Abstract
OBJECTIVES
To examine the association between hypertension and cognitive decline in older adults.
DESIGN
Prospective observational study.
SETTING
Four rural counties in China.
PARTICIPANTS
Two thousand rural Chinese aged 65 and older (median age 70, range 65–92) participated in a baseline evaluation. A follow-up evaluation of 1,737 subjects was conducted 2.5 years after baseline.
MEASUREMENTS
Cognitive function was assessed using the Community Screening Instrument for Dementia (CSID), Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Word List Learning and Recall Tests, Indiana University (IU) Story Recall Test, Animal Fluency Test, and IU Token Test. Hypertension was defined as the mean of two readings of systolic blood pressure (BP) of 140 mmHg or greater, diastolic BP of 90 mmHg or greater, or according to self-report. Cognitive decline was derived as the difference between baseline and follow-up scores. Analysis of covariance models were used to estimate the association between hypertension, BP, and cognitive decline, adjusting for other covariates.
RESULTS
Greater decline was found on the CERAD 10-Word List Learning (P<.001) and Recall (P =.01) scores for subjects with hypertension than for those without. In particular, significantly greater decline was seen in the group with hypertension that was not taking medication than in the group without hypertension. No significant difference on cognitive decline was found between subjects with hypertension who were taking medication and those without hypertention.
CONCLUSION
Untreated hypertension was associated with greater cognitive decline in this Chinese cohort. Better hypertension detection and treatment in elderly people, especially in developing countries, may offer protection against cognitive decline
Keywords: cognitive aging, cardiovascular risk factor, elderly
Hypertension is a leading cause of cardiovascular disease, but results from longitudinal studies on hypertension and cognitive decline have been inconsistent. There is a consensus that midlife high blood pressure (BP) is associated with late-life low cognitive function.1,2 A comprehensive review of the relationship between BP, hypertension, and cognitive decline indicates that many large cohort studies report no association between late-life BP and cognitive decline or dementia.2 Among plausible explanations for this null effect is the possibly protective effects of antihypertensive medications taken by substantial number of individuals in the study samples.
The relationship between late-life BP and cognitive decline deserves further examination because of the sharp increase of hypertension prevalence with increase in age. It has been reported that there is a 90% lifetime risk of developing hypertension for people who are not hypertensive at age 55.3 Because hypertension is a chronic disease that can be managed using medications, controlling hypertension may lead to potential prevention strategies for dementia and cognitive decline if the relationship between late-life BP and cognitive decline exists. Few of the published studies on hypertension and cognitive decline have included populations from developing countries, with the exception of one newly published clinical trial that included patients from China, Eastern Europe, and North Africa.4 The tripling of older persons living in developing countries from 249 million in 2000 to an estimated 690 million in 20305 and that many of these elderly persons do not have adequate access to medical diagnosis or treatment of chronic diseases underscores the projected significance of the relationship between late-life BP and cognitive decline. Given the increasing prevalence of hypertension with age and the paucity of research conducted in developing countries, it is of great public health interest to examine the relationship between hypertension and cognitive decline in these developing countries. This article reports findings from a prospective study in a rural elderly Chinese cohort.
METHODS
Study Population
Two thousand Chinese aged 65 and older from four counties in China were enrolled at study baseline (December 2003 to May 2005). Two counties were from Sichuan province in southwestern China and the other two from Shandong province in eastern China. For each village included in the study, Chinese investigators and a team of interviewers who were employees of provincial and county centers for disease control traveled to the area, established temporary headquarters, and conducted a complete census of residents aged 65 and older in the area. They enrolled eligible residents by going door to door, obtaining informed consent before conducting the interview. There were no refusals, although a few subjects with hearing problems were not enrolled. Details on site selection process were described previously.6 The Indiana University institutional review board and the Institute for Environmental Health and Related Product Safety, Chinese Center for Disease Control and Prevention approved the study.
