Abstract
Objective
To evaluate the clinical outcome of a new sutureless approach for a temporary amniotic membrane patch (ProKera; Bio-Tissue, Inc, Miami, Florida) in eyes with acute burns.
Methods
Retrospective review of 5 eyes of 5 patients with grades I to III acute alkaline burns, receiving ProKera insertion within 8 days of injury.
Results
These eyes had either total (2 cases) or extensive (60%–75%, 3 cases) corneal epithelial defects with limbal (120°–360°) and conjunctival (30%–60%) epithelial defects. ProKera was inserted within a mean (SD) of 3.7 (3.1) days after burn and repeated 1 to 3 times for 3 cases. Conjunctival defects reepithelialized in 8.2 (5) days (range, 5–17 days), while limbal and corneal defects healed in 13.6 (8.3) days (range, 5–25 days). The latter was completed with circumferential closure of limbal defects followed by centripetal healing of corneal defects. In 3 eyes, early peripheral corneal neovascularization was followed by marked regression on completion of healing. During 16.8 (10.8) months of follow-up, all eyes retained a stable surface with improved corneal clarity, and without limbal deficiency or symblepharon.
Conclusion
This sutureless application of an amniotic membrane patch allows for early delivery of its biologic actions, which may help preserve remaining limbal stem cells for rapid expansion and prevent late cicatricial complications in eyes with mild and moderate acute alkaline burns.
Chemical injury is one of the most difficult ocular emergencies physicians face. The prognosis for an injured eye depends not only on the severity of injury, but also on the rapidity and mode of treatment. Conventional acute stage management is focused on promoting epithelialization and reducing inflammation to prevent progressive tissue melting in the acute phase and cicatricial complications in the chronic phase.1 Various medical and surgical therapies have been proposed to achieve this objective. These include topical and systemic uses of ascorbate, citrate, tetracycline, progesterone, and steroids, application of a glued-on hard contact lens, tenonplasty to correct severe ischemia, oral mucosa graft, and application of amniotic membrane patch (AMP).2–9
Clinically, transplantation of amniotic membrane (AM) as a permanent surgical graft has been shown to promote epithelialization, and reduce inflammation, scarring, and neovascularization.10–12 Amniotic membrane has also been used as a temporary patch or biological bandage for acute chemical burns, with overall encouraging results.9,13–21 It has been demonstrated that early intervention with AMP in mild or moderate chemical burns results in a marked reduction of symptoms, rapid restoration of the ocular surface, and improved visual acuity, while preventing cicatricial complications in the chronic stage. However, because sutures were required in AMP, it could only be performed in the operating room, resulting in relatively high costs and potentially unnecessary delays that might affect the clinical outcome. Furthermore, multiple sessions of AMP may be required for a single eye with acute chemical burn, owing to a relatively long course of ocular surface healing.13,20,21 Therefore, a simple bedside or office method of AMP is needed to obviate these problems.
ProKera (Bio-Tissue, Inc, Miami, Florida) is a class II medical device approved by the Food and Drug Administration in 2003 to be used as a temporary AMP for delivering the biological actions of AM to the corneal surface without using sutures. It contains a piece of cryopreserved AM clipped into a concave poly-carbonate dual-ring system, like a symblepharon ring, that conforms to the corneal and limbal surface like a contact lens (Figure 1A). The ring system has an inner diameter of 15 or 16 mm. Because ProKera can be easily inserted, removed, and reapplied in the office or bedside without sutures, we used it in 5 patients with acute alkaline burns. Here, the results of this intervention and the unique healing patterns observed in these patients are reported.
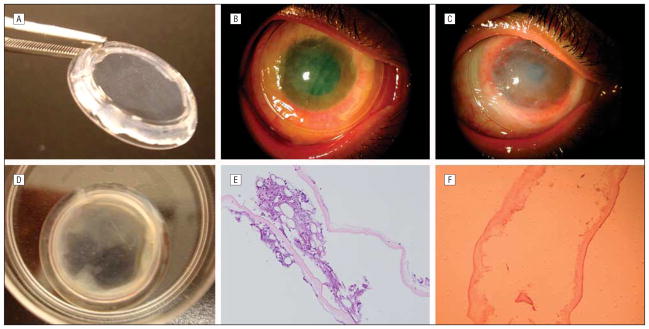
Figure 1.
Temporary sutureless amniotic membrane (AM) patch for acute alkaline burn. A, ProKera (Bio-Tissue, Inc, Miami, Florida) contains a piece of AM clipped into a concave dual-ring system, like a symblepharon ring, and conforms to the cornea like a contact lens. B, In this eye with grade II acute alkaline burn (case 4), ProKera was easily inserted into the patient’s eye in the office. Five days later, although there was marked reduction of conjunctival inflammation under ProKera, the membrane itself before (C) and after (D) removal showed cloudiness due to accumulation of inflammatory debris. E, Histopathologic examination of this cloudy AM showed entrapment of acute inflammatory cells in the AM stroma. F, With progressive healing of the ocular surface, subsequent membranes showed a marked decrease in inflammatory cells.
