Abstract
Objective
Therapeutic hypothermia after cardiac arrest improves survival and functional outcomes, whereas hyperthermia is harmful. The optimal method of tracking the effect of temperature on neurologic recovery after cardiac arrest has not been elucidated. We studied the recovery of cortical electrical function by quantitative electroencephalography after 7-min asphyxial cardiac arrest, using information quantity (IQ).
Design
Laboratory investigation.
Setting
University medical school and animal research facility.
Subjects
A total of 28 male Wistar rats.
Interventions
Using an asphyxial cardiac arrest rodent model, we tracked quantitative electroencephalography of 6-hr immediate postresuscitation hypothermia (at 33°C), normothermia (37°C), or hyperthermia (39°C) (N=8 per group). Neurological recovery was evaluated using the Neurological Deficit Score. Four rats were included as a sham control group.
Measurements and Main Results
Greater recovery of IQ was found in rats treated with hypothermia (IQ=0.74), compared to normothermia (IQ=0.60) and hyperthermia (IQ=0.56) (p<.001). Analysis at different intervals demonstrated a significant separation of IQ scores among the temperature groups within the first 2 hrs postresuscitation (p<.01). IQ values of >0.523 at 60 mins postresuscitation predicted good neurological outcome (72-hr Neurological Deficit Score of≥60) with a specificity of 100% and sensitivity of 81.8%. IQ was also significantly lower in rats that died prematurely compared to survivors (p<.001). IQ values correlated strongly with 72-hr Neurological Deficit Score as early as 30 mins post-cardiac arrest (Pearson correlation 0.735, p<.01) and maintained a significant association throughout the 72-hr experiment. No IQ difference was noted in sham rats with temperature manipulation.
Conclusions
The enhanced recovery provided by hypothermia and the detrimental effect by hyperthermia were robustly detected by early quantitative electroencephalography markers. IQ values during the first 2 hrs after cardiac arrest accurately predicted neurological outcome at 72 hrs.
Keywords: cardiac arrest, hypothermia, hyperthermia, electrophysiology, functional outcome, ischemia
Approximately 164,600 cardiac arrests (CAs) occur in the United States each year (1). Among initial survivors, 80% remain comatose after resuscitation (2) and neurological complications represent the leading cause of disability (3, 4). The ischemic brain is sensitive to temperature, such that small differences can critically influence neuropathological outcomes (5). Hyperthermia has been demonstrated to worsen ischemic outcome and is associated with increased brain injury in animal models (5, 6) and clinical studies (7-9). On the other hand, induced hypothermia to 32-34°C is recommended for comatose survivors of CA (10, 11) and was recently shown to significantly mitigate brain injury in animal models (12-14) and clinical trials (15-18).
Neurological monitoring of comatose CA survivors is complicated by the requirement for sedative and paralytic agents, particularly in patients who are treated with hypothermia. Comatose CA survivors are typically cared for by nurses and physicians in general or cardiac intensive care units with little specialized training in neurological examination. In addition, the ability to detect even major changes in brain function in comatose patients is limited. Electroencephalography (EEG) is frequently employed for neuromonitoring and prognostication (19-21). A recent publication from our group demonstrated that physicians were more likely to withdraw supportive measures in patients with negative neurologic predictors (22). As a diagnostic tool, however, waveform-based EEG analysis is subjective and laborious, with results depending on the interpreter’s expertise(23). Previous attempts to use early quantitative EEG (qEEG) as a measure of neurological recovery after CA have utilized power spectral analysis (24). A readily translatable tool for tracking the effect of temperature on recovery of cortical electrical function has not been thoroughly elucidated.
We have previously established and validated a rodent model for global ischemic brain injury after CA (25, 26) using a standardized Neurological Deficit Scale (NDS) that was adapted from human and animal scales (12, 14, 17, 27-29). We developed the theoretical measure information quantity (IQ) to provide an objective measure of entropy in EEG amplitude (30). Our subsequent work showed that greater injury was associated with lower entropy and reduced IQ values. This methodology tracked functional outcome (23, 25, 26, 31-36) and accurately differentiated EEG recovery between rats treated with hypothermia and normothermia after cardiac arrest (23, 33). To expand on these findings, we evaluated the impact of qEEG-IQ marker for monitoring the effect of temperature on neurological recovery.
Materials and Methods
A total of 24 adult male Wistar rats (300-350g, Charles River, Wilmington MA) were randomly assigned to 7-min asphyxial CA and resuscitation with either hypothermia (hypothermia group), normothermia (normothermia group), and hyperthermia (hyperthermia group) (n=8 per group). Another four anesthetized rats were included as a sham control group for evaluation of the effect of temperature on qEEG in the absence of CA injury. The experimental protocol was approved by the Johns Hopkins Animal Care and Use Committee.
Experimental asphyxia-cardiac arrest model
The asphyxial CA and cardiopulmonary resuscitation (CPR) model used previously validated protocols (23, 26, 30). In brief (detail described by Jia et al. (23)), rats were mechanically ventilated with 1.0% Halothane in N2/O2 (50%/50%). The femoral artery and vein were cannulated to monitor mean arterial pressure sample arterial blood gases, and administer fluid and drugs. Five minutes of baseline recording with halothane was followed by a 5-min washout to ensure no significant residual effect of halothane on qEEG (25). Cardiac arrest was initiated via asphyxia with paralysis and cessation of mechanical ventilation for 7 minutes. Cardiopulmonary resuscitation was performed with sternal compressions (200/min) until return of spontaneous circulation (ROSC). arterial blood gases were obtained four times per animal: at baseline, and 10, 20, and 40mins after resuscitation. Sedative agents were not used after CA to avoid confounding influences on EEG (37).
Immediate temperature manipulation after Cardiac arrest
An intraperitoneal sensor (G2 E-mitter 870-0010-01, Mini Mitter, Sun River, OR) implanted 1 wk before experiments was used to monitor core temperature (23). Hypothermia or hyperthermia was induced immediately after ROSC. Hypothermia was achieved through surface cooling with misted water to achieve the target temperature of 33°C within 15 mins (23, 30). The core temperature was maintained between 32 and 34°C for 6 hours. Rats were then gradually warmed from 33.0° to 37.0° C over 2 hrs.