Cognitive Assessment
Cognitive assessment was conducted in face-to-face interviews using the Community Screening Instrument for Dementia (CSID), the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Word List Learning and Recall,7 the Indiana University (IU) Story Immediate Recall Task, the Animal Fluency Test,8 and the IU Token Test. The CSID was developed as a screening tool for dementia in populations with various cultural backgrounds and literacy levels. The CSID has demonstrated good test–retest reliability, interrater reliability, and validity in detecting dementia when used for dementia screening in various populations.9,10 CSID scores range from 0 to 30. The CERAD Word List Learning test is one of the measures from the CERAD neuropsychological assessment battery designed to assess cognitive skills in elderly people. It consists of a 10-item, three-trial word list in which free recall is taken after each learning trial and after a brief delay (~ 5 minutes). The score is the total number of words recalled across the three learning trials (range 0–30) and after a delay (range 0–10). The research team created the IU Story Immediate Recall Task to be suitable for the Chinese culture and the rural population. The story has 14 units of information that are gist scored (range 0–14). The story was tested in 1,500 elderly Chinese in a previous pilot study and was found to be acceptable to the villagers and produced a normally distributed range of scores.11 The Animal Fluency Test requires a subject to name as many animals as possible in 60 seconds. This kind of naming task is associated with executive, linguistic, and semantic components.12 The IU Token Test is a brief measure of language comprehension and working memory, with scores ranging from 0 to 24.13 The validity of the CSID, the CERAD Word List Learning and Recall, and the Animal Fluency Test have been previously established in Chinese populations and elsewhere.14
The questionnaires were harmonized, translated into Chinese, and back-translated into English. To avoid potential bias, this process was accomplished using lay persons from outside the research team who were not familiar with the goals of the interview. Intensive training sessions for the interviewers were held before the start of the first site, and refresher training was held before interview at each of the other three sites. High interrater reliability was achieved after each interviewer training course using volunteers from the community as study subjects.
Follow-Up Evaluation
Follow-up evaluation of the cohort was conducted from June 2005 to November 2007 (mean follow-up time 2.4 years, range 2.3–2.6 years). Cognitive assessment was conducted using the same instruments used for the baseline evaluation.
Hypertension and Blood Pressure Measurements
At the baseline interview, participants were asked whether a doctor or healthcare professional ever told them that they had hypertension or high BP, defined as systolic BP of 140 mmHg or higher or diastolic BP of 90 mmHg or higher. A follow-up question of whether they were taking any antihypertensive medications was asked if they answered yes to the hypertension question. At the end of the interview, a physical examination was conducted. Trained interviewers took two consecutive measurements of systolic and diastolic BP using a mercury sphygmomanometer while the participant was seated following the protocol outlined in the Hypertension Detection and Follow-up Program and used in other studies.15,16 The average of the two BP measurements was used in all analyses.
Apolipoprotein E Genotype
Blood spots on filter paper were collected from all study participants during the baseline evaluation. Apolipoprotein E (APOE) genotype was determined by eluting deoxyribonucleic acid (DNA) from a dried blood spot17 followed by Haemophilus haemolyticus digestion of amplified products.18
Other Information
Other information collected during the baseline evaluation included age, sex, whether the participant attended school and years of schooling, marital status, household composition, alcohol consumption and smoking history, history of cancer, Parkinson’s disease, diabetes mellitus, hypertension, stroke, heart attack, head injury, and fracture. Participants’ height and weight were also measured during the interview. Body mass index (BMI; body weight in kilograms divided by height in meters squared) was derived from height and weight measurements.
Statistical Analysis
Characteristics of participants with follow-up evaluations were compared with characteristics of those lost to follow-up using t-tests for continuous variables and chi-square tests or Fisher exact tests for categorical variables.
For each participant with a follow-up evaluation, cognitive decline was derived as the difference between baseline score and follow-up score on each cognitive instrument. For ease of result interpretation, standardized decline scores (defined as the difference between the raw cognitive decline score and the mean decline score divided by the standard deviation of decline score) were used in all subsequent analyses.
Hypertension was defined as the mean of two readings of systolic BP of 140 mmHg or greater, diastolic BP of 90 mmHg of greater, or self-report according to established criteria.3 Analysis of covariance (ANCOVA) models were used to determine the association between hypertension and standardized cognitive decline, adjusting for covariates. Separate ANCOVA models were used first to identify factors associated with cognitive decline while controlling for age and education. All factors with P<.10 for at least one cognitive outcome were included in the final ANCOVA models for cognitive decline.