METHODS
We retrospectively reviewed 5 eyes of 5 patients (3 men and 2 women), with a mean (SD) age of 30.6 (18.3) years (range, 3–53 years), whose clinical characteristics are summarized in the Table. All patients received insertion of ProKera within a mean (SD) interval of 3.7 (3.1) days (range, 16 hours to 8 days) following the onset of chemical burn. The injuries were unilateral in 2 patients (cases 1 and 2) and bilateral in 3 patients (cases 3, 4, and 5). Because of asymmetric involvement in the 3 bilateral cases, the eyes with less involvement were managed medically and only the eyes with more severe involvement were included. The burns were caused by alkaline agents in all 5 patients (Table).
Table.
Clinical Characteristics and Outcomes of Temporary Sutureless Amniotic Membrane Patch in Patients With Acute Alkaline Burn
| Patient No./Sex/Age, y/Eye | Agent | Grade | Key Findings | Time Between Burn and AMP | Day(s)of Change or Removal of ProKeraa | Reepithelialization Time, d | Visual Acuity, Before (After) | Clinical Outcome | Follow-up, mo |
|---|---|---|---|---|---|---|---|---|---|
| 1/F/53/L | Bleach | I | Co: Clear, 70% ED Li: 120° ED, no ischemia Cj: 30% ED, severe inflammation |
16 h | 3 | Co: 5 Cj: 5 |
20/50 (20/20) | Co: Clear Li: No LSCD Cj: No symblepharon |
28 |
| 2/M/32/R | Lime | II | Co: mild haze, 60% ED Li: 150° ED, 75° ischemia Cj: 30% ED, moderate inflammation |
2 d | 6 | Co: 6 Cj: 6 |
20/30 (20/20) | Co: Clear Li: No LSCD Cj: No symblepharon |
9 |
| 3/M/38/L | Caustic soda | II | Co: 100% ED, moderate haze Li: 360° ED, no ischemia Cj: 50% ED, moderate inflammation |
2 d | 6, 11, 17, 21 | Co: 25 Cj: 6 |
20/400 (20/200)b | Co: Faint central haziness, stationary inferior superficial NV Li: No LSCD Cj: No symblepharon |
7 |
| 4/F/27/R | Floor cleaner | II | Co: 100% ED, mild haze Li: 360° ED, 75° ischemia Cj: 60% ED, moderate inflammation |
8 d | 5, 10, 15 | Co: 17 Cj: 17 |
20/200 (20/25) | Co: Faint central haziness, stationary peripheral NV Li: No LSCD Cj: Localized early inflammation and granulation outside ProKera resolved by steroid injection, no symblepharon |
29 |
| 5/M/3/R | Cement | III | Co: 75% ED, severe haze Li: 140° ED, 150° ischemia Cj: 30% ED, moderate inflammation, early symblepharon (x2) |
4 d | 7, 14 | Co: 15 Cj: 7 |
CSM (CSM) | Co: Faint inferior haziness, stationary inferior superficial NV Li: No LSCD Cj: No symblepharon |
11 |
Abbreviations: AMP, amniotic membrane patch; Cj, conjunctiva; Co, cornea; CSM, central steady maintain; ED, epithelial defect; F, female; L, left; Li, limbus; LSCD, limbal stem cell deficiency; M, male; NV, neovascularization; R, right.
Bio-Tissue, Inc, Miami, Florida.
Amblyopic eye.
On presentation, patients complained of significant pain, light sensitivity, and blurred vision. Corneal epithelial defects were total (cases 2 and 3) or extensive (60%–75%, cases 1, 4, and 5), and accompanied by various degrees of epithelial defect in the limbal (120°–360°) and conjunctival (30%–60%) regions (Table) (Figures 2 and 3). In addition, 3 eyes had limbal ischemia ranging from 75° to 150°. The severity was classified as grade I (n=1), grade II (n=3), and grade III (n=1), based on the criteria defined by Roper-Hall.22
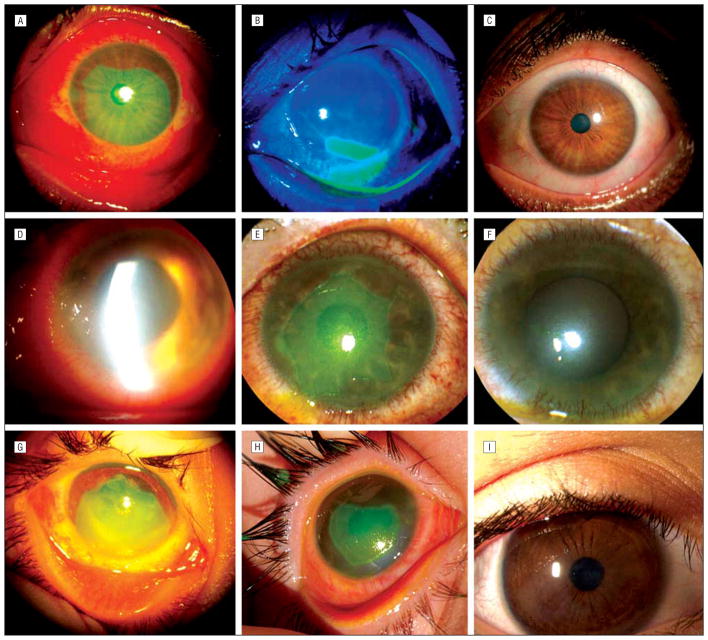
Figure 2.
