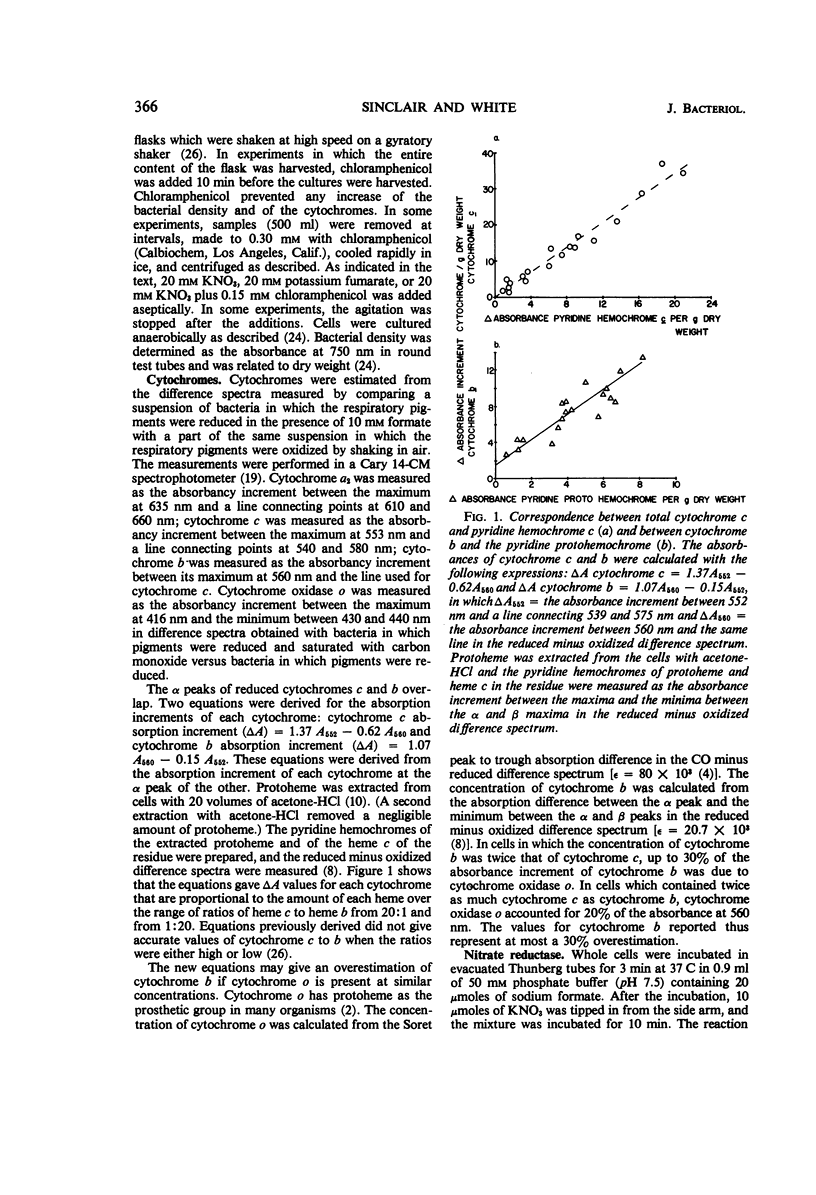
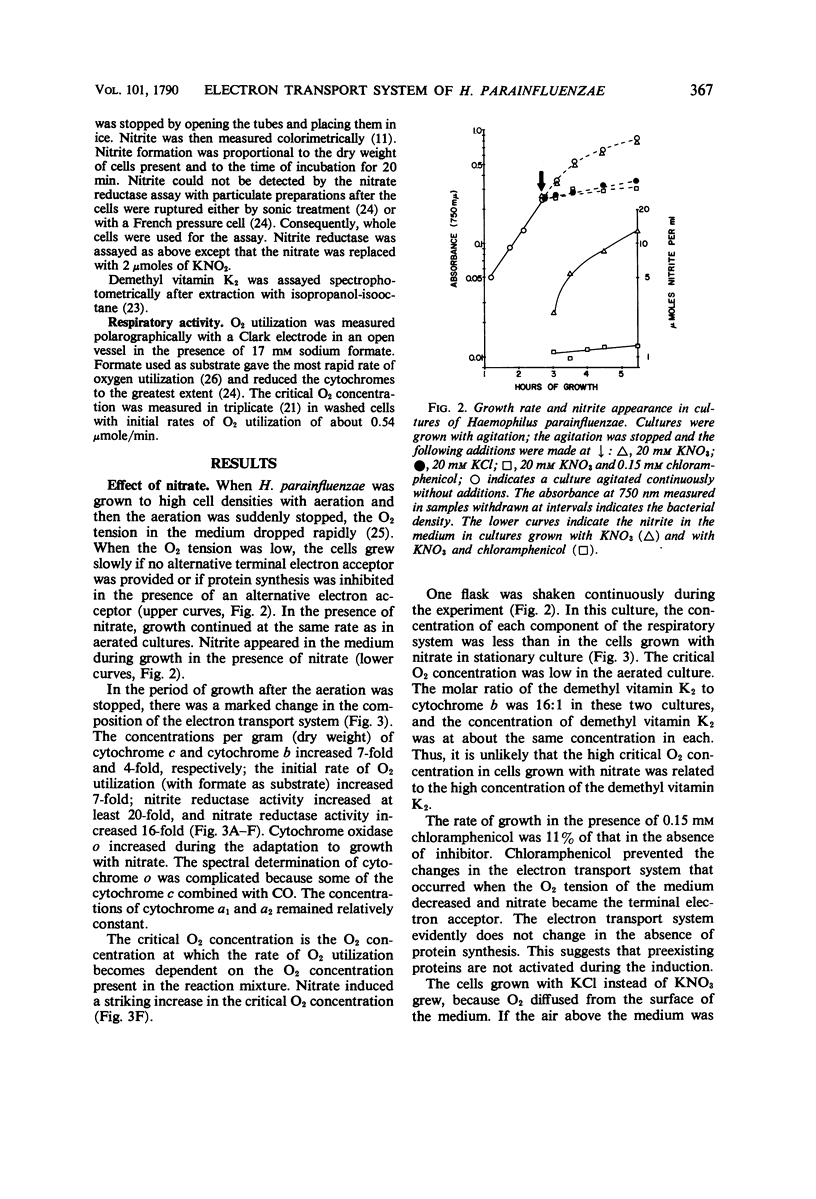
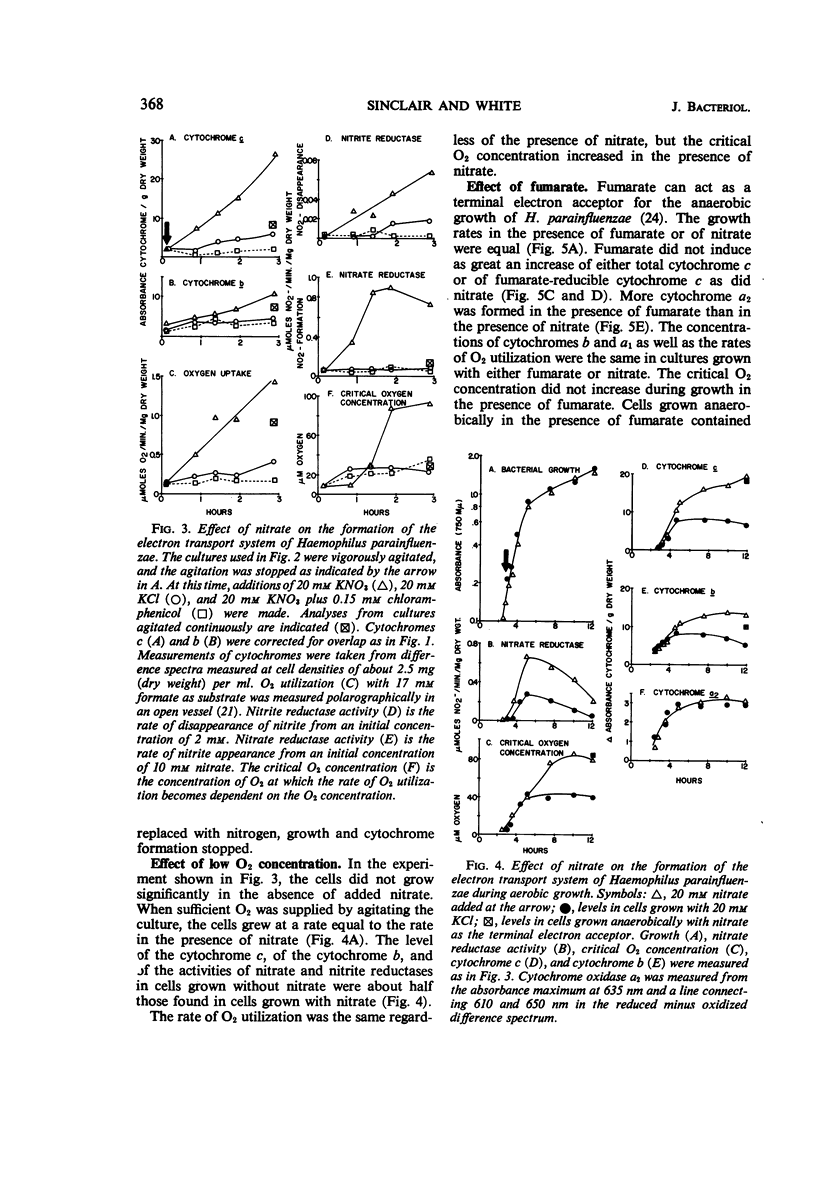
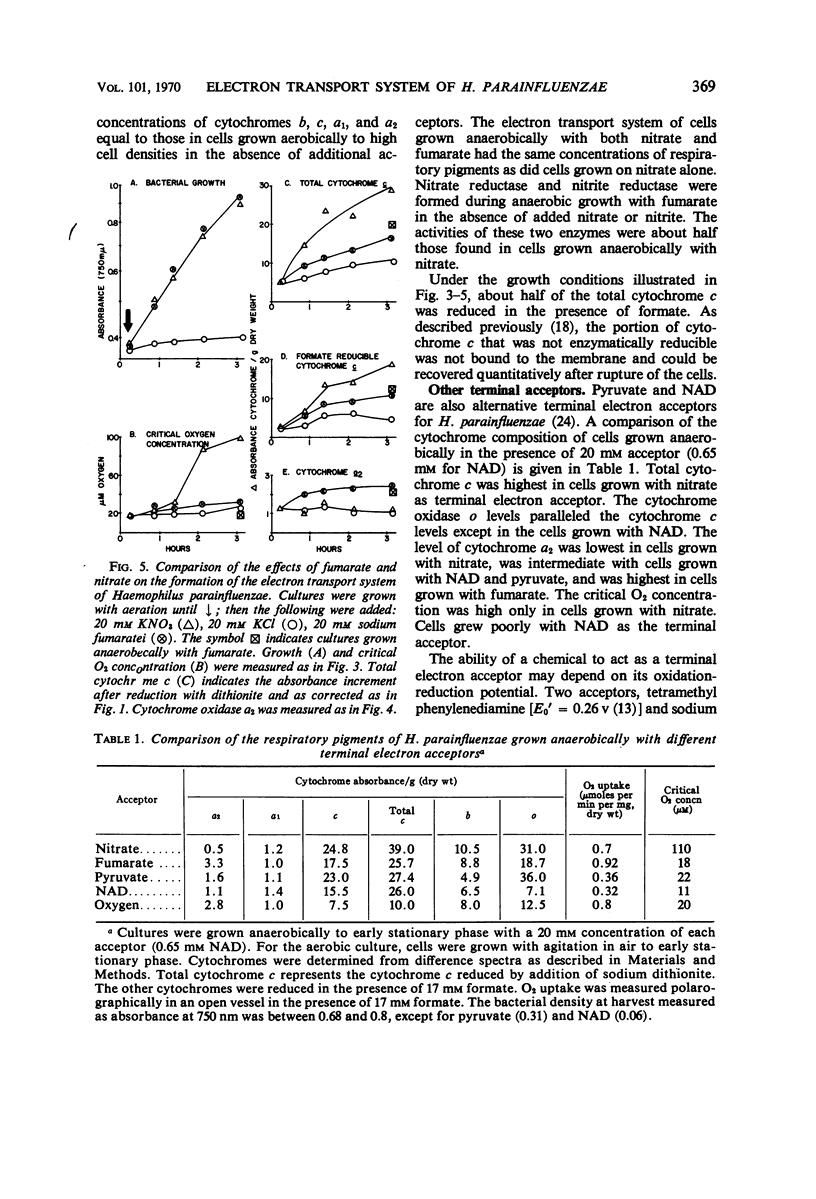
Abstract
The composition of the membrane-bound electron transport system of Haemophilus parainfluenzae underwent modification in response to the terminal electron acceptor in the growth medium. H. parainfluenzae was able to grow with O2, nitrate, fumarate, pyruvate, and substrate amounts of nicotinamide adenine dinucleotide (NAD) as electron acceptors. When O2 served as the electron acceptor and its concentration was lowered below 20 μm, the bacteria formed more cytochromes b, c, a1, a2, and o than were present in the cells grown at 150 to 200 μm O2. Nitrate and nitrite reductase activities also appeared during growth at the low O2 concentrations in the absence of added nitrate. Cytochrome levels in cells grown anaerobically with fumarate, pyruvate, or NAD as terminal acceptors were similar to those formed in cells grown at low O2 concentrations. Cells grown with nitrate had higher levels of cytochromes c, b, and o, and of nitrate and nitrite reductases, than did cells grown with the other acceptors. The formation of cytochrome oxidase a2 was repressed by the presence of nitrate in the growth medium. The critical O2 concentration (the O2 concentration at which the rate of O2 uptake becomes demonstrably dependent on the O2 concentration) was about 100 