The association between hypertension and cognitive decline was examined in separate ANCOVA models including self-reported history of hypertension, hypertension defined using BP measure and self-report, and in three groups of participants: hypertension no medication, hypertension on medication. and normal BP. The association between continuous and categorized BP measures was then examined. The groupings for the categorization may help detect potential nonlinear trends, and they were chosen based on previous studies.19 The ANCOVA model was also used with pulse pressure, defined as the difference between systolic and diastolic BPs, as the independent variable while adjusting for other covariates. The ANCOVA models were repeated excluding participants whose baseline composite cognitive scores were below 10% of the cohort.
RESULTS
Of the 2,000 participants enrolled at baseline, 1,737 participated in the follow-up evaluation. In the subjects with follow-up, mean age ± standard deviation (SD) was 71.4 ±5.2, 922 (53.1%) were female, and 1,061 (61.1%) had never attended school, and mean BMI was 22.0 ± 3.5. Reasons for loss to follow-up were deceased (n = 144), too sick to be interviewed (n = 51), moved out of study area (n = 29), living in another location at time of interview (n = 18), severe hearing impairment (n = 12), refused (n = 3), and other (n = 6). Those lost to follow-up were older, less likely to have attended school, and more likely to be widowed and had lower BMI than the remaining participants. In addition, those lost to follow-up had significantly lower baseline scores on all six cognitive measures than those with follow-up evaluations. No significant differences were found in proportions of self-reported medical conditions between these two groups.
Of the 1,737 participants who were evaluated in the follow-up wave, 288 (16.6%) reported a history of hypertension, and 190 (10.9%) reported taking antihypertensive medications. According to BP measures, 1,063 (61.2%) individuals had systolic BP of 140 mmHg or greater or diastolic BP of 90 mmHg of greater. Thus, 1,099 (63.2%) individuals met the criteria for hypertension.
Table 1 shows participants’ characteristics collected at baseline according to whether they had hypertension but were not taking medication, had hypertension and were taking medication, or did not have hypertension. Significant differences were seen in alcohol consumed per week; smoking and pack-years; BMI; systolic and diastolic BP measures; and self-reported history of diabetes mellitus, stroke, and heart attack. When mean decline in cognitive scores in the three groups were compared in univariate analyses without adjustment for covariates, a significant difference was found for the CERAD Word List Learning score (P =.003), and marginally significant difference was seen for the CERAD Word List Recall score (P =.06).
Table 1.
Participants’ Baseline Characteristics According to Baseline Hypertension Status
| Baseline Characteristic | No Hypertension (n 5638) | Hypertension Not Taking Medication (n 5909) | Hypertension Taking Medicine (n 5190) | P-Value |
|---|---|---|---|---|
| Age, mean ±SD | 71.1 ± 5.3 | 71.7 ±5.3 | 71.0 ±5.0 | .04 |
| Age, % | .31 | |||
| 65–74 | 75.4 | 72.3 | 77.4 | |
| 75–84 | 22.4 | 26.1 | 21.6 | |
| ≥85 | 2.2 | 1.7 | 1.1 | |
| Female, % | 52.0 | 52.4 | 60.0 | .13 |