Outcome of sutureless amniotic membrane patch in grades I to III of acute alkaline burn. Case 1, grade I, with extensive corneal, limbal, and conjunctival epithelial defects (A) showed marked circumferential and centripetal reepithelialization 3 days after insertion of ProKera (Bio-Tissue, Inc, Miami, Florida) (B), and a smooth and stable surface 2 weeks later (C). Case 3, grade II, with total corneal, limbal, and extensive perilimbal conjunctival epithelial defects (D) showed significant improvement of corneal edema, complete epithelialization of the conjunctival defect, closure of the limbal defect by circumferential movement 6 days after the insertion (E), and a smooth and stable ocular surface 25 days later (F). Case 5, grade III, with extensive surface defects and limbal ischemia (G) showed healing of the conjunctival defect and the closure of limbal epithelial defect 7 days after insertion (H), and a stable surface with faint corneal haziness 11 months later (I).
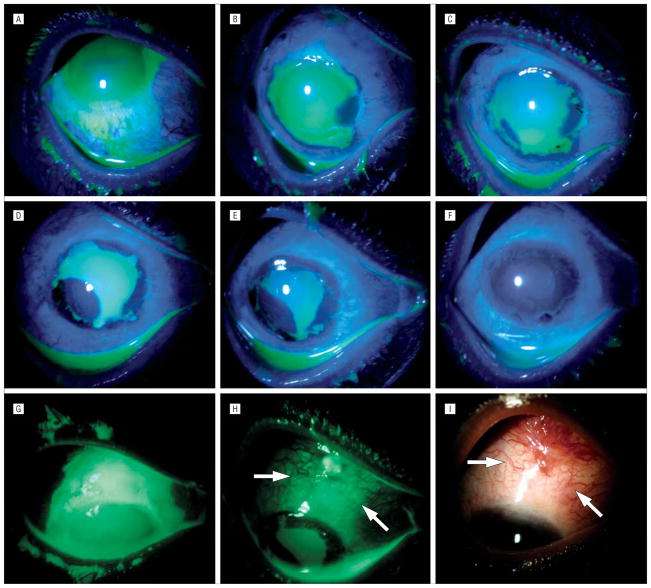
Figure 3.
Unique healing pattern under ProKera (Bio-Tissue, Inc, Miami, Florida). Case 4, grade II, with superior limbal ischemia, total corneal and limbal epithelial defects, and associated perilimbal conjunctival epithelial defect (A and G), showed rapid perilimbal conjunctival healing and epithelialization of the cornea from the 4-o’clock position 5 days after insertion of ProKera (B). At day 7, the expanded epithelial mass at the 4-o’clock position had circumferentially moved to the limbal region while a limbal epithelial mass emerged from the 7-o’clock position where the conjunctival defect had closed (C). At day 10, the epithelial mass from the 7-o’clock position had enlarged (D), and again moved circumferentially toward the limbal region at day 12 (E). At day 17, the corneal surface was completely healed (F). A large conjunctival epithelial defect that extended to the superior bulbar area was noted before amniotic membrane patching (G). This defect was not healed by day 14 in the area outside of ProKera (H, arrows mark the skirt), and by day 17 had evolved into a granulation tissue where hyperemic blood vessels emanated from the fornix but not from the amniotic membrane–covered limbus (I).
All patients had initially been treated with conventional medical therapies, including saline/water irrigation, removal of remaining particulate materials, topical antibiotics, lubricants, steroids and cycloplegics, oral vitamin C, doxycycline, or a combination thereof. Because of persistent and/or extensive epithelial breakdown of the ocular surface without any sign of healing, all patients were given detailed information about the clinical course of chemical burns of the eye, alternative treatments, and the advantages and disadvantages of ProKera insertion. Institutional review board approval was obtained from Baptist Hospital of Miami/South Miami Hospital, Inc (Florida) for cases treated in the Ocular Surface Center (Miami, Florida).
After written consent or assent was obtained, ProKera, in a frozen form, was thawed at room temperature for a few minutes, rinsed with saline, and inserted under general anesthesia in the eye of a 3-year-old patient (case 5), and in the office under topical anesthesia with 0.5% proparacaine hydrochloride eye drops and 2% lidocaine hydrochloride gel for all others. It was first inserted into the superior fornix while the patient looked down and then was slid under the lower eyelid. ProKera with an inner diameter of 16 mm was used in the 4 adult patients, and ProKera with a diameter of 15 mm was inserted in the pediatric patient.
After insertion, patients continued all prior medical therapies without an eye patch, and were followed up 1, 3, and 5 to 7 days later, and every 5 to 7 days thereafter. At each visit, patients were asked about changes in their symptoms. Although ProKera does not need to be removed for fluorescein staining, the device was removed for photographic documentation of the healing pattern and then reinserted. In the event of heavy accumulation of inflammatory debris on the AM (Figures 1B to 1D), ProKera was replaced with a new device. After significant healing of the ocular surface was noted, ProKera was removed and use of topical medication was tapered off. For cases 3 and 4, a bandage contact lens was then inserted. After removal of ProKera, its AM was submitted for histopathologic evaluation with hematoxylin-eosin staining.
