Abstract
Background:
Induction of heme oxygenase-1 (HO-1) attenuates the development of angiotensin II (Ang II)-dependent hypertension in mice. However, the mechanism by which HO-1 lowers blood pressure in this model is not clear. This study was designed to determine if induction of HO-1 resulted in an improvement in vascular relaxation in Ang II hypertensive mice.
Methods:
Mice were treated with either vehicle (Ctrl), the HO-1 inducer cobalt protoporphyrin (CoPP, 50 mg/kg), Ang II (1 μg/kg/min, 14 days), or Ang II + CoPP. CoPP was administered as a single bolus dose two days prior to subcutaneous implantation of the osmotic minipump containing Ang II. Vascular relaxation was examined in isolated carotid arteries pre-contracted with the thromboxane mimetic U46619 (0.4 μg/ml).
Results:
Endothelial dependent relaxation to acetylcholine (ACh, 1 μM) was significantly impaired in Ang II treated mice compared to Ctrl (56±3 vs. 40±4%, p<0.05, n≥6). Similarly, endothelial independent relaxation to sodium nitroprusside (SNP, 1 μM) was significantly impaired in Ang II mice (56±6 vs. 28±6%, p<0.05, n≥6). Relaxation in response to the carbon monoxide donor, CORM-A1 (100 μM), was attenuated after Ang II treatment (75±7 vs. 59±7%,p<0.05, n≥6). CoPP treatment induced HO-1 but not HO-2 protein in the aorta as measured by Western blot. CoPP treatment had no effect on vascular responses in Ctrl mice and did not improve ACh (26±5%, n=15), SNP (23±4%, n=15), or CORMA1 (46±7%, n=10) dependent relaxation in Ang II treated mice.
Conclusions:
These results suggest that induction of HO-1 improves Ang II dependent hypertension through a mechanism independent of improved vascular relaxation.
Keywords: heme oxygenase-1, Angiotensin II, vascular relaxation, carbon monoxide
Introduction
We have recently demonstrated that induction of HO-1 with cobalt protoporphyrin (CoPP) lowers blood pressure in Ang II dependent hypertensive mice 1. Several other studies have also reported that induction of HO-1 either chemically or genetically can reduce blood pressure in spontaneously hypertensive rats (SHR), and in renovascular and angiotensin II (Ang II)-dependent hypertension 2,3. Despite the efficacy of HO-1 induction to lower blood pressure in these models, the mechanism responsible for this effect is not clear. One potential mechanism for the anti-hypertensive actions of HO-1 may be increased production of CO in the vasculature.
CO causes vasodilatation by activating both soluble guanylate cyclase (sGC) and high conductance Ca2+ activated K+ channels 4,5. In the vasculature, CO is an important regulator of basal tone and modulates constriction caused by vasoactive agents and increases in pressure 6,7. Recent studies demonstrated that increases in vascular HO and CO can improve acetylcholine (ACh) mediated relaxation in diabetes 8,9.
Ang II-dependent hypertension in mice is associated with impaired vasodilatory responses to ACh 10. Since studies in human as well as in animal models have linked increases in vascular stiffness with hypertension, we hypothesized that improved vascular relaxation may be associated with the blood pressure lowering actions of HO-1 induction in Ang II-dependent hypertension. Therefore, the goal of the present study was to determine if the blood pressure lowering actions of HO-1 induction resulted in an improvement of vascular relaxation in Ang II infused mice.
Methods
Animals
Studies were performed on 16-24 week male C57BL/6J mice purchased from Jackson Labs (Bar Harbor, ME). All mice were housed under standard conditions and allowed full access to food and water. HO-1 was induced by treatment with cobalt protoporphyrin (CoPP, 50 mg/kg body weight, sc, Frontier Scientific, Logan, UT) as previously described 2. Mice were pretreated with CoPP or vehicle (0.1 M NaOH, pH 8.3) two days prior to Ang II administration as previously described 1. Ang II (1 μg/kg/min, sc) was delivered using an osmotic minipump which was subcutaneously implanted. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Mississippi Medical Center.