μm in cells grown with nitrate and about 15 μm in cells grown with the other acceptors. A mutant of H. parainfluenzae was found to make about 10% as much cytochrome c as the wild type, and its formation of cytochrome a2 was not repressed by nitrate. The critical O2 concentration of the mutant was high when it was grown with nitrate, suggesting that the high levels of cytochrome c and the absence of cytochrome a2 from the wild type are not responsible for the high critical O2 concentration. The modifications of the respiratory system induced by changing the terminal electron acceptor were inhibited by the presence of chloramphenicol, which suggests that protein synthesis is involved.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arima K., Oka T. Cyanide Resistance in Achromobacter I. Induced Formation of Cytochrome a(2) and Its Role in Cyanide-Resistant Respiration. J Bacteriol. 1965 Sep;90(3):734–743. doi: 10.1128/jb.90.3.734-743.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch R. G. Bacterial cytochromes. Annu Rev Microbiol. 1968;22:181–200. doi: 10.1146/annurev.mi.22.100168.001145. [DOI] [PubMed] [Google Scholar]
- Cavari B. Z., Avi-Dor Y., Grossowicz N. Induction by oxygen of respiration and phosphorylation of anaerobically grown Escherichia coli. J Bacteriol. 1968 Sep;96(3):751–759. doi: 10.1128/jb.96.3.751-759.