RESULTS
Insertions, exchanges, and removals of ProKera were easily done in all patients without any complications. On the first day after its insertion, all adult patients reported significant relief of pain and light sensitivity; the pediatric patient (case 5) also became more comfortable. Inflammatory coagulum progressively accumulated in all cases, with increasing cloudiness of the AM (Figures 1B–1D), prompting the need for insertion of a new device. Cases 3, 4, and 5 eventually needed a total of 4, 3, and 2 ProKera insertions, respectively, every 5 to 7 days before healing was complete. With time, there was less accumulation of inflammatory debris. Histopathology showed entrapment of acute inflammatory cells in the AM stroma (Figure 1E) and a progressive decrease in the number of inflammatory cells in the subsequent devices (Figure 1F).
Despite the variable extents of corneal-limbal-conjunctival epithelial defects, rapid and progressive epithelialization was observed in all eyes (Table and Figure 2). For the grade I injury (case 1) with less limbal damage (Figure 2A), the epithelialization was completed in both circumferential and centripetal manners toward the limbus and the central cornea, respectively (Figure 2B). However, for the grade II and III injuries (cases 2–5) with more extensive limbal damage (Figures 2D and 2G), the epithelialization proceeded initially in a circumferential manner to close the limbal defect before moving centripetally to close the corneal defect (Figures 2E and 2H). The healing of the conjunctival defects was complete first, in a mean (SD) of 8.2 (5) days (range, 5–17 days), which was overall faster than the 13.6(8.3) days (range, 5–25 days) for the corneal epithelial defects. Moreover, perilimbal and conjunctival inflammation covered under ProKera were also rapidly reduced in all eyes. Three eyes (cases 3, 4, and 5) displayed early peripheral corneal neovascularization that markedly regressed on completion of epithelialization (Figure 4).
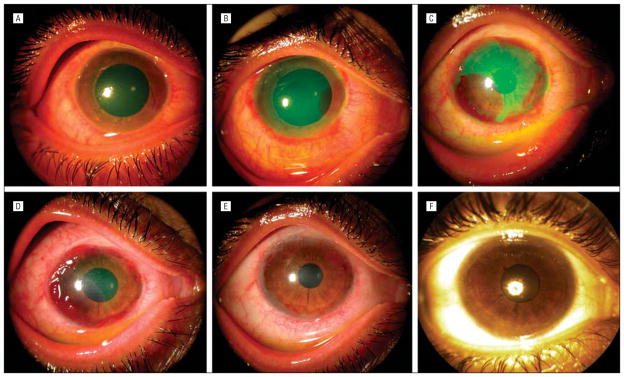
Figure 4.
Unique neovascularization pattern under ProKera (Bio-Tissue, Inc, Miami, Florida). Case 4 with moderate diffuse conjunctival inflammation, superior limbal ischemia, total corneal epithelial defect, and mild stromal haziness (A) showed progressive superficial neovascularization in the area where epithelialization took place at days 5 (B), 10 (C), and 14 (D). Such vascularization regressed following complete epithelialization of the cornea (E), resulting in a faint corneal haze and stationary pannus in the peripheral cornea 29 months later (F).
During a mean (SD) follow-up period of 16.8(10.8) months (range, 7–29 months), all corneas initially showing edema and haze became clear (cases 1 and 2) (Figure 2C) or were left with a mild haze without edema (cases 3–5) (Figures 2F and 2I). Three eyes (cases 3, 4, and 5) were left with stationary perilimbal superficial corneal neovascularization. No eyes developed limbal stem cell deficiency, as judged by impression cytology in 3 cases and by clinical evidence in the other 2 cases, nor cicatricial complication, such as symblepharon, at the chronic stage. For adult cases, mean (SD) visual acuity improved 3.5 (2.6) Snellen lines (range, 1–7 Snellen lines).
CASE EXAMPLE
A 27-year-old woman (case 4) sustained a splash of floor cleaner (alkaline) solution into both eyes, with the right eye more severely involved. After initial irrigation, she was treated with topical prednisolone acetate, homatropine and ofloxacin eye drops, and oral vitamin C and doxycycline for 8 days, and referred for further management owing to lack of epithelialization. On presentation, her best-corrected visual acuity was 20/200 OD and 20/20 OS. The left eye had recovered well. The right eye showed diffuse moderate inflammation in both the bulbar and tarsal conjunctiva, mild corneal edema and haziness, a total corneal epithelial defect, and a 360° (3–4 mm) limbal epithelial defect extending to the bulbar conjunctiva more than 8 mm, from the 11-o’clock to 2-o’clock positions, and from the 4-o’clock to 7-o’clock positions (Figures 3A and 3G). There was notable limbal ischemia from the 11:30- to the 2-o’clock positions. Intraocular pressure was normal in both eyes.