| Education (any schooling) % | 35.6 | 41.4 | 38.4 | .07 |
| Marital status, % | .60 | |||
| Married | 64.1 | 63.9 | 61.1 | |
| Widowed | 34.5 | 35.2 | 36.8 | |
| Other | 1.4 | 0.9 | 2.1 | |
| Household composition, % | .49 | |||
| Live with spouse | 52.9 | 51.6 | 51.6 | |
| Live with children | 33.5 | 32.9 | 29.8 | |
| Live alone | 13.5 | 15.5 | 18.6 | |
| Consume alcohol, % | 44.3 | 45.0 | 38.4 | .24 |
| Drinks/week, mean ± SD | 10.6 ± 9.2 | 12.5 ±9.9 | 13.4 ±10.5 | .02 |
| Smoking, % | <.001 | |||
| Current smoker | 37.6 | 34.7 | 23.7 | |
| Ex-smoker | 10.2 | 11.8 | 21.6 | |
| Pack-years, × 1,000, mean ±SD | 8.0 ± 6.1 | 9.5 ±8.0 | 1.1 ±7.7 | .001 |
| Body mass index, kg/m2, mean ± SD | 21.0 ± 3.1 | 22.2 ±3.5 | 24.3 ±3.6 | <.001 |
| Systolic BP, mmHg, mean ± SD | 123.3 ± 10.2 | 157.0 ±19.8 | 166.8 ±26.3 | <.001 |
| Diastolic BP, mmHg, mean ±SD | 75.0 ± 7.7 | 87.8 ±11.5 | 92.0 ±14.3 | <.001 |
| Apolipoprotein E ε4 carrier, % | 16.6 | 16.6 | 17.9 | .90 |
| Self-reported history, % | ||||
| Cancer | 0.5 | 0.8 | 0.5 | .82 |
| Parkinson’s disease | 0.6 | 1.1 | 0.5 | .64 |
| Diabetes mellitus | 1.7 | 2.3 | 6.3 | .004 |
| Stroke | 0.9 | 2.2 | 12.1 | <.001 |
| Heart attack | 2.5 | 3.5 | 6.8 | .02 |
| Head injury | 6.0 | 5.5 | 6.3 | .84 |
| Fracture | 2.4 | 2.3 | 3.2 | .73 |
| Baseline cognitive score, mean ± SD | ||||
| Community Screening Instrument for Dementia | 25.4 ±3.3 | 25.5 ±3.5 | 26.2 ±3.1 | .01 |
| IU Story Recall | 5.3 ±2.8 | 5.4 ±2.9 | 6.2 ±3.3 | .001 |
| Animal Fluency Test | 12.8 ± 12.8 | 13.2 ±5.0 | 12.7 ±5.2 | .20 |
| CERAD Word List Learning | 13.0 ± 13.0 | 13.3 ±3.9 | 13.6 ±4.0 | .19 |
| CERAD Word List Recall | 4.7 ± 4.7 | 4.7 ±1.9 | 4.9 ±2.1 | .29 |
| IU Token Test | 15.9 ± 15.9 | 16.3 ±5.2 | 17.6 ±4.9 | .001 |
Hypertension was defined as the mean of two readings of systolic blood pressure (BP) ≥140 mmHg or diastolic BP ≥90 mmHg or according to self-report. SD =standard deviation; CERAD = Consortium to Establish a Registry for Alzheimer’s Disease; IU = Indiana University.
Results from separate ANCOVA models are presented in Table 2 examining the relationship between various hypertension variables and cognitive decline on the two CERAD Word List scores adjusting for age, sex, education, APOE status, BMI, and history of stroke. When self-reported hypertension was used in the model (Model 1), no significant association with decline score on CERAD Word List Learning (P =.93) or Recall (P.= .67) score was found, but when measured BP was used to define hypertension, significantly greater decline was found on the CERAD Word List Learning (P= .001) and Recall (P=.007) score for individuals with hypertension than for those without (Model 2). In particular, when the group with hypertension was further divided into two groups, those taken antihypertensive medication and those not taking medication, significantly greater decline was seen in those not taking medication than in those without hypertension. In particular, those not taking medication had 0.17 SD more decline on the Learning score than those without hypertension (Model 3). In comparison, in the same model, APOE ε4 carriers showed 0.14SD more decline than the noncarriers (P =.01). The group taking antihypertensive medication had a greater, but nonsignificant, decline than those without hypertension.
Table 2.
