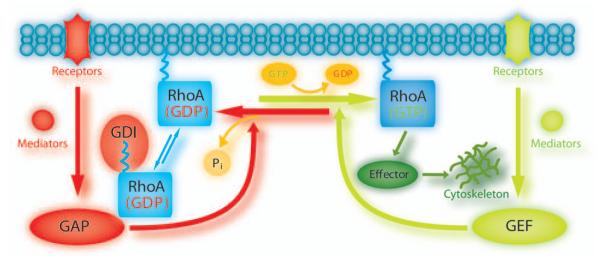
As illustrated in Figure 5, Rho GTPases are inactive when bound to guanosine diphosphate (GDP) but change into their active conformation after binding to GTP. Their activity in mammalian cells is known to be regulated by a family of at least 85 guanine nucleotide exchange factors (GEFs), 80 GTPase-activating proteins (GAPs), and three guanine nucleotide dissociation inhibitors (GDIs) (Jaffe and Hall 2005). GEFs are activators for downstream signaling—they turn on the switch by catalyzing the exchange of GTP for GDP (Schmidt and Hall 2002). On the other hand, GAPs inhibit downstream signaling by stimulation of the intrinsic GTPase activity leading to hydrolysis of GTP to GDP (Bernards 2003; Moon and Zheng 2003). In resting cells, Rho GDIs form a complex with Rho GTPases protecting them from GDP-release and—by shielding their isoprenylated hydrophobic tails from aqueous solvent—prevent them from membrane association (Olofsson 1999).
Fig. 5.
GTPase cycle. The guanine nucleotide bound to RhoA determines its activation state. Upstream regulators and downstream effector pathways are shown in relationship to guanine nucleotide binding state. See text for description. GAP = GTPase-activating protein; GDI = guanine nucleotide dissociation inhibitors; GDP = guanosine diphosphate; GEF = guanine nucleotide exchange factor; GTP = guanosine triphosphate.
RhoA GDIs
Unlike GEFs and GAPs, there are only a few GDIs known to regulate Rho GTPases: RhoGDI-1 (α), RhoGDI-2 (D4/LyGDI/β), and RhoGDI-3 (γ). In addition, there are several sequence-unrelated proteins that may exert GDI activity for Rho GTPases (Anastasiadis and others 2000). RhoGDI-1 is the most extensively studied GDI and is known to function as a GDI for RhoA, Cdc42, and Rac1. With an intracellular concentration equal to that of the GTPases themselves (Michaelson and others 2001), RhoGDI-1 is able to regulate RhoA, Cdc42, or Rac1 stoichiometrically. RhoGDI-3 has its highest affinity toward RhoB and RhoG, with reduced binding affinity for RhoA and Cdc42 (Adra and others 1997). RhoGDI-2 binds only very weakly to RhoA (Olofsson 1999), and thus is not thought to play a major role in RhoA regulation.
With its high expression level, RhoGDI-1’s binding to RhoA needs to be regulated—a function that is carried out by various modulators including Protein kinase A, which stabilizes the complex through phosphorylation of a C-terminal serine residue in RhoA (Lang and others 1996). A recently discovered Src-mediated phosphorylation of Tyr156 decreases the level of RhoA/RhoGDI-1 complex drastically and thereby presents another regulatory mechanism for Rho GTPase activity (DerMardirossian and others 2006).
In addition to regulating the activity levels of Rho GTPases, RhoGDI-1 is essential for their subcellular localization. Instead of targeting to cell membranes like the closely related RhoB, RhoA is largely cytosolic (Michaelson and others 2001). This difference in localization is attributable to the differences in RhoGDI binding. The C-terminal CAAX sequence of Rho GTPases is the site of different posttranslational modifications, including isoprenylation of the highly conserved cysteine residue (Hakoshima and others 2003). Through this hydrophobic tail, Rho GTPases are able to anchor in membranes. RhoGDIs target this anchor and sequester Rho proteins in the cytosol by folding into an immunoglobulin-like β-sandwich capable of capturing the geranylgeranyl chain of RhoA or other GTPases through hydrophobic interactions (Hoffman and others 2000; Scheffzek and others 2000; Grizot and others 2001). The different types of isoprenylation alone do not lead to distinct localizations of the GTPases. Instead, second C-terminal signals such as palmitate in RhoB (Michaelson and others 2001) or polybasic regions in RhoA (van Hennik and others 2003) have been shown to contribute.
RhoA GEFs and GAPs
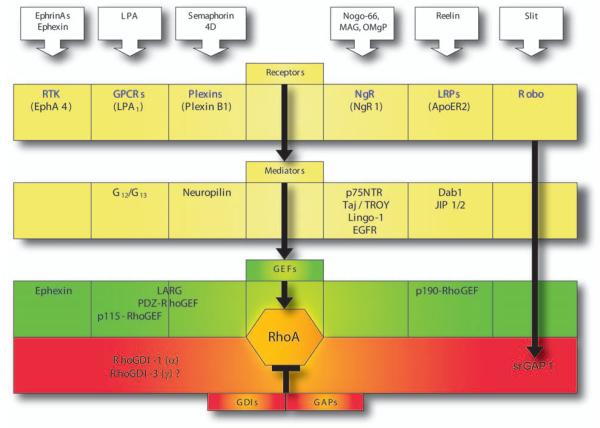
Many distinct GEFs and GAPs regulate RhoGTPase activity in the cell. Concerning RhoA, one may distinguish between GEFs/GAPs that are being activated through direct or indirect downstream effects of various ligand/receptor systems at the cell surface. To illustrate the diversity of signaling mechanisms, this review concentrates on a selected group of receptors regulating neuronal RhoA-GTPases: EphA-, ApoER2-, NgR-, amino-NogoR-, PlexinB1-, LPA1-, and Robo-receptors (Fig. 6).
Fig. 6.
RhoA activation/inactivation in neuronal tissues. Steps in receptor signaling pathways regulating neuronal RhoA are illustrated. See text for description. Dab1 = mammalian disabled 1; Jip1/2 = c-Jun N-terminal kinase (JNK) interacting proteins 1 and 2; EGFR = epidermal growth factor receptor; RTK = receptor tyrosine kinase; GPCR = G-protein-coupled receptor; Robo = roundabout receptor; NgR = Nogo receptor; OMgp = oligodendrocyte-mylein glycoprotein; MAG = myelin-associated glycoprotein; LRP = lipoprotein receptor; Larg = leukemia-associated Rho guanine; GEF = guanine nucleotide exchange factor; GDI = guanine nucleotide dissociation inhibitors; GAP = GTPase-activating protein.
RhoA Activation by Receptor Tyrosine Kinase Signaling
Several receptor tyrosine kinases (RTKs) have been shown to regulate Rho GTPase activity (Table 2). Eph receptors are prominent among RTKs in their influence on RhoA-signaling. After binding of their ephrin-ligands, Eph receptors become activated by formation of higher-order signaling clusters (Himanen and others 2001; Wimmer-Kleikamp and others 2004). They participate in the regulation of attraction/repulsion, adhesion/de-adhesion, and migration and thereby directly regulate cell fate, morphogenesis, and organogenesis during development and neuronal plasticity such as dendritic spine formation during adulthood (Klein 2004). Anchored in the plasma membrane with its transmembrane domain, an Eph receptor possesses an extracellular ligand binding domain that recognizes ephrin stimuli and an intracellular catalytic domain that transmits these signals into the cell. The extracellular domain is composed of a globular domain (ephrin-binding region), a cysteine-rich region, and Fibronectin-type III repeats. The intracellular region contains a juxtamembrane domain, a kinase domain, a SAM domain, and a PDZ-binding motif.
Table 2.
RhoA Regulating TRKs (based on Schiller 2006)
| Subgroup | Receptor | RhoA Activating GEFs | Other GEFs | Other Rho-GTPases |
|---|---|---|---|---|
| EphA | EphA3 | ? | ? | ? |
| EphA4 | Vsm, Ephexin | Vav2 → Rac1 | Rac1, RhoB | |
| INSR | IGF1R | Larg | Vav3 | |
| PDGFR | PDGFR-A/B? | ? | αPix → Rac1 | Rac1,Cdc42 |
| EGFR | EGFR | Vav2 → Rac1, Rho B | Rac1, RhoB | |
| Vav3, βPix, Sos1 α Rac1 | ||||
| P-Rex → Rac1 | ||||
| RET | RET | ? | ? | Rac1 |
| MET | MET | ? | ? | Rac1, Cdc42 |
| TIE | TEK | Sos | Rac1 | |
| VEGFR | VEGFR2 | Vav2 → Cdc42 | Rac1, Cdc42 |
GEF = guanine nucleotide exchange factor; Vsm = vascular smooth muscle; Larg = leukemia-associated Rho guanine; INSR = insulin receptor; IGF1R = insulin-like growth factor-1 receptor; PDGFR = platelet-derived growth factor receptor; EGFR = epidermal growth factor receptor; αPix = PAK-interacting exchange factor alpha; βPix = PAK-interacting exchange factor beta; Sos = son-of-sevenless; P-Rex = phosphatidylinositol (3,4,5)-trisphosphate-dependent Rac exchanger; RET = REarranged during transfection; MET = met proto-oncogene (hepatocyte growth factor receptor); TIE = tyrosine kinase with Ig and EGF homology domains; VEGFR = vascular endothelial growth factor receptor.