ProKera was inserted in the right eye, in the office, under topical anesthesia. The patient’s pain rapidly subsided, and conjunctival inflammation was reduced 3 days later. At this time the conjunctival healing reached the limbal region, and corneal epithelialization started at the 4-o’clock position, where the conjunctival defect closed first. At day 5 post-insertion, healing had progressed more centripetally into the cornea from the 4-o’clock position (Figure 3B). A new ProKera was inserted because of heavy coating with inflammatory debris. At day 7, the epithelial mass noted at the 4-o’clock position on day 5 (Figure 3B) moved circumferentially to the adjacent limbal region, while a new limbal epithelial mass emerged from the 7-o’clock position where the conjunctival defect had closed (Figure 3C). At day 10, the latter epithelial mass from the 7-o’clock position enlarged and moved centripetally (Figure 3D); a new ProKera was inserted. At day 12, the epithelial mass emerging from the 7-o’clock position moved circumferentially to fill in the surrounding limbal region (Figure 3E). At day 14, the limbal epithelialization was complete, and ProKera was replaced with a therapeutic bandage contact lens. At day 17, the corneal surface had healed completely (Figure 3F), and the patient’s visual acuity had improved to 20/25 OD.
Initially, there was a large conjunctival defect that extended superiorly and inferiorly (Figures 3A and 3G) beyond what ProKera could cover. The area located outside of the device skirt healed slowly (Figure 3H). By day 17, the corneal epithelial defect had already healed (Figure 3F), but the conjunctiva outside the ProKera was still not completely healed, and turned into inflamed granulation tissue where hyperemic blood vessels emanated from the fornix (Figure 3I). Thus, subconjunctival injection of triamcinolone acetonide was given that helped reduce inflammation. At the limbal-corneal surface under ProKera, prominent neovascularization was noted distributing in the area where epithelialization had developed and expanded (Figures 4B–4D). However, such vascularization receded following complete epithelialization of the cornea (Figure 4E).
During 29 months of follow-up, the patient retained her visual acuity of 20/25 OD and 20/20 OS. The right central cornea had only faint haziness and the peripheral cornea had stationary pannus, with regression of its blood vessels (Figure 4F). There was no limbal stem cell deficiency (LSCD) or symblepharon.
COMMENT
This was the first study reporting our joint experiences in using temporary sutureless AMP via ProKera as an early intervention for acute alkaline burns. Although not controlled, our overall results suggest its effectiveness in rapidly relieving symptoms, reducing inflammation, and promoting epithelialization. Consequently, it also prevented LSCD and symblepharon at the chronic stage.
Amniotic membrane has been used as a temporary patch for acute chemical burns in the past. Encouraging results were noted by some,9,16–18,21,23 but not by others.18,19,24 We speculate that the inconsistency might be due to 2 factors: the severity of the injury, and the rapidity of delivering AMP. Consistent with previous studies using conventional AMP with sutures,9,16–18,21 our study demonstrated the effectiveness of the sutureless approach in 5 eyes with grades I to III alkaline burns. In grade IV acute burn, although the use of AMP has been shown to reduce limbal stromal inflammation,23 it is not sufficient to prevent LSCD, presumably because of severe limbal ischemia.9,18,19,24 To combat limbal ischemia in this grade of acute burn, additional procedures such as tenonplasty are required to prevent corneal and scleral melt.7,25,26 In addition to the severity of the burns, the lapse in time between chemical injury and AMP is also an important factor that may affect the outcome. Previously, AMP has been performed from less than a day to 4 weeks after the injury.9,14–21 Recently, Prabhasawat et al21 showed that AMP performed within 5 days of grades II and III chemical burns resulted in faster epithelial healing and less corneal haze and LSCD than AMP performed after 5 days. Herein, we noted a similar positive outcome when AMP was performed within 8 days of the injury. Such early intervention became feasible in part because of the ease of insertion of ProKera in the office. Future prospective controlled studies with a large sample size should be conducted by stratifying disease severity and by considering early intervention of AMP in acute burns.
Accumulation of inflammatory debris explained why the AM could become cloudy, and potentially less effective. This phenomenon was observed in the AM of Pro-Kera (Figures 1B and 1D), and confirmed by histopathologic study (Figures 1E). A similar finding has been observed in prior studies, showing that polymorpho-nuclear neutrophils are entrapped by AM stroma in rabbit models of alkaline burn14 or excimer laser ablation,27,28 and in a rat model of Herpes Simplex Virus 1 keratitis.29 Because entrapped neutrophils, lymphocytes, or macrophages undergo rapid apoptosis,27,28,30,31 such inflammatory responses are swiftly quelled. Additionally, expressions of interleukin 1α(IL-1α), IL-1β, IL-8, transforming growth factor β1,32,33 and metalloproteinases34,35 are also markedly suppressed when cells are in contact with AM stroma. These actions explain why AM used as a temporary patch exerts potent antiinflammatory effects.10 In 3 cases, a new ProKera was inserted every 5 to 7 days to refresh the aforementioned antiinflammatory effects (Figure 1F). Therefore, ProKera offers significant convenience for future investigation of the benefit and the timing of AMP in suppressing inflammatory responses in various other ocular surface diseases.