Hyperthermia was achieved using a warming blanket and an automatic warming lamp (Thermalet TH-5, model 6333, Phyritemp Instrumentd, Clifton, NJ) to achieve a target temperature of 39° C within 15 mins, and this temperature was maintained at 38.5-39.5°C for 6 hours. Rats were then passively cooled to 37.0° C in the course of 2 hrs.
The normothermia group was maintained at 36.5-37.5°C for 8 hrs after ROSC. To ensure that no temperature fluctuation occurred after the resuscitation, such as spontaneous hypothermia (6), all animals were then kept inside a neonatal incubator (Isolette infant incubator model C-86, Air-shields Inc, Hatboro, PA) for the first 24 hours post-ROSC.
For the purpose of comparing the direct effect of temperature on IQ, we collected EEGs in four sham animals. Sham rats underwent identical surgical preparation without CA. Anesthesia was maintained with 1.0% Halothane in N2/O2 (50%/50%) throughout the experiment. Continuous EEG was recorded. Four rats were monitored during normothermic baseline for 30 mins, then a target temperature of 32-34°C was maintained for 1 hr. During the course of 30 mins, the rats were allowed to return to normothermia (36.5-37.5°C). After 30 minutes, a temperature of 38.5-39.5°C was maintained for 1 hour.
EEG recording and Quantitative EEG analysis
EEG signals were recorded from both hemispheres at a sampling rate of 250 Hz (23). Serial 30-min EEG recordings were then performed at 24, 48, and 72hrs after ROSC in each group. Signals were examined for noise, both in time domain using WinDaq software, and in frequency spectrum using MATLAB (Mathworks, Natick, MA). Signals that were contaminated by artifact were not included in the final analysis.
We determined the amount of information in the EEG using our previously reported IQ algorithm. First, the EEG waveform was divided into a series of windows of equal length. For the EEG signal in each window, wavelet coefficients were computed using a discrete wavelet transform. The statistical distribution of the wavelet coefficients within a window was then determined by constructing a histogram. Using the frequency of wavelet coefficients in each bin of the histogram, the information or disorder was calculated using the formula for entropy. The final value of the entropy of wavelet coefficients for a single window of EEG is called the Information Quantity (IQ). To quantify how entropy evolves over time, IQ was averaged over several intervals. To compare IQ between multiple rats, the IQ time averages were normalized to a baseline mean, which is the average IQ for the time window preceding CA.
As a result of this analyses, which has been described previously (23, 30), we obtained normalized EEG values that range from 1.0 (control) to 0.0 (isoelectricity). IQ is normalized to baseline values, such that higher values reflect greater EEG entropy relative to baseline. We selected eight segments in each rat and calculated IQ values: baseline (IQ1), and 30 mins (IQ2), 1 hr (IQ3), 2 hrs (IQ4), 4 hrs (IQ5), 24 hrs (IQ6), 48 hrs (IQ7), and 72 hrs (IQ8) post-ROSC.
Neurological evaluation
NDS was determined after the recovery period on the first day, and then repeated at 24, 48, and 72hrs after ROSC. The NDS measures level of arousal, cranial nerve reflexes, motor function, and simple behavioral responses and has a range of 0-80 (Table 1) (23, 26). The NDS examination was performed by a trained examiner blinded to temperature group and the primary outcome measure of this experiment was defined as the 72-hr NDS score. We pre-specified the NDS cut-off for good (NDS≥60) and poor (NDS<60) outcome (26, 31) which represents a level of neurologic function required for independent function.
Table 1.
NEURODEFICIT SCORING FOR RATS (Best function = 80; worst function = 0)
| A)General Behavioral deficit | Total Score : 19 |
| Consciousness | Normal 10/ Stuporous 5 / Comatose or unresponsive 0 |
| Arousal: | Eyes open spontaneously 3/ Eyes open to pain 1/ No Eye Opening 0 |
| Respiration: | Normal 6/ Abnormal (hypo or hyperventilation) 3/ Absent 0 |
| B) Brain-stem Function: | Total Score : 21 |
| Olfaction: response to smell of food | Present 3/Absent 0 |
| Vision: head movement to light | Present 3/Absent 0 |
| Pupillary Reflex: pupillary light reflex | Present 3/Absent 0 |
| Corneal Reflex:, | Present 3/Absent 0 |
| Startle Reflex: | Present 3/Absent 0 |
| Whisker Stimulation: | Present 3/Absent 0 |
| Swallowing: swallowing liquids or solids | Present 3/Absent 0 |
| C) Motor Assessment: Strength: Total Score : 6 |
Normal 3/ Stiff or Weak 1 / No movement/Paralyzed 0. |
| Left and Right side tested and scored separately. |
|
| D) Sensory Assessment: Pain: Total Score : 6 |
Brisk Withdrawal with pain 3/ Weak or abnormal response (extension or flexion posture) 1 /No Withdrawal 0. |
| Left and Right side tested and scored separately. |
|
| E) Motor behavior: | Total Score : 6 |
| Gait coordination: | Normal 3 / Abnormal 1 / Absent 0 |
| Balance on Beam: | Normal 3 /Abnormal 1 / Absent 0 |
| F) Behavior: | Total Score : 12 |
| Righting reflex: | Normal 3 / Abnormal 1 / Absent 0 |
| Negative Geotaxis: | Normal 3 / Abnormal 1 / Absent 0 |
| Visual Placing: | Normal 3 / Abnormal 1 / Absent 0 |
| Turning Alley: | No rmal 3 / Abnormal 1 /Absent 0 |
| G) Seizures(convulsive or non-convulsive): Total Score : 10 |
No Seizure 10 / Focal Seizure 5 / General Seizure 0 |
Statistical methods
Statistical analysis was performed using a computerized statistical package (Statistics Program for the Social Sciences version 14, Chicago, IL). Group values that were parametric were reported as mean±SEM and non-parametric variables were reported as median (interquartile range). Univariate analysis was performed for parametric data with Student’s t-test for continuous variables, chi-square for categorical variables, and least significant difference for multiple comparisons. The multivariate general linear model was used for comparison of aggregate data to account for influencing factors, such as temperature. Nonparametric analysis of variance was used to test for differences in rank order NDS as a repeated measure. The mortality rate was analyzed by Fisher’s exact test (crosstabs) and survival was analyzed by a Kaplan-Meier test. Pearson correlation of bivariate analysis was used to analyze the correlation between 72-hr NDS score with serial IQ. A receiver operating characteristic curve was constructed to determine the IQ cutpoint with optimal sensitivity and specificity for good neurological outcome (72-hr NDS ≥60). A level of p<.05 was selected to consider differences significant.
