Abstract
A potential anti-HIV and HCV drug candidate is highly desirable as coinfection has become a worldwide public health challenge. A potent compound based on a tetrabutoxy-calix[4]arene scaffold that possesses dual inhibition for both HIV and is described. Structural activity relationship studies demonstrate the effects of lower-rim alkylation in maintaining cone conformation and upper-rim interating head groups on the calix[4]arene play key roles for its potent dual antivial activities.
Coinfection with both human immunodeficiency virus 1 (HIV) and hepatitis C virus (HCV) has become a public health challenge, afflicting more than ten million people worldwide.1, 2 Complicating the challenge is the realization that with the advancement in highly active antiretroviral therapy (HAART), liver disease has emerged as a leading cause of death among HIVinfected patients.3 Treatment of coinfected patients with HAART has resulted in more liver toxicity4, creating a major health problem as there is currently no specific anti-HCV drug available. Therefore, the discovery of a dual compound as a potential drug candidate that can block HIV and HCV infection would be desirable. Herein, we report the first synthetic dual inhibitor of HIV and HCV infection in vitro based on a new class of compounds derived from a tetrabutoxy-calix[4]arene scaffold.
The secretion of vascular endothelial growth factor (VEGF) is elevated in HIV infected T-cells and is a major factor in the development of Kaposi’s sarcoma.5 In addition, platelet derived growth factor receptor (PDGFR) is expressed by uninfected T-cell lines, in which secretion of PDGF is also observed6. Although the impact of these processes is known for the development of Kaposi’s sarcoma, the effect of these growth factors and receptors on HIV replication itself is still under investigation. The discovery of calix[4]arene derivatives that block VEGF and PDGF with their respective receptors7–10 provided an opportunity to investigate the effect of this compound series on the replication of HIV. Moreover, a number of studies have shown that certain calixarene derivatives have interesting activities against viruses, enveloped viruses, bacteria, fungi, and cancerous cells.11 In these reports12,13, the macrocyclic scaffolds are based on pyrogallol calixarenes, calixarenes and resorcarenes of different ring sizes (4, 6, and 8 units) without functionality at the lower rim and with sulfite, phosphite or simple carboxylic acid groups on the top rim. These studies suggested that the antiviral activity was derived from the compounds binding to the viral envelope and inhibiting its interaction with cells.
In this study we utilized the series of calix[4]arene derivatives that were previously shown to inhibit the binding of VEGF, PDGF to their respective receptors.9 The three initial compounds tested for antiviral activity and cytotoxicity 1–3 contained a lower ring n-butyl group and an upper ring carboxamide-linked isophthalic group with a benzylester, free carboxylic acid and cyclohexylamide arm, respectively, on one of the carboxylic acids. Compound 2 showed the most promising anti-HIV activity (Table 1) with an EC50 value of 0.3 µM and an ID50 value of 90 µM in an MT-2 cellular assay. Compounds 1 and 3 showed little to no antiviral activity. Notably, these results showed no correlation between antiviral activity and the ability of the compounds to inhibit the interaction of VEGF and PDGF with their receptors. Compound 2 was equally potent against three different laboratory HIV strains: HIV-IIIB, NL-43, and LaiM184V. Moreover, antiviral activity was determined in different cell lines including MT-2, which are human T-cells with HTLV-1 virus, CEMx174, which is a human T cell line, and TZM-bl, which is a HeLa cell line with a firefly lucifarase that is controlled by an HIV promoter. These cell lines are different in their acquired viruses, extracellular proteins, and cytoplasm contents. However, antiviral activity was retained in all these cell lines, which suggests that the anti-HIV activity of compound 2 is independent of cell type. Compound 2 was further investigated against other viruses and shown to have no effect on the replication of herpes simplex virus-1 (HSV-1), HSV-2, and hepatitis B virus (HBV). However, an EC50 of 1.8 µM against hepatitis C virus (HCV) was observed.
Table 1.
