Abstract
Alzheimer disease (AD) is the most common cause of age-related dementia, affecting more than 25 million people worldwide. The accumulation of insoluble β-amyloid (Aβ) plaques in the brain has long been considered central to the pathogenesis of AD. However, recent evidence suggests that soluble oligomeric assemblies of Aβ may be of greater importance. β-Amyloid oligomers have been found to be potent synaptotoxins, but the mechanism by which they exert their action has remained elusive. Herein, we review the recently published finding that cellular prion protein (PrPc) is a high-affinity receptor for Aβ oligomers, mediating their toxic effects on synaptic plasticity. We further discuss the relationship between AD and PrPc and the potential clinical implications. Cellular prion protein may provide a novel target for therapeutic intervention in AD.
Alzheimer disease (AD) is a debilitating neurodegenerative disorder, estimated to affect at least 26.6 million people worldwide.1 Over the next 40 years, this number is predicted to increase 4-fold,1 stressing the need for more effective therapeutics. For many years, the leading hypothesis for the pathogenesis of AD has involved the toxic effects of a gradual buildup of β-amyloid (Aβ) plaques in the brain.2 β-Amyloid is produced after sequential processing of the amyloid precursor protein (APP),2 and increased levels of the Aβ1-42 species are thought to be a key early event in AD.3 A major criticism of the amyloid hypothesis has been that the burden of fibrillar Aβ, the insoluble species constituting plaques, correlates poorly with the degree of dementia4 and brain atrophy,5 and cognitively normal individuals may have significant plaque deposition at autopsy.6 However, more recent findings have elucidated different assemblies of Aβ with varying degrees of toxic effects. β-Amyloid can exist as soluble monomers and oligomers, intermediate protofibrils, and insoluble fibrillar aggregates.7 Of these, soluble Aβ oligomers are potent synaptotoxins,8 and their concentration correlates with disease severity.9
Oligomers are small peptide polymers, and various toxic Aβ oligomers have been reported, ranging from dimers and trimers10 to 100mers.11 Nanomolar concentrations of Aβ oligomers strongly inhibit long-term potentiation (LTP),12,13 a leading experimental model for the synaptic changes underlying learning and memory. Recent evidence also suggests that synapse degeneration, likely central to the memory impairment in AD,14 first involves the single synaptic contact points between axons and dendrites found at dendritic spines.15 Consistent with their role in neurodegeneration, Aβ oligomers cause significant decrease in dendritic spine density in cultured hippocampal neurons.15 Behavioral assessments in rodents have confirmed these findings, complementing a growing number of in vitro investigations. β-Amyloid oligomers greatly impair spatial memory when administered to otherwise healthy rats.12,16
The concept of oligomeric Aβ synaptotoxins has helped strengthen the amyloid hypothesis. In transgenic mice, levels of specific Aβ oligomers closely match deficits in spatial memory,16 and clinically the amount of soluble Aβ in the brain correlates well with the degree of dementia.9 Moreover, Aβ oligomers isolated directly from human AD brains have been found to be equally toxic as synthetically derived Aβ.17 Despite these recent breakthroughs, the mechanisms by which Aβ oligomers exert their action have not been well understood. The effects on neurons are rapid and specific, and together the accumulated data have suggested the presence of a high-affinity Aβ oligomer receptor. This putative receptor would likely be central to the pathogenesis of AD and provide an attractive target for therapeutic intervention. Herein, we review the recent finding that cellular prion protein (PrPc) is a high-affinity binding site for Aβ oligomers, mediating their potent inhibitory effects on synaptic plasticity.11 We further discuss the association between PrPc and AD and the potential clinical relevance of these findings.