Results of Separate Analysis of Covariance Models on the Association Between Various Hypertension Variables and Cognitive Decline Measured According to the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Word List Learning and Recall Scores
| Parameter Estimate (Standard Error) P-Value |
||
|---|---|---|
| Model | CERAD Word List Learning | CERAD Word List Recall |
| 1: Hypertension according to self-report | ||
| Yes | −0.01 (0.06) .93 | −0.03 (0.06) .67 |
| No | Reference | Reference |
| 2: Hypertension according to BP or self-report* | ||
| Yes | 0.15 (0.05) .001 | 0.12 (0.05) .007 |
| No | Reference | Reference |
| 3: Hypertension and medication use, overall P-value | .002 | .01 |
| Taking antihypertensive medicine | 0.09 (0.08) .26 | 0.06 (0.08) .44 |
| Hypertension not taking medicine* | 0.17 (0.05) .001 | 0.13 (0.05) .004 |
| No hypertension | Reference | Reference |
| 4: Continuous BP measures, × 10 mmHg | ||
| Systolic | 0.01 (0.01) .61 | −0.00 (0.01) .95 |
| Diastolic | 0.04 (0.02) .049 | 0.06 (0.02) .002 |
| 5: Categorized BP measures | ||
| Systolic blood pressure, mmHg, overall P-value | .30 | .89 |
| <130 | 0.10 (0.07) .13 | 0.05 (0.07) .50 |
| 130–139 | Reference | Reference |
| 140–149 | 0.15 (0.07) .049 | 0.02 (0.07) .80 |
| 150–159 | 0.15 (0.08) .07 | 0.07 (0.08) .37 |
| ≥160 | 0.11 (0.07) .12 | 0.05 (0.07) .47 |
| Diastolic blood pressure, mmHg, overall P-value | .08 | .005 |
| <70 | 0.03 (0.09) .73 | 0.05 (0.08) .56 |
| 70–79 | Reference | Reference |
| 80–89 | 0.06 (0.06) .28 | 0.13 (0.06) .02 |
| ≥90 | 0.17 (0.07) .01 | 0.23 (0.07) .001 |
| 6: Pulse pressure, × 10 mmHg | 0.01 (0.01) .47 | 0.00 (0.01) .82 |
All models adjusted for age, sex, education, Apolipoprotein E ε4 status, history of stroke, baseline body mass index, and baseline cognitive scores as covariates. Parameter estimates indicate relative differences in cognitive decline (expressed in units of standard deviation) between a particular group and the reference group controlling for all covariates. Positive parameter estimates indicate greater cognitive decline than in the reference group.
Hypertension defined as the mean of two readings of systolic blood pressure measure ≥140 mmHg or diastolic blood pressure ≥90 mmHg or according to self-report.
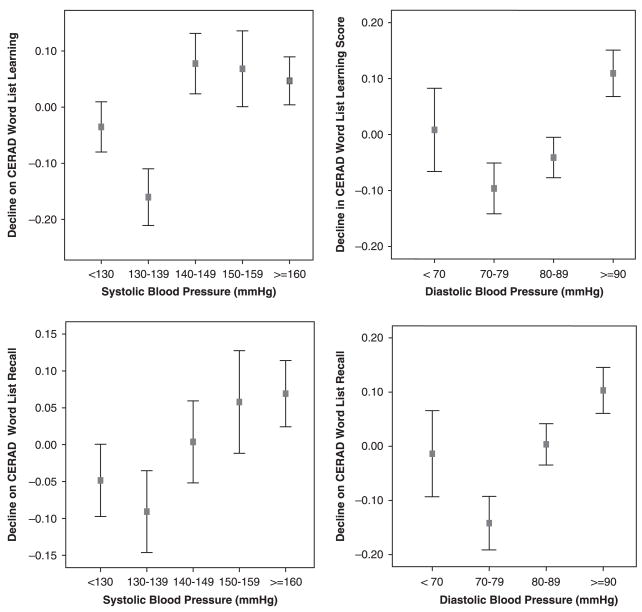
When systolic and diastolic BP were included as continuous variables (Model 4), diastolic BP was significantly related to decline on CERAD Word List Learning (P =.049) and Recall (P =.002), whereas systolic BP was not. Using categorized BP measures, it was found that individuals with diastolic BP of 80 mmHg or higher had significantly more decline on the Recall score (Model 5). There was no significant difference between the groups defined according to systolic BP in the decline of either score. Predicted mean decline scores are presented according to BP groups in Figure 1, in which a potential nonlinear (quadratic) trend between diastolic BP and both CERAD decline scores can be seen but was not statistically significant. No significant association was seen with Learning or Recall scores when pulse pressure was used in the ANCOVA models (Model 6). Similar results were obtained when analyses were repeated using BP measures excluding individuals taking antihypertensive medications.
Figure 1.
Relationship between baseline blood pressure and decline in Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Word List Learning and Recall scores adjusted for age, sex, education, apolipoprotein E status, body mass index, history of stroke, and baseline scores. The horizontal axis represents participants grouped according to their blood pressure measures. The vertical axis displays the means and 95% confidence intervals of decline in the two CERAD scores, defined as the difference in scores between baseline and follow-up. Higher values on the vertical axis indicate greater cognitive decline.
All models in Table 2 were repeated in a subsample excluding participants who may have had cognitive impairment at baseline (defined as baseline composite cognitive score <10%). Conclusions from all models were unchanged from those based on the entire sample.