Correlation studies of anti-HIV activities to growth factor inhibition studies.
| Compds | HIV | Cytotoxicity | VEGF | PDGF |
|---|---|---|---|---|
| Inhibition | IC50, µMa | IC50, µMa | IC50, µMa | |
| IC50, µMa | ||||
| 1 | >50 | >50 | 0.48 (±0.31) | 0.19 (±0.06) |
| 2 | 0.36 (±0.12) | >50 | 4.96 (±0.34) | 2.62 (±0.5) |
| 3 | >50 | >50 | >10 | 0.29 (±0.08) |
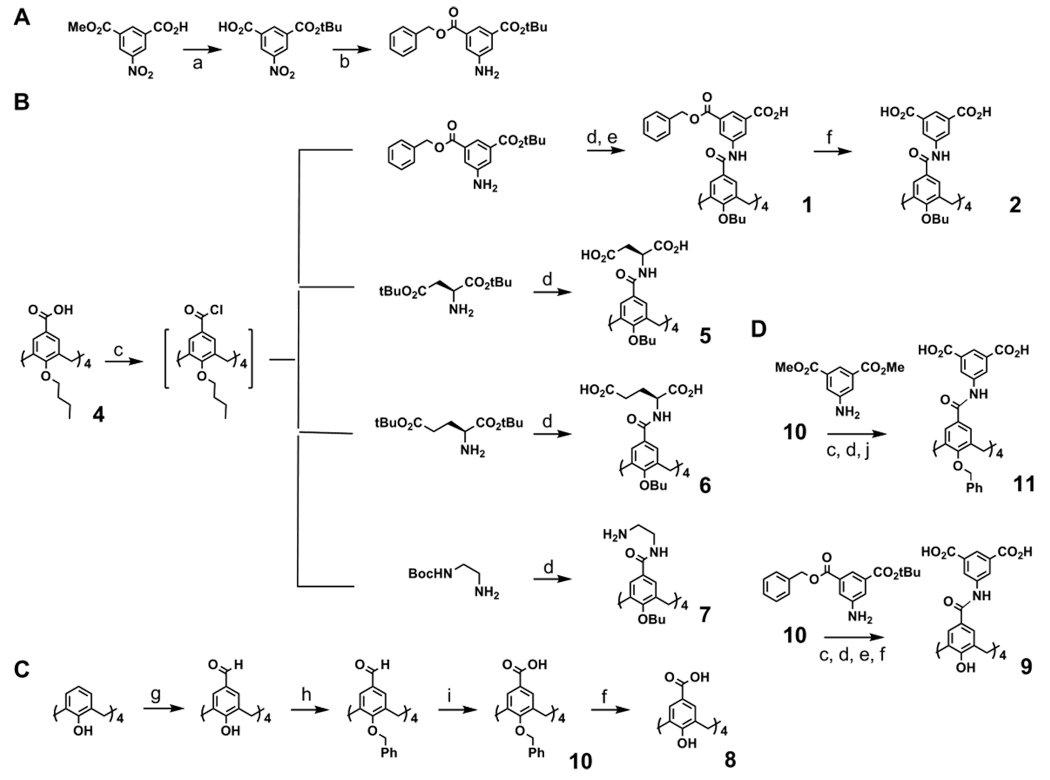
These observations propelled us to prepare a small library of compounds (Figure 1) and screen for their antiviral activity against HIV and HCV (Table 2). In particular, we investigated the effect of a range of upper and lower rim modifications on the activity of core scaffold 2. Calix[4]arenes with different diacid functionalities, such as glutamate, aspartate and isophthalate were synthesized by reacting the acyl chloride form of tetraacid derivative of tetrabutoxy calix[4]arene with L-glutamic acid di-tertbutyl ester, Laspartic acid di-tertbutyl ester and monobenzyl monotertbutyl 5-aminoisophthalate, respectively. The diacid funcationalites were then prepared by either benzyl ester deprotection with H2, Pd–C or tertbutyl ester removal with TFA. The role of alkylation at the lower rim was also studied through compounds 2, 9 and 11, allowing a comparison of benzyl versus n-butyl group at the lower ring hydroxyl.