EXPRESSION CLONING IDENTIFIES PrPc AS A HIGH-AFFINITY BINDING SITE FOR Aβ OLIGOMERS
To detect Aβ oligomer binding sites, a biotin-tagged Aβ1-42 peptide was first synthesized.11 Biotin is frequently used as a molecular marker because of its extraordinarily high affinity for the protein avidin that, in turn, can be used for detection. The Aβ was oligomerized and applied to COS-7 cells expressing different complementary DNA (cDNA) obtained from an adult mouse brain library. At baseline, COS-7 cells show minimal binding of Aβ oligomers compared with hippocampal neurons, making these cells ideal for this type of screen. Biotin-Aβ oligomers potently bind hippocampal neurons, whereas monomeric Aβ does not, suggesting a necessary conformation of Aβ conferred by oligomerization.
Of 225 000 clones from the adult mouse cDNA library screened in the assay, 2 were found to bind biotin-Aβ oligomers. Both of these individual clones were subsequently shown to encode for full-length mouse PrPc. In addition to this unbiased genome-wide screen, a more specific library of known transmembrane proteins at lower stringency was assessed. As summarized in the Table, some were found to exhibit Aβ oligomer binding but with lesser affinity and selectivity than for PrPc. Several putative receptors for Aβ have previously been reported, including the receptor for advanced glycation end products,18 α7 nicotinic acetylcholine receptor,19 and tumor necrosis factor receptor 1.20 In the binding assay reviewed herein, only PrPc had high affinity and high specificity for Aβ oligomers.
Table.
β-Amyloid (Aβ) Oligomer Protein Binding
| Cell Surface Protein | Aβ Oligomer Binding | Oligomer Specificitya |
|---|---|---|
| PrPc | ++++ | ++++ |
| APLP1 | ++ | ++ |
| TMEM30A | ++ | + |
| TMEM30B | ++ | + |
| RAGE | ++ | ? |
| APP | 0 | 0 |
| Doppel | 0 | 0 |
| SPRN | 0 | 0 |
| α7 Nicotinic acetylcholine receptor | 0 | 0 |
| APLP2 | 0 | 0 |
Abbreviations: APLP, amyloid precursor–like protein; APP, amyloid precursor protein; PrPc, cellular prion protein; RAGE, receptor for advanced glycation end products; SPRN, shadow of prion protein; TMEM, transmembrane protein; +, slight; ++, low; ++++, high; ?, unknown.
Refers to the preferential binding of oligomeric Aβ over freshly prepared Aβ.
Aβ OLIGOMERS BIND TO A SPECIFIC REGION OF PrPc
Cellular prion protein is a glycosylphosphatidylinositol-linked extracellular membrane protein of approximately 250 amino acids that is abundantly expressed in the nervous system.21 It is likely best known to clinicians because of its role in human prion disease. Using PrPc deletion mutants, the exact binding site of Aβ oligomers was mapped to the region of amino acids 95 to 110. This area is within the unstructured central domain of PrPc (amino acids 95-134), which has been implicated in neuronal toxic effects in vitro22 and in extensive neurodegeneration in mice.23 α-Secretase, which cleaves APP within the Aβ domain (precluding the toxic Aβ peptide), also cleaves PrPc within its unstructured central domain at amino acids 110 to 111.24 Therefore, α-secretase may act to counter neurodegeneration by preventing the production of Aβ and by keeping potentially detrimental regions of PrPc in check. It is unknown whether cleavage of PrPc by α-secretase also affects Aβ oligomer binding.
PrPc MEDIATES INHIBITION OF LTP BY Aβ OLIGOMERS
To evaluate the functional role of the Aβ oligomer–×-PrPc interaction, the effects of Aβ oligomers on LTP were measured in brain sections from mice in which the PrPc gene was knocked out (PRNP–/–) (OMIM 176640). In wild-type hippocampal sections, a brief train of pulses in the theta frequency to Schaffer collateral fibers results in an increase in the excitatory postsynaptic potentials that can last for hours. This change in synaptic strength in response to a specific stimulus is believed to be a fundamental mechanism underlying learning and memory. When nanomolar concentrations of Aβ oligomers were applied to wild-type sections, LTP was strongly inhibited, as has been reported by numerous laboratories.10,13,17,20 In contrast, no inhibitory effects on LTP were seen when the same Aβ preparation was applied to brain sections from PRNP–/– animals, suggesting that Aβ oligomer signaling is mediated through PrPc.11 To confirm these findings, wild-type brain sections were pretreated with 6D11, a specific antibody to PrPc found to block Aβ oligomer binding. When these pretreated sections were exposed to the Aβ solution, LTP was unaffected, further suggesting a critical role of PrPc in Aβ oligomer–induced synaptotoxic effects.