With overlapping expression patterns and multiple binding partners, Eph receptors’ functions are often redundant (Klein 2004). Based on sequence homologies and the membrane anchoring of ligands, these proteins are grouped as EphA receptors (type 1-8 in mammals), EphB receptors (type 1-4 and 6), ephrinA GPI-anchored ligands (type 1-5), and ephrinB transmembrane ligands (type 1-3). Whereas EphAs bind to most or all ephrinAs, EphBs bind to most or all ephrinBs. The only known exceptions to this rule are EphA4 and ephrinA5. EphA4 binds to ephrinAs and ephrinBs (Kullander and Klein 2002), and ephrinA5 binds not only to EphAs but also to EphB2 (Himanen and others 2004; Pasquale 2004). Ephrin-Eph interactions may lead to forward signaling into the Ephexpressing cell but can also lead to reverse signaling into the ephrin-expressing cell, or bi-directional signaling into both cells (Klein 2004).
Forward signaling in the Eph/ephrin pathway proceeds by one of two pathways, a direct interaction with certain GEFs such as Ephexin (see below) (Shamah and others 2001) or the initiation of a phosphorylation cascade. The first step in this cascade is the interdigitation of the fibronectin type III repeats and SAM domains of the receptors with the ligands’ amino-terminal domains to cluster a signaling complex (Lackmann and others 1998; Stapleton and others 1999; Thanos and others 1999). The receptors’ PDZ domain proteins also contribute to clustering (Himanen and others 2001). Aggregation leads to autophosphorylation of the Eph receptors on several cytoplasmic tyrosine residues (Kalo and Pasquale 1999). Initial phosphorylation disrupts inhibitory interactions between receptor juxtamembrane and kinase domain, begetting further activation of kinase activity and an open SH2-domain binding site (Binns and others 2000; Zisch and others 2000; Wybenga-Groot and others 2001). The latter then interacts with downstream signaling molecules.
The GEFs Ephexin and Vms-Rho activate RhoA downstream of EphA receptors, whereas EphB receptors signal to Rac and Cdc42 through Kalirin and Intersectin. As an EphA-GEF in neurons, Ephexin activates primarily RhoA, and to a lesser extent Cdc42 (Rodrigues and others 2000; Shamah and others 2001). Ephexin’s tandem Dbl homology domain (DH) and pleckstrin homology domain (PH) are required for the catalytic activity of Dbl family exchange factors; the amino-terminal hydrophobic region may promote membrane localization. Ephexin also contains an SH3 domain and an acidic box (Noren and Pasquale 2004). Vms-RhoGEF is closely related to ephexin by sequence and mechanisms of action but is expressed principally in EphA4-positive vascular smooth muscle cells (Shamah and others 2001; Ogita and others 2003). Vms-RhoGEF interacts with EphA4 to assemble actin stress fibers and to regulate vascular contractility. Little is known about the mechanisms by which EphA receptors activate these two GEFs. However, it has been shown that ephrin-A1/EphA4 binding leads to phosphorylation of Vms-RhoGEF tyrosine residues (Ogita and others 2003), suggesting a regulation by tyrosine phosphorylation (Noren and Pasquale 2004). Since Ephexin is a member of a family of five GEFs (Ephexin, Vms-RhoGEF, TIM/Arhgef5, Neuroblastoma, SGEF/FLJ12822), similar signaling mechanisms and functions in its close relatives are suspected. Future studies employing knockout models for these GEFs and EphAs might be helpful to answer these questions.
RhoA Activation by G-Protein Coupled Receptor–Signaling
The seven transmembrane domain G-protein coupled receptors (GPCRs) are the most common receptor type in mammalian proteomes and are known to activate G-proteins that regulate a variety of downstream effector pathways, including the RhoA signaling pathway. After binding its ligand, a GPCR promotes the exchange of GDP for GTP on a coupled heterotrimeric Gα subunit within the cell. When GTP is bound, Gα is in an activated state that dissociates from the Gβγ subunit. One or both subunits then regulate specific downstream effectors such as adenylate cyclases, phospholipases, or ion channels. In the case of RhoA signaling, relevant GPCRs couple to heterotrimeric G12 /G13 and then link to several RhoGEFs such as LARG, PDZ-RhoGEF, or p115-RhoGEF (Vogt and others 2003). These RhoGEFs are able to associate with G12 /G13 via their N-terminal “regulators of G-protein signaling” (RGS) domains (Fukuhara and others 1999; Fukuhara and others 2000).
One GPCR influencing RhoA activity is the LPA1 receptor. Its ligand, lysophosphatidic acid, induces neurite retraction and activates RhoA (Jalink and others 1993; Jalink and others 1994; Tigyi and others 1996; Kranenburg and others 1999). Inhibition of RhoA or ROCK blocks LPA-induced growth cone collapse. Interestingly, receptor tyrosine kinase inhibitors were also shown to decrease the LPA-induced retraction effect, leading to the conclusion that tyrosine kinases as well as ROCKs must be involved in the signaling cascade (Sayas and others 2006). Pyk2 is a Ca2+-sensitive tyrosine kinase known to phosphorylate/activate glycogen synthase kinase-3 (GSK-3), which acts as a multifunctional serine/threonine kinase to target microtubule-binding proteins, promoting microtubule destabilization and neurite retraction. LPA1 receptors couple through G12 /G13 to activate RhoA and also couple through Gi/Gq to phospholipase C activation, Ca2+ mobilization, and Pyk2 (Sayas and others 2006). Thus, parallel pathways ensure robust neurite retraction.
RhoA Activation by Plexin Receptor–Signaling
Plexins are transmembrane proteins that in the nervous system mediate the axon repellant actions of semaphorins. Human plexins comprise 4 major subfamilies: plexin-A-D (Tamagnone and others 1999). In general, membrane-associated semaphorins directly activate plexins. In contrast, most secreted semaphorins bind with highest affinity to neuropilins (Takahashi and others 1999). Subsequently, neuropilins complex with plexinAs to transmit signals to the cytosol (Nakamura and others 1998; Takahashi and others 1999; Waimey and Cheng 2006). Through their PDZ-interacting C termini, plexin-Bs, but not other plexins, were shown to associate with PDZ-RhoGEF and LARG (Swiercz and others 2002). Semaphorin 4D activation of plexin-B1 was found to activate PDZ-RhoGEF and LARG leading to RhoA activation and downstream signaling (Aurandt and others 2002; Perrot and others 2002; Swiercz and others 2002). For other plexins, there is evidence that coupling to Rho family GTPases occurs by direct protein/protein interactions without an intervening GEF or GAP protein (Oinuma and others 2004).
RhoA Activation by Nogo Receptor–Signaling
In the adult CNS, axonal growth is limited and myelin proteins play a role in inhibiting axonal extension. Within the neuron, axonal RhoA plays a role in mediating this inhibition. Three proteins in CNS myelin, Nogo-A, Myelin-associated glycoprotein (MAG), and oligodendrocyte-myelin glycoprotein (OMgp), are recognized as ligands for axonal receptors mediating outgrowth inhibition (McGee and Strittmatter 2003; Liu and others 2006). The axonal Nogo-66 receptor (NgR) was identified based on its ability to bind a fragment of Nogo-A (Fournier and others 2001; Hunt and others 2002). However, NgR also binds MAG (Domeniconi and others 2002; Liu and others 2002) and OMgp (Wang, Koprivica, and others 2002). Although Nogo, MAG, and OMgp do not show sequence homologies, these three proteins do compete for NgR binding (Domeniconi and others 2002; Wang, Koprivica, and others 2002), suggesting similar signaling through the same active site by using different but overlapping binding sites at Nogo receptor.
NgR is a glycosylphosphatidylinositol (GPI)-anchored membrane protein, so a transmembrane protein is thought to mediate communication to the cell interior (Fournier and others 2001). At least in some cells, the low-affinity neurotrophin receptor p75NTR serves as a signal transducing co-receptor (Wang, Kim, and others 2002). Another transmembrane protein, LINGO-1, is reported to facilitate the coupling of p75NTR to NgR (Wang, Kim, and others 2002; Wong and others 2002; Mi and others 2004), and TAJ/TROY (an orphan member of the tumor necrosis factor receptor family) may substitute for p75NTR as a co-receptor (Park and others 2005; Shao and others 2005). Several studies have confirmed that a NgR complex activates RhoA to inhibit axonal growth (Jin and Strittmatter 1997; Lehmann and others 1999; Borisoff and others 2003; Fournier and others 2003), but how the complex is coupled to RhoA is less clear. One report suggests that ligand binding to NgR suppresses a RhoGDI activity within the intracellular domain of p75NTR (Yamashita and Tohyama 2003).
RhoA Activation by Lipoprotein Receptor–Signaling
ApoER2 was identified as an LDL receptor-related gene (Takahashi and others 1992; Kim and others 1996; Novak and others 1996; Herz 2001). Although ApoER2 was initially thought to play a role in lipid metabolism, it was soon found to be expressed solely in neural and testicular tissues (Stockinger and others 1998; Stockinger and others 2000). Double knockout mice for ApoER2 and another LDL receptor family gene, VLDL (Trommsdorff and others 1999), develop profound ataxia secondary to cerebellar dysplasia. Defects of neuronal cell positioning in the forebrain resembled those in the reelin knockout mouse, “reeler,” and the Dab1 (Disabled-1) knockout mouse, “scrambler” (Falconer 1951; Sweet and others 1996). This observation led to the suggestion that ApoER2 and VLDL receptors might function as reelin receptors coupled to Dab-1 intracellularly. Binding assays of reelin with the two receptors’ extracellular domains supported this hypothesis (D’Arcangelo and others 1999; Hiesberger and others 1999). Dab1 was found to interact with the cytoplasmic NPxY motif of ApoER2 and VLDL receptors, and extracellular reelin binding led to tyrosine phosphorylation of Dab1 (D’Arcangelo and others 1999; Hiesberger and others 1999; Trommsdorff and others 1999; Hoe and others 2006).