There were 163 participants aged 80 and older at baseline; only 13 of them reported taking hypertension medicine. When the models in Table 2 were repeated in the samples of participants aged 80 and older, no significant association was found between the three groups of participants on the CERAD Word List Learning score (P =.78) or the CERAD Word List Recall score (P =.16), although similar parameter estimates in the models were obtained. The relationships between BP and cognitive decline were not significant.
DISCUSSION
In this rural elderly Chinese cohort, a significant association was found between hypertension and cognitive decline. In particular, individuals with hypertension who were not taking medications were found to have greater decline than those without hypertension. It was also found that individuals with higher diastolic BP had greater cognitive decline than those with diastolic BP of 70 to 79 mmHg.
High proportions of undetected and untreated hypertension were found in this cohort. The proportion of individuals meeting clinical definition of hypertension (63.2%) was comparable with that reported in the United States (65.4% for aged ≥60)20 but lower than in European populations (78% for aged 65–74)21 or an urban population in Beijing, China (73.4%).22 However, in this cohort, only 26.2% of individuals meeting the clinical definition of hypertension reported a doctor or another health professional ever telling them that they had hypertension, and 17.3% reported taking antihypertensive medications. These rates were much lower than the 69% “awareness” rate and the 58% treatment rate found in the 2000 National Health and Nutrition Examination Survey in the United States.20 The large numbers of elderly individuals with undetected and untreated hypertension underscores the enormous public health challenge facing the medical care system in many developing countries, but they offer an opportunity to untangle the potentially confounded relationship between hypertension, antihypertensive medications, and cognitive decline.
In this cohort, individuals taking antihypertensive medications did not show a significantly greater risk of cognitive decline, despite higher BP levels than those with hypertension who were not taking medication and those without hypertension. The nonelevated risk in the medication group in this cohort could be due to a protective effect of some antihypertensive medications on cognitive decline, as previously reported,2 but it could also be due to a potential selection bias in hypertension detection and treatment in this cohort. The data suggest that people taking medications seem to have more comorbid conditions and higher BMI than other participants, yet their cognitive decline did not differ significantly from those without hypertension. Information was not available on the types of antihypertensive medications the participants were taken.
Hypertension had been established as one of the leading risk factors for cardiovascular disease. There is moderate evidence supporting a relationship between midlife BP and cognition in late life, but the reported relationship between late-life BP and cognitive decline is less consistent.15,18,22 The effects of hypertension on cognition developing later in life deserves further attention, because studies on hypertension show sharp increases in the prevalence of hypertension from midlife to late life.20,21 In addition, high BP can be managed and controlled using medication.20 One explanation for the inconsistent results of the effects of late-life hypertension and cognition has been that a substantial proportion of individuals in these studies were taking antihypertensive medications, which are potentially protective. The relationship between BP measurements, treatment for hypertension, and cognitive decline for participants aged 80 and older is not significant in this cohort, but the number of participants in this category was small.
Several large randomized trials on hypertension treatment and incident dementia have been published.4,23–25 Although none of the trials established significant results by itself, the combined meta-analysis was borderline significant, with an estimated relative risk of 0.87 (95% confidence interval = 0.76–1.00), suggesting that hypertension treatment in elderly people may be protective of dementia risk.4
In the current cohort, individuals with hypertension who were not taking medication showed 0.17 SD more decline on the learning score than participants without hypertension during the 2.5 years of follow-up evaluation. Although this difference was statistically significant, the absolute difference in the change scores may be small during a relatively short follow-up. The clinical significance of this difference is yet to be determined with further addition of clinical diagnosis in this cohort. Nevertheless, the difference in decline between the untreated individuals with hypertension and those without hypertension is of a magnitude greater than carrying an APOE ε4 allele. Larger differences in decline may be anticipated with longer follow-up time.
This study had the strength of examining cognitive decline using multiple instruments, each targeting a slightly different cognitive domain. The tests measuring new learning and formation of new memories, such as the CERAD Word List Learning and Recall, were significantly associated with hypertension, whereas those targeting executive functions were not. It is possible that memory domains are more sensitive to age-related cognitive decline than other cognitive domains.26,27 Studies with similar length of follow-up have also shown that the most sensitive cognitive domains to age-related decline are those involved in the acquisition and early retrieval of new information.27 With longer follow-up, association between hypertension and declines on other domains may also be detected.