Blood Pressure
Blood pressure was directly measured via microrenathane catheters implanted into the carotid artery using aseptic surgical technique as previously described 11. Surgeries were performed 12 days after implantation of the minipumps and the mice were allowed 1 day to recover from surgery. Mean arterial blood pressure (MAP) was recorded from conscious, freely-moving mice for 3 hours and the data averaged.
Vascular Ring Preparation
Mouse carotid arteries were removed and prepared for vessel reactivity studies as previously published 10. Resting tension was adjusted step-wise to reach a final tension of 0.25 grams. For each animal, at least two vessel segments were studied with the averaged response equal to an n of 1. Concentration dependent relaxation (10−8 −10−4 mol/L) to acetylcholine (ACh) and sodium nitroprusside (SNP) were assessed in vessel segments pre-contracted with the thromboxane A2 mimetic U46619 (0.4 μg/ml). CO mediated relaxation was tested in pre-contracted vessels in a time dependent manner (15 and 30 minutes after 100 μM CORM-A1 administration). This concentration was chosen based on previously published data from isolated vessels12.
Heme Oxygenase Assay
Heme oxygenase assay was performed on lysates prepared from the aorta. The protein concentration was measured using a Bio-rad protein assay with BSA standards. Reactions were carried out in 1.2 ml containing: 2 mM glucose-6-phosphate, 0.2 units glucose-6-phosphate dehydrogenase, 0.8 mM NADPH, 20 μM hemin, and 0.5 mg of lysates as previously described 1. The reactions were incubated for 1 hour at 37°C in the dark. The formed bilirubin was extracted with chloroform, and the change in optical density (ΔOD) at 464-530 nm was measured using an extinction coefficient of 40 mM/cm for bilirubin. HO activity was expressed as picomoles of bilirubin formed per hour per milligram of total protein and presented as percent of control values.
Western Blot
Western blots were performed on lysates prepared from aortas collected at the end of the experimental protocol. Samples of 50 μg of protein were boiled in Laemmli sample buffer (Bio-Rad, Hercules, CA) for 5 min and electrophoresed on 10% SDS-polyacrylamide gels and blotted onto nitrocellulose membrane. Membranes were blocked with Odyssey blocking buffer (LI-COR, Lincoln, NE) for 2 hours at room temperature, then incubated with rabbit anti-HO-1 or HO-2 polyclonal antibody (StressGen, Vancouver, Canada 1:2000) as well as a mouse anti-β-actin antibody (Gentest,1:5,000) overnight at 4°C. Membranes were incubated with Alex 680 goat anti-rabbit IgG (Molecular Probes) and IRDye 800 goat anti-mouse IgG (Rockland, Gilbertsville, PA) for 1 hour at room temperature. Membranes were visualized using an Odyssey infrared imager (Li-COR, Lincoln, NE) which allows for the simultaneous detection of two proteins. Densitometry analysis was performed using Odyssey software (LI-COR, Lincoln, NE).
Statistics
Mean values ± SEM are presented. Concentration dependent relaxation curves were analyzed using a Repeated Measures ANOVA with a Student-Newman-Keuls Method post hoc test. HO activity assays and Western blots were analyzed by ANOVA followed by Dunnett's post hoc test. Data were considered statistically different at p<0.05.
Results
Pretreatment with CoPP attenuates Ang II-dependent hypertension
Mice were treated with one dose of CoPP (50 mg/kg body weight, sc) 2 days prior to the implantation of an osmotic minipump which delivered Ang II at a rate of 1 μg/kg/min. Mean arterial pressure (MAP) measured on day 14 post implantation of the Ang II minipump. MAP was significantly lower in mice treated with CoPP and averaged 122 ± 4 mmHg versus 144 ± 4 mmHg in control Ang II infused mice, n=5. The effect of CoPP on Ang II-dependent hypertension was identical to what was previously reported using this treatment protocol in mice 1. In the previous study, treatment with CoPP alone had no effect on blood pressure 1. Heart rate was similar in both groups averaging 575 ± 25 vs. 558 ± 15 beats per minute in Ang II + CoPP vs. Ang II infused mice.