Reelin/ApoER2/Dab-1 signaling to control cell positioning and cell survival occurs through several mechanisms (May and others 2005). Important for the coupling to RhoA activation is the binding of the c-Jun N-terminal kinase (JNK) interacting proteins 1 and 2 (Jip1/2) to an alternatively spliced, ApoER2-specific insert (May and others 2005). Jip1/2 assembles components of a MAP kinase signaling module (Yasuda and others 1999) and is thought to bind RhoGEF, which then activates RhoA (Meyer and others 1999). The interaction of JIP-1 with RhoGEF was confirmed by coimmunoprecipitation of these proteins from lysates of transiently transfected HEK 293 cells (Meyer and others 1999).
RhoA Deactivation by Roundabout (Robo) Receptor–Signaling
Roundabout proteins function as Slit receptors. Their role in midline axonal guidance is evolutionarily conserved from C elegans and Drosophila (Kidd and others 1998; Zallen and others 1998) to mouse (Taguchi and others 1996; Brose and others 1999; Yuan and others 1999) and humans (Kidd and others 1998). Transmembrane Robo proteins are composed of five immunoglobulin (Ig) domains, three extracellular fibronectin III repeats, and a large intracellular region that contains several conserved CC motifs (Kidd and others 1998; Zallen and others 1998). The Robo Ig domains bind to the leucine-rich repeat (LRR) domains of Slits (Howitt and others 2004), and the intracellular motifs are responsible for Robo-specific responses (Bashaw and Goodman 1999; Bashaw and others 2000). Robo/Slit signaling can direct both axon repulsion (Seeger and others 1993; Kidd and others 1998; Zallen and others 1998; Brose and others 1999; Challa and others 2001; Lee and others 2001) and neuronal migration (Wu and others 1999; Zhu and others 1999). Signaling by the SAX-3/Robo receptor of C elegans requires a complex of the juxtamembrane and CC1 region with the VAB-1 tyrosine kinase (Ghenea and others 2005).
Ligand binding to Robo receptors suppresses the level of the GTP-bound form of a Rho family GTPase, Cdc42. This action is mediated via slit-robo-GAP (srGAP) proteins, which contain an SH3 domain that binds to the CC3 motif in Robo and a GAP domain that inactivates Cdc42 (Wong and others 2001).
Conclusions
The Rho family GTPases regulate the actin cytoskeleton and play a multitude of roles in CNS development and function. A principal effector of RhoA activity is the ROCK enzyme, and potent inhibitors of this kinase exist. A wide variety of cell surface signaling systems impinge on the Rho/ROCK pathway. Further analysis of these pathways is likely to accelerate molecular understanding in neuroscience and provide the potential for novel therapeutics in neurology.
Acknowledgments
This work was supported by grants to SMS from the Christopher Reeve Paralysis Foundation and the NIH.
References
- Adamson P, Marshall CJ, Hall A, Tilbrook PA. Posttranslational modifications of P21 (Rho) proteins. J Biol Chem. 1992;267(28):20033–8. [PubMed] [Google Scholar]
- Adra CN, Manor D, Ko JL, Zhu S, Horiuchi T, Van Aelst L. RhoGDIgamma: a GDP-dissociation inhibitor for Rho proteins with preferential expression in brain and pancreas. Proc Natl Acad Sci U S A. 1997;94(9):4279–84. doi: 10.1073/pnas.94.9.4279. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizawa H, Wakatsuki S, Ishii A, Moriyama K, Sasaki Y, Ohashi K. Phosphorylation of cofilin by LIM-kinase is necessary for semaphorin 3A-induced growth cone collapse. Nat Neurosci. 2001;4(4):367–73. doi: 10.1038/86011. others. [DOI] [PubMed] [Google Scholar]
- Amano M, Chihara K, Nakamura N, Fukata Y, Yano T, Shibata M. Myosin II activation promotes neurite retraction during the action of Rho and Rho-kinase. Genes Cells. 1998;3(3):177–88. doi: 10.1046/j.1365-2443.1998.00181.x. others. [DOI] [PubMed] [Google Scholar]
- Amano M, Kaneko T, Maeda A, Nakayama M, Ito M, Yamauchi T. Identification of Tau and MAP2 as novel substrates of Rho-kinase and myosin phosphatase. J Neurochem. 2003;87(3):780–90. doi: 10.1046/j.1471-4159.2003.02054.x. others. [DOI] [PubMed] [Google Scholar]
- Anastasiadis PZ, Moon SY, Thoreson MA, Mariner DJ, Crawford HC, Zheng Y. Inhibition of RhoA by p120 catenin. Nat Cell Biol. 2000;2(9):637–44. doi: 10.1038/35023588. others. [DOI] [PubMed] [Google Scholar]
- Arimura N, Inagaki N, Chihara K, Menager C, Nakamura N, Amano M. Phosphorylation of collapsin response mediator protein-2 by Rho-kinase. Evidence for two separate signaling pathways for growth cone collapse. J Biol Chem. 2000;275(31):23973–80. doi: 10.1074/jbc.M001032200. others. [DOI] [PubMed] [Google Scholar]
- Aspenstrom P, Fransson A, Saras J. Rho GTPases have diverse effects on the organization of the actin filament system. Biochem J. 2004;377(2):327–37. doi: 10.1042/BJ20031041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurandt J, Vikis HG, Gutkind JS, Ahn N, Guan KL. The semaphorin receptor plexin-B1 signals through a direct interaction with the Rho-specific nucleotide exchange factor, LARG. Proc Natl Acad Sci U S A. 2002;99(19):12085–90. doi: 10.1073/pnas.142433199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashaw GJ, Goodman CS. Chimeric axon guidance receptors: the cytoplasmic domains of slit and netrin receptors specify attraction versus repulsion. Cell. 1999;97(7):917–26. doi: 10.1016/s0092-8674(00)80803-x. [DOI] [PubMed] [Google Scholar]
- Bashaw GJ, Kidd T, Murray D, Pawson T, Goodman CS. Repulsive axon guidance: Abelson and enabled play opposing roles downstream of the roundabout receptor. Cell. 2000;101(7):703–15. doi: 10.1016/s0092-8674(00)80883-1. [DOI] [PubMed] [Google Scholar]
- Bernards A. GAPs galore! A survey of putative Ras superfamily GTPase activating proteins in man and Drosophila. Biochim Biophys Acta. 2003;1603(2):47–82. doi: 10.1016/s0304-419x(02)00082-3. [DOI] [PubMed] [Google Scholar]
- Binns KL, Taylor PP, Sicheri F, Pawson T, Holland SJ. Phosphorylation of tyrosine residues in the kinase domain and juxtamembrane region regulates the biological and catalytic activities of Eph receptors. Mol Cell Biol. 2000;20(13):4791–805. doi: 10.1128/mcb.20.13.4791-4805.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenfeld J, Betz H, Roth D. Inhibition of neurite extension by overexpression of individual domains of LIM kinase 1. J Neurochem. 2001;78(4):924–7. doi: 10.1046/j.1471-4159.2001.00500.x. [DOI] [PubMed] [Google Scholar]
- Blumenstein L. Rho-Effektor-Interaktion: Struktur-Funktionsbeziehungen [Inaugural-Dissertation] Ruhr-Universitaet Bochum; Heidelberg: 2004. [Google Scholar]
- Borisoff JF, Chan CC, Hiebert GW, Oschipok L, Robertson GS, Zamboni R. Suppression of Rho-kinase activity promotes axonal growth on inhibitory CNS substrates. Mol Cell Neurosci. 2003;22(3):405–16. doi: 10.1016/s1044-7431(02)00032-5. others. [DOI] [PubMed] [Google Scholar]
- Boureux A, Vignal E, Faure S, Fort P. Evolution of the Rho family of Ras-like GTPases in eukaryotes. Mol Biol Evol. 2007;24(1):203–16. doi: 10.1093/molbev/msl145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brose K, Bland KS, Wang KH, Arnott D, Henzel W, Goodman CS. Slit proteins bind robe receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell. 1999;96(6):795–806. doi: 10.1016/s0092-8674(00)80590-5. others. [DOI] [PubMed] [Google Scholar]
- Buss JE, Quilliam LA, Kato K, Casey PJ, Solski PA, Wong G. The COOH-terminal domain of the Rap1A (Krev-1) protein is isoprenylated and supports transformation by an H-Ras:Rap1A chimeric protein. Mol Cell Biol. 1991;11(3):1523–30. doi: 10.1128/mcb.11.3.1523. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerione RA. Cdc42: new roads to travel. Trends Cell Biol. 2004;14(3):127–32. doi: 10.1016/j.tcb.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Challa AK, Beattie CE, Seeger MA. Identification and characterization of roundabout orthologs in zebrafish. Mech Dev. 2001;101(12):249–53. doi: 10.1016/s0925-4773(00)00570-0. [DOI] [PubMed] [Google Scholar]
- Chen XQ, Tan I, Ng CH, Hall C, Lim L, Leung T. Characterization of RhoA-binding kinase ROKalpha implication of the pleckstrin homology domain in ROKalpha function using region-specific antibodies. J Biol Chem. 2002;277(15):12680–8. doi: 10.1074/jbc.M109839200. [DOI] [PubMed] [Google Scholar]