Although AM does not preclude fluorescein staining,36 easy removal of ProKera allowed us to unravel unique patterns of corneal and limbal reepithelialization under AM. It should be realized that positive fluorescein staining does not always mean the total loss of epithelial cells, including stem cells. Dua and Forrester37 first reported the pattern of limbal and corneal reepithelialization in 17 eyes with large ocular surface abrasions without using AM. These eyes had 32% to 86% corneal abrasions with 0° to 240° (ie, not total) limbal involvement; 10 of 17 cases were caused by acid or alkaline burns. They noted that a preferential circumferential migration of cells from both ends of the limbal defect closed the limbal defect before centripetal healing of the corneal defect and closure of the perilimbal conjunctival defect. They interpreted such a healing pattern as evidence of limbal epithelial stem cell regeneration. However, conjunctivalization occurred in 3 of 17 eyes before completion of limbal healing. Herein, we noted that the healing was completed by both circumferential and centripetal epithelial movements toward the limbus and the central cornea, respectively, when there was less limbal damage, eg, case 1 (grade I injury) with 120° limbal epithelial defect without ischemia (Figure 2B). We also noted that a circumferential epithelial movement to close the limbal defect preceded the corneal epithelialization by centripetal movement in cases 2 and 5 (grade II and III injuries) where there was more extensive limbal damage, including 140° to 150° limbal epithelial defect with 75° to 150° limbal ischemia (Figure 2H). However, to our surprise, we observed a similar circumferential limbal closure in 2 cases (cases 3 and 4) where there was total limbal epithelial defect with or without regional ischemia (Figures 2E and 3).
We thus believe that there was rapid limbal (stem cell) recovery under the AM, even in eyes with total limbal damage. Furthermore, limbal reepithelialization started only in the region where the perilimbal conjunctiva was healed under the AM (Figure 3). That explained why healing of the perilimbal conjunctival defects was complete in a mean (SD) of 8.2 (5) days (range, 5–17 days), overall faster than the mean (SD) of 13.6 (8.3) days (range, 5–25 days) for the healing of the corneal epithelial defects. Because the healed surface did not show any evidence of conjunctivalization clinically and/or by impression cytology, we believe that perilimbal healing resulted from the expansion of the remaining few limbal epithelial stem cells after the acute insult. Moreover, the notion that AMP resurrected and promoted expansion of the remaining limbal stem cells was supported by a rapid increase of epithelial cell mass at the 4-o’clock position at day 5 (Figure 3B) and at the 7-o’clock position at day 10 (Figure 3D) in case 4. Before moving centripetally, both masses first moved to heal the adjacent limbal regions (Figures 3C and 3E). Furthermore, pronounced superficial neovascularization in the limbal and peripheral corneal regions in 3 eyes (cases 2, 3, and 4, Figure 4) accompanied the aforementioned unique epithelial healing pattern. Interestingly, these neovascular tufts showed marked regression on completion of ocular surface healing. This phenomenon was due in part to the rapid suppression of inflammation, as evidenced by the lack of hyperemia in the AM-covered perilimbal conjunctiva (Figure 3I). In contrast, the conjunctiva not covered by AM remained unhealed, and resulted in inflammation-associated granulation tissue (Figures 3H and 3I).
Amniotic membrane’s therapeutic effects of suppressing inflammation and promoting epithelialization has also been recognized when AM, as a temporary patch, was used during the acute stage of Stevens-Johnson syndrome/toxic epidermal necrolysis.38–41 Future studies to identify the factor(s) in AM responsible for the aforementioned actions will undoubtedly reveal new therapeutics in regenerative medicine.
Acknowledgments
Funding/Support: The development of ProKera is supported by grant EY014768 from the National Institutes of Health, National Eye Institute. This study was also supported in part by research funding from TissueTech, Inc, Research to Prevent Blindness, and a Joseph Swiger fellowship grant from the Ocular Surface Research & Education Foundation, Miami, Florida. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Financial Disclosure: Dr Tseng and his family are greater than 5% shareholders of TissueTech, Inc, which owns US patents 6,152,142 and 6,326,019 on the method of preparation and clinical uses of human amniotic membrane and ProKera, distributed by Bio-Tissue, Inc. No other author has any proprietary interest in any material mentioned in this study.