Figure 1.
Synthetic routes for: A. Upper rim modifications, mono-t-butyl-5-aminoisophthalate. B. Lower rim n-butyl modifications on compounds 1, 2 and 4–7. C. Lower rim benzylated compound 10 and tetrahydroxy calix[4]arene 8. D. Compounds 9 and 11.
Table 2.
HIV and HCV antiviral activities and cytotoxicity assay results for compounds 1–11.
 | ||||
|---|---|---|---|---|
| Compds | HIV | Cytotoxicity | HCV | CEM |
| Inhibition | IC50, µMa | Inhibition | IC50, µMa | |
| IC50, µMa | IC50, µMa | |||
| 1 | >50 | >50 | 25.6 | 12.5 |
| 2 | 0.36 (±0.12) | >50 | 1.8 (±0.3) | 50 |
| 3 | >50 | >50 | 4.1 | >50 |
| 4 | 2.9 (±1.4) | 13.0 (±1.4) | 30.1 | 12.5 |
| 5 | 3.0 (±2.8) | >50 | 1.5 (±0.3) | 82 |
| 6 | 2.4 (±1.4) | >50 | 4.1 (±1.8) | 100 |
| 7 | <16 | 16 | 5.7 | <12.5 |
| 8 | >50 | >50 | >50 | >50 |
| 9 | 6.7 (±2.9) | >50 | >50 | ND |
| 10 | 37.1 (±6.1) | >50 | >50 | 50 |
| 11 | 0.3 | 18.0 | 1.4 | 0.46 |
50 µM is the cutoff for assays. Standard deviation is given in parentheses.
The results (Table 2) display some interesting dependence of antiviral activity on the shape and structure of the calix[4]arene. Introduction of n-butyl groups at the lower rim has the effect of locking the calix[4]arene scaffold into a cone conformation as the bulky substitutions are unable to invert through the ring. Benzylated compound 11 also exists in a well-defined cone conformation as indicated by the pair of doublets from the bridging methylene protons in the 1H NMR spectrum.14 Notably, compound 11 had an EC50 value of 0.3 µM for anti-HIV and 1.4 µM for anti-HCV activities, similar in potency to 2. However, the ID50 value was 18 µM suggesting toxicity at a significant lower concentration than that of compound 2 as it showed toxicity at a lower concentration than that of compound 2. Tetrahydroxy calix[4]arene derivative 9, which contains an identical upper periphery to that of 2 exhibited a drop in the EC50 value from 0.3 µM to 6.7 µM for HIV and from 1.8 µM to >50 µM for HCV. Computational studies suggest that a calix[4]arene with four hydroxyl groups at the lower rim can stabilize the scaffold into a cone conformation through intramolecular hydrogen bonding. However, the cone conformation is in equilibrium with the inverted cone conformation through a partial cone intermediate.14 Preorganization of the scaffold into a cone conformation for projection of the recognition groups appears to be important for both anti-HIV and anti-HCV activities.
While alkylation at the bottom rim was essential for activity, the importance of projected diacid groups on the top rim was also clear. Among the seven compounds with a butyl chain at the bottom rim, compound 2 exhibited the most potent anti-HIV activity. When the isophthalic derivative was replaced with aspartic acid in compound 5, anti-HIV activity decreases about ten-fold but anti-HCV activity slightly improved, while cytotoxicity remained the same. This suggested that aromatic substitutions are superior to aliphatic ones at the upper rim for HIV inhibition, while anti-HCV activity appears not to be as sensitive to this change. Compounds 1 and 3 contained benzyl ester and cyclohexylamide derivatives of the isophthalate linkers respectively, suggesting that substituting both acid groups are required for potent antiviral activity. Overall, these observations suggest that four charges on the top rim are important for anti- HIV and anti-HCV activities. The lack of antiviral activity in positively charged 7, confirms the importance of the negative charges on the projected periphery of the compounds.