- Chevrier V, Piel M, Collomb N, Saoudi Y, Frank R, Paintrand M. The Rho-associated protein kinase p160ROCK is required for centrosome positioning. J Cell Biol. 2002;157(5):807–17. doi: 10.1083/jcb.200203034. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A, Olson MF. Membrane blebbing during apoptosis results from caspasemediated activation of ROCK I. Nat Cell Biol. 2001;3(4):339–45. doi: 10.1038/35070009. [DOI] [PubMed] [Google Scholar]
- D’Arcangelo G, Homayouni R, Keshvara L, Rice DS, Sheldon M, Curran T. Reelin is a ligand for lipoprotein receptors. Neuron. 1999;24(2):471–9. doi: 10.1016/s0896-6273(00)80860-0. [DOI] [PubMed] [Google Scholar]
- Denker SP, Barber DL. Cell migration requires both ion translocation and cytoskeletal anchoring by the Na-H exchanger NHE1. J Cell Biol. 2002;159(6):1087–96. doi: 10.1083/jcb.200208050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denker SP, Huang DC, Orlowski J, Furthmayr H, Barber DL. Direct binding of the Na–H exchanger NHE1 to ERM proteins regulates the cortical cytoskeleton and cell shape independently of H (+) translocation. Mol Cell. 2000;6(6):1425–36. doi: 10.1016/s1097-2765(00)00139-8. [DOI] [PubMed] [Google Scholar]
- DerMardirossian C, Rocklin G, Seo JY, Bokoch GM. Phosphorylation of RhoGDI by Src regulates Rho GTPase binding and cytosol-membrane cycling. Mol Biol Cell. 2006;17(11):4760–8. doi: 10.1091/mbc.E06-06-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cunto F, Imarisio S, Hirsch E, Broccoli V, Bulfone A, Migheli A. Defective neurogenesis in citron kinase knockout mice by altered cytokinesis and massive apoptosis. Neuron. 2000;28(1):115–27. doi: 10.1016/s0896-6273(00)00090-8. others. [DOI] [PubMed] [Google Scholar]
- Domeniconi M, Cao ZU, Spencer T, Sivasankaran R, Wang KC, Nikulina E. Myelin-associated glycoprotein interacts with the Nogo66 receptor to inhibit neurite outgrowth. Neuron. 2002;35(2):283–90. doi: 10.1016/s0896-6273(02)00770-5. others. [DOI] [PubMed] [Google Scholar]
- Dvorsky R, Blumenstein L, Vetter IR, Ahmadian MR. Structural insights into the interaction of ROCKI with the switch regions of RhoA. J Biol Chem. 2004;279(8):7098–104. doi: 10.1074/jbc.M311911200. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420(6916):629–35. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- Falconer DS. 2 New mutants, Trembler and Reeler, with neurological actions in the house mouse (Mus-Musculus L) J Genet. 1951;50(2):192–201. doi: 10.1007/BF02996215. [DOI] [PubMed] [Google Scholar]
- Farah S, Agazie Y, Ohan N, Ngsee JK, Liu XJ. A rho-associated protein kinase, ROKalpha, binds insulin receptor substrate-1 and modulates insulin signaling. J Biol Chem. 1998;273(8):4740–6. doi: 10.1074/jbc.273.8.4740. [DOI] [PubMed] [Google Scholar]
- Fournier AE, GrandPre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001;409(6818):341–6. doi: 10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- Fournier AE, Takizawa BT, Strittmatter SM. Rho kinase inhibition enhances axonal regeneration in the injured CNS. J Neurosci. 2003;23(4):1416–23. doi: 10.1523/JNEUROSCI.23-04-01416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Gong MC, Jia T, Somlyo AV, Somlyo AP. The effects of the Rho-kinase inhibitor Y-27632 on arachidonic acid-, GTPgammaS-, and phorbol ester-induced Ca2+-sensitization of smooth muscle. FEBS Lett. 1998;440(1–2):183–7. doi: 10.1016/s0014-5793(98)01455-0. [DOI] [PubMed] [Google Scholar]
- Fujisawa K, Fujita A, Ishizaki T, Saito Y, Narumiya S. Identification of the Rho-binding domain of p160ROCK, a Rho-associated coiled-coil containing protein kinase. J Biol Chem. 1996;271(38):23022–8. doi: 10.1074/jbc.271.38.23022. [DOI] [PubMed] [Google Scholar]
- Fujita A, Hattori Y, Takeuchi T, Kamata Y, Hata F. NGF induces neurite outgrowth via a decrease in phosphorylation of myosin light chain in PC12 cells. Neuroreport. 2001;12(16):3599–602. doi: 10.1097/00001756-200111160-00045. [DOI] [PubMed] [Google Scholar]
- Fukata Y, Oshiro N, Kinoshita N, Kawano Y, Matsuoka Y, Bennett V. Phosphorylation of adducin by Rho-kinase plays a crucial role in cell motility. J Cell Biol. 1999;145(2):347–61. doi: 10.1083/jcb.145.2.347. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara S, Chikumi H, Gutkind JS. Leukemia-associated Rho guanine nucleotide exchange factor (LARG) links heterotrimeric G proteins of the G (12) family to Rho. FEBS Lett. 2000;485(2–3):183–8. doi: 10.1016/s0014-5793(00)02224-9. [DOI] [PubMed] [Google Scholar]
- Fukuhara S, Murga C, Zohar M, Igishi T, Gutkind JS. A novel PDZ domain containing guanine nucleotide exchange factor links heterotrimeric G proteins to Rho. J Biol Chem. 1999;274(9):5868–79. doi: 10.1074/jbc.274.9.5868. [DOI] [PubMed] [Google Scholar]
- Ghenea S, Boudreau JR, Lague NP, Chin-Sang ID. The VAB-1 Eph receptor tyrosine kinase and SAX-3/Robo neuronal receptors function together during C. elegans embryonic morphogenesis. Development. 2005;132(16):3679–90. doi: 10.1242/dev.01947. [DOI] [PubMed] [Google Scholar]
- Goshima Y, Nakamura F, Strittmatter P, Strittmatter SM. Collapsin-induced growth cone collapse mediated by an intracellular protein related to UNC-33. Nature. 1995;376(6540):509–14. doi: 10.1038/376509a0. [DOI] [PubMed] [Google Scholar]
- Goto H, Kosako H, Inagaki M. Regulation of intermediate filament organization during cytokinesis: possible roles of Rho-associated kinase. Microsc Res Tech. 2000;49(2):173–82. doi: 10.1002/(SICI)1097-0029(20000415)49:2<173::AID-JEMT10>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Goto H, Kosako H, Tanabe K, Yanagida M, Sakurai M, Amano M. Phosphorylation of vimentin by Rho-associated kinase at a unique amino-terminal site that is specifically phosphorylated during cytokinesis. J Biol Chem. 1998;273(19):11728–36. doi: 10.1074/jbc.273.19.11728. others. [DOI] [PubMed] [Google Scholar]
- Grizot S, Faure J, Fieschi F, Vignais PV, Dagher MC, Pebay-Peyroula E. Crystal structure of the Rac1-RhoGDI complex involved in nadph oxidase activation. Biochemistry. 2001;40(34):10007–13. doi: 10.1021/bi010288k. [DOI] [PubMed] [Google Scholar]
- Hakoshima T, Shimizu T, Maesaki R. Structural basis of the Rho GTPase signaling. J Biochem (Tokyo) 2003;134(3):327–31. doi: 10.1093/jb/mvg149. [DOI] [PubMed] [Google Scholar]
- Hancock JF, Cadwallader K, Marshall CJ. Methylation and proteolysis are essential for efficient membrane binding of prenylated p21K-ras (B) Embo J. 1991;10(3):641–6. doi: 10.1002/j.1460-2075.1991.tb07992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock JF, Paterson H, Marshall CJ. A polybasic domain or palmitoylation is required in addition to the CAAX motif to localize p21ras to the plasma membrane. Cell. 1990;63(1):133–9. doi: 10.1016/0092-8674(90)90294-o. [DOI] [PubMed] [Google Scholar]
- Hartshorne DJ, Ito M, Erdodi F. Myosin light chain phosphatase: subunit composition, interactions and regulation. J Muscle Res Cell Motil. 1998;19(4):325–41. doi: 10.1023/a:1005385302064. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Nakamura Y, Goto H, Wada Y, Sakoda S, Kaibuchi K. Domain- and site-specific phosphorylation of bovine NF-L by Rho-associated kinase. Biochem Biophys Res Commun. 1998;245(2):407–11. doi: 10.1006/bbrc.1998.8446. others. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Nakamura Y, Kosako H, Amano M, Kaibuchi K, Inagaki M. Distribution of Rho-kinase in the bovine brain. Biochem Biophys Res Commun. 1999;263(2):575–9. doi: 10.1006/bbrc.1999.1409. others. [DOI] [PubMed] [Google Scholar]
- Herz J. The LDL receptor gene family: (un)expected signal transducers in the brain. Neuron. 2001;29(3):571–81. doi: 10.1016/s0896-6273(01)00234-3. [DOI] [PubMed] [Google Scholar]
- Hiesberger T, Trommsdorff M, Howell BW, Goffinet A, Mumby MC, Cooper JA. Direct binding of Reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of disabled-1 and modulates tau phosphorylation. Neuron. 1999;24(2):481–9. doi: 10.1016/s0896-6273(00)80861-2. others. [DOI] [PubMed] [Google Scholar]