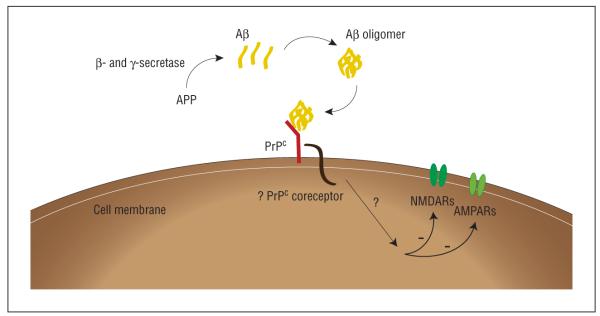
Because PrPc is an extracellular protein, downstream Aβ oligomer signaling is likely mediated via a putative PrPc-associated transmembrane coreceptor (Figure). This putative coreceptor may have a critical role in AD-related neurodegeneration and, once identified, could provide an additional potential therapeutic target.
Figure.
β-Amyloid (Aβ) oligomer signaling. The amyloid precursor protein (APP) is cleaved by β-secretase and γ-secretase to produce monomeric Aβ peptides that, in turn, assemble into toxic Aβ oligomers. β-Amyloid oligomers bind to cellular prion protein (PrPc), which likely signals via a putative transmembrane PrPc coreceptor. This binding mediates the inhibition of synaptic plasticity as measured by long-term potentiation and could be involved in other deleterious effects of Aβ oligomers, including synapse degeneration, rodent spatial memory impairments, and the neurodegeneration seen in human Alzheimer disease. Long-term potentiation may be suppressed by altering neurotransmission through N-methyl-D-aspartate receptors (NMDARs) and α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) receptors (AMPARs), as their distribution has been shown to be regulated by Aβ oligomers.
PRION PROTEIN AND AD
For at least a decade, an indirect association between AD and PrPc has been lingering in the literature. Early interest was fueled mostly by the fact that AD and human prion disease involve aggregates of pathologic proteins in the brain25 and that the amyloid precursor–like protein was reported to bind PrPc.26 Recently, more specific evidence has emerged linking PrPc to AD. From a genetic perspective, interest has been growing in the PRNP methionine/valine polymorphism (M129V) and its relationship to sporadic AD. Homozygosity at this codon, which is located within the unstructured central domain of PrPc, is associated with an increased risk of sporadic Creutzfeldt-Jakob disease.27 Others have found that this genotype confers an increased risk for sporadic AD in certain populations.28 Whether polymorphisms within the PRNP gene may affect Aβ oligomer binding to PrPc and alter susceptibility to AD is unknown, but it remains an intriguing possibility.
Cellular prion protein has also been reported to regulate APP processing by modulating β-secretase (BACE1) activity, but the results have been conflicting. Cells coexpressing APP and PrPc have a significant reduction in secreted Aβ compared with cells expressing APP alone.29 The region of PrPc responsible for this action was found to be in the N-terminus (amino acids 23-26), as no effects on BACE1 or Aβ levels were seen when this segment was removed.29 However, overexpressing PrPc in transgenic APP mice caused a modest increase of Aβ plaques in the brain,30 in contrast to the lowering effect of PrPc found in vitro. It is unclear whether binding of Aβ oligomers to PrPc might affect its proposed ability to regulate BACE1, setting up a potential feedback loop in response to Aβ levels, and this is an issue worth investigating further as the field moves forward.