References
- 1.Wagoner MD. Chemical injuries of the eye: current concepts in pathophysiology and therapy. Surv Ophthalmol. 1997;41(4):275–313. doi: 10.1016/s0039-6257(96)00007-0. [DOI] [PubMed] [Google Scholar]
- 2.Levinson RA, Paterson CA, Pfister RR. Ascorbic acid prevents corneal ulceration and perforation following experimental alkali burns. Invest Ophthalmol Vis Sci. 1976;15(12):986–993. [PubMed] [Google Scholar]
- 3.Pfister RR, Nicolaro ML, Paterson CA. Sodium citrate reduces the incidence of corneal ulcerations and perforations in extreme alkali-burned eyes—acetylcysteine and ascorbate have no favorable effect. Invest Ophthalmol Vis Sci. 1981;21(3):486–490. [PubMed] [Google Scholar]
- 4.Seedor JA, Perry HD, McNamara TF, et al. Systemic tetracycline treatment of alkali-induced corneal ulceration in rabbits. Arch Ophthalmol. 1987;105(2):268–271. doi: 10.1001/archopht.1987.01060020122043. [DOI] [PubMed] [Google Scholar]
- 5.Newsome NA, Gross J. Prevention by medroxyprogesterone of perforation in the alkali-burned rabbit cornea: inhibition of collagenolytic activity. Invest Ophthalmol Vis Sci. 1977;16(1):21–31. [PubMed] [Google Scholar]
- 6.Kenyon KR, Berman M, Rose J, Gage J. Prevention of stromal ulceration in the alkali-burned rabbit cornea by glued-on contact lens: evidence for the role of polymorphonuclear leukocytes in collagen degradation. Invest Ophthalmol Vis Sci. 1979;18(6):570–587. [PubMed] [Google Scholar]
- 7.Reim M, Teping C. Surgical procedures in the treatment of most severe eye burns: revival of the artificial epithelium. Acta Ophthalmol (Copenh) 1989;67 (suppl):47–54. doi: 10.1111/j.1755-3768.1989.tb07094.x. [DOI] [PubMed] [Google Scholar]
- 8.Denig R. Circumcorneal transplantation of buccal mucous membrane as a curative measure in diseases of the eye. Arch Ophthalmol. 1929;1:351–357. [Google Scholar]
- 9.Meller D, Pires RTF, Mack RJS, et al. Amniotic membrane transplantation for acute chemical or thermal burns. Ophthalmology. 2000;107(5):980–990. doi: 10.1016/s0161-6420(00)00024-5. [DOI] [PubMed] [Google Scholar]
- 10.Tseng SCG, Espana EM, Kawakita T, et al. How does amniotic membrane work? Ocul Surf. 2004;2(3):177–187. doi: 10.1016/s1542-0124(12)70059-9. [DOI] [PubMed] [Google Scholar]
- 11.Dua HS, Gomes JA, King AJ, Maharajan VS. The amniotic membrane in ophthalmology. Surv Ophthalmol. 2004;49(1):51–77. doi: 10.1016/j.survophthal.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Bouchard CS, John T. Amniotic membrane transplantation in the management of severe ocular surface disease: indications and outcomes. Ocul Surf. 2004;2(3):201–211. doi: 10.1016/s1542-0124(12)70062-9. [DOI] [PubMed] [Google Scholar]
- 13.Sorsby A, Symons HM. Amniotic membrane grafts in caustic burns of the eye. Br J Ophthalmol. 1946;30(6):337–345. [PMC free article] [PubMed] [Google Scholar]
- 14.Kim JS, Kim JC, Na BK, et al. Amniotic membrane patching promotes healing and inhibits protease activity on wound healing following acute corneal alkali burns. Exp Eye Res. 2000;70(3):329–337. doi: 10.1006/exer.1999.0794. [DOI] [PubMed] [Google Scholar]
- 15.Sridhar MS, Bansal AK, Sangwan VS, Rao GN. Amniotic membrane transplantation in acute chemical and thermal injury. Am J Ophthalmol. 2000;130(1):134–137. doi: 10.1016/s0002-9394(00)00500-6. [DOI] [PubMed] [Google Scholar]
- 16.Uçakhan OO, Kökluü G, Firat E. Nonpreserved human amniotic membrane transplantation in acute and chronic chemical eye injuries. Cornea. 2002;21(2):169–172. doi: 10.1097/00003226-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi A, Shirao Y, Yoshita T, et al. Temporary amniotic membrane patching for acute chemical burns. Eye. 2003;17(2):149–158. doi: 10.1038/sj.eye.6700316. [DOI] [PubMed] [Google Scholar]
- 18.Arora R, Mehta D, Jain V. Amniotic membrane transplantation in acute chemical burns. Eye. 2005;19(3):273–278. doi: 10.1038/sj.eye.6701490. [DOI] [PubMed] [Google Scholar]
- 19.Tamhane A, Vajpayee RB, Biswas NR, et al. Evaluation of amniotic membrane transplantation as an adjunct to medical therapy as compared with medical therapy alone in acute ocular burns. Ophthalmology. 2005;112(11):1963–1969. doi: 10.1016/j.ophtha.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 20.Tejwani S, Kolari RS, Sangwan VS, Rao GN. Role of amniotic membrane graft for ocular chemical and thermal injuries. Cornea. 2007;26(1):21–26. doi: 10.1097/ICO.0b013e31802b4201. [DOI] [PubMed] [Google Scholar]
- 21.Prabhasawat P, Tesavibul N, Prakairungthong N, Booranapong W. Efficacy of amniotic membrane patching for acute chemical and thermal ocular burns. J Med Assoc Thai. 2007;90(2):319–326. [PubMed] [Google Scholar]