Results
Temperature and Arterial Blood Gas monitoring
The target temperature was readily achieved and maintained for the defined duration for each of the three groups, as shown in Figure 1. Arterial blood gas data including arterial pH, HCO3−, PCO2, PO2 and oxygen saturation in hypothermic, normothermic and hyperthermic groups, were similar (Table 2).
Figure 1.
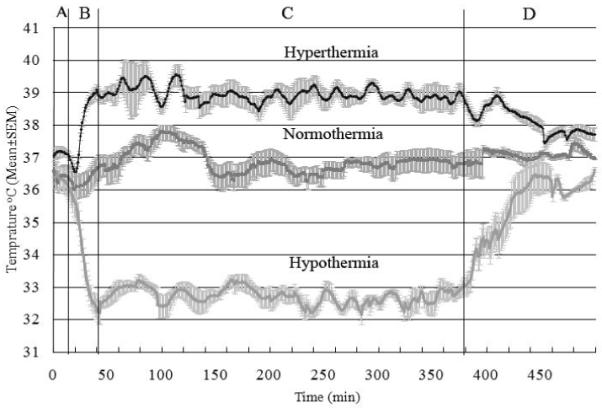
Temperature recording of rats subjected to 7 mins of asphyxic cardiac arrest. These different cohorts received hyperthermia (upper), normothermia (middle), and hypothermia (bottom). The plots show temperatures during four different phases of the experiment. A: baseline and asphyxial cardiac arrest period with cardiaoplumonary resuscitation at 17 mins, B: temperature manipulation induction period, C: temperature manipulation maintenance period, and D: Temperature manipulation recovery period. The solid black line is mean temperature and gray field is SEM.
Table 2.
Arterial blood gasdata in cardiac arrest experiment among 3 groups
| Hypothermia | Normothermia | Hypert hermia | p value | |
|---|---|---|---|---|
| Baseline | ||||
|
| ||||
| pH | 7.42±0.02 | 7.43±0.00 | 7.44±0.01 | 0.79 |
| PCO2 (mmHg) | 39±4 | 37±1 | 33±2 | 0.61 |
| PO2 (mmHg) | 383±25 | 342±41 | 362±37 | 0.71 |
| HCO3 (mmol/L) | 24±5 | 24±1 | 22±1 | 0.54 |
| O2 SAT(%) | 100±0 | 100±0 | 100±0 | 1.00 |
|
| ||||
| 10min post-resuscitation | ||||
|
| ||||
| pH | 7.35±0.02 | 7.35±0.04 | 7.36±0.02 | 0.52 |
| PCO2 (mmHg) | 36±2 | 37±2 | 35±1 | 0.48 |
| PO2 (mmHg) | 403±52 | 496±19 | 449±46 | 0.13 |
| HCO3 (mmol/L) | 19±1 | 20±2 | 19±1 | 0.32 |
| O2SAT (%) | 100±0 | 100±0 | 100±0 | 0.44 |
|
| ||||
| 20min post-resuscitation | ||||
|
| ||||
| pH | 7.37±0.01 | 7.34±0.03 | 7.39±0.02 | 0.06 |
| PCO2 (mmHg) | 35±2 | 41±2 | 34±2 | 0.04* |
| PO2 (mmHg) | 496±19 | 442±36 | 494±29 | 0.36 |
| HCO3 (mmol/L) | 20±1 | 22±1 | 20±2 | 0.61 |
| O2SAT (%) | 100±0 | 100±0 | 100±0 | 1.00 |
|
| ||||
| 40min post-resuscitation | ||||
|
| ||||
| pH | 7.31±0.02 | 7.37±0.01 | 7.41±0.02 | 0.30 |
| PCO2 (mmHg) | 44±4 | 41±2 | 36±3 | 0.51 |
| PO2 (mmHg) | 455±39 | 462±44 | 470±22 | 0.37 |
| HCO3 (mmol/L) | 22±1 | 23±1 | 22±1 | 0.58 |
| O2SAT (%) | 100±0 | 100±0 | 100±0 | 1.00 |
Statistically significant difference was noted but was minimal to cause any significant change in pH.
Functional outcome, survival rates and mean survival duration
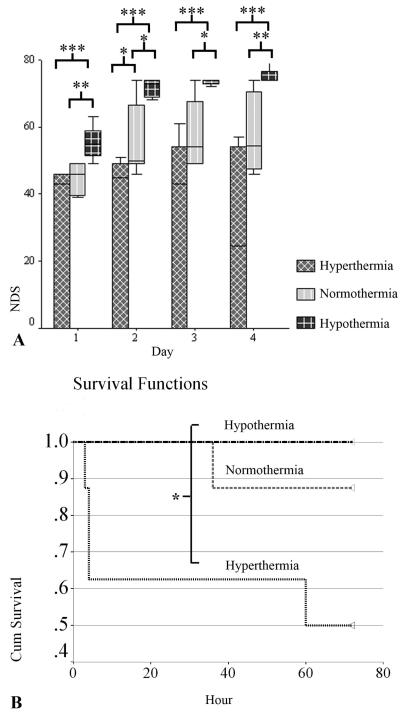
During the 72-hr experiment, aggregate analysis of NDS in all animals showed significant differences (p<.001) among the three groups. The hypothermia group consistently had better recovery, with higher NDS scores (Median [interquartile range], 74[ 61-74]) compared to the normothermia group (49 [47-61]) (p<.001), which was significantly higher at all time periods than the hyperthermia (43[0-50]) group (p=.001) (Fig. 2A). There were significantly more good outcomes (NDS≥60) among hypothermia rats (eight of eight) than the hyperthermia (zero of eight) (p<.001) and normothermia (three of eight) groups (p=.026) while no significant differences existed between the normothermia and hyperthermia groups.
Figure 2.