- Himanen JP, Chumley MJ, Lackmann M, Li C, Barton WA, Jeffrey PD. Repelling class discrimination: ephrin-A5 binds to and activates EphB2 receptor signaling. Nat Neurosci. 2004;7(5):501–9. doi: 10.1038/nn1237. others. [DOI] [PubMed] [Google Scholar]
- Himanen JP, Rajashankar KR, Lackmann M, Cowan CA, Henkemeyer M, Nikolov DB. Crystal structure of an Eph receptor-ephrin complex. Nature. 2001;414(6866):933–8. doi: 10.1038/414933a. [DOI] [PubMed] [Google Scholar]
- Hirokawa N. Microtubule organization and dynamics dependent on microtubule-associated proteins. Curr Opin Cell Biol. 1994;6(1):74–81. doi: 10.1016/0955-0674(94)90119-8. [DOI] [PubMed] [Google Scholar]
- Hirose M, Ishizaki T, Watanabe N, Uehata M, Kranenburg O, Moolenaar WH. Molecular dissection of the Rho-associated protein kinase (p160ROCK)-regulated neurite remodeling in neuroblastoma N1E-115 cells. J Cell Biol. 1998;141(7):1625–36. doi: 10.1083/jcb.141.7.1625. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoe HS, Tran TS, Matsuoka Y, Howell BW, Rebeck GW. DAB1 and Reelin effects on amyloid precursor protein and ApoE receptor 2 trafficking and processing. J Biol Chem. 2006;281(46):35176–85. doi: 10.1074/jbc.M602162200. [DOI] [PubMed] [Google Scholar]
- Hoffman GR, Nassar N, Cerione RA. Structure of the Rho family GTP-binding protein Cdc42 in complex with the multifunctional regulator RhoGDI. Cell. 2000;100(3):345–56. doi: 10.1016/s0092-8674(00)80670-4. [DOI] [PubMed] [Google Scholar]
- Howitt JA, Clout NJ, Hohenester E. Binding site for Robo receptors revealed by dissection of the leucine-rich repeat region of Slit. Embo J. 2004;23(22):4406–12. doi: 10.1038/sj.emboj.7600446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt D, Coffin RS, Anderson PN. The Nogo receptor, its ligands and axonal regeneration in the spinal cord; a review. J Neurocytol. 2002;31(2):93–120. doi: 10.1023/a:1023941421781. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Chihara K, Arimura N, Menager C, Kawano Y, Matsuo N. CRMP-2 induces axons in cultured hippocampal neurons. Nat Neurosci. 2001;4(8):781–2. doi: 10.1038/90476. others. [DOI] [PubMed] [Google Scholar]
- Ishizaki T, Maekawa M, Fujisawa K, Okawa K, Iwamatsu A, Fujita A. The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. Embo J. 1996;15(8):1885–93. others. [PMC free article] [PubMed] [Google Scholar]
- Izawa T, Fukata Y, Kimura T, Iwamatsu A, Dohi K, Kaibuchi K. Elongation factor-1 alpha is a novel substrate of rho-associated kinase. Biochem Biophys Res Commun. 2000;278(1):72–8. doi: 10.1006/bbrc.2000.3772. [DOI] [PubMed] [Google Scholar]
- Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–69. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- Jalink K, Eichholtz T, Postma FR, van Corven EJ, Moolenaar WH. Lysophosphatidic acid induces neuronal shape changes via a novel, receptor-mediated signaling pathway: similarity to thrombin action. Cell Growth Differ. 1993;4(4):247–55. [PubMed] [Google Scholar]
- Jalink K, van Corven EJ, Hengeveld T, Morii N, Narumiya S, Moolenaar WH. Inhibition of lysophosphatidate- and thrombin-induced neurite retraction and neuronal cell rounding by ADP ribosylation of the small GTP-binding protein Rho. J Cell Biol. 1994;126(3):801–10. doi: 10.1083/jcb.126.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z, Strittmatter SM. Rac1 mediates collapsin-1-induced growth cone collapse. J Neurosci. 1997;17(16):6256–63. doi: 10.1523/JNEUROSCI.17-16-06256.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DI. Cdc42: an essential Rho-type GTPase controlling eukaryotic cell polarity. Microbiol Mol Biol Rev. 1999;63(1):54–105. doi: 10.1128/mmbr.63.1.54-105.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalo MS, Pasquale EB. Multiple in vivo tyrosine phosphorylation sites in EphB receptors. Biochemistry. 1999;38(43):14396–408. doi: 10.1021/bi991628t. [DOI] [PubMed] [Google Scholar]
- Kamai T, Yamanishi T, Shirataki H, Takagi K, Asami H, Ito Y. Overexpression of RhoA, Rac1, and Cdc42 GTPases is associated with progression in testicular cancer. Clin Cancer Res. 2004;10(14):4799–805. doi: 10.1158/1078-0432.CCR-0436-03. others. [DOI] [PubMed] [Google Scholar]
- Katayama M, Kawata M, Yoshida Y, Horiuchi H, Yamamoto T, Matsuura Y. The posttranslationally modified C-terminal structure of bovine aortic smooth muscle rhoA p21. J Biol Chem. 1991;266(19):12639–45. others. [PubMed] [Google Scholar]
- Katoh H, Aoki J, Ichikawa A, Negishi M. p160 RhoA-binding kinase ROKalpha induces neurite retraction. J Biol Chem. 1998;273(5):2489–92. doi: 10.1074/jbc.273.5.2489. [DOI] [PubMed] [Google Scholar]
- Katoh K, Kano Y, Amano M, Onishi H, Kaibuchi K, Fujiwara K. Rho-kinase–mediated contraction of isolated stress fibers. J Cell Biol. 2001;153(3):569–84. doi: 10.1083/jcb.153.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano Y, Fukata Y, Oshiro N, Amano M, Nakamura T, Ito M. Phosphorylation of myosin-binding subunit (MBS) of myosin phosphatase by Rho-kinase in vivo. J Cell Biol. 1999;147(5):1023–38. doi: 10.1083/jcb.147.5.1023. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawata M, Farnsworth CC, Yoshida Y, Gelb MH, Glomset JA, Takai Y. Posttranslationally processed structure of the human platelet protein smg p21B: evidence for geranylgeranylation and carboxyl methylation of the C-terminal cysteine. Proc Natl Acad Sci U S A. 1990;87(22):8960–4. doi: 10.1073/pnas.87.22.8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd T, Brose K, Mitchell KJ, Fetter RD, Tessier-Lavigne M, Goodman CS. Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionarily conserved guidance receptors. Cell. 1998;92(2):205–15. doi: 10.1016/s0092-8674(00)80915-0. others. [DOI] [PubMed] [Google Scholar]
- Kim DH, Iijima H, Goto K, Sakai J, Ishii H, Kim HJ. Human apolipoprotein E receptor 2. A novel lipoprotein receptor of the low density lipoprotein receptor family predominantly expressed in brain. J Biol Chem. 1996;271(14):8373–80. doi: 10.1074/jbc.271.14.8373. others. [DOI] [PubMed] [Google Scholar]
- Kimura K, Fukata Y, Matsuoka Y, Bennett V, Matsuura Y, Okawa K. Regulation of the association of adducin with actin filaments by Rho-associated kinase (Rho-kinase) and myosin phosphatase. J Biol Chem. 1998;273(10):5542–8. doi: 10.1074/jbc.273.10.5542. others. [DOI] [PubMed] [Google Scholar]
- Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273(5272):245–8. doi: 10.1126/science.273.5272.245. others. [DOI] [PubMed] [Google Scholar]
- Klein R. Eph/ephrin signaling in morphogenesis, neural development and plasticity. Curr Opin Cell Biol. 2004;16(5):580–9. doi: 10.1016/j.ceb.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Kosako H, Amano M, Yanagida M, Tanabe K, Nishi Y, Kaibuchi K. Phosphorylation of glial fibrillary acidic protein at the same sites by cleavage furrow kinase and Rho-associated kinase. J Biol Chem. 1997;272(16):10333–6. doi: 10.1074/jbc.272.16.10333. others. [DOI] [PubMed] [Google Scholar]
- Kranenburg O, Poland M, van Horck FP, Drechsel D, Hall A, Moolenaar WH. Activation of RhoA by lysophosphatidic acid and Galpha12/13 subunits in neuronal cells: induction of neurite retraction. Mol Biol Cell. 1999;10(6):1851–7. doi: 10.1091/mbc.10.6.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullander K, Klein R. Mechanisms and functions of Eph and ephrin signalling. Nat Rev Mol Cell Biol. 2002;3(7):475–86. doi: 10.1038/nrm856. [DOI] [PubMed] [Google Scholar]
- Lackmann M, Oates AC, Dottori M, Smith FM, Do C, Power M. Distinct subdomains of the EphA3 receptor mediate ligand binding and receptor dimerization. J Biol Chem. 1998;273(32):20228–37. doi: 10.1074/jbc.273.32.20228. others. [DOI] [PubMed] [Google Scholar]
- Lane KT, Beese LS. Thematic review series: lipid posttranslational modifications. Structural biology of protein farnesyltransferase and geranylgeranyltransferase type I. J Lipid Res. 2006;47(4):681–99. doi: 10.1194/jlr.R600002-JLR200. [DOI] [PubMed] [Google Scholar]
- Lang P, Gesbert F, Delespine-Carmagnat M, Stancou R, Pouchelet M, Bertoglio J. Protein kinase A phosphorylation of RhoA mediates the morphological and functional effects of cyclic AMP in cytotoxic lymphocytes. Embo J. 1996;15(3):510–9. [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Ray R, Chien CB. Cloning and expression of three zebrafish roundabout homologs suggest roles in axon guidance and cell migration. Dev Dyn. 2001;221(2):216–30. doi: 10.1002/dvdy.1136. [DOI] [PubMed] [Google Scholar]