This study also had important limitations. Two BP measurements were measured on the same occasion, an approach adopted by most large epidemiological studies,15,16 rather than two measurements on each of two separate occasions defined for clinical practice.3 Therefore, it is possible that there may be misclassification of hypertension cases, yet the effect of the potential misclassification would be to bias the results toward the null hypothesis, meaning that an even stronger association would have been found without the potential misclassification. In addition, accurate information was not available on the onset time of hypertension in this cohort, making it impossible to separate midlife from late-life cases. What the results demonstrate is that hypertension measured in late life is associated with cognitive decline. Information collected on hypertensive medication was limited. Information was not collected on the type of medication or the duration of treatment. In addition, it is possible that the way information on antihypertensive medication was collected may have misclassified participants who were taking BP medication but not for the indication of hypertension treatment. Nevertheless, the presence of these potential misclassifications would have led to an even stronger association between hypertension and cognitive decline than the absence of these misclassifications. There is also the possibility that untreated subjects with hypertension may have suffered from memory impairment and had forgotten to report history of hypertension or had stopped their medication, although similar associations between hypertension and cognitive decline were also found after excluding participants who may have cognitive impairment at baseline, suggesting that subjects who were cognitively impaired at baseline were unlikely to have affected the robust association between hypertension and cognitive decline. A final limitation is that the study did not have information on the clinical diagnosis of dementia and was not able to examine the association between hypertension and dementia.
The finding of a significant association between hypertension, in particular untreated hypertension, and cognitive decline may have important public health relevance. Increasing detection and treatment of hypertension in the millions of elderly persons around the world, the majority of whom are in developing countries, may not only result in reduced cardiovascular events, it may also provide protective benefit on cognitive decline, thus prolonging the state of healthy aging and maintaining the quality of life for many elderly individuals.
Acknowledgments
The research is supported by National Institutes of Health Grants R01 AG019181, R01 AG09956, and P30 AG10133.
Footnotes
Conflict of Interest: The authors report no conflicts of interest.
Author Contributions: Conception and design: Gao, Jin, Hall, Liang, Unverzagt, Murrell, and Hendrie. Acquisition of data: Gao, Jin, Hall, Liang, Unverzagt, Murrell, Ma, Matesan, Cheng, Bian, Li, and Hendrie. Analysis and interpretation of data: Gao and Hendrie. Drafting of the manuscript: Gao, Jin, Liang, and Hendrie. Critical revision of the manuscript for important intellectual content: Gao, Jin, Hall, Liang, Unverzagt, Murrell, Ma, Matesan, Cheng, Bian, Li, and Hendrie. Statistical expertise: Gao. Obtaining funding: Gao, Jin, Hall, Liang, Unverzagt, Murrell, and Hendrie. Administrative, technical or material support: Jin, Liang, Ma, Cheng, Li, Bian, and Matesan. Supervision: Gao, Jin, Liang, and Hendrie.
References
- 1.Stampfer MJ. Cardiovascular disease and Alzheimer’s disease: Common links. J Intern Med. 2006;260:211–223. doi: 10.1111/j.1365-2796.2006.01687.x. [DOI] [PubMed] [Google Scholar]
- 2.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4:487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- 3.Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 4.Peters R, Beckett N, Forette F, et al. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): A double-blind, placebo controlled trial. Lancet Neurol. 2008;7:683–689. doi: 10.1016/S1474-4422(08)70143-1. [DOI] [PubMed] [Google Scholar]
- 5.Center for Disease Control and Prevention. Public health and aging: Trends in aging—United States and worldwide. JAMA. 2003;289:1371–1373. [PubMed] [Google Scholar]