Outcomes by temperature manipulation groups measured by Neurological Deficit Score (NDS) (median [interquartile range]) (A) and survival (B). A significant difference in NDS was noted during the 72-hr experiment after asphyxial cardiac arrest (CA) between hypothermia and normothermia (p<.001) and between normothermia and hyperthermia (p=.001) groups. Significant differences existed in all periods between hypothermia and normothermia and at 2 days post-CA between normothermia and hyperthermia groups. The hypothermia group had a better survival rate and mean survival duration than the hyperthermia group. (*p< .05, **p< .01, ***p< .001)
Kaplan-Meier analysis demonstrated improved survival and mean duration of survival hours (both p<.05) in rats treated with hypothermia (eight of eight, 100%, 72 hrs) compared to the hyperthermia group (four of eight, 50%, 45 hrs), whereas the differences between the normothermia (seven of eight, 87.5%, 68 hos) and other groups were nonsignificant (Fig. 2B).
Electrophysiologic Monitoring
Conventional EEG assessment and qEEG analysis
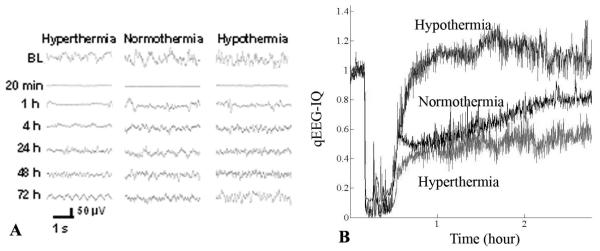
Within seconds of CA, EEG became isoelectric. Recovery was marked by several typical waveform patterns, of variable duration. Using cursory subjective visual review alone, differences in raw EEG tracings (Fig. 3A) were not readily discernable among groups. Using IQ (Fig. 3B), differences among temperature groups were made more apparent.
Figure 3.
Raw electroencephalography (EEG) (A) and information quantity (IQ) (B) in the representative rats by temperature manipulation after cardiac arrest injury. The effect of temperature manipulation on the EEG signal recovery was not evident with conventional EEG assessment whereas quantitative EEG analysis with IQ showed apparent differences during the recovery phase relative to baseline between temperature groups.
qEEG markers track functional recovery after cardiac arrest
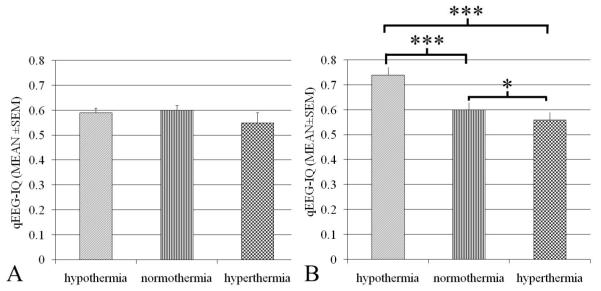
Among four sham rats that did not undergo CA, no significant difference was observed in IQ values during the periods of hypothermia (0.59±0.02, Mean±SEM), normothermia (0.60±0.02), and hyperthermia (0.55±0.04) (p=.932) (Fig. 4A). Unnormalized IQ values showed no significant differences between baseline recordings with halothane anesthesia (0.60±0.01) and the washout period (0.61±0.01) (p=.589), suggesting minimal effects of halothane on IQ.
Figure 4.
Quantitative electroencephalography (EEG) information quantity (IQ) of animals with temperature manipulation in sham control (A) and 7-min cardiac arrest group (B). No significant difference existed in IQ in sham rats between the periods of hypothermia, normothermia and hyperthermia (p= .932). Greater recovery of IQ was found in rats treated with hypothermia compared to normothermia and higher IQ was found in normothermia over hyperthermia rats after return of spontaneous circulation (ROSC) (* p< .05, **p< .01, and *** p< .001).
Aggregate analysis of IQ showed significant differences (p<.001) among the three groups. Greater recovery of IQ was found in rats treated with hypothermia (0.74±0.03) compared to normothermia (0.60±0.03) (p<.001) an in those treated with normothermia over hyperthermia (0.56±0.03) (p=.016) (Fig. 4B).
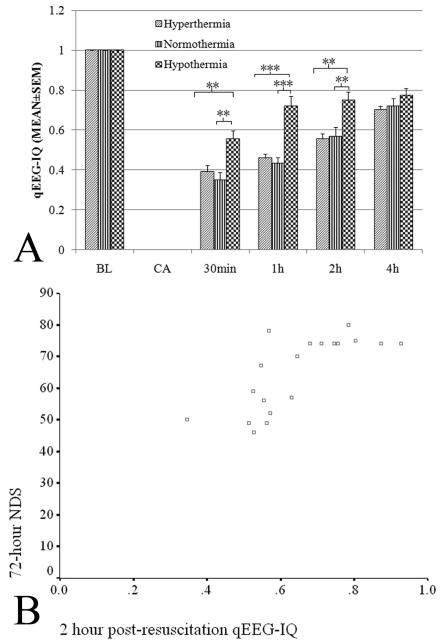
Early qEEG markers tracked functional recovery with temperature manipulation
There was a significant separation of IQ scores between the three groups within the first 2 hours after ROSC (p<.01) (Fig. 5A). Bivariate analyses revealed significant correlations between the 72-hr NDS and IQ values at 30 mins (Pearson correlation, 0.735; p< .01), 1 hr (Pearson correlation, 0.746; p< .01), 2 hours (Pearson correlation, 0.746; p< .01), 4 hours (Pearson correlation, 0.686; p< .01), 24 hours (Pearson correlation, 0.540, p<.05), 48 hours (Pearson correlation, 0.577; p< .01), and 72 hours (Pearson correlation, 0.639; p< .01) post-resuscitation. The IQ value correlated well with 72-hr NDS as early as 30 minutes after ROSC (Fig. 5B).
Figure 5.