- 22.Roper-Hall MJ. Thermal and chemical burns. Trans Ophthalmol Soc U K. 1965;85:631–640. [PubMed] [Google Scholar]
- 23.López-García JS, Rivas Jara L, García-Lozano I, Murube J. Histopathologic limbus evolution after alkaline burns. Cornea. 2007;26(9):1043–1048. doi: 10.1097/ICO.0b013e31812375fd. [DOI] [PubMed] [Google Scholar]
- 24.Joseph A, Dua HS, King AJ. Failure of amniotic membrane transplantation in the treatment of acute ocular burns. Br J Ophthalmol. 2001;85(9):1065–1069. doi: 10.1136/bjo.85.9.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teping C, Reim M. Tenonplasty as a new surgical principle in the early treatment of the most severe chemical eye burns. Klin Monatsbl Augenheilkd. 1989;194 (1):1–5. doi: 10.1055/s-2008-1046325. [DOI] [PubMed] [Google Scholar]
- 26.Casas VE, Kheirkhah A, Blanco G, Tseng SCG. Scleral approach for scleral ischemia and melt. Cornea. 2008;27(2):196. doi: 10.1097/ICO.0b013e31815ba1ae. [DOI] [PubMed] [Google Scholar]
- 27.Wang MX, Gray TB, Parks WC, et al. Corneal haze and apoptosis is reduced by amniotic membrane matrix in excimer laser photoablation in rabbits. J Cataract Refract Surg. 2001;27(2):310–319. doi: 10.1016/s0886-3350(00)00467-3. [DOI] [PubMed] [Google Scholar]
- 28.Park WC, Tseng SCG. Modulation of acute inflammation and keratocyte death by suturing, blood and amniotic membrane in PRK. Invest Ophthalmol Vis Sci. 2000;41(10):2906–2914. [PubMed] [Google Scholar]
- 29.Heiligenhaus A, Meller D, Meller D, et al. Improvement of HSV-1 necrotizing keratitis with amniotic membrane transplantation. Invest Ophthalmol Vis Sci. 2001;42(9):1969–1974. [PubMed] [Google Scholar]
- 30.Shimmura S, Shimazaki J, Ohashi Y, Tsubota K. Antiinflammatory effects of amniotic membrane transplantation in ocular surface disorders. Cornea. 2001;20(4):408–413. doi: 10.1097/00003226-200105000-00015. [DOI] [PubMed] [Google Scholar]
- 31.Li W, He H, Kawakita T, et al. Amniotic membrane induces apoptosis of interferon-gamma activated macrophages in vitro. Exp Eye Res. 2006;82(2):282–292. doi: 10.1016/j.exer.2005.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Solomon A, Rosenblatt M, Monroy DC, et al. Suppression of interleukin-1a and interleukin-1b in the human corneal epithelial cells cultured on the amniotic membrane matrix. Br J Ophthalmol. 2001;85(4):444–449. doi: 10.1136/bjo.85.4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Solomon A, Wajngarten M, Alviano F, et al. Suppression of inflammatory and fibrotic responses in allergic inflammation by the amniotic membrane stromal matrix. Clin Exp Allergy. 2005;35(7):941–948. doi: 10.1111/j.1365-2222.2005.02285.x. [DOI] [PubMed] [Google Scholar]
- 34.Rigal-Sastourné JC, Tixier JM, Renard JP, et al. Corneal burns and matrix metalloproteinases (MMP-2 and -9): the effects of human amniotic membrane transplantation [in French] J Fr Ophtalmol. 2002;25(7):685–693. [PubMed] [Google Scholar]
- 35.Heiligenhaus A, Li H, Yang Y, et al. Amniotic membrane transplantation improves experimental herpetic keratitis: modulation of matrix metalloproteinase-9 [in German] Ophthalmologe. 2004;101(1):59–65. doi: 10.1007/s00347-003-0872-5. [DOI] [PubMed] [Google Scholar]
- 36.Kobayashi A, Ijiri S, Sugiyama K, et al. Detection of corneal epithelial defect through amniotic membrane patch by fluorescein. Cornea. 2005;24(3):359–360. doi: 10.1097/01.ico.0000138855.72073.b6. [DOI] [PubMed] [Google Scholar]
- 37.Dua HS, Forrester JV. Clinical patterns of corneal epithelial wound healing. Am J Ophthalmol. 1987;104(5):481–489. doi: 10.1016/s0002-9394(14)74105-4. [DOI] [PubMed] [Google Scholar]
- 38.John T, Foulks GN, John ME, et al. Amniotic membrane in the surgical management of acute toxic epidermal necrolysis. Ophthalmology. 2002;109(2):351–360. doi: 10.1016/s0161-6420(01)00900-9. [DOI] [PubMed] [Google Scholar]
- 39.Di Pascuale MA, Espana EM, Liu DT, et al. Correlation of corneal complications with eyelid cicatricial pathologies in patients with Stevens-Johnson syndrome and toxic epidermal necrolysis syndrome. Ophthalmology. 2005;112(5):904–912. doi: 10.1016/j.ophtha.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi A, Yoshita T, Sugiyama K, et al. Amniotic membrane transplantation in acute phase of toxic epidermal necrolysis with severe corneal involvement. Ophthalmology. 2006;113(1):126–132. doi: 10.1016/j.ophtha.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 41.Tandon A, Cackett P, Mulvihill A, Fleck B. Amniotic membrane grafting for conjunctival and lid surface disease in the acute phase of toxic epidermal necrolysis. J AAPOS. 2007;11(6):612. doi: 10.1016/j.jaapos.2007.04.020. [DOI] [PubMed] [Google Scholar]