CoPP treatment does not improve endothelial dependent or independent relaxation to nitric oxide in chronic Ang II infused mice
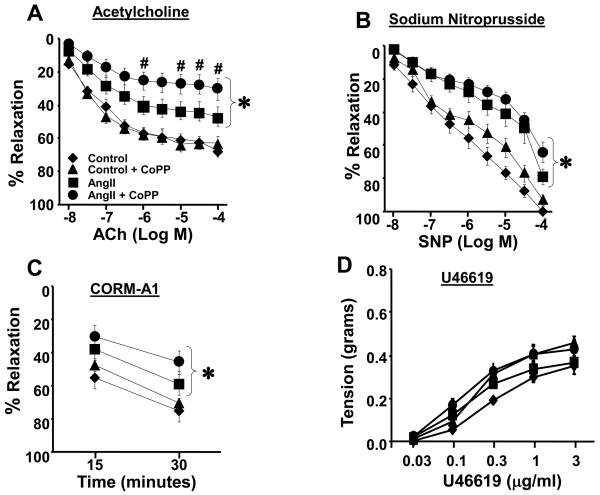
First, we examined whether chronic Ang II infusion for two weeks resulted in significantly impaired ACh mediated relaxation, as a measure of endothelial function. Consistent with earlier studies, ACh dependent relaxation was significantly impaired in Ang II treated mice at all concentrations relative to control animals (Figure 1A). ACh mediated relaxation was not improved in Ang II infused animals pretreated with CoPP and in fact was further impaired at higher concentration of ACh (Figure 1A). CoPP by itself did not change ACh mediated relaxation. In order to determine whether chronic Ang II infusion impairs smooth muscle dependent relaxation to nitric oxide directly, we examined relaxation responses to the nitric oxide donor, SNP. Chronic Ang II infusion resulted in significant attenuation of relaxation to SNP compared to control and CoPP treated mice at all concentrations examined (Figure 1B). These data suggest that both the endothelium and underlying smooth muscle are damaged by Ang II infusion over 14 days. Pre-treatment with CoPP did not improve the smooth muscle response to nitric oxide in Ang II infused mice. Relaxation to SNP was not altered by pre-treatment with CoPP alone (Figure 1B). The carbon monoxide donor, CORM-A1, caused a time dependent relaxation in carotid arteries from all mice studied (Figure 1C). Mice treated with Ang II or Ang II + CoPP had a significantly impaired response to CORM-A1 at both 15 and 30 minutes. In order to determine if differences in the vasoconstrictor response to U46619 in the groups can account for the lack improved vascular relaxation after HO-1 induction in the present study; we performed a dose response curve to concentrations of U46619 over a 100 fold range in carotid arteries of mice from each group (Figure 1D). Ang II infusion did not change the response to U46619 and CoPP treatment did not have any appreciable effect on this response. This finding indicates that alteration in the vasoconstrictor response to U46619 is not a mechanism for the altered relaxation in vessels from Ang II and Ang II + CoPP treated mice.
Figure 1.
A) Relaxation to ACh in the carotid artery from Control (n=10), CoPP (n=10), Ang II (n=16), and Ang II + CoPP (n=15) treated mice. ACh mediated relaxation was impaired by Ang II and this response was not improved by pre-treatment with CoPP. B) SNP mediated relaxation in the carotid artery of Control (n=10), CoPP (n=10), Ang II (n=16), and Ang II + CoPP (n=15) treated mice. The response to SNP was impaired in Ang II treated mice and was not improved by pre-treatment with CoPP. C) Response to to the CO donor CORM-A1 (100 uM) in the carotid artery from Control (n=6), CoPP (n=6), Ang II (n=6), and Ang II + CoPP (n=10) treated mice. Impaired dilatory response to CORM-A1 at both time points in Ang II treated mice. Pre-treatment with CoPP did not improve CO mediated relaxation in Ang II infused mice. * p<0.05 vs Control and Control + CoPP, # p<0.05 vs Control. D) Contraction to the thromboxane A2 mimetic, U46619, in Control (n=6), CoPP (n=6), Ang II (n=10), and Ang II + CoPP (n=10) treated mice. No differences were observed in the constrictor response to U46619 between the groups.