Comparison of quantitative electroencephalography (qEEG)-information quantity (IQ) at different intervals by temperature groups (A) and correlation between 2-hr qEEG-IQ and 72-hr Neurological Deficit Score (NDS) (B). (*p< .05, **p< .01, ***p< .001). Aggregate comparisons show that IQ was significantly different among hypothermia (0.74±0.03), normothermia (0.60±0.03), and hyperthermia (0.56±0.03) groups (p< .001). IQ values correlated well with 72-hour NDS at 2 hrs after cardiac arrest (B).
qEEG markers predict survival
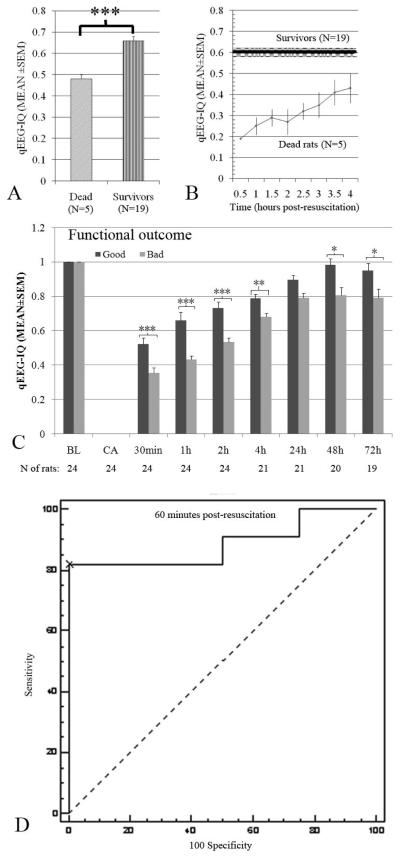
Compared to survivors, rats that died prematurely had lower aggregate IQ values (dead/survivors: 0.48±0.04/0.66±0.02, p<.001) (Fig. 6A). Because a majority (three of five) of rats died within 4 hrs of ROSC, we calculated IQ every 30 minutes starting from 30 minutes post-ROSC, up until 4 hrs. Rats that died prematurely showed significantly lower IQ during each 30-minute interval (0.19±0, 0.25±0.04, 0.29±0.04, 0.27±0.06, 0.32±0.04, 0.35±0.06, 0.41±0.06 and 0.43±0.07 respectively) compared with an average of the first 4 hours (0.60±0.02) for survivors (p<0.05) (Fig. 6B).
Figure 6.
Comparison of quantitative electroencephalography (qEEG)-information quantity (IQ) at different intervals by survival in all animals (A), first 4-hr postresuscitation intervals (B), by functional outcome (C) and 1 hr postresuscitation receiver operating characteristics (ROC) curve demonstrating the IQ value with optimal sensitivity and specificity for good neurological outcomes (D). (*p< .05, **p< .01, ***p< .001). Rats that died within 72 hrs postresuscitation had lower IQ values during the 72-hr experiment than survivors. Rats that died prematurely showed significantly lower IQ during each 30 min interval compared with an average of the first 4 hours (0.60±0.02, black line is MEAN and shadow is SEM in B) for survivors (p< .05). Rats with a bad functional outcome (Neurological Deficit Score (NDS) of <60) had significantly lower qEEG-IQ values in the course of 72 hrs than those with a good functional outcome (NDS of ≥60). A cutpoint of >0.523 yielded 81.8% sensitivity and 100% specificity for good outcomes, with an area under the ROC curve of 0.886.
qEEG markers predict functional outcome
Rats with bad functional outcomes (NDS<60) had significantly lower aggregate IQ values than those with good functional outcomes (NDS≥60) (bad/good: 0.56±0.02/0.73±0.02, p<.001). Significant IQ differences were noted between animals with bad and good outcomes beginning at 30 minutes after ROSC (bad/good: 0.35±0.03/0.52±0.03, p<.001) and at 1 hr (0.43±0.02/0.66±0.05, p<.001), 2 hrs (0.53±0.02/0.73±0.04, p<.001), 4 hrs (0.68±0.02/0.79±0.03, p=.003), 48 hrs (0.81±0.05/0.98±0.05, p=.019), and 72 hrs (0.79±0.07/0.95±0.04, p=.048) (Fig. 6C). Mean IQ scores were significantly correlated with good outcome at 30 mins (mean IQ 0.43, Pearson correlation 0.685, two-sided p<.01), 1 hr (mean IQ 0.54, Pearson correlation 0.809, two-sided p<.01), 2 hrs (mean IQ 0.62, Pearson correlation 0.685, two-sided p<.01) and 4 hrs (mean IQ 0.73, Pearson correlation 0.511, two-sided p<.05).
Receiver operating characteristic curves were constructed to determine IQ cutpoints at various intervals with optimal sensitivity and specificity for good neurological outcome. Using this methodology, accurate cutpoints could be determined with areas under the ROC curve >0.80 at 30-mins, 60-mins, 2 hrs, and 4 hrs after ROSC. The most accurate cutpoint occurred at 60-mins post-ROSC, where an IQ value >0.523 had 81.8% sensitivity and 100% specificity for good outcomes with an area under the receiver operating characteristic curve of 0.864 (Fig. 6D).
Discussion
Our experiment demonstrates that the entropy measure IQ is an early marker of injury and neurologic recovery after asphyxial CA. IQ accurately predicted the effect of temperature on recovery of cortical electrical activity, good/bad functional outcomes and mortality soon after resuscitation.
This study further validated that IQ can accurately predict 72 hr NDS as early as 30 mins post-ROSC. The most significant differences occurred within the first 2 hrs when rats were unresponsive and clinical evaluation is least reliable. An receiver operating characteristic curve was constructed to determine the optimal IQ cutpoint to predict good neurological outcomes at 72 hrs. Good outcomes were predefined as a 72-hr NDS of >60 based on previous experience showing that those animals with scores >60 were capable of functional independence. An IQ value >0.523 at 60 mins from ROSC had excellent sensitivity and specificity for good outcomes. Cutpoints with similar predictive accuracy could be determined for IQ values at 2 and 4 hrs, as well. These data demonstrate that IQ thresholds can be determined as early as 60 mins after ROSC that reliably predict neurological outcomes. If similar cutpoints can be determined in human studies, this technology may greatly enhance the predictive value of early EEG after CA.