- Lehmann M, Fournier A, Selles-Navarro I, Dergham P, Sebok A, Leclerc N. Inactivation of Rho signaling pathway promotes CNS axon regeneration. J Neurosci. 1999;19(17):7537–47. doi: 10.1523/JNEUROSCI.19-17-07537.1999. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung KF, Baron R, Ali BR, Magee AI, Seabra MC. Rab GTPases containing a CAAX motif are processed postgeranylgeranylation by proteolysis and methylation. J Biol Chem. 2007;282(2):1487–97. doi: 10.1074/jbc.M605557200. [DOI] [PubMed] [Google Scholar]
- Leung KF, Baron R, Seabra MC. Thematic review series: lipid posttranslational modifications. Geranylgeranylation of Rab GTPases. J Lipid Res. 2006;47(3):467–75. doi: 10.1194/jlr.R500017-JLR200. [DOI] [PubMed] [Google Scholar]
- Leung T, Chen XQ, Manser E, Lim L. The p160 RhoA-binding kinase ROK alpha is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol. 1996;16(10):5313–27. doi: 10.1128/mcb.16.10.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung T, Manser E, Tan L, Lim L. A novel serine/threonine kinase binding the Ras-related RhoA GTPase which translocates the kinase to peripheral membranes. J Biol Chem. 1995;270(49):29051–4. doi: 10.1074/jbc.270.49.29051. [DOI] [PubMed] [Google Scholar]
- Liu BP, Cafferty WB, Budel SO, Strittmatter SM. Extracellular regulators of axonal growth in the adult central nervous system. Philos Trans R Soc Lond B Biol Sci. 2006;361(1473):1593–610. doi: 10.1098/rstb.2006.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu BP, Fournier A, GrandPre T, Strittmatter SM. Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. Science. 2002;297(5584):1190–3. doi: 10.1126/science.1073031. [DOI] [PubMed] [Google Scholar]
- Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 1999;285(5429):895–8. doi: 10.1126/science.285.5429.895. others. [DOI] [PubMed] [Google Scholar]
- Maltese WA, Sheridan KM. Isoprenoid modification of G25K (Gp), a low molecular mass GTP-binding protein distinct from p21ras. J Biol Chem. 1990;265(29):17883–90. [PubMed] [Google Scholar]
- Maltese WA, Sheridan KM, Repko EM, Erdman RA. Posttranslational modification of low molecular mass GTP-binding proteins by isoprenoid. J Biol Chem. 1990;265(4):2148–55. [PubMed] [Google Scholar]
- Mandelkow E, Mandelkow EM. Microtubules and microtubule-associated proteins. Curr Opin Cell Biol. 1995;7(1):72–81. doi: 10.1016/0955-0674(95)80047-6. [DOI] [PubMed] [Google Scholar]
- Matsui T, Amano M, Yamamoto T, Chihara K, Nakafuku M, Ito M. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. Embo J. 1996;15(9):2208–16. others. [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Maeda M, Doi Y, Yonemura S, Amano M, Kaibuchi K. Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J Cell Biol. 1998;140(3):647–57. doi: 10.1083/jcb.140.3.647. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May P, Herz J, Bock HH. Molecular mechanisms of lipoprotein receptor signalling. Cell Mol Life Sci. 2005;62(1920):2325–38. doi: 10.1007/s00018-005-5231-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee AW, Strittmatter SM. The Nogo-66 receptor: focusing myelin inhibition of axon regeneration. Trends Neurosci. 2003;26(4):193–8. doi: 10.1016/S0166-2236(03)00062-6. [DOI] [PubMed] [Google Scholar]
- Meyer D, Liu A, Margolis B. Interaction of c-Jun amino-terminal kinase interacting protein-1 with p190 rhoGEF and its localization in differentiated neurons. J Biol Chem. 1999;274(49):35113–8. doi: 10.1074/jbc.274.49.35113. [DOI] [PubMed] [Google Scholar]
- Mi S, Lee X, Shao ZH, Thill G, Ji BX, Relton J. LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nat Neurosci. 2004;7(3):221–8. doi: 10.1038/nn1188. others. [DOI] [PubMed] [Google Scholar]
- Michaelson D, Silletti J, Murphy G, D’Eustachio P, Rush M, Philips MR. Differential localization of Rho GTPases in live cells: regulation by hypervariable regions and RhoGDI binding. J Cell Biol. 2001;152(1):111–26. doi: 10.1083/jcb.152.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming XF, Viswambharan H, Barandier C, Ruffieux J, Kaibuchi K, Rusconi S. Rho GTPase/Rho kinase negatively regulates endothelial nitric oxide synthase phosphorylation through the inhibition of protein kinase B/Akt in human endothelial cells. Mol Cell Biol. 2002;22(24):8467–77. doi: 10.1128/MCB.22.24.8467-8477.2002. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon SY, Zheng Y. Rho GTPase-activating proteins in cell regulation. Trends Cell Biol. 2003;13(1):13–22. doi: 10.1016/s0962-8924(02)00004-1. [DOI] [PubMed] [Google Scholar]
- Mueller BK, Mack H, Teusch N. Rho kinase, a promising drug target for neurological disorders. Nat Rev Drug Discov. 2005;4(5):387–98. doi: 10.1038/nrd1719. [DOI] [PubMed] [Google Scholar]
- Nagumo H, Ikenoya M, Sakurada K, Furuya K, Ikuhara T, Hiraoka H. Rho-associated kinase phosphorylates MARCKS in human neuronal cells. Biochem Biophys Res Commun. 2001;280(3):605–9. doi: 10.1006/bbrc.2000.4179. others. [DOI] [PubMed] [Google Scholar]
- Nakagawa O, Fujisawa K, Ishizaki T, Saito Y, Nakao K, Narumiya S. ROCK-I and ROCK-II, two isoforms of Rho-associated coiled-coil forming protein serine/threonine kinase in mice. FEBS Lett. 1996;392(2):189–93. doi: 10.1016/0014-5793(96)00811-3. [DOI] [PubMed] [Google Scholar]
- Nakamura F, Tanaka M, Takahashi T, Kalb RG, Strittmatter SM. Neuropilin-1 extracellular domains mediate semaphorin D/III-induced growth cone collapse. Neuron. 1998;21(5):1093–100. doi: 10.1016/s0896-6273(00)80626-1. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Oceguera-Yanez F, Yasuda S. A new look at Rho GTPases in cell cycle: role in kinetochore-microtubule attachment. Cell Cycle. 2004;3(7):855–7. [PubMed] [Google Scholar]
- Negishi M, Katoh H. Rho family GTPases and dendrite plasticity. Neuroscientist. 2005;11(3):187–91. doi: 10.1177/1073858404268768. [DOI] [PubMed] [Google Scholar]
- Noda M, Yasuda-Fukazawa C, Moriishi K, Kato T, Okuda T, Kurokawa K. Involvement of rho in GTP gamma S-induced enhancement of phosphorylation of 20 kDa myosin light chain in vascular smooth muscle cells: inhibition of phosphatase activity. FEBS Lett. 1995;367(3):246–50. doi: 10.1016/0014-5793(95)00573-r. others. [DOI] [PubMed] [Google Scholar]
- Noren NK, Pasquale EB. Eph receptor-ephrin bidirectional signals that target Ras and Rho proteins. Cell Signal. 2004;16(6):655–66. doi: 10.1016/j.cellsig.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Novak S, Hiesberger T, Schneider WJ, Nimpf J. A new low density lipoprotein receptor homologue with 8 ligand binding repeats in brain of chicken and mouse. J Biol Chem. 1996;271(20):11732–6. doi: 10.1074/jbc.271.20.11732. [DOI] [PubMed] [Google Scholar]
- Ogita H, Kunimoto S, Kamioka Y, Sawa H, Masuda M, Mochizuki N. EphA4-mediated Rho activation via Vsm-RhoGEF expressed specifically in vascular smooth muscle cells. Circ Res. 2003;93(1):23–31. doi: 10.1161/01.RES.0000079310.81429.C8. [DOI] [PubMed] [Google Scholar]
- Ohashi K, Nagata K, Maekawa M, Ishizaki T, Narumiya S, Mizuno K. Rho-associated kinase ROCK activates LIM-kinase 1 by phosphorylation at threonine 508 within the activation loop. J Biol Chem. 2000;275(5):3577–82. doi: 10.1074/jbc.275.5.3577. [DOI] [PubMed] [Google Scholar]
- Oinuma I, Ishikawa Y, Katoh H, Negishi M. The Semaphorin 4D receptor Plexin-B1 is a GTPase activating protein for R-Ras. Science. 2004;305(5685):862–5. doi: 10.1126/science.1097545. [DOI] [PubMed] [Google Scholar]
- Olofsson B. Rho guanine dissociation inhibitors: pivotal molecules in cellular signalling. Cell Signal. 1999;11(8):545–54. doi: 10.1016/s0898-6568(98)00063-1. [DOI] [PubMed] [Google Scholar]
- Palmer RH, Ridden J, Parker PJ. Identification of multiple, novel, protein kinase C-related gene products. FEBS Lett. 1994;356(1):5–8. doi: 10.1016/0014-5793(94)01202-4. [DOI] [PubMed] [Google Scholar]
- Parish CA, Smrcka AV, Rando RR. Functional significance of beta gamma-subunit carboxymethylation for the activation of phospholipase C and phosphoinositide 3-kinase. Biochemistry. 1995;34(23):7722–7. doi: 10.1021/bi00023a019. [DOI] [PubMed] [Google Scholar]