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chippaux M., Pichinoty F. Altération par mutation de la régulation de la biosynthèse des cytochromes par les cultures anaérobies d'une bactérie anaérobie facultative. Eur J Biochem. 1968 Oct 17;6(1):131–134. doi: 10.1111/j.1432-1033.1968.tb00429.x. [DOI] [PubMed] [Google Scholar]
- Clark-Walker G. D., Rittenberg B., Lascelles J. Cytochrome synthesis and its regulation in Spirillum itersonii. J Bacteriol. 1967 Nov;94(5):1648–1655. doi: 10.1128/jb.94.5.1648-1655.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J. A., Wimpenny J. W. Metabolic pathways for nitrate reduction in Escherichia coli. Biochim Biophys Acta. 1968 Jul 16;162(1):39–48. doi: 10.1016/0005-2728(68)90212-0. [DOI] [PubMed] [Google Scholar]
- Harrison D. E., Pirt S. J. The influence of dissolved oxygen concentration on the respiration and glucose metabolism of Klebsiella aerogenes during growth. J Gen Microbiol. 1967 Feb;46(2):193–211. doi: 10.1099/00221287-46-2-193. [DOI] [PubMed] [Google Scholar]
- Hill G. C., White D. C. Respiratory pigments of Crithidia fasciculata. J Bacteriol. 1968 Jun;95(6):2151–2157. doi: 10.1128/jb.95.6.2151-2157.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBS E. E., SANADI D. R. Phosphorylation coupled to electron transport mediated by high potential electron carriers. Biochim Biophys Acta. 1960 Feb 12;38:12–34. doi: 10.1016/0006-3002(60)91192-6. [DOI] [PubMed] [Google Scholar]