EEG is a specific indicator of poor neurological outcome in comatose survivors of CA when characteristic changes occur (19-21, 38). Whereas the manual determination of continuous EEG is laborious, subjective, and requires specialized knowledge, persistence of burst-suppression can be readily monitored with the help of entropy-based EEG analysis. From a neuromonitoring perspective, this study highlights the importance of the immediate post-resuscitation period when brain injury may be most amenable to therapeutic interventions(39). The rapid detection of brain response by qEEG during the first 2 hrs postresuscitation points to the importance of this period in the effort to protect the brain. Given that one of the primary goals is to improve functional outcome, we have to create a paradigm shift to include detection and management of brain injury in the first 2 hrs. As a continuous, non-invasive strategy, qEEG may also allow physicians to monitor the response to potential neuroprotective strategies by translating complicated and subjective waveform analysis into an objective measure. If the same relationship is seen in humans, changes in EEG entropy after CA may be helpful as a real-time monitor of recovery and to tailor therapies such as hypothermia.
With the use of sham animals, we also demonstrated that it is not the temperature itself that alters EEG but the response of the injured brain to hypothermia or hyperthermia as manifested in the qEEG. The temperature manipulation in these experiments was patterned after clinical trials(15, 18) that utilized external cooling and extracerebral temperature monitoring. In addition, previous animal studies have shown that the thermal curves are similar in the brain and peritoneum, independent of the thermal state (40) and most studies have shown that differences in brain and core body temperature are not significant (41). As a translational experiment, we chose this methodology because brain temperature monitoring and invasive cooling are uncommon in clinical practice.
Previous animal studies of induced hyperthermia at 40 °C (5, 6) to 42.0 °C (42, 43) led to poor outcomes, and human studies showed adverse outcomes with temperatures between 38.3 and 39 °C (8, 9, 44). In this study, we chose the target temperature of mild hyperthermia as 39°C to simulate clinical hyperthermia. Our results did not show a significant difference in early IQ and NDS between the hyperthermia and normothermia groups likely, due to a low target temperature range (38.5-39.5°C). The lack of difference between these groups, however, was consistent in IQ and NDS, which lends reassurance that the early IQ values are an accurate predictor of recovery.
Absence of histopathological data is a potential limitation of this study. While we have previously demonstrated that histopathological markers for ischemic cell death correlate with qEEG and NDS measures (26, 37, 45), we also acknowledge that postmortem histological markers in rats have been a poor indicator of clinical significance in human trials of the same agents and have been less predictive than early behavioral assessments (46-48). As such, we put more emphasis on functional outcome and realtime biological measures of recovery (qEEG) as a means to clinical translation.
In this EEG analysis, we used an IQ measure that relies on relative entropy compared to baseline. Although we recognize this as a limitation, we anticipate that similar results will be produced using standardized normal controls as a baseline. We acknowledge that the use of sedatives in clinical practice may affect EEG in ways that were not addressed in this experiment (49, 50), however, sedatives have shown equivocal effects on EEG in other studies (51-53). Our laboratory results have also shown that halothane did not have a significant effect on IQ using this particular animal model (25, 35). Since the predictive effect of IQ is most robust in the first few hours and sedation is rarely required during this period due to coma, we are optimistic that sedatives will have minimal effect on the translation of this technology. To translate these findings to the clinical arena, the impact of EEG artifacts brought about by the clinical environment and patient movement must also be taken into consideration. We anticipate that movement artifact will be minimized with induced hypothermia because pharmacological paralysis is typically employed to eliminate shivering. Translation of this technology to the clinical environment, however, must occur in parallel with development of innovative signal processing algorithms for artifact rejection without compromising important EEG information.
Conclusions
Early IQ was sensitive to the benefit of hypothermia and to the harmful effect of hyperthermia postresuscitation and predicted neurologic recovery at 72 hrs. This qEEG method was able to monitor brain recovery and predict functional outcome and mortality. The predictive value was particularly robust during the first 2 hours after CA. With clinical translation, these experiments have the potential to produce a major shift in how brain recovery is monitored.
Acknowledgements
This research was supported by NIH Grants R01 HL 071568 and R21 NS054146.
All work was performed at the Johns Hopkins University School of Medicine.
Financial support used for the study: NIH Grants R01 HL071568 and R21 NS054146 The authors request no reprints.
Footnotes
Portions of this work were presented in the Annual Meeting of the American Heart Association, Orlando, FL, November, 2007 and Society of Critical Care medicine’s 37th Critical Care Congress, Honolulu, HI, February, 2008..
Disclosures
None.
References
- 1.Rosamond W, Flegal K, Friday G, et al. Heart Disease and Stroke Statistics-2007 Update: A Report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 2.Safar P. Cerebral resuscitation after cardiac arrest: a review. Circulation. 1986;74:IV138–153. [PubMed] [Google Scholar]