CONCLUSIONS
In summary, we have reviewed the recent finding that PrPc functions as a high-affinity receptor to mediate the deleterious effects of Aβ oligomers on synaptic plasticity. Although much work remains to assess whether targeting PrPc or its putative coreceptor can be effective treatments in human AD, the potential is readily apparent. Cellular prior protein represents the first possible target in AD directly disrupting the signaling pathway of Aβ and might provide a more functional approach to relieving AD symptoms. Most important, modulating PrPc function does not seem to produce serious adverse effects, as PRNP knockout mice have minimal phenotypic changes and wild-type animals treated with PrPc antibodies do well. A large body of work on PrPc already exists because of its role in prion diseases. This knowledge could greatly facilitate drug discovery and our understanding of the downstream mechanisms of the Aβ oligomer–×-PrPc interaction.
Acknowledgments
Funding/Support: This study was supported by grants from the Cure Alzheimer’s Fund, National Institutes of Health, and Falk Medical Research Trust (Dr Strittmatter).
Footnotes
Financial Disclosure: None reported.
REFERENCES
- 1.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement. 2007;3(3):186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 2.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [published correction appears in Science. 2002;297(5590):2209] [DOI] [PubMed] [Google Scholar]
- 3.Younkin SG. The role of Aβ42 in Alzheimer’s disease. J Physiol Paris. 1998;92(34):289–292. doi: 10.1016/s0928-4257(98)80035-1. [DOI] [PubMed] [Google Scholar]
- 4.Terry RD, Masliah E, Salmon DP, et al. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30(4):572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 5.Josephs KA, Whitwell JL, Ahmed Z, et al. β-Amyloid burden is not associated with rates of brain atrophy. Ann Neurol. 2008;63(2):204–212. doi: 10.1002/ana.21223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katzman R, Terry R, DeTeresa R, et al. Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol. 1988;23(2):138–144. doi: 10.1002/ana.410230206. [DOI] [PubMed] [Google Scholar]
- 7.Klein WL. Aβ toxicity in Alzheimer’s disease: globular oligomers (ADDLs) as new vaccine and drug targets. Neurochem Int. 2002;41(5):345–352. doi: 10.1016/s0197-0186(02)00050-5. [DOI] [PubMed] [Google Scholar]
- 8.Lambert MP, Barlow AK, Chromy BA, et al. Diffusible, nonfibrillar ligands derived from Aβ1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A. 1998;95(11):6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLean CA, Cherny RA, Fraser FW, et al. Soluble pool of Aβ amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann Neurol. 1999;46(6):860–866. doi: 10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 10.Townsend M, Shankar GM, Mehta T, Walsh DM, Selkoe DJ. Effects of secreted oligomers of amyloid β-protein on hippocampal synaptic plasticity: a potent role for trimers. J Physiol. 2006;572(pt 2):477–492. doi: 10.1113/jphysiol.2005.103754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laurén J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-β oligomers. Nature. 2009;457(7233):1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walsh DM, Klyubin I, Fadeeva JV, et al. Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416(6880):535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 13.Wang HW, Pasternak JF, Kuo H, et al. Soluble oligomers of β amyloid (1-42) inhibit long-term potentiation but not long-term depression in rat dentate gyrus. Brain Res. 2002;924(2):133–140. doi: 10.1016/s0006-8993(01)03058-x. [DOI] [PubMed] [Google Scholar]