CoPP induces HO-1 protein in the vasculature of chronic Ang II infused mice
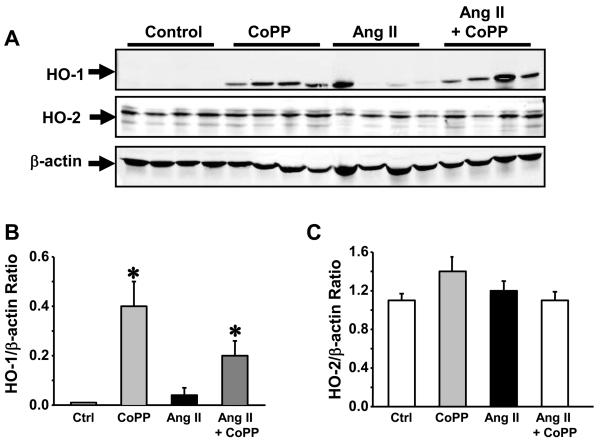
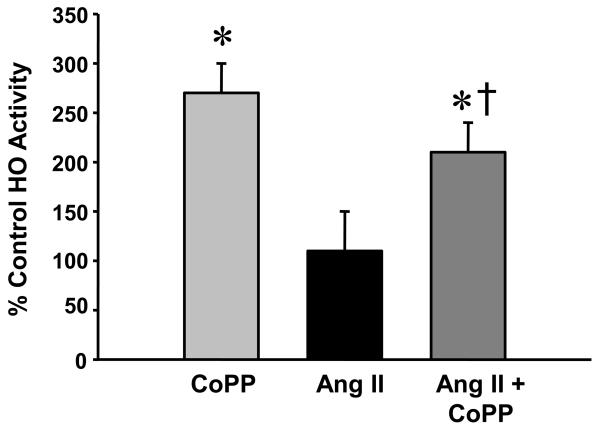
We examined induction of HO-1 by performing a Western blot on lysates prepared from the aorta of control, CoPP, Ang II and Ang II + CoPP treated mice. CoPP treatment resulted in a significant increase in the level of HO-1 protein in the aorta (Figure 2A). Ang II treatment resulted in a slight increase in the levels of HO-1 protein in aorta but this increase was not statistically different from control. Neither CoPP nor Ang II treatment had any effect on the level of HO-2 protein in the aorta (Figure 2B). CoPP treatment significantly increased HO activity measured in the aorta as compared with control mice (Figure 3). CoPP treatment in Ang II infused mice caused an increase in HO-1 activity as compared to mice infused with Ang II (Figure 3).
Figure 2.
A) Representative Western blot of HO-1 and HO-2 in the aorta of Control, CoPP, Ang II and Ang II + CoPP treated mice. B) Densitometric quantification of HO-1 protein. A significant increase in the levels of HO-1 protein were observed in the aorta of CoPP and Ang II + CoPP treated mice, n=4. C) Densitometric quantification of HO-2 protein. No significant differences in the levels of HO-2 protein were observed between the groups, n=4.
Figure 3.
Heme oxygenase activity in the aorta of CoPP (n=9), Ang II (n=11) and Ang II + CoPP (n=11) treated mice. CoPP treatment significantly increased HO activity in the aorta. * p<0.05 as compared to Control. †p<0.05 as compared to Ang II.
Time course of HO-1 induction following CoPP administration
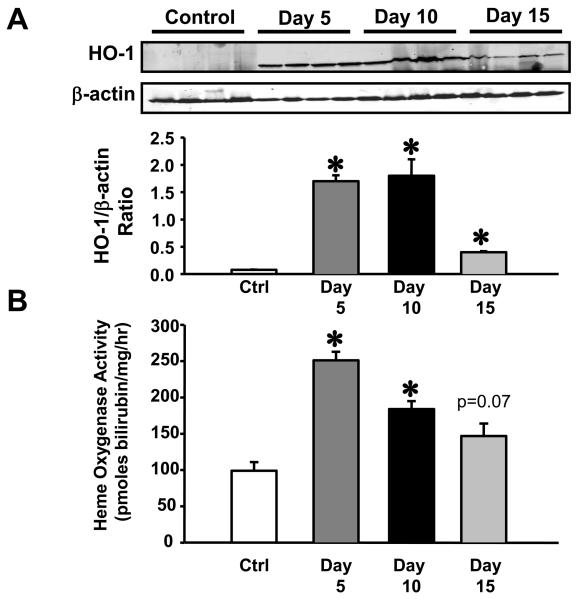
The time course of HO-1 induction in the aorta following CoPP administration was determined by sacrificing groups of mice 5, 10, and 15 days post CoPP administration. HO-1 protein levels were significantly increased in the aorta at all time points examined (Figure 4A). HO activity was also significantly increased at days 5 and 10 post CoPP administration and was still elevated above control levels on day 15 post administration although this increase did not achieve statistical difference (Figure 4B).