- Park JB, Yiu G, Kaneko S, Wang J, Chang JF, He XLL. A TNF receptor family member, TROY, is a coreceptor with nogo receptor in mediating the inhibitory activity of myelin inhibitors. Neuron. 2005;45(3):345–51. doi: 10.1016/j.neuron.2004.12.040. others. [DOI] [PubMed] [Google Scholar]
- Pasquale EB. Eph-ephrin promiscuity is now crystal clear. Nat Neurosci. 2004;7(5):417–18. doi: 10.1038/nn0504-417. [DOI] [PubMed] [Google Scholar]
- Perez-Sala D, Gilbert BA, Tan EW, Rando RR. Prenylated protein methyltransferases do not distinguish between farnesylated and geranylgeranylated substrates. Biochem J. 1992;284(Pt 3):835–40. doi: 10.1042/bj2840835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrot V, Vazquez-Prado J, Gutkind JS. Plexin B regulates Rho through the guanine nucleotide exchange factors leukemia-associated rho GEF (LARG) and PDZ-RhoGEF. J Biol Chem. 2002;277(45):43115–20. doi: 10.1074/jbc.M206005200. [DOI] [PubMed] [Google Scholar]
- Proud CG. A sharper instrument for dissecting signalling events: a specific AGC kinase inhibitor. Biochem J. 2007;401(1):e1–3. doi: 10.1042/BJ20061691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006;16(10):522–9. doi: 10.1016/j.tcb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Riento K, Guasch RM, Garg R, Jin B, Ridley AJ. RhoE binds to ROCK I and inhibits downstream signaling. Mol Cell Biol. 2003;23(12):4219–29. doi: 10.1128/MCB.23.12.4219-4229.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4(6):446–56. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- Rodrigues NR, Theodosiou AM, Nesbit MA, Campbell L, Tandle AT, Saranath D. Characterization of Ngef, a novel member of the Dbl family of genes expressed predominantly in the caudate nucleus. Genomics. 2000;65(1):53–61. doi: 10.1006/geno.2000.6138. others. [DOI] [PubMed] [Google Scholar]
- Sayas CL, Ariaens A, Ponsioen B, Moolenaar WH. GSK-3 is activated by the tyrosine kinase Pyk2 during LPA1-mediated neurite retraction. Mol Biol Cell. 2006;17(4):1834–44. doi: 10.1091/mbc.E05-07-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffzek K, Stephan I, Jensen ON, Illenberger D, Gierschik P. The Rac-RhoGDI complex and the structural basis for the regulation of Rho proteins by RhoGDI. Nat Struct Biol. 2000;7(2):122–6. doi: 10.1038/72392. [DOI] [PubMed] [Google Scholar]
- Schiller MR. Coupling receptor tyrosine kinases to Rho GTPases-GEFs what’s the link. Cell Signal. 2006;18(11):1834–43. doi: 10.1016/j.cellsig.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Hall A. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 2002;16(13):1587–609. doi: 10.1101/gad.1003302. [DOI] [PubMed] [Google Scholar]
- Seabra MC, Reiss Y, Casey PJ, Brown MS, Goldstein JL. Protein farnesyltransferase and geranylgeranyltransferase share a common alpha subunit. Cell. 1991;65(3):429–34. doi: 10.1016/0092-8674(91)90460-g. [DOI] [PubMed] [Google Scholar]
- Sebbagh M, Renvoize C, Hamelin J, Riche N, Bertoglio J, Breard J. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat Cell Biol. 2001;3(4):346–52. doi: 10.1038/35070019. [DOI] [PubMed] [Google Scholar]
- Seeger M, Tear G, Ferres-Marco D, Goodman CS. Mutations affecting growth cone guidance in Drosophila: genes necessary for guidance toward or away from the midline. Neuron. 1993;10(3):409–26. doi: 10.1016/0896-6273(93)90330-t. [DOI] [PubMed] [Google Scholar]
- Shamah SM, Lin MZ, Goldberg JL, Estrach S, Sahin M, Hu L. EphA receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell. 2001;105(2):233–44. doi: 10.1016/s0092-8674(01)00314-2. others. [DOI] [PubMed] [Google Scholar]
- Shao ZH, Browning JL, Lee XH, Scott ML, Shulga-Morskaya S, Allaire N. TAJ/TROY, an orphan TNF receptor family member, binds Nogo-66 receptor 1 and regulates axonal regeneration. Neuron. 2005;45(3):353–9. doi: 10.1016/j.neuron.2004.12.050. others. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Ihara K, Maesaki R, Amano M, Kaibuchi K, Hakoshima T. Parallel coiled-coil association of the RhoA-binding domain in Rho-kinase. J Biol Chem. 2003;278(46):46046–51. doi: 10.1074/jbc.M306458200. [DOI] [PubMed] [Google Scholar]
- Shirao S, Kashiwagi S, Sato M, Miwa S, Nakao F, Kurokawa T. Sphingosylphosphorylcholine is a novel messenger for Rho-kinase-mediated Ca2+ sensitization in the bovine cerebral artery: unimportant role for protein kinase C. Circ Res. 2002;91(2):112–9. doi: 10.1161/01.res.0000026057.13161.42. others. [DOI] [PubMed] [Google Scholar]
- Silvius JR, l’Heureux F. Fluorimetric evaluation of the affinities of isoprenylated peptides for lipid bilayers. Biochemistry. 1994;33(10):3014–22. doi: 10.1021/bi00176a034. [DOI] [PubMed] [Google Scholar]
- Sin WC, Chen XQ, Leung T, Lim L. RhoA-binding kinase alpha translocation is facilitated by the collapse of the vimentin intermediate filament network. Mol Cell Biol. 1998;18(11):6325–39. doi: 10.1128/mcb.18.11.6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol. 2000;522(Pt 2):177–85. doi: 10.1111/j.1469-7793.2000.t01-2-00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanyon CA, Bernard O. LIM-kinase1. Int J Biochem Cell Biol. 1999;31(34):389–94. doi: 10.1016/s1357-2725(98)00116-2. [DOI] [PubMed] [Google Scholar]
- Stapleton D, Balan I, Pawson T, Sicheri F. The crystal structure of an Eph receptor SAM domain reveals a mechanism for modular dimerization. Nat Struct Biol. 1999;6(1):44–9. doi: 10.1038/4917. [DOI] [PubMed] [Google Scholar]
- Stephenson RC, Clarke S. Identification of a C-terminal protein carboxyl methyltransferase in rat liver membranes utilizing a synthetic farnesyl cysteine-containing peptide substrate. J Biol Chem. 1990;265(27):16248–54. [PubMed] [Google Scholar]
- Stockinger W, Brandes C, Fasching D, Hermann M, Gotthardt M, Herz J. The reelin receptor ApoER2 recruits JNK-interacting proteins-1 and -2. J Biol Chem. 2000;275(33):25625–32. doi: 10.1074/jbc.M004119200. others. [DOI] [PubMed] [Google Scholar]
- Stockinger W, Hengstschlager-Ottnad E, Novak S, Matus A, Huttinger M, Bauer J. The low density lipoprotein receptor gene family. Differential expression of two alpha2-macroglobulin receptors in the brain. J Biol Chem. 1998;273(48):32213–21. doi: 10.1074/jbc.273.48.32213. others. [DOI] [PubMed] [Google Scholar]
- Sumi T, Matsumoto K, Nakamura T. Specific activation of LIM kinase 2 via phosphorylation of threonine 505 by ROCK, a Rho-dependent protein kinase. J Biol Chem. 2001;276(1):670–6. doi: 10.1074/jbc.M007074200. [DOI] [PubMed] [Google Scholar]
- Sweet HO, Bronson RT, Johnson KR, Cook SA, Davisson MT. Scrambler, a new neurological mutation of the mouse with abnormalities of neuronal migration. Mammal Genome. 1996;7(11):798–802. doi: 10.1007/s003359900240. [DOI] [PubMed] [Google Scholar]
- Swiercz JM, Kuner R, Behrens J, Offermanns S. Plexin-B1 directly interacts with PDZ-RhoGEF/LARG to regulate RhoA and growth cone morphology. Neuron. 2002;35(1):51–63. doi: 10.1016/s0896-6273(02)00750-x. [DOI] [PubMed] [Google Scholar]
- Taguchi A, Wanaka A, Mori T, Matsumoto K, Imai Y, Tagaki T. Molecular cloning of novel leucine-rich repeat proteins and their expression in the developing mouse nervous system. Mol Brain Res. 1996;35(1–2):31–40. doi: 10.1016/0169-328x(95)00178-u. others. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Kawarabayasi Y, Nakai T, Sakai J, Yamamoto T. Rabbit very low density lipoprotein receptor: a low density lipoprotein receptor-like protein with distinct ligand specificity. Proc Natl Acad Sci U S A. 1992;89(19):9252–6. doi: 10.1073/pnas.89.19.9252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Fournier A, Nakamura F, Wang LH, Murakami Y, Kalb RG. Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell. 1999;99(1):59–69. doi: 10.1016/s0092-8674(00)80062-8. others. [DOI] [PubMed] [Google Scholar]
- Tamagnone L, Artigiani S, Chen H, He Z, Ming Gl, Song H. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell. 1999;99(1):71–80. doi: 10.1016/s0092-8674(00)80063-x. others. [DOI] [PubMed] [Google Scholar]
- Thanos CD, Goodwill KE, Bowie JU. Oligomeric structure of the human EphB2 receptor SAM domain. Science. 1999;283(5403):833–6. doi: 10.1126/science.283.5403.833. [DOI] [PubMed] [Google Scholar]