In conclusion, the results demonstrate remarkable anti-HIV and anti-HCV activities for a series of compounds based on the tetrabutyl-calix[4]arene scaffold. We have shown that maintaining the cone conformation of the scaffold is important for antiviral activity. In addition, aromatic isophthalate spacers at the upper rim are essential for anti-HIV activities and the diacid groups are also necessary for the observed anti-HCV effects. Furthermore, we have identified a potent compound that possesses dual inhibition for both HIV and HCV in vitro. Moreover, it retains potency against different HIV strains in different cell lines while maintaining low cytotoxicity. The molecular targets and mechanisms for anti-HIV and anti-HCV activities with these calix[4]arene compounds are under investigation and will provide valuable insight for future attempts to improve potency.
Acknowledgment
We thank the National Institutes of Health (GM 35208 to A.D.H) and (AI 38204 to Y-C.C.) for financial support of this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kim AY, Chung RT. Gastroenterology. 2009;137:795. doi: 10.1053/j.gastro.2009.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singal AK, Anand BS. World J Gastroenterol. 2009;15:3713. doi: 10.3748/wjg.15.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weber R, Sabin CA, Friis-Moller N, Reiss P, El-Sadr WM, Kirk O, Dabis F, Law MG, Pradier C, De Wit S, Akerlund B, Calvo G, Monforte A, Rickenbach M, Ledergerber B, Phillips AN, Lundgren JD. Archives of internal medicine. 2006;166:1632. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 4.Velasco M, Guijarro C. Jama. 2000;283:2526. doi: 10.1001/jama.283.19.2526. [DOI] [PubMed] [Google Scholar]
- 5.Ascherl G, Hohenadl C, Schatz O, Shumay E, Bogner J, Eckhart L, Tschachler E, Monini P, Ensoli B, Sturzl M. Blood. 1999;93:4232. [PubMed] [Google Scholar]
- 6.Chi KD, McPhee RA, Wagner AS, Dietz JJ, Pantazis P, Goustin AS. Oncogene. 1997;15:1051. doi: 10.1038/sj.onc.1201267. [DOI] [PubMed] [Google Scholar]
- 7.Blaskovich MA, Lin Q, Delarue FL, Sun J, Park HS, Coppola D, Hamilton AD, Sebti SM. Nat Biotechnol. 2000;18:1065. doi: 10.1038/80257. [DOI] [PubMed] [Google Scholar]
- 8.Sun J, Blaskovich MA, Jain RK, Delarue F, Paris D, Brem S, Wotoczek-Obadia M, Lin Q, Coppola D, Choi K, Mullan M, Hamilton AD, Sebti SM. Cancer Res. 2004;64:3586. doi: 10.1158/0008-5472.CAN-03-2673. [DOI] [PubMed] [Google Scholar]
- 9.Sun J, Wang DA, Jain RK, Carie A, Paquette S, Ennis E, Blaskovich MA, Baldini L, Coppola D, Hamilton AD, Sebti SM. Oncogene. 2005;24:4701. doi: 10.1038/sj.onc.1208391. [DOI] [PubMed] [Google Scholar]
- 10.Zhou H, Wang DA, Baldini L, Ennis E, Jain R, Carie A, Sebti SM, Hamilton AD. Org Biomol Chem. 2006;4:2376. doi: 10.1039/b515483a. [DOI] [PubMed] [Google Scholar]
- 11.Perret F, Lazar AN, Coleman AW. Chemical communications (Cambridge, England) 2006:2425. doi: 10.1039/b600720c. [DOI] [PubMed] [Google Scholar]
- 12.Hwang KMQ, You Mao, Liu Su Ying, Choy William, Chen Jen. USA: Genelabs Technologies, I; 1994. [Google Scholar]
- 13.Coveney DC. Benjamin: AIDS Care Pharma Limited, I; 2005. [Google Scholar]
- 14.Veggel FCJMv. Calixarenes in Action. London: Imperial College Press; 2000. [Google Scholar]