Figure 4.
A) Representative Western blot of HO-1 protein in the aorta of mice 5, 10, 15 days following a single administration of CoPP. HO-1 protein levels were increased at all time points examined following CoPP administration, n=4. B) Heme oxygenase activity in the aorta 5, 10, 15 days following CoPP administration. HO activity was increased at all time points examined, n=4. * = p<0.05 as compared to Control.
Discussion
The results from the present study, and our previous report, demonstrate that induction of HO-1 with CoPP prevents Ang II dependent hypertension in mice 1. This finding is in agreement with other studies that have demonstrated the antihypertensive effects of HO-1 induction in several experimental models of hypertension 2,3,13. Despite the blood pressure lowering actions of HO-1 induction, the mechanisms responsible for this are not clear. Since HO-1 induction and increased CO production have both been reported to improve vascular relaxation 8,14,15, we tested whether improvement of vascular relaxation was a contributing mechanism for the blood pressure lowering actions of HO-1 induction in Ang II dependent hypertension. In the present study, we found that two week treatment with Ang II significantly attenuated relaxation to ACh in carotid artery segments. These findings are in agreement with previous studies which have examined ACh mediated relaxation in models of Ang II dependent hypertension 10,16. This defect is likely due to both impaired endothelial function as well as diminished smooth muscle responsiveness to NO as Ang II infused mice exhibited attenuated relaxation to both ACh and SNP. HO-1 induction by CoPP did not improve vascular relaxation to either ACh or SNP despite the normalization of blood pressure and markedly increased vascular HO activity in CoPP treated Ang II infused mice. Unexpectedly, the dilatory response to ACh was further impaired in chronic Ang II infused mice pre-treated with CoPP suggesting that increases in vascular CO levels may decrease NO bioavailability in the endothelium. This is in agreement with a previous report that transgenic mice specifically over-expressing HO-1 in vascular smooth muscle cells exhibit decreased vascular responsiveness to nitric oxide 17. However, an alternative explanation for the reduced responsiveness to ACh and SNP in Ang II + CoPP treated mice could be increases in endogenous NO production. CoPP treatment can result in increased aortic eNOS protein levels 9 and previous studies in transgenic mice overexpressing eNOS have demonstrated that these mice exhibit increased basal NO release but attenuated responses to ACh and SNP 18.
It is also surprising that induction of HO-1 with CoPP did not improve the vascular relaxation to ACh in Ang II-infused mice given the antioxidant properties of HO metabolites, CO and bilirubin 19,20. Studies in the hyperbilirubinemic Gunn rat have demonstrated that these rats do not exhibit significant elevations in Ang II-mediated vascular superoxide production which preserves relaxation to ACh as compared to control rats 21. Since Ang II is able to directly stimulate NAD(P)H oxidase in vascular smooth muscle cells to increase superoxide anion production, this increase in superoxide anion production can result in decreased bioavailability of NO resulting in an attenuated response to ACh 22,23. Bilirubin is a potent scavenger of reactive oxygen species but it is unclear if the protection from Ang II derived superoxide anion production observed in the hyperbilirubinemic Gunn rat results from increases in intracellular bilirubin generation or is the result of increased extracellular bilirubin.