- Tigyi G, Fischer DJ, Sebok A, Yang C, Dyer DL, Miledi R. Lysophosphatidic acid-induced neurite retraction in PC12 cells: control by phosphoinositide-Ca2+ signaling and Rho. J Neurochem. 1996;66(2):537–48. doi: 10.1046/j.1471-4159.1996.66020537.x. [DOI] [PubMed] [Google Scholar]
- Tominaga T, Barber DL. Na-H exchange acts downstream of RhoA to regulate integrin-induced cell adhesion and spreading. Mol Biol Cell. 1998;9(8):2287–303. doi: 10.1091/mbc.9.8.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trommsdorff M, Gotthardt M, Hiesberger T, Shelton J, Stockinger W, Nimpf J. Reeler/disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell. 1999;97(6):689–701. doi: 10.1016/s0092-8674(00)80782-5. others. [DOI] [PubMed] [Google Scholar]
- Van Aelst L, D’Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11(18):2295–322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- van Hennik PB, ten Klooster JP, Halstead JR, Voermans C, Anthony EC, Divecha N. The C-terminal domain of Rac1 contains two motifs that control targeting and signaling specificity. J Biol Chem. 2003;278(40):39166–75. doi: 10.1074/jbc.M307001200. others. [DOI] [PubMed] [Google Scholar]
- Vardouli L, Moustakas A, Stournaras C. LIM-kinase 2 and cofilin phosphorylation mediate actin cytoskeleton reorganization induced by transforming growth factor-beta. J Biol Chem. 2005;280(12):11448–57. doi: 10.1074/jbc.M402651200. [DOI] [PubMed] [Google Scholar]
- Vogt S, Grosse R, Schultz G, Offermanns S. Receptor-dependent RhoA activation in G12/G13-deficient cells: genetic evidence for an involvement of Gq/G11. J Biol Chem. 2003;278(31):28743–9. doi: 10.1074/jbc.M304570200. [DOI] [PubMed] [Google Scholar]
- Volker C, Lane P, Kwee C, Johnson M, Stock J. A single activity carboxyl methylates both farnesyl and geranylgeranyl cysteine residues. FEBS Lett. 1991;295(1–3):189–94. doi: 10.1016/0014-5793(91)81415-5. [DOI] [PubMed] [Google Scholar]
- Voncken JW, van Schaick H, Kaartinen V, Deemer K, Coates T, Landing B. Increased neutrophil respiratory burst in bcr-null mutants. Cell. 1995;80(5):719–28. doi: 10.1016/0092-8674(95)90350-x. others. [DOI] [PubMed] [Google Scholar]
- Waimey KE, Cheng HJ. Axon pruning and synaptic development: how are they per-plexin? Neuroscientist. 2006;12(5):398–409. doi: 10.1177/1073858406292631. [DOI] [PubMed] [Google Scholar]
- Wang KC, Kim JA, Sivasankaran R, Segal R, He ZG. p75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature. 2002;420(6911):74–8. doi: 10.1038/nature01176. [DOI] [PubMed] [Google Scholar]
- Wang KC, Koprivica V, Kim JA, Sivasankaran R, Guo Y, Neve RL. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002;417(6892):941–4. doi: 10.1038/nature00867. others. [DOI] [PubMed] [Google Scholar]
- Ward Y, Yap SF, Ravichandran V, Matsumura F, Ito M, Spinelli B. The GTP binding proteins Gem and Rad are negative regulators of the Rho-Rho kinase pathway. J Cell Biol. 2002;157(2):291–302. doi: 10.1083/jcb.200111026. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Roberts W, Wang L, Yamada M, Zhang S, Zhao Z. Rho kinases play an obligatory role in vertebrate embryonic organogenesis. Development. 2001;128(15):2953–62. doi: 10.1242/dev.128.15.2953. others. [DOI] [PubMed] [Google Scholar]
- Wennerberg K, Der CJ. Rho-family GTPases: it’s not only Rac and Rho (and I like it) J Cell Sci. 2004;117(Pt 8):1301–12. doi: 10.1242/jcs.01118. [DOI] [PubMed] [Google Scholar]
- Wimmer-Kleikamp SH, Janes PW, Squire A, Bastiaens PI, Lackmann M. Recruitment of Eph receptors into signaling clusters does not require ephrin contact. J Cell Biol. 2004;164(5):661–6. doi: 10.1083/jcb.200312001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder SJ, Walsh MP. Smooth muscle calponin. Inhibition of actomyosin MgATPase and regulation by phosphorylation. J Biol Chem. 1990;265(17):10148–55. [PubMed] [Google Scholar]
- Wolfrum S, Dendorfer A, Rikitake Y, Stalker TJ, Gong Y, Scalia R. Inhibition of Rho-kinase leads to rapid activation of phosphatidylinositol 3-kinase/protein kinase Akt and cardiovascular protection. Arterioscler Thromb Vasc Biol. 2004;24(10):1842–7. doi: 10.1161/01.ATV.0000142813.33538.82. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K, Ren XR, Huang YZ, Xie Y, Liu G, Saito H. Signal transduction in neuronal migration: roles of GTPase activating proteins and the small GTPase Cdc42 in the Slit-Robo pathway. Cell. 2001;107(2):209–21. doi: 10.1016/s0092-8674(01)00530-x. others. [DOI] [PubMed] [Google Scholar]
- Wong ST, Henley JR, Kanning KC, Huang KH, Bothwell M, Poo M. A p75 (NTR) and Nogo receptor complex mediates repulsive signaling by myelin-associated glycoprotein. Nat Neurosci. 2002;5(12):1302–8. doi: 10.1038/nn975. [DOI] [PubMed] [Google Scholar]
- Wu W, Wong K, Chen JH, Jiang ZH, Dupuls S, Wu JY. Directional guidance of neuronal migration in the olfactory system by the protein Slit. Nature. 1999;400(6742):331–6. doi: 10.1038/22477. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wybenga-Groot LE, Baskin B, Ong SH, Tong J, Pawson T, Sicheri F. Structural basis for autoinhibition of the Ephb2 receptor tyrosine kinase by the unphosphorylated juxtamembrane region. Cell. 2001;106(6):745–57. doi: 10.1016/s0092-8674(01)00496-2. [DOI] [PubMed] [Google Scholar]
- Yamane HK, Farnsworth CC, Xie HY, Evans T, Howald WN, Gelb MH. Membrane-binding domain of the small G protein G25K contains an S- (all-trans-geranylgeranyl) cysteine methyl ester at its carboxyl terminus. Proc Natl Acad Sci U S A. 1991;88(1):286–90. doi: 10.1073/pnas.88.1.286. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro S, Totsukawa G, Yamakita Y, Sasaki Y, Madaule P, Ishizaki T. Citron kinase, a Rho-dependent kinase, induces di-phosphorylation of regulatory light chain of myosin II. Mol Biol Cell. 2003;14(5):1745–56. doi: 10.1091/mbc.E02-07-0427. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Tohyama M. The p75 receptor acts as a displacement factor that releases Rho from Rho-GDI. Nat Neurosci. 2003;6(5):435–6. doi: 10.1038/nn1045. [DOI] [PubMed] [Google Scholar]
- Yamazaki M, Miyazaki H, Watanabe H, Sasaki T, Maehama T, Frohman MA. Phosphatidylinositol 4-phosphate 5-kinase is essential for ROCK-mediated neurite remodeling. J Biol Chem. 2002;277(19):17226–30. doi: 10.1074/jbc.M109795200. others. [DOI] [PubMed] [Google Scholar]
- Yasuda J, Whitmarsh AJ, Cavanagh J, Sharma M, Davis RJ. The JIP group of mitogen-activated protein kinase scaffold proteins. Mol Cell Biol. 1999;19(10):7245–54. doi: 10.1128/mcb.19.10.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan WL, Zhou LJ, Chen TH, Wu JY, Rao Y, Ornitz DM. The mouse SLIT family: secreted ligands for ROBO expressed in patterns that suggest a role in morphogenesis and axon guidance. Dev Biol. 1999;212(2):290–306. doi: 10.1006/dbio.1999.9371. [DOI] [PubMed] [Google Scholar]
- Zallen JA, Yi BA, Bargmann CI. The conserved immunoglobulin superfamily member SAX-3/Robo directs multiple aspects of axon guidance in C-elegans. Cell. 1998;92(2):217–27. doi: 10.1016/s0092-8674(00)80916-2. [DOI] [PubMed] [Google Scholar]
- Zhao ZS, Manser E. PAK and other Rho-associated kinases—effectors with surprisingly diverse mechanisms of regulation. Biochem J. 2005;386(Pt 2):201–14. doi: 10.1042/BJ20041638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Li H, Zhou L, Wu JY, Rao Y. Cellular and molecular guidance of GABAergic neuronal migration from an extracortical origin to the neocortex. Neuron. 1999;23(3):473–85. doi: 10.1016/s0896-6273(00)80801-6. [DOI] [PubMed] [Google Scholar]
- Zisch AH, Pazzagli C, Freeman AL, Schneller M, Hadman M, Smith JW. Replacing two conserved tyrosines of the EphB2 receptor with glutamic acid prevents binding of SH2 domains without abrogating kinase activity and biological responses. Oncogene. 2000;19(2):177–87. doi: 10.1038/sj.onc.1203304. others. [DOI] [PubMed] [Google Scholar]
- Zohn IM, Campbell SL, Khosravi-Far R, Rossman KL, Der CJ. Rho family proteins and Ras transformation: the RHOad less traveled gets congested. Oncogene. 1998;17(11 Reviews):1415–38. doi: 10.1038/sj.onc.1202181. [DOI] [PubMed] [Google Scholar]