- 3.Bedell SE, Delbanco TL, Cook EF, et al. Survival after cardiopulmonary resuscitation in the hospital. N Engl J Med. 1983;309:569–576. doi: 10.1056/NEJM198309083091001. [DOI] [PubMed] [Google Scholar]
- 4.Vaagenes P, Ginsberg M, Ebmeyer U, et al. Cerebral resuscitation from cardiac arrest: pathophysiologic mechanisms. Crit Care Med. 1996;24:S57–68. [PubMed] [Google Scholar]
- 5.Kim Y, Busto R, Dietrich WD, et al. Delayed postischemic hyperthermia in awake rats worsens the histopathological outcome of transient focal cerebral ischemia. Stroke; a journal of cerebral circulation. 1996;27:2274–2280. doi: 10.1161/01.str.27.12.2274. discussion 2281. [DOI] [PubMed] [Google Scholar]
- 6.Hickey RW, Kochanek PM, Ferimer H, et al. Induced hyperthermia exacerbates neurologic neuronal histologic damage after asphyxial cardiac arrest in rats. Critical care medicine. 2003;31:531–535. doi: 10.1097/01.CCM.0000050323.84293.11. [DOI] [PubMed] [Google Scholar]
- 7.Takino M, Okada Y. Hyperthermia following cardiopulmonary resuscitation. Intensive care medicine. 1991;17:419–420. doi: 10.1007/BF01720680. [DOI] [PubMed] [Google Scholar]
- 8.Zeiner A, Holzer M, Sterz F, et al. Hyperthermia after cardiac arrest is associated with an unfavorable neurologic outcome. Arch Intern Med. 2001;161:2007–2012. doi: 10.1001/archinte.161.16.2007. [DOI] [PubMed] [Google Scholar]
- 9.Takasu A, Saitoh D, Kaneko N, et al. Hyperthermia: is it an ominous sign after cardiac arrest? Resuscitation. 2001;49:273–277. doi: 10.1016/s0300-9572(00)00360-9. [DOI] [PubMed] [Google Scholar]
- 10.Nolan JP, Morley PT, Hoek TL, et al. Therapeutic hypothermia after cardiac arrest. An advisory statement by the Advancement Life support Task Force of the International Liaison committee on Resuscitation. Resuscitation. 2003;57:231–235. doi: 10.1016/s0300-9572(03)00184-9. [DOI] [PubMed] [Google Scholar]
- 11.2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2005;112:IV1–203. doi: 10.1161/CIRCULATIONAHA.105.166550. [DOI] [PubMed] [Google Scholar]
- 12.Xiao F, Safar P, Radovsky A. Mild protective and resuscitative hypothermia for asphyxial cardiac arrest in rats. Am J Emerg Med. 1998;16:17–25. doi: 10.1016/s0735-6757(98)90059-6. [DOI] [PubMed] [Google Scholar]
- 13.Kuboyama K, Safar P, Radovsky A, et al. Delay in cooling negates the beneficial effect of mild resuscitative cerebral hypothermia after cardiac arrest in dogs: a prospective, randomized study. Crit Care Med. 1993;21:1348–1358. doi: 10.1097/00003246-199309000-00019. [DOI] [PubMed] [Google Scholar]
- 14.Ao H, Tanimoto H, Yoshitake A, et al. Long-term mild hypothermia with extracorporeal lung and heart assist improves survival from prolonged cardiac arrest in dogs. Resuscitation. 2001;48:163–174. doi: 10.1016/s0300-9572(00)00252-5. [DOI] [PubMed] [Google Scholar]
- 15.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 16.Bernard SA, Jones BM, Horne MK. Clinical trial of induced hypothermia in comatose survivors of out-of-hospital cardiac arrest. Ann Emerg Med. 1997;30:146–153. doi: 10.1016/s0196-0644(97)70133-1. [DOI] [PubMed] [Google Scholar]
- 17.Zeiner A, Holzer M, Sterz F, et al. Hypothermia After Cardiac Arrest (HACA) Study Group Mild resuscitative hypothermia to improve neurological outcome after cardiac arrest. A clinical feasibility trial. Stroke. 2000;31:86–94. doi: 10.1161/01.str.31.1.86. [DOI] [PubMed] [Google Scholar]
- 18.Hypothermia After Cardiac Arrest (HACA) Study Group Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 19.Jorgensen EO, Malchow-Moller A. Natural history of global and critical brain ischaemia. Part II: EEG and neurological signs in patients remaining unconscious after cardiopulmonary resuscitation. Resuscitation. 1981;9:155–174. doi: 10.1016/0300-9572(81)90024-1. [DOI] [PubMed] [Google Scholar]
- 20.Moller M, Holm B, Sindrup E, et al. Electroencephalographic prediction of anoxic brain damage after resuscitation from cardiac arrest in patients with acute myocardial infarction. Acta Med Scand. 1978;203:31–37. doi: 10.1111/j.0954-6820.1978.tb14827.x. [DOI] [PubMed] [Google Scholar]
- 21.Thomassen A, Sorensen K, Wernberg M. The prognostic value of EEG in coma survivors after cardiac arrest. Acta Anaesthesiol Scand. 1978;22:483–490. doi: 10.1111/j.1399-6576.1978.tb01327.x. [DOI] [PubMed] [Google Scholar]
- 22.Geocadin RG, Buitrago MM, Torbey MT, et al. Neurologic prognosis and withdrawal of life support after resuscitation from cardiac arrest. Neurology. 2006;67:105–108. doi: 10.1212/01.wnl.0000223335.86166.b4. [DOI] [PubMed] [Google Scholar]
- 23.Jia X, Koenig MA, Shin HC, et al. Quantitative EEG and neurological recovery with therapeutic hypothermia after asphyxial cardiac arrest in rats. Brain Res. 2006;1111:166–175. doi: 10.1016/j.brainres.2006.04.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cerchiari EL, Sclabassi RJ, Safar P, et al. Effects of combined superoxide dismutase and deferoxamine on recovery of brainstem auditory evoked potentials and EEG after asphyxial cardiac arrest in dogs. Resuscitation. 1990;19:25–40. doi: 10.1016/0300-9572(90)90096-w. [DOI] [PubMed] [Google Scholar]
- 25.Geocadin RG, Ghodadra R, Kimura T, et al. A novel quantitative EEG injury measure of global cerebral ischemia. Clin Neurophysiol. 2000;111:1779–1787. doi: 10.1016/s1388-2457(00)00379-5. [DOI] [PubMed] [Google Scholar]
- 26.Geocadin RG, Muthuswamy J, Sherman DL, et al. Early electrophysiological and histologic changes after global cerebral ischemia in rats. Mov Disord. 2000;15(Suppl 1):14–21. doi: 10.1002/mds.870150704. [DOI] [PubMed] [Google Scholar]
- 27.Katz L, Ebmeyer U, Safar P, et al. Outcome model of asphyxial cardiac arrest in rats. J Cereb Blood Flow Metab. 1995;15:1032–1039. doi: 10.1038/jcbfm.1995.129. [DOI] [PubMed] [Google Scholar]