- MOSS F. Adaptation of the cytochromes of Aerobacter aerogenes in response to environmental oxygen tension. Aust J Exp Biol Med Sci. 1956 Oct;34(5):395–405. doi: 10.1038/icb.1956.48. [DOI] [PubMed] [Google Scholar]
- MOSS F. The influence of oxygen tension on respiration and cytochrome a2 formation of Escherichia coli. Aust J Exp Biol Med Sci. 1952 Dec;30(6):531–540. doi: 10.1038/icb.1952.51. [DOI] [PubMed] [Google Scholar]
- PICHINOTY F., d' ORNANO [Influence of the culture conditions on the formation of nitrate reductase of Aerobacter aerogenes]. Biochim Biophys Acta. 1961 Mar 18;48:218–220. doi: 10.1016/0006-3002(61)90783-1. [DOI] [PubMed] [Google Scholar]
- SMITH L., WHITE D. C. Structure of the respiratory chain system as indicated by studies with Hemophilus parainfluenzae. J Biol Chem. 1962 Apr;237:1337–1341. [PubMed] [Google Scholar]
- Showe M. K., DeMoss J. A. Localization and regulation of synthesis of nitrate reductase in Escherichia coli. J Bacteriol. 1968 Apr;95(4):1305–1313. doi: 10.1128/jb.95.4.1305-1313.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE D. C. Cytochrome and catalase patterns during growth of Haemophilus parainfluenzae. J Bacteriol. 1962 Apr;83:851–859. doi: 10.1128/jb.83.4.851-859.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE D. C. DIFFERENTIAL SYNTHESIS OF FIVE PRIMARY ELECTRON TRANSPORT DEHYDROGENASES IN HEMOPHILUS PARAINFLUENZAE. J Biol Chem. 1964 Jun;239:2055–2060. [PubMed] [Google Scholar]