Our conclusion that CoPP does not prevent Ang II mediated hypertension through improved vascular function is based upon studies in which we examined relaxation in carotid artery segments. This approach is not without its limitations. Since resistance vessels are generally considered to have a larger role in blood pressure regulation than larger conduit vessels such as the carotid artery, it is possible that induction of HO-1 in these vessels may have a different action on vascular relaxation in Ang II infused mice. However, it is important to note that there is a direct correlation between carotid artery hemodynamics and blood pressure 24. Moreover, as in most studies examining the potential role for vascular function in blood pressure control, whether using conduit or micorvessels, the end result is the well established association between endothelial function and blood pressure. We believe the approach used in the current study adequately addresses our hypothesis.
In the present study, we report an impaired relaxation response to the CO donor, CORM-A1 (in addition to ACh and SNP). Although determining the cellular mechanism for the attenuated CO response is beyond the scope of the present study, we hypothesize based on our previous study that cGMP signaling may be impaired in Ang II infused mice 25. Similar to nitric oxide, CO has been shown to act through cGMP mediated mechanism to cause vessel relaxation 26.
While acutely, CoPP can inhibit HO-1 27, it has been shown by several investigators to significantly induce HO-1 in vivo such that total HO activity is increased chronically 1,2,28. Importantly, the data from our study show that a single bolus dose of CoPP significantly increases HO-1 protein and activity in the aorta up to 2 weeks post administration. This observation is consistent with previous studies which have reported increased tissue levels of cobalt 4 weeks after administration of CoPP 29. While the mechanism responsible for the slow clearance of CoPP is not fully known, recent studies have indicated an important role for increased degradation of the HO-1 supressor, Bach 1, in the induction of HO-1 by CoPP 30.
In summary, our data demonstrate that chronic Ang II hypertension in the mouse is associated with a significant attenuation in the relaxation to ACh, SNP, and CO which may be mediated by alterations in the vasodilatory response in vascular smooth muscle cells. Induction of HO-1 with CoPP does not improve the vascular relaxation in chronic Ang II treated mice despite significantly decreasing blood pressure and increasing vascular HO-1. While the current study cannot entirely rule out the possibility that vascular relaxation may be improved in resistance type vessels, our results demonstrate that induction of HO-1 in conduit vessels is not associated with an improvement in NO mediated vascular relaxation in the carotid artery of Ang II infused mice despite a significant decrease in blood pressure. Future experiments will be important to determine whether other mechanisms such as central or renal are responsible for the blood pressure lowering effects of HO-1 induction in Ang II-dependent hypertension.
Acknowledgements
These studies were supported by grants from the American Heart Association (0460046Z, M.J.R. and 0430094N, 0755330B D.E.S.) as well as the National Institutes of Health (1R01HL085907-01, M.J.R., and PO1HL-5197). T. Vera was supported by postdoctoral fellowships from the American Heart Association Southeast Affiliate (0525494B).
Footnotes
Conflict of Interest
None of the authors have any conflict of interest to declare.
References