- 14.Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298(5594):789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 15.Lacor PN, Buniel MC, Furlow PW, et al. Aβ oligomer–induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J Neurosci. 2007;27(4):796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lesné S, Koh MT, Kotilinek L, et al. A specific amyloid-β protein assembly in the brain impairs memory. Nature. 2006;440(7082):352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- 17.Shankar GM, Li S, Mehta TH, et al. Amyloid-β protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14(8):837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan SD, Chen X, Fu J, et al. RAGE and amyloid-β peptide neurotoxicity in Alzheimer’s disease. Nature. 1996;382(6593):685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 19.Wang HY, Lee DH, D’Andrea MR, Peterson PA, Shank RP, Reitz AB. β-Amyloid1-42 binds to α7 nicotinic acetylcholine receptor with high affinity: implications for Alzheimer’s disease pathology. JBiol Chem. 2000;275(8):5626–5632. doi: 10.1074/jbc.275.8.5626. [DOI] [PubMed] [Google Scholar]
- 20.Wang Q, Wu J, Rowan MJ, Anwyl R. β-amyloid inhibition of long-term potentiation is mediated via tumor necrosis factor. Eur J Neurosci. 2005;22(11):2827–2832. doi: 10.1111/j.1460-9568.2005.04457.x. [DOI] [PubMed] [Google Scholar]
- 21.Aguzzi A, Baumann F, Bremer J. The prion’s elusive reason for being. Annu Rev Neurosci. 2008;31:439–477. doi: 10.1146/annurev.neuro.31.060407.125620. [DOI] [PubMed] [Google Scholar]
- 22.Forloni G, Angeretti N, Chiesa R, et al. Neurotoxicity of a prion protein fragment. Nature. 1993;362(6420):543–546. doi: 10.1038/362543a0. [DOI] [PubMed] [Google Scholar]
- 23.Baumann F, Tolnay M, Brabeck C, et al. Lethal recessive myelin toxicity of prion protein lacking its central domain. EMBO J. 2007;26(2):538–547. doi: 10.1038/sj.emboj.7601510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vincent B, Cisse MA, Sunyach C, Guillot-Sestier MV, Checler F. Regulation of βAPP and PrPc cleavage by α-secretase: mechanistic and therapeutic perspectives. Curr Alzheimer Res. 2008;5(2):202–211. doi: 10.2174/156720508783954749. [DOI] [PubMed] [Google Scholar]
- 25.Aguzzi A, Haass C. Games played by rogue proteins in prion disorders and Alzheimer’s disease. Science. 2003;302(5646):814–818. doi: 10.1126/science.1087348. [DOI] [PubMed] [Google Scholar]
- 26.Yehiely F, Bamborough P, Da Costa M, et al. Identification of candidate proteins binding to prion protein. Neurobiol Dis. 1997;3(4):339–355. doi: 10.1006/nbdi.1997.0130. [published correction appears in Neurobiol Dis. 2002;10(1):67-68] [DOI] [PubMed] [Google Scholar]
- 27.Palmer MS, Dryden AJ, Hughes JT, Collinge J. Homozygous prion protein genotype predisposes to sporadic Creutzfeldt-Jakob disease. Nature. 1991;352(6333):340–342. doi: 10.1038/352340a0. [published correction appears in Nature. 1991;352(6335):547] [DOI] [PubMed] [Google Scholar]
- 28.Del Bo R, Scarlato M, Ghezzi S, et al. Is M129V of PRNP gene associated with Alzheimer’s disease? a case-control study and a meta-analysis. Neurobiol Aging. 2006;27(5):770.e1–770.e5. doi: 10.1016/j.neurobiolaging.2005.05.025. http://www.journals.elsevierhealth.com/periodicals/nba/article/PIIS0187458005001612/abstract. Accessed May 1, 2009. [DOI] [PubMed] [Google Scholar]
- 29.Parkin ET, Watt NT, Hussain I, et al. Cellular prion protein regulates β-secretase cleavage of the Alzheimer’s amyloid precursor protein. Proc Natl Acad Sci U S A. 2007;104(26):11062–11067. doi: 10.1073/pnas.0609621104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwarze-Eicker K, Keyvani K, Görtz N, Westaway D, Sachser N, Paulus W. Prion protein (PrPc) promotes β-amyloid plaque formation. Neurobiol Aging. 2005;26(8):1177–1182. doi: 10.1016/j.neurobiolaging.2004.10.004. [DOI] [PubMed] [Google Scholar]