- WHITE D. C. FACTORS AFFECTING THE AFFINITY FOR OXYGEN OF CYTOCHROME OXIDASES IN HEMOPHILUS PARAINFLUENZAE. J Biol Chem. 1963 Nov;238:3757–3761. [PubMed] [Google Scholar]
- WHITE D. C. Respiratory systems in the hemin-requiring Haemophilus species. J Bacteriol. 1963 Jan;85:84–96. doi: 10.1128/jb.85.1.84-96.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE D. C., SMITH L. LOCALIZATION OF THE ENZYMES THAT CATALYZE HYDROGEN AND ELECTRON TRANSPORT IN HEMOPHILUS PARAINFLUENZAE AND THE NATURE OF THE RESPIRATORY CHAIN SYSTEM. J Biol Chem. 1964 Nov;239:3956–3963. [PubMed] [Google Scholar]
- WHITE D. C. THE FUNCTION OF 2-DEMETHYL VITAMIN K2 IN THE ELECTRON TRANSPORT SYSTEM OF HEMOPHILUS PARAINFLUENZAE. J Biol Chem. 1965 Mar;240:1387–1394. [PubMed] [Google Scholar]
- White D. C. Effect of glucose on the formation of the membrane-bound electron transport system in Haemophilus parainfluenzae. J Bacteriol. 1967 Feb;93(2):567–573. doi: 10.1128/jb.93.2.567-573.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C. The obligatory involvement of the electron transport system in the catabolic metabolism of Haemophilus parainfluenzae. Antonie Van Leeuwenhoek. 1966;32(2):139–158. doi: 10.1007/BF02097454. [DOI] [PubMed] [Google Scholar]
- Wimpenny J. W., Cole J. A. The regulation of metabolism in facultative bacteria. 3. The effect of nitrate. Biochim Biophys Acta. 1967 Oct 9;148(1):233–242. doi: 10.1016/0304-4165(67)90298-x. [DOI] [PubMed] [Google Scholar]