- 1.Vera T, Kelsen S, Yanes LL, Reckelhoff JF, Stec DE. HO-1 induction lowers blood pressure and superoxide production in the renal medulla of angiotensin II hypertensive mice. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1472–R1478. doi: 10.1152/ajpregu.00601.2006. [DOI] [PubMed] [Google Scholar]
- 2.Abraham NG, Botros FT, Rezzani R, Rodella L, Bianchi R, Goodman AI. Differential effect of cobalt protoporphyrin on distributions of heme oxygenase in renal structure and on blood pressure in SHR. Cell Mol Biol (Noisy -le-grand) 2002;48:895–902. [PubMed] [Google Scholar]
- 3.Botros FT, Schwartzman ML, Stier CT, Jr., Goodman AI, Abraham NG. Increase in heme oxygenase-1 levels ameliorates renovascular hypertension. Kidney Int. 2005;68:2745–2755. doi: 10.1111/j.1523-1755.2005.00745.x. [DOI] [PubMed] [Google Scholar]
- 4.Coceani F. Carbon monoxide and dilation of blood vessels. Science. 1993;260:739. doi: 10.1126/science.8484109. [DOI] [PubMed] [Google Scholar]
- 5.Wang R, Wu L. The chemical modification of KCa channels by carbon monoxide in vascular smooth muscle cells. J Biol Chem. 1997;272:8222–8226. doi: 10.1074/jbc.272.13.8222. [DOI] [PubMed] [Google Scholar]
- 6.Kaide JI, Zhang F, Wei Y, Jiang H, Yu C, Wang WH, Balazy M, Abraham NG, Nasjletti A. Carbon monoxide of vascular origin attenuates the sensitivity of renal arterial vessels to vasoconstrictors. J Clin Invest. 2001;107:1163–1171. doi: 10.1172/JCI11218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang F, Kaide J, Wei Y, Jiang H, Yu C, Balazy M, Abraham NG, Wang W, Nasjletti A. Carbon monoxide produced by isolated arterioles attenuates pressure-induced vasoconstriction. Am J Physiol Heart Circ Physiol. 2001;281:H350–H358. doi: 10.1152/ajpheart.2001.281.1.H350. [DOI] [PubMed] [Google Scholar]
- 8.Di Pascoli M, Rodella L, Sacerdoti D, Bolognesi M, Turkseven S, Abraham NG. Chronic CO levels have [corrected] a beneficial effect on vascular relaxation in diabetes. Biochem Biophys Res Commun. 2006;340:935–943. doi: 10.1016/j.bbrc.2005.12.082. [DOI] [PubMed] [Google Scholar]
- 9.Ahmad M, Turkseven S, Mingone CJ, Gupte SA, Wolin MS, Abraham NG. Heme oxygenase-1 gene expression increases vascular relaxation and decreases inducible nitric oxide synthase in diabetic rats. Cell Mol Biol (Noisy -le-grand) 2005;51:371–376. [PubMed] [Google Scholar]
- 10.Ryan MJ, Didion SP, Mathur S, Faraci FM, Sigmund CD. Angiotensin II-induced vascular dysfunction is mediated by the AT1A receptor in mice. Hypertension. 2004;43:1074–1079. doi: 10.1161/01.HYP.0000123074.89717.3d. [DOI] [PubMed] [Google Scholar]
- 11.Vera T, Taylor M, Bohman Q, Flasch A, Roman RJ, Stec DE. Fenofibrate prevents the development of angiotensin II-dependent hypertension in mice. Hypertension. 2005;45:730–735. doi: 10.1161/01.HYP.0000153317.06072.2e. [DOI] [PubMed] [Google Scholar]
- 12.Motterlini R, Sawle P, Hammad J, Bains S, Alberto R, Foresti R, Green CJ. CORM-A1: a new pharmacologically active carbon monoxide-releasing molecule. FASEB J. 2005;19:284–286. doi: 10.1096/fj.04-2169fje. [DOI] [PubMed] [Google Scholar]
- 13.Wang R, Shamloul R, Wang X, Meng Q, Wu L. Sustained normalization of high blood pressure in spontaneously hypertensive rats by implanted hemin pump. Hypertension. 2006;48:685–692. doi: 10.1161/01.HYP.0000239673.80332.2f. [DOI] [PubMed] [Google Scholar]
- 14.Abraham NG, Kappas A. Heme oxygenase and the cardiovascular-renal system. Free Radic Biol Med. 2005;39:1–25. doi: 10.1016/j.freeradbiomed.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Turkseven S, Kruger A, Mingone CJ, Kaminski P, Inaba M, Rodella LF, Ikehara S, Wolin MS, Abraham NG. Antioxidant mechanism of heme oxygenase-1 involves an increase in superoxide dismutase and catalase in experimental diabetes. Am J Physiol Heart Circ Physiol. 2005;289:H701–H707. doi: 10.1152/ajpheart.00024.2005. [DOI] [PubMed] [Google Scholar]