- 6.Gao S, Jin Y, Hall KS, et al. Selenium level and cognitive function in rural elderly Chinese. Am J Epidemiol. 2007;165:955–965. doi: 10.1093/aje/kwk073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morris JC, Heyman A, Mohs RC, et al. The consortium to establish a registry for Alzheimer’s disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 8.Isaacs B, Akhtar AJ. The set test: A rapid test of mental function in old people. Age Ageing. 1972;1:222–226. doi: 10.1093/ageing/1.4.222. [DOI] [PubMed] [Google Scholar]
- 9.Hall KS, Ogunniyi AO, Hendrie HC, et al. A cross-cultural community based study of dementias: Methods and performance of the survey instrument: Indianapolis, U.S.A. and Ibadan, Nigeria. Int J Methods Psychiatr Res. 1996;6:129–142. [Google Scholar]
- 10.Hall KS, Gao S, Emsley CL, et al. Community screening interview for dementia (CSI ‘D’); performance in five disparate study sites. Int J Geriatr Psychiatry. 2000;15:521–531. doi: 10.1002/1099-1166(200006)15:6<521::aid-gps182>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 11.Emsley CL, Gao S, Li Y, et al. Trace element levels in drinking water and cognitive function among elderly Chinese. Am J Epidemiol. 2000;151:913–920. doi: 10.1093/oxfordjournals.aje.a010295. [DOI] [PubMed] [Google Scholar]
- 12.Lezak M, Howieson D, Loring D, et al. Neuropsychological Assessment. 4. New York: Oxford University Press; 2004. [Google Scholar]
- 13.Yamamoto K, Evans JD, Johnson KE, et al. Clinical utility of IU token test in the diagnosis of dementia. J Int Neuropsych Soc. 2003;9:316. [Google Scholar]
- 14.Prince M, Acosta D, Chiu H, et al. Dementia diagnosis in developing countries: A cross-cultural validation study. Lancet. 2003;361:909–917. doi: 10.1016/S0140-6736(03)12772-9. [DOI] [PubMed] [Google Scholar]
- 15.Race, education and prevalence of hypertension. Am J Epidemiol. 1977;106:351–361. [PubMed] [Google Scholar]
- 16.Hebert LE, Scherr PA, Bennett DA, et al. Blood pressure and late-life cognitive function change: A biracial longitudinal population study. Neurology. 2004;62:2021–2024. doi: 10.1212/01.wnl.0000129258.93137.4b. [DOI] [PubMed] [Google Scholar]
- 17.Yang M, Hendrie HC, Hall KS, et al. Improved procedure for eluting DNA from dried blood spots. Clin Chem. 1996;42:1115–1116. [PubMed] [Google Scholar]
- 18.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31:545–548. [PubMed] [Google Scholar]
- 19.Glynn RJ, Beckett LA, Hebert LE, et al. Current and remote blood pressure and cognitive decline. JAMA. 1999;281:438–445. doi: 10.1001/jama.281.5.438. [DOI] [PubMed] [Google Scholar]
- 20.Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA. 2003;290:199–206. doi: 10.1001/jama.290.2.199. [DOI] [PubMed] [Google Scholar]
- 21.Wolf-Maier K, Cooper RS, Banegas JR, et al. Hypertension prevalence and blood pressure levels in 6 European countries, Canada, and the United States. JAMA. 2003;289:2363–2369. doi: 10.1001/jama.289.18.2363. [DOI] [PubMed] [Google Scholar]
- 22.He Y, Jiang B, Wang J, et al. Prevalence of the metabolic syndrome and its relation to cardiovascular disease in an elderly Chinese population. J Am Coll Cardiol. 2006;47:1588–1594. doi: 10.1016/j.jacc.2005.11.074. [DOI] [PubMed] [Google Scholar]
- 23.SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP) JAMA. 1991;265:3255–3264. [PubMed] [Google Scholar]
- 24.Forette F, Seux ML, Staessen JA, et al. Prevention of dementia in randomised double-blind placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial. Lancet. 1998;352:1347–1351. doi: 10.1016/s0140-6736(98)03086-4. [DOI] [PubMed] [Google Scholar]
- 25.Tzourio C, Anderson C, Chapman N, et al. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Intern Med. 2003;163:1069–1075. doi: 10.1001/archinte.163.9.1069. [DOI] [PubMed] [Google Scholar]
- 26.Salloway S, Correia S, Richardson S. Key lessons learned from short-term treatment trials of cholinesterase inhibitors for amnestic MCI. Int Psychogeriatr. 2008;20:40–46. doi: 10.1017/S1041610207005650. [DOI] [PubMed] [Google Scholar]
- 27.Small SA, Stern Y, Tang M, et al. Selective decline in memory function among healthy elderly. Neurology. 1999;52:1392–1396. doi: 10.1212/wnl.52.7.1392. [DOI] [PubMed] [Google Scholar]