- 28.Vaagenes P, Cantadore R, Safar P, et al. Amelioration of brain damage by lidoflazine after prolonged ventricular fibrillation cardiac arrest in dogs. Crit Care Med. 1984;12:846–855. doi: 10.1097/00003246-198410000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Sherman DL, Brambrink AM, Ichord RN, et al. Quantitative EEG during early recovery from hypoxic-ischemic injury in immature piglets: burst occurrence and duration. Clin Electroencephalogr. 1999;30:175–183. doi: 10.1177/155005949903000410. [DOI] [PubMed] [Google Scholar]
- 30.Shin HC, Tong S, Yamashita S, et al. Quantitative EEG and effect of hypothermia on brain recovery after cardiac arrest. IEEE Trans Biomed Eng. 2006;53:1016–1023. doi: 10.1109/TBME.2006.873394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geocadin RG, Sherman DL, Christian Hansen H, et al. Neurological recovery by EEG bursting after resuscitation from cardiac arrest in rats. Resuscitation. 2002;55:193–200. doi: 10.1016/s0300-9572(02)00196-x. [DOI] [PubMed] [Google Scholar]
- 32.Thakor NV, Tong S. Advances in quantitative electroencephalogram analysis methods. Annu Rev Biomed Eng. 2004;6:453–495. doi: 10.1146/annurev.bioeng.5.040202.121601. [DOI] [PubMed] [Google Scholar]
- 33.Jia X, Koenig MA, Shin HC, et al. Detection and Monitoring of Brain Recovery after Therapeutic Hypothermia in a Post-cardiac Arrest Rodent Model: A Quantitative EEG Study. Circulation Research. 2006:99 E45P156. [Google Scholar]
- 34.Jia X, Koenig MA, Shin H-C, et al. Earlier initiation of therapeutic hypothermia increases neurological recovery after asphyxial cardiac arrest in rats; The 4th Annual Meeting of Neurocritical Care Society: Nov 4-5 2006; Baltimore, MD, USA. 2006. [Google Scholar]
- 35.Jia X, Koenig MA, Shin HC, et al. Improving neurologic outcomes post-cardiac arrest in a rat model: immediate hypothermia and quantitative EEG monitoring. Resuscitation. 2008;76:431–442. doi: 10.1016/j.resuscitation.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jia X, Koenig MA, Venkatraman A, et al. Post-cardiac arrest temperature manipulation alters early EEG bursting in rats. Resuscitation. 2008 doi: 10.1016/j.resuscitation.2008.04.011. doi:10.1016/j.resuscitation.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luft AR, Buitrago MM, Paul JS, et al. Early restitution of electrocorticogram predicts subsequent behavioral recovery from cardiac arrest. J Clin Neurophysiol. 2002;19:540–546. doi: 10.1097/00004691-200212000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Wijdicks EF, Hijdra A, Young GB, et al. Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;67:203–210. doi: 10.1212/01.wnl.0000227183.21314.cd. [DOI] [PubMed] [Google Scholar]
- 39.Higashida RT, Furlan AJ, Roberts H, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003;34:e109–137. doi: 10.1161/01.STR.0000082721.62796.09. [DOI] [PubMed] [Google Scholar]
- 40.Sundgren-Andersson AK, Ostlund P, Bartfai T. Simultaneous measurement of brain and core temperature in the rat during fever, hyperthermia, hypothermia and sleep. Neuroimmunomodulation. 1998;5:241–247. doi: 10.1159/000026344. [DOI] [PubMed] [Google Scholar]
- 41.McIlvoy L. Comparison of brain temperature to core temperature: a review of the literature. J Neurosci Nurs. 2004;36:23–31. doi: 10.1097/01376517-200402000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Vogt S, Troitzsch D, Abdul-Khaliq H, et al. Heat stress attenuates ATP-depletion and pH-decrease during cardioplegic arrest. J Surg Res. 2007;139:176–181. doi: 10.1016/j.jss.2006.07.041. [DOI] [PubMed] [Google Scholar]
- 43.Eshel G, Safar P, Sassano J, et al. Hyperthermia-induced cardiac arrest in dogs and monkeys. Resuscitation. 1990;20:129–143. doi: 10.1016/0300-9572(90)90048-j. [DOI] [PubMed] [Google Scholar]
- 44.Hickey RW, Kochanek PM, Ferimer H, et al. Hypothermia and hyperthermia in children after resuscitation from cardiac arrest. Pediatrics. 2000;106:118–122. doi: 10.1542/peds.106.1.118. [DOI] [PubMed] [Google Scholar]
- 45.Geocadin RG, Malhotra AD, Tong S, et al. Effect of acute hypoxic preconditioning on qEEG and functional recovery after cardiac arrest in rats. Brain Res. 2005;1064:146–154. doi: 10.1016/j.brainres.2005.04.046. [DOI] [PubMed] [Google Scholar]
- 46.Gladstone DJ, Black SE, Hakim AM. Toward wisdom from failure: lessons from neuroprotective stroke trials and new therapeutic directions. Stroke. 2002;33:2123–2136. doi: 10.1161/01.str.0000025518.34157.51. [DOI] [PubMed] [Google Scholar]
- 47.Corbett D, Nurse S. The problem of assessing effective neuroprotection in experimental cerebral ischemia. Prog Neurobiol. 1998;54:531–548. doi: 10.1016/s0301-0082(97)00078-6. [DOI] [PubMed] [Google Scholar]
- 48.Hunter AJ, Mackay KB, Rogers DC. To what extent have functional studies of ischaemia in animals been useful in the assessment of potential neuroprotective agents? Trends Pharmacol Sci. 1998;19:59–66. doi: 10.1016/s0165-6147(97)01157-7. [DOI] [PubMed] [Google Scholar]
- 49.Ypparila H, Korhonen I, Westeren-Punnonen S, et al. Assessment of postoperative sedation level with spectral EEG parameters. Clin Neurophysiol. 2002;113:1633–1639. doi: 10.1016/s1388-2457(02)00217-1. [DOI] [PubMed] [Google Scholar]
- 50.Griffiths MJ, Preece AW, Green JL. Monitoring sedation levels by EEG spectral analysis. Anesth Prog. 1991;38:227–231. [PMC free article] [PubMed] [Google Scholar]
- 51.Quante M, Scharein E, Zimmermann R, et al. Dissociation of morphine analgesia and sedation evaluated by EEG measures in healthy volunteers. Arzneimittelforschung. 2004;54:143–151. doi: 10.1055/s-0031-1296951. [DOI] [PubMed] [Google Scholar]
- 52.Mehta UC, Patel I, Castello FV. EEG sedation for children with autism. J Dev Behav Pediatr. 2004;25:102–104. doi: 10.1097/00004703-200404000-00005. [DOI] [PubMed] [Google Scholar]
- 53.Spencer EM, Green JL, Willatts SM. Continuous monitoring of depth of sedation by EEG spectral analysis in patients requiring mechanical ventilation. Br J Anaesth. 1994;73:649–654. doi: 10.1093/bja/73.5.649. [DOI] [PubMed] [Google Scholar]