- 16.Ryan MJ, Didion SP, Mathur S, Faraci FM, Sigmund CD. PPAR(gamma) agonist rosiglitazone improves vascular function and lowers blood pressure in hypertensive transgenic mice. Hypertension. 2004;43:661–666. doi: 10.1161/01.HYP.0000116303.71408.c2. [DOI] [PubMed] [Google Scholar]
- 17.Imai T, Morita T, Shindo T, Nagai R, Yazaki Y, Kurihara H, Suematsu M, Katayama S. Vascular smooth muscle cell-directed overexpression of heme oxygenase-1 elevates blood pressure through attenuation of nitric oxide-induced vasodilation in mice. Circ Res. 2001;89:55–62. doi: 10.1161/hh1301.092679. [DOI] [PubMed] [Google Scholar]
- 18.Ohashi Y, Kawashima S, Hirata K, Yamashita T, Ishida T, Inoue N, Sakoda T, Kurihara H, Yazaki Y, Yokoyama M. Hypotension and reduced nitric oxide-elicited vasorelaxation in transgenic mice overexpressing endothelial nitric oxide synthase. J Clin Invest. 1998;102:2061–2071. doi: 10.1172/JCI4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Llesuy SF, Tomaro ML. Heme oxygenase and oxidative stress. Evidence of involvement of bilirubin as physiological protector against oxidative damage. Biochim Biophys Acta. 1994;1223:9–14. doi: 10.1016/0167-4889(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 20.Taille C, El Benna J, Lanone S, Boczkowski J, Motterlini R. Mitochondrial respiratory chain and NAD(P)H oxidase are targets for the antiproliferative effect of carbon monoxide in human airway smooth muscle. J Biol Chem. 2005;280:25350–25360. doi: 10.1074/jbc.M503512200. [DOI] [PubMed] [Google Scholar]
- 21.Pflueger A, Croatt AJ, Peterson TE, Smith LA, d'Uscio LV, Katusic ZS, Nath KA. The hyperbilirubinemic Gunn rat is resistant to the pressor effects of angiotensin II. Am J Physiol Renal Physiol. 2005;288:F552–F558. doi: 10.1152/ajprenal.00278.2004. [DOI] [PubMed] [Google Scholar]
- 22.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 23.Hanna IR, Taniyama Y, Szocs K, Rocic P, Griendling KK. NAD(P)H oxidase-derived reactive oxygen species as mediators of angiotensin II signaling. Antioxid Redox Signal. 2002;4:899–914. doi: 10.1089/152308602762197443. [DOI] [PubMed] [Google Scholar]
- 24.Manabe S, Okura T, Watanabe S, Higaki J. Association between carotid haemodynamics and inflammation in patients with essential hypertension. J Hum Hypertens. 2005;19:787–791. doi: 10.1038/sj.jhh.1001898. [DOI] [PubMed] [Google Scholar]
- 25.Ryan MJ, Jernigan NL, Drummond HA, McLemore GR, Jr., Rimoldi JM, Poreddy SR, Gadepalli RS, Stec DE. Renal vascular responses to CORM-A1 in the mouse. Pharmacol Res. 2006;54:24–29. doi: 10.1016/j.phrs.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 26.Wang R, Wang Z, Wu L. Carbon monoxide-induced vasorelaxation and the underlying mechanisms. Br J Pharmacol. 1997;121:927–934. doi: 10.1038/sj.bjp.0701222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sardana MK, Kappas A. Dual control mechanism for heme oxygenase: tin(IV)-protoporphyrin potently inhibits enzyme activity while markedly increasing content of enzyme protein in liver. Proc Natl Acad Sci U S A. 1987;84:2464–2468. doi: 10.1073/pnas.84.8.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kato H, Amersi F, Buelow R, Melinek J, Coito AJ, Ke B, Busuttil RW, Kupiec-Weglinski JW. Heme oxygenase-1 overexpression protects rat livers from ischemia/reperfusion injury with extended cold preservation. Am J Transplant. 2001;1:121–128. [PubMed] [Google Scholar]
- 29.Rosenberg DW. Pharmacokinetics of cobalt chloride and cobalt-protoporphyrin. Drug Metab Dispos. 1993;21:846–849. [PubMed] [Google Scholar]
- 30.Shan Y, Lambrecht RW, Donohue SE, Bonkovsky HL. Role of Bach1 and Nrf2 in up-regulation of the heme oxygenase-1 gene by cobalt protoporphyrin. FASEB J. 2006;20:2651–2653. doi: 10.1096/fj.06-6346fje. [DOI] [PubMed] [Google Scholar]